Abstract
The Forkhead box O (FoxO) family has recently been highlighted as an important transcriptional regulator of crucial proteins associated with the many diverse functions of cells. So far, FoxO1, FoxO3a, FoxO4 and FoxO6 proteins have been identified in humans. Although each FoxO family member has its own role, unlike the other FoxO families, FoxO3a has been extensively studied because of its rather unique and pivotal regulation of cell proliferation, apoptosis, metabolism, stress management and longevity. FoxO3a alteration is closely linked to the progression of several types of cancers, fibrosis and other types of diseases. In this review, we will examine the function of FoxO3a in disease progression and also explore FoxO3a’s regulatory mechanisms. We will also discuss FoxO3a as a potential target for the treatment of several types of disease.
Keywords: Forkhead box O, Cell proliferation, Apoptosis, Stress, Aging
Core tip: Forkhead box O (FoxO)3a has recently been highlighted as a critical protein that regulates numerous cell functions from proliferation/apoptosis to stress-resistance and aging. FoxO3a has been found to be deregulated in several diseases and FoxO3a targeting approaches are currently underway to treat various types of cancers. This review will describe the current concept of FoxO3a’s pathological role in various diseases and elucidate the regulatory mechanisms involved. It will also provide the clinical significance and strategies to target FoxO3a to limit the progression of human diseases.
INTRODUCTION
Forkhead box O (FoxO) transcription factors are the human homologues of the C. elegans transcription factor DAF-16 and share a highly conserved 110-amino acid DNA binding domain, forkhead box or winged-helix domain[1,2]. Forkhead box proteins comprise more than 100 members in humans, classified from FOXA to FOXR[3-5]. Members of class O share the characteristic of being regulated by the insulin/PI3K/Akt signaling pathway[4]. Four principal members of the mammalian FoxO subfamily, FoxO1, FoxO3a, FoxO4 and FoxO6 have been previously described[3]. Although they seem to bind a common set of DNA sites, FoxO6 is mainly specific to neurons, while the other 3 FoxO family members are expressed in most tissues. These FoxO members are linked to cell survival, cellular proliferation and DNA damage repair response[5,6]. Among them, FoxO3a has recently been studied extensively as a crucial protein that is involved in the regulation of several essential cellular functions (see page 349). Prior studies have shown that FoxO3a functions as a tumor suppressor by regulating expression of genes involved in apoptosis, cell cycle arrest, oxidative stress resistance and autophagy[3,7-9] (Figure 1). In general, FoxO3a is known to suppress cell cycle progression and promote cell death. Thus, it has been thought that FoxO3a can be an important target to inhibit cancer cell progression. However, recent studies have discovered other functions of FoxO3a, such as stress response and longevity, as described on page 349. FoxO3a alteration is also linked to many different types of disease. Interestingly, FoxO3a increases autophagy to protect cells from environmental stresses[10,11]. Thus, under this situation, unlike the general concept of FoxO3a’s role, FoxO3a potentially has a protective role in maintaining a cell’s homeostasis. Perhaps the most interesting feature of FoxO3a is its biological role associated with longevity (page 349). Based on this, it becomes clear that FoxO3a has diverse roles in response to many environmental stimuli and these recent findings certainly change our view on the previous roles of FoxO3a. Therefore, from the perspective of disease progression, it is imperative to define the potential role of FoxO3a in cells and elucidate how alteration of FoxO3a is linked to the development of several types of disease.
Figure 1.
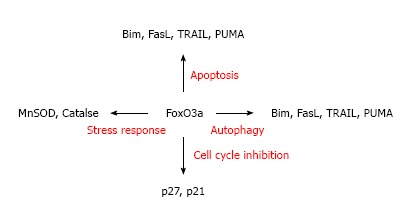
Forkhead box O3a target genes. Forkhead box O (FoxO)3a transcriptionally activates several target genes. FoxO3a binds to the promoter of apoptosis inducing genes, such as Bim, FasL and TRAIL, and to the promoter of cell cycle inhibitors, such as p27 and p21. FoxO3a also activates autophagy genes Gabarapl1, ATG12, etc. A recent study showed that FoxO3a also participates in the activation of stress response genes, such as MnSOD and catalase in response to oxidative stress.
FOXO3A STRUCTURE
Recent technologies have revealed that the primary structure of FoxO3a contains highly conserved residues of the helix H3 (motif NXXRHXXS/T), which is the main DNA recognition element that binds into a major groove, which comprises the majority of the direct base-specific contacts[1,6]. Recent studies further revealed that FoxO proteins recognize two consensus sequences, 5’-GTAAA(T/C)AA-3’ known as the Daf-16 family member-binding element[6,7] and 5’-(C/A)(A/C)AAA(C/T)AA-3’ known as the insulin-responsive sequence (IRE)[8,9]. Crystal structure revealed that the recognition helix H3 docked perpendicular to the major groove making extensive contacts with the DNA[7]. FoxO3a contains several crucial domains[12] (Figure 2) such as a nuclear localization signal (NLS), a nuclear export signal (NES) and a transactivation domain (TA).
Figure 2.
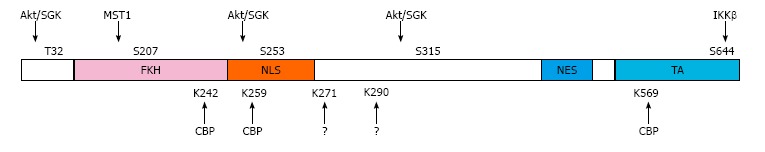
Major phosphorylation and acetylation residues of FoxO3a. Post-translational modification sites of FoxO3a. Shown are sites of serine/threonine phosphorylation by Akt/SGK, MST1, IKKβ or the residues acetylated by CBP or unidentified acetyl transferases (?) on FoxO3a domains[12]. FKH: Forkhead DNA binding domain; NLS: Nuclear localization signal; NES: Nuclear export sequence; TA: Transactivation domain; Akt: Protein kinase B; MST1: Mammalian sterile 20 like kinase-1; CBP: The cyclic–AMP responsive element binding (CREB) binding protein, IKKβ: Ikβ kinase; SGK: Serum-and glucocorticoid-induced protein kinase.
FOXO3A REGULATORY MECHANISMS
Phosphorylation and dephosphorylation
FoxO3a is regulated by posttranslational modifications such as phosphorylation, acetylation and ubiquitination, each of which affects the transcriptional activity of FoxO proteins[11-16] (Figure 2). The potency of FoxO3a is carefully regulated by phosphorylation. The phosphorylation of FoxO3a by several kinases is well established. Among them, protein kinase B (Akt) is an important kinase that directly phosphorylates FoxOs. In the case of FoxO3a, T32, S253 and S315 residues are phosphorylated by Akt and, in particular, the phosphorylation of S253 is a crucial residue regulating the nuclear/cytoplasmic shuttling of FoxO3a. For example, when cells are cultured in the presence of growth factors or insulin, FoxO3a is phosphorylated by Akt and mainly localized to the cytoplasm, which prevents its transcriptional activity. The phosphorylation event of FoxO3a by Akt facilitates FoxO3a interaction with the 14-3-3 nuclear export protein, further preventing nuclear re-import by concealing nuclear localization signals[13]. Furthermore, the phosphorylation of FoxO3a by activated Akt promotes an association with an ubiquitin E3 ligase, subsequently polyubiquitinating FoxO3a, which facilitates FoxO3a degradation by proteasomes[13-17]. Thus, the activation of Akt is thought to be critical in FoxO3a regulation. However, in some tumors, FoxO3a remains in the cytoplasm even in the absence of active Akt[14]. It has been found that IkB kinase (IKK) phosphorylates FoxO3a at serine 644, thereby inhibiting its transcriptional activity in an Akt-independent manner[15]. The phosphorylation of FoxO3a by IKK also leads to its cytoplasmic localization, although the underlying export mechanism is not understood. The insulin/IGF-1 and integrin-dependent signaling pathways activate Akt via PTEN suppression which phosphorylates FoxO3a, thereby rendering it functionally inactive. In contrast, FoxO3a is localized to the nucleus to activate its target genes when growth factors or serum are deprived. Additionally, serum and glucocorticoid regulated kinase (SGK), casein kinase 1 (CK1), dual specificity tyrosine-phosphorylated and regulated kinase 1A (DYRK1A), janus N-terminal kinase (JNK), mitogen-activated protein kinases (MAPKs), IkappaB kinase (IKKβ), mammalian sterile 20-like kinase 1 (MST1) and AMP activated protein kinase (AMPK) are also known to regulate FoxO3a and other family members[18-23] by phosphorylating multiple residues. Interestingly, SGK1 is transcriptionally up-regulated in response to a variety of external stimuli, including growth factors. SGK1 is also known to phosphorylate the pivotal ser 253 residue, which triggers its location to the cytoplasm, thereby inhibiting its function[19]. In contrast, AMPK activates FoxO3a function. 6 threonine/serine residues (T179, S399, S413, S555, S588 and S626) in mammalian FoxO3a are found to be phosphorylated by AMPK[24,25]. Mutation of these phosphorylation residues to alanine severely impairs its function, yet it does not alter its ability to bind to cognate sequences or to participate in nucleocytoplasmic shuttling depending on external cues[25]. Likewise, JNK also phosphorylates FoxO3a, activating FoxO3a function by enhancing its location into the nucleus which subsequently increases its transcriptional activity[18,22].
Unlike kinases, very few phosphatases have been found to regulate FoxO3a. One particular phosphatase, protein phosphatase-2A (PP2A), has been shown to regulate FoxO3a function. Nho et al[26] showed that when fibroblasts attach to 2D type collagen coated plates, PP2A activity is suppressed, which facilitates FoxO3a inactivation by enhanced Akt, promoting fibroblast proliferation. But the over-expression of PP2A reverses this inactivation and increases dephosphorylated FoxO3a, thereby suppressing their proliferation. Singh et al[24] also demonstrated that FoxO3a interacts with PP2A C/A subunits in HeLA cells, dephosphorylating its T32/S253 residues, which subsequently inhibits the interaction of the 14-3-3 protein to FoxO3a by Akt. This study showed that PP2A is required for the reactivation of FoxO3a by promoting its translocation to the nucleus (Figure 3). Interestingly, recent studies also showed that the adenovirus E1A stabilizes FoxO3a by inducing the expression of PP2A/C, which suppresses βTrCP-mediated degradation of FoxO3a[25]. Thus, these studies clearly suggest that the imbalance between kinases and phosphatase(s) can greatly affect a cell’s fate by curbing FoxO3a function and the alteration of these kinases and phosphatases are directly linked to certain disease progression.
Figure 3.
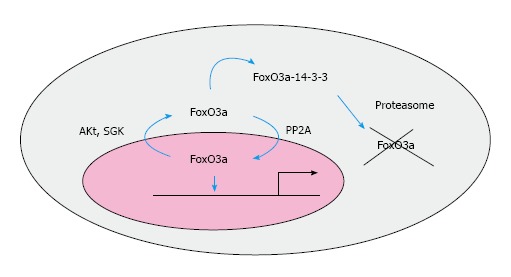
Forkhead box O3a localization by phosphorylation and dephosphorylation. Forkhead box O (FoxO)3a becomes translocated to the cytoplasm when phosphorylated on ser 253 residue by Akt or SGK. FoxO3a is then bound to 14-3-3 and this interaction promotes its degradation by the proteasome. In contrast, FoxO3a is dephosphorylated by protein phosphatase-2A and this opposite event facilitates its re-location into the nucleus, thereby activating its target genes. SGK: Serum-and glucocorticoid-induced protein kinase; Akt: Protein kinase B.
Ubiquitin proteasome degradation
As we briefly described above, FoxO3a degradation is also an important step to regulate its function. The single molecule RING-finger E3 ligase murine double minute 2 (MDM2) promotes ubiquitination of FoxO3a as well as FoxO1 and FoxO4, facilitating their degradation[27]. Intriguingly, knockout or knockdown of MDM2 alone increases FoxO3a protein levels. This effect was shown to be mediated by MDM2-induced polyubiquitination of FoxO proteins[27,28], whereas another study showed that MDM2 catalyzes multiple monoubiquitination of FoxO4 rather than polyubiquitination[28]. When FoxO3a is located to the cytoplasm by Akt, FoxO3a becomes ubiquitinated and this event triggers a proteasome-dependent degradation process. Like MDM2, FoxO3a phosphorylation by IKK also leads to its ubiquitination and degradation[15]. Thus, these studies document that FoxO3a localization in the cytoplasm not only deactivates FoxO3a function but also becomes a crucial step leading to FoxO3a degradation.
Acetylation, transcriptional regulation, microRNA and others
Acetylation also plays an important role in regulating FoxO3a. Oxidative stress triggers FoxO3a acetylation/deacetylation and affects the localization of FoxO3a. For example, protein acetylase CREB binding protein (CBP)[29-31], p300[32,33] and deacetylase Sirt are known to modulate FoxO3a function[34-38], although a precise mechanism describing the effects of acetylation and deacetylation is not known. A recent piece of evidence suggests that the FoxO family is also regulated by microRNA. mir155, mir96 and mir21 are thought to directly regulate FoxO3a, while mir205 regulates FoxO3a via its upstream target PTEN[39-43]. FoxO3a is also known to be regulated by a transcription factor. E2F-1 can bind to the promoter region of FoxO1 and FoxO3a, thereby regulating FoxO3a at the mRNA level[44]. FoxO3a mRNAs are modulated as a function of age in rat muscle, peaking at 6 and 23 mo, suggesting that FoxO3a may also affect longevity in mammals[45].
FOXO3A FUNCTION
Cell proliferation and apoptosis
Perhaps the two most significant cellular processes that are regulated by FoxO transcription factor are the suppression of cell cycle progression and the promotion of apoptosis[46-50]. FoxO3a activation increases cell cycle inhibitor proteins p21 and p27, both of which subsequently suppress G1 to S cell cycle transition[51-54]. Although p27 is transcriptionally regulated by FoxO3a via the PI3K/Akt-dependent axis, it has been shown that p27 is also regulated via the FoxO3a/NF-κB/c-Myc-dependent pathway. Chandramohan et al[55] showed that in WEHI 231 cells, the suppression of PI3K activity promotes a decrease in c-Myc dependent p27 expression via NF-κB inhibition. Since NF-κB is frequently altered in many types of cancers and NF-κB transcriptionally activates c-Myc gene expression, this finding suggests that p27 is reciprocally regulated by FoxO3a and c-Myc. A recent study further suggests that FoxO3a inhibits NF-κB function and that the alteration of FoxO3a is associated with hyper-proliferative helper T cells, cigarette smoke-induced inflammation, airspace enlargement and chronic obstructive pulmonary disease[56,57]. Likewise, FoxO3a also increases several target genes, such as Bim, TRAIL, PUMA and Fas ligand, which all promote cell apoptosis. For example, FoxO3a directly binds to the promoter region of Bim, causing sympathetic neuron cell death[44]. The activation of the transcription factor FoxO3a led to increased TRAIL transcription and induction of G1 arrest in the absence of v-Abl inhibition; this effect could be inhibited by the expression of a constitutively active Akt mutant in BCR-Abl-transformed human cells. Ghaffari et al[49] also demonstrated that cytokine and BCR-Abl suppression of TRAIL transcription is mediated through phosphorylation and inhibition of the FoxO3a transcription factor. This study showed that BCR-Abl-induced inhibition of TRAIL transcription is linked to the tumorigenicity in chronic myeloid leukemia[50]. FoxO3a is also associated with the regulation of PUMA and Noxa proteins in lymphoid and neuroblastoma cells, respectively[58,59]. Thus, these findings clearly demonstrate that FoxO3a-dependent cell cycle arrest and apoptosis induction are important for tumor suppression (Table 1) and further indicate that the pathological alteration of FoxO3a can potentially contribute to the acquisition of uncontrolled cell proliferation and an apoptosis-resistant cell phenotype.
Table 1.
FoxO3a target genes in various cell types
| FoxO3a target genes | Cell types |
| Bim | Neuron cells[48] |
| TRAIL | Bcr/Abl transformed cells[57] |
| TRAIL | Chronic myeloid leukemia[46] |
| PUMA | Lymphoid cells[58] |
| Noxa | Neuroblastoma[59] |
| FasL | Glomerular mesangial cells[102] |
| p27, Caveolin-1 | Glomerular mesangial cells[102] |
| p21 | Glomerular mesangial cells[102] |
Shown are previously known FoxO3a target genes that regulate cell proliferation and apoptosis in different cell types.
Stress resistant effect
The most recent discovery regarding FoxO3a’s function is that it is also associated with stress response and longevity. In contrast to FoxO3a’s better known functions of inhibiting cell proliferation and promoting apoptosis as described above, FoxO3a also participates in protecting cells when exposed to unfavorable conditions. This seemingly contradictory effect of FoxO3a has been observed in various cell models and it has been found that the reactive oxygen species (ROS) are linked to the activation of FoxO3a to protect cells from a stress inducing environment[60,61]. In C. elegans, DAF-16 is thought to regulate 230 genes on the ablated germ cell line background and most of these genes are related to the resistance of external stress[62,63]. Deregulated ROS induce apoptosis and are associated with various diseases and aging. Sirtuin-1 (Sirt1) decreases ROS levels and promotes cell survival under oxidative stress conditions. Interestingly, FoxO3a and other FoxO family members increase superoxide dismutase (SOD) and protect cells from oxidative stress in a Sirt1-dependent manner[34,38]. A Sirt1/FoxO3a-dependent cell regulatory function that has been linked to stress management was previously studied. Brunet et al showed that Sirt1 and FoxO3a form a complex in cells in response to oxidative stress and Sirt1 increases the ability of FoxO3a to induce cell cycle arrest and resistance to oxidative stress but inhibited FoxO3a’s function to induce cell death[38]. These results showed that FoxO3a deacetylation by Sirt1 in response to ROS can be an important self defense mechanism to detoxifying harmful reactive molecules, further suggesting that Sirt1 is linked to protect cells from a stress inducing environment by tipping FoxO dependent response away from apoptosis and toward stress resistance[38]. Studies also found that Sirt3, which belongs to class III of HDACs, is linked to the resistance of stress inducing environments by detoxifying ROS. The role of Sirt3 and FoxO3a function is particularly well described in myocytes[64]. At the cellular level, when cardiomyocytes are exposed to stressful stimuli, Sirt3 levels are elevated, which subsequently deacetylase FoxO3a and facilitate its location into the nucleus to activate anti-oxidant genes[65]. Among them, catalase (Cat) and manganese superoxide dismutase (MnSOD) are direct targets of detoxifying enzymes by FoxO3a. Thus, the increased level of Cat and MsSoD by FoxO3a activation may efficiently and effectively manage ROS, which can be beneficial for reducing stress induced by ROS. Interestingly, a prior study found a potential FoxO activator as a way to protect cells from oxidative stress. Resveratrol, a polyphenolic flavonoid abundant in red wine with potent antioxidant activity, is known to up-regulate the FoxO family and block caspase 3, 8, and 9 activation, protecting photoreceptor cells from oxidative stress[66]. Thus, it is believed that when cells are exposed to a stress inducing environment, FoxO3a protects cells by utilizing SOD, catalase, etc., and this action is ultimately beneficial to cells. Given the fact that FoxO3a is linked to stress response and cells utilize FoxO3a to respond to ROS, it is a plausible scenario that the activation of FoxO3a under stress inducing conditions triggers the cell’s defense system, which can protect cells from harmful environments.
Longevity
However, perhaps the most intriguing recent discovery in FoxO3a function is that the FoxO3a gene is associated with aging. Because FoxO3a is regulated by insulin-IGF1 signaling (IIS) which influences metabolism and lifespan in model organisms[67], FoxO3a had been proposed to be an ideal candidate to study longevity as the link between FoxO3a and longevity that has previously been described. Willcox et al[68] described 3 single nucleotide polymorphisms (SNPs) in the FoxO3a gene that were statistically significantly associated with longevity and different aging phenotypes in a sample of long-lived Americans of Japanese ancestry. Furthermore, Flachsbart et al[69] found that not only were certain FoxO3a variants very common in 90 year olds, they were even more common in 100 year olds, emphasizing the importance of genetics for aging well. It becomes clear that increases in cellular ROS levels are known to be associated with aging[70-75]. Increased cellular oxidative stress regulates FoxO post-translational modifications and the activation of the FoxO family has been shown to regulate cellular oxidative-stress resistance[76-81]. Interestingly, to support these findings, recent studies suggest a possibility that Sirt3 and FoxO3a have been linked to an extended life span in humans[75-78,82].
FOXO3A IN CLINICAL APPLICATION
FoxO3, FoxO1 and FoxO4 are present at chromosomal translocation break points in cells of rhabdomyosarcomas and acute myeloid leukemia. Among the FoxO family, FoxO3a has been shown to be deregulated in several tumor types, including breast cancer[83-85], prostate cancer[86-88], glioblastoma[89] and leukemia[90,91]. Therefore, FoxO3a has been targeted as a way to treat several types of cancers. Interestingly, Akt, IKK and Erk are three commonly activated oncogenic kinases in human cancers and all three kinases target FoxO3a in an identical manner to inhibit its tumor suppressor function[92]. All three kinase-mediated phosphorylations stimulate FoxO3a ubiquitination, resulting in its proteasomal degradation. Thus, a FoxO3a targeting approach via the modulation of above kinases is currently underway. For example, the chemotherapeutic drugs paclitaxel[93] and KP372-1 (a multiple kinase inhibitor)[30], currently used in the treatment of breast carcinoma, activate FoxO3a by reducing Akt activity. Doxorubicin activates FoxO3a to induce the expression of the multidrug resistance gene ABCB1 (MDR1) in K562 doxorubicin-sensitive leukemic cells[94]. Imatinib activates FoxO3a and induces Bim-dependent apoptosis through inhibition of BCR-ABL in chronic myeloid leukemia[95]. Imatinib also induces erythroid differentiation through repressing ID1 gene transcription by FoxO3a activation[96]. BMS-345541, a selective IKK inhibitor, promotes apoptosis in T-cell acute lymphoblastic leukemia (T-ALL) cell lines[97]. Several pieces of evidence in recent years further suggest that a FoxO3a targeting approach may be helpful for the treatment of other types of human diseases. For example, FoxO3a causes the induction of apoptosis in prostate cancer cells via up-regulating PUMA[98]. Low levels of FoxO3a may link to chemotherapy resistance in liver cancer and FoxO3a appears to present antitumor properties in hepatocellular carcinoma[99-101]. FoxO3a also plays a role in the neuroprotective effect of the erythropoietin (EPO) role in Parkinson’s disease via Akt[102]. Thus, all these studies indicate that as our knowledge for FoxO3a targeting approaches continuously develop, the clinical application of FoxO3 is potentially promising to limit the progression of human diseases in the future.
FUTURE APPLICATION OF FOXO3A
FoxO3a has recently been recognized as a promising therapeutic target to treat cancers and other types of diseases. To improve therapeutic outcomes, FoxO3a-dependent chemosensitization is being currently tested. Studies suggest that precise FoxO3a regulation is essential for homeostasis and if there is deregulation of FoxO3a by environmental factors, such as chronic exposure to ROS or genetic/epigenetic alteration, this pathological condition can directly lead to abnormal proliferation or changes in apoptotic signals, which subsequently are responsible for disease progression. In particular, age-dependent FoxO3a modulation is an interesting concept to help understand the pathogenesis of certain types of disease models. If FoxO3a is a crucial protein mainly deregulated by aging, maintaining optimum FoxO3a activity in a patient’s specific clinical condition can be beneficial to minimize age-dependent disease. For example, the preservation of optimum FoxO3a activity using drugs such as paclitaxel may be helpful for patients with age-related diseases. Clearly, more studies are required to elucidate FoxO3a’s function as an effective and useful target capable of preventing or limiting the progression of diseases without clinical compromise.
Footnotes
Supported by the National Institutes of Health R01 HL 114662 to Nho R
P- Reviewer: Moreno JJ, Pospelov VA, Yew PR S- Editor: Wen LL L- Editor: Roemmele A E- Editor: Lu YJ
References
- 1.Clark KL, Halay ED, Lai E, Burley SK. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364:412–420. doi: 10.1038/364412a0. [DOI] [PubMed] [Google Scholar]
- 2.Arden KC. Multiple roles of FOXO transcription factors in mammalian cells point to multiple roles in cancer. Exp Gerontol. 2006;41:709–717. doi: 10.1016/j.exger.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Huang H, Tindall DJ. Dynamic FoxO transcription factors. J Cell Sci. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 4.Dillin A, Crawford DK, Kenyon C. Timing requirements for insulin/IGF-1 signaling in C. elegans. Science. 2002;298:830–834. doi: 10.1126/science.1074240. [DOI] [PubMed] [Google Scholar]
- 5.Monsalve M, Olmos Y. The complex biology of FOXO. Curr Drug Targets. 2011;12:1322–1350. doi: 10.2174/138945011796150307. [DOI] [PubMed] [Google Scholar]
- 6.Biggs WH, Cavenee WK, Arden KC. Identification and characterization of members of the FKHR (FOX O) subclass of winged-helix transcription factors in the mouse. Mamm Genome. 2001;12:416–425. doi: 10.1007/s003350020002. [DOI] [PubMed] [Google Scholar]
- 7.Furuyama T, Nakazawa T, Nakano I, Mori N. Identification of the differential distribution patterns of mRNAs and consensus binding sequences for mouse DAF-16 homologues. Biochem J. 2000;349:629–634. doi: 10.1042/0264-6021:3490629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall RK, Yamasaki T, Kucera T, Waltner-Law M, O’Brien R, Granner DK. Regulation of phosphoenolpyruvate carboxykinase and insulin-like growth factor-binding protein-1 gene expression by insulin. The role of winged helix/forkhead proteins. J Biol Chem. 2000;275:30169–30175. doi: 10.1074/jbc.M004898200. [DOI] [PubMed] [Google Scholar]
- 9.Hribal ML, Nakae J, Kitamura T, Shutter JR, Accili D. Regulation of insulin-like growth factor-dependent myoblast differentiation by Foxo forkhead transcription factors. J Cell Biol. 2003;162:535–541. doi: 10.1083/jcb.200212107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Warr MR, Binnewies M, Flach J, Reynaud D, Garg T, Malhotra R, Debnath J, Passegué E. FOXO3A directs a protective autophagy program in haematopoietic stem cells. Nature. 2013;494:323–327. doi: 10.1038/nature11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xia N, Strand S, Schlufter F, Siuda D, Reifenberg G, Kleinert H, Förstermann U, Li H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide. 2013;32:29–35. doi: 10.1016/j.niox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 13.Plas DR, Thompson CB. Akt activation promotes degradation of tuberin and FOXO3a via the proteasome. J Biol Chem. 2003;278:12361–12366. doi: 10.1074/jbc.M213069200. [DOI] [PubMed] [Google Scholar]
- 14.Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a) Mol Cell Biol. 2001;21:952–965. doi: 10.1128/MCB.21.3.952-965.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu MC, Lee DF, Xia W, Golfman LS, Ou-Yang F, Yang JY, Zou Y, Bao S, Hanada N, Saso H, et al. IkappaB kinase promotes tumorigenesis through inhibition of forkhead FOXO3a. Cell. 2004;117:225–237. doi: 10.1016/s0092-8674(04)00302-2. [DOI] [PubMed] [Google Scholar]
- 16.Vogt PK, Jiang H, Aoki M. Triple layer control: phosphorylation, acetylation and ubiquitination of FOXO proteins. Cell Cycle. 2005;4:908–913. doi: 10.4161/cc.4.7.1796. [DOI] [PubMed] [Google Scholar]
- 17.Fu W, Ma Q, Chen L, Li P, Zhang M, Ramamoorthy S, Nawaz Z, Shimojima T, Wang H, Yang Y, et al. MDM2 acts downstream of p53 as an E3 ligase to promote FOXO ubiquitination and degradation. J Biol Chem. 2009;284:13987–14000. doi: 10.1074/jbc.M901758200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunters A, Madureira PA, Pomeranz KM, Aubert M, Brosens JJ, Cook SJ, Burgering BM, Coombes RC, Lam EW. Paclitaxel-induced nuclear translocation of FOXO3a in breast cancer cells is mediated by c-Jun NH2-terminal kinase and Akt. Cancer Res. 2006;66:212–220. doi: 10.1158/0008-5472.CAN-05-1997. [DOI] [PubMed] [Google Scholar]
- 19.Huang H, Tindall DJ. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim Biophys Acta. 2011;1813:1961–1964. doi: 10.1016/j.bbamcr.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chatterjee A, Chatterjee U, Ghosh MK. Activation of protein kinase CK2 attenuates FOXO3a functioning in a PML-dependent manner: implications in human prostate cancer. Cell Death Dis. 2013;4:e543. doi: 10.1038/cddis.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao J, Yang X, Yin P, Hu W, Liao H, Miao Z, Pan C, Li N. The involvement of FoxO in cell survival and chemosensitivity mediated by Mirk/Dyrk1B in ovarian cancer. Int J Oncol. 2012;40:1203–1209. doi: 10.3892/ijo.2011.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Chen WR, Xing D. A pathway from JNK through decreased ERK and Akt activities for FOXO3a nuclear translocation in response to UV irradiation. J Cell Physiol. 2012;227:1168–1178. doi: 10.1002/jcp.22839. [DOI] [PubMed] [Google Scholar]
- 23.Clavel S, Siffroi-Fernandez S, Coldefy AS, Boulukos K, Pisani DF, Dérijard B. Regulation of the intracellular localization of Foxo3a by stress-activated protein kinase signaling pathways in skeletal muscle cells. Mol Cell Biol. 2010;30:470–480. doi: 10.1128/MCB.00666-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nho RS, Kahm J. beta1-Integrin-collagen interaction suppresses FoxO3a by the coordination of Akt and PP2A. J Biol Chem. 2010;285:14195–14209. doi: 10.1074/jbc.M109.052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh A, Ye M, Bucur O, Zhu S, Tanya Santos M, Rabinovitz I, Wei W, Gao D, Hahn WC, Khosravi-Far R. Protein phosphatase 2A reactivates FOXO3a through a dynamic interplay with 14-3-3 and AKT. Mol Biol Cell. 2010;21:1140–1152. doi: 10.1091/mbc.E09-09-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiacchiera F, Simone C. The AMPK-FoxO3A axis as a target for cancer treatment. Cell Cycle. 2010;9:1091–1096. doi: 10.4161/cc.9.6.11035. [DOI] [PubMed] [Google Scholar]
- 27.Milkiewicz M, Roudier E, Doyle JL, Trifonova A, Birot O, Haas TL. Identification of a mechanism underlying regulation of the anti-angiogenic forkhead transcription factor FoxO1 in cultured endothelial cells and ischemic muscle. Am J Pathol. 2011;178:935–944. doi: 10.1016/j.ajpath.2010.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brenkman AB, de Keizer PL, van den Broek NJ, Jochemsen AG, Burgering BM. Mdm2 induces mono-ubiquitination of FOXO4. PLoS One. 2008;3:e2819. doi: 10.1371/journal.pone.0002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang F, Marshall CB, Li GY, Yamamoto K, Mak TW, Ikura M. Synergistic interplay between promoter recognition and CBP/p300 coactivator recruitment by FOXO3a. ACS Chem Biol. 2009;4:1017–1027. doi: 10.1021/cb900190u. [DOI] [PubMed] [Google Scholar]
- 30.Zeng Z, Samudio IJ, Zhang W, Estrov Z, Pelicano H, Harris D, Frolova O, Hail N, Chen W, Kornblau SM, et al. Simultaneous inhibition of PDK1/AKT and Fms-like tyrosine kinase 3 signaling by a small-molecule KP372-1 induces mitochondrial dysfunction and apoptosis in acute myelogenous leukemia. Cancer Res. 2006;66:3737–3746. doi: 10.1158/0008-5472.CAN-05-1278. [DOI] [PubMed] [Google Scholar]
- 31.Wang F, Marshall CB, Yamamoto K, Li GY, Gasmi-Seabrook GM, Okada H, Mak TW, Ikura M. Structures of KIX domain of CBP in complex with two FOXO3a transactivation domains reveal promiscuity and plasticity in coactivator recruitment. Proc Natl Acad Sci USA. 2012;109:6078–6083. doi: 10.1073/pnas.1119073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emerling BM, Weinberg F, Liu JL, Mak TW, Chandel NS. PTEN regulates p300-dependent hypoxia-inducible factor 1 transcriptional activity through Forkhead transcription factor 3a (FOXO3a) Proc Natl Acad Sci USA. 2008;105:2622–2627. doi: 10.1073/pnas.0706790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Senf SM, Sandesara PB, Reed SA, Judge AR. p300 Acetyltransferase activity differentially regulates the localization and activity of the FOXO homologues in skeletal muscle. Am J Physiol Cell Physiol. 2011;300:C1490–C1501. doi: 10.1152/ajpcell.00255.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hori YS, Kuno A, Hosoda R, Horio Y. Regulation of FOXOs and p53 by SIRT1 modulators under oxidative stress. PLoS One. 2013;8:e73875. doi: 10.1371/journal.pone.0073875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim MJ, Ahn K, Park SH, Kang HJ, Jang BG, Oh SJ, Oh SM, Jeong YJ, Heo JI, Suh JG, et al. SIRT1 regulates tyrosine hydroxylase expression and differentiation of neuroblastoma cells via FOXO3a. FEBS Lett. 2009;583:1183–1188. doi: 10.1016/j.febslet.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 36.Ni HM, Du K, You M, Ding WX. Critical role of FoxO3a in alcohol-induced autophagy and hepatotoxicity. Am J Pathol. 2013;183:1815–1825. doi: 10.1016/j.ajpath.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai L, Yan L, Gao S, Hu CL, Ge H, Davidow A, Park M, Bravo C, Iwatsubo K, Ishikawa Y, et al. Type 5 adenylyl cyclase increases oxidative stress by transcriptional regulation of manganese superoxide dismutase via the SIRT1/FoxO3a pathway. Circulation. 2013;127:1692–1701. doi: 10.1161/CIRCULATIONAHA.112.001212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–2015. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 39.Babar IA, Czochor J, Steinmetz A, Weidhaas JB, Glazer PM, Slack FJ. Inhibition of hypoxia-induced miR-155 radiosensitizes hypoxic lung cancer cells. Cancer Biol Ther. 2011;12:908–914. doi: 10.4161/cbt.12.10.17681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin H, Dai T, Xiong H, Zhao X, Chen X, Yu C, Li J, Wang X, Song L. Unregulated miR-96 induces cell proliferation in human breast cancer by downregulating transcriptional factor FOXO3a. PLoS One. 2010;5:e15797. doi: 10.1371/journal.pone.0015797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ling N, Gu J, Lei Z, Li M, Zhao J, Zhang HT, Li X. microRNA-155 regulates cell proliferation and invasion by targeting FOXO3a in glioma. Oncol Rep. 2013;30:2111–2118. doi: 10.3892/or.2013.2685. [DOI] [PubMed] [Google Scholar]
- 42.Wang K, Li PF. Foxo3a regulates apoptosis by negatively targeting miR-21. J Biol Chem. 2010;285:16958–16966. doi: 10.1074/jbc.M109.093005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai J, Fang L, Huang Y, Li R, Yuan J, Yang Y, Zhu X, Chen B, Wu J, Li M. miR-205 targets PTEN and PHLPP2 to augment AKT signaling and drive malignant phenotypes in non-small cell lung cancer. Cancer Res. 2013;73:5402–5415. doi: 10.1158/0008-5472.CAN-13-0297. [DOI] [PubMed] [Google Scholar]
- 44.Nowak K, Killmer K, Gessner C, Lutz W. E2F-1 regulates expression of FOXO1 and FOXO3a. Biochim Biophys Acta. 2007;1769:244–252. doi: 10.1016/j.bbaexp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Furuyama T, Yamashita H, Kitayama K, Higami Y, Shimokawa I, Mori N. Effects of aging and caloric restriction on the gene expression of Foxo1, 3, and 4 (FKHR, FKHRL1, and AFX) in the rat skeletal muscles. Microsc Res Tech. 2002;59:331–334. doi: 10.1002/jemt.10213. [DOI] [PubMed] [Google Scholar]
- 46.Rathbone CR, Booth FW, Lees SJ. FoxO3a preferentially induces p27Kip1 expression while impairing muscle precursor cell-cycle progression. Muscle Nerve. 2008;37:84–89. doi: 10.1002/mus.20897. [DOI] [PubMed] [Google Scholar]
- 47.Roy SK, Chen Q, Fu J, Shankar S, Srivastava RK. Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors. PLoS One. 2011;6:e25166. doi: 10.1371/journal.pone.0025166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilley J, Coffer PJ, Ham J. FOXO transcription factors directly activate bim gene expression and promote apoptosis in sympathetic neurons. J Cell Biol. 2003;162:613–622. doi: 10.1083/jcb.200303026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson MK, McWhirter SM, Amin RH, Huang D, Schlissel MS. Abelson virus transformation prevents TRAIL expression by inhibiting FoxO3a and NF-kappaB. Mol Cells. 2010;29:333–341. doi: 10.1007/s10059-010-0029-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghaffari S, Jagani Z, Kitidis C, Lodish HF, Khosravi-Far R. Cytokines and BCR-ABL mediate suppression of TRAIL-induced apoptosis through inhibition of forkhead FOXO3a transcription factor. Proc Natl Acad Sci USA. 2003;100:6523–6528. doi: 10.1073/pnas.0731871100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Heuvel AP, Schulze A, Burgering BM. Direct control of caveolin-1 expression by FOXO transcription factors. Biochem J. 2005;385:795–802. doi: 10.1042/BJ20041449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kops GJ, Medema RH, Glassford J, Essers MA, Dijkers PF, Coffer PJ, Lam EW, Burgering BM. Control of cell cycle exit and entry by protein kinase B-regulated forkhead transcription factors. Mol Cell Biol. 2002;22:2025–2036. doi: 10.1128/MCB.22.7.2025-2036.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hauck L, Harms C, Grothe D, An J, Gertz K, Kronenberg G, Dietz R, Endres M, von Harsdorf R. Critical role for FoxO3a-dependent regulation of p21CIP1/WAF1 in response to statin signaling in cardiac myocytes. Circ Res. 2007;100:50–60. doi: 10.1161/01.RES.0000254704.92532.b9. [DOI] [PubMed] [Google Scholar]
- 54.Miyauchi H, Minamino T, Tateno K, Kunieda T, Toko H, Komuro I. Akt negatively regulates the in vitro lifespan of human endothelial cells via a p53/p21-dependent pathway. EMBO J. 2004;23:212–220. doi: 10.1038/sj.emboj.7600045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chandramohan V, Jeay S, Pianetti S, Sonenshein GE. Reciprocal control of Forkhead box O 3a and c-Myc via the phosphatidylinositol 3-kinase pathway coordinately regulates p27Kip1 levels. J Immunol. 2004;172:5522–5527. doi: 10.4049/jimmunol.172.9.5522. [DOI] [PubMed] [Google Scholar]
- 56.Lin L, Hron JD, Peng SL. Regulation of NF-kappaB, Th activation, and autoinflammation by the forkhead transcription factor Foxo3a. Immunity. 2004;21:203–213. doi: 10.1016/j.immuni.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 57.Hwang JW, Rajendrasozhan S, Yao H, Chung S, Sundar IK, Huyck HL, Pryhuber GS, Kinnula VL, Rahman I. FOXO3 deficiency leads to increased susceptibility to cigarette smoke-induced inflammation, airspace enlargement, and chronic obstructive pulmonary disease. J Immunol. 2011;187:987–998. doi: 10.4049/jimmunol.1001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Obexer P, Geiger K, Ambros PF, Meister B, Ausserlechner MJ. FKHRL1-mediated expression of Noxa and Bim induces apoptosis via the mitochondria in neuroblastoma cells. Cell Death Differ. 2007;14:534–547. doi: 10.1038/sj.cdd.4402017. [DOI] [PubMed] [Google Scholar]
- 60.Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J Biol Chem. 2011;286:7468–7478. doi: 10.1074/jbc.M110.179242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu JW, Chandra D, Rudd MD, Butler AP, Pallotta V, Brown D, Coffer PJ, Tang DG. Induction of prosurvival molecules by apoptotic stimuli: involvement of FOXO3a and ROS. Oncogene. 2005;24:2020–2031. doi: 10.1038/sj.onc.1208385. [DOI] [PubMed] [Google Scholar]
- 62.Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- 63.McCormick M, Chen K, Ramaswamy P, Kenyon C. New genes that extend Caenorhabditis elegans’ lifespan in response to reproductive signals. Aging Cell. 2012;11:192–202. doi: 10.1111/j.1474-9726.2011.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sengupta A, Molkentin JD, Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem. 2009;284:28319–28331. doi: 10.1074/jbc.M109.024406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 2009;119:2758–2771. doi: 10.1172/JCI39162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang W, Li G, Qiu J, Gonzalez P, Challa P. Protective effects of resveratrol in experimental retinal detachment. PLoS One. 2013;8:e75735. doi: 10.1371/journal.pone.0075735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li M, Chiu JF, Gagne J, Fukagawa NK. Age-related differences in insulin-like growth factor-1 receptor signaling regulates Akt/FOXO3a and ERK/Fos pathways in vascular smooth muscle cells. J Cell Physiol. 2008;217:377–387. doi: 10.1002/jcp.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Willcox BJ, Donlon TA, He Q, Chen R, Grove JS, Yano K, Masaki KH, Willcox DC, Rodriguez B, Curb JD. FOXO3A genotype is strongly associated with human longevity. Proc Natl Acad Sci USA. 2008;105:13987–13992. doi: 10.1073/pnas.0801030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Flachsbart F, Caliebe A, Kleindorp R, Blanché H, von Eller-Eberstein H, Nikolaus S, Schreiber S, Nebel A. Association of FOXO3A variation with human longevity confirmed in German centenarians. Proc Natl Acad Sci USA. 2009;106:2700–2705. doi: 10.1073/pnas.0809594106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morris BJ. A forkhead in the road to longevity: the molecular basis of lifespan becomes clearer. J Hypertens. 2005;23:1285–1309. doi: 10.1097/01.hjh.0000173509.45363.dd. [DOI] [PubMed] [Google Scholar]
- 71.Eelen G, Verlinden L, Meyer MB, Gijsbers R, Pike JW, Bouillon R, Verstuyf A. 1,25-Dihydroxyvitamin D3 and the aging-related forkhead box O and sestrin proteins in osteoblasts. J Steroid Biochem Mol Biol. 2013;136:112–119. doi: 10.1016/j.jsbmb.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 72.Kops GJ, Dansen TB, Polderman PE, Saarloos I, Wirtz KW, Coffer PJ, Huang TT, Bos JL, Medema RH, Burgering BM. Forkhead transcription factor FOXO3a protects quiescent cells from oxidative stress. Nature. 2002;419:316–321. doi: 10.1038/nature01036. [DOI] [PubMed] [Google Scholar]
- 73.Giordano S, Darley-Usmar V, Zhang J. Autophagy as an essential cellular antioxidant pathway in neurodegenerative disease. Redox Biol. 2014;2:82–90. doi: 10.1016/j.redox.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.El Assar M, Angulo J, Rodríguez-Mañas L. Oxidative stress and vascular inflammation in aging. Free Radic Biol Med. 2013;65:380–401. doi: 10.1016/j.freeradbiomed.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 75.Liochev SI. Reactive oxygen species and the free radical theory of aging. Free Radic Biol Med. 2013;60:1–4. doi: 10.1016/j.freeradbiomed.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 76.van der Horst A, Burgering BM. Stressing the role of FoxO proteins in lifespan and disease. Nat Rev Mol Cell Biol. 2007;8:440–450. doi: 10.1038/nrm2190. [DOI] [PubMed] [Google Scholar]
- 77.Chen IC, Chiang WF, Chen PF, Chiang HC. STRESS-responsive deacetylase SIRT3 is up-regulated by areca nut extract-induced oxidative stress in human oral keratinocytes. J Cell Biochem. 2014;115:328–339. doi: 10.1002/jcb.24667. [DOI] [PubMed] [Google Scholar]
- 78.Zhang S, Zhao Y, Xu M, Yu L, Zhao Y, Chen J, Yuan Y, Zheng Q, Niu X. FoxO3a modulates hypoxia stress induced oxidative stress and apoptosis in cardiac microvascular endothelial cells. PLoS One. 2013;8:e80342. doi: 10.1371/journal.pone.0080342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raju I, Kannan K, Abraham EC. FoxO3a Serves as a Biomarker of Oxidative Stress in Human Lens Epithelial Cells under Conditions of Hyperglycemia. PLoS One. 2013;8:e67126. doi: 10.1371/journal.pone.0067126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen SJ, Zhang W, Tong Q, Conrad K, Hirschler-Laszkiewicz I, Bayerl M, Kim JK, Cheung JY, Miller BA. Role of TRPM2 in cell proliferation and susceptibility to oxidative stress. Am J Physiol Cell Physiol. 2013;304:C548–C560. doi: 10.1152/ajpcell.00069.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li Z, Zhang H, Chen Y, Fan L, Fang J. Forkhead transcription factor FOXO3a protein activates nuclear factor κB through B-cell lymphoma/leukemia 10 (BCL10) protein and promotes tumor cell survival in serum deprivation. J Biol Chem. 2012;287:17737–17745. doi: 10.1074/jbc.M111.291708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tseng AH, Shieh SS, Wang DL. SIRT3 deacetylates FOXO3 to protect mitochondria against oxidative damage. Free Radic Biol Med. 2013;63:222–234. doi: 10.1016/j.freeradbiomed.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 83.Yang JY, Chang CJ, Xia W, Wang Y, Wong KK, Engelman JA, Du Y, Andreeff M, Hortobagyi GN, Hung MC. Activation of FOXO3a is sufficient to reverse mitogen-activated protein/extracellular signal-regulated kinase kinase inhibitor chemoresistance in human cancer. Cancer Res. 2010;70:4709–4718. doi: 10.1158/0008-5472.CAN-09-4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen J, Gomes AR, Monteiro LJ, Wong SY, Wu LH, Ng TT, Karadedou CT, Millour J, Ip YC, Cheung YN, Sunters A, Chan KY, Lam EW, Khoo US. Constitutively nuclear FOXO3a localization predicts poor survival and promotes Akt phosphorylation in breast cancer. PLoS One. 2010;5:e12293. doi: 10.1371/journal.pone.0012293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lam M, Carmichael AR, Griffiths HR. An aqueous extract of Fagonia cretica induces DNA damage, cell cycle arrest and apoptosis in breast cancer cells via FOXO3a and p53 expression. PLoS One. 2012;7:e40152. doi: 10.1371/journal.pone.0040152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shukla S, Bhaskaran N, Maclennan GT, Gupta S. Deregulation of FoxO3a accelerates prostate cancer progression in TRAMP mice. Prostate. 2013;73:1507–1517. doi: 10.1002/pros.22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shukla S, Bhaskaran N, Babcook MA, Fu P, Maclennan GT, Gupta S. Apigenin inhibits prostate cancer progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway. Carcinogenesis. 2014;35:452–460. doi: 10.1093/carcin/bgt316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen Q, Ganapathy S, Singh KP, Shankar S, Srivastava RK. Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells. PLoS One. 2010;5:e15288. doi: 10.1371/journal.pone.0015288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sunayama J, Sato A, Matsuda K, Tachibana K, Watanabe E, Seino S, Suzuki K, Narita Y, Shibui S, Sakurada K, et al. FoxO3a functions as a key integrator of cellular signals that control glioblastoma stem-like cell differentiation and tumorigenicity. Stem Cells. 2011;29:1327–1337. doi: 10.1002/stem.696. [DOI] [PubMed] [Google Scholar]
- 90.Ruvolo PP. The Herculean task of killing cancer cells: suppression of FOXO3A in acute leukemia involves a hydra of multiple survival kinases. Cell Cycle. 2012;11:2589. doi: 10.4161/cc.21233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang W, Li NN, Du Y, Lv FF, Lin GQ. FoxO3a and nilotinib-induced erythroid differentiation of CML-BC cells. Leuk Res. 2013;37:1309–1314. doi: 10.1016/j.leukres.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 92.Yang W, Dolloff NG, El-Deiry WS. ERK and MDM2 prey on FOXO3a. Nat Cell Biol. 2008;10:125–126. doi: 10.1038/ncb0208-125. [DOI] [PubMed] [Google Scholar]
- 93.Sunters A, Fernández de Mattos S, Stahl M, Brosens JJ, Zoumpoulidou G, Saunders CA, Coffer PJ, Medema RH, Coombes RC, Lam EW. FoxO3a transcriptional regulation of Bim controls apoptosis in paclitaxel-treated breast cancer cell lines. J Biol Chem. 2003;278:49795–49805. doi: 10.1074/jbc.M309523200. [DOI] [PubMed] [Google Scholar]
- 94.Hui RC, Francis RE, Guest SK, Costa JR, Gomes AR, Myatt SS, Brosens JJ, Lam EW. Doxorubicin activates FOXO3a to induce the expression of multidrug resistance gene ABCB1 (MDR1) in K562 leukemic cells. Mol Cancer Ther. 2008;7:670–678. doi: 10.1158/1535-7163.MCT-07-0397. [DOI] [PubMed] [Google Scholar]
- 95.Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, Nakao S, Motoyama N, Hirao A. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature. 2010;463:676–680. doi: 10.1038/nature08734. [DOI] [PubMed] [Google Scholar]
- 96.Birkenkamp KU, Essafi A, van der Vos KE, da Costa M, Hui RC, Holstege F, Koenderman L, Lam EW, Coffer PJ. FOXO3a induces differentiation of Bcr-Abl-transformed cells through transcriptional down-regulation of Id1. J Biol Chem. 2007;282:2211–2220. doi: 10.1074/jbc.M606669200. [DOI] [PubMed] [Google Scholar]
- 97.Buontempo F, Chiarini F, Bressanin D, Tabellini G, Melchionda F, Pession A, Fini M, Neri LM, McCubrey JA, Martelli AM. Activity of the selective IκB kinase inhibitor BMS-345541 against T-cell acute lymphoblastic leukemia: involvement of FOXO3a. Cell Cycle. 2012;11:2467–2475. doi: 10.4161/cc.20859. [DOI] [PubMed] [Google Scholar]
- 98.Dey P, Ström A, Gustafsson JÅ. Estrogen receptor β upregulates FOXO3a and causes induction of apoptosis through PUMA in prostate cancer. Oncogene. 2014;33:4213–4225. doi: 10.1038/onc.2013.384. [DOI] [PubMed] [Google Scholar]
- 99.Jia Y, Mo SJ, Feng QQ, Zhan ML, OuYang LS, Chen JC, Ma YX, Wu JJ, Lei WL. EPO-dependent activation of PI3K/Akt/FoxO3a signalling mediates neuroprotection in in vitro and in vivo models of Parkinson’s disease. J Mol Neurosci. 2014;53:117–124. doi: 10.1007/s12031-013-0208-0. [DOI] [PubMed] [Google Scholar]
- 100.Carbajo-Pescador S, Mauriz JL, García-Palomo A, González-Gallego J. FoxO proteins: regulation and molecular targets in liver cancer. Curr Med Chem. 2014;21:1231–1246. doi: 10.2174/0929867321666131228205703. [DOI] [PubMed] [Google Scholar]
- 101.Liang C, Chen W, Zhi X, Ma T, Xia X, Liu H, Zhang Q, Hu Q, Zhang Y, Bai X, et al. Serotonin promotes the proliferation of serum-deprived hepatocellular carcinoma cells via upregulation of FOXO3a. Mol Cancer. 2013;12:14. doi: 10.1186/1476-4598-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ghosh Choudhury G, Lenin M, Calhaun C, Zhang JH, Abboud HE. PDGF inactivates forkhead family transcription factor by activation of Akt in glomerular mesangial cells. Cell Signal. 2003;15:161–170. doi: 10.1016/s0898-6568(02)00057-8. [DOI] [PubMed] [Google Scholar]


