Abstract
Understanding the targets and mechanisms of human immunity to malaria caused by Plasmodium falciparum is crucial for advancing effective vaccines and developing tools for measuring immunity and exposure in populations. Acquired immunity to malaria predominantly targets the blood stage of infection when merozoites of Plasmodium spp. infect erythrocytes and replicate within them. During the intra-erythrocytic development of P. falciparum, numerous parasite-derived antigens are expressed on the surface of infected erythrocytes (IEs). These antigens enable P. falciparum-IEs to adhere in the vasculature and accumulate in multiple organs, which is a key process in the pathogenesis of disease. IE surface antigens, often referred to as variant surface antigens, are important targets of acquired protective immunity and include PfEMP1, RIFIN, STEVOR and SURFIN. These antigens are highly polymorphic and encoded by multigene families, which generate substantial antigenic diversity to mediate immune evasion. The most important immune target appears to be PfEMP1, which is a major ligand for vascular adhesion and sequestration of IEs. Studies are beginning to identify specific variants of PfEMP1 linked to disease pathogenesis that may be suitable for vaccine development, but overcoming antigenic diversity in PfEMP1 remains a major challenge. Much less is known about other surface antigens, or antigens on the surface of gametocyte-IEs, the effector mechanisms that mediate immunity, and how immunity is acquired and maintained over time; these are important topics for future research.
Electronic supplementary material
The online version of this article (doi:10.1007/s00018-014-1614-3) contains supplementary material, which is available to authorized users.
Keywords: Plasmodium falciparum, Variant surface antigens, Antibodies, PfEMP1, Malaria, Vaccines
Introduction
Plasmodium falciparum is the most virulent form of human malaria and is a leading cause of mortality among children under 5 years [1]. Plasmodium falciparum has a complex lifecycle involving a mosquito vector and a human host. The on-going asexual reproduction during the blood stage leads to clinical symptoms of malaria [2]. The pathogenesis of human malaria stems from various host and parasite factors that concurrently influence the severity and outcome of disease. Key pathophysiological features include the sequestration of P. falciparum-infected erythrocytes (IEs) in the microvasculature, the induction of proinflammatory cytokines and anemia resulting from the suppression of erythropoiesis [2, 3]. The destruction of uninfected erythrocytes and IEs further compromises oxygen delivery and exacerbates disease pathogenesis [4]. An important virulence property of P. falciparum is the expression of parasite-derived antigens on the surface of IEs, generally known as variant surface antigens (VSAs; Fig. 1), and its strong propensity to adhere in the vasculature. VSAs are comprised of novel parasite-derived proteins and include P. falciparum erythrocyte membrane protein 1 (PfEMP1) [5], repetitive interspersed family (RIFIN) proteins [6–8], sub-telomeric variable open reading frame (STEVOR) proteins [9–11], surface-associated interspersed gene family (SURFIN) proteins [12] and possibly others such as P. falciparum Maurer’s cleft two transmembrane (PfMC-2TM) proteins [13, 14]. Parasite-modified erythrocyte band 3 has also been proposed as a surface antigen or ligand for IE sequestration [15, 16]. These IE surface proteins are antigenically diverse and undergo clonal antigenic variation because of the selective pressure exerted by human immunity. The significance of VSAs as targets of naturally acquired immunity and their potential as vaccine candidates is the focus of this review. Acquired immunity to blood stage P. falciparum will be addressed, followed by a summary of the VSAs expressed on the IE surface and finally human antibodies to different VSA families.
Fig. 1.
Parasite-induced modifications to P. falciparum-infected erythrocytes. A. During intra-erythrocytic development, P. falciparum expresses knob structures and VSAs on the surface of pigmented trophozoite IEs. PfEMP1, P. falciparum erythrocyte membrane protein 1; RIFIN, repetitive interspersed family; STEVOR, subtelomeric variable open reading frame; SURFIN, surface-associated interspersed gene family; KAHRP, knob-associated histidine-rich protein. B. Scanning (left) and transmission (right) electron microscopy (EM) shows the ultrastructural features of the IE membrane. The IE membrane is distorted by surface knob protrusions (arrows) that present the major virulence factor, PfEMP1
Plasmodium falciparum sequestration and cytoadhesion
The virulence of P. falciparum malaria is attributed to the adhesion of IEs to the vascular endothelium or to uninfected erythrocytes to form rosettes [17–19]. Mature P. falciparum disappear from the peripheral circulation and are sequestered in various organs throughout the body. The importance of splenic clearance of IEs in controlling disease severity has been demonstrated by numerous studies. For example, a study conducted with Aotus monkeys showed that splenectomised animals developed virulent infections, presumably because of enhanced accumulation of IEs in the microvasculature [20, 21]. IE sequestration contributes to the pathogenesis of severe disease syndromes such as cerebral [17, 22, 23] and placental complications [24, 25]. An important feature of IEs that enables P. falciparum to sequester is the expression of knob structures on the IE membrane [26–28]. The major structural component of knobs is the knob-associated histidine-rich protein (KAHRP) [27, 29–32]. Other parasite-encoded proteins such as P. falciparum erythrocyte membrane protein 3 (PfEMP3) [33] and mature IE surface antigen (MESA; also known as PfEMP2) [34, 35] also contribute to knob assembly. KAHRP interacts with cytoskeletal components of the erythrocyte such as spectrin and actin [36–38], resulting in reduced membrane deformability [39]. Knobs present the major virulence factor, PfEMP1 [5], on the external surface of the IE membrane, where it mediates IE cytoadhesion to the host endothelium under physiological flow conditions [40, 41]. Disruption of the kahrp gene impairs proper knob formation and leads to a decrease in surface-exposed PfEMP1 and reduced cytoadhesion [42]. However, the presence of knobs may not necessarily result in sequestration [43]; P. malariae has knob structures but does not sequester, while P. chabaudi sequesters without knobs [16, 43].
A diverse range of host receptors that mediate IE cytoadhesion has been identified [44–46]. The main parasite ligand responsible for cytoadhesion is PfEMP1 and it binds to a range of endothelial and erythrocyte molecules including CD36 [47], ICAM-1 [48], chondroitin sulphate A (CSA) [49, 50], complement receptor 1 (CR1) [51], heparan sulfate (HS) [52] and others. IEs are capable of binding via multiple receptors [53] therefore creating a synergistic effect on IE adhesion [54]. Most P. falciparum isolates adhere to both ICAM-1 and CD36, which are widely distributed in the vasculature [53, 55, 56], but parasites isolated from infected placentas mainly adhere to specific receptors expressed by the syncytiotrophoblasts of the infected placenta [57, 58], particularly CSA [56, 59, 60], and possibly secondary receptors such as hyaluronic acid (HA) [61–63] and non-immune IgM [64–66] and IgG [67]. The differential expression of endothelial cell receptors in various tissues leads to the preferential binding of IEs. For example, it is proposed that ICAM-1-binding parasites are more likely to sequester in the brain [46, 68] as the brain endothelium expresses ICAM-1. While it has been speculated that receptor-specific adhesion (e.g. ICAM-1) predisposes to a particular pattern of disease (e.g. cerebral malaria), studies to date have been inconclusive. In one study, cerebral malaria patients did not show a significant association between disease and ICAM-1 binding [69], and another study reported that ICAM-1-binding was lowest in children with severe malaria [70]. In contrast, post-mortem histopathological analyses of infected cerebral vessels proposed a role for ICAM-1 in the manifestation of severe disease [68], and another study demonstrated that ICAM-1-binding was greater in cerebral malaria patients compared to patients with uncomplicated malaria [71]. This may propose a role for ICAM-1 in the pathogenesis of cerebral malaria but additional studies are necessary to further validate this association. A recent study identified endothelial protein receptor C as a likely mediator of cerebral sequestration [72]. Other host receptors implicated in IE cytoadherence include thrombospondin (TSP) [73], platelet/endothelial cell adhesion molecule (PECAM/CD31) [74], P-selectin [75] and vascular cell adhesion molecule-1 (VCAM-1) [53], but the significance of these receptors in disease pathogenesis remains unclear.
The clustering of mature IEs to uninfected erythrocytes, known as rosetting [76], is also thought to contribute to excessive microvasculature obstruction [77, 78]. Rosetting is associated with severe malaria in African children, suggesting that it contributes to disease pathogenesis [79–83]. However, a study with Malawian [70] and PNG [84] children reported that the rosetting occurred at a similar rate between children with severe and uncomplicated malaria, suggesting that rosette formation is not always associated with severe clinical outcomes. The parasite ligand for rosetting in P. falciparum has been identified as a specific PfEMP1 variant that binds to CR1 [51] or HS proteoglycans [85] expressed by the host erythrocyte.
Plasmodium falciparum also causes vascular obstruction through the clumping of IEs, a feature that was first reported as autoagglutination [86]. This adhesive phenotype is distinct from rosetting, as autoagglutinating parasites do not form rosettes and rosetting parasites do not autoagglutinate [86]. Autoagglutination was a common feature of infection, although more autoagglutinates were observed in children with severe malaria compared to those with mild malaria. This suggests that autoagglutination is more frequently observed in, but not restricted to, severe disease [87]. It was later reported that autoagglutination is mediated by platelets and required the expression of the platelet glycoprotein CD36 [88]. Scanning electron microscopy of platelet-mediated clumping of IEs showed that this interaction occurred at the IE knob structures [88]. Studies with Kenyan children [88] and patients from Thailand [89] established that platelet-mediated clumping of IEs was associated with severe disease, presumably through local disruptions of blood flow. Conversely, binding of platelets to IEs has also been implicated in protection against P. falciparum by directly inhibiting intra-erythrocytic parasite growth [90, 91].
VSAs of P. falciparum
The most extensively studied VSA is the major virulence factor PfEMP1, an important target of naturally acquired immunity [92, 93]. The var genes that encode PfEMP1 appear to be unique to P. falciparum, but the P. knowlesi schizont-infected cell agglutination (SICA) antigens encoded by the SICAvar multigene family [94, 95] have been described as conceptually similar to PfEMP1 [96]. Orthologs of rif and stevor genes have been identified in other Plasmodium species, known collectively as the pir multigene family (Plasmodium interspersed repeats). These include the vir multigene family in P. vivax [97], kir multigene family in P. knowlesi, yir multigene family in P. yoelii, bir multigene family in P. berghei and cir multigene family in P. chabaudi [98, 99].
Currently, little is known regarding the mechanisms that regulate gene transcription in P. falciparum other than the involvement of specific transcription factors and promoter interactions. Exploiting the mutually exclusive transcription of var genes has allowed for the suppression of the entire endogenous var multigene family [100, 101], thus enabling the specific study of PfEMP1 [102]. Conversely, disrupting up to 150 genes such as in the rif family is not currently feasible, and knockdowns of rif, stevor and pfmc2tm gene families have not been achieved. These multigene families share a common activation factor necessary for gene expression, and it has been proposed that the downregulation of one multigene family may affect the expression of members of other multigene families in some conditions [103]. A transcriptionally active rif promoter co-localised with an active var promoter and the downregulation of members in the stevor multigene family appeared to increase transcription of the pfmc-2tm multigene family [103]. Further studies must be employed to dissect the functional roles of these multigene families.
It is hypothesised that a large proportion of parasite proteins exported into the host erythrocyte supports the correct trafficking and surface display of PfEMP1 and other erythrocyte surface proteins. This also includes alterations in the spectrin network and knob protrusions at IE membrane [104]. Many exported proteins contain a pentameric sequence, known as the Plasmodium export element (PEXEL) [105] or vacuolar translocation signal [106], required for the translocation of proteins across the PV membrane. Exported parasite proteins such as PfEMP1 are trafficked via the translocon complex (PTEX; Plasmodium translocon of exported proteins) located at the parasitophorous vacuole (PV) [107, reviewed in 108]. Most parasite proteins are destined for the IE cytosol, and only a small portion is exposed on the IE surface. Interestingly, recent studies have showed that exported parasite proteins may play a role in cellular communication between IEs through microvesicles [109] and exosome-like vesicles [110]. Microvesicles lack components of the knob structure like KAHRP and PfEMP1, suggesting that they originate from MC structures or regions of the erythrocyte membrane that exclude knobs [109]. It was further demonstrated that PfEMP1 is not required for efficient intercellular communication as modified parasites with inhibited PfEMP1 expression were still able to receive exosome-like vesicles [110].
PfEMP1 and var genes
PfEMP1 was first identified by immunoprecipitation with immune sera from infected Aotus monkeys [5] and is encoded by the highly polymorphic var multigene family (~60 genes per genome) [40, 111, 112]. Through mutually exclusive transcription of var genes, only a single PfEMP1 variant is generally expressed on the IE surface at a given time [52, 113]. However, a recent study reported the potential expression of more than one PfEMP1 variant on the IE surface as demonstrated by live confocal microscopy, in vitro adhesion assays and cell sorting by flow cytometry [114]. PfEMP1 is a high-molecular-weight protein (200–350 kDa) and is highly sensitive to cleavage by mild trypsin treatment of intact IEs (10 μg/ml) [5]. The biochemical properties of PfEMP1 (Triton X-100-insoluble and SDS-soluble) demonstrate its anchorage to the IE membrane [115].
The export of PfEMP1 is a highly complex process due to its large size, number of membranes to traverse before reaching the IE surface and the involvement of various chaperone proteins (reviewed in [116, 117]). PfEMP1 molecules are associated with the Maurer’s clefts (MCs) and are ultimately presented by the knob structures at the IE surface at approximately 18 h post-invasion [5, 32, 115, 118–120]. The mechanism of transport of PfEMP1 from the parasite to the IE surface as well as its partner proteins involved remains a process that is poorly defined.
An important MC-resident protein essential for PfEMP1 trafficking is the 48 kDa P. falciparum skeleton binding protein 1 (SBP1) [121–123]. Disruption of the pfsbp1 gene impaired the loading of PfEMP1 molecules into the MCs and resulted in the loss of surface-exposed PfEMP1, but the trafficking of other MC proteins such as KAHRP, MAHRP1 and REX1 was unaffected in the transgenic parasites [122, 123]. A large-scale gene knockout screen further identified MC proteins termed P. falciparum PfEMP1 trafficking protein (PfPTP) involved in the trafficking of PfEMP1 to the IE surface [124]. Of the 83 P. falciparum genes that were disrupted, 6 genes were specifically found to affect the export and surface display of PfEMP1, and 2 were found to disrupt proper knob formation. In these transgenic parasites, PfEMP1 export was arrested either at the PVM or the MC structures in the IE cytosol [124]. Among the proteins involved in the trafficking of PfEMP1 are members of the HSP40/DNAJ and PHIST family [124]. Recently, an exported parasite-encoded HSP70 known as PfHSP70-x was found to complex with HSP40s and colocalised with PfEMP1 in the IE cytosol [125]. Other MC-associated proteins that have been proposed to play a role in the trafficking of PfEMP1 include MAHRP [126], REX1 [127] and Pf332 [128].
Transcription of var genes is epigenetically regulated by the SIR complex as gene disruption of PfSIR2 results in activation of multiple members of this multigene family [129–131]. The rapid switching rate of var genes, of up to 2 % per generation [86], was demonstrated to correlate with changes in IE adhesive and antigenic phenotypes [112, 132]. The typical structure of PfEMP1 includes the variable N-terminal segment (NTS) exposed on the IE surface to interact with host receptors [133], a transmembrane domain and a conserved acidic terminal segment (ATS) [48]. The cytoplasmic ATS domain interacts with KAHRP [134–136], thus anchoring PfEMP1 to the IE membrane. The extracellular portion of PfEMP1 consists of Duffy binding-like (DBL) adhesive domains, the C2 domain and the cysteine-rich interdomain regions (CIDR) [48]. DBL domains are grouped as five sequence classes (α, β, γ, δ, ε), and CIDR domains are grouped as three distinct classes (α, β, γ). While the number of CIDR and DBL domains may vary between different PfEMP1 variants, certain domain architectures such as DBLαCIDRα or DBLδCIDRβ are preferred. This conservation may reflect the biological function of PfEMP1 [48]. The binding site of ICAM-1 resides within the DBL2β and c2 regions of PfEMP1 [137, 138], while the binding site of CD36 is mapped to the CIDRα region [47, 139, 140]. The binding site of CSA in pregnancy-associated parasites lies within the DBL1-DBL3 domain of PfEMP1 [141].
The var genes can be classified into three main subgroups based on their upstream promoter regions (upsA, upsB and upsC) and the single-copy conserved intergenomic genes var1 and var2csa [142–145]. The var gene repertoires of clinical isolates can also be classified according to short sequence tags amplified from the DBLα domain [146, 147]. Analyses of the DBLα sequences from Kenya showed the presence of two or four cysteine residues, with a minority containing one, three, five or six cysteines; therefore, var genes were sub-grouped according to cys2, cys4 and cysX, respectively [147]. These DBLα sequences were further classified according to the amino acid motifs occurring at four fixed positions within the sequenced region, known as “positions of limited variations” (PoLV1-4) [147]. Expression of var genes from the cys2 group was associated with severe malaria in young children [148, 149]. The differential transcription of var gene subgroups has been linked to clinical disease. Transcription of group A var genes was also associated with rosetting parasites [150] and severe malaria in African children [151–153]. Furthermore, the elevated expression of group A-like var genes was associated with impaired consciousness, a key feature of severe disease [154]. Recent studies also reported the expression of a restricted subset of var genes encoding PfEMP1 variants that bind human brain endothelial cells [155, 156]. These var genes belong to group B/A genes that are expressed in early childhood infections [148, 157] and are associated with more severe infections [149, 151]. PfEMP1 variants from group B and C var genes are also associated with autoagglutination and ICAM-1-binding, features that contribute to severe disease [133, 138]. The lack of association between transcription of var group C and clinical presentation suggests that perhaps these var genes are involved in the establishment of chronic infections [158]. These findings support the correlation between var gene expression patterns and clinical presentations, thus suggesting that protective immunity could be conferred by antibodies to key var gene subgroups [147, 158].
A specific var gene, var2csa, is relatively conserved in sequence and is present as a single-copy gene in most isolates. However, some isolates have more than one copy of var2csa (e.g. HB3 has two copies of var2csa) [159–161]. This gene is upregulated in placental isolates and mediates IE adhesion to CSA and other placental receptors such as HA and immunoglobulins [162–165]. Polyclonal antibodies generated against recombinant domains of VAR2CSA recognised the IE surface of parasites isolated from infected placental tissue [166]. Furthermore, sera from pregnant women recognised the IE surface of VAR2CSA-expressing parasites in a parity-dependent manner [164, 167]. Pregnant women with elevated levels of these antibodies had a reduced risk of delivering low-birth-weight babies [164]. Targeted gene disruption of var2csa in the isolates FCR3 [168] and 3D7 [169] inhibited CSA adhesion, suggesting the central role of var2csa in mediating placental adhesion. In contrast, disruption of var2csa in CS2 parasites also ablated CSA binding but repeated selection on CSA restored their binding ability [169] presumably through the expression of other PfEMP1 variants proposed to bind CSA [49]. The use of different parasite lines may reflect the discrepancies between these studies and that the FCR3 or 3D7 isolates lack the PfEMP1 variants thought to rescue the CSA-binding ability.
The var transcripts have also been detected by RT-PCR in both immature (I–II) and mature stage gametocyte-IEs (IIB–V). Initial studies suggested that the var genes transcribed in gametocyte-IEs were similar or identical to those expressed by asexual parasites [170]. However, it was later discovered that the var transcript profile was unlinked to their asexual progenitors. Furthermore, it appears that the most abundant var transcripts found in gametocyte-IEs (generated in vitro) belong to the non-subtelomeric group C var genes [171]. Data regarding the pattern of PfEMP1 expression in gametocyte-IEs are conflicting. Early studies reported the expression of PfEMP1 in all five stages of gametocyte development but with stage-specific patterns. PfEMP1 staining was visualised at the IE membrane of immature gametocyte-IEs (stages I–IIA) but not of mature gametocyte-IEs (IIB–V) [170]. A recent study reported low levels of PfEMP1 expression on the surface of immature gametocyte-IEs, which was absent in mature gametocyte-IEs [172]. Thus, it is thought that sequestration of immature gametocyte-IEs is mediated by PfEMP1, after which its role is replaced by an alternative ligand present on the surface of mature gametocyte-IEs or through mechanical effects [173].
RIFIN proteins
The rif multigene family (150–200 genes per genome) encoding a group of clonally variant RIFIN proteins represents the largest multigene family identified in P. falciparum [6–8]. Transcription of rif genes occurs approximately 12 h post-invasion, but RIFIN proteins are thought to appear on the IE surface at the same time as PfEMP1 [174]. In contrast to var genes, a single parasite simultaneously transcribes several rif genes, resulting in the expression of multiple RIFIN variants on the IE surface [8]. All RIFIN sequences contain the PEXEL motif required for correct export [105]. Surface exposure of RIFIN was evident from immunoprecipitation and Western blot analyses. Bands of expected size corresponding to RIFIN (30–45 kDa) were absent after IEs were treated with high concentrations of trypsin (>100 μg/ml), a concentration much greater than that needed to cleave the highly trypsin-sensitive PfEMP1 [7, 8]. Some variants of the large RIFIN family are also expressed in other developmental stages such as merozoites, sporozoites and gametocytes [175–177]. Bioinformatic analyses of RIFIN sequences revealed two major subgroups of the RIFIN family [178] that are simultaneously expressed in a single parasite. A-type RIFINs associate with the MCs and are destined for the IE surface, whereas B-type RIFINs remain confined within the parasite [176]. Although the biological function of RIFIN remains unknown, the exposure of their highly polymorphic V2 epitope on the IE surface suggests they contribute to antigenic variation of P. falciparum [6, 179]. Although direct evidence is lacking, RIFIN was proposed to play a role in rosetting [7, 8].
STEVOR proteins
The stevor multigene family, encoding STEVOR proteins (~30–40 kDa), is the third largest identified in P. falciparum (reviewed in [10]). First described as 7h8, stevor was detected by a monoclonal antibody as an expressed sequence [6]. Each parasite genome is predicted to contain approximately 30–40 copies of stevor genes. Like var and rif, stevor genes are located at the telomeres of most P. falciparum chromosomes [6]. Similar to rif, multiple stevor transcripts were detected in a single parasite. Peak stevor transcription occurs at 28 h post-invasion during late trophozoites and early schizonts, where they appear to localise in the IE cytosol. As the parasite matures, STEVOR co-localises with PfSBP1 and PfEMP3 at the MCs [9, 180] in immunofluorescence microscopy with fixed IEs [10, 11]. Immunofluorescence microscopy with live, intact schizont stage IEs suggests the surface localisation of STEVOR, which was removed upon IE trypsinisation [11, 181]. A recent study demonstrated that stevor overexpression contributes to increased IE rigidity [182], together with other IE cytoskeletal members such as RESA [183]. It is proposed that the STEVOR-increased stiffness of IEs enhances PfEMP1-mediated IE sequestration [182]. The biological function of STEVOR remains unclear. Because STEVOR is clonally variant [13, 184], it may be involved in immune evasion concurrently with PfEMP1 and RIFIN [10, 11, 13]. In addition, STEVOR has been proposed to play a role in parasite invasion [185]. STEVOR proteins are also expressed in merozoites [10, 186, 187], sporozoites [175] and gametocytes [9]. Interestingly, the same STEVOR variants are transcribed in gametocytes and their asexual progenitors, suggesting that perhaps STEVOR plays a similar role in these lifecycle stages [171].
SURFIN proteins
Little is known about the surf multigene family (10 genes), which encodes high-molecular-weight antigens (~280–300 kDa) known as SURFIN proteins [12]. The expression of surf genes is differentially transcribed according to different stages of the intra-erythrocytic parasite. The expression of surf 1.3, surf 4.2 and surf 8.3 genes was detected throughout parasite development while other surf genes were either not detected or restricted to later developmental stages [188]. A variant expressed by 3D7 and FCR3 parasites, SURFIN4.2 was identified by mass spectrometric analysis of proteins cleaved off the surface of intact IEs by trypsin [12]. SURFIN4.2 was only detected in a subpopulation of cultured IEs (~25 %) with increasing protein expression during mature developmental stages (24–44 h post-invasion). Immunoelectron microscopy showed the presence of SURFIN4.2 at the knob structures suggesting its co-localisation with PfEMP1 at the IE surface [12]. However, attempts to verify the surface localisation of SURFIN4.2 proteins with live, intact IEs were inconclusive. Another variant, SURFIN4.1, localised to the parasitophorous vacuole (PV) but not within the erythrocyte cytosol in mature IEs (>30 h post-invasion) [188]. SURFIN antibodies did not agglutinate mature IEs and no fluorescence was observed with live IEs, suggesting that SURFIN4.1 is not exposed on the IE surface [188]. Whether SURFIN proteins potentially elicit humoral immunity or mediate immune evasion has not been determined.
Other membrane proteins
Plasmodium falciparum Maurer’s clefts two-transmembrane protein (PfMC-2TM) is encoded by a novel gene family (~13 members) located at the subtelomeric regions of several P. falciparum chromosomes [14, 142]. PfMC-2TM is highly conserved within the N-terminus, both transmembrane domains and the C-terminus. The short loop between the transmembrane domains is highly polymorphic, similar to that proposed for RIFIN and STEVOR [13, 184]. The diversity within this loop region proposed the inclusion of pfmc-2tm as a variant multigene family together with var, rif and stevor [13, 14]. It has not been determined whether PfMC-2TM is associated with the IE membrane or exposed on the IE surface.
Another IE membrane protein is modified erythrocyte band 3, which has been proposed as a ligand for IE adhesion to CD36 [189] and thrombospondin [190, 191]. Chemical modifications of band 3 led to a reduction in CD36 binding but not thrombospondin, thus supporting its role in CD36 adhesion [189]. Synthetic peptides based on the exofacial loops of band 3 and antibodies generated against these peptides were capable of inhibiting IE adhesion to C32 amelanotic melanoma cells. Additionally, intravenous infusion of these peptides into Aotus and Saimiri monkeys infected with P. falciparum isolates prevented IE sequestration [192]. However, its significance in relation to PfEMP1 as an adhesive ligand remains unclear. In our recent study, parasites with suppressed PfEMP1 expression were found to retain a substantial proportion of CD36 binding, but not ICAM-1, thus raising the possibility that additional surface antigens contribute to CD36 adhesion [102].
Naturally acquired immunity to malaria
Protective immunity to malaria is elicited through complex interactions between both humoral and cell-mediated responses [193–195]. This protection against symptomatic malaria in humans develops gradually after repeated exposure to P. falciparum infections (reviewed in [196]). In malaria-endemic areas, the risk of severe disease is greatest during the first few years of life, after which the risk rapidly declines as children begin to acquire natural immunity. A study of young African children reported that immunity to non-cerebral severe malaria may develop after several infections and is almost complete by the age of 5 [197]. Adolescents and adults eventually develop protection from severe illness and death, although sterile immunity is rarely or perhaps never achieved [198]. Maternal antibodies transferred across the placenta are also thought to confer protection in young infants [199, 200]. It is becoming increasingly clear that effective immunity to malaria involves immune responses to multiple antigens expressed at different parasite stages and requires multiple immune effector mechanisms [195]. It is likely that the development of a highly effective malaria vaccine will require the inclusion of multiple antigens and that single-antigen vaccines will not be optimally efficacious.
Cell-mediated immunity to VSAs
While the significance of humoral immunity to P. falciparum is well established in humans, the role of cell-mediated immunity (CMI) remains poorly understood. CMI acts through complex interactions with the innate and adaptive immune response (reviewed in [195, 201]). Most studies of CMI have been based on murine models of malaria (reviewed in [202]). Early studies showed that mice incapable of making B cells have the ability to control infection [203], suggesting the importance of CMI in protection against malaria. Antigen-presenting cells process parasite antigens for display on major histocompatibility complex molecules to recruit antigen-specific CD4+ T cells. Th1 cells produce proinflammatory cytokines such as TNF-α and IFN-γ, which lead to monocyte activation and the release of toxic mediators that limit P. falciparum growth [204]. In a study of Gabonese children, IFN-γ responses to erythrocytic antigens were associated with lower rates of P. falciparum reinfection [205]. Despite this protective potential, CMI responses have also been implicated in disease pathogenesis and the development of severe malaria (reviewed in [206]).
Although data on CMI responses to VSAs are limited, studies have reported that parasites may modulate CMI to evade host immune responses. For example, the maturation of dendritic cells cultured in vitro was suppressed following exposure to erythrocytes infected with P. falciparum [207]. It was later reported that the modulation of dendritic cells was not dependent on the interaction between PfEMP1 and CD36 [208]. Interaction between IEs and natural killer (NK) cells leads to their activation, including production of IFN-γ [209]. A recent study using a humanised mouse model has reported that NK cell binding of IEs leads to the activation of NK cells and the elimination of IEs [210]. Using parasites with modified PfEMP1 expression, others reported that PfEMP1 appeared to suppress innate IFN-γ production by naïve CD4+ T cells and NK cells [211]. The CIDR1α domain of PfEMP1 was found to induce polyclonal B cell activation that contributes to the evasion of host immune responses [212–214] and stimulates CD4+ T cells from both malaria-exposed and non-exposed individuals [215]. The addition of recombinant CIDR1α to naïve human peripheral blood mononuclear cells resulted in the activation of CD4+ T cells and NK cells, leading to IFN-γ production [216]. It appears that a fine balance between protective immunity and immunopathology must be achieved in CMI. The lack of CMI-related studies in human malaria and the difficulty of inferring results from murine models are continuing obstacles in our understanding of the role of CMI and a priority topic for further research.
Human antibodies to VSAs
The development of protective immunity to P. falciparum is characterised by a decrease in disease severity over several years after repeated infections [217]. Sterile immunity to P. falciparum is rarely achieved, as adults living in malaria-endemic regions remain susceptible to asymptomatic infection and often experience persistent low levels of parasitaemia without clinical disease [196, 198]. The passive transfer of gamma-globulin from immune individuals to P. falciparum-infected individuals confers protection against malaria infection [218]. Antibodies to both merozoite antigens [219–221] and VSAs appear to play an important role in mediating acquired immunity. The focus of this review is on VSAs, and the significance of merozoite antigens as immune targets is reviewed in detail elsewhere [222]. In brief, numerous antigens on the surface of merozoites (e.g. merozoite surface protein 1, 2, and 3) and erythrocyte invasion ligands (e.g. erythrocyte-binding antigens, PfRh invasion ligands and apical membrane antigen 1) have been identified as important targets of acquired immunity and promising vaccine candidates [219–221, 223, 224]. Although it is highly likely that antibodies to VSA and merozoite antigens contribute to immunity and a strong response to both types of antigens may be essential for highly effective immunity, there are few reports on the relationship between these responses and how they may interact to mediate immunity [225]; this is an important question for further research.
Naturally acquired antibodies against VSAs typically demonstrate a high degree of strain specificity [132, 226]. Antigenic diversity by P. falciparum enables repeated infections to occur over time, as new infections appear to exploit gaps in the repertoire of previously acquired variant-specific antibodies [226]. Antibodies to polymorphic VSAs expressed on the IE surface, such as PfEMP1, have been proposed to play a key role in mediating protective immunity [93, 226–228]. Most published studies used agglutination assays to describe antigenic variation in PfEMP1 as switches in the agglutination phenotype are correlated with switches in var gene expression [112] or PfEMP1 [132]. However, it is difficult to determine PfEMP1-specific antibody responses because of the number of antigens expressed on the IE surface; therefore, antibodies measured to the IE surface are hereafter classified as antibodies to all VSAs (studies summarised in Table S1).
Early studies that measured agglutination antibody responses to P. falciparum infections in Pakistan [229], Papua New Guinea [230] and Africa [226, 231] reported that children developed isolate-specific antibodies to VSAs after infection. Mixed agglutination assays that allowed the determination of shared epitopes on the IE surface also showed that acquired human antibodies are predominantly variant specific while cross-reactive antibodies are rare [231]. However, antibodies from convalescent sera from adults were capable of agglutinating diverse P. falciparum isolates [232], and antibodies acquired towards placental-binding parasites expressing VAR2CSA have a significant amount of cross-reactivity against different isolates, despite polymorphisms in VAR2CSA [159, 233]. Furthermore, an acute P. falciparum infection in returned travellers was sufficient to induce broadly cross-reactive antibodies that were relatively long lived (>20 weeks post-infection) [208], thus suggesting that cross-reactive antibodies are prevalent following infection. The molecular basis for this remains unclear but may be due to extensive sharing of polymorphic epitopes between PfEMP1 variants [159, 234]. In an early study, parasite isolates from ten Gambian children were tested in a checkerboard manner with the acute and convalescent sera collected from each child [226]. Most of the acute sera were not reactive towards the parasite isolate from that same child, whereas each of the serum samples collected during convalescence were highly reactive to the IE surface of the isolate from the same child but not from other children [226]. This study suggested that the VSAs expressed on the IE surface are highly diverse and children tend to acquire antibodies towards the variants expressed by the parasite causing that particular episode. Moreover, hyperimmune sera from Gambian adults agglutinated IEs from those ten children, suggesting that by adulthood most individuals have acquired a broad range of antibodies that protect against numerous parasite variants [226], and the presence of cross-reactive antibodies was also proposed. A large prospective study of young Kenyan children further showed that parasite variants expressed during episodes of clinical infection were less likely to be recognised by homologous sera collected before infection. This suggests that the variants are exploiting gaps in the pre-existing antibody repertoire in order to cause subsequent infections [227]. Consistent with that observation, antibodies from Sudanese children measured after the malaria season could agglutinate a broader range of isolates tested compared with antibodies measured before the season [235]. This suggests that natural P. falciparum infections are capable of inducing high antibody titres directed towards VSAs of the infecting parasite. In a cross-sectional study of Kenyan children during the low transmission season, antibodies to VSAs were higher in parasitaemic individuals, suggesting that these antibodies were induced by current infections [236]. Furthermore, plasma antibody levels were positively correlated with age, indicating an age-related and exposure-related component in the acquisition of VSA-specific antibodies [237].
The importance of antibodies to VSAs is evident through their role in mediating protective immunity to malaria (studies are summarised in Table 1). For example, in a longitudinal study conducted with young Gambian children, acquired antibodies to VSAs from several different isolates were consistently associated with protection from clinical malaria [228]. In Gabonese children, higher levels of IgG to VSAs were associated with lower rates of malaria [238]. A study of Ghanian children demonstrated that those with pre-existing antibodies before the malaria season were less likely to contract malaria than those with low levels of antibodies [239]. Furthermore, the presence of antibodies to a Ghanian isolate was significantly associated with protection from malaria [240]. However, the ability of sera from Kenyan children to recognise VSAs expressed by a Kenyan isolate was not associated with protection from malaria [227]. It appears that anti-VSA antibodies to some but not all parasite isolates are associated with protection [236, 239, 240], and presumably this depends on the prevalence of the parasite variant and its virulence properties. Nonetheless, evidence from all of the studies presented above supports the important contribution of anti-VSA antibodies to protection against malaria.
Table 1.
Studies examining the association between human antibodies to VSAs and protection against malaria
| Province, Country | Study | Population (n) | Age | Parasite isolates | Findingsa |
|---|---|---|---|---|---|
| Farafenni, The Gambia | Marsh et al. [228] | Children (134) | <11 years | Gambian isolate (GAM83/1) | Antibodies to the IE surface were prospectively associated with protection against clinical episodes of malaria |
| Lambarene, Gabon | Tebo et al. [238] | Children (200) | 6 months–11 years | IEs from 3 donor children | Convalescent sera from children with mild malaria had higher anti-VSA IgG compared to matched children with severe malaria |
| Dodowa, Ghana | Dodoo et al. [239] | Children (118) | 3–15 years | IEs isolated from children | IgG to VSAs correlated with protection from clinical malaria |
| Daraweesh, Sudan | Giha et al. [240] | Adults and children (39) | 5–50 years | IEs isolated from children | Antibodies to Ghanian isolate were significantly associated with protection |
| Kilifi, Kenya | Bull et al. [227] | Children (65) | 1–5 years | Kilifi isolate (1759) | Lack of association between antibodies to the Kilifi isolate and protection from malaria |
| Kilifi, Kenya | Bull et al. [236] | Children (84) | 1–5 years | IEs isolated from children | No association between anti-VSA antibodies or parasite positivity and protection from malaria |
| Lambarene, Gabon | Yone et al. [288] | Children (100) | 1–8 years | IEs isolated from 6 donor children | Convalescent-phase IgG1 was associated with clinical protection |
| Kilifi, Kenya | Mackintosh et al. [289] |
Children (272) Children (39) |
6 months–10 years | Reference parasites (A4, 3D7), 1 clinical isolate from a Kenyan child | Failure to mount antibodies against these isolates was associated with malaria susceptibility in children with asymptomatic parasitaemia |
| Kilifi, Kenya | Chan et al. [102] | Children (296) | 1–10 years | Reference parasites (3D7, E8B) and genetically-modified parasites | PfEMP1 is a dominant target of antibodies and PfEMP1-specific antibodies were associated with protection against symptomatic malaria |
PubMed was searched for studies that examined the association between acquired human antibodies to total VSAs and protection against malaria, without an exclusion criterion, and attempts were made to include most studies
aNot all findings are listed for all studies
Despite the apparent role of PfEMP1 antibodies in mediating protection against malaria, the immense diversity of PfEMP1 limits its potential as a vaccine candidate. Although the repertoire of PfEMP1 variants is large, studies have suggested the expression of a dominant subset of variants that are restricted by their biological function in clinical disease. Immune sera from distinct geographic regions agglutinated IEs from other populations, suggesting that antibodies targeted cross-reactive epitopes expressed by many isolates [241]. Another study demonstrated that plasma antibodies were capable of recognising various parasite isolates regardless of the geographic origin of those IEs, suggesting that the repertoire of VSA-specific antibodies may be conserved over different populations [242]. A study in Kenya demonstrated that parasites isolated from children presenting with severe malaria were recognised by heterologous plasma antibodies, suggesting the expression of common PfEMP1 variants in this population [92]. Similarly, IEs from young Ghanian children with severe malaria were more commonly recognised by plasma antibodies from other children than those with uncomplicated malaria [243]. This suggests that a restricted subset of variants is expressed during severe disease to which antibodies are rapidly acquired [197]. These studies propose that the infecting parasites causing severe disease may be expressing a commonly expressed subset of VSA variants [236, 237]. A limitation of these studies is that they were only able to measure antibodies directed towards all VSAs expressed on the IE surface and not the proportion of antibodies to individual VSAs such as PfEMP1.
Little is known about antibody responses directed at antigens expressed on the surface of erythrocytes infected with gametocytes during their development in the human host. Such antigens could potentially elicit immune responses, similar to those of asexual parasites, which may result in the clearance of gametocytes in the host. Sera from Gambian children were reported to be highly reactive towards the surface of mature stage gametocyte-IEs but not towards immature stage gametocyte-IEs [244]. This may suggest that the antigens recognised by the serum antibodies in mature gametocyte-IEs are distinct from those expressed by immature gametocyte-IEs [244]. However, another study showed that sera from children in Papua New Guinea (PNG) were highly reactive to the surface of immature gametocyte-IE, similar to that observed with asexual trophozoite IEs, but not towards the surface of mature gametocyte-IEs [245]. These conflicting results demonstrate that further work is needed to better understand the antibody response directed towards antigens on the surface of gametocyte-IEs. Antibodies targeting these surface antigens represent potential vaccine candidates as they may mediate gametocyte clearance from the circulation, thus leading to reduced malaria transmission. More importantly, understanding the humoral response elicited by antigens on the surface of gametocyte-IEs will shed light on how these antibodies potentially act in synchrony with antibodies to other parasite stages to clear parasitaemia and reduce transmission.
Human antibodies to PfEMP1
Epidemiological data have demonstrated that naturally acquired antibodies predominantly target variant-specific epitopes on the IE surface and PfEMP1 is thought to be a major antibody target. Lacking the molecular tools required to evaluate the significance of PfEMP1 independently of other VSAs, most studies have relied on the use of recombinant purified PfEMP1 domains to study human antibody responses to PfEMP1 (studies summarised in Table S2). A recent study in PNG used a DBLα protein microarray to demonstrate that the magnitude of the anti-PfEMP1 response was limited and variant specific in young children (<3 years of age), after which a broader spectrum of antibody recognition was achieved. By adulthood, serum antibodies were capable of recognising at least 20 different variants indicating an expansion of the PfEMP1 antibody repertoire [246]. Consistent with the preferential expression of PfEMP1 variants in severe disease [151, 152], the acquisition of anti-PfEMP1 antibodies by Tanzanian children was reported to be highly structured. Antibodies to different recombinant PfEMP1 domains were sequentially acquired, with children first acquiring antibodies to particular variants encoded by group A var genes [157, 247]. These findings were supported by another study whereby PfEMP1 DBLα domains were linked to young host age, disease severity and low levels of immunity [148]. Furthermore, they complement the finding that immunity to severe disease may be rapidly acquired after several infections [197].
Others have reported that while sera from Gabonese adults recognised most of the recombinant PfEMP1 based on the DBLα region, sera from children were less reactive to the different variants [248]. Interestingly, they also showed that antibodies from highly reactive adults chosen from previous assays were capable of recognising synthetic peptides based on conserved regions of DBLα [248], suggesting that antibodies to both variant-specific and conserved regions of PfEMP1 are co-acquired. Similarly, serum antibodies from Ghanian [239] and Sudanese [249] children recognised a recombinant peptide derived from a conserved epitope of the DBLα domain, with higher antibody levels observed in asymptomatic individuals compared to those with febrile malaria suggesting that antibodies against conserved epitopes of PfEMP1 may play a role in protective immunity. However, there was no association observed between these antibodies and protection from malaria in Ghanian children, because the recombinant peptide originated from a domain that is inaccessible to antibodies [239]. Since conserved epitopes are not consistent with being key antibody targets, the role of these antibodies in protective immunity remains unclear. Few studies have evaluated the protective effect of anti-PfEMP1 antibodies (studies summarised in Table 2) and results have been inconsistent. A longitudinal study with Ghanian children did not find a correlation between protection and antibodies to the DBLα domain of PfEMP1 [239]. No protective association was observed with antibodies to the recombinant PfEMP1 domains derived from the A4 parasite line [250].
Table 2.
Studies examining the association between human antibodies to PfEMP1 and protection against malaria
| Province, country | Study | Population (n) | Age | Antigena | Findingsb |
|---|---|---|---|---|---|
| Dodowa, Ghana | Dodoo et al. [239] | Children (118) | 3–15 years | Recombinant DBLα domain |
Plasma samples from most children recognised recombinant PfEMP1 No association between IgG to recombinant PfEMP1 and protection |
| Kilifi, Kenya | Mackintosh et al. [289] | Children | <10 years | Recombinant A4 PfEMP1 domains |
Anti-DBLα antibodies in those who were parasite negative at baseline were associated with protection No association between antibodies to other domains and protection |
| Sudan | Staalsoe et al. [252] | Children | – |
Synthetic peptides to conserved regions of PfEMP1 (same epitope as Dodoo et al. 2001) |
IgG levels higher in asymptomatic infection compared to febrile malaria |
| Tanga, Tanzania | Magistrado et al. [290] | Children | 0–19 years | Recombinant DBLα, DBL2γ, CIDR2β (3D7) | In children (4–9 years), the presence of antibodies were associated with reduced numbers of malaria episodes |
| Kilifi, Kenya | Chan et al. [102] | Children (296) | 1–10 years | Reference parasites (3D7, E8B) and genetically-modified parasites | PfEMP1 is a dominant target of antibodies and PfEMP1-specific antibodies were associated with protection against symptomatic malaria |
PubMed was searched for studies that examined the association between acquired human antibodies to recombinant PfEMP1 and protection against malaria, without an exclusion criterion, and attempts were made to include most studies
aAntibodies were measured by ELISA except for Chan et al. where antibodies were measured to native PfEMP1 by flow cytometry
bNot all findings are listed for all studies
Quantifying the importance of different VSAs as targets of human antibodies is important for understanding immunity to malaria, but has been challenging to achieve. Recently, we developed a novel approach using genetically modified P. falciparum with inhibited PfEMP1 expression to evaluate the significance of PfEMP1 and other antigens as targets of acquired antibodies [102]. Suppressed PfEMP1 surface expression was achieved by the transfection of P. falciparum with a construct that encodes a var promoter without a downstream var gene [100, 101]. During in vitro culture with drug-selectable markers, the var promoter is expressed, which causes the silencing of all the other endogenous var promoters and thus suppresses PfEMP1 expression [100, 101] (Fig. 2). This approach was applied to human studies in Kenya to quantify serum antibodies to VSAs. We found that among malaria-exposed individuals, IgG binding to the surface of erythrocytes infected with the transgenic parasites was markedly reduced compared to that seen with parental parasites expressing PfEMP1. This suggests that the majority of the acquired human antibody response to the IE surface targets PfEMP1, while other VSAs appear to play a minor role as antibody targets. Our longitudinal studies further showed that individuals with PfEMP1-specific antibodies had a reduced risk of symptomatic disease while antibodies to other VSAs were not associated with protective immunity. Together, our findings demonstrate the significance of PfEMP1 as a major target of humoral immunity to malaria [102].
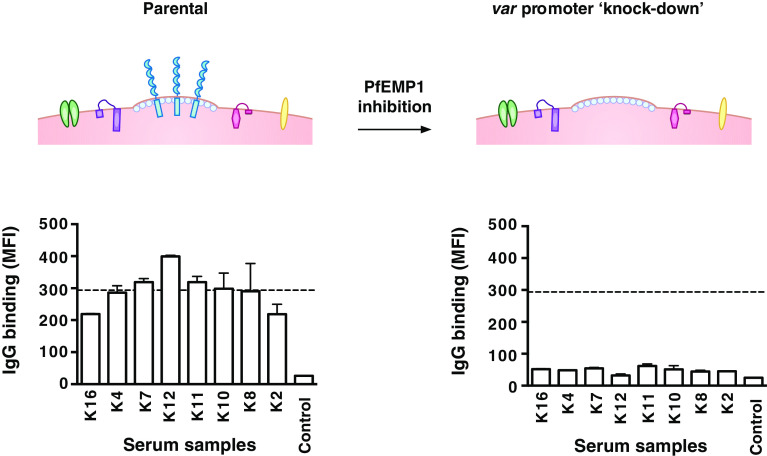
Fig. 2.
Evaluating the antibody response to PfEMP1 using transgenic P. falciparum P. falciparum-infected erythrocytes transfected with a construct that inhibits PfEMP1 expression but does not appear to have an impact on the expression of other VSAs (referred to as ‘var promoter knockdown’). This provides a novel approach to quantify antibodies to PfEMP1 and assess its importance as an immune target. The figure shows a representative selection of serum samples that were tested for IgG binding to parental and transgenic parasites [102]. Samples were from malaria-exposed Kenyan adults (K2-K16) and non-exposed Melbourne residents (Control). IgG binding to the surface of erythrocytes infected with the transgenic parasites was markedly reduced compared to parental parasites as previously reported [102]. The horizontal dotted line represents the mean level of IgG binding to parental parasites (n = 8); bars represent mean and range of samples tested in duplicate; IgG levels are expressed as geometric mean fluorescence intensity for both graphs
Human antibodies to RIFIN and STEVOR
Data suggest that RIFIN and STEVOR may play significant roles as targets of malaria immunity; however, they have been little studied compared to PfEMP1. In an area of intense malaria transmission in Gabon, high levels of antibodies to recombinant RIFIN were detected in a majority of the adult population. Although RIFIN antibodies were also detected in children, the prevalence of these antibodies was much lower [179]. Despite the high copy number of rif genes, most adult sera were capable of recognising more than one RIFIN variant suggesting the generation of a large anti-RIFIN repertoire. In addition, elevated levels of RIFIN antibodies were associated with rapid parasite clearance in children [251]. Longitudinal studies with these children showed that although RIFIN antibodies were not correlated with a reduced rate of reinfection, RIFIN antibodies were long lived (~2 years) [251]. Furthermore, higher levels of RIFIN antibodies were detected in asymptomatic children than in those with severe disease, suggesting a protective effect of these antibodies [251]. The preadsorption of immune sera on recombinant RIFIN resulted in a marked reduction in the overall antibody reactivity to the IE surface [252]. This study proposed that in addition to PfEMP1, RIFIN is a key contributor to the overall anti-VSA response [252]. Others have shown that severe malaria patients in Ghana had substantially higher antibody levels to recombinant RIFIN than asymptomatic controls, suggesting the effect of antibody boosting during a malaria episode [253].
Little has been done on naturally acquired antibodies to STEVOR. Adult plasma had elevated levels of STEVOR antibodies suggesting the immunogenicity of STEVOR during a natural infection [254]. A longitudinal study with 9-month-old infants found no correlation between STEVOR antibodies and protective immunity, but revealed an increase in the frequency of parasitaemic episodes in those with high levels of antibodies [254]. The explanation for this observation remains unclear, but it is speculated that STEVOR is not involved in mediating immunity and acts as a marker of malaria exposure instead [254]. Further studies are necessary to elucidate the importance of antibodies against native STEVOR to fully understand its biological role. As noted above, our study using parasites with suppressed PfEMP1 expression demonstrated that PfEMP1 is the dominant target of human antibodies. However, a proportion of antibody reactivity to the transgenic parasites was observed, suggesting that antibodies to other VSAs, such as RIFIN and STEVOR, may still play an important role in immunity [102].
Function of antibodies to VSAs
The mechanism by which antibodies to VSAs mediate protective immunity is only partially understood. Antibodies targeting VSAs are also thought to confer protection by interfering with IE sequestration or rosetting, features that contribute to malaria pathogenesis [79, 255]. Immune sera from infected Aotus monkeys blocked the binding of IEs to endothelial cells [18, 256]. Serum samples from pregnant women were capable of inhibiting IE adhesion to CSA [156, 257–259] and these antibodies were associated with improved birth outcomes in some studies [58, 260, 261]. In contrast, few studies have addressed adhesion inhibition in non-pregnant individuals. Convalescent serum from PNG children with symptomatic malaria inhibited the binding of homologous isolates to melanoma cells [230]. Antibodies from immune African adults inhibited the binding of a recombinant PfEMP1 domain to ICAM-1 [262]. Taken together, these results suggest the importance of antibodies that inhibit adhesion. Plasma antibodies from children presenting with mild malaria were capable of disrupting rosette formation in vitro, whereas those from children with severe malaria could not, suggesting that acquired antibodies are protective through rosette inhibition [79, 80, 150]. Furthermore, polyclonal antibodies against recombinant PfEMP1 domains disrupted existing rosettes and inhibited the formation of new rosettes [263].
Antibodies to VSAs also play a role in opsonising IEs for phagocytosis, an important mechanism of parasite clearance [264, 265]. A study with pregnant Malawian women showed that high levels of opsonising antibodies targeting VSAs correlated with parasite clearance and a decreased risk of maternal anaemia [266]. Immunisation of rabbits with recombinant PfEMP1 domains generated antibodies capable of inducing the opsonic phagocytosis of IEs [263]. Studies have also found that co-infection with HIV impaired the opsonic activity of antibodies for phagocytosis, thus leading to an increased risk of clinical malaria [267, 268]. Our recent data further identified PfEMP1 as a major target of naturally acquired antibodies that function to opsonise IEs for phagocytic clearance [102]. Individuals with high levels of antibodies to native PfEMP1 expressed on the IE surface promoted opsonic phagocytosis activity compared to transgenic parasites with inhibited PfEMP1 expression [102].
Vaccine studies on PfEMP1
The importance of PfEMP1 as an immune target strongly supports the development of PfEMP1 as a major vaccine candidate. However, a major challenge to its development as a vaccine is substantial antigenic diversity. Studies with animal models have provided evidence that recombinant PfEMP1 is capable of mounting a protective immune response. Immunisation of Aotus monkeys with the CIDRα domain of PfEMP1 protected against a lethal parasite challenge with the homologous, but not the heterologous parasite strain [269]. To overcome the variant-specific limitations of PfEMP1 antibodies, studies have used different combinations of PfEMP1 domains to elicit a broader antibody response. Mice immunised with a combination of CIDRα domains developed antibodies capable of agglutinating IEs using various parasite lines [270, 271]. Using an in vivo model of P. falciparum-IE sequestration, rats immunised with diverse NTS-DBLα domains induced protective antibodies that reduced IE sequestration [272]. This was supported by a recent study in The Netherlands where naïve volunteers, who were infected with P. falciparum, generated cross-reactive antibodies that recognised PfEMP1 from different parasite genomes [273]. Taken together, these studies suggest that it may be possible to induce sufficient cross-reactive antibodies to protect against several PfEMP1 variants, provided that the specific combinations of domains are known. A recent study further demonstrated that rabbits immunised with different recombinant proteins based on the extracellular domains of PfEMP1 recognised native PfEMP1 on intact IEs [263]. These antibodies were also capable of inhibiting rosette formation and promoting the opsonic phagocytosis of IEs [263], suggesting that the inclusion of multiple domains is necessary for effective immunity.
Research efforts on PfEMP1-specific vaccines have centred on the DBLα domain because it is one of the most conserved domains of PfEMP1 and is involved in rosetting [274]. Immunisation of rats with recombinant protein based on the DBLα domain induced antibodies that recognised conserved PfEMP1 peptides [275]. However, these antibodies were not reactive towards the IE surface of intact, mature trophozoites or towards full-length PfEMP1 from different laboratory strains [275]. They were also unable to agglutinate different parasite lines or disrupt rosette formation [275]. Antibodies against recombinant DBLα were reported to inhibit rosette formation in another study [276]. The discrepancy between these two studies [275, 276] may be reflected by different methods of protein expression as the latter, but not the former, utilised protein refolding techniques to obtain conformational-dependent epitopes that may be necessary for antibody recognition [276]. Moreover, antibodies induced by a recombinant mini-PfEMP1 (DBLα-TM-ATS) disrupted preformed rosettes and prevented in vivo sequestration [277]. The importance of the DBLα domain was further supported by the marked reduction in IE sequestration in DBLα-immunised animal models [278].
The PfEMP1 variant, VAR2CSA, is a vaccine candidate for protection against malaria in pregnancy. High levels of antibodies to multiple VAR2CSA domains in acquired pregnant women through natural exposure were associated with reduced placental infection with P. falciparum [279, 280] in some studies. Furthermore, antibodies generated against full-length [160, 281] and single domains of VAR2CSA [282] by immunisation inhibited adhesion of IEs to CSA, suggesting that vaccine-induced antibodies may have a protective function in vivo.
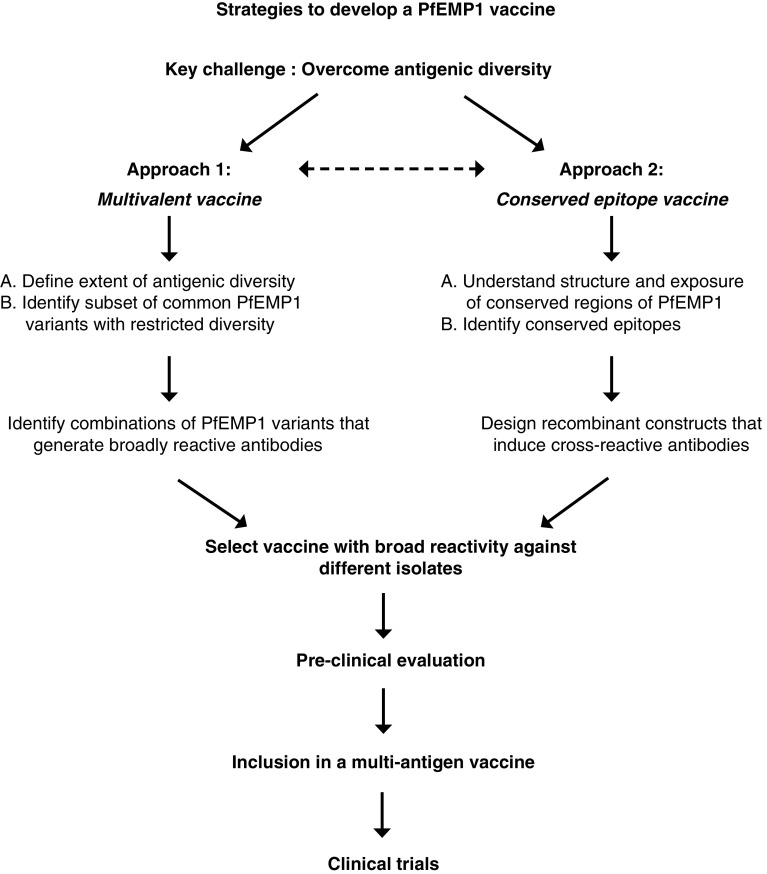
A major obstacle in the development of PfEMP1 as a vaccine against P. falciparum malaria is its substantial level of antigenic diversity. Several strategies can be pursued as an approach to overcome antigenic diversity (reviewed in [283]; Fig. 3). One approach would be to develop a multivalent PfEMP1 vaccine that can induce a broad repertoire of antibodies against most variants. A priority of this approach would be to determine the extent of diversity in PfEMP1 and define a combination of PfEMP1 variants that is needed to generate a broad immune response (Table 3). This approach has been used successfully with the merozoite protein apical membrane antigen 1 to overcome antigenic diversity [284, 285]. Another approach would be to target conserved epitopes of PfEMP1 such that induced antibodies may recognise most PfEMP1 variants expressed. However, identifying conserved epitopes exposed on the IE surface and understanding the tertiary/quaternary structure of PfEMP1 remains highly challenging. Further studies and innovative approaches to target antibody responses towards conserved PfEMP1 epitopes are needed. Studies have demonstrated that naturally acquired cross-reactive antibodies do occur [232, 233] and can be induced by immunisation [271]. Additionally, defining effector mechanisms of PfEMP1 immunity and creating a reference panel of parasite isolates for the evaluation of vaccine candidates must be a priority. Of further importance is a detailed knowledge of the acquisition, boosting and maintenance of antibodies to PfEMP1, as this will impact on vaccine efficacy and durability, but only limited data are currently available. Ideally, malaria vaccines would induce long-lived protection via immune responses that were sustained for an extended period after vaccination and boosted after exposure. One study suggested that antibodies to some VSAs may be short-lived, whereas other responses are sustained [286]. A recent study in pregnant women suggested that antibodies to VAR2CSA may be maintained for several decades, whereas antibodies to merozoite antigens declined more quickly [287]. The durability of vaccine-induced immune responses is not well known, but is an important issue for the development of highly efficacious vaccines against malaria (Table 3).
Fig. 3.
Approaches to overcome antigenic diversity of PfEMP1 in vaccine development. Antigenic diversity is the major challenge to developing PfEMP1 as a vaccine against malaria. The flow chart provides an overview of the two broad approaches to overcoming antigenic diversity in PfEMP1 and the steps involved in progressing vaccine candidates to the clinical trial stage. One approach is to develop a multivalent vaccine comprised of a mixture of common PfEMP1 variants that induces a broad repertoire of antibodies. A second approach is to identify conserved epitopes on PfEMP1 and develop a vaccine that targets these epitopes to induce broadly cross-reactive antibodies. As discussed in the text, there are significant challenges to overcome for each approach. It is likely that any PfEMP1 candidate vaccine antigen(s) would be included in a multi-antigen approach that includes antigens from other parasite life stages to ensure the development of a highly effective vaccine
Table 3.
Research priorities for the development of PfEMP1 vaccines
| General priorities |
Define effector mechanisms of PfEMP1 immune responses and quantify their importance Understand how antibodies to PfEMP1 are acquired, boosted and maintained over time Define antigenically conserved and diverse regions of PfEMP1 Create a reference panel of isolates for testing/evaluating vaccine candidates |
| Development of a multivalent vaccine |
Determine the extent of local/global antigenic diversity in PfEMP1 Understand the evolution of diversity that may lead to vaccine escape Define the number of variants/domains to be included Identify specific domains of PfEMP1 for possible vaccine inclusion |
| Development of a vaccine targeting conserved epitopes |
Understand the tertiary and quaternary structure of PfEMP1 Identify conserved epitopes exposed on the surface of IEs Create innovative approaches/technologies for identifying and targeting conserved epitopes |
It is likely that a highly effective malaria vaccine will require a multi-antigen, multi-stage approach. Therefore, it is anticipated that any PfEMP1-based vaccine antigens would have to be included as part of a vaccine containing antigens from other stages of the parasite life cycle, such as merozoite antigens to enhance blood-stage immunity and circumsporozoite protein for induction of pre-erythrocytic immunity, and the inclusion of gametocyte antigens for transmission-blocking immunity. We suggest that after lead PfEMP1-based vaccine antigens have been identified and prioritised, they will then need to be evaluated in combination with other vaccine antigens before proceeding further to clinical trials.
Conclusion
Understanding the targets and mechanisms of human immunity is crucial for informing and advancing the development of highly effective malaria vaccines and for developing tools for measuring immunity and exposure in populations to help evaluate the impact of malaria control interventions and identify populations at risk of malaria. Multiple studies in different populations now provide strong evidence that IE surface antigens, or VSAs, are important targets of acquired protective immunity. The most important of these antigens is PfEMP1, which is a major virulence factor enabling vascular adhesion and sequestration of IEs. Studies are beginning to identify specific variants of PfEMP1 that may be common in populations or linked to disease pathogenesis and that may be targeted in vaccine development. However, there are still major gaps in our knowledge on this topic, and these are important questions for future research. Little is known about other known or proposed surface antigens and their significance as targets of immunity and new strategies, and approaches are needed to clearly define their significance in immunity. Similarly, knowledge on surface antigens of IEs with P. vivax, the second major cause of malaria, is very limited. The role of surface antigens on gametocyte-IEs needs to be determined, as antibodies to these antigens may help clear gametocyte-IEs and thereby reduce malaria transmission; currently there is great interest globally in transmission-blocking vaccines, but there are few strong candidates in development. A greater understanding of effector mechanisms that mediate immunity is needed, including both humoral and cell-mediated responses, and additional assays to measure antibody functional activity in studies of acquired immunity and in vaccine trials would be valuable. Finally, strategies to overcome antigenic diversity in PfEMP1 would provide an exciting new opportunity in malaria vaccine development.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Thank you to Christine Langer for providing the scanning electron microscopy image of an infected erythrocyte and to Campbell Aitken for helpful comments on the manuscript. The authors gratefully acknowledge funding from the National Health and Medical Research Council of Australia (Fellowship, programme grant and project grant to J. Beeson; infrastructure support scheme), the Australian Research Council (Future Fellowship to J. Beeson), the Australian Government (Australian Postgraduate Award to J. Chan) and the Victorian Operational Infrastructure Support Program.
Abbreviations
- ATS
Acidic terminal segment of PfEMP1
- CD36
Cluster of differentiation 36 receptor
- CIDR
Cysteine-rich interdomain regions
- CMI
Cell-mediated immunity
- CR1
Complement receptor 1
- CSA
Chondroitin sulphate A
- DBL
Duffy binding-like domain
- HA
Hyaluronic acid
- ICAM-1
Intercellular adhesion molecule 1
- IE
P. falciparum infected erythrocyte
- KAHRP
Knob-associated histidine-rich protein
- MAHRP1
Membrane-associated histidine-rich protein 1
- MC
Maurer’s clefts
- MESA
Mature infected erythrocyte surface antigen
- PfEMP1
P. falciparum erythrocyte membrane protein 1
- PfMC-2TM
P. falciparum Maurer’s clefts two transmembrane protein
- REX
Ring exported proteins
- RIFIN
Repetitive interspersed family proteins
- STEVOR
Subtelomeric variable open reading frame proteins
- SURFIN
Surface-associated interspersed gene family proteins
- VSA
Variant surface antigen
References
- 1.Elliott SR, Beeson JG. Estimating the burden of global mortality in children aged < 5 years by pathogen-specific causes. Clin Infect Dis. 2008;46:1794–1795. doi: 10.1086/588049. [DOI] [PubMed] [Google Scholar]
- 2.Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- 3.Beeson JG, Brown GV. Pathogenesis of Plasmodium falciparum malaria: the roles of parasite adhesion and antigenic variation. Cell Mol Life Sci. 2002;59:258–271. doi: 10.1007/s00018-002-8421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang K-H, Stevenson MM. Malarial anaemia: mechanisms and implications of insufficient erythropoiesis during blood-stage malaria. Int J Parasitol. 2004;34:1501–1516. doi: 10.1016/j.ijpara.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Leech JH, Barnwell JW, Miller LH, Howard RJ. Identification of a strain-specific malarial antigen exposed on the surface of Plasmodium falciparum-infected erythrocytes. J Exp Med. 1984;159:1567–1575. doi: 10.1084/jem.159.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Q, Cloonan N, Fischer K, et al. stevor and rif are Plasmodium falciparum multicopy gene families which potentially encode variant antigens. Mol Biochem Parasitol. 1998;97:161–176. doi: 10.1016/s0166-6851(98)00144-3. [DOI] [PubMed] [Google Scholar]
- 7.Kyes SA, Rowe JA, Kriek N, Newbold CI. Rifins: a second family of clonally variant proteins expressed on the surface of red cells infected with Plasmodium falciparum . Proc Natl Acad Sci USA. 1999;96:9333–9338. doi: 10.1073/pnas.96.16.9333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez V, Hommel M, Chen Q, et al. Small, clonally variant antigens expressed on the surface of the Plasmodium falciparum-infected erythrocyte are encoded by the rif gene family and are the target of human immune responses. J Exp Med. 1999;190:1393–1404. doi: 10.1084/jem.190.10.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaviratne M, Khan SM, Jarra W, Preiser PR. Small variant STEVOR antigen is uniquely located within Maurer’s clefts in Plasmodium falciparum-infected red blood cells. Eukaryot Cell. 2002;1:926–935. doi: 10.1128/EC.1.6.926-935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blythe JE, Yan Yam X, Kuss C, et al. Plasmodium falciparum STEVOR proteins are highly expressed in patient isolates and located in the surface membranes of infected red blood cells and the apical tips of merozoites. Infect Immun. 2008;76:3329–3336. doi: 10.1128/IAI.01460-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Niang M, Yam XY, Preiser PR. The Plasmodium falciparum STEVOR multigene family mediates antigenic variation of the infected erythrocyte. PLoS Pathog. 2009;5:e1000307. doi: 10.1371/journal.ppat.1000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winter G, Kawai S, Haeggström M, et al. SURFIN is a polymorphic antigen expressed on Plasmodium falciparum merozoites and infected erythrocytes. J Exp Med. 2005;201:1853–1863. doi: 10.1084/jem.20041392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lavazec C, Sanyal S, Templeton TJ. Hypervariability within the Rifin, Stevor and Pfmc-2TM superfamilies in Plasmodium falciparum . Nucleic Acids Res. 2006;34:6696–6707. doi: 10.1093/nar/gkl942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sam-Yellowe TY, Florens L, Johnson JR, et al. A Plasmodium gene family encoding Maurer’s cleft membrane proteins: structural properties and expression profiling. Genome Res. 2004;14:1052–1059. doi: 10.1101/gr.2126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winograd E, Sherman IW. Malaria infection induces a conformational change in erythrocyte band 3 protein. Mol Biochem Parasitol. 2004;138:83–87. doi: 10.1016/j.molbiopara.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Sherman IW, Crandall IE, Guthrie N, Land KM. The sticky secrets of sequestration. Parasitol Today (Regul Ed) 1995;11:378–384. doi: 10.1016/0169-4758(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 17.MacPherson GG, Warrell MJ, White NJ, et al. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 18.Udeinya IJ, Schmidt JA, Aikawa M, et al. Falciparum malaria-infected erythrocytes specifically bind to cultured human endothelial cells. Science. 1981;213:555–557. doi: 10.1126/science.7017935. [DOI] [PubMed] [Google Scholar]
- 19.Looareesuwan S, Merry AH, Phillips RE, et al. Reduced erythrocyte survival following clearance of malarial parasitaemia in Thai patients. Br J Haematol. 1987;67:473–478. doi: 10.1111/j.1365-2141.1987.tb06171.x. [DOI] [PubMed] [Google Scholar]
- 20.Langreth SG, Peterson E. Pathogenicity, stability, and immunogenicity of a knobless clone of Plasmodium falciparum in Colombian owl monkeys. Infect Immun. 1985;47:760–766. doi: 10.1128/iai.47.3.760-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pye D, O’Brien CM, Franchina P, et al. Plasmodium falciparum infection of splenectomized and intact Guyanan Saimiri monkeys. J Parasitol. 1994;80:558–562. [PubMed] [Google Scholar]
- 22.Aikawa M. Human cerebral malaria. Am J Trop Med Hyg. 1988;39:3–10. doi: 10.4269/ajtmh.1988.39.3. [DOI] [PubMed] [Google Scholar]
- 23.Pongponratn E, Riganti M, Punpoowong B, Aikawa M. Microvascular sequestration of parasitized erythrocytes in human falciparum malaria: a pathological study. Am J Trop Med Hyg. 1991;44:168–175. doi: 10.4269/ajtmh.1991.44.168. [DOI] [PubMed] [Google Scholar]
- 24.Beeson JG, Amin N, Kanjala M, Rogerson SJ. Selective accumulation of mature asexual stages of Plasmodium falciparum-infected erythrocytes in the placenta. Infect Immun. 2002;70:5412–5415. doi: 10.1128/IAI.70.10.5412-5415.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter PR, Garin Y, Blot P. Placental pathologic changes in malaria. A histologic and ultrastructural study. Am J Pathol. 1982;109:330–342. [PMC free article] [PubMed] [Google Scholar]
- 26.Trager W, Rudzinska MA, Bradbury PC. The fine structure of Plasmodium falciparum and its host erythrocytes in natural malarial infections in man. Bull World Health Organ. 1966;35:883–885. [PMC free article] [PubMed] [Google Scholar]
- 27.Kilejian A. Characterization of a protein correlated with the production of knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum . Proc Natl Acad Sci USA. 1979;76:4650–4653. doi: 10.1073/pnas.76.9.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruenberg J, Allred DR, Sherman IW. Scanning electron microscope-analysis of the protrusions (knobs) present on the surface of Plasmodium falciparum-infected erythrocytes. J Cell Biol. 1983;97:795–802. doi: 10.1083/jcb.97.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pologe LG, Pavlovec A, Shio H, Ravetch JV. Primary structure and subcellular localization of the knob-associated histidine-rich protein of Plasmodium falciparum . Proc Natl Acad Sci USA. 1987;84:7139–7143. doi: 10.1073/pnas.84.20.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Culvenor JG, Langford CJ, Crewther PE, et al. Plasmodium falciparum: identification and localization of a knob protein antigen expressed by a cDNA clone. Exp Parasitol. 1987;63:58–67. doi: 10.1016/0014-4894(87)90078-6. [DOI] [PubMed] [Google Scholar]
- 31.Hadley TJ, Leech JH, Green TJ, et al. A comparison of knobby (K+) and knobless (K−) parasites from two strains of Plasmodium falciparum . Mol Biochem Parasitol. 1983;9:271–278. doi: 10.1016/0166-6851(83)90102-0. [DOI] [PubMed] [Google Scholar]
- 32.Leech JH, Barnwell JW, Aikawa M, et al. Plasmodium falciparum malaria: association of knobs on the surface of infected erythrocytes with a histidine-rich protein and the erythrocyte skeleton. J Cell Biol. 1984;98:1256–1264. doi: 10.1083/jcb.98.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pasloske BL, Baruch DI, Van Schravendijk MR, et al. Cloning and characterization of a Plasmodium falciparum gene encoding a novel high-molecular weight host membrane-associated protein, PfEMP3. Mol Biochem Parasitol. 1993;59:59–72. doi: 10.1016/0166-6851(93)90007-k. [DOI] [PubMed] [Google Scholar]
- 34.Coppel RL, Culvenor JG, Bianco AE, et al. Variable antigen associated with the surface of erythrocytes infected with mature stages of Plasmodium falciparum . Mol Biochem Parasitol. 1986;20:265–277. doi: 10.1016/0166-6851(86)90107-6. [DOI] [PubMed] [Google Scholar]
- 35.Howard RJ, Barnwell JW, Rock EP, et al. Two approximately 300 kilodalton Plasmodium falciparum proteins at the surface membrane of infected erythrocytes. Mol Biochem Parasitol. 1988;27:207–223. doi: 10.1016/0166-6851(88)90040-0. [DOI] [PubMed] [Google Scholar]
- 36.Kilejian A, Rashid MA, Parra M, Yang YF. Sequence of the knob protein of Plasmodium falciparum recognized by a monoclonal antibody. Mol Biochem Parasitol. 1991;48:231–233. doi: 10.1016/0166-6851(91)90119-q. [DOI] [PubMed] [Google Scholar]
- 37.Pei X, An X, Guo X, et al. Structural and functional studies of interaction between Plasmodium falciparum knob-associated histidine-rich protein (KAHRP) and erythrocyte spectrin. J Biol Chem. 2005;280:31166–31171. doi: 10.1074/jbc.M505298200. [DOI] [PubMed] [Google Scholar]
- 38.Deitsch KW, Wellems TE. Membrane modifications in erythrocytes parasitized by Plasmodium falciparum . Mol Biochem Parasitol. 1996;76:1–10. doi: 10.1016/0166-6851(95)02575-8. [DOI] [PubMed] [Google Scholar]
- 39.Glenister FK, Coppel RL, Cowman AF, et al. Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells. Blood. 2002;99:1060–1063. doi: 10.1182/blood.v99.3.1060. [DOI] [PubMed] [Google Scholar]
- 40.Baruch DI, Pasloske BL, Singh HB, et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 41.Cooke BM, Glenister FK, Mohandas N, Coppel RL. Assignment of functional roles to parasite proteins in malaria-infected red blood cells by competitive flow-based adhesion assay. Br J Haematol. 2002;117:203–211. doi: 10.1046/j.1365-2141.2002.03404.x. [DOI] [PubMed] [Google Scholar]
- 42.Crabb BS, Cooke BM, Reeder JC, et al. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell. 1997;89:287–296. doi: 10.1016/s0092-8674(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 43.Sherman IW, Eda S, Winograd E. Cytoadherence and sequestration in Plasmodium falciparum: defining the ties that bind. Microbes Infect. 2003;5:897–909. doi: 10.1016/s1286-4579(03)00162-x. [DOI] [PubMed] [Google Scholar]
- 44.Oquendo P, Hundt E, Lawler J, Seed B. CD36 directly mediates cytoadherence of Plasmodium falciparum parasitized erythrocytes. Cell. 1989;58:95–101. doi: 10.1016/0092-8674(89)90406-6. [DOI] [PubMed] [Google Scholar]
- 45.Barnwell JW, Asch AS, Nachman RL, et al. A human 88-kD membrane glycoprotein (CD36) functions in vitro as a receptor for a cytoadherence ligand on Plasmodium falciparum-infected erythrocytes. J Clin Invest. 1989;84:765–772. doi: 10.1172/JCI114234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berendt AR, Simmons DL, Tansey J, et al. Intercellular adhesion molecule-1 is an endothelial cell adhesion receptor for Plasmodium falciparum . Nature. 1989;341:57–59. doi: 10.1038/341057a0. [DOI] [PubMed] [Google Scholar]
- 47.Baruch DI, Ma XC, Singh HB, et al. Identification of a region of PfEMP1 that mediates adherence of Plasmodium falciparum infected erythrocytes to CD36: conserved function with variant sequence. Blood. 1997;90:3766–3775. [PubMed] [Google Scholar]
- 48.Smith JD, Craig AG, Kriek N, et al. Identification of a Plasmodium falciparum intercellular adhesion molecule-1 binding domain: a parasite adhesion trait implicated in cerebral malaria. Proc Natl Acad Sci USA. 2000;97:1766–1771. doi: 10.1073/pnas.040545897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reeder JC, Cowman AF, Davern KM, et al. The adhesion of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A is mediated by P. falciparum erythrocyte membrane protein 1. Proc Natl Acad Sci USA. 1999;96:5198–5202. doi: 10.1073/pnas.96.9.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buffet PA, Gamain B, Scheidig C, et al. Plasmodium falciparum domain mediating adhesion to chondroitin sulfate A: a receptor for human placental infection. Proc Natl Acad Sci USA. 1999;96:12743–12748. doi: 10.1073/pnas.96.22.12743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rowe JA, Moulds JM, Newbold CI, Miller LH. P. falciparum resetting mediated by a parasite-variant erythrocyte membrane protein and complement receptor 1. Nature. 1997;388:292–295. doi: 10.1038/40888. [DOI] [PubMed] [Google Scholar]
- 52.Chen Q, Fernandez V, Sundström A, et al. Developmental selection of var gene expression in Plasmodium falciparum . Nature. 1998;394:392–395. doi: 10.1038/28660. [DOI] [PubMed] [Google Scholar]
- 53.Ockenhouse CF, Tegoshi T, Maeno Y, et al. Human vascular endothelial cell adhesion receptors for Plasmodium falciparum-infected erythrocytes: roles for endothelial leukocyte adhesion molecule 1 and vascular cell adhesion molecule 1. J Exp Med. 1992;176:1183–1189. doi: 10.1084/jem.176.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCormick CJ, Craig A, Roberts D, et al. Intercellular adhesion molecule-1 and CD36 synergize to mediate adherence of Plasmodium falciparum-infected erythrocytes to cultured human microvascular endothelial cells. J Clin Invest. 1997;100:2521–2529. doi: 10.1172/JCI119794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rowe JA, Claessens A, Corrigan RA, Arman M. Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Rev Mol Med. 2009;11:e16. doi: 10.1017/S1462399409001082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beeson JG, Brown GV, Molyneux ME, et al. Plasmodium falciparum isolates from infected pregnant women and children are associated with distinct adhesive and antigenic properties. J Infect Dis. 1999;180:464–472. doi: 10.1086/314899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beeson JG, Rogerson SJ, Elliott SR, Duffy MF. Targets of protective antibodies to malaria during pregnancy. J Infect Dis. 2005;192:1647–1650. doi: 10.1086/496895. [DOI] [PubMed] [Google Scholar]
- 58.Beeson JG, Duffy PE. The immunology and pathogenesis of malaria during pregnancy. Curr Top Microbiol Immunol. 2005;297:187–227. doi: 10.1007/3-540-29967-x_6. [DOI] [PubMed] [Google Scholar]
- 59.Rogerson SJ, Chaiyaroj SC, Ng K, et al. Chondroitin sulfate A is a cell surface receptor for Plasmodium falciparum-infected erythrocytes. J Exp Med. 1995;182:15–20. doi: 10.1084/jem.182.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fried M, Duffy PE. Adherence of Plasmodium falciparum to chondroitin sulfate A in the human placenta. Science. 1996;272:1502–1504. doi: 10.1126/science.272.5267.1502. [DOI] [PubMed] [Google Scholar]
- 61.Beeson JG, Rogerson SJ, Brown GV. Evaluating specific adhesion of Plasmodium falciparum-infected erythrocytes to immobilised hyaluronic acid with comparison to binding of mammalian cells. Int J Parasitol. 2002;32:1245–1252. doi: 10.1016/s0020-7519(02)00097-8. [DOI] [PubMed] [Google Scholar]
- 62.Beeson JG, Rogerson SJ, Cooke BM, et al. Adhesion of Plasmodium falciparum-infected erythrocytes to hyaluronic acid in placental malaria. Nat Med. 2000;6:86–90. doi: 10.1038/71582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Beeson JG, Brown GV. Plasmodium falciparum-infected erythrocytes demonstrate dual specificity for adhesion to hyaluronic acid and chondroitin sulfate A and have distinct adhesive properties. J Infect Dis. 2004;189:169–179. doi: 10.1086/380975. [DOI] [PubMed] [Google Scholar]
- 64.Barfod L, Dalgaard MB, Pleman ST, et al. Evasion of immunity to Plasmodium falciparum malaria by IgM masking of protective IgG epitopes in infected erythrocyte surface-exposed PfEMP1. Proc Natl Acad Sci. 2011;108:12485–12490. doi: 10.1073/pnas.1103708108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rowe JA, Shafi J, Kai OK, et al. Nonimmune IgM, but not IgG binds to the surface of Plasmodium falciparum-infected erythrocytes and correlates with rosetting and severe malaria. Am J Trop Med Hyg. 2002;66:692–699. doi: 10.4269/ajtmh.2002.66.692. [DOI] [PubMed] [Google Scholar]
- 66.Creasey AM, Staalsoe T, Raza A, et al. Nonspecific immunoglobulin M binding and chondroitin sulfate A binding are linked phenotypes of Plasmodium falciparum isolates implicated in malaria during pregnancy. Infect Immun. 2003;71:4767–4771. doi: 10.1128/IAI.71.8.4767-4771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Flick K, Scholander C, Chen Q, et al. Role of nonimmune IgG bound to PfEMP1 in placental malaria. Science. 2001;293:2098–2100. doi: 10.1126/science.1062891. [DOI] [PubMed] [Google Scholar]
- 68.Turner GD, Morrison H, Jones M, et al. An immunohistochemical study of the pathology of fatal malaria. Evidence for widespread endothelial activation and a potential role for intercellular adhesion molecule-1 in cerebral sequestration. Am J Pathol. 1994;145:1057–1069. [PMC free article] [PubMed] [Google Scholar]
- 69.Newbold CI, Craig AG, Kyes S, et al. PfEMP1, polymorphism and pathogenesis. Ann Trop Med Parasitol. 1997;91:551–557. doi: 10.1080/00034989760923. [DOI] [PubMed] [Google Scholar]
- 70.Rogerson SJ, Tembenu R, Dobaño C, et al. Cytoadherence characteristics of Plasmodium falciparum-infected erythrocytes from Malawian children with severe and uncomplicated malaria. Am J Trop Med Hyg. 1999;61:467–472. doi: 10.4269/ajtmh.1999.61.467. [DOI] [PubMed] [Google Scholar]
- 71.Ochola LB, Siddondo BR, Ocholla H, et al. Specific receptor usage in Plasmodium falciparum cytoadherence is associated with disease outcome. PLoS ONE. 2011;6:e14741. doi: 10.1371/journal.pone.0014741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Turner L, Lavstsen T, Berger SS, et al. Severe malaria is associated with parasite binding to endothelial protein C receptor. Nature. 2013 doi: 10.1038/nature12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roberts DD, Sherwood JA, Spitalnik SL, et al. Thrombospondin binds falciparum malaria parasitized erythrocytes and may mediate cytoadherence. Nature. 1985;318:64–66. doi: 10.1038/318064a0. [DOI] [PubMed] [Google Scholar]
- 74.Treutiger CJ, Heddini A, Fernandez V, et al. PECAM-1/CD31, an endothelial receptor for binding Plasmodium falciparum-infected erythrocytes. Nat Med. 1997;3:1405–1408. doi: 10.1038/nm1297-1405. [DOI] [PubMed] [Google Scholar]
- 75.Ho M, Schollaardt T, Niu X, et al. Characterization of Plasmodium falciparum-infected erythrocyte and P-selectin interaction under flow conditions. Blood. 1998;91:4803–4809. [PubMed] [Google Scholar]
- 76.David PH, Handunnetti SM, Leech JH, et al. Rosetting: a new cytoadherence property of malaria-infected erythrocytes. Am J Trop Med Hyg. 1988;38:289–297. doi: 10.4269/ajtmh.1988.38.289. [DOI] [PubMed] [Google Scholar]
- 77.Kaul DK, Roth EF, Nagel RL, et al. Rosetting of Plasmodium falciparum-infected red blood cells with uninfected red blood cells enhances microvascular obstruction under flow conditions. Blood. 1991;78:812–819. [PubMed] [Google Scholar]
- 78.Wahlgren M, Fernandez V, Scholander C, Carlson J. Rosetting. Parasitol Today (Regul Ed) 1994;10:73–79. doi: 10.1016/0169-4758(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 79.Carlson J, Helmby H, Hill AV, et al. Human cerebral malaria: association with erythrocyte rosetting and lack of anti-rosetting antibodies. Lancet. 1990;336:1457–1460. doi: 10.1016/0140-6736(90)93174-n. [DOI] [PubMed] [Google Scholar]
- 80.Treutiger CJ, Hedlund I, Helmby H, et al. Rosette formation in Plasmodium falciparum isolates and anti-rosette activity of sera from Gambians with cerebral or uncomplicated malaria. Am J Trop Med Hyg. 1992;46:503–510. doi: 10.4269/ajtmh.1992.46.503. [DOI] [PubMed] [Google Scholar]
- 81.Rowe A, Obeiro J, Newbold CI, Marsh K. Plasmodium falciparum rosetting is associated with malaria severity in Kenya. Infect Immun. 1995;63:2323–2326. doi: 10.1128/iai.63.6.2323-2326.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rowe JA, Kyes SA, Rogerson SJ, et al. Identification of a conserved Plasmodium falciparum var gene implicated in malaria in pregnancy. J Infect Dis. 2002;185:1207–1211. doi: 10.1086/339684. [DOI] [PubMed] [Google Scholar]
- 83.Doumbo OK, Thera MA, Koné AK, et al. High levels of Plasmodium falciparum rosetting in all clinical forms of severe malaria in African children. Am J Trop Med Hyg. 2009;81:987–993. doi: 10.4269/ajtmh.2009.09-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.al-Yaman F, Genton B, Mokela D, et al. Human cerebral malaria: lack of significant association between erythrocyte rosetting and disease severity. Trans R Soc Trop Med Hyg. 1995;89:55–58. doi: 10.1016/0035-9203(95)90658-4. [DOI] [PubMed] [Google Scholar]
- 85.Chen Q, Barragan A, Fernandez V, et al. Identification of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) as the rosetting ligand of the malaria parasite P. falciparum . J Exp Med. 1998;187:15–23. doi: 10.1084/jem.187.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roberts DJ, Craig AG, Berendt AR, et al. Rapid switching to multiple antigenic and adhesive phenotypes in malaria. Nature. 1992;357:689–692. doi: 10.1038/357689a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roberts DJ, Pain A, Kai O, et al. Autoagglutination of malaria-infected red blood cells and malaria severity. Lancet. 2000;355:1427–1428. doi: 10.1016/S0140-6736(00)02143-7. [DOI] [PubMed] [Google Scholar]
- 88.Pain A, Ferguson DJ, Kai O, et al. Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proc Natl Acad Sci USA. 2001;98:1805–1810. doi: 10.1073/pnas.98.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chotivanich K, Sritabal J, Udomsangpetch R, et al. Platelet-induced autoagglutination of Plasmodium falciparum-infected red blood cells and disease severity in Thailand. J Infect Dis. 2004;189:1052–1055. doi: 10.1086/381900. [DOI] [PubMed] [Google Scholar]
- 90.McMorran BJ, Marshall VM, de Graaf C, et al. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science. 2009;323:797–800. doi: 10.1126/science.1166296. [DOI] [PubMed] [Google Scholar]
- 91.McMorran BJ, Wieczorski L, Drysdale KE, et al. Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum . Science. 2012;338:1348–1351. doi: 10.1126/science.1228892. [DOI] [PubMed] [Google Scholar]
- 92.Bull PC, Lowe BS, Kortok M, Marsh K. Antibody recognition of Plasmodium falciparum erythrocyte surface antigens in Kenya: evidence for rare and prevalent variants. Infect Immun. 1999;67:733–739. doi: 10.1128/iai.67.2.733-739.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Piper KP, Roberts DJ, Day KP. Plasmodium falciparum: analysis of the antibody specificity to the surface of the trophozoite-infected erythrocyte. Exp Parasitol. 1999;91:161–169. doi: 10.1006/expr.1998.4368. [DOI] [PubMed] [Google Scholar]
- 94.Howard RJ, Barnwell JW, Kao V. Antigenic variation of Plasmodium knowlesi malaria: identification of the variant antigen on infected erythrocytes. Proc Natl Acad Sci USA. 1983;80:4129–4133. doi: 10.1073/pnas.80.13.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.al-Khedery B, Barnwell JW, Galinski MR. Antigenic variation in malaria: a 3′ genomic alteration associated with the expression of a P. knowlesi variant antigen. Mol Cell. 1999;3:131–141. doi: 10.1016/s1097-2765(00)80304-4. [DOI] [PubMed] [Google Scholar]
- 96.Korir CC, Galinski MR. Proteomic studies of Plasmodium knowlesi SICA variant antigens demonstrate their relationship with P. falciparum EMP1. Infect Genet Evol. 2006;6:75–79. doi: 10.1016/j.meegid.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 97.del Portillo HA, Fernandez-Becerra C, Bowman S, et al. A superfamily of variant genes encoded in the subtelomeric region of Plasmodium vivax . Nature. 2001;410:839–842. doi: 10.1038/35071118. [DOI] [PubMed] [Google Scholar]
- 98.Janssen CS, Barrett MP, Turner CMR, Phillips RS. A large gene family for putative variant antigens shared by human and rodent malaria parasites. Proc Biol Sci. 2002;269:431–436. doi: 10.1098/rspb.2001.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Janssen CS, Phillips RS, Turner CMR, Barrett MP. Plasmodium interspersed repeats: the major multigene superfamily of malaria parasites. Nucleic Acids Res. 2004;32:5712–5720. doi: 10.1093/nar/gkh907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Voss TS, Healer J, Marty AJ, et al. A var gene promoter controls allelic exclusion of virulence genes in Plasmodium falciparum malaria. Nature. 2006;439:1004–1008. doi: 10.1038/nature04407. [DOI] [PubMed] [Google Scholar]
- 101.Dzikowski R, Frank M, Deitsch K. Mutually exclusive expression of virulence genes by malaria parasites is regulated independently of antigen production. PLoS Pathog. 2006;2:e22. doi: 10.1371/journal.ppat.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chan J-A, Howell KB, Reiling L, et al. Targets of antibodies against Plasmodium falciparum-infected erythrocytes in malaria immunity. J Clin Invest. 2012 doi: 10.1172/JCI62182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Howitt CA, Wilinski D, Llinás M, et al. Clonally variant gene families in Plasmodium falciparum share a common activation factor. Mol Microbiol. 2009;73:1171–1185. doi: 10.1111/j.1365-2958.2009.06846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Garcia CR, Takeuschi M, Yoshioka K, Miyamoto H. Imaging Plasmodium falciparum-infected ghost and parasite by atomic force microscopy. J Struct Biol. 1997;119:92–98. doi: 10.1006/jsbi.1997.3886. [DOI] [PubMed] [Google Scholar]
- 105.Marti M. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science. 2004;306:1930–1933. doi: 10.1126/science.1102452. [DOI] [PubMed] [Google Scholar]
- 106.Hiller NL, Bhattacharjee S, van Ooij C, et al. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science. 2004;306:1934–1937. doi: 10.1126/science.1102737. [DOI] [PubMed] [Google Scholar]
- 107.de Koning-Ward TF, Gilson PR, Boddey JA, et al. A newly discovered protein export machine in malaria parasites. Nature. 2009;459:945–949. doi: 10.1038/nature08104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crabb BS, de Koning-Ward TF, Gilson PR. Protein export in Plasmodium parasites: from the endoplasmic reticulum to the vacuolar export machine. Int J Parasitol. 2010;40:509–513. doi: 10.1016/j.ijpara.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 109.Mantel P-Y, Hoang AN, Goldowitz I, et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe. 2013;13:521–534. doi: 10.1016/j.chom.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Regev-Rudzki N, Wilson DW, Carvalho TG, et al. Cell–cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell. 2013 doi: 10.1016/j.cell.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 111.Su XZ, Heatwole VM, Wertheimer SP, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82:89–100. doi: 10.1016/0092-8674(95)90055-1. [DOI] [PubMed] [Google Scholar]
- 112.Smith JD, Chitnis CE, Craig AG, et al. Switches in expression of Plasmodium falciparum var genes correlate with changes in antigenic and cytoadherent phenotypes of infected erythrocytes. Cell. 1995;82:101–110. doi: 10.1016/0092-8674(95)90056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scherf A, Hernandez-Rivas R, Buffet P, et al. Antigenic variation in malaria: in situ switching, relaxed and mutually exclusive transcription of var genes during intra-erythrocytic development in Plasmodium falciparum . EMBO J. 1998;17:5418–5426. doi: 10.1093/emboj/17.18.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Joergensen L, Bengtsson DC, Bengtsson A, et al. Surface co-expression of two different PfEMP1 antigens on single Plasmodium falciparum-infected erythrocytes facilitates binding to ICAM1 and PECAM1. PLoS Pathog. 2010;6:e1001083. doi: 10.1371/journal.ppat.1001083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kriek N, Tilley L, Horrocks P, et al. Characterization of the pathway for transport of the cytoadherence-mediating protein, PfEMP1, to the host cell surface in malaria parasite-infected erythrocytes. Mol Microbiol. 2003;50:1215–1227. doi: 10.1046/j.1365-2958.2003.03784.x. [DOI] [PubMed] [Google Scholar]
- 116.Boddey JA, Cowman AF. Plasmodium nesting: remaking the erythrocyte from the inside out. Annu Rev Microbiol. 2013;67:243–269. doi: 10.1146/annurev-micro-092412-155730. [DOI] [PubMed] [Google Scholar]
- 117.Elsworth B, Crabb BS, Gilson PR. Protein export in malaria parasites: an update. Cell Microbiol. 2014;16:355–363. doi: 10.1111/cmi.12261. [DOI] [PubMed] [Google Scholar]
- 118.Haeggström M, Kironde F, Berzins K, et al. Common trafficking pathway for variant antigens destined for the surface of the Plasmodium falciparum-infected erythrocyte. Mol Biochem Parasitol. 2004;133:1–14. doi: 10.1016/j.molbiopara.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 119.Horrocks P, Pinches RA, Chakravorty SJ, et al. PfEMP1 expression is reduced on the surface of knobless Plasmodium falciparum infected erythrocytes. J Cell Sci. 2005;118:2507–2518. doi: 10.1242/jcs.02381. [DOI] [PubMed] [Google Scholar]
- 120.Gardner JP, Pinches RA, Roberts DJ, Newbold CI. Variant antigens and endothelial receptor adhesion in Plasmodium falciparum . Proc Natl Acad Sci USA. 1996;93:3503–3508. doi: 10.1073/pnas.93.8.3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Blisnick T, Morales Betoulle ME, Barale JC, et al. Pfsbp1, a Maurer’s cleft Plasmodium falciparum protein, is associated with the erythrocyte skeleton. Mol Biochem Parasitol. 2000;111:107–121. doi: 10.1016/s0166-6851(00)00301-7. [DOI] [PubMed] [Google Scholar]
- 122.Cooke BM, Buckingham DW, Glenister FK, et al. A Maurer’s cleft-associated protein is essential for expression of the major malaria virulence antigen on the surface of infected red blood cells. J Cell Biol. 2006;172:899–908. doi: 10.1083/jcb.200509122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maier AG, Rug M, O’Neill MT, et al. Skeleton-binding protein 1 functions at the parasitophorous vacuole membrane to traffic PfEMP1 to the Plasmodium falciparum-infected erythrocyte surface. Blood. 2007;109:1289–1297. doi: 10.1182/blood-2006-08-043364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Maier AG, Rug M, O’Neill MT, et al. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell. 2008;134:48–61. doi: 10.1016/j.cell.2008.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Külzer S, Charnaud S, Dagan T, et al. Plasmodium falciparum-encoded exported hsp70/hsp40 chaperone/co-chaperone complexes within the host erythrocyte. Cell Microbiol. 2012;14:1784–1795. doi: 10.1111/j.1462-5822.2012.01840.x. [DOI] [PubMed] [Google Scholar]
- 126.Spycher C, Rug M, Pachlatko E, et al. The Maurer’s cleft protein MAHRP1 is essential for trafficking of PfEMP1 to the surface of Plasmodium falciparum-infected erythrocytes. Mol Microbiol. 2008;68:1300–1314. doi: 10.1111/j.1365-2958.2008.06235.x. [DOI] [PubMed] [Google Scholar]
- 127.Dixon MWA, Kenny S, McMillan PJ, et al. Genetic ablation of a Maurer’s cleft protein prevents assembly of the Plasmodium falciparum virulence complex. Mol Microbiol. 2011;81:982–993. doi: 10.1111/j.1365-2958.2011.07740.x. [DOI] [PubMed] [Google Scholar]
- 128.Glenister FK, Fernandez KM, Kats LM, et al. Functional alteration of red blood cells by a megadalton protein of Plasmodium falciparum . Blood. 2009;113:919–928. doi: 10.1182/blood-2008-05-157735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Duraisingh MT, Cowman AF. Contribution of the pfmdr1 gene to antimalarial drug-resistance. Acta Trop. 2005;94:181–190. doi: 10.1016/j.actatropica.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 130.Freitas-Junior LH, Hernández-Rivas R, Ralph SA, et al. Telomeric heterochromatin propagation and histone acetylation control mutually exclusive expression of antigenic variation genes in malaria parasites. Cell. 2005;121:25–36. doi: 10.1016/j.cell.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 131.Tonkin CJ, Carret CK, Duraisingh MT, et al. Sir2 paralogues cooperate to regulate virulence genes and antigenic variation in Plasmodium falciparum . PLoS Biol. 2009;7:e84. doi: 10.1371/journal.pbio.1000084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Biggs BA, Goozé L, Wycherley K, et al. Antigenic variation in Plasmodium falciparum . Proc Natl Acad Sci USA. 1991;88:9171–9174. doi: 10.1073/pnas.88.20.9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chen Q, Heddini A, Barragan A, et al. The semiconserved head structure of Plasmodium falciparum erythrocyte membrane protein 1 mediates binding to multiple independent host receptors. J Exp Med. 2000;192:1–10. doi: 10.1084/jem.192.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Voigt S, Hanspal M, LeRoy PJ, et al. The cytoadherence ligand Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) binds to the P. falciparum knob-associated histidine-rich protein (KAHRP) by electrostatic interactions. Mol Biochem Parasitol. 2000;110:423–428. doi: 10.1016/s0166-6851(00)00281-4. [DOI] [PubMed] [Google Scholar]
- 135.Waller KL, Cooke BM, Nunomura W, et al. Mapping the binding domains involved in the interaction between the Plasmodium falciparum knob-associated histidine-rich protein (KAHRP) and the cytoadherence ligand P. falciparum erythrocyte membrane protein 1 (PfEMP1) J Biol Chem. 1999;274:23808–23813. doi: 10.1074/jbc.274.34.23808. [DOI] [PubMed] [Google Scholar]
- 136.Rug M, Prescott SW, Fernandez KM, et al. The role of KAHRP domains in knob formation and cytoadherence of P. falciparum-infected human erythrocytes. Blood. 2006;108:370–378. doi: 10.1182/blood-2005-11-4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chattopadhyay R, Taneja T, Chakrabarti K, et al. Molecular analysis of the cytoadherence phenotype of a Plasmodium falciparum field isolate that binds intercellular adhesion molecule-1. Mol Biochem Parasitol. 2004;133:255–265. doi: 10.1016/j.molbiopara.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 138.Springer AL, Smith LM, Mackay DQ, et al. Functional interdependence of the DBLbeta domain and c2 region for binding of the Plasmodium falciparum variant antigen to ICAM-1. Mol Biochem Parasitol. 2004;137:55–64. doi: 10.1016/j.molbiopara.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 139.Robinson BA, Welch TL, Smith JD. Widespread functional specialization of Plasmodium falciparum erythrocyte membrane protein 1 family members to bind CD36 analysed across a parasite genome. Mol Microbiol. 2003;47:1265–1278. doi: 10.1046/j.1365-2958.2003.03378.x. [DOI] [PubMed] [Google Scholar]
- 140.Smith JD, Kyes S, Craig AG, et al. Analysis of adhesive domains from the A4VAR Plasmodium falciparum erythrocyte membrane protein-1 identifies a CD36 binding domain. Mol Biochem Parasitol. 1998;97:133–148. doi: 10.1016/s0166-6851(98)00145-5. [DOI] [PubMed] [Google Scholar]
- 141.Srivastava A, Gangnard S, Dechavanne S, et al. Var2CSA minimal CSA binding region is located within the N-terminal region. PLoS ONE. 2011;6:e20270. doi: 10.1371/journal.pone.0020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Gardner MJ, Hall N, Fung E, et al. Genome sequence of the human malaria parasite Plasmodium falciparum . Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kraemer SM, Smith JD. Evidence for the importance of genetic structuring to the structural and functional specialization of the Plasmodium falciparum var gene family. Mol Microbiol. 2003;50:1527–1538. doi: 10.1046/j.1365-2958.2003.03814.x. [DOI] [PubMed] [Google Scholar]
- 144.Lavstsen T, Salanti A, Jensen ATR, et al. Sub-grouping of Plasmodium falciparum 3D7 var genes based on sequence analysis of coding and non-coding regions. Malar J. 2003;2:27. doi: 10.1186/1475-2875-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Voss TS, Thompson JK, Waterkeyn J, et al. Genomic distribution and functional characterisation of two distinct and conserved Plasmodium falciparum var gene 5′ flanking sequences. Mol Biochem Parasitol. 2000;107:103–115. doi: 10.1016/s0166-6851(00)00176-6. [DOI] [PubMed] [Google Scholar]
- 146.Bull PC, Kyes S, Buckee CO, et al. An approach to classifying sequence tags sampled from Plasmodium falciparum var genes. Mol Biochem Parasitol. 2007;154:98–102. doi: 10.1016/j.molbiopara.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bull PC, Berriman M, Kyes S, et al. Plasmodium falciparum variant surface antigen expression patterns during malaria. PLoS Pathog. 2005;1:e26. doi: 10.1371/journal.ppat.0010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Warimwe GM, Keane TM, Fegan G, et al. Plasmodium falciparum var gene expression is modified by host immunity. Proc Natl Acad Sci. 2009;106:21801–21806. doi: 10.1073/pnas.0907590106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kyriacou HM, Stone GN, Challis RJ, et al. Differential var gene transcription in Plasmodium falciparum isolates from patients with cerebral malaria compared to hyperparasitaemia. Mol Biochem Parasitol. 2006;150:211–218. doi: 10.1016/j.molbiopara.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Falk N, Kaestli M, Qi W, et al. Analysis of Plasmodium falciparum var genes expressed in children from Papua New Guinea. J Infect Dis. 2009;200:347–356. doi: 10.1086/600071. [DOI] [PubMed] [Google Scholar]
- 151.Rottmann M, Lavstsen T, Mugasa JP, et al. Differential expression of var gene groups is associated with morbidity caused by Plasmodium falciparum infection in Tanzanian children. Infect Immun. 2006;74:3904–3911. doi: 10.1128/IAI.02073-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jensen ATR, Magistrado P, Sharp S, et al. Plasmodium falciparum associated with severe childhood malaria preferentially expresses PfEMP1 encoded by group A var genes. J Exp Med. 2004;199:1179–1190. doi: 10.1084/jem.20040274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lavstsen T, Turner L, Saguti F, et al. Plasmodium falciparum erythrocyte membrane protein 1 domain cassettes 8 and 13 are associated with severe malaria in children. Proc Natl Acad Sci. 2012 doi: 10.1073/pnas.1120455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Warimwe GM, Fegan G, Musyoki JN, et al. Prognostic indicators of life-threatening malaria are associated with distinct parasite variant antigen profiles. Sci Transl Med. 2012;4:129ra45. doi: 10.1126/scitranslmed.3003247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Avril M, Tripathi AK, Brazier AJ, et al. A restricted subset of var genes mediates adherence of Plasmodium falciparum-infected erythrocytes to brain endothelial cells. Proc Natl Acad Sci. 2012 doi: 10.1073/pnas.1120534109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Claessens A, Adams Y, Ghumra A, et al. A subset of group A-like var genes encodes the malaria parasite ligands for binding to human brain endothelial cells. Proc Natl Acad Sci. 2012 doi: 10.1073/pnas.1120461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cham GKK, Turner L, Lusingu J, et al. Sequential, ordered acquisition of antibodies to Plasmodium falciparum erythrocyte membrane protein 1 domains. J Immunol. 2009;183:3356–3363. doi: 10.4049/jimmunol.0901331. [DOI] [PubMed] [Google Scholar]
- 158.Kaestli M, Cockburn IA, Cortés A, et al. Virulence of malaria is associated with differential expression of Plasmodium falciparum var gene subgroups in a case-control study. J Infect Dis. 2006;193:1567–1574. doi: 10.1086/503776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hommel M, Elliott SR, et al. Evaluation of the antigenic diversity of placenta-binding Plasmodium falciparum variants and the antibody repertoire among pregnant women. Infect Immun. 2010;78:1963–1978. doi: 10.1128/IAI.01365-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Avril M, Hathaway MJ, Srivastava A, et al. Antibodies to a full-length VAR2CSA immunogen are broadly strain-transcendent but do not cross-inhibit different placental-type parasite isolates. PLoS ONE. 2011;6:e16622. doi: 10.1371/journal.pone.0016622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kraemer SM, Kyes SA, Aggarwal G, et al. Patterns of gene recombination shape var gene repertoires in Plasmodium falciparum: comparisons of geographically diverse isolates. BMC Genomics. 2007;8:45. doi: 10.1186/1471-2164-8-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Duffy MF, Byrne TJ, Elliott SR, et al. Broad analysis reveals a consistent pattern of var gene transcription in Plasmodium falciparum repeatedly selected for a defined adhesion phenotype. Mol Microbiol. 2005;56:774–788. doi: 10.1111/j.1365-2958.2005.04577.x. [DOI] [PubMed] [Google Scholar]
- 163.Salanti A, Staalsoe T, Lavstsen T, et al. Selective upregulation of a single distinctly structured var gene in chondroitin sulphate A-adhering Plasmodium falciparum involved in pregnancy-associated malaria. Mol Microbiol. 2003;49:179–191. doi: 10.1046/j.1365-2958.2003.03570.x. [DOI] [PubMed] [Google Scholar]
- 164.Salanti A, Dahlbäck M, Turner L, et al. Evidence for the involvement of VAR2CSA in pregnancy-associated malaria. J Exp Med. 2004;200:1197–1203. doi: 10.1084/jem.20041579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Beeson JG, Andrews KT, Boyle M, et al. Structural basis for binding of Plasmodium falciparum erythrocyte membrane protein 1 to chondroitin sulfate and placental tissue and the influence of protein polymorphisms on binding specificity. J Biol Chem. 2007;282:22426–22436. doi: 10.1074/jbc.M700231200. [DOI] [PubMed] [Google Scholar]
- 166.Magistrado P, Salanti A, Tuikue Ndam NG, et al. VAR2CSA expression on the surface of placenta-derived Plasmodium falciparum-infected erythrocytes. J Infect Dis. 2008;198:1071–1074. doi: 10.1086/591502. [DOI] [PubMed] [Google Scholar]
- 167.Beeson JG, Mann EJ, Elliott SR, et al. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes and adhesion inhibitory antibodies are associated with placental malaria and have overlapping and distinct targets. J Infect Dis. 2004;189:540–551. doi: 10.1086/381186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Viebig NK, Gamain B, Scheidig C, et al. A single member of the Plasmodium falciparum var multigene family determines cytoadhesion to the placental receptor chondroitin sulphate A. EMBO Rep. 2005;6:775–781. doi: 10.1038/sj.embor.7400466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Duffy MF, Maier AG, Byrne TJ, et al. VAR2CSA is the principal ligand for chondroitin sulfate A in two allogeneic isolates of Plasmodium falciparum . Mol Biochem Parasitol. 2006;148:117–124. doi: 10.1016/j.molbiopara.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 170.Hayward RE, Tiwari B, Piper KP, et al. Virulence and transmission success of the malarial parasite Plasmodium falciparum . Proc Natl Acad Sci USA. 1999;96:4563–4568. doi: 10.1073/pnas.96.8.4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sharp S, Lavstsen T, Fivelman QL, et al. Programmed transcription of the var gene family, but not of stevor, in Plasmodium falciparum gametocytes. Eukaryot Cell. 2006;5:1206–1214. doi: 10.1128/EC.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tibùrcio M, Silvestrini F, Bertuccini L, et al. Early gametocytes of the malaria parasite Plasmodium falciparum specifically remodel the adhesive properties of infected erythrocyte surface. Cell Microbiol. 2012 doi: 10.1111/cmi.12062. [DOI] [PubMed] [Google Scholar]
- 173.Aingaran M, Zhang R, Law SK, et al. Host cell deformability is linked to transmission in the human malaria parasite Plasmodium falciparum . Cell Microbiol. 2012;14:983–993. doi: 10.1111/j.1462-5822.2012.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kyes S, Pinches R, Newbold C. A simple RNA analysis method shows var and rif multigene family expression patterns in Plasmodium falciparum . Mol Biochem Parasitol. 2000;105:311–315. doi: 10.1016/s0166-6851(99)00193-0. [DOI] [PubMed] [Google Scholar]
- 175.Florens L, Washburn MP, Raine JD, et al. A proteomic view of the Plasmodium falciparum life cycle. Nature. 2002;419:520–526. doi: 10.1038/nature01107. [DOI] [PubMed] [Google Scholar]
- 176.Petter M, Haeggström M, Khattab A, et al. Variant proteins of the Plasmodium falciparum RIFIN family show distinct subcellular localization and developmental expression patterns. Mol Biochem Parasitol. 2007;156:51–61. doi: 10.1016/j.molbiopara.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 177.Wang CW, Mwakalinga SB, Sutherland CJ, et al. Identification of a major rif transcript common to gametocytes and sporozoites of Plasmodium falciparum . Malar J. 2010;9:147. doi: 10.1186/1475-2875-9-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Joannin N, Abhiman S, Sonnhammer EL, Wahlgren M. Sub-grouping and sub-functionalization of the RIFIN multi-copy protein family. BMC Genomics. 2008;9:19. doi: 10.1186/1471-2164-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Abdel-Latif MS, Khattab A, et al. Recognition of variant Rifin antigens by human antibodies induced during natural Plasmodium falciparum infections. Infect Immun. 2002;70:7013–7021. doi: 10.1128/IAI.70.12.7013-7021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Przyborski JM, Miller SK, Pfahler JM, et al. Trafficking of STEVOR to the Maurer’s clefts in Plasmodium falciparum-infected erythrocytes. EMBO J. 2005;24:2306–2317. doi: 10.1038/sj.emboj.7600720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.McRobert L, Preiser P, Sharp S, et al. Distinct trafficking and localization of STEVOR proteins in three stages of the Plasmodium falciparum life cycle. Infect Immun. 2004;72:6597–6602. doi: 10.1128/IAI.72.11.6597-6602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Sanyal S, Egée S, Bouyer G, et al. Plasmodium falciparum STEVOR proteins impact erythrocyte mechanical properties. Blood. 2012;119:e1–e8. doi: 10.1182/blood-2011-08-370734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mills JP, Diez-Silva M, Quinn DJ, et al. Effect of plasmodial RESA protein on deformability of human red blood cells harboring Plasmodium falciparum . Proc Natl Acad Sci USA. 2007;104:9213–9217. doi: 10.1073/pnas.0703433104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lavazec C, Sanyal S, Templeton TJ. Expression switching in the stevor and Pfmc-2TM superfamilies in Plasmodium falciparum . Mol Microbiol. 2007;64:1621–1634. doi: 10.1111/j.1365-2958.2007.05767.x. [DOI] [PubMed] [Google Scholar]
- 185.García JE, Puentes A, Curtidor H, et al. Peptides from the Plasmodium falciparum STEVOR putative protein bind with high affinity to normal human red blood cells. Peptides. 2005;26:1133–1143. doi: 10.1016/j.peptides.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 186.Khattab A, Meri S. Exposure of the Plasmodium falciparum clonally variant STEVOR proteins on the merozoite surface. Malar J. 2011;10:58. doi: 10.1186/1475-2875-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Khattab A, Bonow I, Schreiber N, et al. Plasmodium falciparum variant STEVOR antigens are expressed in merozoites and possibly associated with erythrocyte invasion. Malar J. 2008;7:137. doi: 10.1186/1475-2875-7-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Mphande FA, Ribacke U, Kaneko O, et al. SURFIN4.1, a schizont-merozoite associated protein in the SURFIN family of Plasmodium falciparum . Malar J. 2008;7:116. doi: 10.1186/1475-2875-7-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Winograd E, Eda S, Sherman IW. Chemical modifications of band 3 protein affect the adhesion of Plasmodium falciparum-infected erythrocytes to CD36. Mol Biochem Parasitol. 2004;136:243–248. doi: 10.1016/j.molbiopara.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 190.Eda S, Lawler J, Sherman IW. Plasmodium falciparum-infected erythrocyte adhesion to the type 3 repeat domain of thrombospondin-1 is mediated by a modified band 3 protein. Mol Biochem Parasitol. 1999;100:195–205. doi: 10.1016/s0166-6851(99)00058-4. [DOI] [PubMed] [Google Scholar]
- 191.Lucas JZ, Sherman IW. Plasmodium falciparum: thrombospondin mediates parasitized erythrocyte band 3-related adhesin binding. Exp Parasitol. 1998;89:78–85. doi: 10.1006/expr.1998.4257. [DOI] [PubMed] [Google Scholar]
- 192.Crandall I, Collins WE, Gysin J, Sherman IW. Synthetic peptides based on motifs present in human band 3 protein inhibit cytoadherence/sequestration of the malaria parasite Plasmodium falciparum. Proc Natl Acad Sci USA. 1993;90:4703–4707. doi: 10.1073/pnas.90.10.4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Langhorne J, Ndungu FM, Sponaas A-M, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–732. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 194.Good MF, Stanisic D, Xu H, et al. The immunological challenge to developing a vaccine to the blood stages of malaria parasites. Immunol Rev. 2004;201:254–267. doi: 10.1111/j.0105-2896.2004.00178.x. [DOI] [PubMed] [Google Scholar]
- 195.Beeson JG, Osier FHA, Engwerda CR. Recent insights into humoral and cellular immune responses against malaria. Trends Parasitol. 2008;24:578–584. doi: 10.1016/j.pt.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 196.Marsh K, Kinyanjui S. Immune effector mechanisms in malaria. Parasite Immunol. 2006;28:51–60. doi: 10.1111/j.1365-3024.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 197.Gupta S, Snow RW, Donnelly CA, et al. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med. 1999;5:340–343. doi: 10.1038/6560. [DOI] [PubMed] [Google Scholar]
- 198.McGregor IA. Studies in the acquisition of immunity of Plasmodium falciparum infections in Africa. Trans R Soc Trop Med Hyg. 1964;58:80–92. doi: 10.1016/0035-9203(64)90073-2. [DOI] [PubMed] [Google Scholar]
- 199.Snow RW, Nahlen B, Palmer A, et al. Risk of severe malaria among African infants: direct evidence of clinical protection during early infancy. J Infect Dis. 1998;177:819–822. doi: 10.1086/517818. [DOI] [PubMed] [Google Scholar]
- 200.Kitua AY, Smith T, Alonso PL, et al. Plasmodium falciparum malaria in the first year of life in an area of intense and perennial transmission. Trop Med Int Health. 1996;1:475–484. doi: 10.1046/j.1365-3156.1996.d01-89.x. [DOI] [PubMed] [Google Scholar]
- 201.Yazdani SS, Mukherjee P, Chauhan VS, Chitnis CE. Immune responses to asexual blood-stages of malaria parasites. Curr Mol Med. 2006;6:187–203. doi: 10.2174/156652406776055212. [DOI] [PubMed] [Google Scholar]
- 202.Good MF, Xu H, Wykes M, Engwerda CR. Development and regulation of cell-mediated immune responses to the blood stages of malaria: implications for vaccine research. Annu Rev Immunol. 2005;23:69–99. doi: 10.1146/annurev.immunol.23.021704.115638. [DOI] [PubMed] [Google Scholar]
- 203.van der Heyde HC, Huszar D, Woodhouse C, et al. The resolution of acute malaria in a definitive model of B cell deficiency, the JHD mouse. J Immunol. 1994;152:4557–4562. [PubMed] [Google Scholar]
- 204.Clark IA, Cowden WB. Why is the pathology of falciparum worse than that of vivax malaria? Parasitol Today (Regul Ed) 1999;15:458–461. doi: 10.1016/s0169-4758(99)01535-5. [DOI] [PubMed] [Google Scholar]
- 205.Luty AJ, Lell B, Schmidt-Ott R, et al. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis. 1999;179:980–988. doi: 10.1086/314689. [DOI] [PubMed] [Google Scholar]
- 206.Hansen DS, Schofield L. Natural regulatory T cells in malaria: host or parasite allies? PLoS Pathog. 2010;6:e1000771. doi: 10.1371/journal.ppat.1000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Urban BC, Ferguson DJ, Pain A, et al. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature. 1999;400:73–77. doi: 10.1038/21900. [DOI] [PubMed] [Google Scholar]
- 208.Elliott SR, Spurck TP, Dodin JM, et al. Inhibition of dendritic cell maturation by malaria is dose dependent and does not require Plasmodium falciparum erythrocyte membrane protein 1. Infect Immun. 2007;75:3621–3632. doi: 10.1128/IAI.00095-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Artavanis-Tsakonas K, Eleme K, McQueen KL, et al. Activation of a subset of human NK cells upon contact with Plasmodium falciparum-infected erythrocytes. J Immunol. 2003;171:5396–5405. doi: 10.4049/jimmunol.171.10.5396. [DOI] [PubMed] [Google Scholar]
- 210.Chen Q, Amaladoss A, Ye W, et al. Human natural killer cells control Plasmodium falciparum infection by eliminating infected red blood cells. Proc Natl Acad Sci. 2014;111:1479–1484. doi: 10.1073/pnas.1323318111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.D’Ombrain MC, Voss TS, Maier AG, et al. Plasmodium falciparum erythrocyte membrane protein-1 specifically suppresses early production of host interferon-gamma. Cell Host Microbe. 2007;2:130–138. doi: 10.1016/j.chom.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 212.Donati D, Zhang LP, Chêne A, et al. Identification of a polyclonal B-cell activator in Plasmodium falciparum . Infect Immun. 2004;72:5412–5418. doi: 10.1128/IAI.72.9.5412-5418.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Donati D, Mok B, Chêne A, et al. Increased B cell survival and preferential activation of the memory compartment by a malaria polyclonal B cell activator. J Immunol. 2006;177:3035–3044. doi: 10.4049/jimmunol.177.5.3035. [DOI] [PubMed] [Google Scholar]
- 214.Simone O, Bejarano MT, Pierce SK, et al. TLRs innate immunereceptors and Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) CIDR1α-driven human polyclonal B-cell activation. Acta Trop. 2011;119:144–150. doi: 10.1016/j.actatropica.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Allsopp CEM, Sanni LA, Reubsaet L, et al. CD4 T cell responses to a variant antigen of the malaria parasite Plasmodium falciparum, erythrocyte membrane protein-1, in individuals living in malaria-endemic areas. J Infect Dis. 2002;185:812–819. doi: 10.1086/339521. [DOI] [PubMed] [Google Scholar]
- 216.Ndungu FM, Sanni L, Urban B, et al. CD4 T cells from malaria-nonexposed individuals respond to the CD36-Binding Domain of Plasmodium falciparum erythrocyte membrane protein-1 via an MHC class II-TCR-independent pathway. J Immunol. 2006;176:5504–5512. doi: 10.4049/jimmunol.176.9.5504. [DOI] [PubMed] [Google Scholar]
- 217.Marsh K. Malaria–a neglected disease? Parasitology. 1992;104(Suppl):S53–S69. doi: 10.1017/s0031182000075247. [DOI] [PubMed] [Google Scholar]
- 218.Cohen S, McGregor IA, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 219.Fowkes FJI, Richards JS, Simpson JA, Beeson JG. The relationship between anti-merozoite antibodies and incidence of Plasmodium falciparum malaria: a systematic review and meta-analysis. Plos Med. 2010;7:e1000218. doi: 10.1371/journal.pmed.1000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Osier FHA, Fegan G, Polley SD, et al. Breadth and magnitude of antibody responses to multiple Plasmodium falciparum merozoite antigens are associated with protection from clinical malaria. Infect Immun. 2008;76:2240–2248. doi: 10.1128/IAI.01585-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Richards JS, Arumugam TU, Reiling L, et al. Identification and prioritization of merozoite antigens as targets of protective human immunity to Plasmodium falciparum malaria for vaccine and biomarker development. J Immunol. 2013;191:795–809. doi: 10.4049/jimmunol.1300778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Richards JS, Beeson JG. The future for blood-stage vaccines against malaria. Immunol Cell Biol. 2009;87:377–390. doi: 10.1038/icb.2009.27. [DOI] [PubMed] [Google Scholar]
- 223.Richards JS, Stanisic DI, Fowkes FJI, et al. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis. 2010;51:e50–e60. doi: 10.1086/656413. [DOI] [PubMed] [Google Scholar]
- 224.Roussilhon C, Oeuvray C, Müller-Graf C, et al. Long-term clinical protection from falciparum malaria is strongly associated with IgG3 antibodies to merozoite surface protein 3. Plos Med. 2007;4:e320. doi: 10.1371/journal.pmed.0040320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Taylor DW, Zhou A, Marsillio LE, et al. Antibodies that inhibit binding of Plasmodium falciparum-infected erythrocytes to chondroitin sulfate A and to the C terminus of merozoite surface protein 1 correlate with reduced placental malaria in Cameroonian women. Infect Immun. 2004;72:1603–1607. doi: 10.1128/IAI.72.3.1603-1607.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Marsh K, Howard RJ. Antigens induced on erythrocytes by P. falciparum: expression of diverse and conserved determinants. Science. 1986;231:150–153. doi: 10.1126/science.2417315. [DOI] [PubMed] [Google Scholar]
- 227.Bull PC, Lowe BS, Kortok M, et al. Parasite antigens on the infected red cell surface are targets for naturally acquired immunity to malaria. Nat Med. 1998;4:358–360. doi: 10.1038/nm0398-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Marsh K, Otoo L, Hayes RJ, et al. Antibodies to blood stage antigens of Plasmodium falciparum in rural Gambians and their relation to protection against infection. Trans R Soc Trop Med Hyg. 1989;83:293–303. doi: 10.1016/0035-9203(89)90478-1. [DOI] [PubMed] [Google Scholar]
- 229.Iqbal J, Perlmann P, Berzins K. Serological diversity of antigens expressed on the surface of erythrocytes infected with Plasmodium falciparum . Trans R Soc Trop Med Hyg. 1993;87:583–588. doi: 10.1016/0035-9203(93)90097-a. [DOI] [PubMed] [Google Scholar]
- 230.Forsyth KP, Philip G, Smith T, et al. Diversity of antigens expressed on the surface of erythrocytes infected with mature Plasmodium falciparum parasites in Papua New Guinea. Am J Trop Med Hyg. 1989;41:259–265. [PubMed] [Google Scholar]
- 231.Newbold CI, Pinches R, Roberts DJ, Marsh K. Plasmodium falciparum: the human agglutinating antibody response to the infected red cell surface is predominantly variant specific. Exp Parasitol. 1992;75:281–292. doi: 10.1016/0014-4894(92)90213-t. [DOI] [PubMed] [Google Scholar]
- 232.Chattopadhyay R, Sharma A, Srivastava VK, et al. Plasmodium falciparum infection elicits both variant-specific and cross-reactive antibodies against variant surface antigens. Infect Immun. 2003;71:597–604. doi: 10.1128/IAI.71.2.597-604.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Beeson JG, Mann EJ, Byrne TJ, et al. Antigenic differences and conservation among placental Plasmodium falciparum-infected erythrocytes and acquisition of variant-specific and cross-reactive antibodies. J Infect Dis. 2006;193:721–730. doi: 10.1086/500145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bockhorst J, Lu F, Janes JH, et al. Structural polymorphism and diversifying selection on the pregnancy malaria vaccine candidate VAR2CSA. Mol Biochem Parasitol. 2007;155:103–112. doi: 10.1016/j.molbiopara.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 235.Giha HA, Theander TG, Staalsø T, et al. Seasonal variation in agglutination of Plasmodium falciparum-infected erythrocytes. Am J Trop Med Hyg. 1998;58:399–405. doi: 10.4269/ajtmh.1998.58.399. [DOI] [PubMed] [Google Scholar]
- 236.Bull PC, Lowe BS, Kaleli N, et al. Plasmodium falciparum infections are associated with agglutinating antibodies to parasite-infected erythrocyte surface antigens among healthy Kenyan children. J Infect Dis. 2002;185:1688–1691. doi: 10.1086/340420. [DOI] [PubMed] [Google Scholar]
- 237.Ofori MF, Dodoo D, Staalsoe T, et al. Malaria-induced acquisition of antibodies to Plasmodium falciparum variant surface antigens. Infect Immun. 2002;70:2982–2988. doi: 10.1128/IAI.70.6.2982-2988.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tebo AE, Kremsner PG, Piper KP, Luty AJF. Low antibody responses to variant surface antigens of Plasmodium falciparum are associated with severe malaria and increased susceptibility to malaria attacks in Gabonese children. Am J Trop Med Hyg. 2002;67:597–603. doi: 10.4269/ajtmh.2002.67.597. [DOI] [PubMed] [Google Scholar]
- 239.Dodoo D, Staalsoe T, Giha H, et al. Antibodies to variant antigens on the surfaces of infected erythrocytes are associated with protection from malaria in Ghanaian children. Infect Immun. 2001;69:3713–3718. doi: 10.1128/IAI.69.6.3713-3718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Giha HA, Staalsoe T, Dodoo D, et al. Antibodies to variable Plasmodium falciparum-infected erythrocyte surface antigens are associated with protection from novel malaria infections. Immunol Lett. 2000;71:117–126. doi: 10.1016/s0165-2478(99)00173-x. [DOI] [PubMed] [Google Scholar]
- 241.Aguiar J, Albrecht G, Cegielski P. Agglutination of Plasmodium falciparum-infected erythrocytes from east and West African. Am J Trop Med Hyg. 1992;47:621–632. doi: 10.4269/ajtmh.1992.47.621. [DOI] [PubMed] [Google Scholar]
- 242.Nielsen MA, Vestergaard LS, Lusingu J, et al. Geographical and temporal conservation of antibody recognition of Plasmodium falciparum variant surface antigens. Infect Immun. 2004;72:3531–3535. doi: 10.1128/IAI.72.6.3531-3535.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Nielsen MA, Staalsoe T, Kurtzhals JAL, et al. Plasmodium falciparum variant surface antigen expression varies between isolates causing severe and nonsevere malaria and is modified by acquired immunity. J Immunol. 2002;168:3444–3450. doi: 10.4049/jimmunol.168.7.3444. [DOI] [PubMed] [Google Scholar]
- 244.Saeed M, Roeffen W, Alexander N, et al. Plasmodium falciparum antigens on the surface of the gametocyte-infected erythrocyte. PLoS ONE. 2008;3:e2280. doi: 10.1371/journal.pone.0002280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Piper KP, Hayward RE, Cox MJ, Day KP. Malaria transmission and naturally acquired immunity to PfEMP1. Infect Immun. 1999;67:6369–6374. doi: 10.1128/iai.67.12.6369-6374.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Barry AW, Trieu A, Fowkes FJI, et al. The stability and complexity of antibody responses to the major surface antigen of Plasmodium falciparum are associated with age in a malaria endemic area. Mol Cell Proteomics. 2011;10(M111):008326. doi: 10.1074/mcp.M111.008326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Cham GKK, Turner L, Kurtis JD, et al. Hierarchical, domain type-specific acquisition of antibodies to Plasmodium falciparum erythrocyte membrane Protein 1 in Tanzanian children. Infect Immun. 2010;78:4653–4659. doi: 10.1128/IAI.00593-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Oguariri RM, Borrmann S, Klinkert MQ, et al. High prevalence of human antibodies to recombinant Duffy binding-like alpha domains of the Plasmodium falciparum-infected erythrocyte membrane protein 1 in semi-immune adults compared to that in nonimmune children. Infect Immun. 2001;69:7603–7609. doi: 10.1128/IAI.69.12.7603-7609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Staalsø T, Khalil EA, Elhassan IM, et al. Antibody reactivity to conserved linear epitopes of Plasmodium falciparum erythrocyte membrane protein 1 (PfEMP1) Immunol Lett. 1998;60:121–126. doi: 10.1016/s0165-2478(97)00143-0. [DOI] [PubMed] [Google Scholar]
- 250.Mackintosh CL, Christodoulou Z, Mwangi TW, et al. Acquisition of naturally occurring antibody responses to recombinant protein domains of Plasmodium falciparum erythrocyte membrane protein 1. Malar J. 2008;7:155. doi: 10.1186/1475-2875-7-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Abdel-Latif MS, Dietz K, Issifou S, et al. Antibodies to Plasmodium falciparum rifin proteins are associated with rapid parasite clearance and asymptomatic infections. Infect Immun. 2003;71:6229–6233. doi: 10.1128/IAI.71.10.6229-6233.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Abdel-Latif MS, Cabrera G, Köhler C, et al. Antibodies to rifin: a component of naturally acquired responses to Plasmodium falciparum variant surface antigens on infected erythrocytes. Am J Trop Med Hyg. 2004;71:179–186. [PubMed] [Google Scholar]
- 253.Schreiber N, Brattig N, Evans J, et al. Cerebral malaria is associated with IgG2 and IgG4 antibody responses to recombinant Plasmodium falciparum RIFIN antigen. Microbes Infect. 2006;8:1269–1276. doi: 10.1016/j.micinf.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 254.Schreiber N, Khattab A, Petter M, et al. Expression of Plasmodium falciparum 3D7 STEVOR proteins for evaluation of antibody responses following malaria infections in naive infants. Parasitology. 2008;135:155. doi: 10.1017/S0031182007003794. [DOI] [PubMed] [Google Scholar]
- 255.Udomsangpetch R, Wahlin B, Carlson J, et al. Plasmodium falciparum-infected erythrocytes form spontaneous erythrocyte rosettes. J Exp Med. 1989;169:1835–1840. doi: 10.1084/jem.169.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Udeinya IJ, Miller LH, McGregor IA, Jensen JB. Plasmodium falciparum strain-specific antibody blocks binding of infected erythrocytes to amelanotic melanoma cells. Nature. 1983;303:429–431. doi: 10.1038/303429a0. [DOI] [PubMed] [Google Scholar]
- 257.Elliott SR, Brennan AK, Beeson JG, et al. Placental malaria induces variant-specific antibodies of the cytophilic subtypes immunoglobulin G1 (IgG1) and IgG3 that correlate with adhesion inhibitory activity. Infect Immun. 2005;73:5903–5907. doi: 10.1128/IAI.73.9.5903-5907.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Ricke CH, Staalsoe T, Koram K, et al. Plasma antibodies from malaria-exposed pregnant women recognize variant surface antigens on Plasmodium falciparum-infected erythrocytes in a parity-dependent manner and block parasite adhesion to chondroitin sulfate A. J Immunol. 2000;165:3309–3316. doi: 10.4049/jimmunol.165.6.3309. [DOI] [PubMed] [Google Scholar]
- 259.Fried M, Nosten F, Brockman A, et al. Maternal antibodies block malaria. Nature. 1998;395:851–852. doi: 10.1038/27570. [DOI] [PubMed] [Google Scholar]
- 260.Duffy PE, Fried M. Antibodies that inhibit Plasmodium falciparum adhesion to chondroitin sulfate A are associated with increased birth weight and the gestational age of newborns. Infect Immun. 2003;71:6620–6623. doi: 10.1128/IAI.71.11.6620-6623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Staalsoe T, Shulman CE, Bulmer JN, et al. Variant surface antigen-specific IgG and protection against clinical consequences of pregnancy-associated Plasmodium falciparum malaria. Lancet. 2004;363:283–289. doi: 10.1016/S0140-6736(03)15386-X. [DOI] [PubMed] [Google Scholar]
- 262.Oleinikov AV, Amos E, Frye IT, et al. High throughput functional assays of the variant antigen PfEMP1 reveal a single domain in the 3D7 Plasmodium falciparum genome that binds ICAM1 with high affinity and is targeted by naturally acquired neutralizing antibodies. PLoS Pathog. 2009;5:e1000386. doi: 10.1371/journal.ppat.1000386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ghumra A, Khunrae P, Ataíde R, et al. Immunisation with recombinant PfEMP1 domains elicits functional rosette-inhibiting and phagocytosis-inducing antibodies to Plasmodium falciparum . PLoS ONE. 2011;6:e16414. doi: 10.1371/journal.pone.0016414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Celada A, Cruchaud A, Perrin LH. Opsonic activity of human immune serum on in vitro phagocytosis of Plasmodium falciparum infected red blood cells by monocytes. Clin Exp Immunol. 1982;47:635–644. [PMC free article] [PubMed] [Google Scholar]
- 265.Bouharoun-Tayoun H, Attanath P, Sabchareon A, et al. Antibodies that protect humans against Plasmodium falciparum blood stages do not on their own inhibit parasite growth and invasion in vitro, but act in cooperation with monocytes. J Exp Med. 1990;172:1633–1641. doi: 10.1084/jem.172.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Feng G, Aitken E, Yosaatmadja F, et al. Antibodies to variant surface antigens of Plasmodium falciparum-infected erythrocytes are associated with protection from treatment failure and the development of anemia in pregnancy. J Infect Dis. 2009;200:299–306. doi: 10.1086/599841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ataíde R, Hasang W, Wilson DW, et al. Using an improved phagocytosis assay to evaluate the effect of HIV on specific antibodies to pregnancy-associated malaria. PLoS ONE. 2010;5:e10807. doi: 10.1371/journal.pone.0010807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Keen J, Serghides L, Ayi K, et al. HIV impairs opsonic phagocytic clearance of pregnancy-associated malaria parasites. Plos Med. 2007;4:e181. doi: 10.1371/journal.pmed.0040181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Baruch DI, Gamain B, Barnwell JW, et al. Immunization of Aotus monkeys with a functional domain of the Plasmodium falciparum variant antigen induces protection against a lethal parasite line. Proc Natl Acad Sci USA. 2002;99:3860–3865. doi: 10.1073/pnas.022018399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Baruch DI, Gamain B, Miller LH. DNA immunization with the cysteine-rich interdomain region 1 of the Plasmodium falciparum variant antigen elicits limited cross-reactive antibody responses. Infect Immun. 2003;71:4536–4543. doi: 10.1128/IAI.71.8.4536-4543.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gratepanche S, Gamain B, Smith JD, et al. Induction of crossreactive antibodies against the Plasmodium falciparum variant protein. Proc Natl Acad Sci USA. 2003;100:13007–13012. doi: 10.1073/pnas.2235588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Ahuja S, Pettersson F, Moll K, et al. Induction of cross-reactive immune responses to NTS-DBL-1alpha/x of PfEMP1 and in vivo protection on challenge with Plasmodium falciparum . Vaccine. 2006;24:6140–6154. doi: 10.1016/j.vaccine.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 273.Turner L, Wang CW, Lavstsen T, et al. Antibodies against PfEMP1, RIFIN, MSP3 and GLURP are acquired during controlled Plasmodium falciparum malaria infections in naïve volunteers. PLoS ONE. 2011;6:e29025. doi: 10.1371/journal.pone.0029025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Flick K, Chen Q. var genes, PfEMP1 and the human host. Mol Biochem Parasitol. 2004;134:3–9. doi: 10.1016/j.molbiopara.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 275.Oguariri RM, Mattei D, Tena-Tomás C, et al. Recombinant Duffy binding- like-alpha domains of Plasmodium falciparum erythrocyte membrane protein 1 elicit antibodies in rats that recognise conserved epitopes. Parasitol Res. 2003;90:467–472. doi: 10.1007/s00436-003-0884-8. [DOI] [PubMed] [Google Scholar]
- 276.Mayor A, Rovira-Vallbona E, Srivastava A, et al. Functional and immunological characterization of a Duffy binding-like alpha domain from Plasmodium falciparum erythrocyte membrane protein 1 that mediates rosetting. Infect Immun. 2009;77:3857–3863. doi: 10.1128/IAI.00049-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Chen Q, Pettersson F, Vogt AM, et al. Immunization with PfEMP1-DBL1alpha generates antibodies that disrupt rosettes and protect against the sequestration of Plasmodium falciparum-infected erythrocytes. Vaccine. 2004;22:2701–2712. doi: 10.1016/j.vaccine.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 278.Moll K, Pettersson F, Vogt AM, et al. Generation of cross-protective antibodies against Plasmodium falciparum sequestration by immunization with an erythrocyte membrane protein 1-duffy binding-like 1 alpha domain. Infect Immun. 2007;75:211–219. doi: 10.1128/IAI.00749-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Tutterrow YL, Salanti A, Avril M, et al. High avidity antibodies to full-length VAR2CSA correlate with absence of placental malaria. PLoS ONE. 2012;7:e40049. doi: 10.1371/journal.pone.0040049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Tutterrow YL, Avril M, Singh K, et al. High levels of antibodies to multiple domains and strains of VAR2CSA correlate with the absence of placental malaria in Cameroonian women living in an area of high Plasmodium falciparum transmission. Infect Immun. 2012;80:1479–1490. doi: 10.1128/IAI.00071-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Bigey P, Gnidehou S, Doritchamou J, et al. The NTS-DBL2X region of VAR2CSA induces cross-reactive antibodies that inhibit adhesion of several Plasmodium falciparum isolates to chondroitin sulfate A. J Infect Dis. 2011;204:1125–1133. doi: 10.1093/infdis/jir499. [DOI] [PubMed] [Google Scholar]
- 282.Nielsen MA, Pinto VV, Resende M, et al. Induction of adhesion-inhibitory antibodies against placental Plasmodium falciparum parasites by using single domains of VAR2CSA. Infect Immun. 2009;77:2482–2487. doi: 10.1128/IAI.00159-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Beeson JG, Chan J-A, Fowkes FJ. PfEMP1 as a target of human immunity and a vaccine candidate against malaria. Expert Rev Vaccines. 2013;12:105–108. doi: 10.1586/erv.12.144. [DOI] [PubMed] [Google Scholar]
- 284.Drew DR, Hodder AN, Wilson DW, et al. Defining the antigenic diversity of Plasmodium falciparum apical membrane antigen 1 and the requirements for a multi-allele vaccine against malaria. PLoS ONE. 2012;7:e51023. doi: 10.1371/journal.pone.0051023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Miura K, Herrera R, Diouf A, et al. Overcoming allelic specificity by immunization with five allelic forms of Plasmodium falciparum apical membrane antigen 1. Infect Immun. 2013;81:1491–1501. doi: 10.1128/IAI.01414-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kinyanjui SM, Bull P, Newbold CI, Marsh K. Kinetics of antibody responses to Plasmodium falciparum-infected erythrocyte variant surface antigens. J Infect Dis. 2003;187:667–674. doi: 10.1086/373994. [DOI] [PubMed] [Google Scholar]
- 287.Fowkes FJI, McGready R, Johnstone-Robertson S, et al. Antibody boosting and longevity following tetanus immunization during pregnancy. Clin Infect Dis. 2012 doi: 10.1093/cid/cis979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Yone CLRP, Kremsner PG, Luty AJF. Immunoglobulin G isotype responses to erythrocyte surface-expressed variant antigens of Plasmodium falciparum predict protection from malaria in African children. Infect Immun. 2005;73:2281–2287. doi: 10.1128/IAI.73.4.2281-2287.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Mackintosh CL, Mwangi T, Kinyanjui SM, et al. Failure to respond to the surface of Plasmodium falciparum infected erythrocytes predicts susceptibility to clinical malaria amongst African children. Int J Parasitol. 2008;38:1445–1454. doi: 10.1016/j.ijpara.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Magistrado PA, Lusingu J, Vestergaard LS, et al. Immunoglobulin G antibody reactivity to a group A Plasmodium falciparum erythrocyte membrane protein 1 and protection from P. falciparum malaria. Infect Immun. 2007;75:2415–2420. doi: 10.1128/IAI.00951-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bull PC, Kortok M, Kai O, et al. Plasmodium falciparum-infected erythrocytes: agglutination by diverse Kenyan plasma is associated with severe disease and young host age. J Infect Dis. 2000;182:252–259. doi: 10.1086/315652. [DOI] [PubMed] [Google Scholar]
- 292.Cabrera G, Yone C, Tebo AE, et al. Immunoglobulin G isotype responses to variant surface antigens of Plasmodium falciparum in healthy Gabonese adults and children during and after successive malaria attacks. Infect Immun. 2004;72:284–294. doi: 10.1128/IAI.72.1.284-294.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Giha HA, Staalsoe T, Dodoo D, et al. Overlapping antigenic repertoires of variant antigens expressed on the surface of erythrocytes infected by Plasmodium falciparum . Parasitology. 1999;119(Pt 1):7–17. doi: 10.1017/s0031182099004485. [DOI] [PubMed] [Google Scholar]
- 294.Reeder JC, Rogerson SJ, al-Yaman F, et al. Diversity of agglutinating phenotype, cytoadherence, and rosette-forming characteristics of Plasmodium falciparum isolates from Papua New Guinean children. Am J Trop Med Hyg. 1994;51:45–55. doi: 10.4269/ajtmh.1994.51.45. [DOI] [PubMed] [Google Scholar]
- 295.Crompton PD, Kayala MA, Traoré B, et al. A prospective analysis of the Ab response to Plasmodium falciparum before and after a malaria season by protein microarray. Proc Natl Acad Sci. 2010;107:6958–6963. doi: 10.1073/pnas.1001323107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.