Abstract
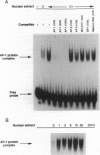
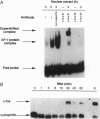
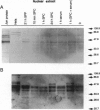
Sphingosylphosphocholine (SPC) is the deacylated derivative of sphingomyelin known to accumulate in neuropathic Niemann-Pick disease type A. SPC is a potent mitogen that increases intracellular free Ca2+ and free arachidonate through pathways that are only partly protein kinase C-dependent. Here we show that SPC increased specific DNA-binding activity of transcription activator AP-1 in electrophoretic mobility-shift assays. Increased DNA-binding activity of AP-1 was detected after only 1-3 min, was maximal after 6 hr, and remained elevated at 12-24 hr. c-Fos was found to be a component of the AP-1 complex. Northern hybridization revealed an increase in c-fos transcripts after 30 min. Since the increase in AP-1 binding activity preceded the increase in c-fos mRNA, posttranslational modifications may be important in mediating the early SPC-induced increases in AP-1 DNA-binding activity. Western analysis detected increases in nuclear c-Jun and c-Fos proteins following SPC treatment. SPC also transactivated a reporter gene construct through the AP-1 recognition site, indicating that SPC can regulate the expression of target genes. Thus, SPC-induced cell proliferation may result from activation of AP-1, linking signal transduction by SPC to gene expression. Since the expression of many proteins with diverse functions is known to be regulated by AP-1, SPC-induced activation of AP-1 may contribute to the pathophysiology of Niemann-Pick disease.
Full text
PDF
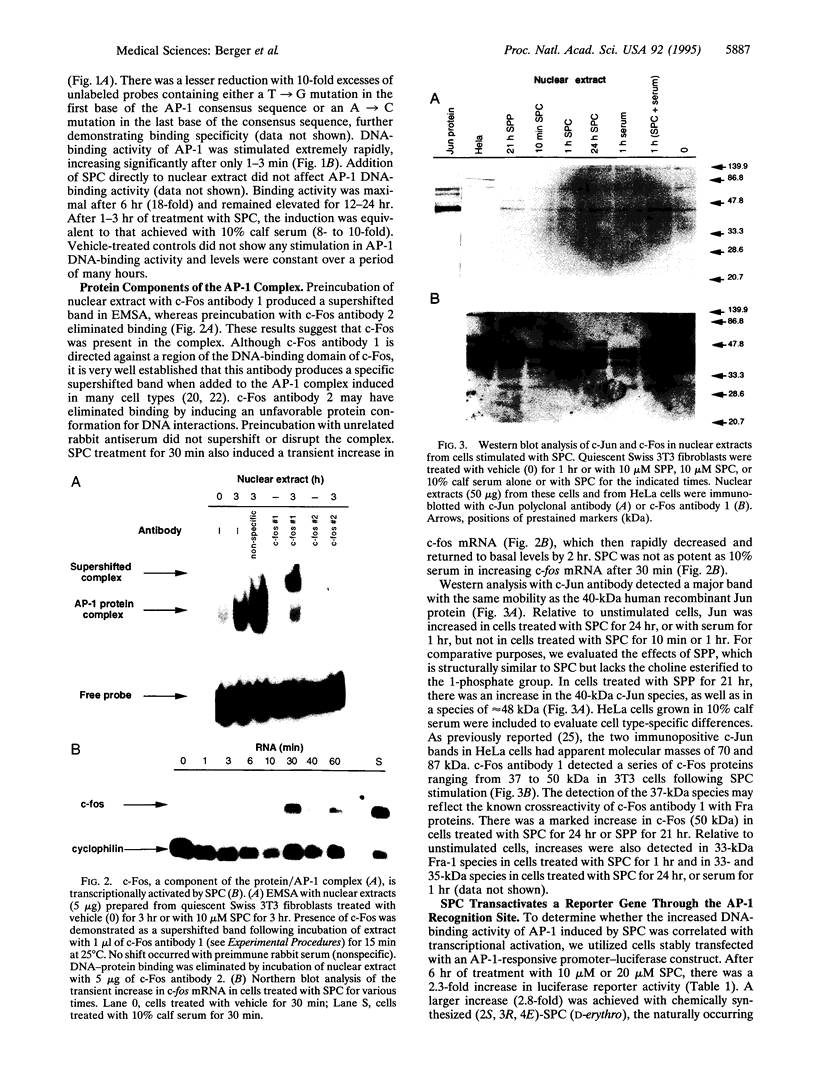
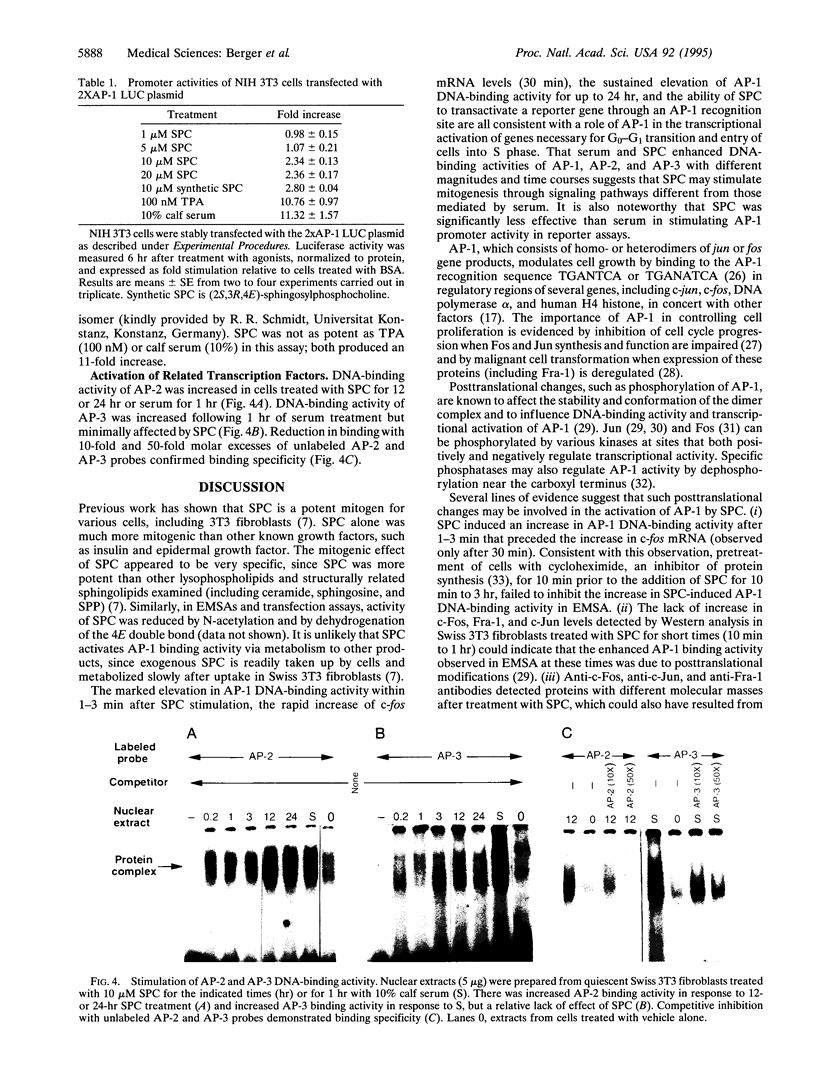

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein L. R., Ferris D. K., Colburn N. H., Sobel M. E. A family of mitogen-activated protein kinase-related proteins interacts in vivo with activator protein-1 transcription factor. J Biol Chem. 1994 Apr 1;269(13):9401–9404. [PubMed] [Google Scholar]
- Chiles T. C., Liu J. L., Rothstein T. L. Cross-linking of surface Ig receptors on murine B lymphocytes stimulates the expression of nuclear tetradecanoyl phorbol acetate-response element-binding proteins. J Immunol. 1991 Mar 15;146(6):1730–1735. [PubMed] [Google Scholar]
- Desai N. N., Carlson R. O., Mattie M. E., Olivera A., Buckley N. E., Seki T., Brooker G., Spiegel S. Signaling pathways for sphingosylphosphorylcholine-mediated mitogenesis in Swiss 3T3 fibroblasts. J Cell Biol. 1993 Jun;121(6):1385–1395. doi: 10.1083/jcb.121.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai N. N., Spiegel S. Sphingosylphosphorylcholine is a remarkably potent mitogen for a variety of cell lines. Biochem Biophys Res Commun. 1991 Nov 27;181(1):361–366. doi: 10.1016/s0006-291x(05)81427-5. [DOI] [PubMed] [Google Scholar]
- Dérijard B., Hibi M., Wu I. H., Barrett T., Su B., Deng T., Karin M., Davis R. J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994 Mar 25;76(6):1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Gabellini N., Facci L., Milani D., Negro A., Callegaro L., Skaper S. D., Leon A. Differences in induction of c-fos transcription by cholera toxin-derived cyclic AMP and Ca2+ signals in astrocytes and 3T3 fibroblasts. Exp Cell Res. 1991 Jun;194(2):210–217. doi: 10.1016/0014-4827(91)90356-y. [DOI] [PubMed] [Google Scholar]
- Gauthier-Rouvière C., Basset M., Blanchard J. M., Cavadore J. C., Fernandez A., Lamb N. J. Casein kinase II induces c-fos expression via the serum response element pathway and p67SRF phosphorylation in living fibroblasts. EMBO J. 1991 Oct;10(10):2921–2930. doi: 10.1002/j.1460-2075.1991.tb07842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Bell R. M. Lysosphingolipids inhibit protein kinase C: implications for the sphingolipidoses. Science. 1987 Feb 6;235(4789):670–674. doi: 10.1126/science.3101176. [DOI] [PubMed] [Google Scholar]
- Kennett F. F., Weglicki W. B. Effects of well-defined ischemia on myocardial lysosomal and microsomal enzymes in a canine model. Circ Res. 1978 Nov;43(5):750–758. doi: 10.1161/01.res.43.5.750. [DOI] [PubMed] [Google Scholar]
- Kindman L. A., Kim S., McDonald T. V., Gardner P. Characterization of a novel intracellular sphingolipid-gated Ca(2+)-permeable channel from rat basophilic leukemia cells. J Biol Chem. 1994 May 6;269(18):13088–13091. [PubMed] [Google Scholar]
- Kovary K., Bravo R. Existence of different Fos/Jun complexes during the G0-to-G1 transition and during exponential growth in mouse fibroblasts: differential role of Fos proteins. Mol Cell Biol. 1992 Nov;12(11):5015–5023. doi: 10.1128/mcb.12.11.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Skelly H., Botteri F., van der Putten H., Barber J. R., Verma I. M., Leffert H. L. Proto-oncogene expression in regenerating liver is simulated in cultures of primary adult rat hepatocytes. J Biol Chem. 1986 Jun 15;261(17):7929–7933. [PubMed] [Google Scholar]
- Lee K. J., Hickey R., Zhu H., Chien K. R. Positive regulatory elements (HF-1a and HF-1b) and a novel negative regulatory element (HF-3) mediate ventricular muscle-specific expression of myosin light-chain 2-luciferase fusion genes in transgenic mice. Mol Cell Biol. 1994 Feb;14(2):1220–1229. doi: 10.1128/mcb.14.2.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. S., Chen J. C., Sheikh M. S., Shao Z. M., Fontana J. A. Retinoic acid inhibition of insulin-like growth factor I stimulation of c-fos mRNA levels in a breast carcinoma cell line. Exp Cell Res. 1994 Mar;211(1):68–73. doi: 10.1006/excr.1994.1060. [DOI] [PubMed] [Google Scholar]
- Mattie M., Brooker G., Spiegel S. Sphingosine-1-phosphate, a putative second messenger, mobilizes calcium from internal stores via an inositol trisphosphate-independent pathway. J Biol Chem. 1994 Feb 4;269(5):3181–3188. [PubMed] [Google Scholar]
- McDonald O. B., Hannun Y. A., Reynolds C. H., Sahyoun N. Activation of casein kinase II by sphingosine. J Biol Chem. 1991 Nov 15;266(32):21773–21776. [PubMed] [Google Scholar]
- Pepperkok R., Herr S., Lorenz P., Pyerin W., Ansorge W. System for quantitation of gene expression in single cells by computerized microimaging: application to c-fos expression after microinjection of anti-casein kinase II antibody. Exp Cell Res. 1993 Feb;204(2):278–285. doi: 10.1006/excr.1993.1034. [DOI] [PubMed] [Google Scholar]
- Perez P., Schönthal A., Aranda A. Repression of c-fos gene expression by thyroid hormone and retinoic acid receptors. J Biol Chem. 1993 Nov 5;268(31):23538–23543. [PubMed] [Google Scholar]
- Pushkareva MYu, Hannun Y. A. Modulation of cytosolic protein phosphorylation by sphingosylphosphorylcholine. Biochim Biophys Acta. 1994 Mar 10;1221(1):54–60. doi: 10.1016/0167-4889(94)90215-1. [DOI] [PubMed] [Google Scholar]
- Quinn J. P., Farina A. R., Gardner K., Krutzsch H., Levens D. Multiple components are required for sequence recognition of the AP1 site in the gibbon ape leukemia virus enhancer. Mol Cell Biol. 1989 Nov;9(11):4713–4721. doi: 10.1128/mcb.9.11.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Lafrasse C., Rousson R., Pentchev P. G., Louisot P., Vanier M. T. Free sphingoid bases in tissues from patients with type C Niemann-Pick disease and other lysosomal storage disorders. Biochim Biophys Acta. 1994 May 25;1226(2):138–144. doi: 10.1016/0925-4439(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Su Y., Rosenthal D., Smulson M., Spiegel S. Sphingosine 1-phosphate, a novel signaling molecule, stimulates DNA binding activity of AP-1 in quiescent Swiss 3T3 fibroblasts. J Biol Chem. 1994 Jun 10;269(23):16512–16517. [PubMed] [Google Scholar]
- Sugiyama E., Uemura K., Hara A., Taketomi T. Effects of various lysosphingolipids on cell growth, morphology and lipid composition in three neuroblastoma cell lines. Biochem Biophys Res Commun. 1990 Jun 15;169(2):673–679. doi: 10.1016/0006-291x(90)90383-x. [DOI] [PubMed] [Google Scholar]
- Sugiyama E., Uemura K., Hara A., Taketomi T. Metabolism and neurite promoting effect of exogenous sphingosylphosphocholine in cultured murine neuroblastoma cells. J Biochem. 1993 Apr;113(4):467–472. doi: 10.1093/oxfordjournals.jbchem.a124068. [DOI] [PubMed] [Google Scholar]
- Trejo J., Massamiri T., Deng T., Dewji N. N., Bayney R. M., Brown J. H. A direct role for protein kinase C and the transcription factor Jun/AP-1 in the regulation of the Alzheimer's beta-amyloid precursor protein gene. J Biol Chem. 1994 Aug 26;269(34):21682–21690. [PubMed] [Google Scholar]
- Tsuchiya H., Fujii M., Niki T., Tokuhara M., Matsui M., Seiki M. Human T-cell leukemia virus type 1 Tax activates transcription of the human fra-1 gene through multiple cis elements responsive to transmembrane signals. J Virol. 1993 Dec;67(12):7001–7007. doi: 10.1128/jvi.67.12.7001-7007.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törnquist K., Ekokoski E. Effect of sphingosine derivatives on calcium fluxes in thyroid FRTL-5 cells. Biochem J. 1994 Apr 1;299(Pt 1):213–218. doi: 10.1042/bj2990213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Foglesong R. J., Bell R. M. A facile enzymatic synthesis of sphingosine-1-phosphate and dihydrosphingosine-1-phosphate. J Lipid Res. 1989 Apr;30(4):611–616. [PubMed] [Google Scholar]
- Wong K., Kwan-Yeung L. Sphingosine mobilizes intracellular calcium in human neutrophils. Cell Calcium. 1993 Jun;14(6):493–505. doi: 10.1016/0143-4160(93)90008-t. [DOI] [PubMed] [Google Scholar]
- Young S. T., Porrino L. J., Iadarola M. J. Cocaine induces striatal c-fos-immunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yule D. I., Wu D., Essington T. E., Shayman J. A., Williams J. A. Sphingosine metabolism induces Ca2+ oscillations in rat pancreatic acinar cells. J Biol Chem. 1993 Jun 15;268(17):12353–12358. [PubMed] [Google Scholar]