Abstract
Background
Postamputation neuroma pain can prevent comfortable prosthesis wear in patients with limb amputations, and currently available treatments are not consistently effective. Targeted muscle reinnervation (TMR) is a decade-old technique that employs a series of novel nerve transfers to permit intuitive control of upper-limb prostheses. Clinical experience suggests that it may also serve as an effective therapy for postamputation neuroma pain; however, this has not been explicitly studied.
Questions/purposes
We evaluated the effect of TMR on residual limb neuroma pain in upper-extremity amputees.
Methods
We conducted a retrospective medical record review of all 28 patients treated with TMR from 2002 to 2012 at Northwestern Memorial Hospital/Rehabilitation Institute of Chicago (Chicago, IL, USA) and San Antonio Military Medical Center (San Antonio, TX, USA). Twenty-six of 28 patients had sufficient (> 6 months) followup for study inclusion. The amputation levels were shoulder disarticulation (10 patients) and transhumeral (16 patients). All patients underwent TMR for the primary purpose of improved myoelectric control. Of the 26 patients included in the study, 15 patients had evidence of postamputation neuroma pain before undergoing TMR.
Results
Of the 15 patients presenting with neuroma pain before TMR, 14 experienced complete resolution of pain in the transferred nerves, and the remaining patient’s pain improved (though did not resolve). None of the patients who presented without evidence of postamputation neuroma pain developed neuroma pain after the TMR procedure. All 26 patients were fitted with a prosthesis, and 23 of the 26 patients were able to operate a TMR-controlled prosthesis.
Conclusions
None of the 26 patients who underwent TMR demonstrated evidence of new neuroma pain after the procedure, and all but one of the 15 patients who presented with preoperative neuroma pain experienced complete relief of pain in the distribution of the transferred nerves. TMR offers a novel and potentially more effective therapy for the management of neuroma pain after limb amputation.
Level of Evidence
Level IV, therapeutic study. See Instructions for Authors for a complete description of levels of evidence.
Introduction
The proximal ends of severed or damaged peripheral nerves form neuromas when the distal nerve scaffold or its neurotrophic factors are deficient or absent. Neuromas can cause focal pain that is often difficult to treat medically or surgically. After traumatic amputation, these neuromas are particularly problematic because of the extent of nerve injury, the number of nerves injured, and the superficial location of these nerves in the residual limb. Painful neuromas often prevent consistent use of a prosthesis, thus further limiting the functional capacity of the amputee. The problem of postamputation neuroma pain has received increased recognition in the wake of the recent military conflicts in Iraq and Afghanistan. Between 2001 and 2010, more than 1000 US military personnel suffered traumatic major limb amputations, prompting the Department of Defense to identify the treatment of postamputation neuroma pain as a primary focus area for funded scientific investigation [33]. Yet, major limb loss is hardly isolated to the combat theater. As of 2005, 1.7 million people in the United States were living with limb amputations, and that number is expected to double by 2050 [39]. Approximately 25% of all major limb amputees will develop chronic localized pain due to symptomatic neuromas in their residual limbs [8, 30]. The proportion of patients with residual limb neuroma pain after traumatic amputation may be even higher; this complication is reported in as many as 71% of those patients [13, 37].
Despite recognition of the problem posed by neuroma pain, the current surgical strategies to inhibit neuroma formation or treat existing neuromas are unpredictable and frequently unsatisfactory [12, 30]. Most of the described techniques aim to cap the proximal transected nerve end with various artificial or autologous materials [1, 3, 11, 15, 26–28, 31, 35]. Others emphasize manipulation of the nearby bone or soft tissues to provide padding for the nerve or to alter its microenvironment [2, 4–7, 14, 20, 26, 32, 34, 38]. In all, more than 150 surgical treatments for end neuromas have been proposed in the literature [36]. This myriad of described approaches highlights the lack of a single definitive treatment.
Targeted muscle reinnervation (TMR) is a decade-old surgical procedure designed to permit intuitive control of upper-limb prostheses through a set of novel nerve transfers [9, 10, 16, 22–24, 29]. By providing both a distal target and a vascularized scaffold on which to guide sprouting nerve axons, TMR may represent a novel technique for the treatment of painful neuromas; however, this has not been explicitly studied and further evaluation of the role of TMR on neuroma pain outcomes is needed. We therefore evaluated TMR in upper-extremity amputees in terms of its efficacy in the management of residual limb neuroma pain (in addition to motor control) and its safety (ability to be performed for motor control purposes without the risk of causing post-TMR neuromas).
Patients and Methods
We performed a comprehensive retrospective medical record review of all patients who underwent TMR between February 2002 and February 2012 at Northwestern Memorial Hospital/Rehabilitation Institute of Chicago (Chicago, IL, USA) and San Antonio Military Medical Center (San Antonio, TX, USA). TMR surgery was performed on 28 patients as an elective procedure after the primary amputation. The surgical technique for both transhumeral and shoulder disarticulation TMR has been previously described in detail [9, 10, 16, 22, 24, 29]. At Northwestern Memorial Hospital, eight transhumeral and nine shoulder disarticulation procedures were performed. At San Antonio Military Medical Center, 10 transhumeral and one shoulder disarticulation procedures were performed. The institutional review boards of both institutions approved this study.
All TMR procedures were performed for the primary purpose of improving control of upper-extremity myoelectric prostheses. Given uncertainty with regard to the effect of TMR on residual limb pain, the presence or absence of residual limb pain was not a deciding factor in determining candidacy for the TMR procedure.
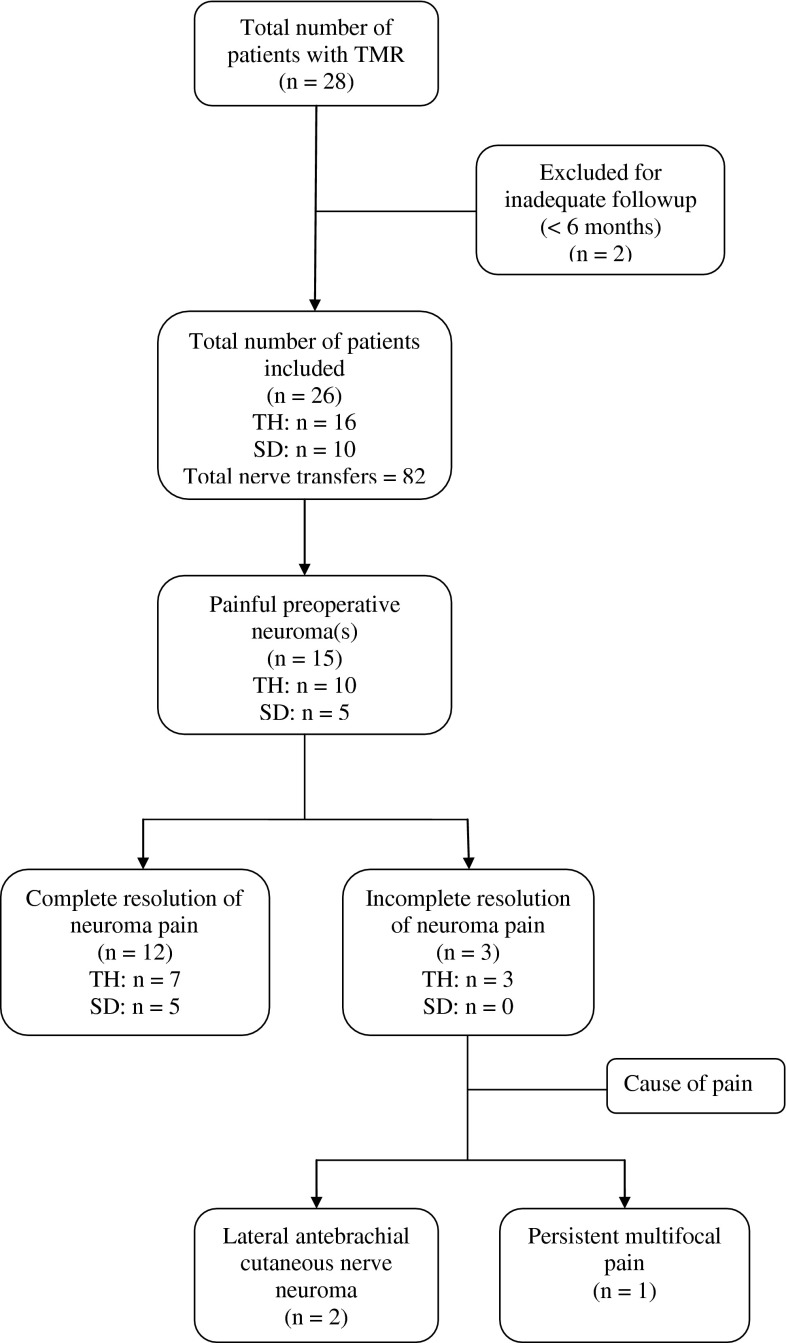
Review of patient characteristics included age, sex, date of amputation, mechanism of injury, level of amputation, and duration from amputation to TMR (Table 1). Procedural details were focused on the number and pattern of nerve transfers performed. Our chart review focused on residual limb pain and prosthetic fitting. Medical record review included hospital and outpatient records of the surgeon, physiatrist, occupational therapist, physical therapist, and prosthetist. The length of followup was defined to be from the date of TMR to the last direct patient contact, and a minimum followup of 6 months was required for inclusion in this report. Of the 28 patients in whom TMR was performed, two patients had insufficient followup (< 6 months) for inclusion in our analysis (Fig. 1).
Table 1.
Patient characteristics
| Variable | Value |
|---|---|
| Number of patients | 26 |
| Sex (number of patients) | |
| Male | 22 (15%) |
| Female | 4 (85%) |
| Age at TMR (years)* | 32.8 ± 11.7 (18–55) |
| Amputation level† (number of patients) | |
| Transhumeral | 16 |
| Shoulder disarticulation | 10 |
| Mechanism of injury (number of patients) | |
| Combat/blast | 11 |
| Motor vehicle accident | 10 |
| Electrical burn | 3 |
| Other | 2 |
| Duration amputation to TMR (months)* | 16.5 ± 14.6 (4.0–73.3) |
| Followup (months)* | 27.6 ± 27.5 (6.1–124.0) |
| Treating institution (number of patients) | |
| NMH/RIC | 15 |
| SAMMC | 11 |
| Number of nerve transfers per procedure‡ | |
| Transhumeral | 2.4 (2–3) |
| Shoulder disarticulation | 4.1 (4–5) |
| Total number of nerve transfers | 82 |
| Number of transfers yielding detectable EMG | 78 |
*Values are expressed as mean ± SD, with range in parentheses; †amputation level determined by pattern of nerve transfers; ‡values are expressed as mean, with range in parentheses; TMR = targeted muscle reinnervation; NMH/RIC = Northwestern Memorial Hospital/Rehabilitation Institute of Chicago; SAMMC = San Antonio Military Medical Center.
Fig. 1.
This flowchart demonstrates the number of patients enrolled in the study, those with sufficient followup, and the results for those included in the study. TH = transhumeral; SD = shoulder disarticulation.
Of the 26 patients included in the final analysis, 22 of the 26 patients were male. The mean patient age was 34 ± 12 years. Ten of 26 patients presented with amputations at the shoulder disarticulation level, with the remaining 16 patients having previously undergone a transhumeral amputation. The mean duration between amputation and TMR surgery was 16 months. Mean followup was 25 months (range, 6–124 months).
Fifteen of the 26 patients complained of focal residual limb pain consistent with neuroma before TMR. All of these patients were documented to have residual limb pain that interfered with fitting or consistent use of an upper-limb prosthesis. While these 15 patients with pain were evaluated to demonstrate the efficacy of TMR for neuroma management (in addition to motor control), the remaining 11 asymptomatic patients were evaluated to demonstrate that the procedure can be safely performed for motor control purposes without the risk of causing post-TMR neuromas.
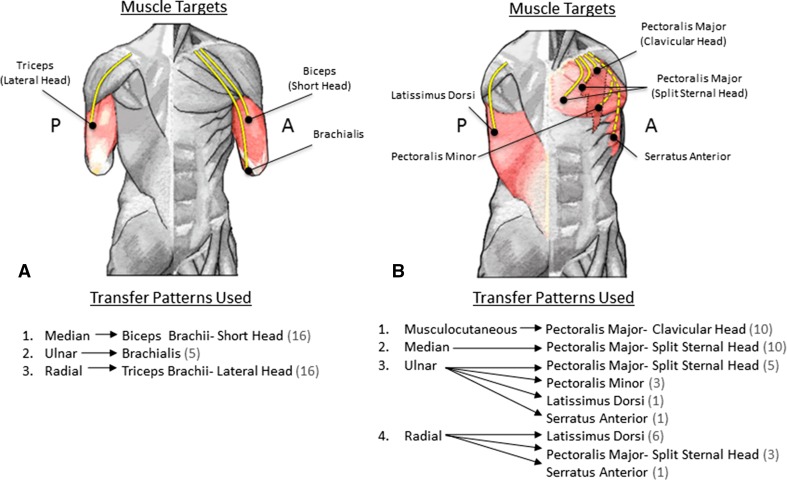
Each patient had between two and five end neuromas excised as part of the TMR procedure, with a total of 82 nerves transferred for the entire cohort (Fig. 1). Seventy-eight (95%) of the nerve transfers produced a detectable EMG signal in the recipient muscle segment. The target muscles and nerve transfer patterns for both the transhumeral and shoulder disarticulation TMR procedures are exhibited, as is the number of each transfer performed in this series (Fig. 2).
Fig. 2A–B.
Diagrams illustrate the nerve transfers employed for the (A) transhumeral and (B) shoulder disarticulation procedures. The left side of each image provides a posterior (P) perspective while the right depicts the anterior (A) side. Donor nerves are coapted to the motor nerves of the target muscles via small recipient motor nerve branches. The target muscles are labeled on the diagrams and the yellow lines demonstrate the donor nerves in their transferred positions. The dashed yellow lines indicate nerve transfers that are less frequently used. The parenthetical numbers indicate the frequency with which each transfer was used in this series.
Given the retrospective nature of the study and the fact that validated pain scales were not consistently used, our end points were the number of patients experiencing complete resolution of pain in the transferred nerves and the number of patients who were fit with a TMR-controlled prosthesis. Pain outcomes were based on pre- and postoperative assessment of localized neuroma pain. Patients were identified as having neuroma pain if the reviewed medical records included both documentation of physical findings consistent with localized nerve pain and a formal diagnosis of neuroma pain in the provider assessments. In all patients deemed to have preoperative pain, neuroma pain was specifically differentiated from phantom limb pain in the provider assessments. The diagnosis of neuroma pain was based solely on clinical findings. Radiographic imaging was not routinely used to support this diagnosis preoperatively, as residual limb pain was not the principal indication for TMR and any symptomatic end neuroma would be excised as part of the nerve transfer procedure. There were no instances of conflicting diagnoses with regard to pain location or etiology, despite independent evaluation by multiple providers. Residual limb pain that precluded prosthetic fitting or routine use was documented by the treating prosthetists. Fitting with a TMR-controlled prosthesis was thus intended as a practical outcome measure to suggest the absence of clinically significant residual limb pain, as TMR-controlled prostheses require more intensive rehabilitation and stricter fitting requirements than conventional devices.
Results
Of the 15 patients with neuroma pain after amputation, 14 experienced complete resolution of pain in the transferred nerves. However, the other patient experienced such significant improvement in his pain that he was able to be fit with a prosthesis despite the fact that pre-TMR attempts at fitting had been precluded by his severe residual limb pain. Two additional patients with transhumeral amputations reported post-TMR neuroma pain; however, the painful sites were localized to the lateral antebrachial cutaneous nerve, which was not manipulated as part of the transhumeral TMR procedure. There were no symptomatic neuromas identified after TMR at the shoulder level, where all transected nerves were transferred to a nearby target muscle segment. In addition, none of the 11 patients who underwent TMR who did not have preoperative evidence of postamputation neuroma pain developed neuroma pain after the procedure.
Overall, 23 of the 26 patients were successfully fit with a TMR myoelectric prosthesis. One patient failed fitting due to persistent residual limb pain; a second patient was found to have a brachial plexopathy intraoperatively that prevented successful reinnervation; and a third patient was not fit due to financial challenges. All three were still able to wear a non-TMR prosthesis.
Discussion
In addition to the functional deficits directly attributable to extremity loss, amputees are often further impaired by postamputation neuroma pain. This residual limb pain diminishes quality of life and serves as an obstacle to prosthetic rehabilitation [30]. Unfortunately, there is no definitive surgical therapy for management of this pain [12, 30]. However, a recent report comparing management strategies for neuroma pain in the intact extremity suggests a benefit to techniques that reconstitute the peripheral nervous anatomy over those that merely excise or bury the damaged nerve [12]. By providing recipient nerves and denervated muscle targets for reinnervation, TMR effectively restores continuity to the peripheral nervous system despite absence of the native distal nerve segments. Based on this theory, TMR provides the elements necessary to favor coordinated nerve regeneration over neuroma formation; however, this has not been explicitly studied and further evaluation of the role of TMR on neuroma pain is needed. The findings of this study suggest that TMR, while initially intended for improved prosthetic control, may also inhibit neuroma recurrence in the transferred nerves.
The most important limitation of this study was the absence of standardized patient-reported pain scores. As TMR was initially intended for prosthetic control, the improvement or resolution of preexisting neuroma pain was an unanticipated benefit. Notably, the possibility of increased pain after neuroma excision and TMR was strongly emphasized as part of the consent process for what was initially an experimental surgical procedure. As our primary focus was functional improvement, no prospective, standardized pain assessment was collected. However, the chart review, which was based on a comprehensive evaluation of the records from all providers involved in the perioperative care of the patient, was fairly consistent in terms of the finding of pain relief, even if the pain end point used was not very granular (complete relief versus incomplete relief). The surgeons, physiatrists, therapists, and prosthetists whose records were queried as part of this review were all attuned to the identification and treatment of amputation-related neuropathic pain and frequently included descriptions of pain location and quality as part of their standard clinical evaluations. Given the retrospective nature of the study, no consistent evaluation template was used to differentiate neuroma pain from that attributable to other causes (ie, phantom pain, pain due to soft tissue problems). However, patients were only deemed to have neuroma pain if formally stated in the provider’s assessment and supported by documented examination findings. While the lack of a standardized pain evaluation is a major limitation of this study, failure to anticipate the beneficial effect of TMR on neuroma pain simultaneously limits the influence of assessor and selection bias on the part of the treating providers. In addition, the inclusion of prosthetists’ records is particularly important, as neuroma pain frequently prohibits successful prosthetic fitting, The TMR-controlled myoelectric devices are heavier and require tighter fitting than conventional prostheses. Thus, successful prosthetic fitting and use can be considered a practical outcome measure to suggest the absence of clinically significant residual limb pain.
After TMR, whereby two to five end neuromas were excised per patient, 12 of the 15 patients with preoperative neuroma pain experienced complete resolution of their pain; one patient did not have his pain completely resolved in the transferred nerves and another two had painful sites localized to a nerve not manipulated in the TMR procedure. In addition, no patient developed new neuroma pain as a result of the procedure. As further evidence of absent residual limb pain, 23 of the 26 patients in this review were successfully fit with a TMR-controlled myoelectric device, with the remaining three patients able to use conventional body-powered devices. Though not specifically intended to address postamputation neuroma pain, TMR yields pain outcomes that compare favorably with those achieved using established techniques [1–7, 11, 14, 15, 20, 26–28, 31–35, 38]. While the patterns of nerve transfers described in this study were chosen based on the need to provide a myoelectric control pattern, a wider range of nerve transfers can be performed for the purpose of neuroma treatment. In fact, any motor nerve branch can serve as a nerve transfer recipient after neuroma excision, provided there is acceptable morbidity associated with sacrifice of the recipient motor branch. The availability of multiple muscle targets and lack of functional donor site morbidity make this technique particularly applicable to patients with major limb amputations.
The clinical observations outlined in this study led our group to hypothesize that distal neurectomy, followed by coaptation of the residual nerve to a recipient motor nerve branch, can be used to prevent neuroma recurrence by encouraging organized nerve regeneration into newly denervated muscle. This clinical finding is realized despite the obvious size mismatch between donor and recipient nerves—a surgical consideration previously thought important in peripheral nerve surgery (Fig. 3). To further evaluate this hypothesis, we performed animal studies to better delineate the role of TMR on neuroma formation [17–19, 21]. Using a novel rabbit amputation-neuroma model [17, 18], we performed nerve transfers between amputated forelimb nerve stumps and the motor nerves of a pedicled rectus abdominis muscle that had been mobilized to the chest. After 10 weeks, the transferred nerves more closely resembled the morphology of their uninjured counterparts than that of previously excised neuromas [19]. This animal model demonstrated that TMR yielded a significant decrease in histologic neuroma formation, even when compared to burial of transected nerve ends into normal muscle, a technique many consider the gold standard for neuroma treatment [25].
Fig. 3A–B.
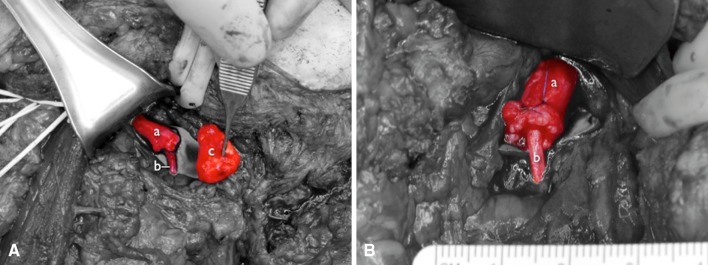
(A) This photograph depicts a shoulder disarticulation amputee undergoing TMR. The neuroma has been excised (c) from the donor radial nerve (a) and the recipient thoracodorsal nerve (b) has been cut near its entry into the latissimus muscle in preparation for coaptation. (B) The recipient thoracodorsal nerve (b) has been sutured into the center of the donor radial nerve (a) using two epineurial mattress sutures. The discrepancy in nerve caliber precludes use of conventional nerve repair techniques. However, despite the obvious size mismatch, the coaptation yielded clinically useful latissimus reinnervation without evidence of neuroma formation.
While recognizing the limitations imposed by our methodology, we believe that the concepts introduced by this study are important given the absence of a single definitive treatment for amputation-related neuroma pain and the increasing utilization of TMR techniques for amputee care. Motivated by these retrospective outcomes and supportive preclinical findings, a large multiinstitutional randomized clinical trial of TMR versus standard neuroma excision and burial is now underway. The study features both civilian and military sites and will use imaging and prospective patient-reported outcomes to more definitively assess the efficacy of TMR for the treatment of postamputation neuroma pain. In summary, TMR offers the exciting potential to treat pain that too frequently proves refractory to current medical and surgical therapies. Simply put, TMR gives the nerves somewhere to go and something to do—elements lacking in other neuroma treatments.
Acknowledgments
We thank Aaron Barrow MD his assistance with data collection. We also thank Christopher Wilson MD and Robert Granville MD for sharing the results of their upper-limb TMR procedures.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Northwestern University Feinberg School of Medicine, Chicago, IL, USA.
References
- 1.Ashley L, Stallings JO. End-to-side nerve flap for treatment of painful neuroma: a 15-year follow-up. J Am Osteopath Assoc. 1988;88:621–624. [PubMed] [Google Scholar]
- 2.Balcin H, Erba P, Wettstein R, Schaefer DJ, Pierer G, Kalbermatten DF. A comparative study of two methods of surgical treatment for painful neuroma. J Bone Joint Surg Br. 2009;91:803–808. doi: 10.1302/0301-620X.91B6.22145. [DOI] [PubMed] [Google Scholar]
- 3.Barberá J, Albert-Pampló R. Centrocentral anastomosis of the proximal nerve stump in the treatment of painful amputation neuromas of major nerves. J Neurosurg. 1993;79:331–334. doi: 10.3171/jns.1993.79.3.0331. [DOI] [PubMed] [Google Scholar]
- 4.Boldrey E. Amputation neuroma in nerves implanted in bone. Ann Surg. 1943;118:1052–1057. doi: 10.1097/00000658-194312000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burchiel KJ, Johans TJ, Ochoa J. The surgical treatment of painful traumatic neuromas. J Neurosurg. 1993;78:714–719. doi: 10.3171/jns.1993.78.5.0714. [DOI] [PubMed] [Google Scholar]
- 6.Dellon AL, Mackinnon SE. Treatment of the painful neuroma by neuroma resection and muscle implantation. Plast Reconstr Surg. 1986;77:427–438. doi: 10.1097/00006534-198603000-00016. [DOI] [PubMed] [Google Scholar]
- 7.Dellon AL, Mackinnon SE, Pestronk A. Implantation of sensory nerve into muscle: preliminary clinical and experimental observations on neuroma formation. Ann Plast Surg. 1984;12:30–40. doi: 10.1097/00000637-198401000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Ducic I, Mesbahi AN, Attinger CE, Graw K. The role of peripheral nerve surgery in the treatment of chronic pain associated with amputation stumps. Plast Reconstr Surg. 2008;121:908–914. doi: 10.1097/01.prs.0000299281.57480.77. [DOI] [PubMed] [Google Scholar]
- 9.Dumanian GA, Ko JH, O’Shaughnessy KD, Kim PS, Wilson CJ, Kuiken TA. Targeted reinnervation for transhumeral amputees: current surgical technique and update on results. Plast Reconstr Surg. 2009;124:863–869. doi: 10.1097/PRS.0b013e3181b038c9. [DOI] [PubMed] [Google Scholar]
- 10.Dumanian GA, Souza JM. Surgical techniques for targeted muscle reinnervation (TMR) In: Kuiken TA, Barlow AK, Schultz AE, editors. Targeted Muscle Reinnervation: A Neural Interface for Artificial Limbs. Boca Raton, FL: CRC Press; 2013. [Google Scholar]
- 11.González-Darder J, Barberá J, Abellán MJ, Mora A. Centrocentral anastomosis in the prevention and treatment of painful terminal neuroma: an experimental study in the rat. J Neurosurg. 1985;63:754–758. doi: 10.3171/jns.1985.63.5.0754. [DOI] [PubMed] [Google Scholar]
- 12.Guse DM, Moran SL. Outcomes of the surgical treatment of peripheral neuromas of the hand and forearm: a 25-year comparative outcome study. Ann Plast Surg. 2013;71:654–658. doi: 10.1097/SAP.0b013e3182583cf9. [DOI] [PubMed] [Google Scholar]
- 13.Hanley MA, Ehde DM, Jensen M, Czerniecki J, Smith DG, Robinson LR. Chronic pain associated with upper-limb loss. Am J Phys Med Rehabil. 2009;88:742–752. doi: 10.1097/PHM.0b013e3181b306ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazari A, Elliot D. Treatment of end-neuromas, neuromas-in-continuity and scarred nerves of the digits by proximal relocation. J Hand Surg Br. 2004;29:338–350. doi: 10.1016/j.jhsb.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Herbert TJ, Filan SL. Vein implantation for treatment of painful cutaneous neuromas: a preliminary report. J Hand Surg Br. 1998;23:220–224. doi: 10.1016/S0266-7681(98)80178-2. [DOI] [PubMed] [Google Scholar]
- 16.Hijjawi JB, Kuiken TA, Lipschutz RD, Miller LA, Stubblefield KA, Dumanian GA. Improved myoelectric prosthesis control accomplished using multiple nerve transfers. Plast Reconstr Surg. 2006;118:1573–1578. doi: 10.1097/01.prs.0000242487.62487.fb. [DOI] [PubMed] [Google Scholar]
- 17.Kim PS, Ko J, O’Shaughnessy KK, Kuiken TA, Dumanian GA. Novel model for end-neuroma formation in the amputated rabbit forelimb. J Brachial Plex Peripher Nerve Inj. 2010;5:6. doi: 10.1186/1749-7221-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim PS, Ko JH, O’Shaughnessy KK, Kuiken TA, Pohlmeyer EA, Dumanian GA. The effects of targeted muscle reinnervation on neuromas in a rabbit rectus abdominis flap model. J Hand Surg Am. 2012;37:1609–1616. doi: 10.1016/j.jhsa.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 19.Ko JH, Kim PS, O’Shaughnessy KD, Ding X, Kuiken TA, Dumanian GA. A quantitative evaluation of gross versus histologic neuroma formation in a rabbit forelimb amputation model: potential implications for the operative treatment and study of neuromas. J Brachial Plex Peripher Nerve Inj. 2011;6:8. doi: 10.1186/1749-7221-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krishnan KG, Pinzer T, Schackert G. Coverage of painful peripheral nerve neuromas with vascularized soft tissue: method and results. Neurosurgery. 2005;56:369–378. doi: 10.1227/01.NEU.0000156881.10388.D8. [DOI] [PubMed] [Google Scholar]
- 21.Kuiken TA, Childress DS, Rymer WZ. The hyper-reinnervation of rat skeletal muscle. Brain Res. 1995;676:113–123. doi: 10.1016/0006-8993(95)00102-V. [DOI] [PubMed] [Google Scholar]
- 22.Kuiken TA, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield KA. The use of targeted muscle reinnervation for improved myoelectric prosthesis control in a bilateral shoulder disarticulation amputee. Prosthet Orthot Int. 2004;28:245–253. doi: 10.3109/03093640409167756. [DOI] [PubMed] [Google Scholar]
- 23.Kuiken TA, Li G, Lock BA, Lipschutz RD, Miller LA, Stubblefield KA, Englehart KB. Targeted muscle reinnervation for real-time myoelectric control of multifunction artificial arms. JAMA. 2009;301:619–628. doi: 10.1001/jama.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuiken TA, Miller LA, Lipschutz RD, Lock BA, Stubblefield KA, Morasco PD, Zhou P, Dumanian GA. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study. Lancet. 2007;369:371–380. doi: 10.1016/S0140-6736(07)60193-7. [DOI] [PubMed] [Google Scholar]
- 25.Mackinnon SE, Dellon AL, Hudson AR, Hunter DA. Alteration of neuroma formation by manipulation of its microenvironment. Plast Reconstr Surg. 1985;76:345–353. doi: 10.1097/00006534-198509000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Mass DP, Ciano MC, Tortosa R, Newmeyer WL, Kilgore ES. Treatment of painful hand neuromas by their transfer into bone. Plast Reconstr Surg. 1984;74:182–185. doi: 10.1097/00006534-198408000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Mobbs RJ, Vonau M, Blum P. Treatment of painful peripheral neuroma by vein implantation. J Clin Neurosci. 2003;10:338–339. doi: 10.1016/S0967-5868(03)00010-9. [DOI] [PubMed] [Google Scholar]
- 28.Muehleman C, Rahimi F. Effectiveness of an epineurial barrier in reducing axonal regeneration and neuroma formation in the rat. J Foot Surg. 1990;29:260–264. [PubMed] [Google Scholar]
- 29.O’Shaughnessy KD, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield K, Kuiken TA. Targeted reinnervation to improve prosthesis control in transhumeral amputees: a report of three cases. J Bone Joint Surg Am. 2008;90:393–400. doi: 10.2106/JBJS.G.00268. [DOI] [PubMed] [Google Scholar]
- 30.Pierce RO, Jr, Kernek CB, Ambrose TA., 2nd The plight of the traumatic amputee. Orthopedics. 1993;16:793–797. doi: 10.3928/0147-7447-19930701-08. [DOI] [PubMed] [Google Scholar]
- 31.Sakai Y, Ochi M, Uchio Y, Ryoke K, Yamamoto S. Prevention and treatment of amputation neuroma by an atelocollagen tube in rat sciatic nerves. J Biomed Mater Res B Appl Biomater. 2005;73:355–360. doi: 10.1002/jbm.b.30219. [DOI] [PubMed] [Google Scholar]
- 32.Stahl S, Rosenberg N. Surgical treatment of painful neuroma in medial antebrachial cutaneous nerve. Ann Plast Surg. 2002;48:154–160. doi: 10.1097/00000637-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 33.Stansbury LG, Lalliss SJ, Branstetter JG, Bagg MR, Holcomb JB. Amputations in U.S. military personnel in the current conflicts in Afghanistan and Iraq. J Orthop Trauma. 2008;22:43–46. doi: 10.1097/BOT.0b013e31815b35aa. [DOI] [PubMed] [Google Scholar]
- 34.Stokvis A, van der Avoort DJ, van Neck JW, Hovius SE, Coert JH. Surgical management of neuroma pain: a prospective follow-up study. Pain. 2010;151:862–869. doi: 10.1016/j.pain.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 35.Swanson AB, Boeve NR, Lumsden RM. The prevention and treatment of amputation neuromata by silicone capping. J Hand Surg Am. 1977;2:70–78. doi: 10.1016/S0363-5023(77)80013-0. [DOI] [PubMed] [Google Scholar]
- 36.Wood VE, Mudge MK. Treatment of neuromas about a major amputation stump. J Hand Surg Am. 1987;12:302–306. doi: 10.1016/S0363-5023(87)80297-6. [DOI] [PubMed] [Google Scholar]
- 37.Tintle SM, Keeling JJ, Shawen SB, Forseberg JA, Potter BK. Traumatic and trauma-related amputations. J Bone Joint Surg Am. 2010;92:2852–2868. doi: 10.2106/JBJS.J.00257. [DOI] [PubMed] [Google Scholar]
- 38.Tupper JW, Booth DM. Treatment of painful neuromas of sensory nerves in the hand: a comparison of traditional and newer methods. J Hand Surg Am. 1976;1:144–151. doi: 10.1016/S0363-5023(76)80008-1. [DOI] [PubMed] [Google Scholar]
- 39.Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States—2005 to 2050. Arch Phys Med Rehabil. 2008;89:422–429. doi: 10.1016/j.apmr.2007.11.005. [DOI] [PubMed] [Google Scholar]