Abstract
Background
Antisocial personality disorder (AsPD) is associated with a wide range of disturbance including persistent rule-breaking, criminality, substance misuse, unemployment, homelessness and relationship difficulties.
Objectives
To evaluate the potential beneficial and adverse effects of pharmacological interventions for people with AsPD.
Search methods
We searched the Cochrane Central Register of Controlled Trials (The Cochrane Library 2009, Issue 3), MEDLINE (1950 to September 2009), EMBASE (1980 to 2009, week 37), CINAHL (1982 to September 2009), PsycINFO (1872 to September 2009), ASSIA (1987 to September 2009), BIOSIS (1985 to September 2009), COPAC (September 2009), National Criminal Justice Reference Service Abstracts (1970 to July 2008), Sociological Abstracts (1963 to September 2009), ISI-Proceedings (1981 to September 2009), Science Citation Index (1981 to September 2009), Social Science Citation Index (1981 to September 2009), SIGLE (1980 to April 2006), Dissertation Abstracts (September 2009), ZETOC (September 2009) and the metaRegister of Controlled Trials (September 2009).
Selection criteria
Controlled trials in which participants with AsPD were randomly allocated to a pharmacological intervention and a placebo control condition. Two trials comparing one drug against another without a placebo control are reported separately.
Data collection and analysis
Three review authors independently selected studies. Two review authors independently extracted data. We calculated mean differences, with odds ratios for dichotomous data.
Main results
Eight studies met the inclusion criteria involving 394 participants with AsPD. Data were available from four studies involving 274 participants with AsPD. No study set out to recruit participants solely on the basis of having AsPD, and in only one study was the sample entirely of AsPD participants. Eight different drugs were examined in eight studies. Study quality was relatively poor. Inadequate reporting meant the data available were generally insufficient to allow any independent statistical analysis. The findings are limited to descriptive summaries based on analyses carried out and reported by the trial investigators. All the available data were derived from unreplicated single reports. Only three drugs (nortriptyline, bromocriptine, phenytoin) were effective compared to placebo in terms of improvement in at least one outcome. Nortriptyline was reported in one study as superior for men with alcohol dependency on mean number of drinking days and on alcohol dependence, but not for severity of alcohol misuse or on the patient’s or clinician’s rating of drinking. In the same study, both nortriptyline and bromocriptine were reported as superior to placebo on anxiety on one scale but not on another. In one study, phenytoin was reported as superior to placebo on the frequency and intensity of aggressive acts in male prisoners with impulsive (but not premeditated) aggression. In the remaining two studies, both amantadine and desipramine were not superior to placebo for adults with opioid and cocaine dependence, and desipramine was not superior to placebo for men with cocaine dependence.
Authors’ conclusions
The body of evidence summarised in this review is insufficient to allow any conclusion to be drawn about the use of pharmacological interventions in the treatment of antisocial personality disorder.
Medical Subject Headings (MeSH): Aggression [drug effects], Alcohol-Related Disorders [drug therapy], Amantadine [therapeutic use], Antisocial Personality Disorder [*drug therapy], Anxiety [drug therapy], Bromocriptine [therapeutic use], Desipramine [therapeutic use], Nortriptyline [therapeutic use], Phenytoin [therapeutic use], Psychotropic Drugs [*therapeutic use], Randomized Controlled Trials as Topic
MeSH check words: Adult, Female, Humans, Male
BACKGROUND
Description of the condition
Antisocial personality disorder (AsPD) is a subcategory of personality disorder. The current edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; APA 1994) defines personality disorder as: “an enduring pattern of inner experience and behaviour that deviates markedly from the expectations of the person’s culture, is pervasive and inflexible, has an onset in adolescence or early adulthood, is stable over time, and leads to distress or impairment.” General criteria for personality disorder according to DSM-IV are given in Table 1 below.
Table 1. DSM-IV general criteria for personality disorder.
|
AsPD is identified by traits that include irresponsible and exploitive behaviour, recklessness, impulsivity, high negative emotionality and deceitfulness. In order to be diagnosed with AsPD according to the DSM-IV, a person must fulfil criteria A, B, C and D shown in Table 2 below as well as fulfilling general criteria for a personality disorder as outlined above.
Table 2. DSM-IV diagnostic criteria for AsPD (APA 1994).
|
Although the focus of this review is on AsPD, this condition is also often classified using the International Classification of Diseases - tenth edition (ICD-10; WHO 1992) as dissocial personality disorder (F60.2). AsPD and dissocial personality disorder are often used interchangeably by clinicians and they describe a very similar presentation. While there is considerable overlap between these two diagnostic systems, they differ in two respects. First, DSM-IV requires those meeting the diagnostic criteria also show evidence of conduct disorder with onset before the age of 15 years and there is no such requirement when making the diagnosis of dissocial personality disorder using ICD-10 criteria. However, Perdikouri 2007 did not find any clinically important differences when they compared subjects meeting full criteria for AsPD with those who otherwise fulfilled criteria for AsPD but who did not demonstrate evidence of childhood conduct disorder. Second, it has been argued that the criteria in ICD-10 are more reflective of the core personality traits of the antisocial with less emphasis on criminal behaviour.
Table 3 below shows the diagnostic criteria in ICD-10 for diagnosing dissocial personality disorder.
Table 3. ICD-10 diagnostic criteria for dissocial personality disorder (F60.2; WHO 1992).
|
Whilst estimates of the prevalence of AsPD in the general population vary across studies and countries, most studies report a prevalence of between 2% and 3% in the general population (Moran 1999). Prevalence rates are consistently higher in men compared with women. For instance, the lifetime prevalence in two North American studies was 4.5% among men and 0.8% among women (Robins 1991) and 6.8% among men and 0.8% in women (Swanson 1994). However, two European studies found lower prevalence rates (i.e. 1.3% in men and 0% in women (Torgensen 2001) and 1% in men and 0.2% in women (Coid 2006)). As would be expected AsPD is especially common in prison settings. In the UK prison population the prevalence of people with AsPD has been identified as 63% in male remand prisoners, 49% in male sentenced prisoners, and 31% in female prisoners (Singleton 1998).
The condition is associated with a wide range of disturbance and is associated with greatly increased rates of criminality, substance misuse, unemployment, homelessness and relationship difficulties. AsPD is generally associated with a negative long-term outcome. Many adults with AsPD are incarcerated in prison at some point during their life. Although follow up studies have demonstrated some improvement over time, particularly in rates of re-offending (Grilo 1998; Weissman 1993), men with AsPD who reduce their offending behaviour over time may nonetheless continue to have major problems in their interpersonal relationships (Paris 2003). Black 1996 found that men with AsPD aged less than 40 years had a strikingly high rate of premature death and obtained a value of 33 for the Standardized Mortality Rate (the age-adjusted ratio of observed deaths to expected deaths). This increased mortality was due not only to an increased rate of suicide, but was also associated with reckless behaviours such as drug misuse and aggression.
Significant comorbidity exists between AsPD and many other Axis I disorders: mood and anxiety disorders are common, although the most frequent co-occurrence is with substance misuse. Men with AsPD have been found to be three to five times more likely to abuse alcohol and illicit drugs than those without the disorder (Robins 1991). The presence of personality disorder co-occurring with an Axis I condition may have a negative impact on the outcome of the latter (Newton-Howes 2006; Skodol 2005).
Description of the intervention
It has been argued that people with personality disorders may respond to pharmacological interventions that target both their state and trait symptoms, highlighting the need to evaluate drug treatments that target the cognitive-perceptual, affective, impulsive-behavioural and anxious-fearful domains of personality disorder (Soloff 1998). A number of authors have reviewed the evidence relating to treatment of personality disorders with antidepressants, benzodiazepines, anticonvulsants, psychostimulants, antipsychotics and mood stabilisers (Dolan 1993; Lieb 2010; Stein 1992; Warren 2001).
Stein 1992 concluded that small doses of neuroleptics may afford some benefits for people with well-defined borderline and schizotypal personality disorders. Dolan 1993 argued that carbamazepine had been shown to improve overactivity, aggression and impulse control in psychopathic and antisocial personality disorders. They also concluded that lithium maintenance treatment may be of benefit to explosive and impulsive individuals, holding out some hope for those with AsPD. Warren 2001 concluded that selective serotonin reuptake inhibitor antidepressants (SSRIs) may improve personality disorder symptoms and anger. They noted, however, that the evidence for pharmacological intervention was very poor, since the studies included in their review suffered serious methodological limitations including small sample sizes, highly selected participants, high drop-out rates, short duration or lack of long-term follow-up.
Overall, these reviews found the evidence base for pharmacological interventions for AsPD to be weak, since the bulk of the studies reviewed had been restricted to individuals with borderline personality disorder (BPD). Therefore, it will be important to consider all relevant studies without restriction on the pharmaceutical agents, and to consider pharmacological interventions where drugs are given not only as monotherapy but also as an adjunctive intervention.
How the intervention might work
Several arguments have been put forward to justify drug treatment for personality disorders (Tyrer 2004), and there is a growing body of evidence that personality disorders are associated with neurochemical abnormalities, whether inherited or arising out of physical or psychological trauma (Coccaro 1998; Skodol 2002). One justification for the use of pharmacotherapy is that it has the potential to modulate neurotransmitter function and so may be able to correct imbalances in the central nervous system of people with personality disorder to a more normal neurochemical state (Markovitz 2004).
The main neurotransmitter system which may be implicated in AsPD is the serotonergic system (Coccaro 1996). For example, impulsive and aggressive features of the disorder have been linked to serotonergic system deficits (Sugden 2006). The serotonergic system has been found to be less responsive to pharmacological challenges (which increase serotonin levels in healthy individuals) in people with AsPD (Moss 1990), and brain activations following such challenges are reduced in AsPD subjects as demonstrated in functional imaging studies (Völlm 2010). The biological factors contributing to both antisocial behaviour and criminality may also include the under-arousal of the autonomic system (Raine 2000). An alternative approach (Soloff 1998) suggests that the likely impact of drugs on the primary symptoms in personality disorder can broadly be predicted from drug effects when used in Axis I disorders. On this basis, medication is matched to the primary symptom group, so that antipsychotic medication would be the preferred drug treatment for cognitive-perceptual symptoms, and mood stabilisers and SSRIs would be indicated for impulsive-behavioural dyscontrol.
In practice, there are reports of behavioural dyscontrol improving in response to lithium (Links 1990) and to anticonvulsants such as carbamazepine (Cowdry 1989), sodium valproate (Stein 1995) and divalproex sodium (Wilcox 1995). There are also a number of reports on the use of SSRIs to reduce aggressive and impulsive behaviour (Bond 2005).
Why it is important to do this review
AsPD is an important condition that has a considerable impact on individuals, families and society more widely. Even by the most conservative estimate, AsPD appears to have the same prevalence in men as schizophrenia, the condition that receives the greatest attention from mental health professionals. Furthermore, AsPD is associated with significant costs, arising from emotional and physical damage to victims, damage to property, use of police time, and involvement from the criminal justice system and prison services. Related costs include increased use of healthcare facilities, lost employment opportunities, family disruption, gambling, and problems related to alcohol and substance misuse (Home Office 1999; Myers 1998), and in one study the lifetime public costs for a group of adults with a history of conduct disorder (of which 50% will go onto develop adult AsPD) were found to be ten times those for a similar group without the disorder (Scott 2001).
Despite this, there is currently a dearth of evidence on how best to treat people diagnosed with AsPD, and to date the few reviews that have been carried out have been inconclusive. Dolan 1993 reviewed the use of numerous drug groups amongst people with AsPD and psychopathic disorder, but identified only a small number of studies and noted that these were limited by poor methodology and lack of long-term follow-up. They found the evidence base for pharmacological treatments for AsPD to be poor, a conclusion endorsed by the Reed committee in 1993 which recommended that the UK Department of Health and the Home Office should encourage further research into this area with added attention to female and ethnic minority groups (Reed 1994). A further review carried out eight years later failed to uncover a more credible evidence base (Warren 2001). There has, however, been increased interest in developing and evaluating pharmacological treatments for personality disorder in recent years, suggesting that a systematic review is now timely.
OBJECTIVES
This review aims to evaluate the potential beneficial effects (e.g. a reduction in reconviction or aggression) and adverse effects of pharmacological interventions compared to placebo in people with a diagnosis of AsPD.
METHODS
Criteria for considering studies for this review
Types of studies
Controlled trials in which participants have been randomly allocated to an experimental group and a placebo control group. We included all relevant randomised controlled trials with or without blinding, published in any language. We report separately on two head-to-head trials which compared one drug against another without a placebo control condition.
Types of participants
Men or women 18 years or over with a diagnosis of AsPD defined by any operational criteria such as DSM-IV, or dissocial personality disorder as defined by operational criteria such as ICD-10. We included studies of people diagnosed with comorbid personality disorders or other mental health problems other than the major functional mental illnesses (i.e. schizophrenia, schizoaffective disorder or bipolar disorder). The decision to exclude persons with co-morbid major functional illness is based on the rationale that the presence of such disorders (and the possible confounding effects of any associated management or treatment) might obscure whatever other psychopathology (including personality disorder) might be present. We placed no restrictions on setting and included studies with participants living in the community as well as those incarcerated in prison or detained in hospital.
Types of interventions
People with personality disorders may respond to pharmacological interventions that target both their state and trait symptoms. Although it has been argued that drug treatments that target the cognitive-perceptual, affective, impulsive-behavioural and anxiousfearful domains of personality disorder need to be evaluated (Soloff 1998), we carried out the review without any a priori assumptions about the effectiveness of certain drugs in a specific domain.
We included in the review studies of any drug(s) with psychotropic properties, including those falling within the following classes of pharmacological interventions (as defined by the British National Formulary, 2008):
hypnotics, anxiolytics and barbiturates;
antipsychotic drugs (including depot injections);
antidepressant drugs; tricyclic and related, monoamineoxidase inhibitors, SSRIs and related, and other antidepressant drugs;
central nervous system stimulants;
antiepileptics, mood stabilising agents/antimanic drugs;
drugs used in essential tremor, chorea, tics and related disorders;
drugs used in substance dependence;
dopaminergic drugs used in parkinsonism;
others.
We included studies evaluating a combination of drug interventions. We included studies in which the drug being evaluated was given as an adjunct to another drug, but only where the comparison was between the adjunct and a placebo adjunct. Studies in which the comparison was between one drug and another drug or between a pharmacological and a psychological intervention are reported separately.
Types of outcome measures
Primary and secondary outcomes are listed below in terms of single constructs. We anticipated that a range of outcome measures will have been used in the studies included in the review (for example, aggression may be measured by a self-report instrument or by an external observer).
Primary outcomes
Aggression: reduction in aggressive behaviour or aggressive feelings; continuous outcome, measured through improvement in scores on the Aggression Questionnaire (AQ; Buss 1992), the Modified Overt Aggression Scale (MOAS; Malone 1994) or similar validated instrument; or as number of observed incidents.
Reconviction: measured as overall reconviction rate for the sample, or as mean time to reconviction.
Global state/functioning: continuous outcome, measured through improvement on the Global Assessment of Functioning numeric scale (GAF; APA 2000)
Social functioning: continuous outcome, measured through improvement in scores on the Social Adjustment Scale (SAS-SR; Weissman 1976), the Social Functioning Questionnaire (SFQ; Tyrer 2005) or similar validated instrument.
Adverse events: measured as the proportion of participants reporting (a) any adverse event, and (b) the three most commonly reported adverse events.
Secondary outcomes
Quality of life: self-reported improvement in overall quality of life; continuous outcome, measured through improvement in scores on the European Quality Of Life instrument (EuroQol; EuroQoL group 1990) or similar validated instrument.
Engagement with services: health-seeking engagement with services measured through improvement in scores on the Service Engagement Scale (SES; Tait 2002), or similar validated instrument.
Satisfaction with treatment: continuous outcome; measured through improvement in scores on the Client Satisfaction Questionnaire (CSQ-8; Attkisson 1982) or similar validated instrument.
Leaving the study early: measured as proportion of participants discontinuing treatment.
Substance misuse: measured as improvement on the Substance Use Rating Scale, patient version (SURSp; Duke 1994) or similar validated instrument.
Employment status: measured as number of days in employment over the assessment period.
Housing/accommodation status: measured as number of days living in independent housing/accommodation over the assessment period.
Economic outcomes: any economic outcome, such as cost-effectiveness measured using cost-benefit ratios or incremental cost-effectiveness ratios (ICERs).
Impulsivity: self-reported improvement in impulsivity; continuous outcome, measured through reduction in scores on the Barratt Impulsivity Scale (BIS; Patton 1995) or similar validated instrument.
Anger: self-reported improvement in anger expression and control; continuous outcome, measured through reduction in scores on the State-Trait Anger Expression Inventory-2 (STAXI-II; Spielberger 1999) or similar validated instrument.
Whilst acknowledging that the nature of the disorder can lead to difficulty in long-term follow-up of individuals with AsPD, we report relevant outcomes without restriction on period of follow-up. We aimed to divide outcomes into immediate (within 6 months), short-term (> 6 months to 24 months), medium-term (> 24 months to 5 years) and long-term (beyond 5 years) if there were sufficient studies to warrant this.
Search methods for identification of studies
Electronic searches
The following electronic databases were searched:
MEDLINE (1950 to September 2009);
EMBASE (1980 to September 2009);
CINAHL (1982 to September 2009);
Cochrane Central Register of Controlled Trials (The Cochrane Library 2009, Issue 3);
PsycINFO (1872 to September 2009);
Cochrane Schizophrenia Group’s register of trials related to forensic mental health;
ASSIA (1987 to September 2009);
BIOSIS (1985 to September 2009);
COPAC (September 2009);
Dissertation Abstracts (September 2009);
ISI-Proceedings (1981 to 12 September 2009):
Science Citation Index (1981 to 12 September 2009);
Social Sciences Citation Index (1981 to 12 September 2009);
OpenSIGLE (1980 to April 2006);
Sociological Abstracts (1963 to September 2009);
ZETOC (September 2009);
National Criminal Justice Reference Service Abstracts (1970 to July 2008);
UK Clinical Trials Gateway*;
Action Medical Research*;
King’s College London (UK)*;
ISRCTN Register*;
The Wellcome Trust Register*;
NHS Trusts Clinical Trials Register*;
NHS R&D Health Technology Assessment Programme Register (HTA)*;
NHS R&D Regional Programmes Register*.
*Searched in September 2009 using the metaRegister of Controlled Trials (http://www.controlled-trials.com/mrct/) .
Detailed search strategies for these databases are in the Appendices. The searches were designed to find records for a series of reviews on personality disorders. From the total number of records which were retrieved, we selected only those studies which were relevant to this review
Searching other resources
For studies reported in a language other than English, we initially examined the English version of the title and abstract, where provided, to decide whether the inclusion criteria were met. We obtained a translation of the full paper where this was necessary for a decision to be made.
Hand searching
We searched the reference lists of included and excluded studies for additional relevant trials. We also examined bibliographies of systematic review articles published in the last five years to identify relevant studies.
Requests for additional data
We contacted authors of relevant studies to enquire about other sources of information and the first author of each included study for information regarding unpublished data. We contacted a representative from all major pharmaceutical companies to request information about any published or unpublished trials.
Data collection and analysis
Selection of studies
Because this review is part of a larger series of reviews of personality disorders, we selected studies in two stages. In the first stage, JS and NH independently read titles and abstracts against the inclusion criteria to identify all studies carried out with participants with personality disorder, regardless of any specific personality disorder(s) diagnosed. In the second stage, NK and BV independently assessed full copies of studies identified in stage one against the inclusion criteria. This second stage assessment identified not only trials with participants diagnosed with AsPD, but also trials with participants having a mix of personality disorders for which data on a subgroup with AsPD may be available.
We included studies with two treatment conditions in which the relevant participants formed a small subgroup only if the trial investigators randomised at least five people with antisocial or dissocial personality disorder. The rationale is that variance and standard deviation cannot be calculated in samples of two or less, and a two-condition study that randomises less than five relevant participants will have at least one arm for which variance or standard deviation cannot be calculated.
We resolved uncertainties concerning the appropriateness of studies for inclusion in the review through consultation with a third review author (CD).
Data extraction and management
Three review authors (NK, MF and NH) independently extracted data using a data extraction form. We entered the data into RevMan 5. Where data were not available in the published trial reports, we attempted to contact the study authors and asked them to supply the missing information.
Assessment of risk of bias in included studies
For each included study, two review authors (MF and NH or NK and NH) independently completed The Cochrane Collaboration’s tool for assessing risk of bias (Higgins 2008a, section 8.5.1). We would have resolved any disagreement through consultation with a third review author (KL). We assessed the degree to which:
the allocation sequence was adequately generated (‘sequence generation’)
the allocation was adequately concealed (‘allocation concealment’)
knowledge of the allocated interventions was adequately prevented during the study (‘blinding’)
incomplete outcome data were adequately addressed
reports of the study were free of suggestion of selective outcome reporting
the study was apparently free of other problems that could put it at high risk of bias
Each domain was allocated one of three possible categories for each of the included studies: ‘Yes’ for low risk of bias, ‘No’ for high risk of bias, and ‘Unclear’ where the risk of bias was uncertain or unknown.
Measures of treatment effect
For dichotomous (binary) data, we used the odds ratio with a 95% confidence interval to summarise results within each study. We chose the odds ratio because it has statistical advantages relating to its sampling distribution and its suitability for modelling, and is a relative measure and therefore can be used to combine studies. For continuous data, such as the measurement of impulsiveness and aggression on a scale, we compared the mean score for each outcome as determined by a standardised tool between the two groups to give a mean difference (MD), again with a 95% confidence interval. Where possible, we made these comparisons at specific follow-up periods: (1) within the first month, (2) between one and six months, and (3) between six and twelve months. Where possible, we presented endpoint data. Where both endpoint and change data were available for the same outcomes, we reported only the former. We used the mean difference (MD) where the same outcome measures were reported in more than one study. We used the standardised mean difference (SMD) where different outcome measures of the same construct were reported.
Continuous data that are skewed were reported in a separate table, and treatment effect sizes were not calculated to minimise the risk of applying parametric statistics to data that depart significantly from a normal distribution. We define skewness as occurring when, for a scale or measure with positive values and a minimum value of zero, the mean is less than twice the standard deviation (Altman 1996).
Unit of analysis issues
(a) Cluster-randomised trials
See Table 5 for information about future updates of this review.
Table 5. Additional methods for future updates.
| Issue | Method |
|---|---|
| Cluster-randomised trials | Where trials use clustered randomisation, study investigators may present their results after appropriately adjusting for clustering effects (robust standard errors or hierarchical linear models). Where it is unclear whether this was done, we will contact the study investigators for further information. If appropriate adjustments were not used, we will request individual participant data and re-analyse using multilevel models which control for clustering. Following this, we will carry out meta-analysis in RevMan5 using the generic inverse method (Higgins 2008a). If appropriate adjustments were not used, we will follow the method described by Donner 2001, imputing an intra-cluster correlation coefficient and adjusting for sample size. If there is insufficient information to adjust for clustering, we will enter outcome data into RevMan5 using the individual as the unit of analysis, and then use sensitivity analysis used to assess the potential biasing effects of inadequately adjusted clustered trials |
| Missing data |
Standard deviations: The standard deviations of the outcome measures should be reported for each group in each trial. If these are not given, we will impute standard deviations using relevant data (for example, standard deviations or correlation coefficients) from other, similar studies (Follman 1992) but only if, after seeking statistical advice, to do so is deemed practical and appropriate. Given that trials in this area are often conducted with small samples, any imputations (and the assumptions behind them) are likely to have an important impact. We will therefore follow, where possible, the method suggested by Higgins 2008b for weighting studies with imputed data. Loss to follow up: We will assess the extent to which the results of the review could be altered by the missing data by, for example, a sensitivity analysis based on consideration of ‘best-case’ and ‘worst-case’ scenarios (Gamble 2005). Here, the ‘best-case’ scenario is that where all participants with missing outcomes in the experimental condition had good outcomes, and all those with missing outcomes in the control condition had poor outcomes; the ‘worst-case’ scenario is the converse (Higgins 2008a, section 16.2.2). |
| Assessment of heterogeneity | We will consider I2 values in the range 0% to 40% as indicating low heterogeneity, values in the range 30% to 60% as indicating moderate heterogeneity, values in the range 50% to 90% as indicating substantial heterogeneity, and values greater than 75% as indicating considerable heterogeneity. We will attempt to identify any significant determinants of heterogeneity categorised at moderate or substantial |
| Assessment of reporting biases | We will draw funnel plots (effect size versus standard error) to assess publication bias. Asymmetry of the plots may indicate publication bias, although they may also represent a true relationship between trial size and effect size. If such a relationship is identified, we will further examine the clinical diversity of the studies as a possible explanation (Egger 1997). |
| Sub-group analysis | We will undertake a subgroup analysis to examine the effect on primary outcomes of:
|
| Sensitivity analysis | We will undertake sensitivity analyses to investigate the robustness of the overall findings in relation to certain study characteristics. A priori sensitivity analyses are planned for:
|
(b) Crossover trials
When conducting a meta-analysis combining the results of crossover trials, we planned to use the inverse variance methods recommended by Elbourne 2002. Where data presented from a crossover trial were restricted (and more information was not available from the original investigators) we planned to use the presented data within the first phase only, up to the point of crossover.
(c) Multi-arm trials
We included all eligible outcome measures for all trial arms in this review.
Dealing with missing data
We attempted to contact the original investigators to request any missing data and information on whether or not it can be assumed to be ‘missing at random’. For dichotomous data, we report missing data and dropouts for each included study and report the number of participants who are included in the final analysis as a proportion of all participants in each study. We provide reasons for the missing data in the narrative summary where these are available. For missing continuous data, we provide a qualitative summary. See Table 5 for information about future updates of this review.
Assessment of heterogeneity
We aimed to assess the extent of between-trial differences and the consistency of results of any meta-analysis in three ways: first, by visual inspection of the forest plots; second, by performing the Chi2 test of heterogeneity (where a significance level less than 0.10 is interpreted as evidence of heterogeneity); and third, by examining the I2 statistic (Higgins 2008a; section 9.5.2). The I2 statistic describes approximately the proportion of variation in point estimates due to heterogeneity rather than sampling error. See Table 5 for information about future updates of this review.
Assessment of reporting biases
See Table 5 for information about future updates of this review.
Data synthesis
We planned to use meta-analyses to combine comparable outcome measures across studies. In carrying out meta-analysis, the weight given to each study is the inverse of the variance so that the more precise estimates (from larger studies with more events) are given more weight. See Table 5 for information about future updates of this review.
Subgroup analysis and investigation of heterogeneity
See Table 5 for information about future updates of this review.
Sensitivity analysis
See Table 5 for information about future updates of this review.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification.
Results of the search
We performed electronic searches over two consecutive time periods to minimise the difficulty in managing large numbers of citations. Searches to December 2006 produced in excess of 10,000 records. Searches from December 2006 to September 2009 produced 6398 records. From inspection of titles and abstracts we identified 26 citations of studies with all or part of the sample potentially meeting diagnostic criteria for AsPD.
Included studies
Of the 26 studies, we identified eight that met fully the inclusion criteria. All included participants were diagnosed with AsPD under DSM criteria, although data from those participants with AsPD were available only for four of the eight studies (Arndt 1994; Barratt 1997; Leal 1994; Powell 1995). These are summarised in this review. Of the remaining four studies, the AsPD data reported by Ralevski 2007 were not split by allocation condition, and no data on the AsPD subgroup were available for Hollander 2003, Stanford 2001 and Stanford 2005 at the time this review was prepared. The eight included studies involved a total of 12 comparisons of a drug against placebo. There were some important differences between the studies. We summarise these differences and the main study characteristics below. Further details are provided in the Characteristics of included studies table.
Design
Six of eight included studies were parallel trials and two were crossover designs. Of the six parallel trials, two were two-condition comparisons of a drug against placebo (Arndt 1994; Hollander 2003), two were three-condition comparison of two drugs against placebo (Leal 1994; Powell 1995), one was a four-condition comparison involving three drugs against placebo (Stanford 2005), and one was a four-condition comparison of two drugs against placebo, both separately and in combination with each other (Ralevski 2007).
Both studies with cross-over designs were of a single drug against placebo (Barratt 1997; Stanford 2001). Both recruited participants with recurrent aggression. When evaluating these cross-over trials for inclusion in the review, we first considered whether the cross-over design was suitable for the condition being studied. Cross-over trials are suitable for evaluating interventions with a temporary effect in the treatment of stable conditions, and where longterm follow up is not required (Higgins 2008a, p.500). On this basis, the cross-over design was considered suitable since both AsPD and recurrent aggression are reasonably stable conditions and longterm follow up, though desirable, is not essential for evaluating the effects of medication on outcomes such as aggression, which is an important feature for many individuals with AsPD.
Sample sizes
There was some variation in sample size between studies. Overall, 365 participants with AsPD were randomised in the seven trials where this number was reported unambiguously, with the size of sample ranging from 9 to 150 (mean 52.1; median 19.0). The number with AsPD randomised in the eighth study was not reported, although 29 AsPD participants completed to study end-point (Arndt 1994).
Data were, however, available to us for only four trials (which include Arndt 1994). In these, 274 participants with AsPD were randomised, with the sample size ranging from 19 to 150 (mean 68.5; median 52.5; calculation based on an assumption that 50% of the Arndt 1994 sample was AsPD). The proportion of participants completing was reported unambiguously in only three studies: 84% for Barratt 1997 in a prison sample; 57.9% for Leal 1994 in an inpatient sample; and 44.6% for Powell 1995 where participants were in an outpatient setting at study endpoint.
Setting
All eight studies were carried out in North America. Four were single-centre trials (Arndt 1994; Leal 1994; Stanford 2001; Stanford 2005). Four were multi-centre trials: Powell 1995 with two sites; Ralevski 2007 with three sites; Hollander 2003 with 19 sites; and Barratt 1997 where the number of sites was not reported. The trials took place in a number of very different settings comprising an outpatient environment (n = 5), an inpatient setting (n = 1), and a prison (n = 1). One study involved participants who were inpatients at baseline but moved to outpatient status during the course of treatment (Powell 1995).
Participants
Participants were restricted to males in five studies (Arndt 1994; Barratt 1997; Powell 1995; Stanford 2001; Stanford 2005). The remaining three studies had a mix of male and female participants. All studies randomised more men than women. The overall mix was 96.6% men as compared to 3.4% women. All of the eight studies involved adult participants with the mean age per study ranging between 28.7 and 45.1 years (average 38.7 years). Four studies focused on participants with substance misuse difficulties. For these, inclusion criteria included cocaine dependency (Arndt 1994), cocaine and opioid dependency (Leal 1994), and alcohol dependency (Powell 1995; Ralevski 2007). The remaining four studies recruited participants on the basis of having displayed recurrent aggression, which was defined as impulsive aggression in three studies (Hollander 2003; Stanford 2001; Stanford 2005), and as impulsive or premeditated aggression in one study (Barratt 1997).
The precise definition of AsPD and the method by which it was assessed varied between the studies. Four used DSM-IV criteria: Hollander 2003 and Ralevski 2007 made assessments using the Structured Clinical Interview-II (SCID-II); Stanford 2001 and Stanford 2005 “assessed by a licensed clinical psychologist” (further details not reported). Three studies used DSM-III-R criteria: Barratt 1997 and Powell 1995 assessed using the Psychiatric Diagnostic Interview-revised (PDI-R); and Leal 1994 assessed using the SCID-II. One study (Arndt 1994) used DSM-III criteria and assessed using the NIMH Diagnostic Interview Schedule (DIS). It is important to note that none of the eight studies set out to recruit participants on the basis of having a diagnosis of AsPD. In seven, participants with AsPD formed a subgroup which accounted for between 4% and 59% of the trial’s sample. In one study (Barratt 1997), participants were recruited on the basis of recurrent aggression and subsequent assessment revealed that 100% met the criteria for AsPD.
Ethnicity of participants was not always reported. For the five studies where it was, 68.2% of randomised participants were described as either ‘white’ or ‘Caucasian’.
Interventions
Eight drugs were compared to placebo in the eight included studies. These were categorised as:
antiepileptics - carbamazepine (one study); phenytoin (three studies); valproate/divalproex (two studies);
antidepressants - desipramine (two studies); nortriptyline (one study);
dopamine agonists - bromocriptine (one study); amantadine (one study); and
opioid antagonists - naltrexone (one study)
In each case, the route of administration was oral (by tablets, capsules or liquid). Studies varied in the way they reported the dose administered to the treatment group: a fixed daily dose (mg/day), or a dose adjusted in an attempt to achieve a target blood serum concentration (ng/ml or mg/ml). Details are provided in the Characteristics of included studies table but can be summarised as follows.
one study involved amantadine (Leal 1994; 300 mg/day for adults with opioid and cocaine dependency)
one study involved bromocriptine (Powell 1995; 15 mg/day for men with alcohol dependency)
one study involved carbamazepine (Stanford 2005; 450 mg/day for men with aggression, but with no data available for the AsPD subgroup)
two studies involved desipramine (Arndt 1994; 250 to 300 mg/day for men with cocaine dependency) (Leal 1994; 150 mg/day for adults with opioid and cocaine dependency)
one study involved naltrexone (Ralevski 2007; 50 mg/day for adults with alcohol dependency)
one study involved nortriptyline (Powell 1995; 25 to 75 mg/day for men with alcohol dependency)
three studies involved phenytoin (Barratt 1997; 300 mg/day for prisoners with aggression) (Stanford 2001 and Stanford 2005; 300 mg/day for outpatient men with aggression, but with no data available for the AsPD subgroup)
two studies involved valproate (full name: sodium valproate) (Stanford 2005; 750 mg/day for men with aggression, but with no data available for the AsPD subgroup) or divalproex (full name: divalproex sodium) (Hollander 2003; max 30 mg/kg/day for outpatients with aggression but with no data available for the AsPD subgroup). Divalproex sodium is an equimolar compound of sodium valproate and valproic acid; because these two drugs are regarded as equivalent in efficacy and have similar side effect profiles, we consider them together in this review.
The duration of the interventions ranged between 6 and 24 weeks (mean 11.3 weeks; median 12.0 weeks). None of the eight studies followed up participants beyond the end of the intervention period. The duration of the trials ranged between 8 and 24 weeks (mean 14.0 weeks; median 12.5 weeks).
Outcomes
Primary outcomes
Studies varied in terms of choice of primary outcomes. Four studies included aggression as an outcome: Barratt 1997 using the Overt Aggression Scale (OAS); Hollander 2003 used the Overt Aggression Scale - Modified (OAS-M; a clinician-rated semi-structured interview), but with no data available for the subgroup with AsPD; Stanford 2001 and Stanford 2005 using the Overt Aggression Scale (OAS), but neither with any data available for the subgroup with AsPD. The outcome of social functioning was considered only by Arndt 1994 using the family-social domain of the Addiction Severity Index (ASI). Two studies included Global state/functioning as an outcome: Powell 1995 using the Global Assessment Scale (GAS) and the general severity index sub scale of the Symptom Check List-90 (SCL-90); Hollander 2003 using the Clinical Global Impression scale (CGI), but with no data available for the subgroup with AsPD. Three studies included adverse events as an outcome: Barratt 1997 using blood cell counts and liver function tests; Ralevski 2007 and Hollander 2003 using self-reported side effects, but neither with any data available for the subgroup with AsPD. No study reported on reconviction.
Secondary outcomes
Studies varied in terms of choice of secondary outcomes. Five studies reported on leaving the study early: Leal 1994 and Powell 1995 reported on the proportion of participants discontinuing treatment; Ralevski 2007, Hollander 2003 and Stanford 2005 reported similarly, but with no data available for the subgroup with AsPD. Two studies reported on the outcome of substance misuse (drugs): Leal 1994 using dollars spent on cocaine per week and cocaine abstinence measured as percentage of cocaine-free urine samples; and Arndt 1994 using cocaine-positive urinalysis results, ASI drug domain, days opiate use, days cocaine use, and cocaine craving scores. Two studies reported on the outcome of substance misuse (alcohol): Powell 1995 using number of drinking days in the last 30 days, alcohol craving scores, self-report of longest period of total abstinence during the 6-month study, abstinence from drinking at endpoint; severity of alcohol misuse as measured with the Addiction Severity Index (ASI), alcohol dependence measured with the Alcohol Dependence Questionnaire (SAD-Q), and both patient and clinical ratings of drinking behaviour; Ralevski 2007 used the Timeline Follow-Back Interview and alcohol craving measured with the Obsessive Compulsive Drinking Scale, but with no allocation group data available for the subgroup with AsPD. The outcome of employment status was considered only by Arndt 1994 using the employment domain on the Addiction Severity Index (ASI), days worked in the last 30 days and employment income. The outcome of anger was considered by two studies: Barratt 1997 and Stanford 2001 both using the anger-hostility sub scale of the Profile of Moods Scale (POMS), but neither with any data available for the subgroup with AsPD. No study reported on quality of life, engagement with services, satisfaction with treatment, housing/accommodation status, economic outcomes, or impulsivity.
Other relevant outcomes
Two studies reported on the outcome of depression: Powell 1995 using the depression sub scale of the Symptom Check List-90 (SCL-90) and the Beck Depression Inventory (BDI); Arndt 1994 using the Beck Depression Inventory (BDI). One study reported on anxiety: Powell 1995 using the anxiety sub scale of the Symptom Check List-90 (SCL-90) and the Beck Anxiety Index (BAI). One study reported on the outcome of illegal activity: Arndt 1994 using days of illegal activity in the last 30 days, illegal income, and the illegal domain on the Addiction Severity Index (ASI).
Studies awaiting classification
We identified two studies in which the sample comprised a mixture of personality disorders where it remains unclear whether the investigators had included a subgroup of participants with a diagnosis of AsPD (Hellerstein 2000; Verkes 1998). Clarification has been sought from the trial investigators but no further information was available at the time this review was prepared. Details are provided in the Characteristics of studies awaiting classification table. The two studies are summarised as follows:
Hellerstein 2000 compared sertraline, imipramine and placebo in outpatients with early-onset dysthymia, and may have recruited a subgroup with AsPD since 48 participants had DSM-III-R cluster B personality disorder.
Verkes 1998 compared paroxetine with placebo in outpatients with repeated suicidal attempts but without major depression, and may have recruited a subgroup with AsPD since at least one cluster B personality disorder was present in 74 out of 91 participants.
Excluded studies
The remaining 16 studies that failed to meet all inclusion criteria were categorised as excluded studies. Six were excluded because there was no placebo control condition. Eight were excluded because on close inspection, and following translation into English and contact with the investigators where necessary, it became clear that the sample did not include a subgroup with antisocial or dissocial personality disorder. A further two were excluded because there were too few participants with AsPD to allow calculation of means and SDs for reasons that are explained in the Selection of studies section. Reasons for exclusion of each of these 16 studies are given in the Characteristics of excluded studies table.
We paid particular attention to the two studies that compared one medication against another (Joyce 2003; Mattes 1990). Both were excluded because there was no placebo control condition. Although neither focused exclusively on AsPD or provided data on their AsPD subgroup, both reported information that we considered would be of interest to a clinician who was seeking treatment options for clients with AsPD. Because of this, we have summarised briefly the characteristics of these two studies and the conclusions drawn by the trial investigators in the Discussion section.
Risk of bias in included studies
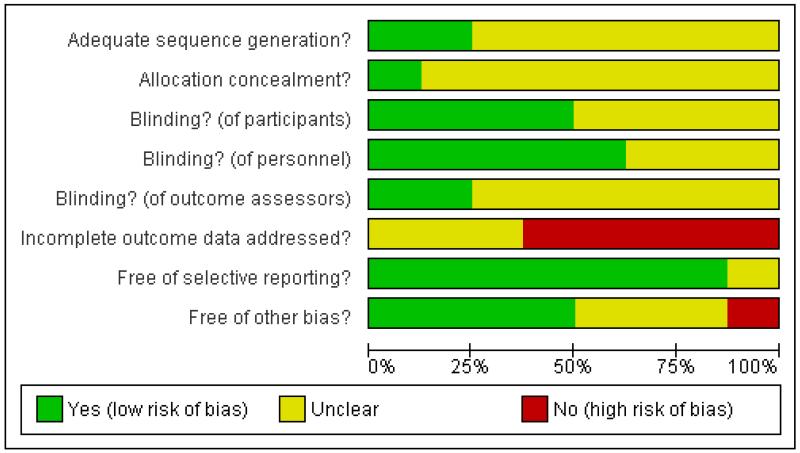
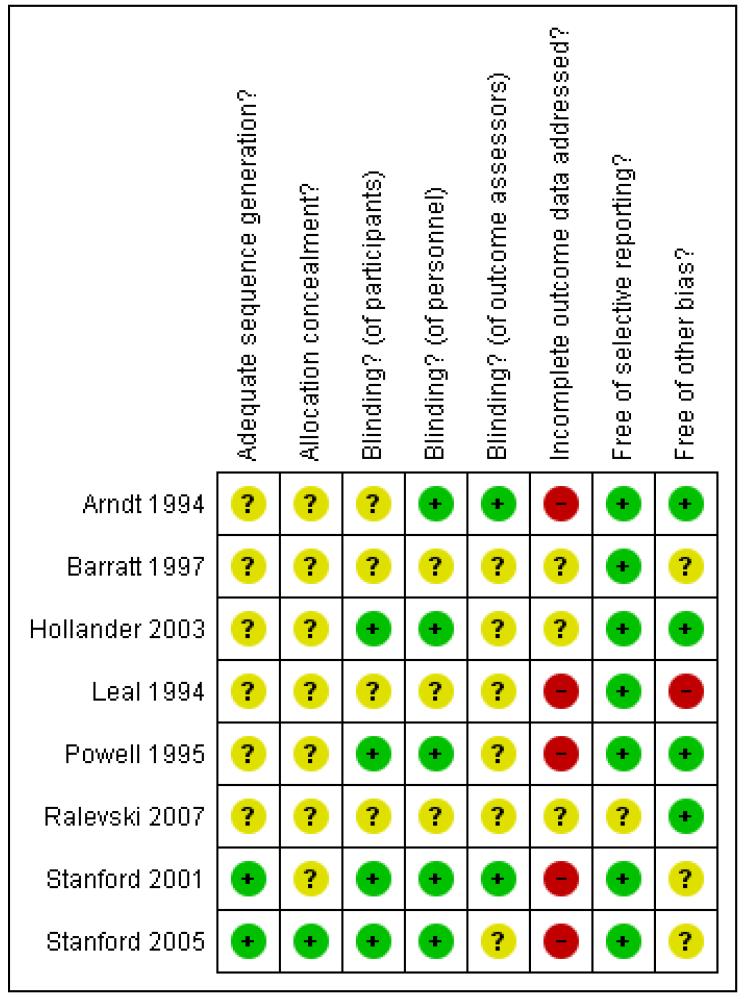
Reporting varied considerably among the included studies. Study quality was poor, overall. We attempted to contact the investigators wherever the available trial reports provided insufficient information for decisions to be made about the likely risk of bias, and were successful in respect of two studies. Full details of our assessment of the risk of bias for each included study are provided within the Characteristics of included studies section. Graphical summaries of methodological quality are presented in Figure 1 and Figure 2.
Figure 1. Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Figure 2. Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Allocation
With data (four studies)
Adequacy of sequence generation was judged by the review authors as ‘unclear’ in all four studies because the investigators reported that participants had been allocated at random but provided no further information on how this had been achieved. Concealment of the allocation sequence was also classified as ‘unclear’ in each of these studies, again because the information available was insufficient to allow a judgment to be made.
Without data (four studies)
The generation of allocation sequence was considered to be adequate in two studies where allocation was performed using a table of random numbers (Stanford 2001; Stanford 2005), but ‘unclear’ in Hollander 2003 and Ralevski 2007 because the information available was insufficient to allow a judgment to be made.
Blinding
With data (four studies)
Blinding of participants was considered by the review authors to be adequate in only one study (Powell 1995), and was considered ‘unclear’ in the other three studies because the information available was insufficient to allow a judgment to be made. Adequacy of blinding of personnel was judged adequate in Arndt 1992 and Powell 1995 but ‘unclear’ in Barratt 1997 and Leal 1994 because insufficient information was provided. Adequacy of blinding of outcome assessors was judged adequate only for Arndt 1994 and ‘unclear’ for the other three studies, again because of insufficient information.
Without data (four studies)
Blinding of participants and of personnel was considered adequate in three studies (Hollander 2003; Stanford 2001; Stanford 2005), but ‘unclear’ in Ralevski 2007 because the information available was insufficient to allow a judgment to be made. Blinding of outcome assessors was judged adequate for Stanford 2001, but ‘unclear’ for the other three studies because of insufficient information.
Incomplete outcome data
With data (four studies)
None of the four studies were judged to have adequately addressed any incomplete outcome data, and the overall proportion of participants completing was reported unambiguously in only three studies: 84% for Barratt 1997 in a prison sample; 57.9% for Leal 1994 in an inpatient sample; 44.6% for Powell 1995 in which participants were outpatients at study endpoint. In reporting their data, Arndt 1994, Barratt 1997 and Powell 1995 provided analysis only for those participants classed by the investigators as ‘completers’.
Without data (four studies)
None of the four studies were judged to have adequately addressed any incomplete outcome data. Two studies were judged ‘unclear’; Hollander 2003 because of insufficient information to judge whether reasons for missing data were balanced across conditions, and Ralevski 2007 because the trial investigators provided no information on either the numbers randomised to each condition or on the extent of the missing data for each condition.
Selective reporting
With data (four studies)
Review authors judged that all four studies appeared to have reported on all the measures they set out to use and at all time scales in as far as could be discerned from the published reports without access to the original protocols.
Without data (four studies)
Three studies were judged to be free of bias from selective reporting. Ralevski 2007 was classed ‘unclear’ because a companion paper (Petrakis 2005) indicated that adverse events were measured weekly via the Hopkins Symptom Checklist, although these were not reported.
Other potential sources of bias
With data (four studies)
Two studies (Arndt 1994; Powell 1995) were judged free of other potential sources of bias. Barratt 1997 was judged ‘unclear’ because of the possibility of bias in the selection by the investigators of two subgroups for analysis. Leal 1994 was judged ‘inadequate’ because of the possibility of false negative results arising from urinanalysis carried out twice-weekly when the detectability window for cocaine is 6 to 8 hours. It is also unclear in the Leal 1994 study whether participants continued to receive contingency management during the trial and if so whether this was similar across the conditions; since the latter involves monetary incentives in return for a clean urine sample, differences in percentages of cocaine-free urine samples may be related to such incentives rather than the effects of medications.
Without data (four studies)
Hollander 2003 and Ralevski 2007 were judged free of other potential sources of bias. Stanford 2001 and Stanford 2005 were judged ‘unclear’ because the investigators declared their research sponsored by the Dreyfus Health Foundation. This Foundation was established to study and disseminate information and to sponsor collaborative, clinical, and basic health research on the benefits of phenytoin. We have insufficient information to assess whether sponsorship from such a foundation constitutes a risk of bias.
Effects of interventions
1. Comparison 1: amantadine versus placebo
One study was included in this comparison: Leal 1994 (methadone-maintained inpatient adults with opioid and cocaine dependency; dose 300 mg/day; n = 12).
1.1 Leaving the study early
Leal 1994 reported data indicating no statistically significant difference between treatment and control conditions for leaving the study early (OR 5.00; 95% CI 0.34 to 72.77, P = 0.24, Analysis 1.1).
1.2 Substance misuse (drugs)
For the outcome of dollars spent on cocaine per week, Leal 1994 reported skewed summary data for amantadine and control conditions at week 1, week 6, and week 12 (Table 1). The trial investigators reported no statistical analysis that compared amantadine and control groups, although they noted that the AsPD participants were spending significantly more money on drugs than the non-AsPD participants at weeks 1 and 6. Leal 1994 separately reported skewed summary data (Table 2) for ‘medicated’ participants (i.e. those receiving either amantadine 300 mg/day or desipramine 150 mg/day; total n = 15) and controls, but again with no statistical analysis that compared treatment and control groups.
For the outcome of cocaine abstinence, Leal 1994 reported the percentage of (twice-weekly) urinalyses that were cocaine-free for first 2 weeks, for weeks 5 and 6, and for the last 2 weeks of the study (Table 3). Trial investigators reported no statistical analysis that compared amantadine and control groups on this outcome, but noted that “when comparing the first and last two weeks of treatment … AsPD … patients treated with placebo showed no difference in the percentage of cocaine-free urines … medicated AsPD patients [those receiving either amantadine 300 mg/day or desipramine 150 mg/day; total n = 15] also showed no change in cocaine-free urines (13% to 14%)” (page 33, col 2).
2. Comparison 2: bromocriptine versus placebo
One study was included in this comparison: Powell 1995 (inpatient, and later outpatient, men with alcohol dependency; dose 15 mg/day; n = 18 completers).
2.1 Global state/functioning
Powell 1995 reported data indicating no statistically significant difference between treatment and control conditions for global functioning as measured with both the Global Assessment Scale and the general severity index sub scale of the Symptom Check List-90 (Table 4; 3-way ANOVA; comorbidity × treatment × time; analysis by trial investigators).
2.2 Leaving the study early
Powell 1995 did not provide data on leaving the study early for treatment and placebo conditions for their AsPD subgroup. They reported, however, that “the dropout rates for the comorbidity and medication subgroups ranged from 52.1% to 55.4%, and were not significantly different” (p.464, col 1).
2.3 Substance misuse (alcohol)
Powell 1995 reported graphical data indicating no statistically significant difference between bromocriptine versus placebo on mean number of drinking days (Table 5; two-way ANOVA; comorbidity × medication: F(4,89) = 2.60; P < 0.05; however, Tukey post-hoc tests did not indicate a statistically significant effect for AsPD/bromocriptine subgroup; completer analysis by trial investigators). Powell 1995 reported graphical data indicating no statistically significant difference between bromocriptine versus placebo on mean alcohol craving scores (Table 6) (three-way ANOVA; comorbidity × medication × time; no significant main effects or interactions; completer analysis by trial investigators).
Powell 1995 reported an analysis indicating no statistically significant difference between medication (bromocriptine or nortriptyline) and placebo conditions on participants’ self-report of longest period of total abstinence during the 6-month study (two-way ANOVA; comorbidity × medication: F(2,90) = 3.02; completer analysis by trial investigators).
Powell 1995 additionally reported that 7 of 11 (64%) completers in the bromocriptine group were abstinent from drinking at 6 months compared to 1 of 9 (11%) of completers in the placebo group.
Powell 1995 reported data indicating no statistically significant difference between treatment and control conditions on severity of alcohol misuse as measured with the Alcohol Severity Scale, alcohol dependence on the Alcohol Dependence Questionnaire (SAD-Q), and both patient and clinical ratings of drinking (Table 7; 3-way ANOVA; comorbidity × treatment × time; analysis by trial investigators).
2.4 Other outcomes
Powell 1995 additionally reported data indicating no statistically significant difference between treatment and control conditions on depressive symptoms as measured with the depression subscale of the Symptom Check List-90 and the Beck Depression Inventory (Table 8; 3-way ANOVA; comorbidity × treatment × time; analysis by trial investigators).
Powell 1995 additionally reported data indicating a statistically significant difference between treatment and control conditions favouring bromocriptine on anxiety symptoms measured using the Beck Anxiety Inventory (Table 8; 3-way ANOVA; comorbidity × treatment × time; P < 0.05; analysis by trial investigators), but no significant difference using the anxiety subscale of the Symptom Check List-90 (Table 8).
3. Comparison 3: desipramine versus placebo
Two studies were included in this comparison: Leal 1994 (methadone-maintained inpatient adults with opioid and cocaine dependency; dose 150 mg/day; n = 11) and Arndt 1994 (methadonemaintained outpatient men with cocaine dependency; dose 250 to 300 mg/day; n = 29 with AsPD completers).
3.1 Social functioning
Arndt 1994 reported data indicating no statistically significant difference between treatment and control conditions on family-social domain scores on the Addiction Severity Index (Table 9; between-groups ANCOVA using baseline value as covariate; analysis by trial investigators).
3.2 Leaving the study early
Leal 1994 reported data indicating no statistically significant difference between treatment and control conditions for leaving the study early (OR 1.20; 95% CI 0.07 to 19.63, P = 0.90, Analysis 3.1).
3.3 Substance misuse (drugs)
For the outcome of dollars spent on cocaine per week, Leal 1994 reported skewed summary data for desipramine and control conditions at week 1, week 6, and week 12 (Table 10). The trial investigators reported no statistical analysis that compares desipramine and control groups, although they note that the AsPD participants were spending significantly more money on drugs than the non-AsPD participants at weeks 1 and 6. Leal 1994 separately reported skewed summary data for ‘medicated’ participants (i.e. those receiving either desipramine 150 mg/day or amantadine 300 mg/day; total n = 15) and controls (Table 2), but again with no statistical analysis that compares treatment and control groups.
For the outcome of cocaine abstinence, Leal 1994 reported the percentage of (twice-weekly) urinalyses that were cocaine-free for first 2 weeks, for weeks 5 and 6, and for the last 2 weeks of the study (Table 11). Trial investigators reported no statistical analysis that compares desipramine and control groups on this outcome, but note that “when comparing the first and last two weeks of treatment … AsPD … patients treated with placebo showed no difference in the percentage of cocaine-free urines … medicated AsPD patients [those receiving either amantadine 300 mg/day or desipramine 150 mg/day; total n = 15] also showed no change in cocaine-free urines (13% to 14%)” (page 33, col 2).
Arndt 1994 reported no statistically significant difference between conditions on mean percentage of cocaine-positive urinalysis results across all 12 weeks of the study (Table 12). The trial investigators note that “the AsPD subjects showed no indication of either a medication group effect (F < 1.0, P > 0.10) or a decrease in the proportion of cocaine-positive urines over time (F < 1.0, P > 0.10)” (page 155, col 2).
Arndt 1994 reported data indicating no statistically significant difference between treatment and control conditions on drug domain scores, days of opiate use, and days of cocaine use from the Addiction Severity Index, and on cocaine craving scores from the Cocaine Craving Scale and Quantitative Cocaine Inventory (Table 13; between-groups ANCOVA using baseline value as covariate; analysis by trial investigators).
3.4 Employment status
Arndt 1994 reported data indicating no statistically significant difference between treatment and control conditions on employment domain scores and days worked in the last 30 days from the Addiction Severity Index (Table 14; between-groups ANCOVA using baseline value as covariate; analysis by trial investigators). They did, however, report results of a similar analysis suggesting a statistically significant greater employment income from the control group in comparison with the desipramine group (Table 14; P < 0.05).
3.5 Other outcomes
Depression: Arndt 1994 reported data indicating no statistically significant difference between treatment and control conditions on depression scores on the Beck Depression Inventory (Table 15; between-groups ANCOVA using baseline value as covariate; analysis by trial investigators).
Illegal activity: Arndt 1994 reported data indicating no statistically significant difference between treatment and control conditions on illegal domain scores, days of illegal activity in the last 30 days and illegal income from the Addiction Severity Index (Table 16; between-groups ANCOVA using baseline value as covariate; analysis by trial investigators).
4. Comparison 4: naltrexone versus placebo
One study was included in this comparison: Ralevski 2007 (outpatient adults with alcohol dependency; dose 50 mg/day; number randomised not reported). This study provides further analysis of a trial reported by Petrakis 2005 in which outpatients with alcohol dependency and an Axis I disorder were randomised to treatment with naltrexone, disulfiram or placebo. Ralevski 2007 compared subgroups of participants with and without AsPD (and with and without borderline personality disorder), but with no indication of numbers allocated to the treatment and control groups and with no statistical comparisons relevant to this review.
It is worth noting, however, that in the original trial (of which those with AsPD formed only a subgroup) the investigators concluded that there was “a high rate of abstinence across groups” and that “subjects treated with an active medication had significantly more consecutive weeks of abstinence and less craving than those treated with placebo, but there were no significant group differences in other measures of alcohol consumption” (Petrakis 2005, p.1128, Abstract). In the subsequent analysis, the investigators concluded that “diagnosis of personality disorder did not adversely affect alcohol outcomes, and patients with AsPD or BPD did not have a poorer response to medication than patients without diagnosis of AsPD or BPD” (Ralevski 2007, p.443, Abstract).
5. Comparison 5: nortriptyline versus placebo
One study was included in this comparison: Powell 1995 (inpatient, and later outpatient, men with alcohol dependency; dose 25 to 75 mg/day; n = 20 completers).
5.1 Global state/functioning
Powell 1995 reported data indicating no statistically significant difference between treatment and control conditions for global functioning as measured with both the Global Assessment Scale and the general severity index subscale of the Symptom Check List-90 (Table 17; 3-way ANOVA; comorbidity × treatment × time; analysis by trial investigators).
5.2 Leaving the study early
Powell 1995 did not provide data on leaving the study early for treatment and placebo conditions for their AsPD subgroup. They reported, however, that “the dropout rates for the comorbidity and medication subgroups ranged from 52.1% to 55.4%, and were not significantly different” (p.464, col 1).
5.3 Substance misuse (alcohol)
Powell 1995 reported graphical data indicating a statistically significant difference for nortriptyline versus placebo on mean number of drinking days, favouring nortriptyline (Table 18; two-way ANOVA; comorbidity × medication: F(4,89) = 2.60; P < 0.05; Tukey post-hoc tests for each comorbidity subgroup indicated a medication effect for AsPD/nortriptyline subgroup only; P < 0.05; completer analysis by trial investigators).
Powell 1995 reported graphical data indicating no statistically significant difference between nortriptyline and placebo conditions on mean alcohol craving scores (Table 19; three-way ANOVA; comorbidity × medication × time; no significant main effects or interactions; completer analysis by trial investigators).
Powell 1995 reported an analysis indicating no statistically significant difference between medication (nortriptyline or bromocriptine) and placebo conditions on participants’ self-report of longest period of total abstinence during the 6-month study (two-way ANOVA; comorbidity × medication: F(2,90) = 3.02; completer analysis by trial investigators).
Powell 1995 additionally reported that 3 of 9 (33%) completers in the nortriptyline group were abstinent from drinking at 6 months compared to 1 of 9 (11%) of completers in the placebo group.
Powell 1995 reported data indicating no statistically significant difference between treatment and control conditions on severity of alcohol misuse on Alcohol Severity Scale scores, on patient’s rating of drinking, and on clinical rating of drinking (Table 20). They did, however, find a significantly greater improvement over time for nortriptyline compared to placebo on alcohol dependence as measured with the Alcohol Dependence Questionnaire (SAD-Q) (Table 20; 3-way ANOVA; comorbidity × treatment × time; P < 0.01; analysis by trial investigators).
5.4 Other outcomes
Powell 1995 reported data indicating no statistically significant difference between treatment and control conditions on depressive symptoms as measured with the depression subscale of the Symptom Check List-90 and the Beck Depression Inventory (Table 21; 3-way ANOVA; comorbidity × treatment × time; analysis by trial investigators).
Powell 1995 reported data indicating a statistically significant difference between treatment and control conditions favouring nor-triptyline on anxiety symptoms measured using the Beck Anxiety Inventory (P < 0.05), but no significant difference using anxiety subscale of the Symptom Check List-90 (Table 21; 3-way ANOVA; comorbidity × treatment × time; analysis by trial investigators).
6. Comparison 6: phenytoin versus placebo
Three studies were included in this comparison, of which data from Barratt 1997 (incarcerated men with aggression; dose 300 mg/day; n = 126 with analysis of 60) are summarised below.
No data were available for the AsPD subgroup in the remaining two studies: Stanford 2001 (outpatient men with aggression; dose 300 mg/day; n = 10); and Stanford 2005 (outpatient men with aggression; dose 300 mg/day; number randomised not reported). The AsPD subgroup formed a substantial proportion (43%) of total sample in the Stanford 2001 trial. It is therefore worth noting that the investigators reported statistically significant lower scores for the phenytoin condition on frequency of impulsive-aggressive outbursts per week and POMS anger-hostility subscale scores at endpoint for the whole sample. Similarly, the AsPD subgroup formed a substantial proportion (59%) of total sample in the study reported by Stanford 2005. It is therefore worth noting that the investigators reported “a significant reduction in impulsive aggression during all three anticonvulsant conditions [phenytoin; carbamazepine; valproate] compared to placebo” (p.72, Abstract).
6.1 Aggression
Barratt 1997 reported skewed summary data (see Table 22) for both impulsive and non-impulsive sub-groups. For the impulsive subgroup, these data indicate a significant difference between conditions at endpoint (6 weeks) for mean frequency of aggressive acts (P < 0.01) and for mean intensity of aggressive acts (P < 0.01), favouring phenytoin in both cases. For the non-impulsive subgroup, the data indicate no significant difference between conditions at endpoint (6 weeks) for either mean frequency or mean intensity of aggressive acts (ANOVA, Geissner-Greenhouse adjusted; completer analysis by the trial investigators).
6.2 Adverse events
Barratt 1997 provided data indicating no statistically significant difference between phenytoin and placebo conditions for the presence of nausea (OR 1.00 [0.06 to 16.76], P = 1.00, Analysis 6.1). Barratt 1997 also reported no significant side effects detectable via blood cell counts or liver enzyme tests (no data provided).
6.3 Anger
Barratt 1997 reported skewed summary data indicating no significant difference between conditions for anger-hostility subscale scores on the Profile of Moods Scale at endpoint (6 weeks) for both impulsive and non-impulsive aggression subgroups (Table 23; ANOVA, Geissner-Greenhouse adjusted; completer analysis by the trial investigators).
7. Comparison 7: valproate/divalproex versus placebo
Two studies were included in this comparison, although for neither were data available for the AsPD subgroup: Stanford 2005 (outpatient men with aggression; valproate; dose 750 mg/day; number randomised not reported); and Hollander 2003 (adult outpatients with aggression; divalproex; dose maximum 30 mg/kg/day; n = 9).
For Stanford 2005, the AsPD subgroup formed a substantial proportion (59%) of total sample in this study. It is therefore worth noting that the investigators reported “a significant reduction in impulsive aggression during all three anticonvulsant conditions [valproate; carbamazepine; phenytoin] compared to placebo” (Stanford 2005, p.72, Abstract), and that a repeated-measures ANOVA of completers conducted by the trial investigators indicated a statistically significant lower overall Overt Aggression Scale score for the valproate condition compared with the placebo condition (P = 0.001). For Hollander 2003, only 9% of those randomised with a cluster B personality disorder had a diagnosis of AsPD. It may therefore be of limited relevance that the investigators reported statistically significant lower aggression scores for the divalproex condition for the cluster B subgroup (P = 0.047) over the last four weeks of the intervention.
8. Comparison 8: carbamazepine versus placebo
One study was included in this comparison, but with no data available for the AsPD subgroup: Stanford 2005 (outpatient men with aggression; dose 450 mg/day; number randomised not reported). The AsPD subgroup formed a substantial proportion (59%) of the total sample in this study and it is therefore worth noting that the investigators reported “a significant reduction in impulsive aggression during all three anticonvulsant conditions [carbamazepine; phenytoin; valproate] compared to placebo” (Stanford 2005, p.72, Abstract).
DISCUSSION
As described in the introduction, AsPD is a prevalent condition associated with considerable personal and societal adverse consequences. It also has major negative economic consequences as it is associated with poor occupational productivity and increased criminal justice costs. Consequently, one might expect that identifying the interventions that might reduce this impact would be a research priority. Unfortunately, the conclusion of this review is similar to many that preceded it in that there is little good quality evidence as to what might (or might not) be effective for this condition. As only eight studies could be included in the review (and only four with usable data), the first point to make is how few studies there were to consider. It is also worth noting here that none of the eight studies set out to recruit participants on the basis of having a diagnosis of AsPD.
The second refers to the design and methodological quality of the few studies that could be included. While the underlying personality structure of AsPD comprises dissociate traits such as impulsivity, lack of remorse and irritability, it is persistent rule-breaking that is its most common behavioural manifestation. Although focusing on behaviour rather than on the underlying personality structure has been frowned upon by some commentators (e.g. Livesley 2007), we argue that persistent rule-breaking is akin to a final common pathway manifestation of the underlying personality structure. If one accepts this argument, it is disappointing that none of the included studies had reconviction as their primary outcome, and only one (Arndt 1994) reported on illegal activity. Furthermore, four of the eight included studies were trials to reduce substance misuse. As many within the sample of substance misusers also satisfied criteria for AsPD, there was an opportunity to report on these separately. Hence, strictly speaking, these were not interventions for AsPD; rather, they were interventions to reduce substance misuse in a sample, some of whom also satisfied criteria for AsPD. While these studies had some limitations, there is some evidence that nortriptyline is effective in reducing some aspects of alcohol misuse in this population, and that both nortriptyline and bromocriptine are effective in reducing anxiety symptoms in individuals with AsPD and alcohol dependency. The remaining four studies focused on aggressive behaviour, although data on AsPD participants were available for only one of these which makes it difficult to draw any robust conclusion. There is some evidence that phenytoin is effective in reducing the frequency and intensity of aggressive acts.
In the light of the important adverse cost consequences of the condition, it was also disappointing that none of the studies considered the economic impact of their intervention. It is also important to note that all included studies were older trials from the mid 90s, with no testing of more recently developed pharmaceutical substances.
Summary of main results
Much of the quantitative data available from the studies included in this review was either inadequately summarised by the trial investigators or else met our criteria for skewed data as described in the section on Measures of treatment effect. In the absence of raw data from the trial investigators, we have therefore presented the majority of the quantitative data as Additional tables and have reported statistics on comparisons between conditions as calculated by the trial investigators rather than performing our own analysis. Where data were skewed, we did not carry out any synthesis of primary or secondary outcome data via meta-analysis because either (a) data for an outcome were available from only one study, or (b) we wanted to minimise the risk of applying parametric statistics to skewed data which are by definition not normally distributed. The summaries that follow below are therefore essentially descriptive. The focus of this review is relatively broad since it seeks evidence on effectiveness of any pharmacological intervention in the treatment of AsPD. We found considerable differences between the studies in terms of participants, size of sample, intervention, and choice of outcome measures. Based on analyses conducted by the trial investigators, we found only three drugs (nortriptyline, bromocriptine, phenytoin) which were effective compared to placebo in terms of improvement in at least one outcome in at least one study. Only Barratt 1997 reported significant change in a specific antisocial behaviour (phenytoin on aggression). No study provided a comprehensive analysis of adverse effects.
bromocriptine was reported as superior to placebo at a dose of 15 mg/day on anxiety symptoms measured using the Beck Anxiety Inventory in inpatient (later outpatient) men with alcohol dependency (Powell 1995), but not when measured with the anxiety subscale of the Symptom Check List-90 (Powell 1995).
nortriptyline was reported as superior to placebo at a dose of 25 to 75 mg/day for inpatient (later outpatient) men with alcohol dependency on mean number of drinking days and on alcohol dependence measured with the Alcohol Dependence Questionnaire (Powell 1995), but not for severity of alcohol use on the Alcohol Severity Scale, or on patient’s rating of drinking or on clinical rating of drinking.
nortriptyline was reported as superior to placebo at a dose of 25 to 75 mg/day on anxiety symptoms measured using the Beck Anxiety Inventory (Powell 1995) for inpatient (later outpatient) men with alcohol dependency, but not when measured with the anxiety subscale of the Symptom Check List-90.
phenytoin at a dose of 300 mg/day, was reported as superior to placebo on the frequency and intensity of aggressive acts in a study of male prisoners with impulsive (but not premeditated) aggression (Barratt 1997) with no significant difference reported between conditions for the presence of nausea.
desipramine was reported as inferior to placebo at a dose of 250 to 300 mg/day on employment income (Arndt 1994) in outpatient men with cocaine dependency, but with no statistically significant difference between drug and placebo in number of days worked nor on employment domain scores on the Addiction Severity Index (Arndt 1994).
As noted in Huband 2010, the finding by Barratt 1997 that phenytoin reduced acts of impulsive but not premeditated aggression, compared to placebo, is in line with evidence from the wider literature on aggression which suggests that different forms of aggression to others are underpinned by different mechanisms. The differences between impulsive or reactive aggression and premeditated or instrumental aggression have been well documented (e.g. Blair 2001). We suggest therefore that studies evaluating the effectiveness of interventions for aggression - both generally and in the context of AsPD - should use outcome measures that enable distinctions to be made according to the evidence-based typologies of aggression.
A note on the two excluded trials that compared one drug against another
We excluded two studies that compared one drug against another because there was no placebo control condition. Both had a small subgroup with AsPD.
Joyce 2003 conducted a randomised trial comparing fluoxetine (mean 28 mg/day) with nortriptyline (mean 93 mg/day) in patients with major depression. Differential drug response was compared in three groups; those with borderline personality disorder (BPD), those with other personality disorder (OPD), and those with no personality disorder. Six of 53 (11.3%) of participants in the OPD group had an AsPD diagnosis. Data on the AsPD subgroup were not reported. For the OPD group as a whole, no statistically significant difference was observed between fluoxetine and nortriptyline in terms of improvement in Montgomery Asberg Depression Rating Scale (MADRS) scores at six weeks.
Mattes 1990 describe a trial comparing carbamazepine (mean 860 mg/day) with propranolol (mean 486 mg/day) for temper outbursts. Eight of the sample of 51 randomised (15.7%) had a diagnosis of AsPD. The investigators concluded that both drugs were beneficial for rage outbursts (on psychiatrist’s rating of benefit), but that a diagnosis of attention deficit disorder predicted preferential response to propranolol whereas a diagnosis of intermittent explosive disorder predicted preferential response to carbamazepine.
Findings from these two trials provide little additional information on potential pharmacological treatment for AsPD, but we summarise them here for completeness.
Overall completeness and applicability of evidence
The evidence obtained from the included studies is relevant to the review question, but is incomplete for the following reasons:
Although eight different pharmacological interventions were compared, none of the studies evaluated the primary outcome of reconviction, only one study with data on AsPD participants reported on adverse effects.
No study reported on the secondary outcomes of quality of life, engagement with services, satisfaction with treatment, housing/accommodation status, economic outcomes, or impulsivity.
The majority of studies did not primarily focus on the treatment of AsPD, and only one recruited a sample in which all participants had this diagnosis.
Four studies focused on participants with substance misuse difficulties. Although drug/alcohol misuse is often relevant to people with AsPD, having a substance misuse problem is not part of the diagnostic criteria for AsPD.
All studies with usable data were trials of older medications, such as the antiepileptic drug phenytoin and tricyclic antidepressants such as nortriptyline, which are no longer widely used and which have been largely superseded by newer drugs with more favourable side-effect profiles.
Quality of the evidence
We identified eight studies that met the criteria for inclusion in this review, involving a total of 394 participants with AsPD. Of these, only four provided usable data, involving 274 participants with AsPD. We judged the overall quality of the evidence from these trials to be relatively poor for the following reasons:
The review relies on data from only four of the eight included studies, despite attempts to contact the trial investigators for information on the AsPD subgroups.
The study samples were heterogeneous, encompassing for example both prisoners and outpatients. In addition, AsPD was diagnosed under three similar but not identical rubrics (DSM-III, DSM-III-R, and DSM-IV).
The data available were generally insufficient to allow any independent statistical analysis.
All the available data were derived from unreplicated single reports.
There was inconsistency in the way primary and secondary outcomes were measured and reported.
The authors consider that the body of evidence summarised in this review is insufficient to allow any conclusion to be drawn about the use of pharmacological interventions in the treatment of AsPD.
Potential biases in the review process
None identified.
Agreements and disagreements with other studies or reviews
An earlier review on the pharmacotherapy of personality disorders (Markovitz 2004) found no controlled trials and only one case report on treatment of AsPD (with risperidone). The most relevant recent review with which to compare our findings is that carried out in the development of the NICE clinical guideline on AsPD (NIHCE 2009).
In reporting their systematic review, the NICE guideline authors observed that “the state of current practice in relation to the use of pharmacological interventions to treat antisocial personality disorder is unclear, but it is likely that pharmacological interventions are used in this population to treat symptoms rather than as an intervention for the disorder” (NIHCE 2009, para 7.5.1, page 232). They also noted three difficulties that arise when attempting to assess the effectiveness of drug interventions within this client group: lack of clarity as to whether the medication is being used to target the AsPD or a comorbid Axis I condition, the possibility that comorbid substance misuse may diminish response rates, and the likelihood that multiple neurotransmitter systems are involved making drug selection difficult. In recognition of this, they chose to consider not only interventions which targeted AsPD itself, but also those which targeted the symptoms or behaviours associated with the diagnosis (such as anger, impulsivity and aggression) as well as interventions specifically for offenders regardless of diagnosis. The review described by NIHCE 2009 thus is much broader than our current review which focuses solely on studies of participants with a diagnosis of AsPD.
Although the two reviews identified the same five studies (Barratt 1997; Hollander 2003; Leal 1994; Powell 1995; Stanford 2005) targeting treatment of AsPD and treatment of comorbid disorder in people with AsPD, there were several differences.
The current review identified three additional studies (Arndt 1994; Ralevski 2007; Stanford 2001) that were not included in the NIHCE review, although only one of these (Arndt 1994) had data available from the AsPD subgroup.
NIHCE 2009 considered three additional studies that were excluded from the current review because there was no indication that AsPD was represented in the sample: Mattes 2005 on oxcarbazepine versus placebo in outpatients with impulsive aggression; Mattes 2008 on levetiracetam versus placebo in outpatients with impulsive aggression; and Nickel 2005 on topiramate versus placebo in male outpatients with aggression.
NIHCE 2009 considered a further two trials looking specifically at offenders (Gottschalk 1973; Sheard 1976). These studies would not have been eligible for inclusion in the current review because the participants had no formal diagnosis of AsPD.
NIHCE 2009 found “no consistent evidence, including that from uncontrolled studies, that supported the use of any pharmacological intervention to treat antisocial personality disorder, or to treat the behaviour and symptoms that underline the specific diagnostic criteria for antisocial personality disorder” (para 7.5.5, page 237). They also found “no evidence on the cost-effectiveness of pharmacological interventions for AsPD with or without substance misuse” (para 7.5.6, page 237).
The overall recommendations from NIHCE 2009 were that (a) “pharmacological interventions should not be routinely used for the treatment of AsPD or associated behaviours of aggression, anger and impulsivity” (para 7.5.8.1, page 238), and (b) “pharmacological interventions for comorbid mental disorders, in particular depression and anxiety, should be in line with recommendations in the relevant NICE guideline” (para 7.5.8.2, page 238). The current review similarly concludes that good quality evidence favouring any pharmacological intervention for AsPD is virtually non-existent.
AUTHORS’ CONCLUSIONS
Implications for practice
There is insufficient evidence upon which to base recommendations for clinical practice. In the absence of good quality trial data, the use of pharmacological interventions to treat people with AsPD in clinical practice remains a matter for the clinician who will wish to weigh the limited evidence of effectiveness against any risk of possible harm; it should ideally be based on consultation with the patient, their carer (subject to their consent and right to confidentiality) and the multi-disciplinary team involved in individual care.
Implications for research
Studies with positive findings reported here require replicating to confirm apparent efficacy. Given the very few studies that could be considered in this review, there is clearly an imperative to conduct well-designed trials using pharmacological approaches. Such trials should recruit sufficient numbers of people on the basis of having the disorder and use outcomes measures that are of particular relevance to this disorder. They should also focus on recently marketed drugs where these have largely replaced older medications which are no longer widely used (for example, nortriptyline and phenytoin).
We are also concerned to note that the four trials whose data could be used in this review were all published more than a decade ago so that interest in trials for pharmacological interventions for this group appears to be diminishing rather than increasing. We speculate that one of the reasons for this reluctance by the industry to develop treatments for this group is a fear of litigation were something to go wrong. Whatever the reason, given the poor evidence base, we recognise that these initial trials are almost inevitably going to be of an active treatment against placebo rather than the more desirable investigation of one active medication against another. A major problem in carrying out such a trial in the community is that this is a notoriously difficult group to retain in treatment, as individuals with AsPD tend to be treatment-rejecting rather than treatment-seeking (NIHCE 2009). However, this caveat does not apply to those in prison or forensic health services where there is a large number of individuals incarcerated with AsPD. If this were the population chosen, then reconviction on release ought to be the outcome as reconviction is a relatively common outcome in many with AsPD with approximately two thirds of those being released from prison reoffending within two years (Home Office 1999; ONS 2004). We therefore recommend that reconviction is chosen as the primary outcome in such a trial, preferably in conjunction with an economic evaluation. If there was a consensus on a single outcome measured across studies, then it would be possible to make cross study comparisons, a task that is difficult to perform at the moment because of the wide range of outcomes and outcome measures that are used.
PLAIN LANGUAGE SUMMARY.
The use of medication to treat people with antisocial personality disorder
Antisocial personality disorder is a condition characterised by persistent social rule-breaking, deceitfulness, offending behaviour, irresponsibility, lack of remorse and failure to plan ahead. People with antisocial personality disorder often present with a range of other problems including alcohol and illicit drug misuse, anxiety, depression, unemployment, homelessness and relationship difficulties. The condition causes a great deal of distress for the individual concerned and to the people around them including family members and friends. Also, it puts a huge financial burden on the society in terms of health and social care expenditure. This review sets out to assess the evidence for the effectiveness of medication used to treat antisocial personality disorder.
We considered eight studies, but none of them recruited participants solely on the basis of having antisocial personality disorder. While most studies included in this review looked at treatments to reduce drug or alcohol misuse in people with antisocial personality disorder, no study focused on treating the disorder itself. Studies varied in terms of choice of outcomes. While some studies reported outcome measures that were originally defined in the review protocol as being of particular importance in this disorder (for example, aggression, social functioning and adverse effects resulting from the use of medication), no study reported on reconviction.
In summary, we were unable to draw any firm conclusions from the existing literature. Nonetheless, there was some evidence that nortriptyline (a drug used to treat depression) could help people with antisocial personality disorder to reduce their misuse of alcohol. There was also some evidence that phenytoin (a drug used to treat epilepsy) could help to reduce the intensity of impulsive aggressive acts in people with antisocial personality disorder. Further research is required to clarify which medications are effective for treating the core features of this disorder. This research is best carried out using carefully designed clinical trials. Such trials should recruit sufficient numbers of people on the basis of having the disorder and use outcome measures that are of particular relevance to this disorder. They should also focus on recently marketed drugs where these have largely replaced older medications (for example, nortriptyline and phenytoin) which are no longer widely used.
Table 4. Summary of inclusion and exclusion criteria.
| Studies | Participants | Interventions | Primary outcomes1 | Secondary outcomes1 |
|---|---|---|---|---|
| Inclusion criteria | ||||
| Controlled studies with random allocation With or without blinding Reported in any language |
Men or women Aged 18 years or over With AsPD diagnosis |
Any drug(s) with psychotropic properties | Aggression Reconviction Global functioning Social functioning Adverse events |
Quality of life Engagement Satisfaction Leaving the study early Substance misuse Employment status Housing status Economic outcomes Impulsivity Anger |
| Exclusion criteria | ||||
| Comorbid major functional mental illness | Studies comparing one drug with another Studies comparing a pharmacological and a psychological intervention | |||
studies reporting on at least one primary or secondary outcome were considered for inclusion
ACKNOWLEDGEMENTS
We gratefully acknowledge: Jo Abbott (Cochrane DPLPG) for running the electronic searches, Jane Dennis for advice on drafting the original protocol, Renate Reniers for translation of a paper from Dutch, and Cathy Bennett for advice and helpful comments on an early draft of this review.
SOURCES OF SUPPORT
Internal sources
Nottinghamshire Healthcare NHS Trust, UK.
German Federal Ministry of Education and Research, grant no. 01KG0812, Germany.
External sources
NHS Cochrane Collaboration Programme Grant Scheme, UK.
Appendix 1. MEDLINE search strategy
We searched MEDLINE (1950 to 11 September 2009) using the following terms:
exp Personality Disorders/
exp Antisocial Personality Disorder/
exp Borderline Personality Disorder/
exp Compulsive Personality Disorder/
exp Dependent Personality Disorder/
exp Histrionic Personality Disorder/
exp Hysteria/
exp Paranoid Personality Disorder/
exp Passive-Aggressive Personality Disorder/
exp Schizoid Personality Disorder/
exp Schizotypal Personality Disorder/
((asocial$ or antisocial$ or dissocial$ or psychopath$ or sadist$ or sociopath$) adj2 person$).tw.
psychopath.tw.
sociopath$.tw.
(moral adj2 insanity).tw.
(DSM and (axis and II)).tw.
or/1-16
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized controlled trials.sh.
random allocation.sh.
double blind method.sh.
single-blind method.sh.
or/18-23
(animal not human).sh.
24 not 25
clinical trial.pt.
exp clinical trials/
(clin$ adj25 trial$).ti,ab.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab.
Placebos.sh.
placebo$.ti,ab.
random$.ti,ab.
research design.sh.
or/27-34
35 not 25
36 not 26
comparative study.sh.
exp evaluation studies/
follow up studies.sh.
prospective studies.sh.
(control$ or prospectiv$ or volunteer$).ti,ab.
or/38-42
43 not 25
44 not (26 or 37)
26 or 37 or 45
17 and 46
Appendix 2. ASSIA search strategy
We searched Applied Social Sciences Index and Abstracts (1987 to September 2009) using the following terms: ((personality near disorder*) or ((antisocial* near disorder*) or (avoidant* near disorder*) or (borderline* near disorder*)) or ((dependent* near disorder*) or (histrionic* near disorder*) or (narcissistic* near disorder*)) or ((obsessive* near disorder*) or (compulsive* near disorder*) or (paranoid* near disorder*)) or (((passive* near disorder*) or (aggress* near disorder*) or (sadomasochistic* near disorder*)) or (schizo* near disorder*)) or (((passive* and disorder*) or (aggress* and disorder*) or (sadomasochistic* and disorder*)) or ((schizo* and disorder*) or (paranoid* and disorder*) or (compulsive* and disorder*)) or ((obsessive* and disorder*) or (narcissistic and disorder*) or (histrionic* and disorder*))) or (((personality and disorder*) or (antisocial* and disorder*) or (avoidant* and disorder*)) or ((borderline* and disorder*) or (dependent* and disorder*)))) and ((AB=randomi* or TI=randomi*) or (DE=(randomi?ed controlled trials) or AB=(double* blind*) or TI=(double* blind*)) or (DE=(double blind studies) or (single* near blind*)))
Appendix 3. BIOSIS search strategy
We searched BIOSIS (1985 to 16 September 2009) using the following terms: ((((al: ((personality and disorder))) or al: ((antisocial and behaviour))) or al: ((antisocial and behavior)) or (((al: ((self and defeating))) or al: ((parano* and person*))) or al: ((gender and identity)) or ((al: ((asocial or antisocial* or dissocial* or psychopath* or sadist* or sociopath*))) and al: ((person*)) and or (al: ((moral and insanity)) or ((al: ((psychopath* or sociopath* or dissocial* or sadis* or schizotypal self-defeating or borderline or avoidant or dependent or depressive))) and al: (person*) or ((al: ((histrionic or multi-impulsive or multiple or narcissistic or passive-aggressive))) and al: (person*) and ((al: ((randomi* or crossover or random-assignment))) or al: (((singl* or doubl* or tripl*or trebl*) and (mask* or blind*)))
Appendix 4. COPAC search strategy
We searched the Consortium of University Research Libraries joint catalogue (in September 2009) using the following terms: randomi* OR ((double OR single OR triple OR treble) and blind) OR prospective OR (clinical and trial) We then downloaded results into a Procite5 database and searched again using the terms: (antisocial* OR asocial* OR avoidant OR borderline OR dependent OR depressive OR dissocial OR dissocial* OR histrionic OR moral OR multi-impulsive OR multiple* OR narcissistic OR parano* OR passive-aggressive OR psychopath* OR sadis* OR schizotypal OR self-defeating OR sociopath*)
Appendix 5. CENTRAL search strategy
We searched CENTRAL (The Cochrane Library 2009, Issue 3) using the following terms: [(antisocial-personality-disorder*:me OR personality-disorders*:me OR sexual-and-gender-disorders*:me OR multiple-personality-disorder*:me OR paraphilias*:me) OR (multi-impulsive and personality) OR (parano* NEAR person*) OR (asocial* NEAR person) OR (dissocial* NEAR person) OR (psychopath* NEAR person) OR (sadist* NEAR person) OR (sociopath* NEAR person*) OR (moral NEAR insanity) OR ((personality and disorder*) and ((((avoidant OR multiimpulsive) OR narcissistic) OR self-defeating) OR personality)]
Appendix 6. CINAHL search strategy
We searched CINHAL (1982 to September 2009) using the following terms:
exp Personality Disorders/
exp Antisocial Personality Disorder/
exp Borderline Personality Disorder/
exp Compulsive Personality Disorder/
exp Dependent Personality Disorder/
exp Impulse Control Disorders/
exp Passive-Aggressive Personality Disorder/
(histrionic$ adj2 person$).tw.
(parano$ adj2 person$).tw.
(schizo$ adj3 person$).tw.
((asocial$ or antisocial$ or dissocial$ or psychopath$ or sadist$ or sociopath$) adj2 person$).tw.
psychopath.tw.
sociopath.tw.
(moral adj2 insanity).tw.
dyssocial.tw.
(DSM and (Axis and II)).tw.
or/1-16
randomi$.mp. [mp=title, subject heading word, abstract, instrumentation]
clin$.mp. [mp=title, subject heading word, abstract, instrumentation]
trial$.mp. [mp=title, subject heading word, abstract, instrumentation]
(clin$ adj3 trial$).mp. [mp=title, subject heading word, abstract, instrumentation]
singl$.mp. [mp=title, subject heading word, abstract, instrumentation]
doubl$.mp. [mp=title, subject heading word, abstract, instrumentation]
tripl$.mp. [mp=title, subject heading word, abstract, instrumentation]
trebl$.mp. [mp=title, subject heading word, abstract, instrumentation]
mask$.mp. [mp=title, subject heading word, abstract, instrumentation]
blind$.mp. [mp=title, subject heading word, abstract, instrumentation]
(22 or 23 or 24 or 25) and (26 or 27)
crossover.mp. [mp=title, subject heading word, abstract, instrumentation]
random$.mp. [mp=title, subject heading word, abstract, instrumentation]
allocate$.mp. [mp=title, subject heading word, abstract, instrumentation]
assign$.mp. [mp=title, subject heading word, abstract, instrumentation]
(random$ adj3 (allocate$ or assign$)).mp.
Random Assignment/
exp Clinical Trials/
exp Meta Analysis/
33 or 29 or 28 or 21 or 18 or 34 or 35 or 36
17 and 37
Appendix 7. EMBASE search strategy
We searched EMBASE (1980 to 37th week 2009) using the following terms:
exp Personality Disorder/
exp Borderline State/
exp Character Disorder/
exp Compulsive Personality Disorder/
exp DELUSION/
exp Dependent Personality Disorder/
exp DEPERSONALIZATION/
exp JEALOUSY/
exp KLEPTOMANIA/
exp Multiple Personality/
exp NARCISSISM/
exp PSYCHOPATHY/
exp SCHIZOIDISM/
exp SOCIOPATHY/
(antisoci$ adj2 person$).tw.
(aggres$ adj2 person$).tw.
(border$ adj2 person$).tw.
histrion$ person$.tw.
paranoid person$.tw.
(passive adj2 aggressive).tw.
((asocial$ or antisocial$ or dissocial$ or psychopath$ or sadist$ or sociopath$) adj person$).tw.
(moral adj2 insan$).tw.
dyssocial.tw.
(DSM and (Axis and II)).tw.
or/1-24
clin$.tw.
trial$.tw.
(clin$ adj3 trial$).tw.
singl$.tw.
doubl$.tw.
trebl$.tw.
tripl$.tw.
blind$.tw.
mask$.tw.
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
randomi$.tw.
random$.tw.
allocat$.tw.
assign$.tw.
(random$ adj3 (allocat$ or assign$)).tw.
crossover.tw.
41 or 40 or 36 or 35 or 28
exp Randomized Controlled Trial/
exp Double Blind Procedure/
exp Crossover Procedure/
exp Single Blind Procedure/
exp RANDOMIZATION/
43 or 44 or 45 or 46 or 47 or 42
25 and 48
Appendix 8. NATIONAL CRIMINAL JUSTICE REFERENCE SERVICE ABSTRACTS search strategy
We searched NCJRS (1970 to July 2008) using the following terms: (randomi* OR double blind) and (antisocial* OR asocial* OR avoidant OR borderline OR dependent OR depressive OR dissocial OR dissocial* OR histrionic OR moral OR multiimpulsive OR multiple* OR narcissistic OR parano* OR passiveaggressive OR psychopath* OR sadis* OR schizotypal OR selfdefeating OR sociopath*)
Appendix 9. PsycINFO search strategy
We searched PsycINFO (1872 to 2nd week September 2009) using the following terms:
Personality Disorders/
exp Antisocial Personality Disorder/
exp Avoidant Personality Disorder/
exp Borderline Personality Disorder/
exp Dependent Personality Disorder/
exp Histrionic Personality Disorder/
exp Narcissistic Personality Disorder/
exp Obsessive Compulsive Personality Disorder/
exp Paranoid Personality Disorder/
exp Passive Aggressive Personality Disorder/
exp Sadomasochistic Personality/
exp Schizoid Personality Disorder/
exp Schizotypal Personality Disorder/
(personality adj disorders).tw.
(antisocial adj personality).tw.
(avoidant adj personality).tw.
(borderline adj personality).tw.
(dependent adj personality).tw.
(histrionic adj (personality and disorder)).tw.
(narcissistic adj personality).tw.
(obsessive adj (compulsive and personality)).tw.
(paranoid adj personality).tw.
(passive adj (aggressive and personality)).tw.
(sadomasochistic adj personality).tw.
(schizoid adj personality).tw.
(schizotypal adj personality).tw.
or/1-26
randomi$.tw.
singl$.tw.
doubl$.tw.
trebl$.tw.
tripl$.tw.
blind$.tw.
mask$.tw.
(or/29-32) adj3 (or/33-34)
clin$.tw.
trial$.tw.
(clin$ adj3 trial$).tw.
placebo$.tw.
exp PLACEBO/
crossover.tw.
exp Treatment Effectiveness Evaluation/
exp Mental Health Program Evaluation/
random$.tw.
assign$.tw.
allocate$.tw.
(random$ adj3 (assign$ or allocate$)).tw.
27 or 35 or 38 or 39 or 40 or 41 or 42 or 43 or 47
27 and 48
Appendix 10. SIGLE search strategy
We searched SIGLE (1980 to April 2006) using the following terms: ((randomisation) OR (randomised) OR (randomisee) OR (randomises) OR (randomize) OR (randomized) OR (randomly) OR ((double AND blind) OR double-blind OR double* blind* OR randomi?ed controlled trials)) AND ((psychopath* OR sociopath* OR dissocial OR sadis* OR schizotypal OR selfdefeating OR borderline OR avoidant OR dependent OR depressive OR histrionic OR multi-impulsive OR multiple OR narcissistic OR passive-aggressive) AND (person*) OR (antisocial AND behaviour) OR (personality AND disorder*) OR (gender AND identity) OR (parano* AND person*) OR (self AND defeating) OR ((asocial* OR antisocial* OR dissocial* OR psychopath* OR sadist* OR sociopath*) AND person*) OR (moral AND insanity))
Appendix 11. SOCIOLOGICAL ABSTRACTS search strategy
We searched SOCIOLOGICAL ABSTRACTS (1963 to September 2009) using the following terms: ((personality near disorder*) or ((antisocial* near disorder*) or (avoidant* near disorder*) or (borderline* near disorder*)) or ((dependent* near disorder*) or (histrionic* near disorder*) or (narcissistic* near disorder*)) or ((obsessive* near disorder*) or (compulsive* near disorder*) or (paranoid* near disorder*)) or (((passive* near disorder*) or (aggress* near disorder*) or (sadomasochistic* near disorder*)) or (schizo* near disorder*)) or (((passive* and disorder*) or (aggress* and disorder*) or (sadomasochistic* and disorder*)) or ((schizo* and disorder*) or (paranoid* and disorder*) or (compulsive* and disorder*)) or ((obsessive* and disorder*) or (narcissistic and disorder*) or (histrionic* and disorder*))) or (((personality and disorder*) or (antisocial* and disorder*) or (avoidant* and disorder*)) or ((borderline* and disorder*) or (dependent* and disorder*)))) and ((AB=randomi* or TI=randomi*) or (DE=(randomi?ed controlled trials) or AB= (double* blind*) or TI=(double* blind*)) or (DE=(double blind studies) or (single* near blind*)))
Appendix 12. ISI-PROCEEDINGS, Science Citation Index and Social Sciences Citation Index strategies
We searched ISI-Proceedings, the Science Citation Index and the Social Sciences Citation Index (1981 to 12 September 2009) using the following terms: (double blind OR randomi*) AND ((passive-aggressive OR psychopath* OR sociopath* OR dissocial OR sadis* OR schizotypal OR self-defeating OR borderline OR avoidant OR dependent OR depressive OR parano* OR asocial* OR antisocial* OR dissocial* OR psychopath* OR sadist* OR sociopath* OR histrionic OR multi-impulsive OR multiple* OR narcissistic) AND personality*) OR ((moral AND insanity) OR (self AND defeating) OR (gender AND identity) OR (personality AND disorder) OR (antisocial AND behaviour))
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Design: placebo-controlled parallel trial | |
| Participants | Participants: methadone-maintained male outpatients with cocaine dependency (AsPD subgroup) Sex: male only Age: (for whole sample): mean 40.5 years; range 29 to 59 years Unit of allocation: individual participant Number randomised: 79 (number with AsPD not reported) (see note 1) Number completing: 29 with AsPD (desipramine, n = 17; control, n = 12) (see note 1) Setting: outpatient; single site; USA (Philadelphia) Inclusion criteria: cocaine dependence lasting at least 3 months (DSM-III; NIMH Diagnostic Interview Schedule); aged 20 to 60 years; cocaine-positive urines over one month prior to being contacted for participation Exclusion criteria: medical condition contraindicating desipramine use; cocaine misuse disorder lasting less than 3 months Ethnicity: (for whole sample): black American (90%) Baseline characteristics: (for whole sample): male service veterans (100%); on methadone maintenance for at least 1 month (100%); average methadone dose 45 mg/day (range 1585 mg/day); reported using cocaine intravenously (83%); reported ‘free basing’ (15%) ; reported intranasal use (11%); employed (79%); educated to high school degree level (53%); some college education (29%); married (35%); never married (35%); separated or divorced (30%) |
|
| Interventions | Two conditions: desipramine/placebo
All participants received standard clinical services including weekly drug counselling, social work services as needed, employment counselling, referral psychiatric and medical care Duration of intervention: 12 weeks Duration of trial: 12 weeks Length of follow up: participants were not followed up beyond the study period Dose adjustment: desipramine 50 mg/day initially increased by 50 mg every 2 to 4 days as tolerated to a target dose of 250 to 300 mg/day; mean blood levels 185 mg/ml (range 85 to 270 mg/ml) |
|
| Outcomes |
Primary outcomes
Social functioning: days family/social problems in past 30 days (Addiction Severity Index) Secondary outcomes Substance misuse: urinanalysis; days opiate use in past 30 days via the Addiction Severity Index (ASI); days cocaine use in past 30 days (ASI); days depressant use in past 30 days (ASI); cocaine craving score (see note 2) Employment status: days worked in past 30 days (ASI); employment income (ASI) Other outcomes Depression (Beck Depression Inventory); days medical problems in past 30 days (ASI) ; days illegal activity in past 30 days (ASI); illegal income (ASI); days psychological problems past 30 days (ASI) |
|
| Notes |
|
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | No information given. Clarification about method of sequence generation has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Allocation concealment? | Unclear | No information given. Clarification about method of sequence generation has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Blinding? of participants |
Unclear | Investigators described the study as “double-blind” and report that an independent researcher “gave directions for changing the dose given to the patients receiving placebo so that the double-blind condition was maintained” (Arndt 1992, p.889, col 2). No further information given. Clarification about method of sequence generation has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Blinding? of personnel |
Yes | Investigators reported that “study physicians were blind to the blood level results [which were] provided to an independent research physician who recommended increases or decreases to achieve the desirable blood level” (Arndt 1994, p.152, col 2), and also that the BDI outcome measure was “administered by trained research technicians who were experimentally blind and independent of the treatment program” (Arndt 1992, p.889, col 1). Review authors judged that appropriate care was taken to ensure blinding of study personnel, and that it was unlikely that this blinding could have been broken |
| Blinding? of outcome assessors |
Yes | Investigators reported that “study physicians were blind to the blood level results [which were] provided to an independent research physician who recommended increases or decreases to achieve the desirable blood level” (Arndt 1994, p.152, col 2), and also that the BDI outcome measure was “administered by trained research technicians who were experimentally blind and independent of the treatment program” (Arndt 1992, p.889, col 1). Review authors judged that appropriate care was taken to ensure blinding ofoutcome assessors, and that it was unlikely that this blinding could have been broken |
| Incomplete outcome data addressed? All outcomes |
No | Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. Investigators did not provide numbers of non-completers for the AsPD subgroup, but supplied (in Arndt 1992) the following data for the whole sample. For the desipramine group, 17 out of 53 (32%) discontinued because of side effects (4), non-compliance with the protocol (4), hospitalisation unrelated to desipramine treatment (3), legal violations (3), or for other reasons (3). For the control group, 3 out of 26 (12%) discontinued (reasons not given) . Numbers of missing data are thus not balanced between experimental conditions for the whole sample. Clarification has been requested from the trial investigators but no further information was available at the time this review was prepared In this review, data from the 29 participants with AsPD who completed the study were included in the analysis (desipramine condition, n = 17; control condition, n = 12) |
| Free of selective reporting? | Yes | Study protocol is not available but it seems clear that the published report included all expected outcomes, including those that were pre-specified |
| Free of other bias? | Yes | The study appeared to be free of other sources of bias. |
| Methods | Design: placebo-controlled crossover trial | |
| Participants | Participants: male prisoners with recurrent aggressive behaviour Sex: male only Age: adults; age not reported Unit of allocation: individual participant Number randomised: 150 Number completing: 126; results reported for 60 (30 with primarily impulsive aggression and 30 with primarily premeditated aggression; remaining 66 had committed mixed types of aggression and were not included) (see note 2) Setting: prisons; multiple sites; USA (Texas) Inclusion criteria: history of at least 3 documented aggressive acts within prison over a 3 months period prior to commencing the study. Aggressive acts classified as impulsive and non-impulsive based on interview and prison reports (see note 1) Exclusion criteria: Verbal and performance IQ of less than 80; DSM-III-Raxis I disorder as measured by Psychiatric Diagnostic Interview-revised (PDI-R); neurological or other serious medical disorder; taking medication Ethnicity: not reported here, but the baseline study paper (Barratt 1997a) reported: African-American (53%); Hispanic (24%); white (24%) Baseline characteristics: DSM-III-R AsPD (100%); history of aggressive behaviour prior to incarceration (98%); participants had mean of 6.2 incidents of aggression recorded against them while in prison; lifetime substance misuse diagnosis (55%). Other baseline measures including ERP, profile of mood states (POMS), neuropsychology measures and personality traits are reported in the baseline study paper (Barratt 1997a), although investigators noted there was only a 90% overlap between the 2 studies |
|
| Interventions | Two conditions: phenytoin/placebo (crossover design)
Duration of intervention: 6 weeks for each condition with 1 week washout between the 2 phases Duration of trial: 13 weeks (cross-over trial; two phases; one-week washout period between phases) Length of follow up: participants were not followed up beyond the end of the intervention period Dose adjustment: no information given |
|
| Outcomes |
Primary outcomes
Aggression: frequency of aggressive acts; intensity of aggressive acts (modified Overt Aggression Scale) Adverse events: blood cell counts; liver function tests Secondary outcomes Anger: Profile of Mood States (POMS) anger-hostility subscale Other outcomes P300 peak amplitude and latency (electroencephalogram, ERP oddball task); phenytoin blood levels (in relation to frequency and intensity of aggressive acts); other subscales of Profile of Mood States (tension-anxiety, vigour, depression-dejection, fatigue inertia, confusion bewilderment) |
|
| Notes |
|
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Investigators reported that participants “were randomly assigned to an initial drug/placebo condition” (p.3) suggesting that the order of treatments was randomised in this cross-over trial. No further details reported. Insufficient information provided to allow judgment to be made. Clarification about method of sequence generation has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Allocation concealment? | Unclear | No details reported. Insufficient information provided to allow judgment to be made. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Blinding? of participants |
Unclear | Investigators describe the study as “double-blind”. No further details reported. Insufficient information provided to allow judgment to be made, although bloods were taken during placebo treatment, possibly to maintain blinding. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Blinding? of personnel |
Unclear | Investigators describe the study as “double-blind”. No further details reported. Insufficient information provided to allow judgment to be made. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Blinding? of outcome assessors |
Unclear | Investigators describe the study as “double-blind”. No further details reported. Insufficient information provided to allow judgment to be made. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Incomplete outcome data addressed? All outcomes |
Unclear | Insufficient reporting of attrition to permit judgement of ‘Yes’ or ‘No’. Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. It appears that 24 did not complete study, and a subgroup of 66 (with ‘mixed’ type of aggression) were excluded by investigators. Clarification has been requested from the trial investigators but no further information was available at the time this review was prepared. In this review, data from the subgroup of 60 completers were included in the analysis |
| Free of selective reporting? | Yes | Study protocol is not available but it seems clear that the published report includes all expected outcomes |
| Free of other bias? | Unclear | There was a one-week placebo washout period between phases in this cross-over trial and the trial investigators reported no significant cross-over effects for the aggression measures for the combined groups suggesting the study was not biased by carryover effects. An important source of bias would be the criminogenic programmes delivered in prison if such programmes were delivered in different proportions to each randomised condition, although no information is provided on this. It is also not clear whether participants were engaged in any of these during the study period. It is not clear what effects the exclusion of ‘mixed aggression group’ would have on the results Of the 150 participants randomised, results were reported for 60 of 126 completers only (30 committing primarily impulsive and 30 committing primarily premeditated aggression; remaining 66 had committed mixed types of aggression and were not included) . Thus there is the possibility of bias arising through excluding the ‘mixed aggression’ group, although it is unclear what effect this would have on the results |
| Methods | Design: placebo-controlled parallel trial | |
| Participants | Participants: adults with impulsive aggression (subgroup with AsPD; see note 1) Sex: mixed (72.5% men in whole sample including non-AsPD participants) Age: mean 40.3 years; range 19 to 67 years (whole sample including non-AsPD participants) Unit of allocation: individual participant Length of followup: Number randomised: 233 in whole sample; 9 with AsPD (see note 1; breakdown by treatment condition not reported) Number completing: not reported Setting: outpatient; 19 sites; USA Inclusion criteria: aged 18 to 65 years; diagnosis of cluster B personality disorder (DSM-IV; SCID-II) or intermittent explosive disorder (IED), or PTSD; average of two episodes of physical or verbal aggressive outbursts per week for at least a month prior to screening, causing marked distress or impairment in occupational or interpersonal function where the aggressive behaviour was judged to be neither premeditated nor committed to achieve a tangible objective; minimum score of 15 on OAS at first screening visit and at either the second screening visit or at randomisation; if receiving psychotherapy, have a stable psychotherapy schedule for at least 3 months prior to screening and maintained throughout the study Exclusion criteria: lifetime bipolar I disorder; bipolar II disorder with hypomania in the last year or a baseline Mania Syndrome Scale Score >= 12; major depressive disorder > 15 on HAM-D; history of schizophrenia or other psychotic disorder; symptoms of dementia; serious homicidal or suicidal ideation; impulsive aggression resulting from previous head trauma or other medical condition; pregnant or lactating females; clinically abnormal laboratory data; unstable medical condition; any underlying condition that would confound the interpretation of study results; concurrent use of psychotropic medication, with exception of SSRIs, tricyclic antidepressants and stimulants if taken at a stable dose for at least 2 months prior to screening and continued at same dose throughout the study; participants specifically prohibited from use of benzodiazepines, mood stabilisers, anticonvulsants, MAOIs and antipsychotic agents (see note 2) Ethnicity: 195 Caucasian, 26 black, 12 other (whole sample including non-AsPD participants) Baseline characteristics: (whole sample including non-AsPD): at least one psychiatric hospitalisation (n = 36); history of alcohol misuse/dependence (n = 75); history of drug misuse/dependence (n = 38); history of incarceration (n = 52) |
|
| Interventions | Two conditions: divalproex sodium / placebo
Duration of intervention: 12 weeks Duration of trial: 15 weeks (treatment preceded by screening period not exceeding 14 days and followed by one-week tapering period) Length offollow up: participants were not followed up beyond the end ofthe intervention period Dose adjustment: initiated at 500 mg/day, and increased by 250 mg every 3 to 7 days during first three weeks of treatment, based on individual clinical response and tolerance. Maximum dose 30 mg/kg/day |
|
| Outcomes |
Primary outcomes
Aggression (self-reported): Overt Aggression Scale-Modified (OAS-M) scores Global state/functioning: Clinical Global Impression (CGI) scores Adverse events: assessment by attending physician Secondary outcomes Leaving the study early: proportion of participants discontinuing treatment Other outcomes None |
|
| Notes |
|
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Investigators reported “patients were randomised in equal numbers, within each of the three diagnostic groups, to receive either divalproex sodium delayed-release tablets … or matching placebo” (col 1, page 1188). No further details given. Insufficient information to permit judgement on adequacy of sequence generation. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Allocation concealment? | Unclear | Insufficient information to permit judgement on adequacy of allocation concealment. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Blinding? of participants |
Yes | Investigators describe study throughout as “double-blind” and that participants received a “matching placebo”. Review authors judged that blinding of participants was adequate and that it was unlikely that this blinding could have been broken |
| Blinding? of personnel |
Yes | Investigators reported “An unblinded person from the central laboratory reported serum valproate levels … to the investigators, so that the dose of the study drug could be adjusted appropriately. In order to preserve the study blind, sham valproate levels were reported for selected placebo patients” (p.1188, col 1). Review authors judged that blinding of personnel was adequate and that it was unlikely that this blinding could have been broken |
| Blinding? of outcome assessors |
Unclear | Insufficient information to permit judgement on adequacy of blinding of outcome assessors |
| Incomplete outcome data addressed? All outcomes |
Unclear | Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. Overall, 54/124 (44%) of the treatment group and 47/122 (39%) of the control group discontinued prematurely, with reasons for non-completion approximately balanced between conditions. Review authors unable to make a judgement unless data from the (small) AsPD subgroup (n = 9) become available |
| Free of selective reporting? | Yes | Study protocol is not available but it seems clear that the published report included all expected outcomes, including those that were pre-specified |
| Free of other bias? | Yes | The investigators note that the mean final valproate serum level was 64.2 μg/ml, which is well below possible therapeutic range (80-120 μg/ml) based on previous studies. The study appeared to be free of other sources of bias |
| Methods | Design: placebo-controlled parallel trial | |
| Participants | Participants: methadone-maintained inpatients meeting DSM-III-R criteria for opioid and cocaine dependence (non-depressed AsPD subgroup) Sex: 11 male; 8 female (for the AsPD subgroup) Age: mean 33.0 (SD 4.5) years (for the AsPD subgroup) Unit of allocation: individual participant Number randomised: 19 (desipramine, n = 7; amantadine, n = 8; control, n = 4) (see note 1) Number completing: 11 (desipramine, n = 5; amantadine, n = 3; control, n = 3) (see note 1) Setting: inpatient; single site; USA (Yale) Inclusion criteria: AsPD diagnosis without depression (DSM-III-R; SCID-II); opioid and cocaine dependency (DSM-III-R) Exclusion criteria: concurrent DSM-III-R depression; zidovudine treatment for AIDS; medical contra-indications including asthma, renal dysfunction, high blood pressure and diabetes; current alcoholism; refusal to use adequate birth control if female Ethnicity: white (68%, n = 13) (for the AsPD subgroup) Baseline characteristics: for AsPD subgroup (see note 1): married (74%, n = 14); mean methadone dose 57 (SD 11) mg/day; mean time on methadone 4.5 (SD 2.7) months; mean time using heroin 12.0 (SD 6.2) years; mean time using cocaine 7.5 (SD 6.1) years; mean expenditure on cocaine 1141 (SD 1379) US Dollars/month; lifetime diagnosis alcohol misuse disorder (58%), mean time intoxicated 1.7 (SD 3.6) days/month; mean Addiction Severity Index factor scores: psychiatric, 4.3 (SD 2.4); medical, 3.3 (SD 2.0) ; job, 5.9 (SD 2.7); alcohol, 3.5 (SD 2.5); drug, 8.2 (SD 0.7); family, 5.6 (SD 2.1) |
|
| Interventions | Three conditions: amantadine/ desipramine/ placebo
Duration of intervention: 12 weeks Duration of trial: 12 weeks Length of follow up: participants were not followed up beyond the end of the intervention period Dose adjustment: no information given |
|
| Outcomes |
Primary outcomes
None Secondary outcomes Leaving the study early: treatment retention in the first and the last 6 weeks of treatment Substance misuse: urinalysis to detect cocaine-free urine samples (on-site enzyme-multiplied immunoassay (EMIT) system); total US Dollars/week spent on cocaine (self report) Other outcomes Depression (Beck Depression Inventory) |
|
| Notes | 1. Study also reported data for an additional 75 participants without AsPD; these data are not included | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Investigators described a “ … randomised, double-blind trial …” (p.32, col 2). No further details reported. Insufficient information to permit judgement on adequacy of sequence generation. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Allocation concealment? | Unclear | Investigators described a “ … randomised, double-blind trial …” (p.32, col 2). No further details reported. Insufficient information to permit judgement on adequacy of allocation concealment. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Blinding? of participants |
Unclear | Investigators described the trial as “ double-blind ” (p.32, col 2). No further details reported. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Blinding? of personnel |
Unclear | Investigators described the trial as “ double-blind ” (p.32, col 2). No further details reported. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Blinding? of outcome assessors |
Unclear | Investigators describe the trial as “ double-blind ” (p.32, col 2). No further details reported. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Incomplete outcome data addressed? All outcomes |
No | Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. The investigators reported that “fifteen patients left the study for medication non-compliance, four for incarceration, and one for medical reasons” (p. 32, col 2), but these figures apply to the whole sample including non-AsPD participants. For the AsPD subgroup, 5/8 (63%) were missing from the amantadine group, 2/7 (29%) were missing from the desipramine group and 1/4 (25%) missing from the control group - all for reasons that are unclear and no breakdown by experimental group was provided. Clarification has been requested from the trial investigators but no further information was available at the time this review was prepared |
| Free of selective reporting? | Yes | Study protocol is not available but it seems clear that the published report includes all expected outcomes, including those that were pre-specified |
| Free of other bias? | No | Two potential sources of bias were identified. First, it is not clear whether patients continued to receive general substance misuse counselling and behavioural contingency management during this trial, and if so whether this was similar for both treatment and control conditions. This is important since the latter involves monetary incentives in return for a clean urine sample. Differences in percentages of cocaine-free urine samples may be related to that rather than the effects of medications. Second, urinanalysis was carried out twice weekly, but the detectability window for cocaine is 6-8 hours (Wolff 1999) which increases the possibility of false negative results. It is noteworthy that four participants meeting criteria for AsPD plus dysthymia were included in the non-ASP group and so their results are not included; investigators justify this because “the diagnosis of depression has been reported to favourably affect the treatment outcome of patients with antisocial personality disorder” (page 32, col 1) |
| Methods | Design: placebo-controlled parallel trial | |
| Participants | Participants: men with alcohol dependence and comorbid psychiatric disorders (subgroup with AsPD) Sex: male only Age: not reported for AsPD subgroup; for the whole sample, mean 41.3 (SD = 9.2) years Unit of allocation: individual participant Number randomised: 65 with AsPD (out of 216; see note 1) Number completing: 29 with AsPD (out of 99; see note 1) Setting: initially inpatient, then outpatient; two sites; USA (Kansas and Topeka) Inclusion criteria: alcohol dependence (DSM-III-R; measured using the PDI-R) Exclusion criteria: medical condition contraindicating use of tricyclic antidepressants or bromocriptine; receiving psychotropic medication (see note 2), living more than 150 miles from treatment site Ethnicity: for the whole sample: white (63%); black (32%); native American (2%); other (3%) Baseline characteristics: for the whole sample: all veterans; college degree (4%); some college or vocational training (39%); high school education or equivalent (47%); less than high school education (10%); married (21%); separated (15%); divorced/widowed (43%); never married (20%); employed full or part time in the month before admission (50%); retired (4%); student (1%); unemployed (45%) |
|
| Interventions | Three conditions: nortriptyline / bromocriptine / placebo nortriptyline arm
Duration of intervention: 6 months Duration of trial: 6 months Length of follow up: participants were not followed up beyond the end of the intervention period Dose adjustment: nortriptyline dose adjusted to therapeutic levels (50 to 150 ng/ml) with corresponding increase in capsules made for placebo patients; bromocriptine initially 2.5 mg three times daily, but increased to 5 mg three times daily from month 4 to month 6; placebo as matching capsules with number adjusted to match a participant in the active treatment group Other notes: participants reimbursed USD 15 for each clinic visit; payment was not made until the participant either dropped out or completed the study |
|
| Outcomes |
Primary outcomes
Global state/functioning: Global Assessment Scale (GAS); global severity index sub scale of the Symptom Check List-90 (SCL-90) Adverse effects: via participant self-report Secondary outcomes Substance misuse: Severity of alcoholism (Alcohol Severity Scale, self report); alcohol craving (visual analogue scale); alcohol dependence (Severity of Alcohol Dependence Questionnaire) Other outcomes depression (Beck Depression Inventory; SCL-90 depression sub scale); anxiety (Beck Anxiety Inventory; SCL-90 anxiety sub scale); problem behaviours (Problem Behavior Checklist) |
|
| Notes |
|
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | A two-stage randomisation process was used; patients within each of the subgroups (alcoholism alone, alcoholism + mood/anxietydis-orders, alcoholism + AsPD) “were first randomly assigned to the bromocriptine or nor-triptyline arm of the study. Patients within each drug arm were then randomised to receive either active drug or placebo” (p.463, col 1). No further information given. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Allocation concealment? | Unclear | A two-stage randomisation process was used (see above). “Patients within each drug arm were then randomised to receive either active drug or placebo” (p.463, col 1). No further information given. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Blinding? of participants |
Yes | Investigators reported that “both active medications and placebo were prepared as identical-appearing capsules … . when the number of pills was increased for a given patient, a corresponding increase in pills was made for a placebo patient” (p.463, col 1). Review authors judged that appropriate care was taken to ensure blinding of participants, and that it was unlikely that this blinding could have been broken |
| Blinding? of personnel |
Yes | Investigators reported that “at each visit, a physician blinded to the patients’ treatment assignment obtained blood samples and systematically recorded pill counts, medication side effects and other medical information” (p.463, col 2). Review authors judged that appropriate care was taken to ensure blinding of trial personnel, and that it was unlikely that this blinding could have been broken |
| Blinding? of outcome assessors |
Unclear | Investigators reported that “a research assistant then recorded number of drinking days and patient rating of alcohol craving since the last follow-up visit” (p.463, col 2), but there was no indication that this research assistant was blinded, other than the statement that the study was “double-blind”. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Incomplete outcome data addressed? All outcomes |
No | Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. The investigators reported significant dropout rates for the comorbidity and medication subgroups which ranged from 52.1% to 55.4%, and note that there were 99 participants (overall) who completed the study and 117 (overall) who did not (p.464, col 1). No details on missing data were provided for the AsPD subgroup. Clarification has been requested from the trial investigators but no further information was available at the time this review was prepared In this review, data from the 29 participants with AsPD who completed the study were included in the analysis (bromocriptine condition, n = 9; nortriptyline condition, n = 11; control condition, n = 9) |
| Free of selective reporting? | Yes | Study protocol is not available but it seems clear that the published report includes all expected outcomes, including those that were pre-specified |
| Free of other bias? | Yes | The study appeared to be free of other sources of bias. |
| Methods | Design: placebo-controlled parallel trial | |
| Participants | Participants: treatment-seeking adults with alcohol dependency and a current Axis I disorder (subgroup with AsPD) Sex: AsPD subgroup: 93 male; 2 female Age: AsPD subgroup: mean 44.2 (SD 6.6) years Unit of allocation: individual participant Number randomised: AsPD subgroup: n = 95 (numbers allocated to treatment and control conditions not reported; see note 1) Number completing: unclear Setting: outpatient; three sites; USA (New England) Inclusion criteria: For whole sample (see note 1): treatment-seeking; alcohol dependency with at least one other current Axis I disorder (DSM-IV; SCID-I); alcohol use within the past 30 days; stable dose of psychiatric medication for at least 2 weeks if on medication. Additionally for AsPD subgroup: presence of AsPD diagnosis (DSM-IV; SCID-II) Exclusion criteria: unstable psychotic symptoms; serious current psychiatric symptoms such as suicidal or homicidal ideation; current opiate dependence; contraindication to the use of naltrexone and disulfiram including liver function tests greater than three times the normal Ethnicity: AsPD subgroup: white (72.6%, n = 69); black (14.7%, n = 14); Hispanic (7. 4%, n = 7); native American (4.2%, n = 4); other (1.0%, n = 1) Baseline characteristics: AsPD subgroup: all veterans; mean duration of alcohol use 25. 3 (SD 8.9) years; mean number of drinking days in last 30 days 14.3 (SD 12.3) days; mean total number of drinks in last 30 days 326.5 (SD 338.7); mean number of heavy drinking days in last 30 days 13.4 (SD 12.1); mean baseline ADS score 23.5 (SD 7.8); any psychiatric medication (82.3%, n = 79); antidepressants (58.5%, n = 55); anxiolytics (6.3%, n = 6); mood stabilizers (40.6%, n = 39); antipsychotics (22.9%, n = 22); taking more than one medication (43.7%, n = 42) |
|
| Interventions | Two conditions: naltrexone / placebo (see note 2)
All participants received weekly Clinical Management / Compliance Enhancement Therapy focused on discussing negative consequences of drinking, relapse prevention, compliance monitoring and psychoeducation plus treatment as usual (rehabilitation with aftercare and supported housing options) Duration of intervention: 12 weeks Duration of trial: 12 weeks (no washout period) Length of follow up: participants were not followed up beyond the end of the intervention period Dose adjustment: no information |
|
| Outcomes |
Primary outcomes
Adverse events: Hopkins Symptom Checklist (self-report) (described in Petrakis 2005 (p.1130, col 1) but no details reported for AsPD subgroup) Secondary outcomes Substance misuse: Alcohol use (Timeline Follow-Back Interview); Alcohol craving (Obsessive Compulsive Drinking Scale) Leaving the study early: treatment retention |
|
| Notes |
|
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | No information provided. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Allocation concealment? | Unclear | No information provided on how allocation sequence was concealed. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Blinding? of participants |
Unclear | Trial investigators reported that naltrexone was given in a double-blinded fashion, but provided no further information. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Blinding? of personnel |
Unclear | Trial investigators reported that naltrexone was given in a double-blinded fashion, but provided no further information. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Blinding? of outcome assessors |
Unclear | Trial investigators reported that naltrexone was given in a double-blinded fashion, but provided no further information. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Incomplete outcome data addressed? All outcomes |
Unclear | Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. Trial investigators provided no information on the numbers randomised to each condition, nor on the extent of missing data. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Free of selective reporting? | Unclear | A companion paper (Petrakis 2005) indicated that adverse events were measured weekly via the Hopkins Symptom Checklist, but these are not reported here or in that paper. Clarification has been requested from the trial investigators, but no further information was available at the time this review was prepared |
| Free of other bias? | Yes | The study appeared free from other sources of potential bias, although the trial investigators acknowledge the possibility of bias arising from the confounding effects of Axis I disorders and their inability to test whether improvement in personality disorder symptoms were related to medication treatment |
| Methods | Design: placebo-controlled cross-over trial | |
| Participants | Participants: men with DSM-IV personality disorder and impulsive aggressive behaviour (subgroup with AsPD; see note 1) Sex: male only Age: mean 45.1 (SD 6.8) years (whole sample including non-AsPD participants) Unit of allocation: individual participant Number randomised: 46 in whole sample, 10 with AsPD (see note 1) Number completing: not reported Setting: outpatient; single site; USA (New Orleans) Inclusion criteria: over past 6 months, several discrete participant-identified episodes of failure to resist aggressive impulses resulting in serious assaultative acts or destruction of property; degree of aggressiveness expressed during the episodes was grossly out of proportion to any precipitating psychosocial stressor; at least two such episodes during the month prior to entering the study; score of 8 or higher on the Irritability sub scale of the Buss-Durkee Hostility Inventory; must have identified an individual willing to document any impulsive-aggressive outbursts that occurred during the study Exclusion criteria: female (due to potential teratogenic effects of phenytoin); verbal IQ < 80; diagnosis of a DSM-IV-TR Axis I psychiatric disorder; present use of medication; medical/neurological problems (including seizures); liver enzymes not within normal limits Ethnicity: not reported Baseline characteristics: mean verbal IQ 105.8 (SD 10.7); mean 14.3 (SD 2.4) years education; DSM-IV personality disorder diagnoses for phase one completers: obsessive-compulsive personality disorder (n = 12), AsPD (n = 10), narcissistic personality disorder (n = l) |
|
| Interventions | Two conditions: phenytoin / placebo
Duration of intervention: 6 weeks Duration of trial: 16 weeks (cross-over trial; two phases, 2-week placebo baseline period, and 2-week placebo washout period between phases) Length of follow up: participants were not followed up beyond the end of the intervention period Dose adjustment: no details reported |
|
| Outcomes |
Primary outcomes
Aggression (observer-reported): Overt Aggression Scale (OAS) scores Secondary outcomes Anger-hostility: Profile of Mood States anger-hostility subscale scores Other outcomes Psychophysiological recordings (including evoked potentials) |
|
| Notes | 1. 43% of 23 participants had AsPD (n = 10). Data from this subgroup not reported | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Investigators report that “subjects were randomly assigned” (p.195, col. 2) suggesting that the order of treatments was randomised in this cross-over trial. Further details obtained from trial investigators (2009 email from M Stanford to J Dennis clarifying trial methods; unreferenced) indicated that sequence generation was achieved by use of computer generated random numbers |
| Allocation concealment? | Unclear | Insufficient information to allow a judgement to be made. Clarification has been requested from the trial investigators but no further information was available at the time this review was prepared |
| Blinding? of participants |
Yes | Investigators described the study as “double-blind”. Response from trial investigators suggests that appropriate care was taken to ensure blinding of participants |
| Blinding? of personnel |
Yes | Investigators described the study as “double-blind”. Response from trial investigators suggests that appropriate care was taken to ensure blinding of personnel |
| Blinding? of outcome assessors |
Yes | Investigators described the study as “double-blind”. Response from trial investigators suggests that appropriate care was taken to ensure blinding of outcome assessors |
| Incomplete outcome data addressed? All outcomes |
No | Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. Overall, 17 of 46 were non-completers and a further 6 were excluded giving a 50% missing data rate. Review authors judged risk of bias to be high pending data from the AsPD subgroup becoming available |
| Free of selective reporting? | Yes | Study protocol is not available but it seems clear that the published report includes all expected outcomes |
| Free of other bias? | Unclear | The investigators declared their research sponsored by the Dreyfus Health Foundation, which is focused on phenytoin and was established “to study, collect, and disseminate information and sponsor collaborative, clinical, and basic health research on its benefits”. The authors have insufficient information to assess whether this constitutes a risk of bias. The trial investigators reported a two-week placebo washout period between phases in this cross-over trial which will have reduced the possibility of carryover effects and the study appeared to be free of other sources of bias |
| Methods | Design: placebo-controlled parallel trial | |
| Participants | Participants: men with recurrent impulsive aggressive behaviour (subgroup with AsPD; see note 1) Sex: male only Age: mean 28.7 years (phenytoin group); mean 34.9 years (carbamazepine group); mean 33.6 years (valproate group); mean 34.8 years (placebo group) (all figures for whole sample including non-AsPD participants) Unit of allocation: individual participant Number randomised: 38 in whole sample, 17 with AsPD (see note 1; breakdown by treatment condition not supplied) Number completing: not known Setting: outpatient; single site; USA (vicinity of New Orleans) Inclusion criteria: over past 6 months, several discrete episodes of failure to resist aggressive impulses resulting in serious assaultative acts or destruction of property; degree of aggressiveness expressed during the episodes was grossly out of proportion to any precipitating psychosocial stressor; at least two such episodes during the month prior to entering the study; score of 8 or higher on the Irritability subscale of the Buss-Durkee Hostility Inventory; must have identified an individual willing to document any impulsive-aggressive outbursts that occurred during the study Exclusion criteria: female (due to potential teratogenic effects of phenytoin); verbal IQ < 80; current bipolar disorder; current thought disorder; present use of psychoactive medication; history of medical/neurological problems (including seizures); non-native English speaker; liver enzymes not within normal limits Ethnicity: not reported Baseline characteristics: for 29 completers overall: at least one Axis I diagnosis (n = 12); major depression (n = 5); alcohol misuse (n = 7); substance misuse (n = 4); at least one Axis II diagnosis (n = 24); AsPD (n = 17); borderline personality disorder (n = 3) |
|
| Interventions | Four conditions: phenytoin / carbamazepine / valproate / placebo
Duration of intervention: 6 weeks Duration of trial: 8 weeks (treatment preceded by 2-week placebo-baseline period) Length of follow up: participants were not followed up beyond the end of the intervention period Dose adjustment: Not reported. Serum blood levels measured after sixth week of administration |
|
| Outcomes |
Primary outcomes
Aggression (observer-reported): Overt Aggression Scale (OAS) scores, averaged over four 2-week periods (placebo-baseline, 0-2 weeks, 2-4 weeks, 4-6 weeks) Secondary outcomes Leaving the study early: proportion of participants discontinuing treatment |
|
| Notes | 1. 59% of 29 participants had AsPD (n = 17). Data from this subgroup not reported | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Sequence generation achieved using a random numbers table. |
| Allocation concealment? | Yes | Investigators state “anticonvulsants and placebo were administered in identical, unmarked capsules obtained from a local pharmacy” (p.74, col 1). The lead author [MS] “was responsible for the random assignment and the maintenance/administrations of all study medication. He was not involved in participant assessment subsequent to the placebo-baseline” (p.73, col 2) |
| Blinding? of participants |
Yes | Investigators state “anticonvulsants and placebo were administered in identical, unmarked capsules obtained from a local pharmacy” (p.74, col 1). Appropriate care appears to have been taken to ensure blinding of participants. Unlikely that this blinding could have been broken |
| Blinding? of personnel |
Yes | Appropriate care appears to have been taken to ensure blinding of personnel. Unlikely that this blinding could have been broken |
| Blinding? of outcome assessors |
Unclear | Insufficient information to allow a judgement to be made. Clarification has been requested from the trial investigators but no further information was available at the time this review was prepared |
| Incomplete outcome data addressed? All outcomes |
No | Incomplete outcome data arise from participants not completing the study and applies to all measured outcomes. Overall, 14 of 43 (33%) were non-completers. Review authors judged risk of bias to be high, pending data from the AsPD subgroup becoming available |
| Free of selective reporting? | Yes | Study protocol is not available but it seems clear that the published report includes all expected outcomes, including those that were pre-specified |
| Free of other bias? | Unclear | Investigators report that this study was sponsored by the Dreyfus Health Foundation which is focused on phenytoin and, according to its website, was established “to study, collect, and disseminate information and sponsor collaboration, clinical, and basic health research into its [phenytoin’s] benefits”. This raises the potential for bias in a study such as this which compares phenytoin with other anticonvulsants as well as against placebo |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aberg-Wisted 2000 | RCT of sertraline versus paroxetine for outpatients with major depression. Investigators examined effects of comorbid Axis II disorder by personality disorder cluster. No indication that any subgroup of participants had AsPD. Excluded because of lack of a placebo control group |
| Agosti 2002 | RCT of fluoxetine versus imipramine versus placebo in outpatient with major depression. Excluded because no participants were reported with a diagnosis of AsPD |
| Allen 1976 | Crossover trial in which 41 “sociopathic” prisoners received a random sequence of four active substances (amphetamine, caffeine, imipramine and chlorpromazine) and one inactive placebo. Excluded because participants were not assessed for possible diagnosis of AsPD |
| Alpert 1990 | RCT of nadolol versus placebo for violent psychiatric patients. Excluded because nadolol is not a drug with known psychotropic properties, because none of the participants had a diagnosis of AsPD, and because most had a major functional mental illness (i.e. schizophrenic disorder, schizoaffective disorder or bipolar disorder) |
| Battaglia 1999 | RCT of depot fluphenazine (‘low dose’ versus ‘ultra low dose’) for multiple suicide attempters in the emergency department. Cluster B personality disorder was represented in the sample, but unclear whether any participants had an AsPD diagnosis. Excluded because of lack of a placebo control group |
| Black 1994 | RCT comparing fluvoxamine, cognitive therapy and placebo for participants with panic disorder. Excluded because no specific personality disorder diagnoses were reported and there was no indication that any subgroup had AsPD |
| Coccaro 1997 | RCT comparing fluoxetine with placebo in adult outpatients with personality disorder and a history of impulsive aggression and irritability. Excluded because only four participants with AsPD were diagnosed, which is too few to allow calculation of means and SDs when randomised to two treatment conditions |
| Ekselius 1998 | RCT of sertraline versus citalopram in depressed patients in primary care. Excluded because no assessment of AsPD was made, and because there was no placebo control condition |
| Fournier 2008 | RCT comparing paroxetine, placebo and cognitive therapy in outpatients with depressive disorder. Excluded because presence of AsPD was an exclusion criterion for the trial |
| Joyce 2003 | RCT comparing fluoxetine with nortriptyline in patients with major depression. Differential drug response was compared in three groups; with BPD, with other personality disorder and with no personality disorder. Six participants had AsPD. Excluded because of lack of a placebo control group |
| Kool 2007 | RCT comparing psychodynamic supportive therapy plus pharmacotherapy with pharmacotherapy alone for depressive disorder in adult patients (article in Dutch). Excluded because there was no placebo control condition and only three participants had an AsPD diagnosis |
| Mattes 1990 | RCT comparing carbamazepine versus propranolol for temper outbursts. A subgroup of participants (n = 8) had AsPD. Excluded because of lack of a placebo control condition |
| Mattes 2005 | RCT comparing oxcarbazepine with placebo in outpatients with impulsive aggression. Excluded because no diagnosis of personality disorder was made |
| Mattes 2008 | RCT comparing levetiracetam with placebo in outpatients with impulsive aggression. Excluded because no diagnosis of personality disorder was made |
| Nickel 2005 | RCT comparing topiramate with placebo in male outpatients with aggression. Excluded because no diagnosis of personality disorder was made |
| Noyes 1991 | RCT comparing alprazolam, diazepam and placebo in patients with panic disorder. Investigators examined the effect of co-morbid personality disorder traits on treatment outcome, but report only mean PDQ (trait) scores. Excluded because no indication that any participants with a diagnosis of AsPD were randomised |
| Patience 1995 | RCT in which 113 participants meeting DSM-III criteria for major depression were randomly assigned to one of four conditions of which one was amitriptyline and one was routine primary care. Excluded because only eight participants were diagnosed with AsPD which is too few to allow calculation of means and SDs when randomised to four treatment conditions |
| Shea 1990 | RCT in which 250 participants with a primary diagnosis of major depressive disorder were randomised to four conditions, of which one was imipramine plus clinical management and one was placebo plus clinical management. AsPD was an exclusion criterion |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Design: placebo-controlled parallel trial. |
| Participants | Participants: outpatients with early-onset dysthymia. Sex: 266 female; 144 male (data not extractable for any AsPD subgroup; see note 1) Age: mean 42.0 (SD 9.0) years (data not extractable for any AsPD subgroup; see note 1) Unit of allocation: individual participant. Number randomised: 410 (sertraline, n = 134; imipramine, n = 136; control, n = 140) (see note 1) Number completing: completion rates: sertraline 84%, imipramine 67%, placebo 76% (data not extractable for any AsPD subgroup; see note 1) Setting: outpatient; multicentre (17 sites), North America. Inclusion criteria: early-onset dysthymia (DSM-III-R) of at least 5 years’ duration; score of 12 or higher on 29-item Hamilton Depression Rating Scale (SAD version) at end of 1-week single-blind placebo washout period Exclusion criteria: major depression; pregnancy or lactation; history of drug or alcohol dependency / misuse within preceding 6 months; serious risk of suicide; current primary diagnosis of panic disorder or generalised anxiety disorder; lifetime diagnosis of bipolar disorder, OCD, or any psychotic disorder; failure to respond in two or more prior antidepressant trials; previous adequate trial of imipramine or sertraline treatment Ethnicity: Caucasian (95%, n = 390) (data not extractable for any AsPD subgroup; see note 1) |
| Interventions | Three conditions: sertraline/ imipramine/ placebo.
Duration of intervention: 10 weeks. Duration of trial: 10 weeks. Length of follow up: participants were not followed up beyond the end of the intervention period Dose adjustment: sertraline initially 50 mg/day and titrated after weeks 4, 6 and 7 to a maximum of 200 mg/day; imipramine initially 50 mg/day and titrated weekly to a maximum of 300 mg/day; all participants received 4 identical capsules containing either placebo, 50 mg sertraline, or 50 or 100 mg imipramine |
| Outcomes |
Primary outcomes
Social functioning: Social Adjustment Scale scores. Secondary outcomes Leaving the study early: Other outcomes Changes in personality dimensions (Tridimensional Personality Questionnaire) |
| Notes | 1. study may have recruited a subgroup with AsPD as 48 participants had DSM-III-R cluster B personality disorder, although this is unclear. No data extractable on any AsPD subgroup. Awaiting clarification from investigators |
| Methods | Design: placebo-controlled parallel trial. |
| Participants | Participants: outpatients with repeated suicidal attempts but without major depression Sex: 37 male; 54 female (data not extractable for any AsPD subgroup; see note 1) Age: mean 34.1 (SD 11.6) and 37.1 (SD 13.0) years (data not extractable for any AsPD subgroup; see note 1) Unit of allocation: individual participant. Number randomised: 91 (paroxetine, n = 46; placebo, n = 44) (see note 1). Number completing: at 8 weeks (paroxetine, n = 28; placebo, n = 30); at 52 weeks (paroxetine, n = 11; placebo, n = 8) (see note 1) Setting: outpatient; 2 sites; Netherlands (Rotterdam, Leiden). Inclusion criteria: at least one previous suicide attempt; aged 18 yrs or older Exclusion criteria: major affective disorder; psychotic disorder; currently taking antidepressant or antipsychotic medication; organic mental disorder; dependency on alcohol or substances; using prohibited medication; serious physical disease; unable to co-operate Ethnicity: not reported. |
| Interventions | Two conditions: paroxetine/ placebo.
In addition to medication, supportive psychotherapy offered weekly to fortnightly to all participants Duration of intervention: up to 52 weeks. Duration of trial: up to 52 weeks. Length of follow up: participants were not followed up beyond the end of the intervention period Dose adjustment: initial placebo washout period of 2 weeks; then 20 mg/day paroxetine for one week followed by a fixed dose of 40 mg/day for up to 52 weeks (or matching placebo) |
| Outcomes |
Primary outcomes
none. Secondary outcomes Leaving the study early: Anger: STAXI scores. Other outcomes subsequent suicide attempts; depression (Beck Depression Inventory; self report); hopelessness (Beck Hopelessness Scale, self-report) |
| Notes | 1. study may have recruited a subgroup with AsPD as 74 participants had DSM-III-R Cluster B personality disorder, although this is unclear. No data extractable on any AsPD subgroup. Awaiting clarification from investigators |
RCT: randomised controlled trial
DATA AND ANALYSES
Comparison 1. amantadine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early | 1 | 12 | Odds Ratio (M-H, Fixed, 95% CI) | 5.0 [0.34, 72.77] |
Comparison 3. desipramine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early | 1 | 11 | Odds Ratio (M-H, Fixed, 95% CI) | 1.2 [0.07, 19.63] |
Comparison 6. phenytoin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events, nausea | 1 | 60 | Odds Ratio (M-H, Fixed, 95% CI) | 1.0 [0.06, 16.76] |
Analysis 1.1. Comparison 1 amantadine versus placebo, Outcome 1 Leaving the study early.
Review: Pharmacological interventions for antisocial personality disorder
Comparison: 1 amantadine versus placebo
Outcome: 1 Leaving the study early
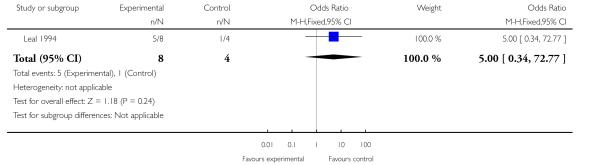
|
Analysis 3.1. Comparison 3 desipramine versus placebo, Outcome 1 Leaving the study early.
Review: Pharmacological interventions for antisocial personality disorder
Comparison: 3 desipramine versus placebo
Outcome: 1 Leaving the study early
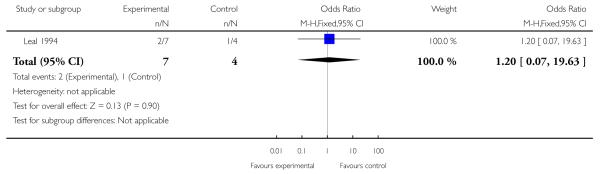
|
Analysis 6.1. Comparison 6 phenytoin versus placebo, Outcome 1 Adverse events, nauseak .
Review: Pharmacological interventions for antisocial personality disorder
Comparison: 6 phenytoin versus placebo
Outcome: 1 Adverse events, nausea
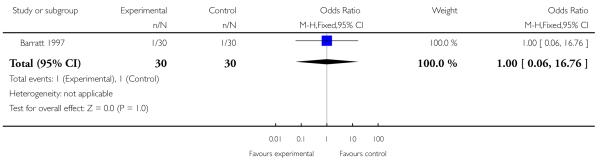
|
ADDITIONAL TABLES
Table 1. Comparison 1: amantadine versus placebo: money spent on cocaine per week (skewed data).
| Study | Outcome | Experimental group (n=8 randomised) (n=3 completed) | Control group (n=4 randomised) (n=3 completed) | Statistic |
|---|---|---|---|---|
| Leal 1994 | mean $ per week spent on cocaine; at week 1 | 162 (SD 138) | 70 (SD 29) | none available (see note 1) |
| Leal 1994 | mean $ per week spent on cocaine; at week 6 | 115 (SD 127) | 32 (SD 33) | none available (see note 1) |
| Leal 1994 | mean $ per week spent on cocaine; at week 12 | 70 (SD 63) | 59 (SD 64) | none available (see note 1) |
SD = standard deviation
1. trial investigators report no statistical analysis that compares amantadine and control groups, although they note that “the AsPD patients were spending significantly more money on drugs than the non-AsPD patients at weeks 1 and 6” (page 33, col 2).
Table 2. Comparison 1a: ‘medicated’ (amantadine or desipramine) versus placebo: money spent on cocaine per week (skewed data).
| Study | Outcome | Experimental group (see note 1) (n=15 randomised) (n=8 completed) | Control group (n=4 randomised) (n=3 completed) | Statistic |
|---|---|---|---|---|
| Leal 1994 | mean $ per week spent on cocaine; at week 1 | 172 (SD 152) | 70 (SD 29) | none available (see note 2) |
| Leal 1994 | mean $ per week spent on cocaine; at week 6 | 119 (SD 121) | 17 (SD 12) | none available (see note 2) |
| Leal 1994 | mean $ per week spent on cocaine; at week 12 | 48 (SD 55) | 52 (SD 76) | none available (see note 2) |
SD = standard deviation
1. data for ‘medicated’ participants (amantadine 300 mg/day or desipramine 150 mg/day; total n=15).
2. trial investigators report no statistical analysis that compares experimental and control groups.
Table 3. Comparison 1: amantadine versus placebo: urine toxicology (cocaine metabolite).
| Study | Outcome (see note 1) | Experimental group (n=8 randomised) (n=3 completed) | Control group (n=4 randomised) (n=3 completed) | Statistic |
|---|---|---|---|---|
| Leal 1994 | % cocaine-free urine samples: during first 2 weeks | 6% | 0% | none available (see note 2) |
| Leal 1994 | % cocaine-free urine samples: during weeks 5 & 6 | 6% | 25% | none available (see note 2) |
| Leal 1994 | % cocaine-free urine samples: during last 2 weeks | 0% | 0% | none available (see note 2) |
1. urinalyses performed twice-weekly throughout study and evaluated for cocaine metabolite (benzoylcognine).
2. trial investigators report no statistical analysis that compares experimental and control groups; however they note that “when comparing the first and last two weeks of treatment … AsPD … patients treated with placebo showed no difference in the percentage of cocaine-free urines … medicated AsPD patients [those receiving either amantadine 300 mg/day or desipramine 150 mg/day; total n=15] also showed no change in cocaine-free urines (13% to 14%)” (page 33, col 2).
Table 4. Comparison 2: bromocriptine versus placebo: global functioning.
| Study | Outcome (mean scores) | Experimental group baseline (see note 1) | Experimental group endpoint (n=9) (see note 1) | Control group baseline (see note 1) | Control group endpoint (n=9) (see note 1) | Statistic (see note 2) |
|---|---|---|---|---|---|---|
| Powell 1995 | Global functioning (GAS-high)b | 55.8 | 76.0 | 57.9 | 75.7 | favours neither condition |
| Powell 1995 | Global functioning (GAS-low)b | 36.0 | 47.4 | 34.8 | 43.0 | favours neither condition |
| Powell 1995 | Global functioning (SCL-90 GSI subscale)a | 0.8 | 0.4 | 0.8 | 0.7 | favours neither condition |
GAS = Global Assessment Scale; SCL-90 = Symptom Check List-90; GSI = global severity index
high scores indicate greater severity.
high scores indicate better functioning.
1. standard deviations not reported.
2. trend-over-time analyses based on measurements at baseline and six months with additional measurements at 2, 4, and 6 weeks, then at 2, 3, 4, and 5 months; analyses conducted by trial investigators.
Table 5. Comparison 2: bromocriptine versus placebo: drinking days.
| Study | Outcome | Experimental group (n=9 completed) | Control group (n=9 completed) | Statistic |
|---|---|---|---|---|
| Powell 1995 | mean number of drinking days over the 6-month study | 19.0 (no SD provided) (see note 1) | 42.2 (no SD provided) (see note 1) | favours neither condition (see note 2) |
SD = standard deviation
1. figures derived from data presented graphically (Powell 1995, p.467, fig 1); no SDs provided.
2. two-way ANOVA; comorbidity × medication: F(4,89)=2.60; P<0.05; however, Tukey post-hoc tests did not indicate a statistically significant effect for AsPD/bromocriptine subgroup; completer analysis by trial investigators.
Table 6. Comparison 2: bromocriptine versus placebo: alcohol craving scores.
| Study | Outcome | Experimental group (n=9 completed) | Control group k (n=9 completed) | Statistic |
|---|---|---|---|---|
| Powell 1995 | mean alcohol craving scores (visual analog scale) over the 6-month study | 6.3 (no SD provided) (see note 1) | 9.5 (no SD provided) (see note 1) | favours neither condition (see note 2) |
SD = standard deviation
1. figures derived from data presented graphically (Powell 1995, p.467, fig 2); no SDs provided.
2. three-way ANOVA; comorbidity × medication × time; no significant main effects or interactions; completer analysis by trial investigators.
Table 7. Comparison 2: bromocriptine versus placebo: severity of alcohol misuse.
| Study | Outcome (mean scores; see note 1) | Experimental group baseline (see note 2) | Experimental group endpoint (n=9) (see note 2) | Control group baseline (see note 2) | Control group endpoint (n=9) (see note 2) | Statistic (see note 3) |
|---|---|---|---|---|---|---|
| Powell 1995 | Alcohol severity (ASS scores) | 22.8 | 10.8 | 20.7 | 14.4 | favours neither condition |
| Powell 1995 | Patient rating of drinking | 4.8 | 2.9 | 4.7 | 3.6 | favours neither condition |
| Powell 1995 | Clinical rating of drinking | 6.6 | 3.6 | 6.7 | 4.6 | favours neither condition |
| Powell 1995 | Alcohol dependence (SADQ scores) | 28.6 | 17.3 | 20.8 | 15.7 | favours neither condition |
ASS = Alcohol Severity Scale; SADQ = Severity of Alcohol Dependence Questionnaire
1. high scores indicate greater severity.
2. standard deviations not reported.
3. trend-over-time analyses based on measurements at baseline and six months with additional measurements at 2, 4, and 6 weeks, then at 2, 3, 4, and 5 months; analyses conducted by trial investigators.
Table 8. Comparison 2: bromocriptine versus placebo: depression and anxiety.
| Study | Outcome (mean scores; see note 1) | Experimental group baseline (see note 2) | Experimental group endpoint (n=9) (see note 2) | Control group baseline (see note 2) | Control group endpoint (n=9) (see note 2) | Statistic (see note 3) |
|---|---|---|---|---|---|---|
| Powell 1995 | BDI scores | 12.7 | 3.3 | 5.7 | 5.4 | favours neither condition |
| Powell 1995 | BAI scores | 6.8 | 1.9 | 4.3 | 9.1 | favours bromocriptine 3-way ANOVA; post-hoc test showed significantly greater improvement over time for bromocrip-tine compared to placebo; P<0.05 |
| Powell 1995 | SCL-90 depression subscale scores | 0.9 | 0.4 | 0.8 | 0.7 | favours neither condition |
| Powell 1995 | SCL-90 anxiety subscale scores | 0.7 | 0.6 | 0.7 | 0.6 | favours neither condition |
BDI = Beck Depression Inventory; BAI = Beck Anxiety Inventory; SCL-90 = Symptom Check List-90
1. high scores indicate greater severity.
2. standard deviations not reported.
3. trend-over-time analyses based on measurements at baseline and six months with additional measurements at 2, 4, and 6 weeks, then at 2, 3, 4, and 5 months; analyses conducted by trial investigators.
Table 9. Comparison 3: desipramine versus placebo: social functioning.
| Study | Outcome (see note 1) | Experimental group (n=17 completed) | Control group (n=12 completed) | Statistic (see note 2) |
|---|---|---|---|---|
| Arndt 1994 | ASI family/social factor scores | 0.185 | 0.207 | favours neither condition (P>0.05) |
ASI = Addiction Severity Index
1. 12-week end of treatment means; ASI factor scores range from 0 to 1 with larger values indicating greater problem severity
2. between-groups ANCOVA using baseline value as covariate; baseline values reflect the 30 days before start of treatment; analysis carried out by trial investigators
Table 10. Comparison 3: desipramine versus placebo: money spent on cocaine per week (skewed data).
| Study | Outcome | Experimental group (n=7 randomised) (n=5 completed) | Control group (n=4 randomised) (n=3 completed) | Statistic |
|---|---|---|---|---|
| Leal 1994 | mean $ per week spent on cocaine; at week 1 | 184 (SD 177) | 70 (SD 29) | none available (see note 1) |
| Leal 1994 | mean $ per week spent on cocaine; at week 6 | 98 (SD 101) | 32 (SD 33) | none available (see note 1) |
| Leal 1994 | mean $ per week spent on cocaine; at week 12 | 76 (SD 69) | 59 (SD 64) | none available (see note 1) |
SD = standard deviation
1. trial investigators report no statistical analysis that compares amantadine and control groups, although they note that “the AsPD patients were spending significantly more money on drugs than the non-AsPD patients at weeks 1 and 6” (page 33, col 2).
Table 11. Comparison 3: desipramine versus placebo: urine toxicology (cocaine metabolite).
| Study | Outcome (see note 1) | Experimental group (n=7 randomised) (n=5 completed) | Control group (n=4 randomised) (n=3 completed) | Statistic |
|---|---|---|---|---|
| Leal 1994 | % cocaine-free urine samples: during first 2 weeks | 21% | 0% | none available (see note 2) |
| Leal 1994 | % cocaine-free urine samples: during weeks 5 & 6 | 18% | 25% | none available (see note 2) |
| Leal 1994 | % cocaine-free urine samples: during last 2 weeks | 20% | 0% | none available (see note 2) |
1. urinalyses performed twice-weekly throughout study and evaluated for cocaine metabolite (benzoylcognine).
2. trial investigators report no statistical analysis that compares experimental and control groups; however they note that “when comparing the first and last two weeks of treatment … AsPD … patients treated with placebo showed no difference in the percentage of cocaine-free urines … medicated AsPD patients [those receiving either amantadine 300 mg/day or desipramine 150 mg/day; total n=15] also showed no change in cocaine-free urines (13% to 14%)” (page 33, col 2).
Table 12. Comparison 3: desipramine versus placebo: urine toxicology (cocaine metabolite).
| Study | Outcome (see note 1) | Experimental group (n=17 completed) | Control group (n=12 completed) | Statistic |
|---|---|---|---|---|
| Arndt 1994 | mean % cocaine-positive urinalysis results: across all 12 weeks of the study | 78% | 77% | favours neither condition (ANOVA carried out by trial investigators showed no indication of treatment group effect; F<1.0, P>0. 10) (see note 2) |
1. urinalyses performed twice-weekly throughout study and evaluated for cocaine metabolite (benzoylcognine); urine samples collected on a random schedule.
2. investigators report that “the AsPD subjects showed no indication of either a medication group effect (F<1.0, P>0.10) or a decrease in the proportion of cocaine-positive urines over time (F<1.0, P>.010)” (page 155, col 2)
Table 13. Comparison 3: desipramine versus placebo: substance misuse.
| Study | Outcome (see note 1) | Experimental group (n=17 completed) | Control group (n=12 completed) | Statistic (see note 2) |
|---|---|---|---|---|
| Arndt 1994 | ASI drug factor scores | 0.259 | 0.234 | favours neither condition (P>0.05) |
| Arndt 1994 | days opiate use | 2 | 2 | favours neither condition (P>0.05) |
| Arndt 1994 | days cocaine use | 9 | 8 | favours neither condition (P>0.05) |
| Arndt 1994 | cocaine craving score | 8 | 7 | favours neither condition (P>0.05) |
ASI = Addiction Severity Index
1. 12-week end of treatment means; ASI factor scores range from 0 to 1 with larger values indicating greater problem severity
2. between-groups ANCOVA using baseline value as covariate; baseline values reflect the 30 days before start of treatment; analysis carried out by trial investigators
Table 14. Comparison 3: desipramine versus placebo: employment status.
| Study | Outcome (see note 1) | Experimental group (n=17 completed) | Control group (n=12 completed) | Statistic (see note 2) |
|---|---|---|---|---|
| Arndt 1994 | ASI employment factor | 0.606 | 0.495 | favours neither condition (P=0.08) |
| Arndt 1994 | days worked in past 30 days | 9 | 10 | favours neither condition (P>0.05) |
| Arndt 1994 | employment income | $479 | $1049 | favours control (P<0.05) |
ASI = Addiction Severity Index
1. 12-week end of treatment means; ASI factor scores range from 0 to 1 with larger values indicating greater problem severity
2. between-groups ANCOVA using baseline value as covariate; baseline values reflect the 30 days before start of treatment; analysis carried out by trial investigators
Table 15. Comparison 3: desipramine versus placebo: depression.
| Study | Outcome (see note 1) | Experimental group (n=17 completed) | Control group (n=12 completed) | Statistic (see note 2) |
|---|---|---|---|---|
| Arndt 1994 | BDI score | 7 | 8 | favours neither condition (P>0.05) |
BDI = Beck Depression Inventory
1. 12-week end of treatment means; larger values indicating greater problem severity
2. between-groups ANCOVA using baseline value as covariate; baseline values reflect the 30 days before start of treatment; analysis carried out by trial investigators
Table 16. Comparison 3: desipramine versus placebo: illegal activity.
| Study | Outcome (see note 1) | Experimental group (n=17 completed) | Control group (n=12 completed) | Statistic (see note 2) |
|---|---|---|---|---|
| Arndt 1994 | ASI legal factor scores | 0.150 | 0.062 | favours neither condition (P>0.05) |
| Arndt 1994 | days illegal activity | 4 | 2 | favours neither condition (P>0.05) |
| Arndt 1994 | illegal income | $251 | $176 | favours neither condition (P>0.05) |
ASI = Addiction Severity Index
1. 12-week end of treatment means; ASI factor scores range from 0 to 1 with larger values indicating greater problem severity
2. between-groups ANCOVA using baseline value as covariate; baseline values reflect the 30 days before start of treatment; analysis carried out by trial investigators
Table 17. Comparison 5: nortriptyline versus placebo: global functioning.
| Study | Outcome (mean scores) | Experimental group baseline (see note 1) | Experimental group endpoint (n=11) (see note 1) | Control group baseline (see note 1) | Control group endpoint (n=9) (see note 1) | Statistic (see note 2) |
|---|---|---|---|---|---|---|
| Powell 1995 | Global functioning (GAS-high)b | 59.3 | 77.1 | 57.9 | 75.7 | favours neither condition |
| Powell 1995 | Global functioning (GAS-low)b | 37.6 | 51.7 | 34.8 | 43.0 | favours neither condition |
| Powell 1995 | Global functioning (SCL-90 GSI subscale)a | 1.0 | 0.3 | 0.8 | 0.7 | favours neither condition |
GAS = Global Assessment Scale; SCL-90 = Symptom Check List-90; GSI = global severity index
high scores indicate greater severity.
high scores indicate better functioning.
1. standard deviations not reported.
2. trend-over-time analyses based on measurements at baseline and six months with additional measurements at 2, 4, and 6 weeks, then at 2, 3, 4, and 5 months; analyses conducted by trial investigators
Table 18. Comparison 5: nortriptyline versus placebo: drinking days.
| Study | Outcome | Experimental group (n=11 completed) | Control group (n=9 completed) | Statistic |
|---|---|---|---|---|
| Powell 1995 | mean number of drinking days over the 6-month study | 9.5 (no SD provided) (see note 1) | 42.2(no SD provided) (see note 1) | P<0.05 Favours nortriptyline (see note 2) |
SD = standard deviation
1. figures derived from data presented graphically (Powell 1995, p.467, fig 1); no SDs provided.
2. two-way ANOVA; comorbidity × medication: F(4,89)=2.60; P<0.05; Tukey post-hoc tests for each comorbidity subgroup indicated a medication effect for AsPD/nortriptyline subgroup only; P<0.05; completer analysis by trial investigators.
Table 19. Comparison 5: nortriptyline versus placebo: alcohol craving scores.
| Study | Outcome | Experimental group (n=11 completed) | Control group (n=9 completed) | Statistic |
|---|---|---|---|---|
| Powell 1995 | mean alcohol craving scores (visual analog scale) over the 6-month study | 5.3 (no SD provided) (see note 1) | 9.5 (no SD provided) (see note 1) | favours neither condition |
SD = standard deviation
1. figures derived from data presented graphically (Powell 1995, p.467, fig 2); no SDs provided.
2. three-way ANOVA; comorbidity × medication × time; no significant main effects or interactions; completer analysis by trial investigators.
Table 20. Comparison 5: nortriptyline versus placebo: severity of alcohol misuse.
| Study | Outcome (mean scores; see note 1) | Experimental group baseline (see note 2) | Experimental group endpoint (n=11) (see note 2) | Control group baseline (see note 2) | Control group endpoint (n=9) (see note 2) | Statistic (see note 3) |
|---|---|---|---|---|---|---|
| Powell 1995 | Alcohol severity (ASS scores) | 20.9 | 4.6 | 20.7 | 14.4 | favours neither condition |
| Powell 1995 | Patient rating of drinking | 4.6 | 1.8 | 4.7 | 3.6 | favours neither condition |
| Powell 1995 | Clinical rating of drinking | 5.9 | 2.4 | 6.7 | 4.6 | favours neither condition |
| Powell 1995 | Alcohol dependence (SAD-Q scores) | 27.3 | 4.6 | 20.8 | 15.7 | favours nortriptyline 3-way ANOVA; post-hoc test showed significantly greater improvement over time for nortriptyline compared to placebo; P<0.01 |
ASS = Alcohol Severity Scale; SAD-Q = Severity of Alcohol Dependence Questionnaire
1. high scores indicate greater severity.
2. standard deviations not reported.
3. trend-over-time analyses based on measurements at baseline and six months with additional measurements at 2, 4, and 6 weeks, then at 2, 3, 4, and 5 months; analyses conducted by trial investigators.
Table 21. Comparison 5: nortriptyline versus placebo: depression and anxiety.
| Study | Outcome (mean scores; see note 1) | Experimental group baseline (see note 2) | Experimental group endpoint (n=11) (see note 2) | Control group baseline (see note 2) | Control group endpoint (n=9) (see note 2) | Statistic (see note 3) |
|---|---|---|---|---|---|---|
| Powell 1995 | BDI scores | 11.2 | 3.1 | 5.7 | 5.4 | favours neither condition |
| Powell 1995 | BAI scores | 9.4 | 4.3 | 4.3 | 9.1 | favours nortriptyline 3-way ANOVA; post-hoc test showed significantly greater improvement over time for nor-triptyline compared to placebo; P<0.05 |
| Powell 1995 | SCL-90 depression subscale scores | 1.3 | 0.4 | 0.8 | 0.7 | favours neither condition |
| Powell 1995 | SCL-90 anxiety subscale scores | 0.8 | 0.2 | 0.7 | 0.6 | favours neither condition |
BDI = Beck Depression Inventory; BAI = Beck Anxiety Inventory; SCL-90 = Symptom Check List-90
1. high scores indicate greater severity.
2. standard deviations not reported.
3. trend-over-time analyses based on measurements at baseline and six months with additional measurements at 2, 4, and 6 weeks, then at 2, 3, 4, and 5 months; analyses conducted by trial investigators.
Table 22. Comparison 6: phenytoin versus placebo: aggression (skewed data).
| Study | outcome | n(Exp) | mean(Exp) | SD(Exp) | n(Cntrl) | mean(Cntrl) | SD(Cntrl) | statistic | notes |
|---|---|---|---|---|---|---|---|---|---|
|
Barratt 1997
300 mg/day |
frequency of aggressive acts per week; at 6 weeks | 60 | 0.33 | no data | 60 | 0.51 | no data | F 1,58 = 9.64 (repeated measure ANOVA, Geissner-Greenhouse adjusted; P<0.001) | Favours phenytoin(see rows below for subgroup analysis) |
|
Barratt 1997
300 mg/day |
intensity of aggressive acts; at 6 weeks | 60 | 2.61 | no data | 60 | 3.96 | no data | F 1,58 = 8.23 (repeated measure ANOVA, Geissner-Greenhouse adjusted; P<0.01) | Favours phenytoin(see rows below for subgroup analysis) |
|
Barratt 1997
300 mg/day |
frequency of aggressive acts per week; impulsive subgroup; at 6 weeks | 30 | 0.20 | 0.19 | 30 | 0.52 | 0.46 | Subgroup effect (impulsive vs. non-impulsive) F 1,58=9.21 (repeated measure ANOVA, Geissner-Greenhouse adjusted; P<0.01) Subgroup by drug-placebo effect F 1,58=9.50 (repeated measure ANOVA, Geissner-Greenhouse adjusted; P<0.01) | Favours phenytoin(impulsive aggression subgroup) |
|
Barratt 1997
300 mg / day |
intensity of aggressive acts; mean; impulsive subgroup; at 6 weeks | 30 | 2.11 | 1.20 | 30 | 4.16 | 1.92 | Subgroup effect (impulsive vs. non-impulsive) F 1, 58=4.78 (repeated measure ANOVA, Geissner-Greenhouse adjusted; P<0.05) Subgroup by drug-placebo effect F 1, 58=9.74 (repeated measure ANOVA, Geissner-Greenhouse adjusted; P<0.01) |
Favours phenytoin
(impulsive aggression subgroup) |
|
Barratt 1997
300 mg/day |
frequency of aggressive acts per week; non-impulsive subgroup; at 6 wks | 30 | 0.42 | 0.24 | 30 | 0.51 | 0.48 | Subgroup effect (impulsive vs. non-impulsive) F 1,58 = 9. 21 (repeated measure ANOVA, Geissner-Greenhouse adjusted; P<0.01) Subgroup by drug-placebo effect F 1,58 = 9.50 (repeated measure ANOVA, Geissner-Greenhouse adjusted; P<0.01) | Favours neither condition (non-impulsive aggression subgroup) |
|
Barratt 1997
300 mg/day |
intensity of aggressive acts; non-impulsive subgroup; at 6 weeks | 30 | 3.40 | 1.29 | 30 | 3.76 | 1.59 | Subgroup effect (impulsive vs. non-impulsive) F 1,58=4.78 (repeated measure ANOVA, Geissner-Greenhouse adjusted; P<0.05) Subgroup by drug-placebo effect Fh 58=9.74 (repeated measure ANOVA, Geissner-Greenhouse adjusted; P<0.01) | Favours neither condition (non-impulsive aggression subgroup) |
SD = standard deviation
Table 23. Comparison 6: phenytoin versus placebo: anger-hostility (skewed data).
| Study | outcome | n(Exp) | mean(Exp) | SD(Exp) | n(Cntrl) | mean(Cntrl) | SD(Cntrl) | statistic | notes |
|---|---|---|---|---|---|---|---|---|---|
|
Barratt 1997
300 mg/day |
POMS anger-hostility sub-scale; impulsive subgroup; at 6 weeks | 30 | 20.4 | no data | 30 | 22.3 | no data | Scores not significantly reduced from baseline (ANOVA, Geissner-Greenhouse adjusted; no further details given) | Favours neither condition (impulsive aggression subgroup) |
|
Barratt 1997
300 mg/day |
POMS anger-hostility sub-scale; non-impulsive subgroup; at 6 weeks | 30 | 11.2 | no data | 30 | 12.5 | no data | Scores not significantly reduced from baseline (ANOVA, Geissner-Greenhouse adjusted; no further details given) | Favours neither condition (non-impulsive aggression subgroup) |
POMS = Profile of Mood States; SD = standard deviation
WHAT’S NEW
Last assessed as up-to-date: 28 February 2010.
| Date | Event | Description |
|---|---|---|
| 4 August 2010 | Amended | Declaration of interest added. |
HISTORY
Protocol first published: Issue 1, 2009
Review first published: Issue 8, 2010
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
The review differs from the original protocol in three ways:
an additional restriction was added to the Selection of studies section to apply to studies in which participants with AsPD formed a small subgroup. This required that studies with two treatment conditions should have randomised at least five people with AsPD to be included in the review. The rationale is that variance and standard deviation cannot be calculated in samples of two or less, and so a two-condition study randomising less than five (relevant) participants will have at least one arm for which standard deviation cannot be calculated.
the outcome of substance misuse (as specified a priori in the section on Secondary outcomes in the protocol) was modified so that a reader would find it easier to differentiate drug misuse outcomes from alcohol misuse outcomes. It has been replaced by two separate categories: substance misuse (drugs) and substance misuse (alcohol).
skewed data is reported in separate tables as specified in the original protocol (see Measures of treatment effect). However, where the trial investigators provide results of their own statistical analysis on such data, we report their results descriptively within the section on Effects of interventions.
The review omits six analyses specified in the original protocol because of insufficient data. These were:
sub-group analysis of effect on primary outcomes of comorbid diagnosis, setting, and class of drug;
sensitivity analysis to investigate the robustness of findings concerning concealment of allocation, blinding of outcome assessors, and extent of dropouts;
assessment of the extent to which the results of the review could be altered by the missing data by sensitivity analysis based on consideration of ‘best-case’ and ‘worst-case’ scenarios;
sensitivity analysis of the impact of including studies with high attrition rates (25% to 50%);
drawing of funnel plots of effect size versus standard error to assess for possible publication bias; and
grouping of outcome measures by length of follow-up.
Footnotes
DECLARATIONS OF INTEREST
Conor Duggan: Chair of the UK National Institute of Clinical Excellence Committee on antisocial personality disorder; advisor to a current randomised controlled trial of schema focused therapy at Ashworth Special Hospital in the UK, and co-applicant on a randomised controlled trial of Selective serotonin re-uptake inhibitors (SSRIs) for antisocial personality disorder.
References to studies included in this review
- Arndt 1994 {published data only} .Arndt IO, Dorozynski L, McLellan AT, Woody GE, O’Brien CP. Controlled study of desipramine treatment of cocaine dependence in methadone treated patients. Archives of General Psychiatry. 1992;49:888–93. doi: 10.1001/archpsyc.1992.01820110052008. [DOI] [PubMed] [Google Scholar]
- *; Arndt IO, McClellan AT, Dorozynsky L, Woody GE, O’Brien CP. Desipramine treatment for cocaine dependence: role of antisocial personality disorder. Journal of Nervous and Mental Disease. 1994;182(3):151–6. doi: 10.1097/00005053-199403000-00004. [DOI] [PubMed] [Google Scholar]
- Barratt 1997 {published data only} .*; Barratt ES, Stanford MS, Felthous AR, Kent TA. The effects of phenytoin on impulsive and premeditated aggression: a controlled study. Journal of Clinical Psychopharmacology. 1997;17(5):341–9. doi: 10.1097/00004714-199710000-00002. 1: 1997461709. [DOI] [PubMed] [Google Scholar]
- Barratt ES, Stanford MS, Kent TA, Felthous A. Neuropsychological and cognitive psychophysiological substrates of impulsive aggression. Biological Psychiatry. 1997;41:1045–61. doi: 10.1016/s0006-3223(96)00175-8. [DOI] [PubMed] [Google Scholar]
- Hollander 2003 {published data only} .Hollander E, Tracy KA, Swann AC, Coccaro EF, McElroy SL, Wozniak P, et al. Divalproex in the treatment of impulsive aggression: efficacy in cluster B personality disorders. Neuropsychopharmacology. 2003;28(6):1186–97. doi: 10.1038/sj.npp.1300153. [DOI] [PubMed] [Google Scholar]
- Leal 1994 {published data only} .Leal J, Ziedonis D, Kosten T. Antisocial personality disorder as a prognostic factor for pharmacotherapy of cocaine dependence. Drug and Alcohol Dependence. 1994;35:31–5. doi: 10.1016/0376-8716(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Powell 1995 {published data only} .Powell BJ, Campbell JL, Landon JF, Liskow BI, Thomas HM, Nickel EJ, et al. A double-blind, placebo-controlled study of nortriptyline and bromocriptine in male alcoholics subtyped by comorbid psychiatric disorders. Alcoholism, Clinical and Experimental Research. 1995;19(2):462–8. doi: 10.1111/j.1530-0277.1995.tb01532.x. [DOI] [PubMed] [Google Scholar]
- Ralevski 2007 {published data only} .*; Petrakis IL, Poling J, Levinson C, Nich C, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biological Psychiatry. 2005;57:1128–37. doi: 10.1016/j.biopsych.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Ralevski E, Ball S, Nich C, Limoncelli D, Petrakis I. The impact of personality disorders on alcohol-use outcomes in a pharmacotherapy trial for alcohol dependence and comorbid Axis I disorders. American Journal on Addictions. 2007;16(6):443–9. doi: 10.1080/10550490701643336. [DOI] [PubMed] [Google Scholar]
- Stanford 2001 {published data only} .Stanford MS, Houston RJ, Mathias CW, Greve KW, Villemarette-Pittman NR, Adams D. A double-blind placebo-controlled crossover study of phenytoin in individuals with impulsive aggression. Psychiatry Research. 2001;103(2-3):193–203. doi: 10.1016/s0165-1781(01)00287-6. [DOI] [PubMed] [Google Scholar]
- Stanford 2005 {published data only} .Stanford MS, Helfritz LE, Conklin SM, Villemarette-Pittman NR, Greve KW, Adams D, et al. A comparison of anticonvulsants in the treatment of impulsive aggression. Experimental and Clinical Psychopharmacology. 2005;13(1):72–7. doi: 10.1037/1064-1297.13.1.72. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Aberg-Wisted 2000 {published data only} .Aberg-Wistedt A, Agren H, Ekselius L, Bengtsson F, Akerblad AC. Sertraline versus paroxetine in major depression: clinical outcome after six months of continuous therapy. Journal of Clinical Psychopharmacology. 2000;20(6):645–53. doi: 10.1097/00004714-200012000-00010. [DOI] [PubMed] [Google Scholar]
- Agosti 2002 {published data only} .Agosti V, McGrath PJ. Comparison of the effects of fluoxetine, imipramine and placebo on personality in atypical depression. Journal of Affective Disorders. 2002;71:113–20. doi: 10.1016/s0165-0327(01)00393-7. [DOI] [PubMed] [Google Scholar]
- Allen 1976 {published data only} .Allen HE, Dinitz S, Foster TW, Goldman H, Lindner LA. Sociopathy: An experiment in internal environmental control. American Behavioural Scientist. 1976;20(2):215–26. doi: 10.1177/000276427602000204. [DOI] [PubMed] [Google Scholar]
- Alpert 1990 {published data only} .Alpert M, Allan ER, Citrome L, Laury G, Sison C, Sudilovsky A. A double-blind placebo-controlled study of adjunctive nadolol in the management of violent psychiatric patients. Psychopharmacology Bulletin. 1990;26(3):367–71. [PubMed] [Google Scholar]
- Battaglia 1999 {published data only} .Battaglia J, Wolff TK, Wagner-Johnson DS, Rush AJ, Carmody TJ, Basco MR. Structured diagnostic assessment and depot fluphenazine treatment of multiple suicide attempters in the emergency department. International Clinical Psychopharmacology. 1999;14(6):361–72. doi: 10.1097/00004850-199911000-00007. [DOI] [PubMed] [Google Scholar]
- Black 1994 {published data only} .Black DW, Wesner RB, Gabal J, Bowers W, Monahan P. Predictors of short-term treatment response in 66 patients with panic disorder. Journal of Affective Disorders. 1994;30:233–41. doi: 10.1016/0165-0327(94)90131-7. [DOI] [PubMed] [Google Scholar]
- Coccaro 1997 {published data only} .Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behaviour in personality-disordered subjects. Archives of General Psychiatry. 1997;54:1081–8. doi: 10.1001/archpsyc.1997.01830240035005. [DOI] [PubMed] [Google Scholar]
- Ekselius 1998 {published data only} .Ekselius L, von Knorring L. Personality disorder comorbidity with major depression and response to treatment with sertraline or citalopram. International Clinical Psychopharmacology. 1998;13(5):205–11. doi: 10.1097/00004850-199809000-00003. [DOI] [PubMed] [Google Scholar]
- Fournier 2008 {published data only} .Fournier JC, DeRubeis RJ, Shelton RC, Gallop R, Amsterdam JD, Hollon SD. Antidepressant medications v. cognitive therapy in people with depression with or withoutpersonality disorder. British Journal of Psychiatry. 2008;192(2):124–9. doi: 10.1192/bjp.bp.107.037234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce 2003 {published data only} .Joyce PR, Mulder RT, Luty SE, Mackenzie JM, Sullivan PF, Cloninger RC. Borderline personality disorder in major depression: symptomatology, temperament, character, differential drug response, and 6-month outcome. Comprehensive Psychiatry. 2003;44(1):35–43. doi: 10.1053/comp.2003.50001. [DOI] [PubMed] [Google Scholar]
- Kool 2007 {published data only} .Kool S, Schoevers R, Duijsens IJ, Peen J, Van AAlst G, De Jonghe F, et al. Treatment of depressive disorder and comorbid personality pathology: combined therapy versus pharmacotherapy [Dutch] Tijdschrift voor Psychiatrie. 2007;49(6):361–72. [PubMed] [Google Scholar]
- Mattes 1990 {published data only} .Mattes JA. Comparative effectiveness of carbamazepine and propranolol for rage outbursts. Journal of Neuropsychiatry and Clinical Neurosciences. 1990;2:159–64. doi: 10.1176/jnp.2.2.159. [DOI] [PubMed] [Google Scholar]
- Mattes 2005 {published data only} .Mattes JA. Oxcarbazepine in patients with impulsive aggression: a double-blind, placebo-controlled trial. Journal of Clinical Psychopharmacology. 2005;25(6):575–9. doi: 10.1097/01.jcp.0000186739.22395.6b. [DOI] [PubMed] [Google Scholar]
- Mattes 2008 {published data only} .Mattes J. Levetiracetam in patients with impulsive aggression: A double-blind, placebo-controlled trial. Journal of Clinical Psychiatry. 2008;69(2):310–15. doi: 10.4088/jcp.v69n0218. [DOI] [PubMed] [Google Scholar]
- Nickel 2005 {published data only} .Nickel M, Nickel C, Kaplan P, Lahmann C, Mühlbacher M, Tritt K, et al. Treatment of aggression with topiramate in male borderline patients: a double-blind, placebo-controlled study. Biological Psychiatry. 2005;57(5):495–9. doi: 10.1016/j.biopsych.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Noyes 1991 {published data only} .Noyes R, Reich JH, Suelzer M, Christiansen J. Personality traits associated with panic disorder: change associated with treatment. Comprehensive Psychiatry. 1991;32(4):283–94. doi: 10.1016/0010-440x(91)90076-o. [DOI] [PubMed] [Google Scholar]
- Patience 1995 {published data only} .Patience DA, McGuire RJ, Freeman CPL. The Edinburgh Primary Care Depression Study: personality disorder and outcome. British Journal of Psychiatry. 1995;167(3):324–30. doi: 10.1192/bjp.167.3.324. [DOI] [PubMed] [Google Scholar]
- Shea 1990 {published data only} .Shea MT, Pilkonis PA, Beckham E, Collins JF, Elkin I, Sotsky SM, et al. Personality disorders and treatment outcome in the NIMH Treatment of Depression Collaborative Research Program. American Journal of Psychiatry. 1990;147(6):711–18. doi: 10.1176/ajp.147.6.711. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Hellerstein 2000 {published data only} .Hellerstein DJ, Kocsis JH, Stewart JW, Harrison W. Double-blind comparison of sertraline, imipramine, and placebo in the treatment of dysthymia: effects on personality. American Journal of Psychiatry. 2000;157(9):1436–44. doi: 10.1176/appi.ajp.157.9.1436. [DOI] [PubMed] [Google Scholar]
- Verkes 1998 {published data only} .Verkes RJ, Van der Mast RC, Hengeveld MW, Tuyl JP, Zwinderman AH, Van Kempen GM. Reduction by paroxetine of suicidal behavior in patients with repeated suicide attempts but not major depression. American Journal of Psychiatry. 1998;155(4):543–7. doi: 10.1176/ajp.155.4.543. [DOI] [PubMed] [Google Scholar]
Additional references
- Altman 1996 .Altman DG, Bland JM. Detecting skewedness from summary information. British Medical Journal. 1996;313(7066):1200. doi: 10.1136/bmj.313.7066.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA 1994 .American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th Edition American Psychiatric Association; Washington DC: 1994. [Google Scholar]
- APA 2000 .American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders -Text Revision (DSM-IV-TR) American Psychiatric Association; Washington DC: 2000. [Google Scholar]
- Arndt 1992 .Arndt IO, Dorozynski L, McLellan AT, Woody GE, O’Brien CP. Controlled study of desipramine treatment of cocaine dependence in methadone treated patients. Archives of General Psychiatry. 1992;49:888–93. doi: 10.1001/archpsyc.1992.01820110052008. [DOI] [PubMed] [Google Scholar]
- Attkisson 1982 .Attkisson CC, Zwick R. The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Evaluation and Program Planning. 1982;5:233–7. doi: 10.1016/0149-7189(82)90074-x. [DOI] [PubMed] [Google Scholar]
- Barratt 1997a .Barratt ES, Stanford MS, Kent TA, Felthous A. Neuropsychological and cognitive psychophysiological substrates of impulsive aggression. Biological Psychiatry. 1997;41:1045–61. doi: 10.1016/s0006-3223(96)00175-8. [DOI] [PubMed] [Google Scholar]
- Black 1996 .Black DW, Baumgard CH, Bell SE, Kao C. Death rates in 71 men with antisocial personality disorder: A comparison with general population mortality. Psychosomatics. 1996;37(2):131–6. doi: 10.1016/S0033-3182(96)71579-7. [DOI] [PubMed] [Google Scholar]
- Blair 2001 .Blair RJR. Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. Journal of Neurology, Neurosurgery and Psychiatry. 2001;71:727–31. doi: 10.1136/jnnp.71.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond 2005 .Bond AJ. The neuropsychopharmacology of aggression and addiction. European Journal of Pharmacology. 2005;526(1-3):218–25. doi: 10.1016/j.ejphar.2005.09.033. [DOI] [PubMed] [Google Scholar]
- Buss 1992 .Buss AH, Perry M. The Aggression Questionnaire. Journal of Personality and Social Psychology. 1992;63:452–9. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Coccaro 1996 .Coccaro EF, Kavoussi RJ, Sheline YI, Lish JD, Csernansky G. Impulsive aggression in personality disorder correlates with tritiated paroxetine binding in the platelet. Archives of General Psychiatry. 1996;53:531–6. doi: 10.1001/archpsyc.1996.01830060075010. [DOI] [PubMed] [Google Scholar]
- Coccaro 1998 .Coccaro EF, Kavoussi RJ, Hauger RL, Cooper TB, Ferris CF. Cerebrospinal fluid vasopressin levels: correlates with aggression and serotonin function in personality-disordered subjects. Archives of General Psychiatry. 1998;55:708–14. doi: 10.1001/archpsyc.55.8.708. [DOI] [PubMed] [Google Scholar]
- Coid 2006 .Coid J, Yang M, Tyrer P, Roberts A, Ullrich S. Prevalence and correlates of personality disorder in Great Britain. British Journal of Psychiatry. 2006;188:423–31. doi: 10.1192/bjp.188.5.423. [DOI] [PubMed] [Google Scholar]
- Cowdry 1989 .Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder: alprazolam, carbamazepine, trifluoperazine and tranylcypromin. Archives of General Psychiatry. 1989;45:111–9. doi: 10.1001/archpsyc.1988.01800260015002. [DOI] [PubMed] [Google Scholar]
- Dolan 1993 .Dolan B, Coid J. Psychopathic and anti-social personality disorders: treatment and research Issues. Gaskell; London: 1993. [Google Scholar]
- Donner 2001 .Donner A, Piaggio G, Villar J. Statistical methods for the meta-analysis of cluster randomization trials. Statistical Methods in Medical Research. 2001;10(5):325–38. doi: 10.1177/096228020101000502. [DOI] [PubMed] [Google Scholar]
- Duke 1994 .Duke PJ, Pantelis C, Barnes TRE. South Westminster Schizophrenia Survey. Alcohol use and its relationship to symptoms, tardive dyskinesia and illness onset. British Journal of Psychiatry. 1994;164:630–636. doi: 10.1192/bjp.164.5.630. [DOI] [PubMed] [Google Scholar]
- Egger 1997 .Egger M, Davey-Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbourne 2002 .Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology. 2002;31(1):140–9. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- EuroQoL group 1990 .EuroQoL Group EuroQoL: a new facility for measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- Follman 1992 .Follmann D, Elliot P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology. 1992;45(7):769–93. doi: 10.1016/0895-4356(92)90054-q. [DOI] [PubMed] [Google Scholar]
- Gamble 2005 .Gamble C, Hollis S. Uncertainty method on best-worst case analysis in a binary meta-analysis. Journal of Clinical Epidemiology. 2005;58(6):579–88. doi: 10.1016/j.jclinepi.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Gottschalk 1973 .Gottschalk LA, Covi L, Uliana R, Bates DE. Effects of diphenylhydantoin on anxiety and hostility in institutionalized prisoners. Comprehensive Psychiatry. 1973;14(6):503–11. doi: 10.1016/0010-440x(73)90036-9. [DOI] [PubMed] [Google Scholar]
- Grilo 1998 .Grilo CM, McGlashan TH, Oldham JM. Course and stability of personality disorders. Journal of Practical Psychiatry and Behavioural Health. 1998;4:61–75. [Google Scholar]
- Higgins 2008a .Higgins JPT, Green S, editors. [accessed 1 April 2008];Cochrane Handbook for Systematic Reviews of Interventions 5.0.0. www.cochrane.org/resources/handbook/hbook.htm.
- Higgins 2008b .Higgins JPT, White IR, Wood AM. Imputation methods for missing outcome data in meta-analysis of clinical trials. Clinical Trials. 2008;5:225–39. doi: 10.1177/1740774508091600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Home Office 1999 .Home Office and Department of Health . Managing people with severe personality disorder. Home Office; London: 1999. [Google Scholar]
- Huband 2010 .Huband N, Ferriter M, Nathan R, Jones H. Antiepileptics for aggression and associated impulsivity. Cochrane Database of Systematic Reviews. 2010;(2) doi: 10.1002/14651858.CD003499.pub3. DOI: 10.1002/14651858.CD003499.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieb 2010 .Lieb K, Völlm B, Rücker G, Timmer A, Stoffers JM. Pharmacotherapy for borderline personality disorder: Cochrane systematic review of randomised trials. British Journal of Psychiatry. 2010;196(1):4–12. doi: 10.1192/bjp.bp.108.062984. [DOI] [PubMed] [Google Scholar]
- Links 1990 .Links P. Lithium therapy for borderline patients. Journal of Personality Disorders. 1990;4:173–81. [Google Scholar]
- Livesley 2007 .Livesley W. A framework for integrating dimensional and categorical classifications of personality disorder. Journal of Personality Disorders. 2007;21(2):199–224. doi: 10.1521/pedi.2007.21.2.199. [DOI] [PubMed] [Google Scholar]
- Malone 1994 .Malone RP, Luebbert RP, Pena-Ariet M, Biesecker K, Delaney MA. The Overt Aggression Scale in the study of lithium in aggressive conduct disorder. Psychopharmacology Bulletin. 1994;30:215–8. [PubMed] [Google Scholar]
- Markovitz 2004 .Markovitz PJ. Recent trends in the pharmacotherapy of personality disorders. Journal of Personality Disorders. 2004;18(1):90–101. doi: 10.1521/pedi.18.1.90.32768. [DOI] [PubMed] [Google Scholar]
- Moran 1999 .Moran P. The epidemiology of antisocial personality disorder. Social Psychiatry and Psychiatric Epidemiology. 1999;34(5):231–42. doi: 10.1007/s001270050138. [DOI] [PubMed] [Google Scholar]
- Moss 1990 .Moss HB, Yao JK, Panzak GL. Serotonergic responsivity and behavioral dimensions in antisocial personality disorder with substance abuse. Biological Psychiatry. 1990;28:325–38. doi: 10.1016/0006-3223(90)90660-t. [DOI] [PubMed] [Google Scholar]
- Myers 1998 .Myers MG, Stewart DG, Brown SA. Progression from conduct disorder to antisocial personality disorder following treatment for adolescent substance abuse. American Journal of Psychiatry. 1998;155:479–85. doi: 10.1176/ajp.155.4.479. [DOI] [PubMed] [Google Scholar]
- Newton-Howes 2006 .Newton-Howes G, Tyrer P, Johnson T. Personality disorder and the outcome of depression: meta-analysis of published studies. British Journal of Psychiatry. 2006;188:13–20. doi: 10.1192/bjp.188.1.13. [DOI] [PubMed] [Google Scholar]
- NIHCE 2009 .National Institute of Health and Clinical Excellence . Antisocial personality disorder: treatment, management and prevention (NICE Guideline 77) National Institute of Health and Clinical Excellence; London: [accessed 28/06/2010]. 2009. www.nice.org.uk/CG77 [Google Scholar]
- ONS 2004 .ONS . Social Trends 34. Office of National Statistics; London: 2004. [Google Scholar]
- Paris 2003 .Paris J. Personality Disorders Over Time: Precursors, Course, and Outcome. American Psychiatric Publishing; Arlington, Va: 2003. [DOI] [PubMed] [Google Scholar]
- Patton 1995 .Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51(6):768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Perdikouri 2007 .Perdikouri M, Rathbone G, Huband N, Duggan C. A comparison of adults with antisocial personality traits with and without childhood conduct disorder. Annals of Clinical Psychiatry. 2007;19(1):17–23. doi: 10.1080/10401230601163501. [DOI] [PubMed] [Google Scholar]
- Petrakis 2005 .Petrakis IL, Poling J, Levinson C, Nich C, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biological Psychiatry. 2005;57:1128–37. doi: 10.1016/j.biopsych.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Raine 2000 .Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder. Archives of General Psychiatry. 2000;57:119–27. doi: 10.1001/archpsyc.57.2.119. [DOI] [PubMed] [Google Scholar]
- Reed 1994 .Reed J. Working Group on Psychopathic Disorder: Report (Chairman Dr John Reed) Department of Health; London: 1994. [Google Scholar]
- Robins 1991 .Robins LN, Tipp J, Przybeck T. Antisocial personality. In: Robins LN, Regier DA, editors. Psychiatric disorders in America. Free Press; New York: 1991. [Google Scholar]
- Scott 2001 .Scott S, Knapp M, Henderson J, Maughan B. Financial cost of social exclusion: follow up study of antisocial children into adulthood. British Medical Journal. 2001;323:191–5. doi: 10.1136/bmj.323.7306.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheard 1976 .Sheard MH, Marini JL, Bridges CI. The effect of lithium on impulsive aggressive behavior in man. American Journal of Psychiatry. 1976;133:1409–13. doi: 10.1176/ajp.133.12.1409. [DOI] [PubMed] [Google Scholar]
- Singleton 1998 .Singleton N, Melzer H, Gatward R. Psychiatric morbidity among prisoners in England and Wales. HM Stationery Office; London: 1998. [Google Scholar]
- Skodol 2002 .Skodol AE, Siever LJ, Livesley WJ, Gunderson JG, Pfohl B, Widiger TA. The borderline diagnosis II: biology, genetics and clinical course. Biological Psychiatry. 2002;51:951–63. doi: 10.1016/s0006-3223(02)01325-2. [DOI] [PubMed] [Google Scholar]
- Skodol 2005 .Skodol AE, Oldham JM, Bender DS, Dyck IR, Stout RL, Morey LC, et al. Dimensional representations of DSM-IV personality disorders: relationships to functional impairment. American Journal of Psychiatry. 2005;162(10):1919–25. doi: 10.1176/appi.ajp.162.10.1919. [DOI] [PubMed] [Google Scholar]
- Soloff 1998 .Soloff PH. Algorithms for pharmacological treatment of personality dimensions. Symptom-specific treatments for cognitive-perceptual, affective, and impulsive-behavioral dysregulation. Bulletin of the Menninger Clinic. 1998;62:195–214. [PubMed] [Google Scholar]
- Spielberger 1999 .Spielberger CD. STAXI-2: State-Trait Anger Expression Inventory-2. Psychological Assessment Resources, Inc.; Odessa, FL: 1999. [Google Scholar]
- Stein 1992 .Stein GS. Drug treatment of the personality disorders. British Journal of Psychiatry. 1992;161:167–84. doi: 10.1192/bjp.161.2.167. [DOI] [PubMed] [Google Scholar]
- Stein 1995 .Stein DJ, Simeon D, Frenkel M, Islan MN, Hollander E. An open trial of valproate in borderline personality disorder. Journal of Clinical Psychiatry. 1995;56:506–10. [PubMed] [Google Scholar]
- Sugden 2006 .Sugden SG, Kile SJ, Hendren R. Neurodevelopmental pathways to aggression: a model to understand and target treatment in youth. Journal of Neuropsychiatry and Clinical Neurosciences. 2006;18(3):302–17. doi: 10.1176/jnp.2006.18.3.302. [DOI] [PubMed] [Google Scholar]
- Swanson 1994 .Swanson MC, Bland RC, Newman SC. Epidemiology of psychiatric disorders in Edmonton: antisocial personality disorders. Acta Psychiatrica Scandinavica. Supplementum. 1994;376:63–70. [PubMed] [Google Scholar]
- Tait 2002 .Tait L, Birchwood M, Trower P. A new scale (SES) to measure engagement with community mental health services. Journal of Mental Health. 2002;11(1):191–8. doi: 10.1080/09638230020023570-2. [DOI] [PubMed] [Google Scholar]
- Torgensen 2001 .Torgensen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Archives of General Psychiatry. 2001;58:590–6. doi: 10.1001/archpsyc.58.6.590. [DOI] [PubMed] [Google Scholar]
- Tyrer 2004 .Tyrer P, Bateman AW. Drug treatment for personality disorders. Advances in Psychiatric Treatment. 2004;10:389–98. [Google Scholar]
- Tyrer 2005 .Tyrer P, Nur U, Crawford M. The Social Functioning Questionniare: a rapid and robust measure of perceived functioning. International Journal of Social Psychiatry. 2005;51:265–75. [PubMed] [Google Scholar]
- Völlm 2010 .Völlm B, Richardson P, McKie S, Reniers R, Elliott R, Anderson I, et al. Neuronal correlates and serotonergic modulation of behavioural inhibition and reward in healthy and antisocial individuals. Journal of Psychiatric Research. 2010;44:123–31. doi: 10.1016/j.jpsychires.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Warren 2001 .Warren F, Preedy-Fayers K, McGauley G, Pickering A, Norton K, Geddes JR, et al. Review of treatments for severe personality disorder. Home Office; London: 2001. [Google Scholar]
- Weissman 1976 .Weissman MM, Bothwell S. Assessment of social adjustment by patient self-report. Archives of General Psychiatry. 1976;33:1111–15. doi: 10.1001/archpsyc.1976.01770090101010. [DOI] [PubMed] [Google Scholar]
- Weissman 1993 .Weissman MM. The epidemiology of personality disorders: a 1990 update. Journal of Personality Disorders. 1993;(Suppl):44–62. [Google Scholar]
- WHO 1992 .World Health Organization (WHO) The ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. World Health Organization; Geneva: 1992. [Google Scholar]
- Wilcox 1995 .Wilcox JA. Divalproex sodium as a treatment for borderline personality disorder. Annals of Clinical Psychiatry. 1995;7(1):33–7. doi: 10.3109/10401239509149022. [DOI] [PubMed] [Google Scholar]
- Wolff 1999 .Wolff K, Farrell M, Marsden J, Monteiro MG, Ali R, Welch S, et al. A review of biological indicators of illicit drug use: practical considerations and clinical usefulness. Addiction. 1999;94(9):1279–98. doi: 10.1046/j.1360-0443.1999.94912792.x. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study