Abstract
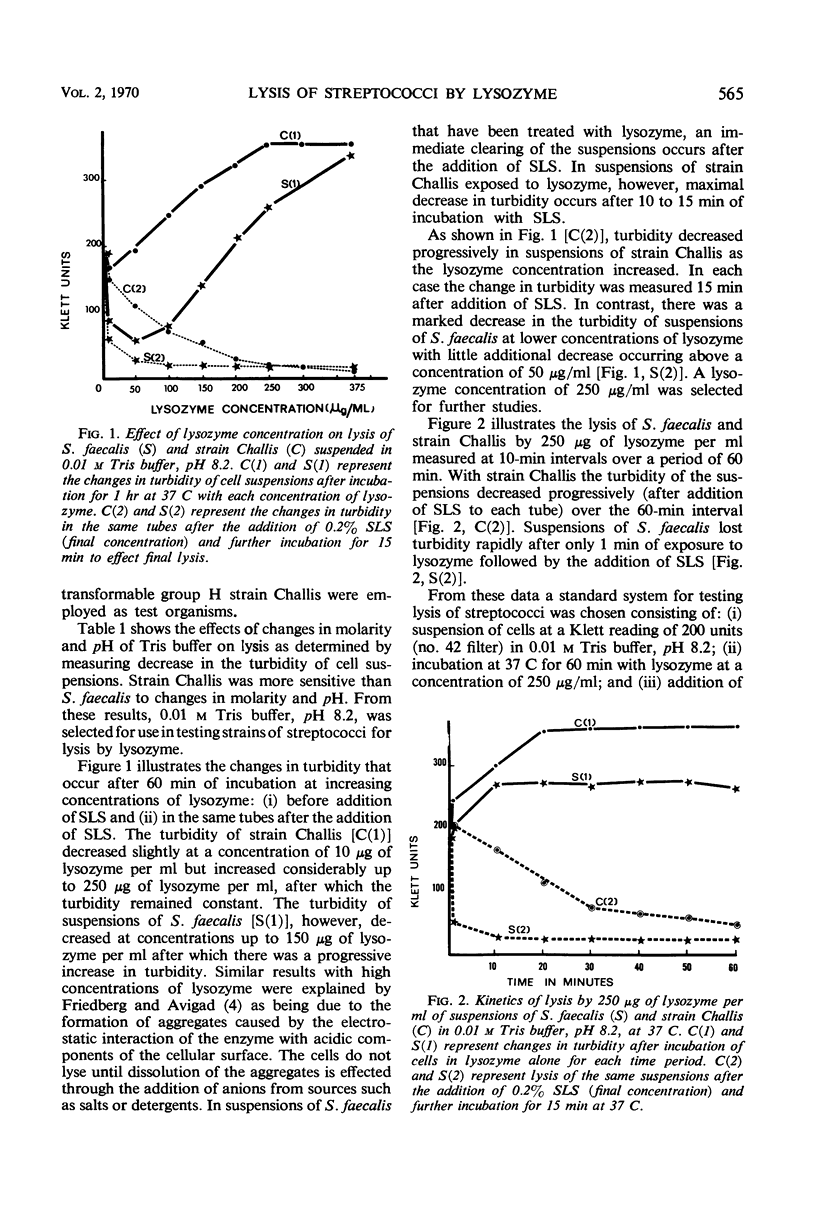
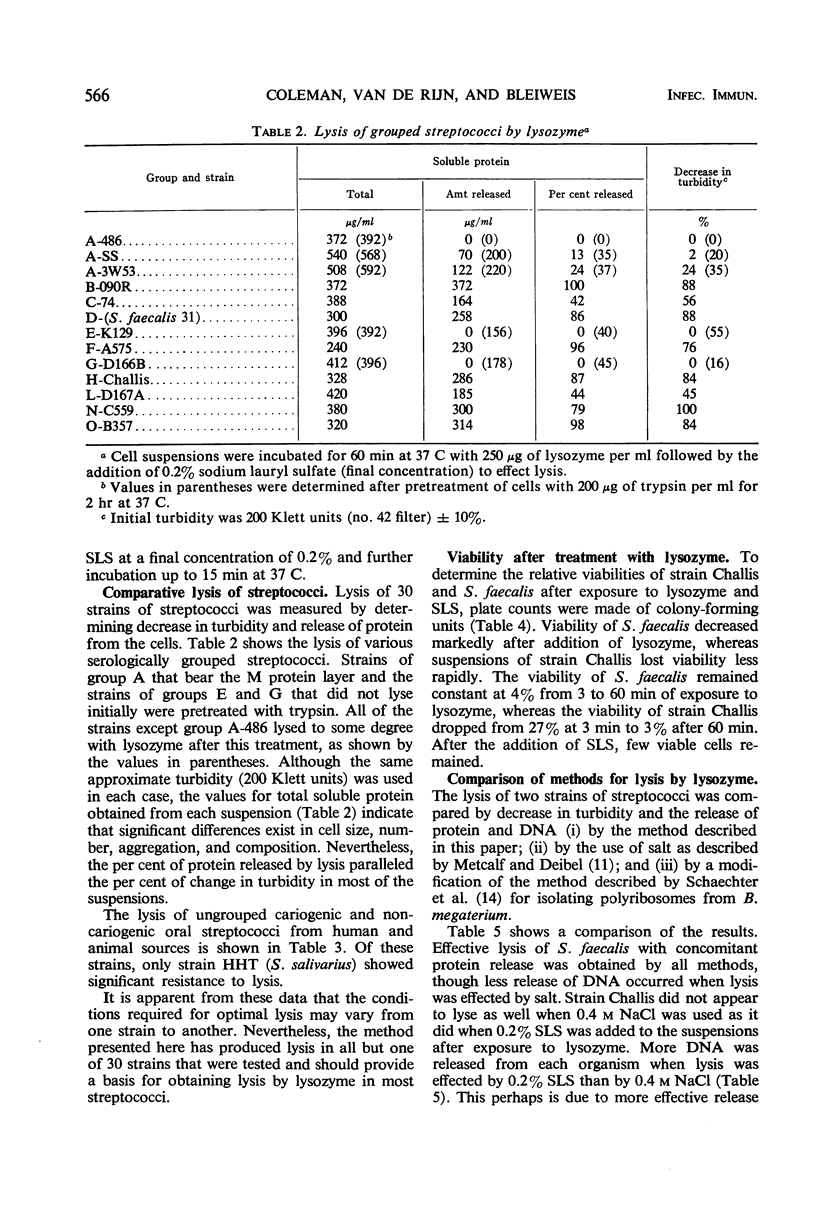
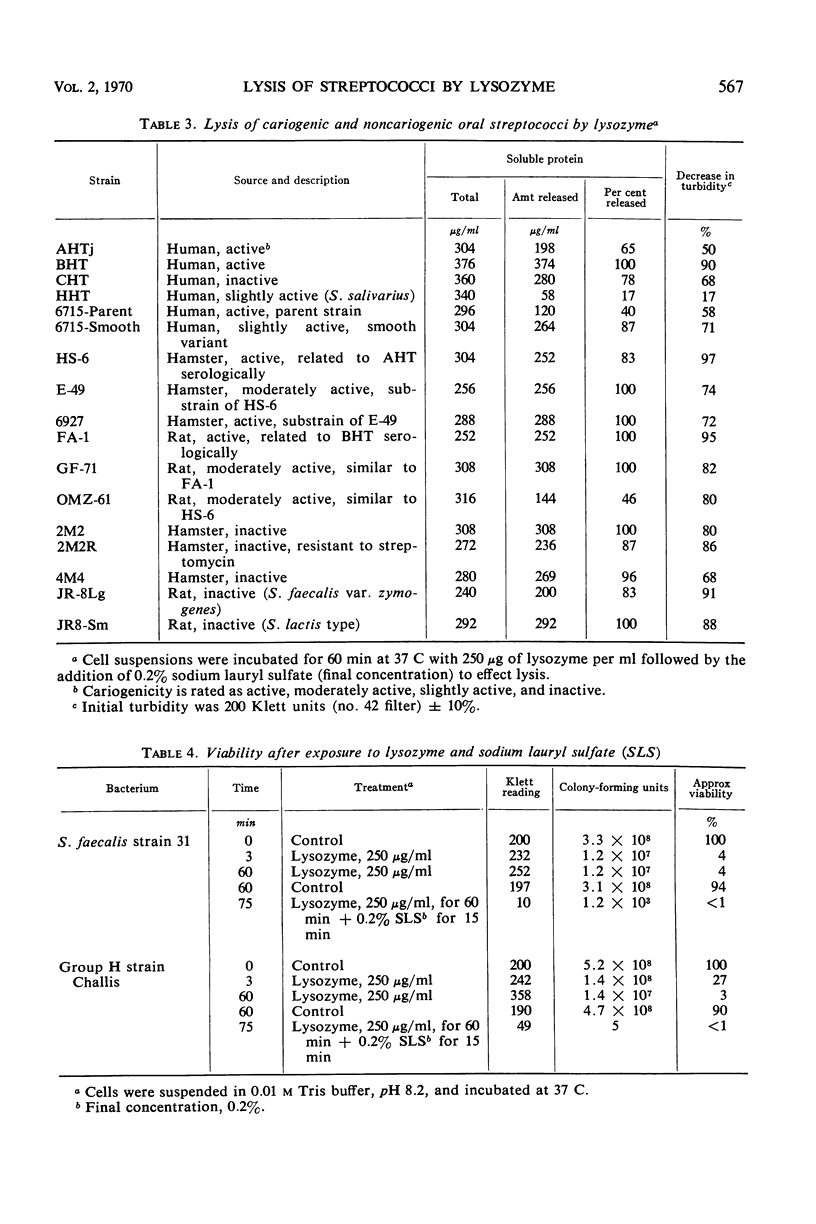
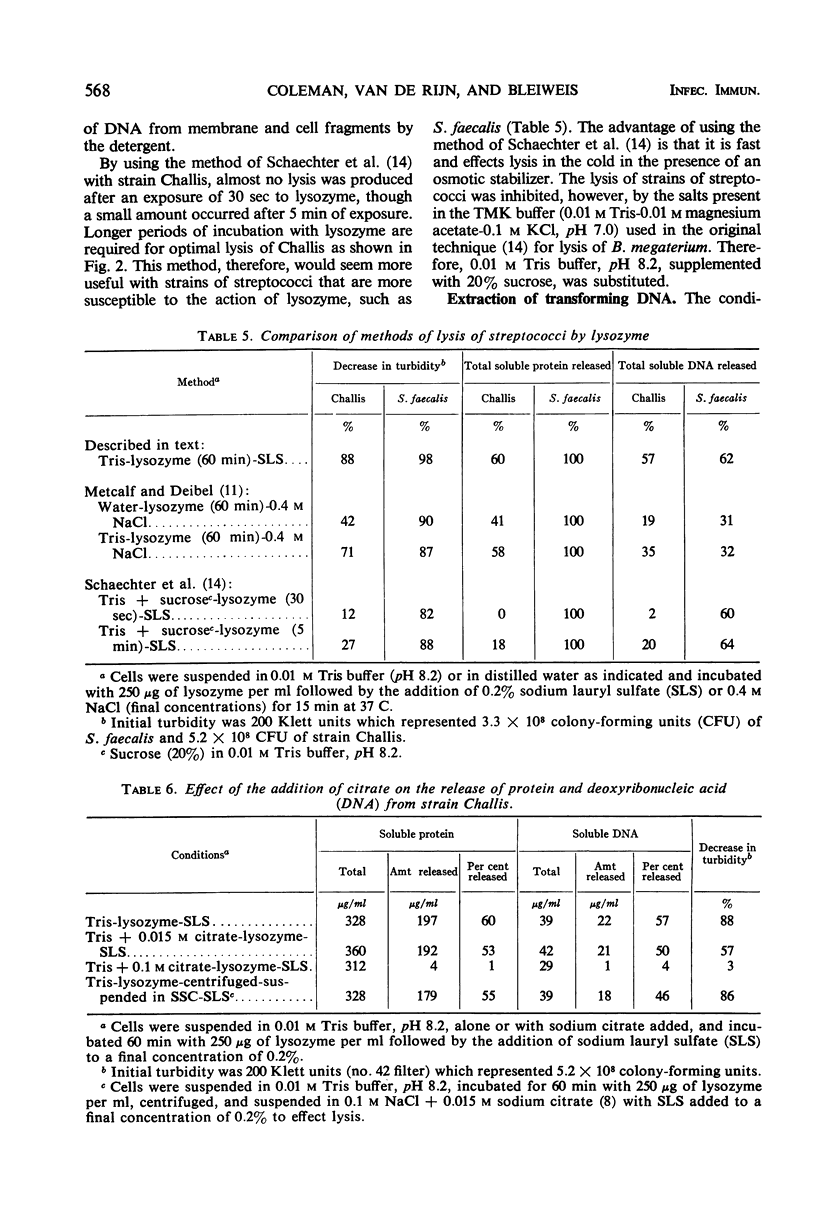
Thirty strains of streptococci were tested for lysis with lysozyme, and 29 of these could be lysed by the following method: (i) suspension of the cells to a Klett reading of 200 units (no. 42 filter) in 0.01 m tris(hydroxymethyl)aminomethane buffer, pH 8.2, after washing twice with the buffer; (ii) addition of lysozyme to a final concentration of 250 μg/ml with incubation for 60 min at 37 C; (iii) addition of sodium lauryl sulfate (SLS) to a final concentration of 0.2% and incubation up to an additional 15 min at 37 C. Significant lysis was obtained only after the addition of SLS. (Strains of groups A, E, and G were treated with trypsin at a concentration of 200 μg/ml for 2 hr at 37 C before exposure to lysozyme.) These parameters for optimal lysis of streptococci by lysozyme were established by testing the group D Streptococcus faecalis strain 31 which lyses readily with lysozyme and the group H strain Challis which is less susceptible to the action of the enzyme. Viability of S. faecalis decreased 96% after 3 min of exposure to 250 μg of lysozyme per ml, whereas the more resistant strain Challis retained 27% of the initial viability after the same period. After 60 min, there was almost total loss of viability in each case. Variations of three methods of lysing streptococci with lysozyme were compared with respect to the decrease in turbidity and the release of protein and deoxyribonucleic acid (DNA) effected by each variation. The method presented in this paper allowed the greatest release of these cytoplasmic constituents from S. faecalis and strain Challis. Transformation experiments using DNA obtained from strain Challis (streptomycinresistant) by this method showed that the DNA released is biologically active.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CERIOTTI G. A microchemical determination of desoxyribonucleic acid. J Biol Chem. 1952 Sep;198(1):297–303. [PubMed] [Google Scholar]
- Colman G. Transformation of viridans-like streptococci. J Gen Microbiol. 1969 Aug;57(2):247–255. doi: 10.1099/00221287-57-2-247. [DOI] [PubMed] [Google Scholar]
- Friedberg I., Avigad G. High lysozyme concentration and lysis of Micrococcus lysodeikticus. Biochim Biophys Acta. 1966 Oct 31;127(2):532–535. doi: 10.1016/0304-4165(66)90409-0. [DOI] [PubMed] [Google Scholar]
- KECK K. An ultramicro technique for the determination of deoxypentose nucleic acid. Arch Biochem Biophys. 1956 Aug;63(2):446–451. doi: 10.1016/0003-9861(56)90059-5. [DOI] [PubMed] [Google Scholar]
- KRAUSE R. M. Studies on bacteriophages of hemolytic streptococci. I. Factors influencing the interaction of phage and susceptible host cell. J Exp Med. 1957 Sep 1;106(3):365–384. doi: 10.1084/jem.106.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAXTED W. R. The active agent in nascent phage lysis of streptococci. J Gen Microbiol. 1957 Jun;16(3):584–595. doi: 10.1099/00221287-16-3-584. [DOI] [PubMed] [Google Scholar]
- MCCARTY M. The lysis of group A hemolytic streptococci by extracellular enzymes of Streptomyces albus. I. Production and fractionation of the lytic enzymes. J Exp Med. 1952 Dec;96(6):555–568. doi: 10.1084/jem.96.6.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf R. H., Deibel R. H. Differential lytic response of enterococci associated with addition order of lysozyme and anions. J Bacteriol. 1969 Sep;99(3):674–680. doi: 10.1128/jb.99.3.674-680.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY D., SLADE H. D. INTRASPECIFIC AND INTERSPECIFIC TRANSFORMATION IN STREPTOCOCCI. J Bacteriol. 1964 Sep;88:595–601. doi: 10.1128/jb.88.3.595-601.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry D., Slade H. D. Effect of filtrates from transformable and nontransformable streptococci on the transformation of streptococci. J Bacteriol. 1966 Jun;91(6):2216–2222. doi: 10.1128/jb.91.6.2216-2222.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAECHTER M., PREVIC E. P., GILLESPIE M. E. MESSENGER RNA AND POLYRIBOSOMES IN BACILLUS MEGATERIUM. J Mol Biol. 1965 May;12:119–129. doi: 10.1016/s0022-2836(65)80286-8. [DOI] [PubMed] [Google Scholar]
- SHUGAR D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta. 1952 Mar;8(3):302–309. doi: 10.1016/0006-3002(52)90045-0. [DOI] [PubMed] [Google Scholar]


