Abstract
Background
The current perception threshold (CPT) could be quantified by stimulating Aβ and C fibers at 2,000 and 5 Hz, respectively. C fibers play a role in the autonomic nervous system and are involved in temperature and pain sensation. We evaluated the usefulness of CPT for diagnosing distal polyneuropathy (DPN) and cardiovascular autonomic neuropathy (CAN) in diabetic patients.
Methods
The CPT was measured in the index finger (C7 level) and in the third toe (L5 level) in diabetic patients aged 30 to 69 years. We assessed DPN according to the neuropathy total symptom score-6 (NTSS-6) and 10-g monofilament pressure sensation. Subjects with a NTSS-6 >6 or with abnormal 10-g monofilament sensation were defined to have DPN. CAN was evaluated by spectral analysis of heart rate variability and by Ewing's traditional tests.
Results
The subjects with DPN had significantly higher CPT at all of the frequencies than the subjects without DPN (P<0.05). Abnormal 10-g monofilament sensation and NTSS-6 >6 could be most precisely predicted by CPT at 2,000 and 5 Hz, respectively. However, only 6.5% and 19.6% of subjects with DPN had an abnormal CPT at 2,000 Hz at the C7 and L5 levels. Although CPT at 5 Hz showed a negative correlation with the power of low and high frequency in the spectral analysis (P<0.05), only 16.7% of subjects with CAN exhibited an abnormal CPT at the same frequency.
Conclusion
Although the CPT is significantly associated with neuropathic symptoms or signs corresponding to the nerve fiber stimulated, it provides little additional information compared with conventional evaluations.
Keywords: Cardiac autonomic neuropathy, Current perception threshold, Diabetic neuropathy
INTRODUCTION
The early recognition and appropriate management of neuropathy in patients with diabetes is important because a number of treatment options exist for symptomatic diabetic neuropathy [1,2]. Moreover, screening for distal polyneuropathy (DPN) is important because up to 50% of patients with diabetic DPN are asymptomatic and are at risk for insensate injuries to their feet [2,3]. DPN screening could be performed using tests such as vibration perception (using a 128-Hz tuning fork), 10-g monofilament pressure sensation, and the assessment of ankle reflexes. Loss of 10-g monofilament perception and reduced vibration perception could predict foot ulcers [3].
Current perception threshold (CPT) testing is also a useful technique for assessing diabetic sensory neuropathy [4,5]. CPT might provide additional information because it is able to test different types of nerve fibers by using different electric stimulus frequencies; Aβ, Aδ, and C fibers could be stimulated at 2,000, 250, and 5 Hz, respectively. Aβ fibers are large myelinated nerve fibers associated with touch and pressure sensation. Meanwhile, C fibers primarily conduct impulses for temperature and pain and play a role in the autonomic nervous system. A few studies involving a small number of patients with diabetes have validated the use of CPT to detect DPN [5,6,7,8,9]. In the present study, we evaluated the usefulness of CPT for the diagnosis of DPN and for the diagnosis of cardiovascular autonomic neuropathy (CAN) compared with conventional tests in a large number of Korean patients with diabetes.
METHODS
Patients
We retrospectively reviewed the medical records of patients with diabetes in the Boramae Diabetes Center who underwent CPT evaluation from April 2011 to November 2012. The inclusion criteria were as follows: 1) diagnosis of diabetes mellitus according to the World Health Organization 1999 criteria; 2) age 30 to 69 years old; 3) aspartate aminotransferase and alanine aminotransferase levels <120 IU/L; and 4) no active foot disease, such as infection. Subjects with 1) spine disease, 2) history of stroke, or 3) other causes of peripheral neuropathy, such as chronic alcoholism, were excluded.
The hemoglobin A1c (HbA1c), lipid profile, serum creatinine, spot urine microalbuminuria to creatinine ratio, and diabetic retinopathy status were evaluated upon study enrollment. This study was approved by the Institutional Review Board of Boramae Medical Center and conformed to the provisions of the Declaration of Helsinki revised in 2000.
Evaluation of peripheral neuropathy and CAN
Neuropathic symptoms were evaluated according to the neuropathy total symptom score-6 (NTSS-6), whose reliability, consistency, and clinical validity have been previously confirmed [10]. The subjects with NTSS-6 >6 were defined as symptomatic subjects with neuropathy [10]. Light pressure sensation was evaluated using a 5.07 Semmes-Weinstein monofilament (US Neurologicals, Poulsbo, WA, USA; 10-g monofilament test) at 10 points including the plantar surface of the big toe and the first, third, and fifth metatarsal heads in each foot. Intact sensation at seven or more sites in each foot was considered normal. The subjects with NTSS-6 >6 or abnormal 10-g monofilament test results were defined to have DPN.
CAN was evaluated by Ewing's traditional five simple tests [3]; changes in the R-R with deep breathing, standing, and the Valsalva maneuver as well as changes in blood pressure in response to standing up and sustained handgrip were evaluated. Subjects with ≥2 abnormalities among these five tests were considered to have CAN. Spectral analysis of heart rate variation (HRV), the standard deviation of all normal R-R intervals (SDNN), and the root-mean square of the difference of successive R-R intervals (rMSSD) were also evaluated using DiCAN (Medicore, Seoul, Korea). The SDNN is thought to represent joint sympathetic and parasympathetic modulation of HRV, whereas the rMSSD is specific for the parasympathetic limb [11].
CPT measurement
The Neurometer (Neurotron Inc., Baltimore, MD, USA) generates a constant alternating current (AC) stimulus, which was applied to two different test sites, the index finger (C7 level) and the third toe (L5 level). The electrodes are positioned at the test site and held in place with tape. Contacts were optimized using electroconductive gel. The CPT was measured by a well-trained technologist from the Boramae Diabetes Center; the subject was instructed to press a button until a stimulus is detected at the site of the electrode and then to release the button. The standard method of CPT testing has been well described [8]. At this point, the CPT measure is verified using Compliance Guard software (Neurotron Inc.); 1 CPT unit corresponded to 0.01 mA. At each test site, three different frequencies of AC current-2000, 250, and 5 Hz-were applied to stimulate large myelinated Aβ fibers, small myelinated Aδ fibers, and small unmyelinated C fibers, respectively [9]. At each frequency, a CPT below or above the reference range obtained from healthy subjects provided by the manufacturer [12] was defined as hyperesthesia or hypoesthesia, respectively. The CPT of each group is presented as the median (interquartile range) because the data were not normally distributed.
Statistical analyses
The statistical analyses were performed using SPSS version 16 (SPSS Inc., Chicago, IL, USA). Independent t-tests and chi-square tests were performed where appropriate. Logarithmic transformation was performed before the statistical analysis for variables that were not normally distributed. Linear-by-linear analysis was used to compare the trends of categorical variables between the groups with and without neuropathy. Because the CPT was not normally distributed even after logarithmic transformation, the Mann-Whitney U test and Spearman rank correlation test were used to identify intergroup differences in CPT and to investigate the association between CPT and other markers of neuropathy. A receiver operating characteristic (ROC) curve analysis was performed to investigate the sensitivity of the CPT for predicting diabetic neuropathy. The level of significance was set at P<0.05.
RESULTS
A total of 439 patients underwent CPT evaluations during the study period. After excluding 1) subjects suspected of having other causes of neuropathy or cerebrovascular disease, 2) subjects who did not undergo a 10-g monofilament test, and 3) subjects who did not respond to a questionnaire for NTSS-6, 241 patients with diabetes aged 30 to 69 years (124 men and 117 women) were included in our final analysis. The mean age was 56±9 years, the median diabetes duration was 8 years (range, 0 to 27 years), and the mean HbA1c was 8.8%±2.3%. Of the patients, 23.9% and 25.4% had diabetic retinopathy and proteinuria ranging from microalbuminuria to overt proteinuria, respectively (Table 1).
Table 1.
Clinical characteristics of the study subjects
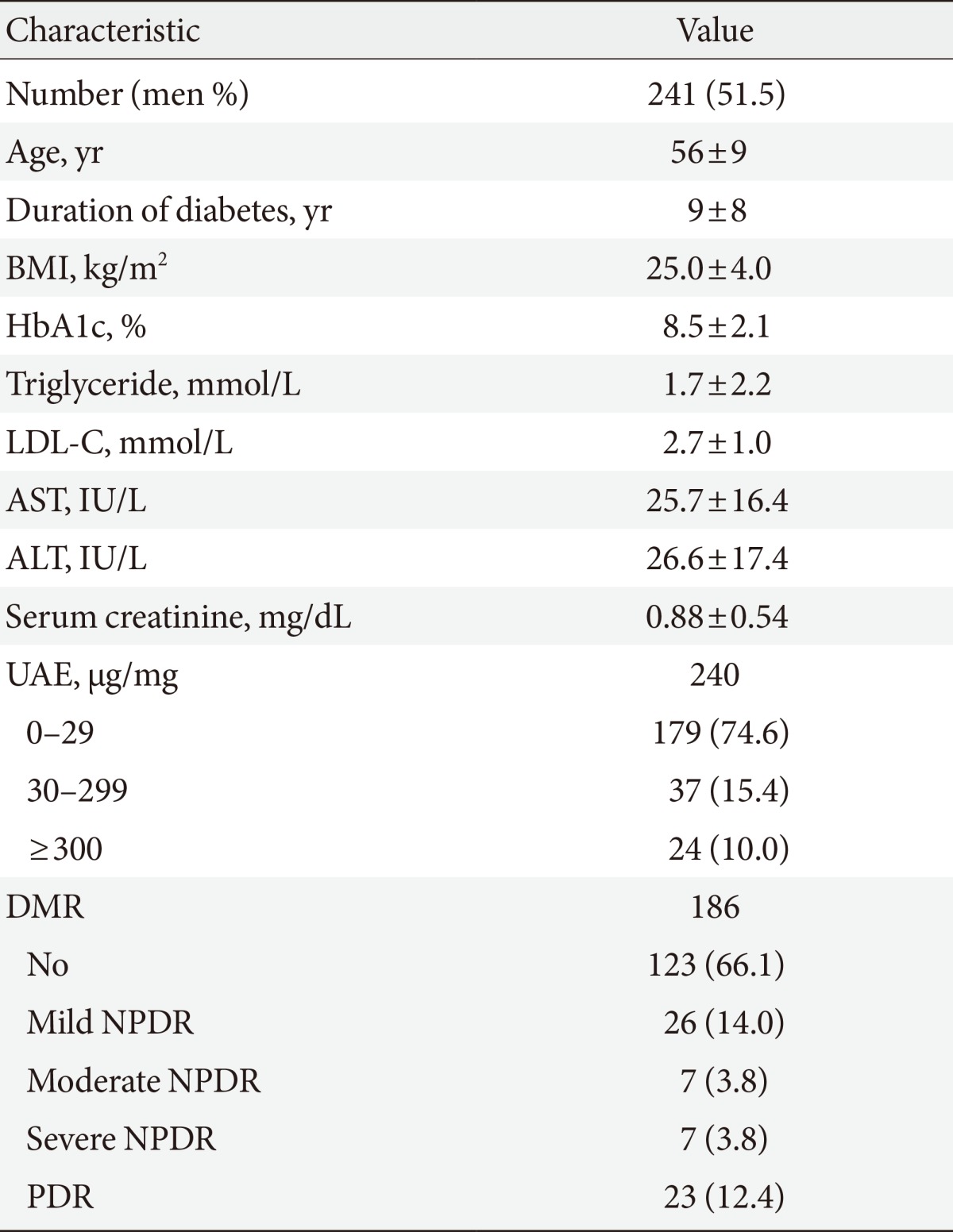
Values are presented as number (%) or mean±standard deviation.
BMI, body mass index; HbA1c, hemoglobin A1c; LDL-C, low density lipoprotein cholesterol; AST, aspartate aminotransferase; ALT, alanine aminotransferase; UAE, urine albumin excretion ratio; DMR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy.
The prevalence of DPN in the study subjects was 19.1%, whereas the prevalence of CAN was 18.8%. The presence of DPN and CAN were significantly associated with the severity of diabetic retinopathy (P=0.002 and P<0.001, respectively) (Table 2). The severity of proteinuria and diabetes duration were also significantly associated with DPN (P=0.015 and P<0.001, respectively). In addition, female sex was a significant risk factor for DPN (P=0.014). However, there was no significant difference in the HbA1c or lipid profile between the groups with and without each neuropathy (Table 2).
Table 2.
CPT according to diabetic neuropathy
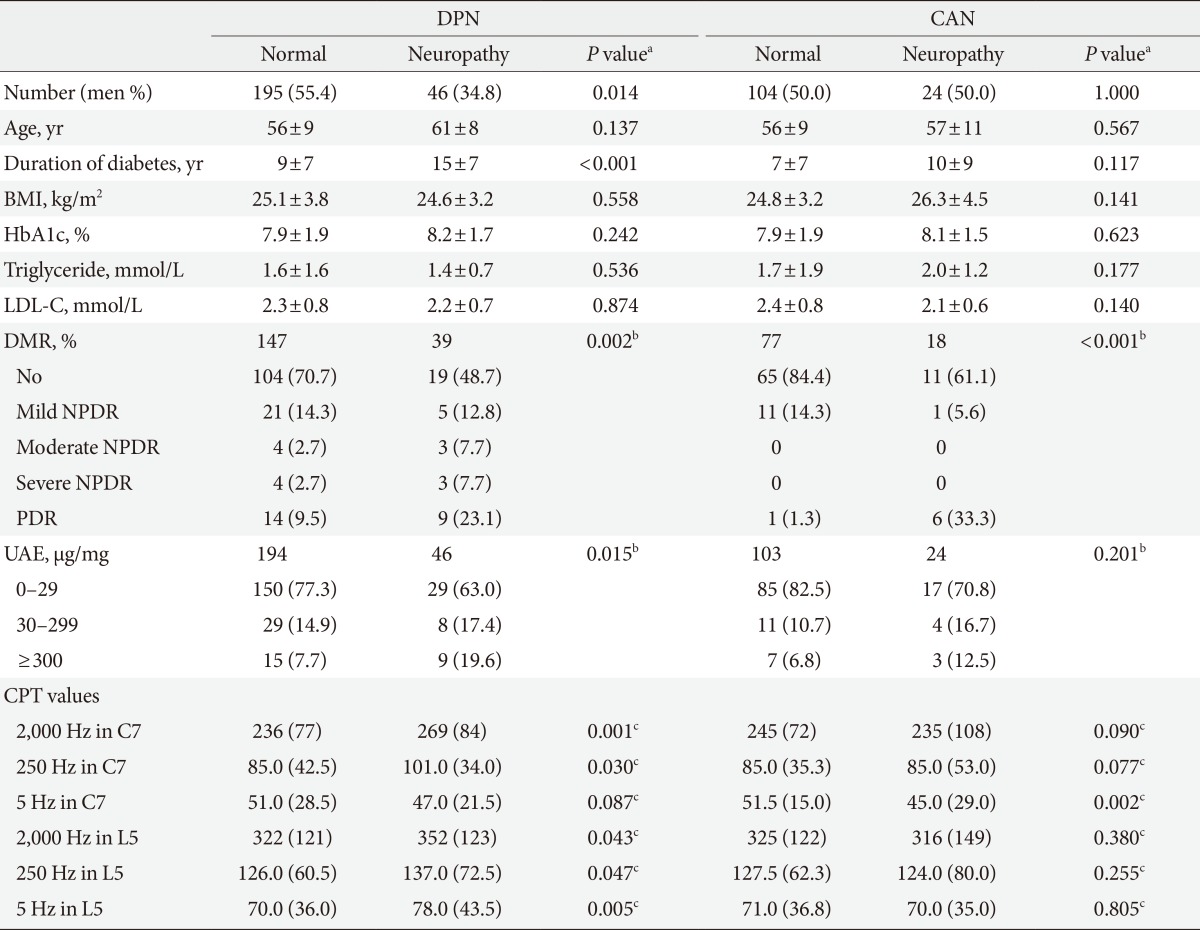
Values are presented as number (%) or mean±standard deviation. The CPT are presented as median (interquartile range).
CPT, current perception threshold; DPN, distal polyneuropathy; CAN, cardiovascular autonomic neuropathy; BMI, body mass index; HbA1c, hemoglobin A1c; LDL-C, low density lipoprotein cholesterol; DMR, diabetic retinopathy; NPDR, nonproliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; UAE, urine albumin excretion ratio.
aP value from comparisons between the subjects with or without neuropathy, bP value from linear-by-linear analysis, cP values from Mann-Whitney test, the number of subjects with normal vs. (hypoesthesia+hyperesthesia) range of CPT according to the group was compared with the chi-square test.
Association between CPT and DPN
The subjects with DPN had significantly higher CPT at 2,000 Hz (P=0.001 at C7 and P=0.043 at L5) and at 250 Hz at both the C7 and L5 levels (P=0.030 at C7 and P=0.047 at L5) compared with the subjects without DPN; at the L5 level, the DNP subjects also had a higher CPT at 5 Hz (P=0.005) (Table 2). If the hyperesthesia or hypoesthesia range of CPT was defined as below or above the reference range provided by the manufacturer [12], the subjects with DPN had a significantly higher prevalence of the hypoesthetic CPT at 2,000 Hz (P=0.019 at C7 level and P=0.003 at L5 level) at both the C7 and L5 levels compared with the subjects without DPN; however, only 6.5% and 19.6% of the subjects with DPN had an abnormal CPT at 2,000 Hz at the C7 and L5 levels, respectively. The prevalence of an abnormal CPT at 250 Hz was very low in the subjects with DPN, although there was a significant difference in the prevalence of abnormal CPTs between the subjects with and without DPN (P=0.004 at C7 level and P=0.034 at L5 level) (Table 3).
Table 3.
Prevalence of an abnormal current perception threshold according to diabetic neuropathy
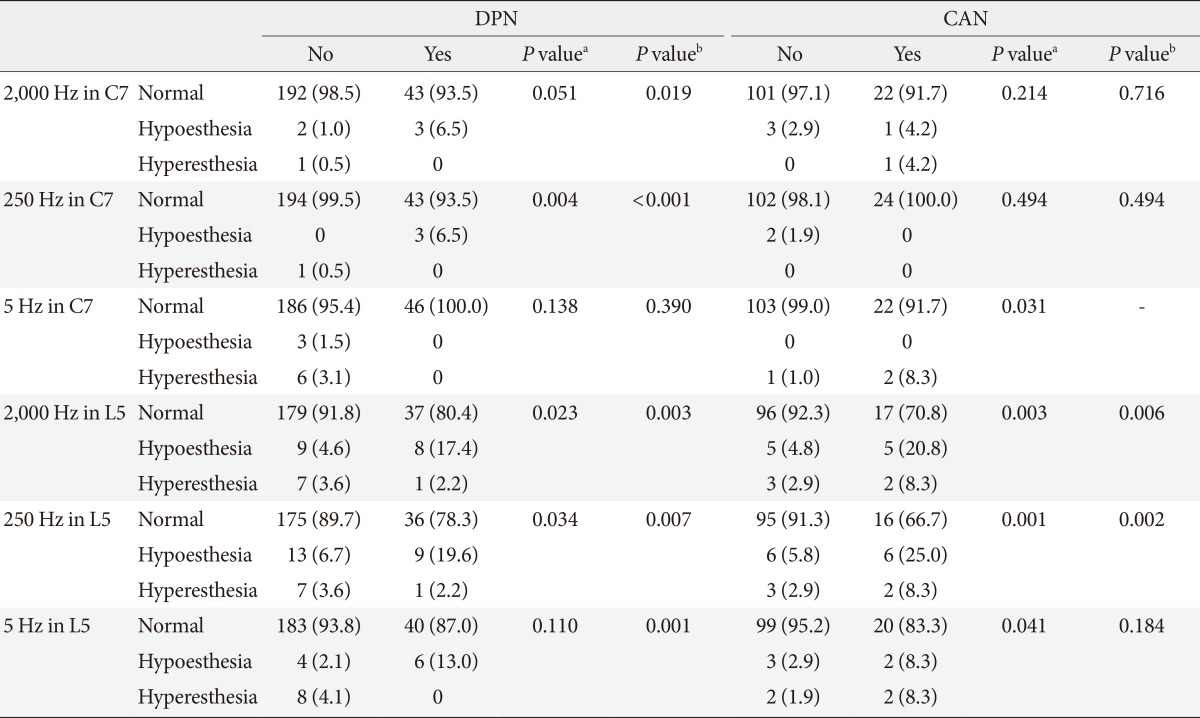
Values are presented as number (%). A current perception threshold (CPT) below or above the reference range obtained from healthy CPT values which were provided by manufacturer [12] was defined as hyperesthesia or hypoesthesia, respectively.
DPN, distal polyneuropathy; CAN, cardiovascular autonomic neuropathy.
aP values from the chi-square test to compare the frequency of normal vs. (hypoesthesia+hyperesthesia), bP values from the chi-square test to compare the frequency of normal vs. hypoesthesia.
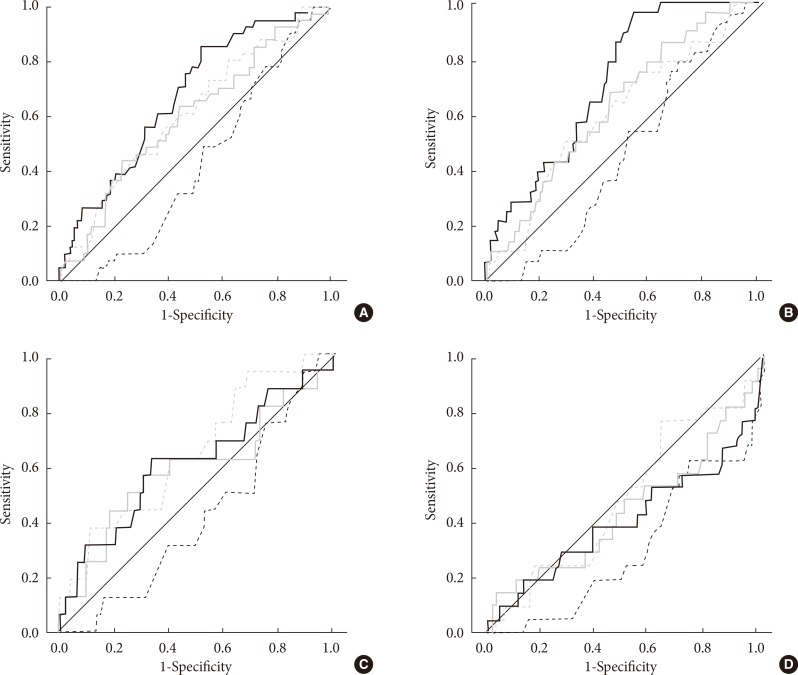
The ROC analysis showed that CPT at 2,000 Hz at the C7 and L5 levels, 250 Hz at the C7 level, and 5 Hz at the L5 level could predict the presence of DPN; the areas under the curve (AUC) were 0.676 (P<0.001), 0.601 (P=0.042), 0.605 (P=0.036), and 0.607 (P=0.019), respectively (Fig. 1A). More specifically, abnormal 10-g monofilament test results could be well predicted by CPT at 2,000 Hz at the C7 and L5 levels (AUC=0.721, P<0.001 at the C7 level; ACU=0.632, P=0.024 at the L5 level), whereas NTSS-6 >6 could be predicted most precisely by CPT at 5 Hz at the L5 level (AUC=0.647, P=0.049) (Fig. 1B and C). The current maximal reference values of CPT at 2,000 Hz to detect hypoesthesia provided by the manufacturer [12] were 398 and 523 at the C7 and L5 levels, respectively; their sensitivities for predicting abnormal 10-g monofilament test results were 7.1% (with a specificity of 99.0%) and 10.7% (with a specificity of 96.1%), respectively.
Fig. 1.
Receiver operating characteristic (ROC) curve of the current perception threshold (CPT) for predicting neuropathy. (A) ROC curves of CPT for diabetic polyneuropathy, (B) results of the 10-g monofilament test, (C) abnormality of the neuropathy total symptom score-6 (NTSS-6), and (D) autonomic neuropathy. The solid and dashed lines represent the ROC curves of CPT at 2,000 Hz and 5 Hz, respectively, and the black and gray lines represent the ROC curves at the C7 and L5 levels, respectively.
Because CPT at 5 Hz at the L5 level most precisely predicted the NTSS-6 >6 with ROC analysis, we further analyzed the correlation between CPT at 5 Hz at the L5 level and the symptom score of each component of the NTSS-6. We analyzed the symptom score of each of the six components (prickling/tingling, aching/tightness, sharp/shooting/lancinating, burning, paresthesia, and numbness) according to the hyperesthesia/normal/hypoesthesia ranges of CPT at 5 Hz at the L5 level. Independent of the NTSS-6 component, the subjects with the hypoesthesia range of CPT at 5 Hz at the L5 level showed the highest score compared with the patients with hyperesthesia and normal CPTs. In particular, the subjects with hypoesthetic ranges of the CPT reported statistically higher symptom scores of sharp/shooting/lancinating pain (P=0.011), paresthesias (P=0.008), and numbness (P=0.012). Spearman rank correlation tests also showed that CPT at 5 Hz at the L5 level was significantly correlated with the symptom scores of aching/tightness (ρ=0.160, P=0.021), sharp/shooting/lancinating pain (ρ=0.150, P=0.031), paresthesia (ρ=0.118, P=0.042), and numbness (ρ=0.182, P=0.002).
Association between CPT and CAN
The subjects with CAN had significantly lower CPTs at 5 Hz at the C7 level (P=0.002) (Table 2), and they showed a significantly higher prevalence of abnormal CPTs (hyperesthesia) at the same frequency at the C7 level (P=0.031) (Table 3). Otherwise, the prevalence of an abnormal CPT at the C7 level was very low and was not significantly different between the subjects with and without CAN at any of the frequencies (Table 3). An abnormal CPT at the L5 level was more frequent in the subjects with CAN at all of the frequencies (P=0.003 at 2,000 Hz, P=0.001 at 250 Hz, and P=0.041 at 5 Hz); 29.2%, 33.3%, and 16.7% of the subjects with CAN exhibited an abnormal CPT at 2,000, 250, and 5 Hz, respectively (Table 3). However, the ROC analysis showed that CPT could not predict the presence of CAN (Fig. 1D).
The analysis of the R-R interval showed that the CPT at 5 Hz at the L5 level was significantly correlated with the SDNN (r=-0.175, P=0.009) (Table 4). The CPT at 5 Hz at the L5 level showed a negative correlation with the power of low and high frequencies in the spectral analysis (ρ=-0.161, P=0.047 and ρ=-0.207, P=0.011, respectively). There was no correlation between the rMSSD and CPT (Table 4).
Table 4.
Correlation between the current perception threshold and markers of cardiac autonomic neuropathy
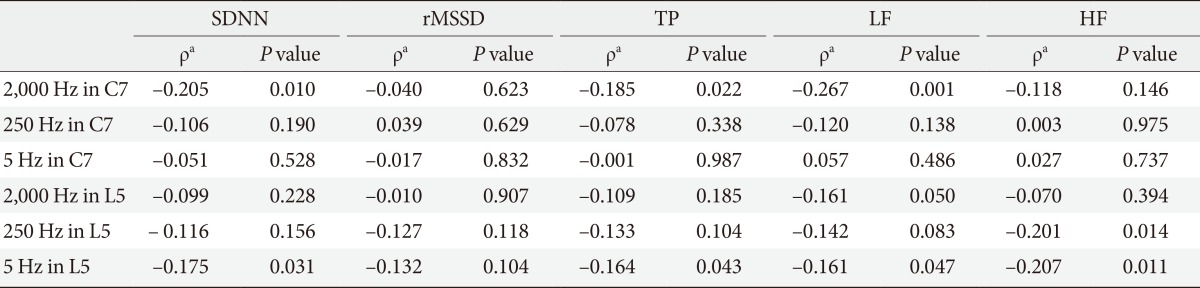
SDNN, standard deviation of all normal R-R intervals; rMSSD, root-mean square of the difference of successive R-R intervals; TP, total power from spectral analysis of heart rate variability; LF, power of low frequency from spectral analysis; HF, power of high frequency from spectral analysis.
aSpearman rank correlation test.
DISCUSSION
The results of our study show that the CPT was significantly associated with DPN; the CPT at all of the frequencies was significantly higher in the subjects with DPN compared with the subjects without DPN. Furthermore, CPT could predict the presence of significant neuropathic symptoms and light pressure sensation; the ROC analysis showed that the neuropathic symptom score and pressure sensation were related to the CPT at 5 and 2,000 Hz, respectively. Pain is conducted via C and Aδ fibers, which are theoretically stimulated at 5 and 250 Hz, respectively. In contrast, pressure impulses are conducted via Aβ fibers stimulated at a frequency of 2,000 Hz, suggesting that subjects with an abnormal neuropathic symptom score or abnormal pressure sensation have abnormal CPTs at the corresponding current frequency.
However, our study also revealed that the low sensitivity of CPT for detecting diabetic neuropathy might be a clinical limitation. Although there was a significant difference in the CPT between the subjects with and without neuropathy, only 10% to 20% of the subjects with DPN could be classified as having an abnormal CPT; 87% of the subjects with abnormal 10-g monofilament test results had a normal CPT at 2,000 Hz at the L5 level, which is corroborated by the results of a previous study [4,13]. The sensitivity of CPT at the C7 level was more limited; less than 10% of the neuropathic patients had an abnormal CPT at all of the frequencies at the C7 level. The ROC curve analysis showed that the area under the curve of the CPT at 2,000 Hz for detecting abnormal 10-g monofilament test results was approximately 0.7, which did not show sufficient performance as a screening test.
There are conflicting data showing that the incidence of sensory neuropathy detected by the neurometer is significantly higher than that detected by the 10-g monofilament test [6,7] and that CPT could be used to distinguish neuropathic from nonneuropathic patients with diabetes [9]. Our study population exhibited a relatively low prevalence of diabetic retinopathy and nephropathy-approximately half of the prevalence reported in a previous study [8]-which might be the cause of the different results in the present and previous studies. Only 14% of the study subjects had an abnormal 10-g monofilament test result; therefore, the small number of patients with neuropathy might reduce the discriminatory power of CPT. In addition, the CPT might be more clinically useful in subjects with more advanced diabetic complications. However, the Centers for Disease Control and Prevention reported that the incidence of diabetic complications has decreased since the 2000s, irrespective of ethnicity [14]; the low prevalence in our study population might reflect these trends. A recent cross-sectional hospital-based study in China showed that the prevalence of diabetic neuropathy was 17.8% [15], which is corroborated by our results.
The 5-Hz current stimulates C fibers, and C fibers play a role in the autonomic nervous system, suggesting that the CPT at 5 Hz might be associated with CAN. An association between CPT and autonomic neuropathy has been reported in a limited number of studies conducted in patients with diabetes [16] and without diabetes [17,18]. In our study, an abnormal CPT at 5 Hz at the L5 level was more frequent in subjects with CAN. Furthermore, autonomic nerve system capacity evaluated by HRV showed that the SDNN and the power of low and high frequencies from the HRV analysis showed a significantly negative correlation with CPT at 5 Hz at the L5 level, which is in agreement with the results of a previous study in subjects without diabetes [17]. The SDNN is thought to represent joint sympathetic and parasympathetic modulation of HRV, and the power of low frequency of HRV is a marker of sympathetic nerve function [11]. However, the selectivity of nerve fibers stimulated by specific frequencies might be limited; an abnormal CPT at the L5 level was more frequent at 250 and 5 Hz in subjects with CAN and SDNN, and the power of the HRV analysis showed a significantly negative correlation with the CPT at 2,000 Hz at the C7 level. However, subjects with CAN diagnosed according to Ewing's criteria did not have high CPTs. Furthermore, the sensitivity to detect CAN was also low; although an abnormal CPT at the L5 level was more frequent in subjects with CAN at 2,000, 250, and 5 Hz, only 29.2%, 33.3%, and 26.7% of subjects with CAN had abnormal CPTs at those respective current frequencies at the L5 level.
The main limitation of our study is that definition of DPN is not confirmed by nerve conduction tests or the quantification of intraepidermal nerve fibers [19]. We defined subjects with NTSS-6 >6 or abnormal 10-g monofilament test results to have DPN, which might result in underestimation of DPN. In our study, the prevalence of DPN was 19.1%, which is somewhat lower than in previous studies [2,3]. Furthermore, medications associated with neuropathy, such as antidepressants, anticonvulsants and opioids, might be associated with the symptom score; however, those medications were not considered in our analysis. In addition, the CPT does rely on patient's subjective responses and might be influenced by skin resistance associated with dehydration, although a limited number of studies have shown the ability of the neurometer to maintain the current despite alterations in skin resistance [9]. The reference values of CPT were from the neurometer manufacturer rather than from normal Korean subjects. Therefore, an additional study investigating the CPT in the normal Korean population is warranted. The results from our study population, which had low prevalences of neuropathy and other microvascular complications, should be interpreted cautiously when being applied to general patients with diabetes.
However, the current guidelines for screening for DPN state that tests using a 128-Hz tuning fork and 10-g monofilament and the assessment of ankle reflexes are sufficient to detect DPN because combinations of more than one test have 87% sensitivity for detecting DPN [2]. We investigated the clinical usefulness of CPT compared with the neuropathic symptom score and conventional tests for diabetic neuropathy in a relatively large number of patients with diabetes. To the best of our knowledge, this study is the first to analyze the association between CPT and CAN using spectral analysis in patients with diabetes.
We conclude that although the CPT at each frequency is significantly associated with neuropathic symptoms or signs corresponding to the nerve fiber stimulated, the CPT provides little additional information compared with conventional neuropathic evaluations. Furthermore, the current reference range of the CPT should be re-evaluated because of its low sensitivity for detecting diabetic neuropathy.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, Feldman E, Iverson DJ, Perkins B, Russell JW, Zochodne D American Academy of Neurology; American Association of Neuromuscular and Electrodiagnostic Medicine; American Academy of Physical Medicine and Rehabilitation. Evidence-based guideline: treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76:1758–1765. doi: 10.1212/WNL.0b013e3182166ebe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association. Standards of medical care in diabetes: 2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 4.Rendell MS, Dovgan DJ, Bergman TF, O'Donnell GP, Drobny EP, Katims JJ. Mapping diabetic sensory neuropathy by current perception threshold testing. Diabetes Care. 1989;12:636–640. doi: 10.2337/diacare.12.9.636. [DOI] [PubMed] [Google Scholar]
- 5.Pitei DL, Watkins PJ, Stevens MJ, Edmonds ME. The value of the neurometer in assessing diabetic neuropathy by measurement of the current perception threshold. Diabet Med. 1994;11:872–876. doi: 10.1111/j.1464-5491.1994.tb00371.x. [DOI] [PubMed] [Google Scholar]
- 6.Cheng WY, Jiang YD, Chuang LM, Huang CN, Heng LT, Wu HP, Tai TY, Lin BJ. Quantitative sensory testing and risk factors of diabetic sensory neuropathy. J Neurol. 1999;246:394–398. doi: 10.1007/s004150050370. [DOI] [PubMed] [Google Scholar]
- 7.Nather A, Neo SH, Chionh SB, Liew SC, Sim EY, Chew JL. Assessment of sensory neuropathy in diabetic patients without diabetic foot problems. J Diabetes Complications. 2008;22:126–131. doi: 10.1016/j.jdiacomp.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Matsutomo R, Takebayashi K, Aso Y. Assessment of peripheral neuropathy using measurement of the current perception threshold with the neurometer in patients with type 2 diabetes mellitus. J Int Med Res. 2005;33:442–453. doi: 10.1177/147323000503300410. [DOI] [PubMed] [Google Scholar]
- 9.Masson EA, Veves A, Fernando D, Boulton AJ. Current perception thresholds: a new, quick, and reproducible method for the assessment of peripheral neuropathy in diabetes mellitus. Diabetologia. 1989;32:724–728. doi: 10.1007/BF00274531. [DOI] [PubMed] [Google Scholar]
- 10.Bastyr EJ, 3rd, Price KL, Bril V MBBQ Study Group. Development and validity testing of the neuropathy total symptom score-6: questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy. Clin Ther. 2005;27:1278–1294. doi: 10.1016/j.clinthera.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–1065. [PubMed] [Google Scholar]
- 12.Neurotron, Incorporated Innovative Medical Technology: Neurometer® CPT® Painless Electrodiagnostic Clinical and Laboratory Sensory Nerve Testing Equipment. [updated 2013 Dec 2]. Available from: http://www.neurotron.com/downloads/Professional_Feasibility_Report_Neurology.pdf.
- 13.Veves A, Young MJ, Manes C, Boulton AJ. Differences in peripheral and autonomic nerve function measurements in painful and painless neuropathy. A clinical study. Diabetes Care. 1994;17:1200–1202. doi: 10.2337/diacare.17.10.1200. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention: Age-adjusted incidence of end-stage renal disease related to diabetes mellitus (ESRD-DM) per 100,000 diabetic population, by race, ethnicity, and sex, United States, 1980-2008. [updated 2013 Oct 21]. Available from: http://www.cdc.gov/diabetes/statistics/esrd/fig5.htm.
- 15.Liu Z, Fu C, Wang W, Xu B. Prevalence of chronic complications of type 2 diabetes mellitus in outpatients: a cross-sectional hospital based survey in urban China. Health Qual Life Outcomes. 2010;8:62. doi: 10.1186/1477-7525-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Legrady P, Bajcsi D, Lengyel C, Varkonyi TT, Fejes I, Kempler P, Abraham G. Investigation of cardiac autonomic and peripheral sensory neuropathy in diabetic and nondiabetic patients with hypertension. Clin Exp Hypertens. 2013;35:465–469. doi: 10.3109/10641963.2012.758272. [DOI] [PubMed] [Google Scholar]
- 17.Keresztes K, Istenes I, Folhoffer A, Lakatos PL, Horvath A, Csak T, Varga P, Kempler P, Szalay F. Autonomic and sensory nerve dysfunction in primary biliary cirrhosis. World J Gastroenterol. 2004;10:3039–3043. doi: 10.3748/wjg.v10.i20.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Putz Z, Tabak AG, Toth N, Istenes I, Nemeth N, Gandhi RA, Hermanyi Z, Keresztes K, Jermendy G, Tesfaye S, Kempler P. Noninvasive evaluation of neural impairment in subjects with impaired glucose tolerance. Diabetes Care. 2009;32:181–183. doi: 10.2337/dc08-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G, Malik RA, Spallone V, Vinik A, Bernardi L, Valensi P Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]