Abstract
Background
Acute hyperglycemia in the perioperative period is associated with significantly increased complications. In few human studies the effects of propofol and inhalational anesthetic on the glucose metabolism were compared. In this study we evaluated the effect of propofol and isoflurane on blood glucose during abdominal hysterectomy in diabetic patients.
Methods
After approval by the Ethical Committee and written informed consent, thirty 35 to 65 years old diabetic women underwent for elective abdominal hysterectomy under general anesthesia were studied in this randomized single blind clinical trial study. The plasma glucose was maintained at 100 to 180 mg/dL during the operation. Anesthesia protocol was similar in two groups except maintenance of anesthesia that was with infusion of propofol in the propofol group and with isoflurane in the isoflurane group. Blood glucose level and the rate of insulin intake during surgery compared between two groups.
Results
Mean blood glucose before induction of anesthesia did not have significant difference between two groups, but 60 and 90 minutes after starting the operation blood glucose in the propofol group was significantly lower than isoflurane group. Also with using Repeated Measure test, two groups was significantly different according to blood glucose (P=0.045). Mean of administration of insulin during the surgery did not have significant difference between two groups by using repeated measure test and P=0.271. Also mean of bispectral index in different times during the surgery between two groups didn't have significant difference (P=0.35 repeated measure test).
Conclusion
Blood glucose increased during maintenance of anesthesia with isoflurane compared to propofol during the surgery.
Keywords: Blood glucose, Isoflurane, Propofol
INTRODUCTION
One of the most important metabolic reactions during surgery is resistance to insulin and hyperglycemia [1]. Hyperglycemia in the perioperative period is due to stress hormones such as epinephrine, cortisol, and inflammatory mediators. Even short-term Hyperglycemia causes immunosuppression and is associated with significantly increased infectious complications and patient mortality [2]. Acute hyperglycemia during surgery worsens prognosis even in the patients who had normal glucose tolerance test [3,4]. The effect of surgical stress on the blood glucose during the surgery is more prominent in diabetic patients. Hyperglycemic reaction to the surgical stress is more during inhalation anesthesia. Some studies showed impaired glucose tolerance and hyperglycemia even without surgical stress during isoflurane anesthesia [5,6]. In few human studies the effects of propofol and inhalational anesthetic on the glucose metabolism were compared [7,8]. In this study we evaluated the effect of propofol and isoflurane on concentration of plasma glucose during abdominal hysterectomy in diabetic patients.
METHODS
After approval by the Ethical Committee and written informed consent, 30 women were studied in this randomized single blind clinical trial study. Patients aged 35 to 65 years old with American Society of Anesthesia physical status II underwent for elective abdominal hysterectomy under general anesthesia were studied. The study was registered in the Iranian registry of clinical trials (http://irct.ir); IRCT201203032963N5. Patients with Renal failure due to diabetic nephropathy, severe liver disease, ischemic heart disease, history of allergy to propofol or the patients who received any medications known to affect blood glucose or insulin release; i.e., steroids, β adrenergic blocking agents at least 1 week preoperatively were excluded from the study. All the patients received insulin before the surgery as plasma glucose at the night before the operation and fasting blood glucose in the morning were <200 mg/dL. All the patients received 2/3 of their neutral protamine Hagedorn and regular insulin at the night before the operation but they did not receive insulin in the morning of the operation day. Plasma glucose checked preoperatively and recorded, and then infusion of glucose 5%+0.45% normal saline in the rate of 100 mL/hr was started. Also regular insulin infused in the rate of 0.02 IU/kg/hr during the surgery. Regulation of blood glucose was done based on the protocol in Table 1. This protocol is obtained from a national guideline for inpatient blood glucose control [9]. The rate of infusion of glucose 5%+0.45% normal saline was 100 mL/hr and the rate of infusion of regular insulin was based on the plasma glucose. The plasma glucose was maintained at 100 to 180 mg/dL during the operation. Blood glucose and the rate of insulin intake were checked before the induction of anesthesia, after surgical incision, and then every 30 minutes during the operation. Blood glucose was checked by the glucometer (Accu-chek; Roche Diagnostics GmbH, Mannheim, Germany). Electrocardiograms, pulse oximetry, bispectral index (BIS), capnography, and noninvasive blood pressure monitored during surgery. All patients were premedicated with midazolam 2 mg, 30 minutes before the operation. Induction of anaesthesia was with propofol (2 mg/kg IV) and after administration of atracurium (0.5 mg/kg IV), laryngoscopy and tracheal intubation was performed. The patients assigned into one of the two groups according to random-number table. Maintenance of anesthesia was with infusion of propofol (100 µg/kg/min) in the propofol group (group P) and with isoflurane 0.5-1 MAC (minimum alveolar concentration) in the isoflurane group (group I). Fentanyl (2 µg/kg IV) was administered two minutes before laryngoscopy and intubation. The 0.1 mg/kg morphine (IV) was injected to the patients after intubation of trachea. The patients received 50% N2O in oxygen (O2) during the operation. BIS was maintained between 40 to 60 during the operation. At the end of the operation residual neuromascular blockade was reversed with neostigmine (0.04 mg/kg IV) and atropine (0.02 mg/kg IV). All data analyzed using SPSS version 15 (SPSS Inc., Chicago, IL, USA) and statistical tests. Blood glucose level, the rate of insulin intake compared between two groups by using t-test and repeated measure. P<0.05 was considered significant.
Table 1.
The rate of infusion of insulin based on concentration of blood glucose

RESULTS
Thirty-eight patients entered in this study but eight of them were excluded and finally 30 patients were compared between two groups (15 patients in propofol group and 15 patients in isoflurane group).
Mean age was 56.73±5.28 years in the propofol group and 56.93±6.68 years in the isoflurane group (P=0.59). Mean weight for the propofol group was 69.46±11.88 and 75.73±13.20 kg for the isoflurane group (P=0.184). Two groups did not have significant differences according to age and weight.
Mean blood glucose before induction of anesthesia did not have significant difference between two groups, but 60 and 90 minutes after starting the operation blood glucose in the propofol group was significantly lower than isoflurane group (Table 2). Also with using repeated measure test, two groups was significantly different according to blood glucose at different times during the operation (P=0.045). Table 3 shows the differences between blood glucose at different times in comparison to the preoperative value in each group. In isoflurane group blood glucose increased significantly 60 and 90 minutes after starting the operation in comparison to preoperative value but in propofol group blood glucose didn't change during the surgery (Table 3). Mean administration of insulin during the surgery was not significantly different between study groups (P=0.271). Also mean of BIS in different times during the surgery between had no significant difference (P=0.35).
Table 2.
Mean of blood glucose in different times in two groups
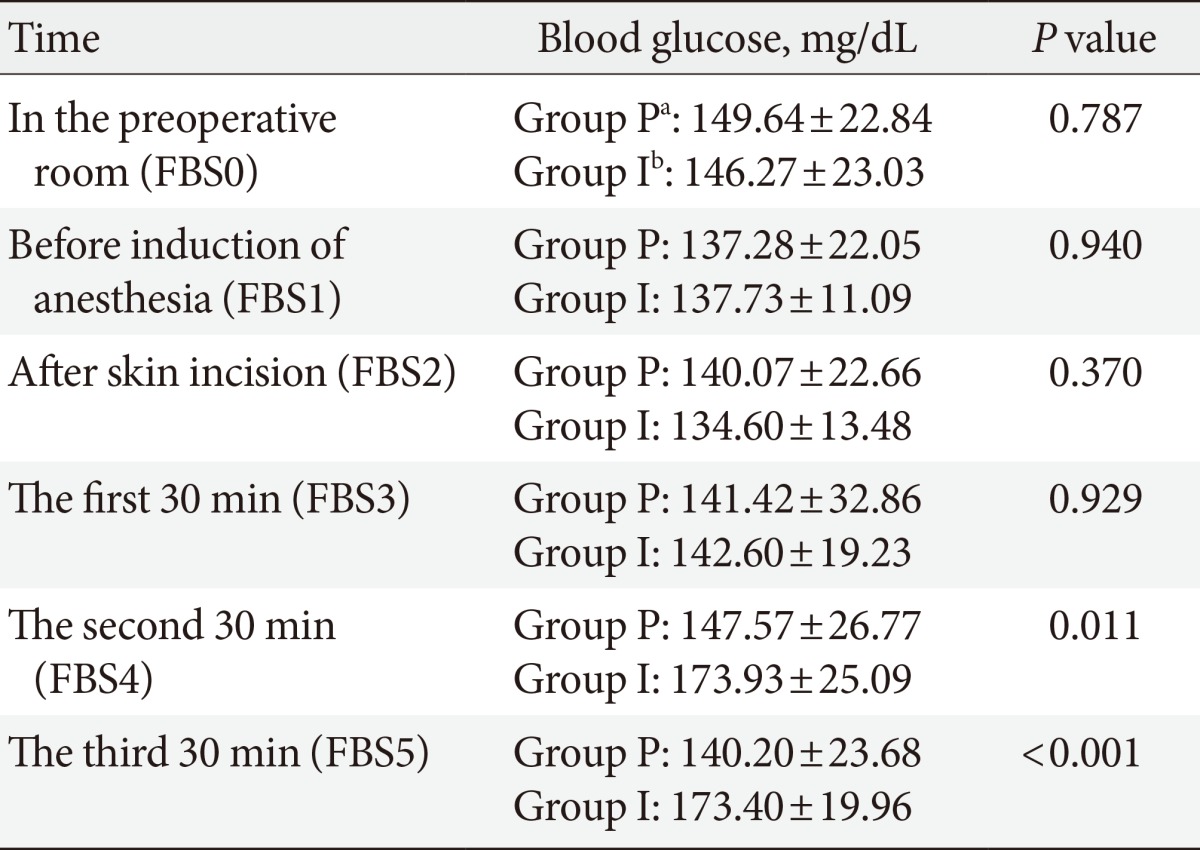
Values are presented as mean±standard deviation.
FBS, fasting blood sugar.
aGroup P: propofol, bGroup I: isoflurane
Table 3.
The differences of blood glucoses at different times in comparison to preoperative value in two groups
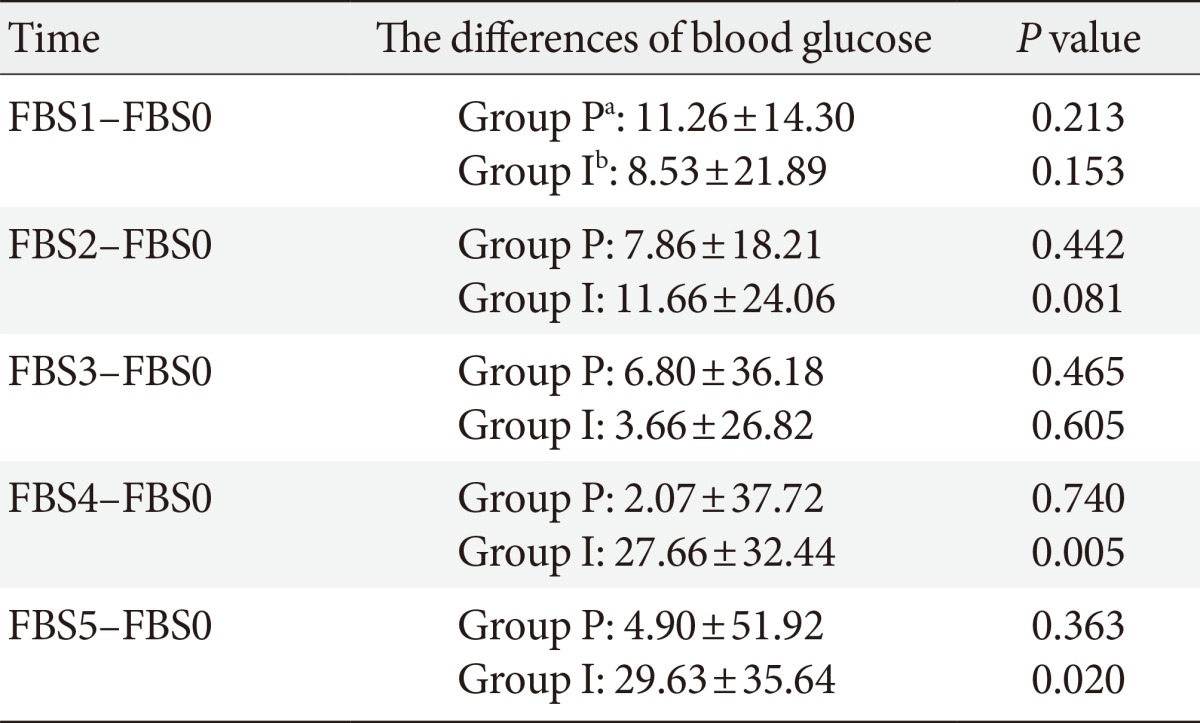
Values are presented as mean±standard deviation.
FBS, fasting blood sugar; FBS0, preoperative room; FBS1, before induction of anesthesia; FBS2, after skin incision; FBS3, the first 30 minutes; FBS4, the second 30 minutes; FBS5, the third 30 minutes.
aGroup P: propofol, bGroup I: isoflurane.
DISCUSSION
Results of present study showed that blood glucose increases during isoflurane anesthesia in the patients undergoing abdominal hysterectomy but anesthesia with propofol maintain blood glucose without changing during the surgery.
Increasing secretion of adrenaline, noradrenaline, and resistance to insulin due to surgical stress causes hyperglycemia. Even short term hyperglycemia is associated with increasing infectious complications, exacerbation of inflammatory reactions, and poor surgical outcome. The results of Turina et al. [2] study showed that monocyte human leukocyte antigens-DR (HLA-DR) expression is correlated with infection and patients' mortality. They showed that hyperglycemia during the surgery is associated with decreased monocyte HLA-DR expression and increasing infection and mortality in surgical patients [2]. Tanaka et al. [10] showed that insulin secretion and glucose utilization are impaired during isoflurane anesthesia even without surgical stress. Also hyperglycemia during craniotomy under isoflurane anesthesia was shown in Cok et al. [11] study. According to the results of the Lattermann et al. [12] study increased blood glucose during isoflurane anesthesia is due to impaired glucose clearance and increased production of glucose. In Diltoer and Camu [13] study, the effect of isoflurane anesthesia on glucose tolerance test was evaluated in two states of with or without surgical stress. This study concluded that growth hormone and norepinephrine concentrations increases and insulin secretion in response to hyperglycemia impaired during isoflurane anesthesia without surgical stress, but during surgery under isoflurane anesthesia cortisol, growth hormone, norepinephrine, and epinephrine concentrations increases and due to insulin resistance and/or increased production of glucose, glucose tolerance impaired more [13]. Akavipat et al. [14] study demonstrated that isoflurane causes hyperglycemia during surgery because of incomplete control of metabolic response to surgical stress during inhalation anesthesia by isoflurane.
Tanaka et al. [15] evaluated the Mechanisms of impaired glucose tolerance and insulin secretion during isoflurane anesthesia. The results showed that isoflurane anesthesia impairs adenosine triphosphate-sensitive potassium channel activity in pancreatic β-cells; so secretion of insulin decreases and hyperglycemia occurs [15]. Schricker et al. [16] demonstrated that enough analgesia with remifentanil or epidural analgesia can prevent hyperglycemia during the surgery. So it can be concluded that hyperglycemia during inhalation anesthesia may be due to surgical pain and stimulation of sympathic system. The results of Baldini et al. [17] study confirm this concept as this study showed that the depth of anesthesia with desflurane has no effect on the blood glucose during the surgery and even deep anesthesia with desflurane doesn't prevent hyperglycemia. In fact the reason of hyperglycemia during surgery may be surgical pain and metabolic response to surgical stress that even deep anesthesia cannot block these responses, but with enough analgesia we can maintain blood glucose in normal limits and prevent hyperglycemia and its complications during perioperative period. The results of Lattermann et al. [18,19] studies confirm this concept too. In these studies epidural analgesia during general anesthesia could prevent hyperglycemia in the patients during the surgery. In another study Lattermann et al. [20] concluded that combined spinal-epidural technique can prevent hyperglycemia compared to general anesthesia in the patients undergoing surgery.
In few human studies the effect of propofol on blood glucose during surgery was evaluated. Jeong et al. [21] studied on the effect of propofol and enflurane on blood glucose. This study showed that propofol maintains normoglycemia during surgery compared to enflurane. Yasuda et al. [22] studied about insulin resistance during propofol anesthesia in rat. They concluded that propofol induces systemic insulin resistance and decreased glucose uptake in skeletal and heart muscle. Also the results of this study showed that hepatic glucose output increases during propofol anesthesia in rats [22]. In this study rats received propofol without opioids analgesics, but in Zuurbier et al. [23] study, anesthesia with combination of sufentanil-propofol-morphine in rats prevented hyperglycemia comparing to inhalation anesthesia. Differences in these two animal studies may be because of using opiods in the Zuurbier et al. [23] study. Kitamura et al. [7] evaluated the effect of propofol on the glucose metabolism compared to sevoflurane in rats and concluded enough doses of propofol prevent hyperglycemia in rats in comparison to sevoflurane. Also this study showed that adding buprenorphine to propofol prevents hyperglycemia if enough doses of propofol is not used in rat. In fact hyperglycemia could be prevented with using a potent analgesic with low dose propofol but it is not true about sevoflurane. In another study, Kitamura et al. [8] compared concentration of blood glucose during propofol and sevoflurane anesthesia. This study suggested the increased level of blood glucose in sevoflurane anesthesia compared to anesthesia with propofol. This human study confirms our results in present study that the effect of propofol on glucose metabolism is less than inhalational anesthetics. Zhu et al. [24] study showed that propofol protects endothelial cells against hyperglycemia induced insults. Ishii et al. [25] studied the mechanism of the protective effect of propofol on the cerebral cells during ischemia. This animal study showed that cerebral cell injury due to ischemia is exaggerated during hyperglycemia. This effect of hyperglycemia on the function of cerebral cells during ischemia is due to lactic acidosis and its effect on cellular mitochondria. The results of Ishii et al. [25] study showed that propofol prevents lactic acidosis and its impacts on the cerebral cells. Propofol also decrease cerebral edema and cell injury after period of ischemia. Nakahata et al. [26] study concluded that hyperglycemia impairs on dilatation of cerebral arteriols and enough doses of propofol improve function of cerebral microvasculature due to decreasing level of superoxides in the brain.
This study is done on diabetic women were candidate for abdominal hysterectomy. Although by this way we tried to avoid gender bias but the results may be different in men or in other surgeries and further studies are necessary to confirm such efficacies among all diabetic patients and all surgeries.
In conclusion the results of present study confirmed the other few human studies that propofol prevent increasing in blood glucose during the surgery in comparison to isoflurane anesthesia. According to the results of the present study and the other similar studies and the protective effects of propofol on the ischemic cerebral cells it is reasonable to use propofol for general anesthesia in diabetic patients. More clinical trials are needed to confirm this issue.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the financial support for this work that was provided by Shahid Sadoghi University of Medical Sciences, Yazd, Iran as dissertation of Dr. Abolghasem Mortazavizadeh to be graduated as anesthesiologist. We also are thankful to Mrs. Sousan Mahabadi because of her help in collection of data in this project.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ljungqvist O, Jonathan E. Rhoads lecture 2011: insulin resistance and enhanced recovery after surgery. JPEN J Parenter Enteral Nutr. 2012;36:389–398. doi: 10.1177/0148607112445580. [DOI] [PubMed] [Google Scholar]
- 2.Turina M, Miller FN, Tucker CF, Polk HC. Short-term hyperglycemia in surgical patients and a study of related cellular mechanisms. Ann Surg. 2006;243:845–851. doi: 10.1097/01.sla.0000220041.68156.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Puskas F, Grocott HP, White WD, Mathew JP, Newman MF, Bar-Yosef S. Intraoperative hyperglycemia and cognitive decline after CABG. Ann Thorac Surg. 2007;84:1467–1473. doi: 10.1016/j.athoracsur.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 4.Bochicchio GV, Sung J, Joshi M, Bochicchio K, Johnson SB, Meyer W, Scalea TM. Persistent hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;58:921–924. doi: 10.1097/01.ta.0000162141.26392.07. [DOI] [PubMed] [Google Scholar]
- 5.Oyama T, Latto P, Holaday DA. Effect of isoflurane anaesthesia and surgery on carbohydrate metabolism and plasma cortisol levels in man. Can Anaesth Soc J. 1975;22:696–702. doi: 10.1007/BF03013318. [DOI] [PubMed] [Google Scholar]
- 6.Horber FF, Krayer S, Miles J, Cryer P, Rehder K, Haymond MW. Isoflurane and whole body leucine, glucose, and fatty acid metabolism in dogs. Anesthesiology. 1990;73:82–92. doi: 10.1097/00000542-199007000-00013. [DOI] [PubMed] [Google Scholar]
- 7.Kitamura T, Ogawa M, Kawamura G, Sato K, Yamada Y. The effects of sevoflurane and propofol on glucose metabolism under aerobic conditions in fed rats. Anesth Analg. 2009;109:1479–1485. doi: 10.1213/ANE.0b013e3181b8554a. [DOI] [PubMed] [Google Scholar]
- 8.Kitamura T, Kawamura G, Ogawa M, Yamada Y. Comparison of the changes in blood glucose levels during anesthetic management using sevoflurane and propofol. Masui. 2009;58:81–84. [PubMed] [Google Scholar]
- 9.Faghih-Imani E, Amini M, Amin-al-Roaya A, Rezvanian H, Kacouei A, Siavash M, Khalili N Diabetes and Metabolism Research Center, Isfahan University of Medical Sciences. Clinical guideline for inpatient blood glucose control. Isafahan: Isfahan University of Medical Sciences publications; 2009. [Google Scholar]
- 10.Tanaka T, Nabatame H, Tanifuji Y. Insulin secretion and glucose utilization are impaired under general anesthesia with sevoflurane as well as isoflurane in a concentration-independent manner. J Anesth. 2005;19:277–281. doi: 10.1007/s00540-005-0341-1. [DOI] [PubMed] [Google Scholar]
- 11.Cok OY, Ozkose Z, Pasaoglu H, Yardim S. Glucose response during craniotomy: propofol-remifentanil versus isoflurane-remifentanil. Minerva Anestesiol. 2011;77:1141–1148. [PubMed] [Google Scholar]
- 12.Lattermann R, Schricker T, Wachter U, Georgieff M, Goertz A. Understanding the mechanisms by which isoflurane modifies the hyperglycemic response to surgery. Anesth Analg. 2001;93:121–127. doi: 10.1097/00000539-200107000-00026. [DOI] [PubMed] [Google Scholar]
- 13.Diltoer M, Camu F. Glucose homeostasis and insulin secretion during isoflurane anesthesia in humans. Anesthesiology. 1988;68:880–886. doi: 10.1097/00000542-198806000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Akavipat P, Polsayom N, Pannak S, Punkla W. Blood glucose level in neurosurgery. Is it different between isoflurane and desflurane anesthesia? Acta Med Indones. 2009;41:121–125. [PubMed] [Google Scholar]
- 15.Tanaka K, Kawano T, Tomino T, Kawano H, Okada T, Oshita S, Takahashi A, NaKaya Y. Mechanisms of impaired glucose tolerance and insulin secretion during isoflurane anesthesia. Anesthesiology. 2009;111:1044–1051. doi: 10.1097/ALN.0b013e3181bbcb0d. [DOI] [PubMed] [Google Scholar]
- 16.Schricker T, Galeone M, Wykes L, Carli F. Effect of desflurane/remifentanil anaesthesia on glucose metabolism during surgery: a comparison with desflurane/epidural anaesthesia. Acta Anaesthesiol Scand. 2004;48:169–173. doi: 10.1111/j.0001-5172.2004.00297.x. [DOI] [PubMed] [Google Scholar]
- 17.Baldini G, Bagry H, Carli F. Depth of anesthesia with desflurane does not influence the endocrine-metabolic response to pelvic surgery. Acta Anaesthesiol Scand. 2008;52:99–105. doi: 10.1111/j.1399-6576.2007.01470.x. [DOI] [PubMed] [Google Scholar]
- 18.Lattermann R, Carli F, Wykes L, Schricker T. Epidural blockade modifies perioperative glucose production without affecting protein catabolism. Anesthesiology. 2002;97:374–381. doi: 10.1097/00000542-200208000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Lattermann R, Carli F, Wykes L, Schricker T. Perioperative glucose infusion and the catabolic response to surgery: the effect of epidural block. Anesth Analg. 2003;96:555–562. doi: 10.1097/00000539-200302000-00047. [DOI] [PubMed] [Google Scholar]
- 20.Lattermann R, Belohlavek G, Wittmann S, Fuchtmeier B, Gruber M. The anticatabolic effect of neuraxial blockade after hip surgery. Anesth Analg. 2005;101:1202–1208. doi: 10.1213/01.ane.0000167282.65352.e7. [DOI] [PubMed] [Google Scholar]
- 21.Jeong JS, Oh SW, Koo GH. Comparison of effects of propofol and enflurane on blood glucose level. Korean J Anesthesiol. 1998;34:323–328. [Google Scholar]
- 22.Yasuda Y, Fukushima Y, Kaneki M, Martyn JA. Anesthesia with propofol induces insulin resistance systemically in skeletal and cardiac muscles and liver of rats. Biochem Biophys Res Commun. 2013;431:81–85. doi: 10.1016/j.bbrc.2012.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuurbier CJ, Keijzers PJ, Koeman A, Van Wezel HB, Hollmann MW. Anesthesia's effects on plasma glucose and insulin and cardiac hexokinase at similar hemodynamics and without major surgical stress in fed rats. Anesth Analg. 2008;106:135–142. doi: 10.1213/01.ane.0000297299.91527.74. [DOI] [PubMed] [Google Scholar]
- 24.Zhu M, Chen J, Jiang H, Miao C. Propofol protects against high glucose-induced endothelial adhesion molecules expression in human umbilical vein endothelial cells. Cardiovasc Diabetol. 2013;12:13. doi: 10.1186/1475-2840-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishii H, Arai T, Segawa H, Morikawa S, Inubushi T, Fukuda K. Effects of propofol on lactate accumulation and oedema formation in focal cerebral ischaemia in hyperglycaemic rats. Br J Anaesth. 2002;88:412–417. doi: 10.1093/bja/88.3.412. [DOI] [PubMed] [Google Scholar]
- 26.Nakahata K, Kinoshita H, Azma T, Matsuda N, Hama-Tomioka K, Haba M, Hatano Y. Propofol restores brain microvascular function impaired by high glucose via the decrease in oxidative stress. Anesthesiology. 2008;108:269–275. doi: 10.1097/01.anes.0000299830.13203.60. [DOI] [PubMed] [Google Scholar]


