Abstract
Ovarian cancer is a lethal gynecologic malignancy with greater than 70% of women presenting with advanced stage disease. Despite new treatments, long term outcomes have not significantly changed in the past 30 years with the five-year overall survival remaining between 20% and 40% for stage III and IV disease. In contrast patients with stage I disease have a greater than 90% five-year overall survival. Detection of ovarian cancer at an early stage would likely have significant impact on mortality rate. Screening biomarkers discovered at the bench have not translated to success in clinical trials. Existing screening modalities have not demonstrated survival benefit in completed prospective trials. Advances in high throughput screening are making it possible to evaluate the development of ovarian cancer in ways never before imagined. Data in the form of human “-omes” including the proteome, genome, metabolome, and transcriptome are now available in various packaged forms. With the correct pooling of resources including prospective collection of patient specimens, integration of high throughput screening, and use of molecular heterogeneity in biomarker discovery, we are poised to make progress in ovarian cancer screening. This review will summarize current biomarkers, imaging, and multimodality screening strategies in the context of emerging technologies.
Keywords: Ovarian cancer, Screening, Biomarker, Detection, Diagnostic imaging, Proteomics, Adnexal mass
Core tip: Ovarian cancer is a lethal gynecologic malignancy with five-year survival of only 20% to 40% for advanced stage disease. Detection at an early stage would likely have significant impact on mortality rate. Advances in high throughput screening with the human “-omes” including the proteome, genome, metabolome, and transcriptome are now available in various packaged forms. To make progress in screening we need greater emphasis on prospective collection of patient specimens, integration of high throughput screening, and use of molecular heterogeneity in biomarker discovery.
INTRODUCTION
Ovarian cancer is a lethal gynecologic malignancy with greater than 70% of women presenting with advanced stage disease[1]. Worldwide it is estimated there are 225500 new cases of ovarian cancer and 140200 deaths every year including 14030 deaths in the United States alone[2,3]. Primary treatment for advanced stage disease involves both surgery and chemotherapy.
Despite new treatments, long term outcomes have not significantly changed in the past 30 years with the five-year overall survival remaining between 30 and 40%[3]. Greater than 60% of advanced stage patients will develop recurrent disease[4]. Patients with advanced stage disease have a five-year overall survival between 20% and 40%, in stark contrast to the greater than 90% five-year overall survival of patients identified and treated with stage I disease[5-7].
Given the poor prognosis for patients with advanced stage disease, effective screening modalities are needed to identify patients with early stage disease. The majority of women with early stage disease are asymptomatic, and unfortunately when they do present for diagnosis, three quarters are found to have regional or distant metastases[7]. Preliminary evaluations of screening with serum markers, pelvic ultrasounds, and multimodality strategies have demonstrated potential benefit in the earlier identification of ovarian cancer[8,9]. With these encouraging results, prospective screening trials have been undertaken as the scientific community continues to increase the number of potential biomarkers and imaging tests which might assist with identification of early stage ovarian cancer in asymptomatic women.
In the United States a woman’s lifetime risk of developing ovarian cancer is 1 in 70 and the prevalence of ovarian cancer in postmenopausal women over the age of 50 is 1 in 2500[10]. To minimize harms while identifying women at risk, a positive predictive value (PPV) of 10% is needed, requiring a sensitivity of greater than 75% and specificity of 99.6% to identify one case of ovarian cancer for every ten operations[10]. It is unlikely that one biomarker test will meet this criteria given the high specificity needed[11].
The ideal biomarker or panel of biomarkers is obtained through noninvasive means such as a bodily fluid: blood, saliva, urine, and cervical mucous are possibilities[12]. Advances in high throughput screening have made it possible to evaluate the human genome with the hope of better understanding genetic and epigenetic changes associated with the development of ovarian cancer. Enormous amounts of data in the form of human “-omes” including the proteome, genome, metabolome, and transcriptome are now available in various packaged forms. The Cancer Genome Atlas (TCGA) recently completed a comprehensive genomic and epigenomic evaluation of over three hundred high-grade serous ovarian cancer samples with microarray analyses and massively parallel sequencing coupled with hybrid affinity capture[13].
Ovarian cancer represents a very diverse group of tumors. Scientific endeavors such as TCGA are now making it possible to better delineate characteristics of various subtypes. The continued quest for a strategy that meets the need to identify asymptomatic women in the general population may depend on the ability to parse out the origins of ovarian cancer. The epithelial category, which accounts for 90% of all ovarian cancers, consists of the following subtypes: (1) serous (50%); (2) endometrioid (10%-25%); (3) mucinous (5%-10%); (4) clear cell (4%-5%); (5) undifferentiated carcinomas (5%); and (6) transitional cells (rare)[14]. These ovarian tumors are likely distinct diseases with different cells of origin and driver mutations, united under one term due to their predilection for dissemination to the ovary and related pelvic organs[15]. A pitfall of the past may be failure to develop screening strategies based on differences among these tumors. It is not yet clear if one screening strategy or separate approaches will be needed to identify patients with these tumor types at an early stage of disease. This review will summarize current biomarkers, imaging, and multimodality screening strategies in the context of emerging technologies.
BIOMARKERS AND EXISTING ALGORITHMS
Initially described by Bast et al[16] in 1981, cancer antigen 125 (CA125) was recognized by the murine monoclonal antibody OC-125 as an antigenic determinant on a high molecular-weight glycoprotein. It is the most widely studied biomarker in ovarian cancer screening. Measurement of CA125 can be performed with different commercial assays resulting in a certain degree of variation. The majority of assays appear to be both clinically reliable and correlative, nonetheless, new quantitative methods including mass spectrometry are under investigation[17,18]. As part of its development, CA125 underwent molecular cloning and was found to have characteristics of mucin, receiving the name MUC16[19].
In adults, CA125 is expressed in tissues derived from coelomic epithelium (mesothelial cells of the peritoneum, pleura, and pericardium) and Mullerian (tubal, endometrial, and endocervical) epithelia, as well as epithelia of the pancreas, colon, gall bladder, lung, kidney, and stomach[20,21]. CA125 can be elevated in a number of conditions unrelated to ovarian cancer, resulting in decreased specificity and PPV. Diverticulitis, endometriosis, liver cirrhosis, uterine fibroids, menstruation, pregnancy, benign ovarian neoplasms, and other malignancies (pancreatic, bladder, breast, liver, lung) can all result in an elevated CA125[11].
When values below 35 U/L are designated as normal, CA125 is elevated in 80% of epithelial ovarian cancers[22]. CA125 is elevated in approximately 50%-60% of stage I epithelial ovarian cancers and 75%-90% of patients with advanced stage disease[21,23]. The sensitivity of CA125 to identify early stage disease is limited as a screening tool. With evaluation of 22000 volunteers and over 50000 serum CA125 samples with a median follow up of 8.6 years, Jacobs et al[24] demonstrated CA125 levels in women without ovarian cancer remained static or decreased over time while levels associated with malignancy tended to increase[8,24]. Based on these findings, the Risk of Ovarian Cancer Algorithm (ROCA) was developed incorporating an individual’s age specific incidence of ovarian cancer and CA125 profile to triage women into various risk categories[8]. ROCA increased the sensitivity of CA125 from 62% to 86% for detection of preclinical ovarian cancer while maintaining a specificity of 98%[25]. A randomized control trial to evaluate ROCA consisted of 13582 postmenopausal women over the age of 50 and demonstrated a specificity of 99.8% (CI: 99.7% to 99.9%) and positive predictive value of 19% (CI: 4.1% to 45.6%)[26]. This model has been incorporated into various multimodality screening strategies in an attempt to optimize sensitivity, specificity, and positive predictive value.
The quest for other biomarker candidates has continued because a single CA125 value at a given time point will not reach a specificity of 99.6%, and approximately 20% of ovarian cancers may not express this antigen. Human epididymis protein (HE4), found primarily in the epithelia of normal genital tissues and made up of two whey acidic protein (WAP) domains and a four disulfide core, is elevated in epithelial ovarian cancer[27,28]. HE4 is overexpressed in 50% of clear cell, 93% of serous, and 100% of endometrioid cancers but is not overexpressed in mucinous tumors[28]. Identified initially as an mRNA transcript specific to the distal epididymal tissue, genomic advances with microarray gene expression profiling demonstrated HE4 is highly-expressed in ovarian cancer[29,30]. HE4 has greater specificity in the premenopausal age group than CA125 given it does not appear to be expressed at high levels in the setting of benign conditions such as endometriomas[31-33]. HE4 represents a victory for genomic strategies in the search for potentially effective biomarkers with microarray gene expression[34].
In a systemic review of women with suspected gynecologic disease HE4 demonstrated a higher specificity (93% vs 78%) and similar sensitivity (79%) to CA125 when distinguishing benign disease from ovarian cancer[35]. Studies have demonstrated a potential benefit in combining HE4 and CA125 when quantifying risk potential malignancy in the evaluation of a pelvic mass[36,37]. Even with new technology, it is unlikely that an individual biomarker will reach a specificity of 99.6%, positive predictive value of 10%, and sensitivity greater than 75% when screening an asymptomatic general population.
In efforts to further triage women in the detection of ovarian cancer, progress has been made in the development of algorithms to delineate malignancy in the setting of an adnexal mass. Woman appropriately referred to a gynecologic oncologist have better outcomes including survival, demonstrating the potential importance of these triage tests[38,39]. The Risk of Malignancy Index (RMI), developed by Jacobs et al[40] in 1990, is a formula which incorporates a woman’s CA125 level, ultrasound score, and menopausal status to determine her likelihood of malignancy in the setting of an adnexal mass. Since that time two other algorithms have been developed for assessment of malignancy risk in women with adnexal masses: the Risk of Malignancy Algorithm (ROMA) and the OVA1 test[41,42]. The ROMA algorithm is based on serum levels of HE4 and CA125 with menopausal status[41]. OVA1, with the exception of CA125, is made up of biomarkers discovered through mass spectrometry: β-2 microglobulin, transferrin, transthyretin, and apolipoprotein[42,43]. Various studies have been published evaluating the effectiveness of RMI, ROMA, and OVA1, as well as other strategies to help delineate the likelihood of malignancy in the setting of a pelvic mass. Table 1 provides a summary of various algorithms and assays used to predict likelihood of malignancy.
Table 1.
Screening algorithms and commercially available assays
| Algorithm or assay (Screening population) | How it works |
| ROCA (asymptomatic general population) | 1 Compares a woman’s longitudinal CA-125 pattern to the change-point CA-125 profile seen in women with ovarian cancer and the flat CA-125 profiles seen in women without ovarian cancer[1] 2 Based on the ROCA result, women get triaged into one of three groups[1]: (1) Low Risk: continue annual CA-125 testing (2) Intermediate Risk: repeat CA-125 test 3 mo later (3) High Risk: receive TVS and referral to a gynecologic oncologist 3 After each additional CA-125 value, ROCA is recalculated and a new recommendation is made[1] |
| ROMA (known pelvic mass) | 1 Uses both HE-4 and CA-125 test levels to evaluate patients as low or high risk for ovarian cancer[8] 2 A predictive index (PI) is calculated using different equations for pre-menopausal and post-menopausal women[8] 3 The PI is then inserted into the ROMA algorithm to predict the probability of ovarian cancer[8] |
| RMI (known pelvic mass) | Uses menopausal status, ultrasound findings, and serum CA-125 levels to determine malignancy risk[40] |
| OVA1 (known pelvic mass) | 1 A multivariate index assay that incorporates CA-125, transferrin, transthyretin (prealbumin), apolipoprotein A1, and beta-2-microglobulin[41] 2 An algorithm is used to generate an ovarian malignancy risk score between 0 and 10[41] 3 OVA1 scores greater than or equal to 5.0 (premenopausal) or 4.4 (postmenopausal) result in high risk stratification and referral to a gynecologic oncologist[41] |
| LR-1 (known pelvic mass) | 1 An ultrasound-based prediction model 2 Twelve variables are used to calculate a probability of malignancy[88]: (1) personal history of ovarian cancer (2) current hormonal therapy (3) age of the patient (4) maximum diameter of the lesion (5) pain during examination (6) ascites (7) blood flow within a solid papillary projection (8) a purely solid tumor (9) maximum diameter of the solid component (10) irregular internal cyst walls (11) acoustic shadows (12) color score |
| LR-2 (known pelvic mass) | 1 An ultrasound-based prediction model 2 Uses six variables to calculate a probability of malignancy[90]: (1) patient’s age (2) presence of ascites (3) presence of blood flow within a papillary projection (4) maximal diameter of solid components (5) irregular internal cyst walls (6) presence of acoustic shadows |
ROCA: Risk of ovarian cancer algorithm; ROMA: Risk of ovarian malignancy algorithm; RMI: Risk of malignancy index; OVA1: Vermillion Inc. OVA1® blood test; LR-1: International ovarian tumor analysis logistic regression model 1;LR-2: International ovarian tumor analysis logistic regression model 2; ROCA: Risk of ovarian cancer algorithm; ROMA: Risk of Malignancy Algorithm.
OVA1 and ROMA each have benefits and disadvantages. Prospective multi-institutional trials and cost-benefit analysis are needed before definitive conclusions can be drawn regarding these tests[34]. Table 2 lists sensitivities and specificities for various modalities in the setting of a pelvic mass. Based on available data, OVA1 and ROMA likely have similar sensitivities, but ROMA appears to have greater specificity (75% vs 43%) which may impact cost-effectiveness and referral patterns from general gynecologists reticent to lose patients with benign masses to gynecologic oncologists[53]. OVA1, based largely on mass spectrometry with proteomics, and ROMA, made possible by the incorporation of a microarray gene-expression based discovery in HE4, represent hopeful advancements in the ability to identify women with malignancy earlier than had been in the past. These are not screening tests for the general population, but represent potential tools to further triage of women to the appropriate providers once the decision for surgical intervention has been made.
Table 2.
Specificity and sensitivity results of various screening strategies in the setting of a pelvic mass
| Algorithm or assay | Ref. | Sensitivity (%) | Specificity (%) |
| ROMA | Karlsen et al[44] | 94.4 | 76.5 |
| Moore et al[45] | 94.3 | 75 | |
| Sandri et al[46] | 91.2 | 75 | |
| 89.3 | 81.7 | ||
| Van Gorp et al[89] | 84.7 | 76.8 | |
| Sandri et al[46] | 84.4 | 90 | |
| Chan et al[47] | 89.2 | 87.3 | |
| Kaijser et al[90] | 84 | 80 | |
| RMI | Karlsen et al[44] | 94.4 | 81.5 |
| Håkansson et al[48] | 92 | 82 | |
| Moore et al[45] | 84.6 | 75 | |
| Van den Akker[49] | 81 | 85 | |
| Van Gorp et al[89] | 76 | 92.4 | |
| OVA1 | Bristow et al[50] | 92.4 | 53.5 |
| Longoria et al[52] | 92.2 | 49.4 | |
| OVA1 + | Bristow et al[50] | 95.7 | 50.7 |
| Clinical assessment | Longoria et al[52] | 95.3 | 44.2 |
| LR-1 | Kaijser et al[88] | 93 | 77 |
| LR-2 | Nunes et al[51] | 97 | 69 |
| Kaijser et al[88] | 92 | 75 | |
| Kaijser et al[90] | 93.8 | 81.9 | |
| TVS | van Nagell et al[8] | 86.4 | 98.8 |
ROMA: Risk of ovarian malignancy algorithm; RMI: Risk of malignancy index; OVA1: Vermillion Inc. OVA1® blood test; LR-1: International ovarian tumor analysis logistic regression model 1; LR-2: International ovarian tumor analysis logistic regression model 2; TVS: Transvaginal ultrasonography.
Various other biomarkers and biomarker panels are currently under development for both the prediction of malignancy in the setting of a pelvic mass and in asymptomatic women. Table 3 lists various single biomarker and multi-biomarker panels with sensitivities and specificities for ovarian cancer detection. An important consideration with all of these tests is the ultimate need to demonstrate benefit for patients through reduction in morbidity and mortality while minimizing harm. The advancements of technology combined with our exponentially growing knowledge of the human “-omes” have outpaced our ability to reliably test these discoveries through clinical settings in a timely fashion.
Table 3.
Results of serum marker panels for the detection of ovarian cancer
| Serum marker(s) | Ref. | Sensitivity (%) | Specificity (%) |
| CA-125 | 1Karlsen et al[44] | 91.7 | 75 |
| 1Chan et al[47] | 90.8 | 67.2 | |
| 1Leung et al[123] | 89 | 90 | |
| 1Sandri et al[46] | 84.4 | 80 | |
| 1Montagnana et al[54] | 83 | 100 | |
| 1Sandri et al[46] | 73.1 | 90 | |
| Yang et al[55] | 62.5 | 80 | |
| Havrilesky et al[56] | 45.9-58.5 | 98.2 | |
| 1Moore et al[37] | 43.3 | 95 | |
| Jacob et al[57] | 12.5 | 90.1-93.9 | |
| HE-4 | 1Montagnana et al[54] | 98 | 100 |
| Yang et al[55] | 96.2 | 83.8 | |
| 1Karlsen et al[44] | 91.3 | 75 | |
| 1Sandri et al[46] | 83.1 | 90 | |
| Havrilesky et al[56] | 82.7-92.5 | 86.3 | |
| 1Moore et al[37] | 72.9 | 95 | |
| Jacob et al[57] | 62.5 | 81.8-85.9 | |
| 1Chan et al[47] | 56.9 | 96.9 | |
| CA-125, HE-4 | 1Moore et al[37] | 76.4 | 95 |
| 1Moore et al[41] | 88.7 | 74.7 | |
| CA 125, leptin, PRL, OPN, IGFII, MIF | Visintin et al[58] | 95.3 | 99.4 |
| CA 125, CRP, SAA, IL-6, IL-8 | Edgell et al[59] | 94.1 | 91.3 |
| CA-125, apoA-I, TTR, TF | Su et al[60] | 89-97 | 91-99 |
| CA 125, HE4, CEA, VCAM-1 | Yurkovetsky et al[61] | 86–93 | 98 |
| CA 125, ApoA1, TTR | Kim et al[62] | 93.9 | 95 |
| Zhang et al[42] | 74 | 97 | |
| CA 125, CA 19-9, EGFR, CRP, myoglobin, ApoA1, ApoCIII, MIP-1a, IL-6, IL-18, tenascin C | 1Amonkar et al[63] | 91.3 | 88.5 |
| CA-125, OVX1r, LASA,CA15-3, CA72-4 | Nossov et al[11] | 90.6 | 93.2 |
| CA 125, CA 72-4, CA 15-3, M-CSF | 1Skates et al[64] | 70 | 98 |
| LPA | Nossov et al[11] | 90-100 | 90 |
| FOLR1 | 1Leung et al[123] | 62 | 90 |
| M-CSF | Nossov et al[11] | 61-68 | 93 |
| SMRP | 1Moore et al[37] | 53.7 | 95 |
Study involved patients presenting with a pelvic mass; CA: Cancer antigen; HE-4: Human epididymis protein 4; PRL: Prolactin; OPN: Osteopontin; IGFII: Insulin-like growth factor II; MIF: Macrophage inhibitory factor; CRP: C-reactive protein; SAA: Serum amyloid A; IL: Interleukin; apoA-I: Apolipoprotein A-I; TTR: Transthyretin; TF: Transferrin; CEA: Carcinoembryonic antigen; VCAM-1: Vascular cell adhesion protein 1; EGFR: Epidermal growth factor receptor; ApoCIII: Apolipoprotein CIII; MIP-1a: Macrophage inflammatory protein-1alph; OVX1: Mouse antibody generated by immunizing mice with antigenic preparations from multiple OC cell lines[21]; LASA: Lipid-associated sialic acid; M-CSF: Macrophage colony-stimulating factor; LPA: Lipoprotein A; FOLR1: Folate receptor 1; SMRP: Soluble mesothelin-related peptide.
AREAS OF GROWTH IN BIOMARKER DISCOVERY
High throughput technology in conjunction with TCGA has now made it possible to combine multiplex assays with data from the proteome, genome, metabolome, and transcriptome. Within proteomics, biomarker panels have been developed in an attempt to increase sensitivity for ovarian cancer detection due to the heterogeneous make up of subtypes (Table 3). Biomarker discovery in proteomics is usually based on two-dimensional gel electrophoresis, mass spectrometry (MS), and/or protein microarrays in combination with bioinformatics analysis[65]. MS with matrix assisted laser desorption and ionization time of flight (MALDI-TOF) and surface-enhanced laser desorption and ionization time of flight (SELDI-TOF) allow for the entire protein complement of a patient sample to be evaluated in rapid high throughput fashion[12,64]. Protein microarrays can be used to profile the proteome of cell populations using antigen-antibody interactions[66]. Protein microarrays are made up of two major classes: (1) forward-phase arrays (FPA) with antibodies arrayed and probed with cell lysates; and (2) reverse-phase arrays (RPA) with cell lysates arrayed and probed with antibodies[65].
Unfortunately, proteomics has not resulted in the major breakthroughs previously anticipated. An important consideration here is the biological samples used when identifying potential biomarkers. Various studies have demonstrated protein biomarkers perform very differently in the detection of ovarian cancer when analyzed in prospectively collected samples from asymptomatic patients[67,68]. Future proteomic discovery may best focus on samples from patients prospectively followed until diagnosis in larger population based trials. Incorporation of methods aimed at depletion of abundant serum proteins such as acute phase reactants, and the use of multiplex bead-based immunossays may allow for identification of low abundance or low concentration proteins not previously identified[12].
MS continues to serve as an important tool to explore the thousands of proteins relevant to ovarian cancer and has now been extended to use in glycomics, metabolomics, MALDI-MS imaging, and autoantibody signatures for biomarker discovery[69]. Glycosylation or the addition of carbohydrates to nascent proteins is a common post-translational modification that is potentially altered in a malignant state[69-71]. There is evidence to indicate various histologic subtypes of ovarian cancer exhibit different glycoproteins[72]. This is encouraging given the significant heterogeneity of ovarian cancer. The differences seen in glycomics may assist in screening algorithms which can be developed with this heterogeneity in mind.
Evaluation of the metabolome through MS has demonstrated differences in metabolites in patients with and without epithelial ovarian cancer[73,74]. Existing concerns with the study of metabolites include the significant variation in metabolic response and extensive biotransformation from the site of malignancy to fluids such as serum or plasma[75]. Study of the peptidome within the low-molecular weight proteome in ovarian cancer has been limited by the potential loss of peptides bound to carrier proteins during sample processing, although attempts have been made to mitigate this with isolation and enrichment of carrier proteins prior to MS evaluation[69,76]. Ovarian cancer diagnoses may also be aided with the uses of anti-tumor autoantibody signatures and MALDI-MS imaging; however, these areas of research are preliminary with MS[77,78].
Separate from the use of MS, there is a growing role for microRNAs in the development of ovarian cancer biomarkers[79]. MicroRNAs are a class of small noncoding RNAs which impact gene expression by targeting multiple messenger RNAs and triggering translation repression and/or RNA degradation[80]. Aberrant expression of microRNAs in ovarian cancer indicate they may act as a novel class of oncogenes or tumor-suppressor genes[79]. Five microRNAs (miR-200a, miR-100, miR-141, miR-200b, and miR-200c) have been found to be consistently differentially regulated in epithelial ovarian cancer and may assist in the development of biomarkers[81]. The future is promising with these techniques; however, validation strategies and appropriate patient samples are vital to improving success in clinical testing. No individual biomarker or biomarker panel has been developed which meets the sensitivity, specificity, and PPV criteria desired for screening in a general population.
IMAGING
There has been an immense effort placed in the evaluation of screening with radiologic technology. A systematic approach to the diagnosis of ovarian tumors with imaging is necessary given the majority of women have benign lesions, and unnecessary interventions should be avoided without placing patients at risks for advanced stage disease[82]. Available imaging modalities include ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET). Pelvic ultrasound has been the most studied imaging modality in ovarian cancer screening. Of 48053 postmenopausal women in the ultrasound group of the United Kingdom Collaborative Trial of Ovarian Cancer Screening (UKCTOCS), 4367 asymptomatic women (9.1%CI: 8.8%-9.3%) had abnormal adnexal morphology with an overall absolute risk of epithelial ovarian cancer of 1.08% (95%CI: 0.79%-1.43%) and a 1 in 22 risk of epithelial ovarian cancer if the abnormal findings included solid elements[83].
In a single arm prospective screening cohort, the University of Kentucky Ovarian Cancer Screening Trial, asymptomatic women 25 years or older with a documented family history of ovarian cancer and asymptomatic women 50 years or older were screened with annual transvaginal ultrasound[84]. Serial ultrasonography in this trial demonstrated many ovarian abnormalities resolve in follow up: 63.2% of women with an initially abnormal ultrasound were found to have resolution on subsequent imaging[85]. Observation with serial imaging may help improve positive predictive value and decrease false positive results in screening trials[85]. Of 37293 women who underwent annual screening, the five-year disease-free survival rate for women with ovarian cancer in the screening group, including those who developed ovarian cancer within one year of a normal ultrasound (false negative), was 74.8% ± 6.6%. In contrast, a group of unscreened women with ovarian cancer treated at the same institution with the same surgical and chemotherapeutic protocols had a five-year disease free survival of 53.7% ± 2.3%, P-value < 0.01[86]. Ultrasound screening does not impact disease-free survival by itself. Ultimately, the goal of ultrasound screening is to identify patients with early stage disease who can be treated before the malignancy becomes advanced. While the results from this study are encouraging, the mortality benefit may have been impacted by a healthy volunteer effect and lead time detection rather than impact on the natural history of ovarian cancer[87].
Although screening in an asymptomatic population ultimately provides the best opportunity to improve survival in women with ovarian cancer, there has been progress made in the development of imaging algorithms designed for those women with a known adnexal mass. The International Ovarian Tumor Analysis (IOTA) group has developed various approaches to characterize adnexal masses as malignant or benign with ultrasound guidelines. These approaches can be divided into two strategies: the first consisting of risk prediction with two logistic regression models (LR1 and LR2) based on demographic and ultrasound variables (Table 1), and the second based on simple ultrasound features that are descriptors of benign or malignant masses[88]. In women with a pelvic mass the sensitivity and specificity of ROMA and the RMI were compared to subjective assessment by skilled ultrasonographers in a prospective cohort study of women[89]. The sensitivity of ROMA, RMI, and expert ultrasonographers were 84.7% (77.9% to 90.0%), 76.0% (68.4% to 82.6%), and 96.7% (92.4% to 98.9%) respectively, and the specificity was 76.8% (70.7% to 82.2%), 92.4% (88.1% to 95.5%), and 90.2% (85.5% to 93.7%) respectively[89]. Generalizability of these results may not be possible based on its location, the cohort, and the ultrasonographers used. The study took place at one single tertiary care center in Europe with experienced ultrasonographers and a high prevalence of malignant disease in the cohort[89].
In a different study, a cross-sectional cohort of 360 patients with adnexal masses undergoing surgery was retrospectively evaluated with ROMA and LR2[90]. This study demonstrated decreased sensitivity and specificity for ROMA vs LR2 in both premenopausal and postmenopausal patients, with overall sensitivity 84.0% vs 93.8%, and specificity 80% vs 81.9%, respectively[90]. While this result indicates LR2 may be a more effective screening test in the setting of adnexal mass, prospective randomized control trials are needed before conclusions can be made regarding the use of algorithms which include biomarkers such as HE4 and CA125 (ROMA) vs ultrasound-based prediction models such as LR2. Table 2 lists sensitivities and specificities for various modalities in the setting of a pelvic mass.
Currently no prospective randomized studies support the use of imaging as a single strategy in screening for ovarian cancer. At this time, given ultrasound is relatively inexpensive, available widely, and can provide tissue specific information with a presumptively risk-free technology; it is the method of choice for initial evaluation of an adnexal mass and estimating risk of malignancy[82]. In asymptomatic postmenopausal women, the ultrasound screening arm results of the UKCTOCS expected in 2015 will help elucidate the role of ultrasound in population-based screening strategies. At this time is it unlikely ultrasound will significantly reduce mortality in primary screening, but it may be extremely important in reducing false positive rates in multimodality screening[91]. Existing ultrasound-based strategies evaluating the likelihood of malignancy in the setting of a known adnexal mass are based on those who have already been scheduled for surgery. Comparative prospective studies are needed to determine efficacy and effect on survival in women who have surgery based on prediction models using proposed ultrasound-based strategies with and without biomarkers such as CA125 and HE4.
MULTIMODALITY SCREENING
The promising results of imaging in population-based screening for ovarian cancer have led to large scale multimodality strategies. Prior prospective studies demonstrating CA125 and ultrasound were feasible screening modalities have given way to prospective randomized multimodality screening trials involving ultrasound, serum biomarkers, and risk calculations using patient demographics[92,93]. The Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) is a multicenter randomized control trial of 78216 asymptomatic women aged 55 to 74 years who underwent multimodality screening or usual care between November 1993 and July 2001 with management of positive screens left to the discretion of the patient’s physician[94]. Multimodality screening consisted of annual testing for three years with transvaginal ultrasound and serum CA125 with a cutoff of 35 U/mL followed by CA125 alone for an additional two years[94]. After four rounds of screening, the PPV and cancer yield per 10000 women screened in the multimodality screening arm remained similar across screening rounds at 1.0% to 1.3% and 4.7 to 6.2 cancers respectively with the overall ratio of surgeries to screen-detected cancers 19.5 to 1[95]. After a median follow up of 12.4 years (25th% to 75th%, 10.9 to 13.0), no mortality benefit was found with combination transvaginal ultrasound and CA125 using an absolute cutoff: 118 deaths due to ovarian cancer (3.1 per 10000 person-years) in the intervention group and 100 deaths (2.6 per 10000 person-years) in the usual care group (mortality rate ratio, 1.18; 95%CI: 0.82-1.71)[96].
The Japanese Shizuoka Cohort Study of Ovarian Cancer Screening is a randomized control trial of 82487 low risk postmenopausal women between 1985 and 1999 with the intervention arm consisting of annual ultrasound and CA125 with a cutoff value[97]. The strategy achieved a sensitivity of 77.1% and specificity of 99.9% with a nonsignificant difference in the proportion of stage I ovarian cancers identified, 63% in the screened group vs 38% in the control group, P-value = 0.2285[97]. Mortality results from this trial have not yet been published and, as such, conclusions cannot be drawn from this trial regarding the benefit of screening in an asymptomatic population.
The UKCTOCS is a randomized prospective multi-arm ovarian cancer screening study in the United Kingdom. This trial, made up of 202638 post-menopausal women aged 50 to 74, randomizes women in a 2:1:1 format to three arms: (1) control; (2) annual screening with ultrasound; and (3) a multimodality strategy that takes advantage of ROCA to triage women to various substrategies[98]. These substrategies include transvaginal ultrasound and/or repeat CA125 at defined time points[98]. In the prevalence screen of the UKCTOCS, ultrasonography alone was compared to multimodality screening (ROCA as a primary test followed by transvaginal ultrasound as a secondary test or repeat CA125 if indicated). With regard to primary invasive epithelial and tubal cancers, the multimodality screening arm demonstrated a higher specificity compared to the ultrasonography arm (99.8% vs 98.2%), P-value < 0.001, while the difference in sensitivity was not statistically significant (89.4% vs 84.9%), P-value = 0.564[98].
A single-arm prospective cohort study of 4051 average-risk postmenopausal women in the United States was performed over 11 years using a two-stage ovarian cancer screening strategy (CA125 interpreted through ROCA with subsequent repeat CA125 or transvaginal ultrasound as indicated) with a PPV of 40% for invasive ovarian cancer and specificity of 99.9% (95%CI: 99.7%-100%)[1]. The results from both the UKCTOCS and two-stage strategy in the United States indicate the use of ROCA to interpret CA125 may be effective in triaging women to subsequent follow-up categories that impact both screening outcomes.
When comparing the UKCTOCS prevalence screen results to the PLCO trial results, both the UKCTOCS multimodality arm (89.4% vs 51.7%) and the ultrasound arm (75.0% vs 67.4%) had higher sensitivities[99]. When CA125 values were retrospectively evaluated with ROCA within the PLCO data set no mortality benefit was seen; best-case and stage-shift scenarios resulted in 25 and 19 deaths prevented with ROCA for relative risks of 0.90 (95%CI: 0.69-1.17) and 0.95 (95%CI: 0.74-1.23), respectively[100]. In addition to the use of absolute cutoff value for CA125, other concerns have been raised regarding the PCLO trial design including leaving management of positive screens to the discretion of the treating physician and 40.6% of ovarian cancer diagnosis took place after the screening ended[101]. Use of an individualized algorithm that tracks a patient over time will likely provide the best combination of sensitivity, specificity, and PPV. For this reason, the UKCTOCS and its incorporation of ROCA in the multimodality screening arm, represents the best opportunity yet to identify a potential screening strategy. The results from the final mortality analysis in the UKCTOCS will be reported in 2015 and provide significant insight into whether population-based screening in asymptomatic women is possible with currently available imaging and biomarkers.
SYMPTOM-BASED SCREENING
Screening efforts in ovarian cancer have largely focused on asymptomatic women in the general population or women with known adnexal masses requiring further dichotomization for treatment purposes. Women with ovarian cancer do have physical symptoms such as abdominal pain, bloating, and bowel irregularity that may serve as a potential trigger for diagnosis. In a case-control study comparing woman with ovarian cancer to age and race matched controls, more than 90% of cases reported at least one symptom and symptoms were cited as the most common reason for the doctor visit leading to diagnosis (74%)[102]. Two feasibility studies have been performed demonstrating symptom-based screening in women is possible[103,104]. A symptom index was created with a sensitivity of 56.7% for early stage disease and 79.5% for advanced stage disease, and a specificity of 90% for women greater than 50 years of age and 86.7% for women less than 50 years of age[105]. Based on patient interviews performed with 812 women with ovarian cancer and 1313 population-based controls, the symptom index and symptoms established in consensus recommendations had a PPV of 0.6%-1.1% overall and less than 0.5% for early-stage disease[106]. The identification of specific symptoms associated with ovarian cancer has value, but recognition of symptoms alone will not significantly improve overall survival from ovarian cancer[107]. A cross sectional study of 160 women evaluated with use of this symptom index found that the addition of CA125, HE4, or the ROMA to a positive symptom index increased PPV when determining malignancy vs benign process in patients with a known adnexal mass[108]. At this time, given no effective screening tool has been proven in a prospective model, physicians should continue to discuss potential symptoms with their patients in an effort to increase self-awareness regarding warning signs for ovarian cancer.
SCREENING IN HIGH RISK PATIENTS
Familial genetic predisposition makes up approximately 10% of ovarian cancers with germline mutations in BRCA1/BRCA2 and mismatch repair (MMR) genes in Lynch syndrome being the most common[109]. Women with BRCA1 and BRCA2 mutations have a cumulative lifetime risk of ovarian cancer of 40%-50% and 20%-30% respectively, while the DNA MMR genes, including those that predispose to Lynch syndrome, result in a cumulative lifetime risk of ovarian cancer ranging from 6.7% to 12%[110]. As seen with improved survival in BRCA-associated ovarian cancers, inherited ovarian cancers may have biological differences which allow treatment at time of screen detection to have significant benefit[111].
Currently, no prospective studies exist which demonstrate a mortality benefit by screening high risk asymptomatic patients. The United Kingdom Familial Ovarian Cancer Screening Study (FOCSS) has recently completed a phase 1 trial in which 3563 women at greater than a 10% risk of ovarian or fallopian tube cancer were screened with annual transvaginal ultrasound and CA125 for a mean of 3.2 years[109]. Sensitivity for detection of incident ovarian and fallopian tube cancers at one year after last annual screen was 81.3% (95%CI: 54.3%-96.0%) if occult cancers were classified as false negatives, and the PPV was 25.5% (95%CI: 14.3%-40.0%) with only four women undergoing surgery for each case of detected cancer[109]. As part of phase II of the FOCSS, screening frequency will increase to every four months, ROCA will be incorporated into the decision tree, and the threshold and work-up for repeat tests will be per protocol. The Gynecologic Oncology Group (GOG) and Cancer Genetics Network have recently completed GOG 199, a prospective study screening women at high risk of ovarian cancer with the use of ROCA and transvaginal ultrasound[112].
For women with Hereditary Breast/Ovarian Cancer Syndrome who have not undergone risk reducing bilateral salpingo-oophorectomy, the National Comprehensive Cancer Network recommends screening with transvaginal ultrasound and CA125 every 6 mo starting at age 30 or 5 to 10 years prior to the earliest age at diagnosis of ovarian cancer in relatives[113]. Given the potential biologic differences associated with high risk patients, screening asymptomatic women within this population may have greater benefit than in the general population. The FOCSS phase II results and GOG 199 will provide evidence regarding potential screening benefits and assist with strategy optimization.
FUTURE CONSIDERATIONS
Despite the technological advances which have been made, our current approach to screening strategies in ovarian cancer has inherent difficulties which need to be overcome. Directly impacting our ability to screen asymptomatic women for ovarian cancer is the evolving reclassification of this heterogeneous group of tumors. Results from the recently completed study of serous ovarian cancer through TCGA demonstrate significant genomic heterogeneity even within one subtype of epithelial ovarian cancer, high grade serous carcinoma[13].
As in colorectal cancer and cervical cancer, identification of a precursor lesion or lesions will improve our ability to screen for the disease. These precursor lesions are likely varied based on the subtype of ovarian cancer. With high grade serous carcinoma, a precursor lesion may develop in the fimbria of the fallopian tube (serous tubal intraepithelial carcinoma also known as a STIC) or in an ovarian cortical inclusion cyst during implantation of fimbrial epithelium on the denuded ovarian surface with ovulation[114]. Genetic evaluation links both clear cell and endometrioid carcinomas to precursor lesions within endometriosis[115]. A new model that considers both morphologic and molecular characteristics separates epithelial ovarian tumors into two categories: type I tumors are low-grade serous, low-grade endometrioid, clear cell, and mucinous tumors which usually present as large cystic masses within one ovary, while type II tumors are composed of high-grade serous, high-grade endometrioid, malignant mixed mesodermal (carcinosarcoma), and undifferentiated carcinomas which commonly present as advanced stage disease[116].
A focus on identification of the origins of these groups of tumors will lead to more effective screening strategies in an asymptomatic population. For example, evaluating blood samples of patients found to have STICs at the time of prophylactic bilateral salpingo-oophorectomy may prove useful in identifying biomarkers for preclinical serous carcinoma[12]. Type I tumors tend to be genetically stable with mutations in various genes including PTEN, BRAF, β-catenin and KRAS, while type II tumors have a high level of genetic instability and commonly have a TP53 mutation[117]. Biomarker panels and multimodality screening may achieve better sensitivity and specificity with screening strategies based on differences in both cell origin and genetics among these varied tumors.
In addition to the varied origin and molecular heterogeneity, the time course of ovarian cancer development still eludes understanding. The time required for development of invasive disease or progression from stage I to stage III remains unknown[118]. This information is likely specific to the various ovarian tumors, and improved categorization through molecular advances will better elucidate the time course of disease. For example, type I tumors appear to follow a developed path of transformation with stepwise progression from a benign lesion to a malignant tumor[119]. It has been proposed that ovarian cancer screening strategies should focus on type II tumors with the goal to identify low volume disease rather than early stage, as high grade serous carcinomas represent 75% of all ovarian cancers and result in the majority of deaths[113,120]. Low volume advanced stage disease may be more easily resectable at the time of tumor debulking, but advanced stage patients still have a worse prognosis than those patients who are treated with early stage disease. Identification of early stage disease will have the greatest benefit on mortality, and will require a shift from current approaches to incorporate advances made in the understanding of tumor heterogeneity in this malignancy.
Five phases of biomarker development have been previously proposed: (1) the preclinical exploratory phase; (2) the clinical assay and validation stage; (3) the retrospective longitudinal study; (4) prospective screening evaluation; and (5) randomized control trials[121]. The preclinical exploratory phase must take advantage of developments in high-throughput screening technologies to more effectively identify potential biomarkers among the thousands of candidate molecules. For example, a biomarker discovery platform which incorporates proteome and transcriptome comparisons of serum, tissue, ascites, cancer cell lines, and animal models through mass spectrometry and microarray technology makes it possible to take advantage of these immense data sets[122]. Folate receptor 1 protein, developed through use of proteomics, transcriptomics, and bioinformatics, demonstrates the incorporation of various technologic platforms that make it possible to identify new biomarkers[123].
Further efforts must be devoted to the collection of appropriate patient specimens in prospective trials. Within ovarian cancer, the majority of biomarkers are evaluated with patient samples taken at the time of diagnosis, usually advanced stage disease. It is not surprising that biomarkers discovered in an advanced disease setting do not perform with the same sensitivity or specificity in a prospective trial in which the goal is diagnosis of early stage disease. Prospectively collected samples in asymptomatic women provide a better understanding of the ability of candidate biomarkers to detect cancer prior to physical symptoms[124]. The prospective specimen collection retrospective blinded evaluation (PRoBE) study design mandates samples are collected prospectively, stored in a similar fashion, and once outcome status is defined, used to validate biomarkers in a blinded fashion with randomly selected cases and controls[125]. Given the low prevalence of ovarian cancer in the general population, pooling of resources is necessary to make advances in biomarker discovery. The National Cancer Institute’s Early Detection Research Network assists with development of prospective patient samples under the PRoBE study design[126]. PLCO samples have been used in this fashion to test potential biomarkers[67,68]. Development of a large scale collection of samples prospectively in asymptomatic women on a national or international level would provide the ability to validate biomarkers and predict lead time in the discovery of ovarian cancer prior to physical symptoms (Figure 1).
Figure 1.
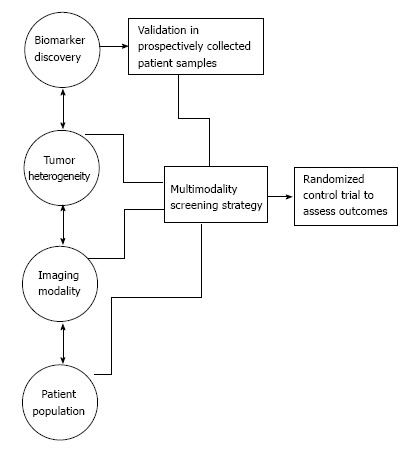
Biomarker candidates must be tested in patient samples collected prior to the onset of physical symptoms in ovarian cancer screening strategies.
The final phase of biomarker design is a randomized control trial, with the goal of ovarian cancer screening to demonstrate a mortality benefit in the studied population. This mortality benefit must be considered in the context of the number needed to treat to reach such a benefit. A systematic review and meta-analysis of available screening trials involving asymptomatic women found no reduction in ovarian cancer-specific or all-cause mortality [relative risk (RR), 1.08; 95%CI: 0.84-1.38; and 1.0; 95%CI: 0.84-1.38 respectively][127]. While this analysis does not include results from the UKCTOCS which will not be available until 2015, it does demonstrate that prospective trials within current paradigms have failed to meet major goals.
In the PLCO trial 1080 women underwent surgery in the setting of false positive results and 163 (15%) experienced a complication[96]. Based on review of available clinical trials, 6% of women with false positive screening results experienced a severe complication while undergoing surgery[127]. These patients underwent potential harm without benefit. A mortality benefit is necessary to justify the potential harm associated with false positives. If the UKCTOCS and/or the Japanese cohort fail to show a benefit in mortality, this may be explained by lead time bias in which slow growing tumors are detected more commonly by screening than fast growing lethal serous epithelial ovarian cancers[91]. Type I tumors, which tend to be slow growing and more indolent than type II tumors, were detected twice as often as type II tumors in the ultrasound arm of the UKCTOCS (32 borderline or type I tumors vs 15 type II tumors) despite a higher prevalence of type II tumors in epithelial ovarian cancer[83]. If this same pattern is seen through the UKCTOCS in 2015, it is unlikely there will be a mortality benefit given the better prognosis associated with the majority of borderline and type I tumors compared to type II tumors.
While further prospective screening trials will take place, ovarian cancer screening in the asymptomatic general population results in potential harms without proven benefit at this time. Guidelines from the American College of Obstetrics and Gynecology, the Society of Gynecologic Oncologists, the United States Preventive Services Task Force, and the American Cancer Society do not recommend screening for ovarian cancer in asymptomatic low-risk women in the general population[128-130].
CONCLUSION
Ovarian cancer is deadly at advanced stage, and as the quest for an optimal screening strategy continues, it is apparent there are risks associated with false positives and invasive tests. When surveyed, 80% of women without risk factors or symptoms for ovarian cancer in the University of Kentucky cohort felt that they would definitely want to participate in ovarian cancer screening starting at age 50[131]. Various avenues continue to be investigated in ovarian cancer screening including imaging, protein profiles, specific symptoms, and combinations of these, as well as other modalities. An expanded and shared biobank of patient specimens collected before development of symptoms and advanced disease is needed. It is with these precious samples that high throughput technology and human “-omes” will have the most positive impact on identification of screening modalities. Emerging technology will allow science to evaluate biological data in ways never imagined. With the correct pooling of resources, including prospective collection of patient specimens, integration of high throughput screening, and use of molecular heterogeneity in biomarker discovery, we are poised to make progress in ovarian cancer screening. If we are prudent in trial design and altruistic in the sharing of resources such as biological samples, identification of an effective screening modality for ovarian cancer is within our capabilities.
Footnotes
P- Reviewer: Wiemer EAC, Wang QE S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Lu KH, Skates S, Hernandez MA, Bedi D, Bevers T, Leeds L, Moore R, Granai C, Harris S, Newland W, et al. A 2-stage ovarian cancer screening strategy using the Risk of Ovarian Cancer Algorithm (ROCA) identifies early-stage incident cancers and demonstrates high positive predictive value. Cancer. 2013;119:3454–3461. doi: 10.1002/cncr.28183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 4.Salani R, Santillan A, Zahurak ML, Giuntoli RL, Gardner GJ, Armstrong DK, Bristow RE. Secondary cytoreductive surgery for localized, recurrent epithelial ovarian cancer: analysis of prognostic factors and survival outcome. Cancer. 2007;109:685–691. doi: 10.1002/cncr.22447. [DOI] [PubMed] [Google Scholar]
- 5.Cannistra SA. Cancer of the ovary. N Engl J Med. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 6.Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, et al. SEER Cancer Statistics Review, 1975-2010. SEER online, cited 2013-04. Available from: http: //seer.cancer.gov/csr/1975_2010/
- 7.Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19:3–10. doi: 10.1002/1098-2388(200007/08)19:1<3::aid-ssu2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs IJ, Skates SJ, MacDonald N, Menon U, Rosenthal AN, Davies AP, Woolas R, Jeyarajah AR, Sibley K, Lowe DG, et al. Screening for ovarian cancer: a pilot randomised controlled trial. Lancet. 1999;353:1207–1210. doi: 10.1016/S0140-6736(98)10261-1. [DOI] [PubMed] [Google Scholar]
- 9.van Nagell JR, DePriest PD, Reedy MB, Gallion HH, Ueland FR, Pavlik EJ, Kryscio RJ. The efficacy of transvaginal sonographic screening in asymptomatic women at risk for ovarian cancer. Gynecol Oncol. 2000;77:350–356. doi: 10.1006/gyno.2000.5816. [DOI] [PubMed] [Google Scholar]
- 10.Bast RC, Brewer M, Zou C, Hernandez MA, Daley M, Ozols R, Lu K, Lu Z, Badgwell D, Mills GB, et al. Prevention and early detection of ovarian cancer: mission impossible? Recent Results Cancer Res. 2007;174:91–100. doi: 10.1007/978-3-540-37696-5_9. [DOI] [PubMed] [Google Scholar]
- 11.Nossov V, Amneus M, Su F, Lang J, Janco JM, Reddy ST, Farias-Eisner R. The early detection of ovarian cancer: from traditional methods to proteomics. Can we really do better than serum CA-125? Am J Obstet Gynecol. 2008;199:215–223. doi: 10.1016/j.ajog.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Nolen BM, Lokshin AE. Protein biomarkers of ovarian cancer: the forest and the trees. Future Oncol. 2012;8:55–71. doi: 10.2217/fon.11.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaughan S, Coward JI, Bast RC, Berchuck A, Berek JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, et al. Rethinking ovarian cancer: recommendations for improving outcomes. Nat Rev Cancer. 2011;11:719–725. doi: 10.1038/nrc3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen VW, Ruiz B, Killeen JL, Coté TR, Wu XC, Correa CN. Pathology and classification of ovarian tumors. Cancer. 2003;97:2631–2642. doi: 10.1002/cncr.11345. [DOI] [PubMed] [Google Scholar]
- 16.Bast RC, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981;68:1331–1337. doi: 10.1172/JCI110380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiland F, Martin K, Oehler MK, Hoffmann P. Deciphering the Molecular Nature of Ovarian Cancer Biomarker CA125. Int J Mol Sci. 2012;13:10568–10582. doi: 10.3390/ijms130810568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davelaar EM, van Kamp GJ, Verstraeten RA, Kenemans P. Comparison of seven immunoassays for the quantification of CA 125 antigen in serum. Clin Chem. 1998;44:1417–1422. [PubMed] [Google Scholar]
- 19.Yin BW, Lloyd KO. Molecular cloning of the CA125 ovarian cancer antigen: identification as a new mucin, MUC16. J Biol Chem. 2001;276:27371–27375. doi: 10.1074/jbc.M103554200. [DOI] [PubMed] [Google Scholar]
- 20.Kabawat SE, Bast RC, Bhan AK, Welch WR, Knapp RC, Colvin RB. Tissue distribution of a coelomic-epithelium-related antigen recognized by the monoclonal antibody OC125. Int J Gynecol Pathol. 1983;2:275–285. doi: 10.1097/00004347-198303000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Jacobs I, Bast RC. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989;4:1–12. doi: 10.1093/oxfordjournals.humrep.a136832. [DOI] [PubMed] [Google Scholar]
- 22.Cramer DW, O’Rourke DJ, Vitonis AF, Matulonis UA, Dijohnson DA, Sluss PM, Crum CP, Liu BC. CA125 immune complexes in ovarian cancer patients with low CA125 concentrations. Clin Chem. 2010;56:1889–1892. doi: 10.1373/clinchem.2010.153122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woolas RP, Xu FJ, Jacobs IJ, Yu YJ, Daly L, Berchuck A, Soper JT, Clarke-Pearson DL, Oram DH, Bast RC Jr. Elevation of multiple serum markers in patients with stage I ovarian cancer. J Natl Cancer Inst. 1993;85:1748–1751. doi: 10.1093/jnci/85.21.1748. [DOI] [PubMed] [Google Scholar]
- 24.Jacobs IJ, Skates S, Davies AP, Woolas RP, Jeyerajah A, Weidemann P, Sibley K, Oram DH. Risk of diagnosis of ovarian cancer after raised serum CA 125 concentration: a prospective cohort study. BMJ. 1996;313:1355–1358. doi: 10.1136/bmj.313.7069.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gentry-Maharaj A, Menon U. Screening for ovarian cancer in the general population. Best Pract Res Clin Obstet Gynaecol. 2012;26:243–256. doi: 10.1016/j.bpobgyn.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Skates SJ, Menon U, MacDonald N, Rosenthal AN, Oram DH, Knapp RC, Jacobs IJ. Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women. J Clin Oncol. 2003;21:206s–210s. doi: 10.1200/JCO.2003.02.955. [DOI] [PubMed] [Google Scholar]
- 27.Menon U, Skates SJ, Lewis S, Rosenthal AN, Rufford B, Sibley K, Macdonald N, Dawnay A, Jeyarajah A, Bast RC, et al. Prospective study using the risk of ovarian cancer algorithm to screen for ovarian cancer. J Clin Oncol. 2005;23:7919–7926. doi: 10.1200/JCO.2005.01.6642. [DOI] [PubMed] [Google Scholar]
- 28.Hellström I, Raycraft J, Hayden-Ledbetter M, Ledbetter JA, Schummer M, McIntosh M, Drescher C, Urban N, Hellström KE. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res. 2003;63:3695–3700. [PubMed] [Google Scholar]
- 29.Drapkin R, von Horsten HH, Lin Y, Mok SC, Crum CP, Welch WR, Hecht JL. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res. 2005;65:2162–2169. doi: 10.1158/0008-5472.CAN-04-3924. [DOI] [PubMed] [Google Scholar]
- 30.Kirchhoff C, Habben I, Ivell R, Krull N. A major human epididymis-specific cDNA encodes a protein with sequence homology to extracellular proteinase inhibitors. Biol Reprod. 1991;45:350–357. doi: 10.1095/biolreprod45.2.350. [DOI] [PubMed] [Google Scholar]
- 31.Moore RG, MacLaughlan S, Bast RC. Current state of biomarker development for clinical application in epithelial ovarian cancer. Gynecol Oncol. 2010;116:240–245. doi: 10.1016/j.ygyno.2009.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huhtinen K, Suvitie P, Hiissa J, Junnila J, Huvila J, Kujari H, Setälä M, Härkki P, Jalkanen J, Fraser J, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer. 2009;100:1315–1319. doi: 10.1038/sj.bjc.6605011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moore RG, Miller MC, Steinhoff MM, Skates SJ, Lu KH, Lambert-Messerlian G, Bast RC. Serum HE4 levels are less frequently elevated than CA125 in women with benign gynecologic disorders. Am J Obstet Gynecol. 2012;206:351.e1–351.e8. doi: 10.1016/j.ajog.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung F, Diamandis EP, Kulasingam V. From bench to bedside: discovery of ovarian cancer biomarkers using high-throughput technologies in the past decade. Biomark Med. 2012;6:613–625. doi: 10.2217/bmm.12.70. [DOI] [PubMed] [Google Scholar]
- 35.Ferraro S, Braga F, Lanzoni M, Boracchi P, Biganzoli EM, Panteghini M. Serum human epididymis protein 4 vs carbohydrate antigen 125 for ovarian cancer diagnosis: a systematic review. J Clin Pathol. 2013;66:273–281. doi: 10.1136/jclinpath-2012-201031. [DOI] [PubMed] [Google Scholar]
- 36.Nolen B, Velikokhatnaya L, Marrangoni A, De Geest K, Lomakin A, Bast RC, Lokshin A. Serum biomarker panels for the discrimination of benign from malignant cases in patients with an adnexal mass. Gynecol Oncol. 2010;117:440–445. doi: 10.1016/j.ygyno.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore RG, Brown AK, Miller MC, Skates S, Allard WJ, Verch T, Steinhoff M, Messerlian G, DiSilvestro P, Granai CO, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol. 2008;108:402–408. doi: 10.1016/j.ygyno.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 38.Engelen MJ, Kos HE, Willemse PH, Aalders JG, de Vries EG, Schaapveld M, Otter R, van der Zee AG. Surgery by consultant gynecologic oncologists improves survival in patients with ovarian carcinoma. Cancer. 2006;106:589–598. doi: 10.1002/cncr.21616. [DOI] [PubMed] [Google Scholar]
- 39.Vernooij F, Heintz P, Witteveen E, van der Graaf Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: a systematic review. Gynecol Oncol. 2007;105:801–812. doi: 10.1016/j.ygyno.2007.02.030. [DOI] [PubMed] [Google Scholar]
- 40.Jacobs I, Oram D, Fairbanks J, Turner J, Frost C, Grudzinskas JG. A risk of malignancy index incorporating CA 125, ultrasound and menopausal status for the accurate preoperative diagnosis of ovarian cancer. Br J Obstet Gynaecol. 1990;97:922–929. doi: 10.1111/j.1471-0528.1990.tb02448.x. [DOI] [PubMed] [Google Scholar]
- 41.Moore RG, McMeekin DS, Brown AK, DiSilvestro P, Miller MC, Allard WJ, Gajewski W, Kurman R, Bast RC, Skates SJ. A novel multiple marker bioassay utilizing HE4 and CA125 for the prediction of ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2009;112:40–46. doi: 10.1016/j.ygyno.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z, Bast RC, Yu Y, Li J, Sokoll LJ, Rai AJ, Rosenzweig JM, Cameron B, Wang YY, Meng XY, et al. Three biomarkers identified from serum proteomic analysis for the detection of early stage ovarian cancer. Cancer Res. 2004;64:5882–5890. doi: 10.1158/0008-5472.CAN-04-0746. [DOI] [PubMed] [Google Scholar]
- 43.Ueland FR, Desimone CP, Seamon LG, Miller RA, Goodrich S, Podzielinski I, Sokoll L, Smith A, van Nagell JR, Zhang Z. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol. 2011;117:1289–1297. doi: 10.1097/AOG.0b013e31821b5118. [DOI] [PubMed] [Google Scholar]
- 44.Karlsen MA, Sandhu N, Høgdall C, Christensen IJ, Nedergaard L, Lundvall L, Engelholm SA, Pedersen AT, Hartwell D, Lydolph M, et al. Evaluation of HE4, CA125, risk of ovarian malignancy algorithm (ROMA) and risk of malignancy index (RMI) as diagnostic tools of epithelial ovarian cancer in patients with a pelvic mass. Gynecol Oncol. 2012;127:379–383. doi: 10.1016/j.ygyno.2012.07.106. [DOI] [PubMed] [Google Scholar]
- 45.Moore RG, Jabre-Raughley M, Brown AK, Robison KM, Miller MC, Allard WJ, Kurman RJ, Bast RC, Skates SJ. Comparison of a novel multiple marker assay vs the Risk of Malignancy Index for the prediction of epithelial ovarian cancer in patients with a pelvic mass. Am J Obstet Gynecol. 2010;203:228.e1–228.e6. doi: 10.1016/j.ajog.2010.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sandri MT, Bottari F, Franchi D, Boveri S, Candiani M, Ronzoni S, Peiretti M, Radice D, Passerini R, Sideri M. Comparison of HE4, CA125 and ROMA algorithm in women with a pelvic mass: correlation with pathological outcome. Gynecol Oncol. 2013;128:233–238. doi: 10.1016/j.ygyno.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 47.Chan KK, Chen CA, Nam JH, Ochiai K, Wilailak S, Choon AT, Sabaratnam S, Hebbar S, Sickan J, Schodin BA, et al. The use of HE4 in the prediction of ovarian cancer in Asian women with a pelvic mass. Gynecol Oncol. 2013;128:239–244. doi: 10.1016/j.ygyno.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 48.Håkansson F, Høgdall EV, Nedergaard L, Lundvall L, Engelholm SA, Pedersen AT, Hartwell D, Høgdall C. Risk of malignancy index used as a diagnostic tool in a tertiary centre for patients with a pelvic mass. Acta Obstet Gynecol Scand. 2012;91:496–502. doi: 10.1111/j.1600-0412.2012.01359.x. [DOI] [PubMed] [Google Scholar]
- 49.van den Akker PA, Aalders AL, Snijders MP, Kluivers KB, Samlal RA, Vollebergh JH, Massuger LF. Evaluation of the Risk of Malignancy Index in daily clinical management of adnexal masses. Gynecol Oncol. 2010;116:384–388. doi: 10.1016/j.ygyno.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 50.Bristow RE, Smith A, Zhang Z, Chan DW, Crutcher G, Fung ET, Munroe DG. Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol Oncol. 2013;128:252–259. doi: 10.1016/j.ygyno.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 51.Nunes N, Yazbek J, Ambler G, Hoo W, Naftalin J, Jurkovic D. Prospective evaluation of the IOTA logistic regression model LR2 for the diagnosis of ovarian cancer. Ultrasound Obstet Gynecol. 2012;40:355–359. doi: 10.1002/uog.11088. [DOI] [PubMed] [Google Scholar]
- 52.Longoria TC, Ueland FR, Zhang Z, Chan DW, Smith A, Fung ET, Munroe DG, Bristow RE. Clinical performance of a multivariate index assay for detecting early-stage ovarian cancer. Am J Obstet Gynecol. 2014;210:78.e1–78.e9. doi: 10.1016/j.ajog.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 53.Nolen BM, Lokshin AE. Biomarker testing for ovarian cancer: clinical utility of multiplex assays. Mol Diagn Ther. 2013;17:139–146. doi: 10.1007/s40291-013-0027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montagnana M, Lippi G, Ruzzenente O, Bresciani V, Danese E, Scevarolli S, Salvagno GL, Giudici S, Franchi M, Guidi GC. The utility of serum human epididymis protein 4 (HE4) in patients with a pelvic mass. J Clin Lab Anal. 2009;23:331–335. doi: 10.1002/jcla.20340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Z, Luo Z, Zhao B, Zhang W, Zhang J, Li Z, Li L. Diagnosis and preoperative predictive value of serum HE4 concentrations for optimal debulking in epithelial ovarian cancer. Oncol Lett. 2013;6:28–34. doi: 10.3892/ol.2013.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Havrilesky LJ, Whitehead CM, Rubatt JM, Cheek RL, Groelke J, He Q, Malinowski DP, Fischer TJ, Berchuck A. Evaluation of biomarker panels for early stage ovarian cancer detection and monitoring for disease recurrence. Gynecol Oncol. 2008;110:374–382. doi: 10.1016/j.ygyno.2008.04.041. [DOI] [PubMed] [Google Scholar]
- 57.Jacob F, Meier M, Caduff R, Goldstein D, Pochechueva T, Hacker N, Fink D, Heinzelmann-Schwarz V. No benefit from combining HE4 and CA125 as ovarian tumor markers in a clinical setting. Gynecol Oncol. 2011;121:487–491. doi: 10.1016/j.ygyno.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 58.Visintin I, Feng Z, Longton G, Ward DC, Alvero AB, Lai Y, Tenthorey J, Leiser A, Flores-Saaib R, Yu H, et al. Diagnostic markers for early detection of ovarian cancer. Clin Cancer Res. 2008;14:1065–1072. doi: 10.1158/1078-0432.CCR-07-1569. [DOI] [PubMed] [Google Scholar]
- 59.Edgell T, Martin-Roussety G, Barker G, Autelitano DJ, Allen D, Grant P, Rice GE. Phase II biomarker trial of a multimarker diagnostic for ovarian cancer. J Cancer Res Clin Oncol. 2010;136:1079–1088. doi: 10.1007/s00432-009-0755-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Su F, Lang J, Kumar A, Ng C, Hsieh B, Suchard MA, Reddy ST, Farias-Eisner R. Validation of candidate serum ovarian cancer biomarkers for early detection. Biomark Insights. 2007;2:369–375. [PMC free article] [PubMed] [Google Scholar]
- 61.Yurkovetsky Z, Skates S, Lomakin A, Nolen B, Pulsipher T, Modugno F, Marks J, Godwin A, Gorelik E, Jacobs I, et al. Development of a multimarker assay for early detection of ovarian cancer. J Clin Oncol. 2010;28:2159–2166. doi: 10.1200/JCO.2008.19.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim YW, Bae SM, Lim H, Kim YJ, Ahn WS. Development of multiplexed bead-based immunoassays for the detection of early stage ovarian cancer using a combination of serum biomarkers. PLoS One. 2012;7:e44960. doi: 10.1371/journal.pone.0044960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amonkar SD, Bertenshaw GP, Chen TH, Bergstrom KJ, Zhao J, Seshaiah P, Yip P, Mansfield BC. Development and preliminary evaluation of a multivariate index assay for ovarian cancer. PLoS One. 2009;4:e4599. doi: 10.1371/journal.pone.0004599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Skates SJ, Horick N, Yu Y, Xu FJ, Berchuck A, Havrilesky LJ, de Bruijn HW, van der Zee AG, Woolas RP, Jacobs IJ, et al. Preoperative sensitivity and specificity for early-stage ovarian cancer when combining cancer antigen CA-125II, CA 15-3, CA 72-4, and macrophage colony-stimulating factor using mixtures of multivariate normal distributions. J Clin Oncol. 2004;22:4059–4066. doi: 10.1200/JCO.2004.03.091. [DOI] [PubMed] [Google Scholar]
- 65.Zhang B, Barekati Z, Kohler C, Radpour R, Asadollahi R, Holzgreve W, Zhong XY. Proteomics and biomarkers for ovarian cancer diagnosis. Ann Clin Lab Sci. 2010;40:218–225. [PubMed] [Google Scholar]
- 66.Moshkovskii SA, Serebryakova MV, Kuteykin-Teplyakov KB, Tikhonova OV, Goufman EI, Zgoda VG, Taranets IN, Makarov OV, Archakov AI. Ovarian cancer marker of 11.7 kDa detected by proteomics is a serum amyloid A1. Proteomics. 2005;5:3790–3797. doi: 10.1002/pmic.200401205. [DOI] [PubMed] [Google Scholar]
- 67.Zhu CS, Pinsky PF, Cramer DW, Ransohoff DF, Hartge P, Pfeiffer RM, Urban N, Mor G, Bast RC, Moore LE, et al. A framework for evaluating biomarkers for early detection: validation of biomarker panels for ovarian cancer. Cancer Prev Res (Phila) 2011;4:375–383. doi: 10.1158/1940-6207.CAPR-10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cramer DW, Bast RC, Berg CD, Diamandis EP, Godwin AK, Hartge P, Lokshin AE, Lu KH, McIntosh MW, Mor G, et al. Ovarian cancer biomarker performance in prostate, lung, colorectal, and ovarian cancer screening trial specimens. Cancer Prev Res (Phila) 2011;4:365–374. doi: 10.1158/1940-6207.CAPR-10-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leung F, Musrap N, Diamandis E, Kulasingam V. Advances in mass spectrometry-based technologies to direct personalized medicine in ovarian cancer. Translational Proteomics. 2013;1:74–86. [Google Scholar]
- 70.Adamczyk B, Tharmalingam T, Rudd PM. Glycans as cancer biomarkers. Biochim Biophys Acta. 2012;1820:1347–1353. doi: 10.1016/j.bbagen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 71.Tian Y, Yao Z, Roden RB, Zhang H. Identification of glycoproteins associated with different histological subtypes of ovarian tumors using quantitative glycoproteomics. Proteomics. 2011;11:4677–4687. doi: 10.1002/pmic.201000811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hua S, Williams CC, Dimapasoc LM, Ro GS, Ozcan S, Miyamoto S, Lebrilla CB, An HJ, Leiserowitz GS. Isomer-specific chromatographic profiling yields highly sensitive and specific potential N-glycan biomarkers for epithelial ovarian cancer. J Chromatogr A. 2013;1279:58–67. doi: 10.1016/j.chroma.2012.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang T, Wu X, Ke C, Yin M, Li Z, Fan L, Zhang W, Zhang H, Zhao F, Zhou X, et al. Identification of potential biomarkers for ovarian cancer by urinary metabolomic profiling. J Proteome Res. 2013;12:505–512. doi: 10.1021/pr3009572. [DOI] [PubMed] [Google Scholar]
- 74.Fan L, Zhang W, Yin M, Zhang T, Wu X, Zhang H, Sun M, Li Z, Hou Y, Zhou X, et al. Identification of metabolic biomarkers to diagnose epithelial ovarian cancer using a UPLC/QTOF/MS platform. Acta Oncol. 2012;51:473–479. doi: 10.3109/0284186X.2011.648338. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt CW. Metabolomics: what’s happening downstream of DNA. Environ Health Perspect. 2004;112:A410–A415. doi: 10.1289/ehp.112-a410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lowenthal MS, Mehta AI, Frogale K, Bandle RW, Araujo RP, Hood BL, Veenstra TD, Conrads TP, Goldsmith P, Fishman D, et al. Analysis of albumin-associated peptides and proteins from ovarian cancer patients. Clin Chem. 2005;51:1933–1945. doi: 10.1373/clinchem.2005.052944. [DOI] [PubMed] [Google Scholar]
- 77.Philip R, Murthy S, Krakover J, Sinnathamby G, Zerfass J, Keller L, Philip M. Shared immunoproteome for ovarian cancer diagnostics and immunotherapy: potential theranostic approach to cancer. J Proteome Res. 2007;6:2509–2517. doi: 10.1021/pr0606777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.El Ayed M, Bonnel D, Longuespée R, Castelier C, Franck J, Vergara D, Desmons A, Tasiemski A, Kenani A, Vinatier D, et al. MALDI imaging mass spectrometry in ovarian cancer for tracking, identifying, and validating biomarkers. Med Sci Monit. 2010;16:BR233–BR245. [PubMed] [Google Scholar]
- 79.Zhang B, Cai FF, Zhong XY. An overview of biomarkers for the ovarian cancer diagnosis. Eur J Obstet Gynecol Reprod Biol. 2011;158:119–123. doi: 10.1016/j.ejogrb.2011.04.023. [DOI] [PubMed] [Google Scholar]
- 80.Iorio MV, Visone R, Di Leva G, Donati V, Petrocca F, Casalini P, Taccioli C, Volinia S, Liu CG, Alder H, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. doi: 10.1158/0008-5472.CAN-07-1936. [DOI] [PubMed] [Google Scholar]
- 81.Chen Y, Zhang L, Hao Q. Candidate microRNA biomarkers in human epithelial ovarian cancer: systematic review profiling studies and experimental validation. Cancer Cell Int. 2013;13:86. doi: 10.1186/1475-2867-13-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Manegold-Brauer G, Bellin AK, Tercanli S, Lapaire O, Heinzelmann-Schwarz V. The special role of ultrasound for screening, staging and surveillance of malignant ovarian tumors: distinction from other methods of diagnostic imaging. Arch Gynecol Obstet. 2014;289:491–498. doi: 10.1007/s00404-013-3081-8. [DOI] [PubMed] [Google Scholar]
- 83.Sharma A, Apostolidou S, Burnell M, Campbell S, Habib M, Gentry-Maharaj A, Amso N, Seif MW, Fletcher G, Singh N, et al. Risk of epithelial ovarian cancer in asymptomatic women with ultrasound-detected ovarian masses: a prospective cohort study within the UK collaborative trial of ovarian cancer screening (UKCTOCS) Ultrasound Obstet Gynecol. 2012;40:338–344. doi: 10.1002/uog.12270. [DOI] [PubMed] [Google Scholar]
- 84.van Nagell JR, DePriest PD, Ueland FR, DeSimone CP, Cooper AL, McDonald JM, Pavlik EJ, Kryscio RJ. Ovarian cancer screening with annual transvaginal sonography: findings of 25,000 women screened. Cancer. 2007;109:1887–1896. doi: 10.1002/cncr.22594. [DOI] [PubMed] [Google Scholar]
- 85.Pavlik EJ, Ueland FR, Miller RW, Ubellacker JM, DeSimone CP, Elder J, Hoff J, Baldwin L, Kryscio RJ, van Nagell JR. Frequency and disposition of ovarian abnormalities followed with serial transvaginal ultrasonography. Obstet Gynecol. 2013;122:210–217. doi: 10.1097/AOG.0b013e318298def5. [DOI] [PubMed] [Google Scholar]
- 86.van Nagell JR, Miller RW, DeSimone CP, Ueland FR, Podzielinski I, Goodrich ST, Elder JW, Huang B, Kryscio RJ, Pavlik EJ. Long-term survival of women with epithelial ovarian cancer detected by ultrasonographic screening. Obstet Gynecol. 2011;118:1212–1221. doi: 10.1097/AOG.0b013e318238d030. [DOI] [PubMed] [Google Scholar]
- 87.Jacobs I, Menon U. Can ovarian cancer screening save lives? The question remains unanswered. Obstet Gynecol. 2011;118:1209–1211. doi: 10.1097/AOG.0b013e31823b49b3. [DOI] [PubMed] [Google Scholar]
- 88.Kaijser J, Bourne T, Valentin L, Sayasneh A, Van Holsbeke C, Vergote I, Testa AC, Franchi D, Van Calster B, Timmerman D. Improving strategies for diagnosing ovarian cancer: a summary of the International Ovarian Tumor Analysis (IOTA) studies. Ultrasound Obstet Gynecol. 2013;41:9–20. doi: 10.1002/uog.12323. [DOI] [PubMed] [Google Scholar]
- 89.Van Gorp T, Veldman J, Van Calster B, Cadron I, Leunen K, Amant F, Timmerman D, Vergote I. Subjective assessment by ultrasound is superior to the risk of malignancy index (RMI) or the risk of ovarian malignancy algorithm (ROMA) in discriminating benign from malignant adnexal masses. Eur J Cancer. 2012;48:1649–1656. doi: 10.1016/j.ejca.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 90.Kaijser J, Van Gorp T, Van Hoorde K, Van Holsbeke C, Sayasneh A, Vergote I, Bourne T, Timmerman D, Van Calster B. A comparison between an ultrasound based prediction model (LR2) and the risk of ovarian malignancy algorithm (ROMA) to assess the risk of malignancy in women with an adnexal mass. Gynecol Oncol. 2013;129:377–383. doi: 10.1016/j.ygyno.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 91.Campbell S. Ovarian cancer: role of ultrasound in preoperative diagnosis and population screening. Ultrasound Obstet Gynecol. 2012;40:245–254. doi: 10.1002/uog.12281. [DOI] [PubMed] [Google Scholar]
- 92.Einhorn N, Sjövall K, Knapp RC, Hall P, Scully RE, Bast RC, Zurawski VR. Prospective evaluation of serum CA 125 levels for early detection of ovarian cancer. Obstet Gynecol. 1992;80:14–18. [PubMed] [Google Scholar]
- 93.Campbell S, Bhan V, Royston P, Whitehead MI, Collins WP. Transabdominal ultrasound screening for early ovarian cancer. BMJ. 1989;299:1363–1367. doi: 10.1136/bmj.299.6712.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buys SS, Partridge E, Greene MH, Prorok PC, Reding D, Riley TL, Hartge P, Fagerstrom RM, Ragard LR, Chia D, et al. Ovarian cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial: findings from the initial screen of a randomized trial. Am J Obstet Gynecol. 2005;193:1630–1639. doi: 10.1016/j.ajog.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 95.Partridge E, Kreimer AR, Greenlee RT, Williams C, Xu JL, Church TR, Kessel B, Johnson CC, Weissfeld JL, Isaacs C, et al. Results from four rounds of ovarian cancer screening in a randomized trial. Obstet Gynecol. 2009;113:775–782. doi: 10.1097/AOG.0b013e31819cda77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buys SS, Partridge E, Black A, Johnson CC, Lamerato L, Isaacs C, Reding DJ, Greenlee RT, Yokochi LA, Kessel B, et al. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305:2295–2303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 97.Klompmaker J, Jansen HW, Veth RP, de Groot JH, Nijenhuis AJ, Pennings AJ. Porous polymer implant for repair of meniscal lesions: a preliminary study in dogs. Biomaterials. 1991;12:810–816. doi: 10.1016/0142-9612(91)90066-j. [DOI] [PubMed] [Google Scholar]
- 98.Menon U, Gentry-Maharaj A, Hallett R, Ryan A, Burnell M, Sharma A, Lewis S, Davies S, Philpott S, Lopes A, et al. Sensitivity and specificity of multimodal and ultrasound screening for ovarian cancer, and stage distribution of detected cancers: results of the prevalence screen of the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS) Lancet Oncol. 2009;10:327–340. doi: 10.1016/S1470-2045(09)70026-9. [DOI] [PubMed] [Google Scholar]
- 99.Menon U, Kalsi J, Jacobs I. The UKCTOCS experience--reasons for hope? Int J Gynecol Cancer. 2012;22 Suppl 1:S18–S20. doi: 10.1097/IGC.0b013e318251cb47. [DOI] [PubMed] [Google Scholar]
- 100.Pinsky PF, Zhu C, Skates SJ, Black A, Partridge E, Buys SS, Berg CD. Potential effect of the risk of ovarian cancer algorithm (ROCA) on the mortality outcome of the Prostate, Lung, Colorectal and Ovarian (PLCO) trial. Int J Cancer. 2013;132:2127–2133. doi: 10.1002/ijc.27909. [DOI] [PubMed] [Google Scholar]
- 101.Menon U, Gentry-Maharaj A, Jacobs I. Ovarian cancer screening and mortality. JAMA. 2011;306:1544; author reply 1544–1545. doi: 10.1001/jama.2011.1461. [DOI] [PubMed] [Google Scholar]
- 102.Vine MF, Calingaert B, Berchuck A, Schildkraut JM. Characterization of prediagnostic symptoms among primary epithelial ovarian cancer cases and controls. Gynecol Oncol. 2003;90:75–82. doi: 10.1016/s0090-8258(03)00175-6. [DOI] [PubMed] [Google Scholar]
- 103.Goff BA, Lowe KA, Kane JC, Robertson MD, Gaul MA, Andersen MR. Symptom triggered screening for ovarian cancer: a pilot study of feasibility and acceptability. Gynecol Oncol. 2012;124:230–235. doi: 10.1016/j.ygyno.2011.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rufford BD, Jacobs IJ, Menon U. Feasibility of screening for ovarian cancer using symptoms as selection criteria. BJOG. 2007;114:59–64. doi: 10.1111/j.1471-0528.2006.01153.x. [DOI] [PubMed] [Google Scholar]
- 105.Goff BA, Mandel LS, Drescher CW, Urban N, Gough S, Schurman KM, Patras J, Mahony BS, Andersen MR. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer. 2007;109:221–227. doi: 10.1002/cncr.22371. [DOI] [PubMed] [Google Scholar]
- 106.Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst. 2010;102:222–229. doi: 10.1093/jnci/djp500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cass I, Karlan BY. Ovarian cancer symptoms speak out--but what are they really saying? J Natl Cancer Inst. 2010;102:211–212. doi: 10.1093/jnci/djp525. [DOI] [PubMed] [Google Scholar]
- 108.Pitta Dda R, Sarian LO, Barreta A, Campos EA, Andrade LL, Fachini AM, Campbell LM, Derchain S. Symptoms, CA125 and HE4 for the preoperative prediction of ovarian malignancy in Brazilian women with ovarian masses. BMC Cancer. 2013;13:423. doi: 10.1186/1471-2407-13-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rosenthal AN, Fraser L, Manchanda R, Badman P, Philpott S, Mozersky J, Hadwin R, Cafferty FH, Benjamin E, Singh N, et al. Results of annual screening in phase I of the United Kingdom familial ovarian cancer screening study highlight the need for strict adherence to screening schedule. J Clin Oncol. 2013;31:49–57. doi: 10.1200/JCO.2011.39.7638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bolton KL, Ganda C, Berchuck A, Pharaoh PD, Gayther SA. Role of common genetic variants in ovarian cancer susceptibility and outcome: progress to date from the Ovarian Cancer Association Consortium (OCAC) J Intern Med. 2012;271:366–378. doi: 10.1111/j.1365-2796.2011.02509.x. [DOI] [PubMed] [Google Scholar]
- 111.Long KC, Kauff ND. Screening for familial ovarian cancer: a ray of hope and a light to steer by. J Clin Oncol. 2013;31:8–10. doi: 10.1200/JCO.2012.45.4678. [DOI] [PubMed] [Google Scholar]
- 112.Greene MH, Piedmonte M, Alberts D, Gail M, Hensley M, Miner Z, Mai PL, Loud J, Rodriguez G, Basil J, et al. A prospective study of risk-reducing salpingo-oophorectomy and longitudinal CA-125 screening among women at increased genetic risk of ovarian cancer: design and baseline characteristics: a Gynecologic Oncology Group study. Cancer Epidemiol Biomarkers Prev. 2008;17:594–604. doi: 10.1158/1055-9965.EPI-07-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology-Genetic/Familial High-Risk Assessment: Breast and Ovarian Cancer. Version 1; 2014. [Google Scholar]
- 114.Nik NN, Vang R, Shih IeM, Kurman RJ. Origin and pathogenesis of pelvic (ovarian, tubal, and primary peritoneal) serous carcinoma. Annu Rev Pathol. 2014;9:27–45. doi: 10.1146/annurev-pathol-020712-163949. [DOI] [PubMed] [Google Scholar]
- 115.Wiegand KC, Shah SP, Al-Agha OM, Zhao Y, Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363:1532–1543. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kurman RJ, Shih IeM. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42:918–931. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kurman RJ, Shih IeM. Pathogenesis of ovarian cancer: lessons from morphology and molecular biology and their clinical implications. Int J Gynecol Pathol. 2008;27:151–160. doi: 10.1097/PGP.0b013e318161e4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rauh-Hain JA, Krivak TC, Del Carmen MG, Olawaiye AB. Ovarian cancer screening and early detection in the general population. Rev Obstet Gynecol. 2011;4:15–21. [PMC free article] [PubMed] [Google Scholar]
- 119.Lim D, Oliva E. Precursors and pathogenesis of ovarian carcinoma. Pathology. 2013;45:229–242. doi: 10.1097/PAT.0b013e32835f2264. [DOI] [PubMed] [Google Scholar]
- 120.Kurman RJ, Visvanathan K, Roden R, Wu TC, Shih IeM. Early detection and treatment of ovarian cancer: shifting from early stage to minimal volume of disease based on a new model of carcinogenesis. Am J Obstet Gynecol. 2008;198:351–356. doi: 10.1016/j.ajog.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, Winget M, Yasui Y. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–1061. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 122.Kulasingam V, Pavlou MP, Diamandis EP. Integrating high-throughput technologies in the quest for effective biomarkers for ovarian cancer. Nat Rev Cancer. 2010;10:371–378. doi: 10.1038/nrc2831. [DOI] [PubMed] [Google Scholar]
- 123.Leung F, Dimitromanolakis A, Kobayashi H, Diamandis EP, Kulasingam V. Folate-receptor 1 (FOLR1) protein is elevated in the serum of ovarian cancer patients. Clin Biochem. 2013;46:1462–1468. doi: 10.1016/j.clinbiochem.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Anderson GL, McIntosh M, Wu L, Barnett M, Goodman G, Thorpe JD, Bergan L, Thornquist MD, Scholler N, Kim N, et al. Assessing lead time of selected ovarian cancer biomarkers: a nested case-control study. J Natl Cancer Inst. 2010;102:26–38. doi: 10.1093/jnci/djp438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pepe MS, Feng Z. Improving biomarker identification with better designs and reporting. Clin Chem. 2011;57:1093–1095. doi: 10.1373/clinchem.2011.164657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Feng Z, Kagan J, Pepe M, Thornquist M, Ann Rinaudo J, Dahlgren J, Krueger K, Zheng Y, Patriotis C, Huang Y, et al. The Early Detection Research Network’s Specimen reference sets: paving the way for rapid evaluation of potential biomarkers. Clin Chem. 2013;59:68–74. doi: 10.1373/clinchem.2012.185140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reade CJ, Riva JJ, Busse JW, Goldsmith CH, Elit L. Risks and benefits of screening asymptomatic women for ovarian cancer: a systematic review and meta-analysis. Gynecol Oncol. 2013;130:674–681. doi: 10.1016/j.ygyno.2013.06.029. [DOI] [PubMed] [Google Scholar]
- 128.American College of Obstetrics and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 477: the role of the obstetrician-gynecologist in the early detection of epithelial ovarian cancer. Obstet Gynecol. 2011;117:742–746. doi: 10.1097/AOG.0b013e31821477db. [DOI] [PubMed] [Google Scholar]
- 129.Moyer VA. Screening for ovarian cancer: U.S. Preventive Services Task Force reaffirmation recommendation statement. Ann Intern Med. 2012;157:900–904. doi: 10.7326/0003-4819-157-11-201212040-00539. [DOI] [PubMed] [Google Scholar]
- 130.Smith RA, Brooks D, Cokkinides V, Saslow D, Brawley OW. Cancer screening in the United States, 2013: a review of current American Cancer Society guidelines, current issues in cancer screening, and new guidance on cervical cancer screening and lung cancer screening. CA Cancer J Clin. 2013;63:88–105. doi: 10.3322/caac.21174. [DOI] [PubMed] [Google Scholar]
- 131.Pavlik EJ, van Nagell JR. Ovarian cancer screening--what women want. Int J Gynecol Cancer. 2012;22 Suppl 1:S21–S23. doi: 10.1097/IGC.0b013e318251cbc2. [DOI] [PubMed] [Google Scholar]


