Abstract
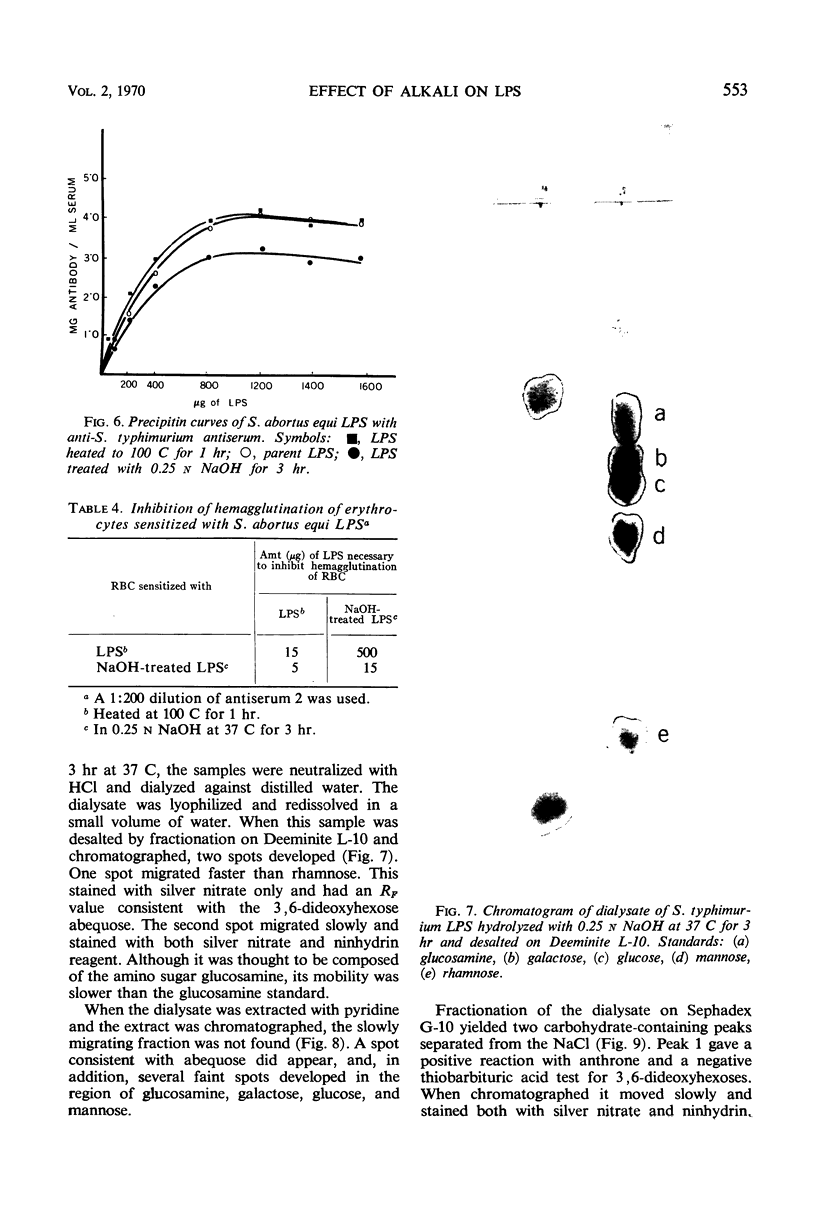
The effect of mild alkaline hydrolysis on the immunological reactivity of Salmonella typhimurium lipopolysaccharide (LPS) was studied. Hydrolysis of LPS at 37 C in 0.01 to 0.25 n NaOH caused a decrease in precipitation of LPS by antiserum. Red cells sensitized with alkali-treated LPS were less responsive to hemagglutination by antiserum than were cells sensitized with heated LPS. Hemagglutination-inhibition studies showed that alkali-treated S. typhimurium LPS was antigenically deficient. Alkaline hydrolysis of S. typhimurium LPS destroys antigen 5 of the Kauffmann-White scheme by cleavage of O-acetyl groups. However, abequose, the immunodominant sugar for antigen 4, is also cleaved from the O side chains by alkali, suggesting the loss of more than one antigenic determinant. It is concluded that alkali treatment of LPS to promote the sensitization of red cells may alter the serological specificity and therefore places limitations on its use in serological studies.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson E. H. Growth Requirements of Virus-Resistant Mutants of Escherichia Coli Strain "B". Proc Natl Acad Sci U S A. 1946 May;32(5):120–128. doi: 10.1073/pnas.32.5.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagdian G., Lüderitz O., Staub A. M. Immunochemical studies on Salmonella. XI. Chemical modification correlated with conversion of group B Salmonella by bacteriophage 27. Ann N Y Acad Sci. 1966 Jun 30;133(2):405–424. doi: 10.1111/j.1749-6632.1966.tb52380.x. [DOI] [PubMed] [Google Scholar]
- CYNKIN M. A., ASHWELL G. Estimation of 3-deoxy sugars by means of the malonaldehyde-thiobarbituric acid reaction. Nature. 1960 Apr 9;186:155–156. doi: 10.1038/186155a0. [DOI] [PubMed] [Google Scholar]
- GORZYNSKI E. A., LUDERITZ O., NETER E., WESTPHAL O. The bacterial hemagglutination test for the demonstration of antibodies to Enterobacteriaceae. Ann N Y Acad Sci. 1956 Aug 10;66(1):141–156. doi: 10.1111/j.1749-6632.1956.tb40113.x. [DOI] [PubMed] [Google Scholar]
- Gmeiner J., Lüderitz O., Westphal O. Biochemical studies on lipopolysaccharides of Salmonella R mutants. 6. Investigations on the structure of the lipid A component. Eur J Biochem. 1969 Jan;7(3):370–379. doi: 10.1111/j.1432-1033.1969.tb19618.x. [DOI] [PubMed] [Google Scholar]
- JENKIN C. R., ROWLEY D. PARTIAL PURIFICATION OF THE "PROTECTIVE" ANTIGEN OF SALMONELLA TYPHIMURIUM AND ITS DISTRIBUTION AMONGST VARIOUS STRAINS OF BACTERIA. Aust J Exp Biol Med Sci. 1965 Feb;43:65–78. doi: 10.1038/icb.1965.5. [DOI] [PubMed] [Google Scholar]
- Jenkin C. R., Karnovsky M. L., Rowley D. Preparation of an artificial antigen and immunity to mouse typhoid. Immunology. 1967 Oct;13(4):361–372. [PMC free article] [PubMed] [Google Scholar]
- KOTELKO K., STAUB A. M., TINELLI R. [Immunochemical study on Salmonella. VIII. Role of O acetyl groupings in the specificity of the O:5 factor]. Ann Inst Pasteur (Paris) 1961 May;100:618–637. [PubMed] [Google Scholar]
- Kenny K., Schlecht S., Westphal O. Antibody response to various single-factor o antigens of salmonella. Infect Immun. 1970 Jan;1(1):41–50. doi: 10.1128/iai.1.1.41-50.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx A., Musetescu M., Sendrea M., Mihalca M. Relationship between particle size and biological activity of Salmonella typhimurium endotoxin. Zentralbl Bakteriol Orig. 1968;207(3):313–316. [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- NETER E. Bacterial hemagglutination and hemolysis. Bacteriol Rev. 1956 Sep;20(3):166–188. doi: 10.1128/br.20.3.166-188.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NETER E., WESTPHAL O., LUDERITZ O., GORZYNSKI E. A., EICHENBERGER E. Studies of enterobacterial lipopolysaccharides; effects of heat and chemicals on erythrocyte-modifying, antigenic, toxic and pyrogenic properties. J Immunol. 1956 May;76(5):377–385. [PubMed] [Google Scholar]
- Springer G. F., Wang E. T., Nichols J. H., Shear J. M. Relations between bacterial lipopolysaccharide structures and those of human cells. Ann N Y Acad Sci. 1966 Jun 30;133(2):566–579. doi: 10.1111/j.1749-6632.1966.tb52389.x. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]