Abstract
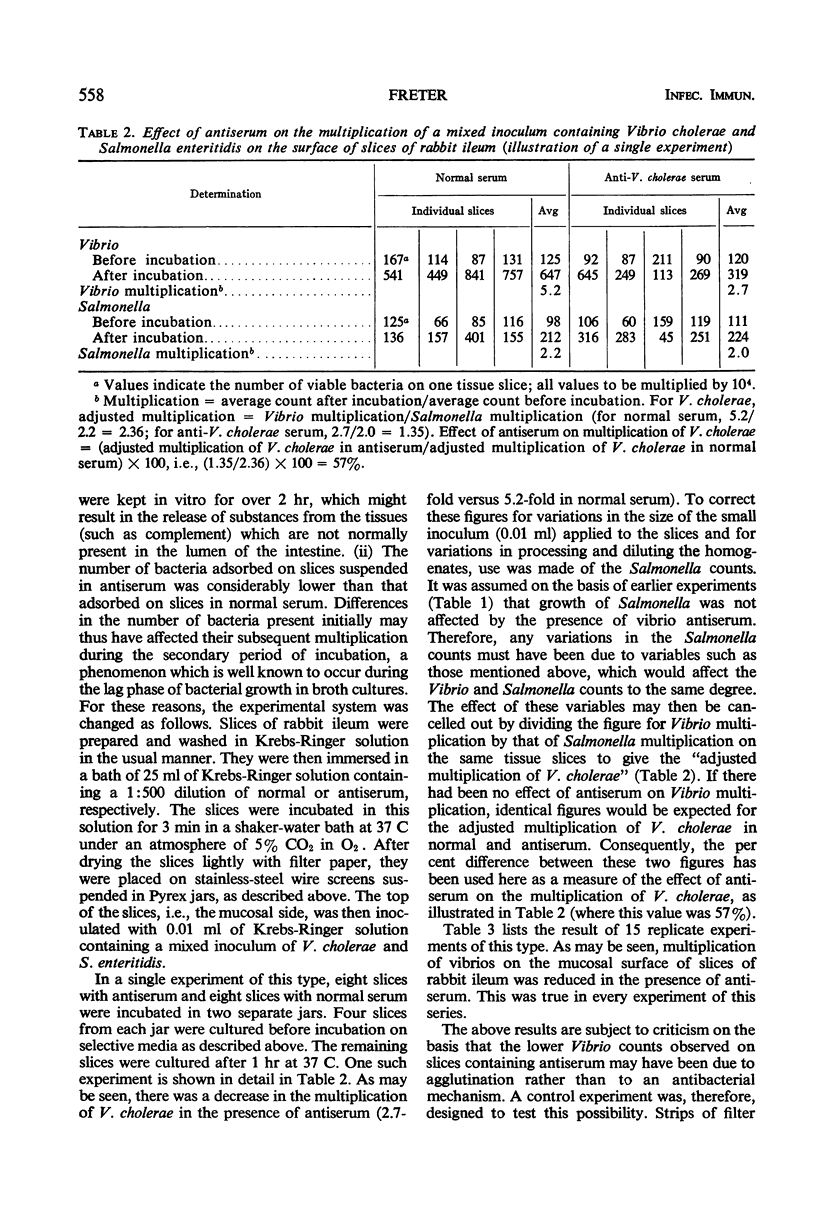
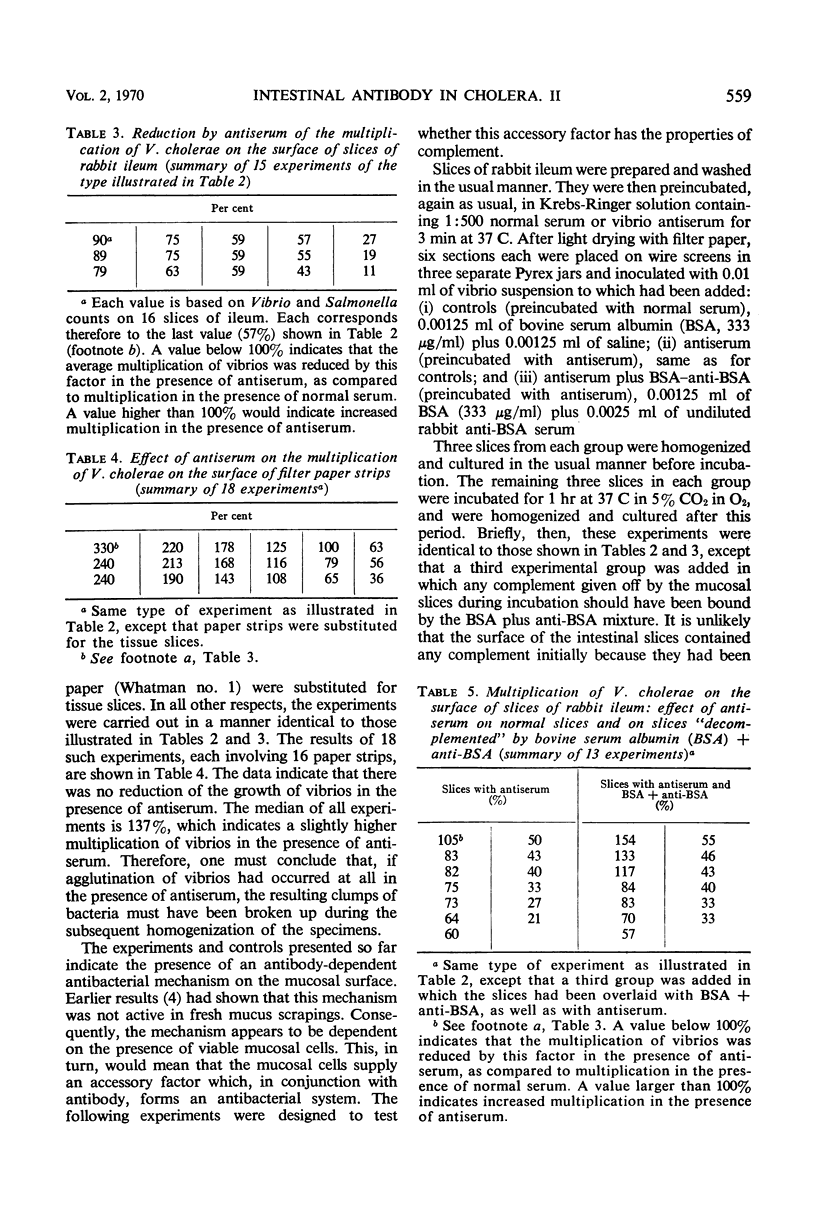
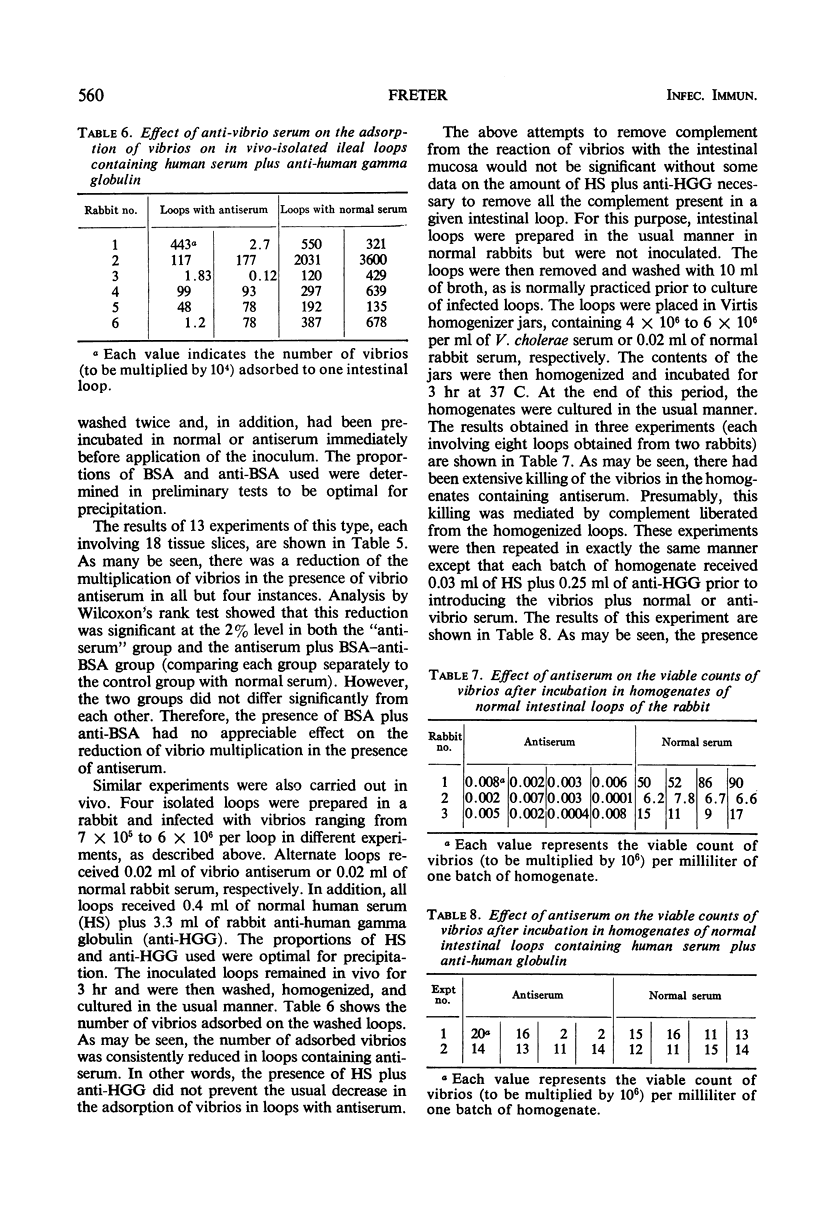
Earlier studies have shown that intestinal antibody (coproantibody) decreases the adsorption of vibrios to the intestinal wall of rabbits. The mechanism underlying this phenomenon was studied by means of an in vitro model in which vibrios were grown on slices of rabbit ileum in a moist chamber. Antibody prevented the adsorption of vibrios onto such slices in the same manner as it did in vivo. Studies of the in vitro growth rate of vibrios on slices of ileum indicated an antibody-dependent antibacterial mechanism on the mucosal surface. This mechanism appeared to require the presence of viable mucosal cells, as it was not present when filter paper was substituted for the tissue slices and it could not be demonstrated in fresh scrapings from the intestinal mucosa. Antigen-antibody mixtures were added to the surface of slices of rabbit ileum or were introduced into the lumen of intestinal loops. Presence of these mixtures did not inhibit the antibody-dependent antibacterial mechanism on the mucosa in either the in vivo or the in vitro system. The amount of antigen-antibody mixture used in vivo was at least 12 times that required to neutralize the complement released by homogenization from an entire intestinal loop. The results obtained support the hypothesis that coproantibody protects by decreasing the adsorption of vibrios on the intestinal mucosa. The mechanism responsible appears to be an antibacterial effect on the mucosal surface which requires antibody plus some additional factor(s) supplied by viable mucosal cells. The postulated factor does not appear to be complement.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bullen J. J., Rogers H. J. Bacterial iron metabolism and immunity to Pasteurella septica and Escherichia coli. Nature. 1969 Oct 25;224(5217):380–382. doi: 10.1038/224380a0. [DOI] [PubMed] [Google Scholar]
- FRETER R. COMPARISON OF IMMUNE MECHANISMS IN VARIOUS EXPERIMENTAL MODELS OF CHOLERA. Bull World Health Organ. 1964;31:825–834. [PMC free article] [PubMed] [Google Scholar]
- Li I. W., Mudd S. Serum effect on the killing of Staphylococcus aureus by human leukocytic extracts. J Immunol. 1966 Jul;97(1):41–45. [PubMed] [Google Scholar]
- MUSCHEL L. H., CAREY W. F., BARON L. S. Formation of bacterial protoplasts by serum components. J Immunol. 1959 Jan;82(1):38–42. [PubMed] [Google Scholar]
- Strominger J. L., Ghuysen J. M. Mechanisms of enzymatic bacteriaolysis. Cell walls of bacteri are solubilized by action of either specific carbohydrases or specific peptidases. Science. 1967 Apr 14;156(3772):213–221. doi: 10.1126/science.156.3772.213. [DOI] [PubMed] [Google Scholar]



