Abstract
Background
Diagnostic ultrasound is used selectively in late pregnancy where there are specific clinical indications. However, the value of routine late pregnancy ultrasound screening in unselected populations is controversial. The rationale for such screening would be the detection of clinical conditions which place the fetus or mother at high risk, which would not necessarily have been detected by other means such as clinical examination, and for which subsequent management would improve perinatal outcome.
Objectives
To assess the effects on obstetric practice and pregnancy outcome of routine late pregnancy ultrasound, defined as greater than 24 weeks’ gestation, in women with either unselected or low-risk pregnancies.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register (February 2008).
Selection criteria
All acceptably controlled trials of routine ultrasound in late pregnancy (defined as after 24 weeks).
Data collection and analysis
All three review authors were involved in assessing trial quality and data extraction.
Main results
Eight trials recruiting 27,024 women were included. The quality of trials overall was satisfactory. There was no difference in antenatal, obstetric and neonatal intervention or morbidity in screened versus control groups. There was a slightly higher caesarean section rate in the screened group, but this difference did not reach statistical significance. Routine late pregnancy ultrasound was not associated with improvements in overall perinatal mortality. Placental grading as an adjunct to third trimester examination scan was associated with a significant reduction in the stillbirth rate in the one trial that assessed it. There is little information on long-term substantive outcomes such as neurodevelopment. There is a lack of data on maternal psychological effects.
Authors’ conclusions
Based on existing evidence, routine late pregnancy ultrasound in low-risk or unselected populations does not confer benefit on mother or baby. It may be associated with a small increase in caesarean section rates. There is a lack of data about the potential psychological effects of routine ultrasound in late pregnancy, and limited data about its effects on both short- and long-term neonatal and childhood outcome. Placental grading in the third trimester may be valuable, but whether reported results are reproducible remains to be seen, and future research of late pregnancy ultrasound should include evaluation of placental textural assessment.
Medical Subject Headings (MeSH): *Ultrasonography, Prenatal; Pregnancy Outcome; Pregnancy Trimester, Second; Pregnancy Trimester, Third; Randomized Controlled Trials as Topic
MeSH check words: Female, Humans, Pregnancy
BACKGROUND
Diagnostic ultrasound is a sophisticated electronic technology, which utilises pulses of high frequency sound. The transducer, which is moved across the area to be examined, emits the pulses of ultrasound which propagate through the tissues, and some are reflected back to the transducer which converts these returning echoes into electronic signals. Tissue interface characteristics determine the strength of the returning echo. Signals are processed by a computer which displays each echo in both strength and position as an image on a screen.
Diagnostic ultrasound is used selectively in late pregnancy where there are specific clinical indications, such as antepartum haemorrhage or clinical concern that the fetus may be poorly grown. However, the value of routine late pregnancy ultrasound screening in unselected populations is controversial. The rationale for such screening would be the detection of clinical conditions which place the fetus or mother at high risk, which would not necessarily have been detected by other means such as clinical examination, and for which subsequent management would improve perinatal outcome.
Fetal growth/size
Small for gestational age (SGA) fetuses are at greater risk of stillbirth, birth hypoxia, neonatal complications in the perinatal period, impaired neurodevelopment and cerebral palsy in childhood, and non-insulin dependent diabetes and hypertension in adult life (Barker 1993). The majority of these small infants are not diagnosed until delivery (Leeson 1997), and detecting these fetuses prenatally remains a priority of antenatal care (Lindqvist 2005). Methods of detecting SGA fetuses include antenatal clinical examination, measurement of symphysis-fundal height, fetal anthropometry and ultrasound estimated fetal weight. Harding 1995 demonstrated that symphysis-fundal height measurements perform relatively poorly compared with ultrasound abdominal circumference measurements, while Hendrix 2000 suggested that, for term pregnancies, clinical examinations provide more accurate estimates of birthweight than ultrasound. A combined approach of screening with symphysis-fundal height measurement, complemented with ultrasound derived fetal abdominal circumference if failing growth is suspected has been advocated. Holmes 1996 cautions that small size should be viewed as a clinical sign, and not a diagnosis, as a number of small fetuses are not at risk of adverse outcome. Furthermore, the use of ultrasound to detect the SGA fetus is dogged by a number of complicating factors, including the lack of defined thresholds for normality versus abnormality, its dependence on accurate gestational dating, the fact that the assessment of growth velocity (serial measurements) may be more valuable clinically than a single estimate of size, and differences due to other factors, namely, maternal ethnicity and parity, fetal gender and environmental factors (Altman 1989). A previous systematic review of routine late pregnancy anthropometry concluded that despite increased intervention (admission to hospital and induction of labour), there was no identifiable benefit in fetal outcome (Neilson 1995).
Another clinical concern is with the large-for-gestational-age (LGA) fetus. These babies are at increased risk of perinatal morbidity and mortality, which arises mainly from birth injury and asphyxia; their mothers are at increased risk of cephalo-pelvic disproportion and its sequelae, and operative delivery and the associated morbidity. Our ability to detect fetal macrosomia antenatally by clinical examination remains limited (Lurie 1995), and the antenatal prediction of fetal macrosomia is associated with a marked increase in caesarean births without a significant reduction in the incidence of shoulder dystocia or fetal injury (Weeks 1995). This is because most cases of shoulder dystocia and birth trauma occur in non-macrosomic infants (Gonen 1996). Hence, the value of detecting LGA fetuses by routine ultrasound in late pregnancy is questionable.
Amniotic fluid
Fetal urine is the major source of amniotic fluid in the latter half of pregnancy (Brace 1989). Decreased amniotic fluid volume (oligohydramnios) in the absence of ruptured membranes or fetal anomalies is thought to be associated with chronic fetal compromise and redistribution of regional blood flow leading to reduction in fetal renal blood flow, fetal oliguria and thus less amniotic fluid. Increased amniotic fluid volume (polyhydramnios) occurs as a result of overproduction (polyuria in fetuses of diabetic mothers, rare placental tumours), decreased turnover (congenital anomalies affecting fetal swallowing), or unknown aetiology. Both oligohydramnios and polyhydramnios can be diagnosed by ultrasound measurement of maximum pool depth, two-diameter amniotic fluid pocket or amniotic fluid index (the sum of the vertical maximum pool depths in four quadrants), and applying the result to normal reference ranges. While in high-risk pregnancies, such as postdates pregnancy, the measurement of amniotic fluid volume may have bearing on management decisions, there is some debate about the best measurement method, and the clinical significance of the available reference ranges, which compounds the uncertainty about the effect on perinatal outcome of detecting amniotic fluid abnormalities.
The placenta
Placenta praevia occurs in 0.5% of pregnancies and is associated with considerable risk to both mother and fetus. Ultrasound is the best available method for locating the placental position (Neilson 1989). Only 10% of low placentas at second trimester scan remain low at term (Rizos 1979). However, in most pregnancies with placenta praevia, a clinical indication for diagnostic ultrasound will arise such as antepartum haemorrhage and fetal malpresentation, and therefore the role of screening for placenta praevia is debatable. Grannum 1979 described a classification system to grade the placental texture appearances on ultrasound imaging, and suggested a correlation between maturational changes of the placenta as seen on ultrasound and fetal pulmonic maturity. This was not confirmed in further study, but an association between ‘mature’ appearances at earlier gestations with maternal smoking and placental dysfunction was postulated. Therefore, the knowledge of placental appearances in late pregnancy could, in theory, result in care leading to improved perinatal outcome (Abramowicz 2007).
Structural fetal abnormalities
A number of structural fetal abnormalities may manifest later in pregnancy. These include craniospinal abnormalities (microcephaly and hydrocephaly), gastrointestinal abnormalities (intestinal obstruction and atresia), urinary tract abnormalities, and some skeletal abnormalities (Chitty 1995). It has been suggested that the value of detecting fetal structural abnormalities before birth allows for the optimal timing and mode of delivery leading to improved management and outcome. However, a report of a Working Party of the Royal College of Obstetricians and Gynaecologists on Ultrasound Screening for Fetal Abnormalities (RCOG 1997) stated that further research is required to evaluate whether prior identification of an abnormality before birth, particularly those amenable to intrauterine procedures and neonatal surgery, is advantageous in both the short and long term.
Fetal presentation
Some fetal malpresentations (e.g. breech) go undetected during routine antenatal care but would be identified by routine ultrasound in late pregnancy. In a retrospective case review, Nwosu 1993 showed that babies undiagnosed as a breech were not subject to increased morbidity and mortality compared with a breech diagnosed prior to labour. This highlights the uncertainty about the clinical value of routine ultrasound screening for fetal malpresentations.
Safety
The use of routine pregnancy ultrasound needs to be considered in the context of potential hazards. Theoretically, some ultrasonic energy propagated through tissue is converted to heat, and biological effects of ultrasound have been observed in laboratory experiments. However, these effects have been produced using continuous wave ultrasound with long ‘dwell’ time (time insonating one area) and high power output. Diagnostic ultrasound is pulsed wave (short pulses of sound propagation), and most modern machines have inbuilt safety features, so that safe power output limits cannot be exceeded. Operators are advised to apply the ALARA principle (as low as reasonably attainable) to the ultrasound power output used (EFSUMB 1995), and to ensure time taken for an examination, including the ‘dwell’ time over a specific target, is kept to a minimum. However, there is some evidence that operators’ knowledge about safety may not be accurate (Sheiner 2007). At present, there is no clear epidemiological evidence that ultrasound examination during pregnancy is harmful, but no firm conclusion has been reached from available data (see Cochrane review: Ultrasound for fetal assessment in early pregnancy (Neilson 1998)), and therefore, continual vigilance is necessary. This is particularly important in a context where scans are increasingly being carried out for non-medical reasons, and where there is a paucity of information on the number of scans women actually receive as a part of their antenatal care (ACOG 2004).
Cost and maternal psychological effects
There have been few studies evaluating the cost effectiveness of ultrasound scans (Henderson 2002). While there is some information on the direct costs of ultrasound examinations, there is much less on indirect cost (e.g. the costs of associated counselling, follow-up tests and related interventions, and indirect costs to women). Further, there is relatively little information on the psychological impact of ultrasound on women and their families (partners are frequently present for scans). Scans are popular with women, but women may not fully understand the purpose of their scan, and may be either falsely reassured or unprepared for adverse findings (Garcia 2002).
OBJECTIVES
To assess the effects on obstetric practice and pregnancy outcome of routine late pregnancy ultrasound, defined as greater than 24 weeks’ gestation, in women with either unselected or low-risk pregnancies.
METHODS
Criteria for considering studies for this review
Types of studies
All acceptably controlled trials of routine ultrasound in late pregnancy (after 24 weeks). Due to an anticipated paucity of randomised controlled trials, we considered quasi-randomised trials for inclusion. Routine ultrasound in early pregnancy (Neilson 1998) has been considered in a previous Cochrane review. Routine doppler ultrasound in pregnancy will be considered in a separate review (Bricker 2007b).
Types of participants
Women in late pregnancy (after 24 weeks’ gestation) in both unselected populations and designated low-risk populations.
Types of interventions
Routine ultrasound examination in late pregnancy (after 24 weeks’ gestation) to assess one, some or all of the following: fetal size; amniotic fluid volume; placental site; placental grading; fetal structural anatomy; fetal presentation.
Types of outcome measures
Primary outcomes
Induction of labour.
Caesarean section.
All deaths (perinatal, neonatal and infant).
Preterm delivery less than 34 weeks.
Neurodevelopment at age two.
Maternal psychological effects.
Secondary outcomes
Interventions
Antenatal admission to hospital.
Antenatal fetal monitoring:
kick count chart;
cardiotocography;
biophysical profile;
Doppler ultrasound;
further ultrasound.
Intention to deliver.
Operative delivery:
elective caesarean section (CS);
emergency CS;
instrumental vaginal delivery;
CS for distress;
CS for distress antepartum;
CS for distress intrapartum.
Perinatal outcome
Gestational age at birth.
Birthweight (mean and standard deviation).
Birthweight less than tenth percentile.
Birthweight less than third percentile.
Preterm delivery less than 37 weeks.
Low birthweight (less than 2.5 kg).
Very low birthweight (less than 1.5 kg).
Need for resuscitation.
Need for ventilation.
Admission to special care baby unit and average stay.
Low Apgar score (less than seven at five minutes).
Neonatal outcome
Acute neonatal problems:
hypoxic ischaemic encephalopathy;
necrotising enterocolitis;
intraventricular haemorrhage (IVH);
IVH with cystic periventricular leukomalacia;
pulmonary haemorrhage.
Early neonatal death (in first week of life).
Late neonatal death (from one to four weeks).
Infant death (one month to one year).
Maternal outcome
Psychological effects (including stress, anxiety, depression, quality of life, satisfaction).
Detection of:
major anomaly before birth;
malpresentation before labour.
Furthermore, the following non-prespecified outcome measures were used:
Post-term delivery greater than 42 weeks.
Birthweight less than 5th percentile.
Moderate neonatal morbidity (includes any of the following: presumed neonatal sepsis, oxygen required greater than 48 hours, necrotising enterocolitis without perforation, grade I or II intraventricular haemorrhage, fracture of clavicle or other bones, facial nerve injury, brachial plexus injury, stay greater than five days in the special care nursery).
Severe neonatal morbidity (includes any of the following: grade IV retinopathy of prematurity, bronchopulmonary dysplasia, mechanical ventilation greater than 48 hours, intestinal perforation due to necrotising enterocolitis, grade III or IV intraventricular haemorrhage, subdural or cerebral haemorrhage, spinal cord injury, neonatal seizures, placement of chest tube, documented neonatal sepsis, stay more than 30 days in the special care nursery).
Perinatal mortality of twins.
Only the six primary outcome measures will be used for subgroup analysis.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co-ordinator (February 2008).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co-ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co-ordinator searches the register for each review using the topic list rather than keywords.
We did not apply any language restrictions.
Data collection and analysis
All potential studies were identified as outlined in the search strategy. All three review authors were involved in assessing studies for inclusion and in data extraction. Two review authors independently assessed the trials for methodological quality. The reason for exclusion of any trial was clearly stated. The trials were not assessed blinded and the review authors knew the author’s name, institution, source of the publication and results when applying inclusion criteria. We resolved disagreements by discussion until consensus was reached. We sought additional information from some of the trialists by personal communication.
In earlier versions of this review, data extraction was performed by a principal review author and double checked for discrepancies with the co-author. For the additional trial included in this update, one author (T Dowswell) carried out data extraction and both the other authors (L Bricker and JP Neilson) checked the data; where data were unclear, we reached consensus by discussion. We performed statistical analysis using the Review Manager software (RevMan 2008).
Assessment of risk of bias
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We resolved any disagreement by discussion.
(1) Sequence generation (checking for possible selection bias)
We described for each included study the methods used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the methods as:
adequate (any truly random method, e.g. random number table; computer random-number generator);
inadequate (odd or even date of birth; hospital or clinic record number); or
unclear.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal the allocation sequence and assessed whether intervention allocation could have been foreseen in advance of, or during, recruitment.
We assessed the methods as:
adequate (e.g. consecutively numbered sealed opaque envelopes);
inadequate (open random allocation; unsealed or nonopaque envelopes, alternation, date of birth);
unclear.
(3) Blinding (checking for possible performance bias)
With interventions such as ultrasonography it may not be feasible to blind women and clinical staff to group allocation. Where there was any attempt to blind outcome assessors, this has been noted.
We have assessed the methods as:
adequate, inadequate or unclear for participants;
adequate, inadequate or unclear for personnel;
adequate, inadequate or unclear for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We have described for each included study the completeness of outcome data for each main outcome, including attrition and exclusions from the analysis. We have stated whether attrition and exclusions were reported, the numbers (compared with the total randomised participants), reasons for attrition or exclusion where reported, and any re-inclusions in analyses which we have undertaken.
We have assessed the methods as:
adequate (e.g. where there were no missing data, or low levels of missing data and where reasons for missing data are balanced across groups);
inadequate (e.g. where there are higher levels of missing data or attrition is not balanced across groups);
unclear (e.g. where there is insufficient reporting of attrition or exclusions to permit a judgement to me made).
(5) Selective reporting bias
We have described for each included study how the possibility of selective outcome reporting bias was examined by us and what we found.
We have assessed the methods as:
adequate (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
inadequate (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear.
(6) Other sources of bias
We have described for each included study any important concerns we had about other possible sources of bias; for example, where there was a potential source of bias associated with a particular aspect of study design.
Measures of treatment effect
We have carried out statistical analysis using the Review Manager software (RevMan 2008). We have used fixed-effect meta-analysis for combining data in the absence of high levels of heterogeneity. Where there was unexplained heterogeneity this has been noted, and we have used a random-effects model in the analysis.
Dichotomous data
For dichotomous data, we have presented results as summary risk ratios with 95% confidence intervals.
Continuous data
For continuous data, we have used the mean difference if outcomes have been measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but using different methods.
Subgroup and sensitivity analysis
If data had been available, we would have performed subgroup analyses as follows.
low-risk versus unselected populations;
purpose of ultrasound examinations (e.g. to detect intrauterine growth retardation, malpresentation, or to carry placental grading or to assess the placental site).
If the findings of any of the above subgroup analyses for the primary outcome measures had been significant, we would have extended the analysis to include all outcome measures.
We performed sensitivity analyses on the basis of quality of randomisation.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
See ‘Characteristics of included studies’ tables below.
Eight trials comprising 27,024 women were included (Alesund 1999; Belfast 2003; Glasgow 1984; New Zealand 1993; Perth 1993; Peterborough 1987; RADIUS 1993; Trondheim 1984). Longer term outcomes from the Alesund 1999 and Trondheim 1984 trials have been combined, and described in a series of papers by Salvesen and colleagues (Norway 1992).
Ultrasound examination options differed between trials, with some offering no routine scans at any time in pregnancy to the control group, some offering routine scans to all participants earlier in pregnancy (before 24 weeks’ gestation), and some offering routine scan at all stages of the trial, but only revealing results of late pregnancy ultrasound (after 24 weeks’ gestation) for the study groups.
Three trials (Alesund 1999 (Norway); RADIUS 1993 (USA);Trondheim 1984 (Norway)) offered routine ultrasound in the second and third trimesters versus selective ultrasound. In the New Zealand 1993 trial, all women had second trimester ultrasound scans, and only the study group underwent further third trimester ultrasound. In the Belfast 2003 (UK) trial all women had a first trimester scan while the study group had additional scans at 30 to 32 weeks and 36 to 37 weeks. In the Glasgow 1984 (UK) trial, all women were offered second and third trimester ultrasound, but the results of third trimester ultrasound were revealed only for the study group. In the Peterborough 1987 (UK) trial, all women had routine second and third trimester ultrasound, but placental grading at third trimester ultrasound was revealed only for the study group. In the Perth 1993 (Australia) trial, all women had routine second trimester ultrasound scans, and only the study group were offered serial ultrasound screening thereafter.
The trials evaluated different aspects of third trimester ultrasound. Two trials (Glasgow 1984 (UK); New Zealand 1993) addressed ultrasound screening for small for dates. The Peterborough 1987 (UK) trial addressed the value of placental grading as an adjunct to routine third trimester ultrasound scan. The Belfast 2003 trial examined the impact of two third trimester scans which assessed liquor volume, fetal weight and placental maturity. The RADIUS 1993 (USA) trial was the only study which reported in detail, detection of fetal abnormalities at routine third trimester ultrasound scan. The Perth 1993 (Australia) trial combined repeated ultrasound scan for fetal biometry and amniotic fluid assessment with Doppler ultrasound, and the data were therefore analysed in a separate comparison (serial ultrasound and Doppler ultrasound versus selective ultrasound), and were also included in another Cochrane review entitled ‘Routine Doppler ultrasound in pregnancy’ (Bricker 2007b).
The results of the review should be considered in the light of these different factors, as the specific nature of the ultrasound regimens may have had some effect on the outcome measures.
Childhood developmental outcomes were measured in the Perth 1993 study and in the Alesund and Trondheim trials (the longer term outcomes from the latter two trials have been combined (Norway 1992)).
Risk of bias in included studies
The methodological quality in general was good. The Glasgow 1984 (UK) study was ‘quasi-randomised’ with allocation according to hospital number, and this had the potential to introduce bias. The assessment of bias in the included studies has been set out in the risk of bias tables following the ‘Characteristics of included studies’ tables.
Effects of interventions
There were no or few data available for some of the prespecified outcome measures, particularly maternal and neonatal outcomes. A number of outcome measures which were not prespecified were included, namely post-term delivery, birthweight less than fifth centile (less than third centile was prespecified, but there were no data), moderate neonatal morbidity, severe neonatal morbidity and perinatal mortality specifically of twin babies. In this update we have also examined longer term outcomes including school performance at eight to nine years of age.
Subgroup analyses were not performed due to the small number of included studies, and hence limited data.
For a number of the comparisons there was considerable heterogeneity. Where heterogeneity was substantial (over 50%), we have used a random-effects model, and in view of high levels of heterogeneity these results should be interpreted with caution (Analysis 1.1; Analysis 1.4; Analysis 1.5; Analysis 1.14; Analysis 1.18; Analysis 1.20; Analysis 1.23; Analysis 1.26).
Routine ultrasound after 24 weeks’ gestation versus no/concealed or selective ultrasound after 24 weeks’ gestation
There were no differences between groups in the rates of women having further ultrasound scans, in antenatal admissions or in other tests of fetal wellbeing. Neither was there a significant difference in obstetric interventions, such as induction of labour and instrumental deliveries. There was a slightly increased caesarean section rate in the screened compared to the comparison group and this finding approached statistical significance (risk ratio (RR) 1.06, 95% confidence interval (CI) 1.00 to 1.13, P = 0.07).
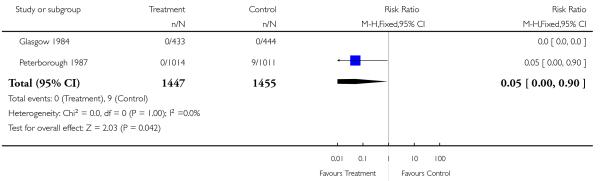
Screened groups were less likely to deliver post-term (after 42 weeks’ gestation) (RR 0.69, 95% CI 0.59 to 0.81). Preterm delivery rates and birthweight data were similar in study and control groups. Overall, perinatal mortality including or not including congenital abnormalities was no different. There was also no difference in perinatal mortality of twins. Only two studies reported separate data for stillbirths and neonatal deaths in congenitally normal fetuses or neonates (Glasgow 1984 (UK); Peterborough 1987 (UK)). The Peterborough 1987 (UK) data have suggested a reduction in the stillbirth rate if placental grading is incorporated into routine third trimester ultrasound scan (RR 0.05, 95% CI 0.00 to 0.90).
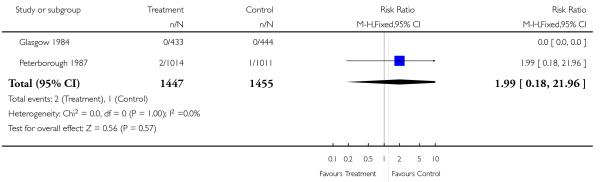
Neonatal interventions such as resuscitation, ventilation and admission to special care, were no different in screened or revealed versus control groups. Neither was there a difference in five minute Apgar scores. The only study reporting moderate and severe neonatal morbidity (RADIUS 1993 (USA)) showed no difference between study groups.
There were no differences between screened and control groups for any childhood development outcomes measured at eight to nine years including physical development, language, speech and intelligence (Perth 1993; Norway 1992). The findings from these studies have not been combined in meta-analysis as outcomes were measured at different time points or using different tools, or both. There was some evidence of increased non-right handedness for children exposed to ultrasound in the follow up from the Alesund and Trondheim trials, but as these findings have not been replicated they may have occurred by chance (Norway 1992).
Psychological and other maternal outcomes were not reported in any of the included studies.
Sensitivity analysis
One of the trials examining ultrasound after 24 weeks’ gestation used a quasi-randomised design with poor allocation concealment (Glasgow 1984). We conducted a sensitivity analysis, removing this trial from each comparison where it contributed data to see if this would make any difference to the direction of findings, or to the size of the treatment effect. Removing this study made little difference to the results; it did not change the direction of findings and, as it was a relatively small study, made little difference to the size of the treatment effect even for those outcomes where only a small number of trials contributed data.
Serial ultrasound and Doppler ultrasound versus selective ultrasound
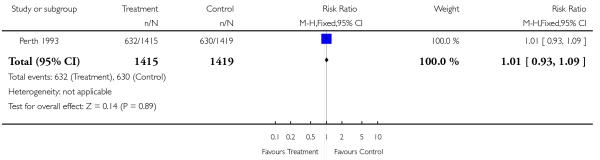
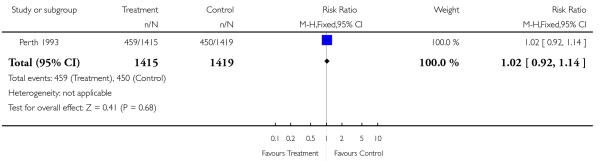
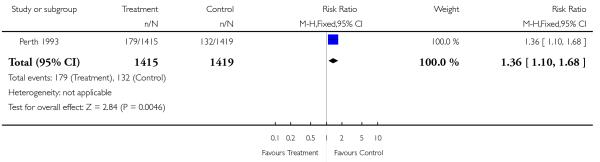
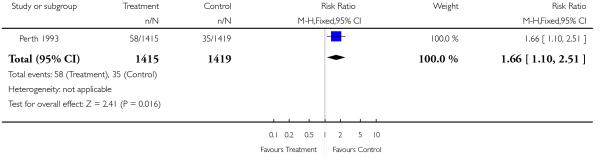
This comparison includes published and unpublished data from the Perth 1993 study (Australia). The results of this trial showed no significant difference between the two groups in antenatal admissions or other tests of fetal wellbeing. Neither was there a significant difference in obstetric interventions, such as induction of labour, instrumental deliveries and caesarean section. More babies in the screened group were of birthweight less than tenth centile (RR 1.36, 95% CI 1.10 to 1.68), and less than third centile (RR 1.66, 95% CI 1.10 to 2.51). There was no difference in other indices of perinatal or neonatal outcome.
DISCUSSION
The ultrasound scan protocols in each trial varied. It is difficult to assess the effect of scans before 24 weeks’ gestation on the outcome measures. For example, the finding of a reduction in post-term delivery in the screened group of the RADIUS 1993 (USA) study is probably due to better gestational age assessment at the 18 to 20 week scan. Furthermore, the reason for routine ultrasound scan after 24 weeks’ gestation differed amongst trials. Ideally, subgroup analyses according to the reason for the scan would resolve the possible difference in outcomes according to the diagnostic approach, but there are not enough studies to perform meaningful subgroup analyses. The results of the meta-analysis should be viewed in this light.
Whilst the most accurate approach to assessment of the effect of routine late pregnancy ultrasound would be trials where the intervention is late pregnancy ultrasound alone, no such trials exist. The fact that assessment of most parameters at late pregnancy ultrasound are based on gestational reference data, which in turn rely on accurate gestational dating in early pregnancy, further compounds this issue. Therefore, it is neither realistic, nor pragmatic, to consider routine late pregnancy ultrasound in isolation, and the included studies probably reflect existing practice.
Meta-analysis of the data shows very little difference between groups in antenatal, obstetric and neonatal interventions apart from a small increase in the caesarean section rate in the screened group. Overall perinatal mortality was no different, for all fetuses or neonates, and twin pregnancies. Although there was some heterogeneity in perinatal mortality overall (I2 = 38%), there was increased heterogeneity in perinatal mortality corrected for abnormality (I2 = 58%). This was due to the Peterborough 1987 (UK) trial data that suggested a significant reduction in the number of congenitally normal stillbirths. This trial is unique in that it is an evaluation of placental grading as an adjunct to routine late pregnancy ultrasound. The authors state that this observation was not a formal prior hypothesis and may be an overestimate of the true effect of the test. In view of the nature of the trial, i.e. single centre and limited power to assess perinatal outcome (2000 participants), and the fact that it was performed over two decades ago (1987), this finding needs to be revisited in future research.
There was no overall difference in the incidence of babies being small-for-gestational age at birth (less than tenth percentile), comparing experimental and control groups (RR 0.98, 95% CI 0.74 to 1.28), but there was high heterogeneity (I2 = 70%), mainly due to the findings of the Belfast 2003 study. Whilst, theoretically, there is some potential scope to decrease the incidence of small-for-dates babies at birth by recognition of fetal growth restriction through ultrasound screening, and early planned delivery, it seems unlikely that this intervention could have a strong effect on this particular outcome.
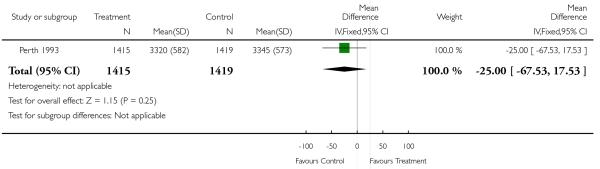
In the Perth 1993 (Australia) trial there was an unexpected finding of significantly higher intrauterine growth restriction in the serial ultrasound and Doppler examination group (i.e. the intensive group). The authors state that while this may have been a chance finding, it is possible that frequent exposure to ultrasound may have influenced fetal growth. This finding was not associated with increased perinatal morbidity and mortality, and follow up of these children at one year of age found that the difference was no longer discernible (Newnham 1996). The authors stress the need for further investigation of the effects of frequent ultrasound exposure on fetal growth. Furthermore, if this were a true effect, the modality responsible (Doppler ultrasound versus realtime ultrasound) would need to be elucidated. More recent data from this trial reporting findings for children at eight years of age found no negative effect associated with serial ultrasound in terms of physical size or speech, language and neurological development (Newnham 2004).
Long-term follow up of children from the Norwegian studies revealed no positive or negative effects of exposure to ultrasound in children aged eight to nine years although there was an association between exposure to ultrasound in utero and non-right handedness. This finding may be a chance one, but merits further investigation (Alesund 1999; Norway 1992; Trondheim 1984).
The RADIUS 1993 (USA) trial was the only study which addressed detection of fetal anomalies in the third trimester. The overall fetal anomaly detection rate in this trial was poor, at 35%. After 24 weeks’ gestation, 34/156 (22%) anomalous fetuses were detected in the screened group, and 10/155 (6.5%) anomalies were detected in the control group. However, the better detection rate in the screened group did not translate into an improvement in infant survival.
Few of the trials addressed long-term neurodevelopmental outcome and none examined maternal psychological outcome, and it is arguable that these are the most important outcomes. Exposure of the expectant mother to uncertainty and possible anxiety about the health of her baby has implications which may be far reaching. In addition, perinatal survival does not automatically translate into long-term success, as little is known about the longterm prognosis of the in utero compromised fetus.
AUTHORS’ CONCLUSIONS
Implications for practice
There is no evidence that routine ultrasound in late pregnancy improves perinatal outcome. Its use may increase the rate of caesarean section, and hence the risk of iatrogenic morbidity. As a result of this review, it is not clear what aspects of late pregnancy ultrasound may be valuable in centres where it is undertaken. However, placental grading may be useful, and perhaps should be considered in late pregnancy ultrasound, whether routine or selective.
Implications for research
There is a lack of data about the potential psychological effects of routine ultrasound in late pregnancy, and the effects on both short- and long-term neonatal and childhood outcome. Future studies should address these issues along with the effect of ultrasound on operative delivery rates.
Based on the available data about the value of placental grading, future research of late pregnancy ultrasound should include assessment of placental texture.
PLAIN LANGUAGE SUMMARY.
Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Ultrasound can be used in late pregnancy to assess the baby’s condition when there are complications, but carrying out scans on all women is controversial. Scans can be used in late pregnancy to detect problems which may not otherwise be apparent, such as abnormalities in the placenta, in the fluid surrounding the baby, or in the baby’s growth. If such problems are identified this may lead to changes in care and improved outcome for babies. At the same time, screening all women may mean that interventions are increased without benefit to mothers or babies. Scans are popular, but women may not fully understand the purpose of their scan and may be either falsely reassured or unprepared for adverse findings. Based on existing evidence, routine ultrasound, after 24 weeks gestation, in low-risk or unselected women does not provide any benefit for mother or baby. Eight studies that randomised 27,024 women to screening or a control group (no or selective ultrasound, or ultrasound with concealed results) contributed to the review. The quality of trials was satisfactory. There were no differences between groups in the rates of women having additional scans, antenatal admissions, preterm delivery, induction of labour, or instrumental deliveries although the rate of caesarean section increased slightly with screening. For babies, birthweight, condition at birth, interventions such as resuscitation, and admission to special care were similar between groups. Infant survival, with or without congenital abnormalities, was no different with and without routine screening, and childhood development at eight to nine years was similar in the three trials that measured it. None of the trials reported on psychological effects for mothers of routine ultrasound in late pregnancy.
ACKNOWLEDGEMENTS
Mrs Lixia Dou, Cochrane Pregnancy and Childbirth Group, The University of Liverpool, provided much valued help with the assessment of risk of bias for the studies included in this updated review.
Professor A M Weindling (Professor of Perinatal Medicine, The University of Liverpool) for advice on neonatal outcome measures.
Prof SH Eik-Nes, Drs KA Salvesen, LJ Vatten and O Okland have provided unpublished results from the Alesund trial. Professor J Newnham and Dr Sharon Evans have provided unpublished results from the Perth trial.
SOURCES OF SUPPORT
Internal sources
NHS R&D Health Technology Assessment Programme, Grant number 93/30/03, UK.
The University of Liverpool, UK.
External sources
National Institute for Health Research, UK.
NIHR NHS Cochrane Collaboration Programme Grant Scheme award for NHS-prioritised centrally-managed, pregnancy and childbirth systematic reviews:CPGS02
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Randomisation by sealed envelopes. | |
| Participants | Nearly all women in that geographical area, including those with ‘high-risk’ pregnancies. Recruitment 1979-1981. 1628 women | |
| Interventions | Routine ultrasound examination at 18 weeks (biparietal diameter measured) and 32 weeks (biparietal diameter and mean abdominal diameter) with additional examination at 36 weeks’ gestation if fetus small-for-gestational age and/or presenting by breech - versus selective examination for clinical indications only | |
| Outcomes | Obstetric interventions (antepartum and intrapartum) for singleton pregnancies only. Perinatal outcome indices for all pregnancies (including multiple pregnancies) | |
| Notes | This trial was reported in letter form only in 1984. It subsequently became clear that there were inconsistencies in results, and the data were subsequently re-analysed. The data entered in this review are derived from more recent unpublished and published reports | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not stated. |
| Allocation concealment? | Unclear | Described as “sealed envelope”. |
| Blinding? Women |
No | Not feasible. |
| Blinding? Clinical staff |
No | Not feasible. |
| Blinding? Outcome assessors |
No | Not feasible. |
| Incomplete outcome data addressed? All outcomes |
Yes | The data could be re-included. |
| Free of selective reporting? | Yes | Selective outcome reporting bias not apparent. |
| Free of other bias? | No | More smokers in screened group; historical study. |
| Methods | Randomised controlled trial. Randomisation by sealed, numbered envelopes | |
| Participants | Women recruited at 30 weeks’ gestation assessed at low risk with singleton pregnancy and dates confirmed by 18-20 weeks’ anomaly scan Exclusion criteria: known medical or obstetric problems or known fetal anomaly 1998 women recruited over a 21 month period. |
|
| Interventions | Assessment 30-32 weeks and at 36-37 weeks by a midwife as part of routine care with midwife estimate of fetal size, presentation, position and amniotic fluid volume. In addition, the study group had ultrasound examinations by the specially trained midwife to assess liquor volume, fetal weight and placental maturity. The comparison group had selective ultrasound examinations if indicated | |
| Outcomes | Small-for-gestation age at birth, admission to special care and antenatal interventions | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Computer generated (restricted to achieve group balance). |
| Allocation concealment? | Yes | Sealed, numbered envelopes. |
| Blinding? Women |
No | Not feasible. |
| Blinding? Clinical staff |
No | Not feasible. |
| Blinding? Outcome assessors |
No | Not feasible. |
| Incomplete outcome data addressed? All outcomes |
Yes | Good follow up for most outcomes. |
| Free of selective reporting? | Unclear | Difficult to interpret the results relating to the main outcome |
| Free of other bias? | Yes | |
| Methods | Pseudorandomisation according to last digit in hospital number | |
| Participants | 877 women attending the hospital antenatal clinic between 34-36.5 weeks’ gestation with uncomplicated singleton pregnancies, i.e. low-risk pregnancies | |
| Interventions | All women had an ultrasound examination <; 24 weeks’ gestation for gestational dating. All had further ultrasound scan at 34-36.5 weeks’ gestation to measure crown rump length and trunk area, but in the study group the two measurements were multiplied and the results plotted and reported in the case notes (i.e. revealed). Further management was the responsibility of the clinical staff. No requests for control group measurements to be revealed occurred, but this option was available to clinicians | |
| Outcomes | Obstetric interventions (antepartum and intrapartum) and perinatal outcome indices | |
| Notes | This study addressed ultrasound screening for small for dates | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | No | Described as allocated “from their hospital index numbers”. |
| Allocation concealment? | No | See above. |
| Blinding? Women |
No | Not feasible. |
| Blinding? Clinical staff |
No | Not feasible. |
| Blinding? Outcome assessors |
No | Not feasible. |
| Incomplete outcome data addressed? All outcomes |
Unclear | Small loss to follow up. |
| Free of selective reporting? | Yes | Selective outcome reporting not detected. |
| Free of other bias? | Unclear | There were more participants from social class V in the reported group |
| Methods | Randomised by women selecting one of a number of envelopes (< 6) containing a computer-generated random 1 or 2 and a study number | |
| Participants | All pregnant women who attended antenatal clinic < 24 weeks’ gestation, i.e. unselected population. Multiple pregancies excluded once diagnosed (and study numbers reused). 1527 women | |
| Interventions | All women had a dating scan 16-24 weeks’ gestation. Study group had a further scan at 32-36 weeks’ gestation (ideally 34 weeks’ gestation) which aimed to detect small-for-gestational-age fetuses, and if estimated fetal weight fell below the 20th centile for gestation, this was reported and additional scans recommended but not arranged. Clinicians were able to order further scans for the control group if clinically indicated | |
| Outcomes | Mainly perinatal outcome indices. Number of further ultrasound scans | |
| Notes | Scan to detect small-for-gestational age. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Described as “randomised controlled trial”. |
| Allocation concealment? | Unclear | Not stated. |
| Blinding? Women |
No | Not feasible. |
| Blinding? Clinical staff |
No | Not feasible. |
| Blinding? Outcome assessors |
No | Not feasible. |
| Incomplete outcome data addressed? All outcomes |
Unclear | Not stated. |
| Free of selective reporting? | Unclear | No information provided. |
| Free of other bias? | Unclear | No information provided. |
| Methods | Combined findings from the Tronheim and Alesund trials for childhood developmental outcomes | |
| Participants | Alesund - nearly all women in that geographical area, including those with ’high-risk’ pregnancies. Recruitment 1979-1981. 1628 women Trondheim - 1009 pregnant women in Trondheim attending for antenatal care between 1979-1980 |
|
| Interventions | Alesund - routine ultrasound examination at 18 weeks (biparietal diameter measured) and 32 weeks (biparietal diameter and mean abdominal diameter) with additional examination at 36 weeks’ gestation if fetus small-for-gestational age and/or presenting by breech - versus selective examination for clinical indications only Trondheim - study group offered ultrasound examinations at 19 weeks’ and 32 weeks’ gestation |
|
| Outcomes | Follow up of singletons at 8-9 years including teacher assessed school performance, along with assessments of reading, speech and intelligence scores | |
| Notes | This is not strictly a separate study, but findings from the Alesund and Trondheim trials were combined for long-term follow up | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not stated (both studies). |
| Allocation concealment? | Unclear | Sealed envelopes. |
| Blinding? Women |
No | Not feasible. |
| Blinding? Clinical staff |
No | Not feasible. |
| Blinding? Outcome assessors |
Unclear | Not stated. |
| Incomplete outcome data addressed? All outcomes |
Unclear | 2011 of 2824 eligible followed up. |
| Methods | Sealed envelopes. | |
| Participants | 2834 singleton pregnancies. Criteria for recruitment were gestational age 16-20 weeks, sufficient proficiency in English, expected to deliver at the hospital and an intention to remain in Western Australia so that childhood follow up would be feasible | |
| Interventions | The ’regular’ group had an ultrasound examination at 18 weeks for fetal biometry, subjective amniotic fluid assessment and placental morphology and location, and any further scans in pregnancy were conducted on clinicians request. The ’intensive group’ had the aforementioned ultrasound examination, plus an amniotic fluid index and continuous wave Doppler ultrasound of the umbilical artery and an arcuate artery within the placental vascular bed at 18, 24, 28, 34 and 38 weeks’ gestation. The Doppler ultrasound parameter reportedwas systolic/diastolic ratio. Results of these examinations were recorded in the hospital chart, but no clinical management guidance was given | |
| Outcomes | Obstetric interventions (antepartum and intrapartum) and perinatal outcome indices | |
| Notes | The published study reports the results overall, but little data are available for extraction. The authors were contacted and provided unpublished data |
|
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Described as “computer-generated random numbers”. |
| Allocation concealment? | Unclear | Described as “sealed envelopes”. |
| Blinding? Women |
No | Not feasible. |
| Blinding? Clinical staff |
No | Not feasible. |
| Blinding? Outcome assessors |
No | Not feasible. |
| Incomplete outcome data addressed? All outcomes |
Unclear | Some loss to follow up in both groups. “13 were lost to follow up in the intensive group of the trial and 20 in the regular” care group |
| Free of selective reporting? | Yes | Selective outcome reporting bias not detected. |
| Free of other bias? | Yes | Other bias not detected. |
| Methods | Randomisation by opaque sealed envelopes. | |
| Participants | 2000 pregnant women attending the ultrasound department for routine third trimester scans, including multiple pregnancies | |
| Interventions | All women were offered routine early pregnancy ultrasound and 2 routine scans in the third trimester. Placental grading was performed at the routine third trimester scan. The results of placental grading in the study group were revealed, and the control group concealed. Clinical management in both groups was left entirely to the clinician responsible for care | |
| Outcomes | Obstetric interventions (antepartum and intrapartum) and perinatal outcome indices | |
| Notes | This study addresses the value of placental grading at routine third trimester ultrasound | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Described as “randomly allocated to 1 of 2 groups”. |
| Allocation concealment? | Yes | Described as “a correspondingly numbered, sealed, opaque envelope” |
| Blinding? Women |
No | Not feasible. |
| Blinding? Clinical staff |
No | Not feasible. |
| Blinding? Outcome assessors |
No | Not feasible. |
| Incomplete outcome data addressed? All outcomes |
Yes | No losses to follow up. |
| Free of selective reporting? | Yes | Selective outcome reporting not detected. |
| Free of other bias? | Yes | No other bias apparent. |
| Methods | Randomisation by microcomputer after stratification by practice site. Intention to treat | |
| Participants | 15151 pregnant women who did not have “an indication for ultrasonography” based on uncertain gestational age, previous or index pregnancy complication, medical disorder. Therefore, those eligible were at low risk of adverse pregnancy outcome, and comprised 40% of the total population | |
| Interventions | Ultrasound screen at 18-20 weeks’ and 31-33 weeks’ gestation, versus selective ultrasonography | |
| Outcomes | Perinatal outcome indices. The primary outcomes were perinatal mortality and moderate/ severe neonatal morbidity | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Described as “computer-generated randomisation sequence”. |
| Allocation concealment? | Unclear | Not stated. |
| Blinding? Women |
No | Not feasible. |
| Blinding? Clinical staff |
No | Not feasible. |
| Blinding? Outcome assessors |
Unclear | Not feasible. |
| Incomplete outcome data addressed? All outcomes |
Unclear | 252 women lost to follow up. |
| Free of selective reporting? | Yes | Selective outcome reporting bias not detected. |
| Free of other bias? | Yes | No other bias detected. |
| Methods | Randomised by sealed-envelope method. | |
| Participants | 1009 pregnant women in Trondheim attending for antenatal care between 1979-1980 | |
| Interventions | Study group offered ultrasound examinations at 19 weeks and 32 weeks” gestation | |
| Outcomes | Obstetric interventions (antepartum and intrapartum) and perinatal outcome indices | |
| Notes | Some data only presented for singletons (mean birthweight, birthweight < 10th centile, low birthweight, neonatal resuscitation, admission to special care, Apgar scores) | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Not stated. |
| Allocation concealment? | Unclear | Described as sealed envelopes. |
| Blinding? Women |
No | Not feasible. |
| Blinding? Clinical staff |
No | Not feasible. |
| Blinding? Outcome assessors |
Yes | Neonatal outcome assessment blinding - yes. Pregnancy outcome assessment blinding - no. |
| Incomplete outcome data addressed? All outcomes |
Unclear | Small loss to follow up. |
| Free of selective reporting? | Yes | Selective outcome reporting bias not detected. |
| Free of other bias? | Yes | Other bias not apparent. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belanger 1996 | Brief abstract. No usable data. |
| Hendrix 2000 | Compared clinical versus ultrasound estimates of birthweight in terms of accuracy. Did not include review outcomes |
| Morrison 1992 | Brief abstract. No usable data. |
| Ong 2001 | Study not undertaken. |
| Owen 1994 | Brief abstract. No usable data. |
| Secher 1986 | The methodology is unclear as all suspected LGA fetuses were to be referred to an obstetrician for further evaluation. However, suspected LGA in 26 such fetuses included in the final analysis was not reported to clinicians primarily because they were part of another randomised study. The other randomised trial (Secher 1987) was also not included - see reasons in this table. |
| Secher 1987 | In this study, third trimester ultrasound was used to identify a group of uncomplicated pregnancies where there was ultrasound suspicion of poor intrauterine growth, but no clinical suspicion of poor growth. Only these pregnancies were randomised. The revealed group underwent serial tests of fetal wellbeing (non-stress CTG and serum oestriol and placental lactogen) and fetal growth and management was planned depending on the results of the tests. Therefore, the study assesses the value of various tests of fetal wellbeing if fetal growth retardation is suspected, rather than the value of routine third trimester ultrasound alone |
| Wladimiroff 1980 | The primary aim of this study was to assess the ability of third trimester ultrasound in detecting small and large-for-dates infants, and no clinical outcomes were evaluated |
CTG: cardiotocograph
LGA: light-for-gestational age
DATA AND ANALYSES
Comparison 1. Routine ultrasound > 24 weeks vs no/concealed/selective ultrasound > 24 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Antenatal admission | 4 | 5396 | Risk Ratio (M-H, Random, 95% CI) | 1.07 [0.80, 1.43] |
| 2 Number of days in hospital (mean, standard deviation (SD)) | 1 | 877 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.07, 0.13] |
| 3 CTG (cardiotocograph) | 1 | 2000 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.97, 1.06] |
| 4 Further ultrasound scan/s | 2 | 2536 | Risk Ratio (M-H, Random, 95% CI) | 0.91 [0.43, 1.89] |
| 5 Induction of labour | 6 | 22663 | Risk Ratio (M-H, Random, 95% CI) | 0.93 [0.81, 1.07] |
| 6 Instrumental delivery | 4 | 5884 | Risk Ratio (M-H, Fixed, 95% CI) | 1.04 [0.92, 1.18] |
| 7 Caesarean section | 5 | 21035 | Risk Ratio (M-H, Fixed, 95% CI) | 1.06 [1.00, 1.13] |
| 8 Elective caesarean section | 4 | 5884 | Risk Ratio (M-H, Fixed, 95% CI) | 1.09 [0.89, 1.34] |
| 9 Emergency caesarean section | 4 | 5884 | Risk Ratio (M-H, Fixed, 95% CI) | 1.11 [0.92, 1.33] |
| 10 Preterm delivery < 37 weeks’ gestation | 2 | 17151 | Risk Ratio (M-H, Fixed, 95% CI) | 0.96 [0.85, 1.08] |
| 11 Post-term delivery >42 weeks’ gestation | 2 | 17151 | Risk Ratio (M-H, Fixed, 95% CI) | 0.69 [0.59, 0.81] |
| 12 Gestation at delivery (mean, SD) | 2 | 2877 | Mean Difference (IV, Fixed, 95% CI) | −0.16 [−0.26, −0.06] |
| 13 Birthweight (mean, SD) | 4 | 19710 | Mean Difference (IV, Fixed, 95% CI) | −0.47 [−15.49, 14.54]. |
| 14 Birthweight < 10th centile | 4 | 20293 | Risk Ratio (M-H, Random, 95% CI) | 0.98 [0.74, 1.28] |
| 15 Birthweight < 5th centile | 2 | 2404 | Risk Ratio (M-H, Fixed, 95% CI) | 1.18 [0.81, 1.74] |
| 16 Low birthweight < 2.5 kg | 3 | 4510 | Risk Ratio (M-H, Fixed, 95% CI) | 0.92 [0.71, 1.18] |
| 17 Neonatal resuscitation | 4 | 6533 | Risk Ratio (M-H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 18 Neonatal ventilation | 2 | 3004 | Risk Ratio (M-H, Random, 95% CI) | 0.64 [0.23, 1.77] |
| 19 Admission to special care baby unit | 4 | 6539 | Risk Ratio (M-H, Fixed, 95% CI) | 0.95 [0.79, 1.14] |
| 20 Apgar score < 7 at 5 minutes | 4 | 5889 | Risk Ratio (M-H, Random, 95% CI) | 0.89 [0.41, 1.93] |
| 21 Moderate neonatal morbidity | 1 | 15281 | Risk Ratio (M-H, Fixed, 95% CI) | 0.97 [0.81, 1.16] |
| 22 Severe neonatal morbidity | 1 | 15281 | Risk Ratio (M-H, Fixed, 95% CI) | 1.03 [0.78, 1.36] |
| 23 Perinatal mortality | 7 | 24276 | Risk Ratio (M-H, Random, 95% CI) | 0.94 [0.55, 1.61] |
| 24 Stillbirths | 5 | 21708 | Risk Ratio (M-H, Random, 95% CI) | 1.11 [0.29, 4.26] |
| 25 Neonatal deaths | 5 | 21708 | Risk Ratio (M-H, Fixed, 95% CI) | 1.04 [0.58, 1.85] |
| 26 Perinatal mortality (excluding congenital abnormalities) | 5 | 21734 | Risk Ratio (M-H, Random, 95% CI) | 0.88 [0.36, 2.17] |
| 27 Stillbirths (excluding congenital abnormalities) | 2 | 2902 | Risk Ratio (M-H, Fixed, 95% CI) | 0.05 [0.00, 0.90] |
| 28 Neonatal deaths (excluding congenital abnormalities) | 2 | 2902 | Risk Ratio (M-H, Fixed, 95% CI) | 1.99 [0.18, 21.96] |
| 29 Perinatal mortality (twins) | 3 | 314 | Risk Ratio (M-H, Fixed, 95% CI) | 0.63 [0.24, 1.66] |
Comparison 2. Serial ultrasound and Doppler ultrasound vs selective ultrasound.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 CTG (cardiograph) | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 1.01 [0.93, 1.09] |
| 2 Induction of labour | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 1.02 [0.92, 1.14] |
| 3 Caesarean section | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 0.89 [0.76, 1.03] |
| 4 Elective caesarean section | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 0.95 [0.77, 1.17] |
| 5 Emergency caesarean section | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 0.82 [0.64, 1.05] |
| 6 Gestation at delivery (mean, SD) | 1 | 2834 | Mean Difference (IV, Fixed, 95% CI) | −0.10 [−1.21, 1.01] |
| 7 Birthweight (mean, SD) | 1 | 2834 | Mean Difference (IV, Fixed, 95% CI) | −25.0 [−67.53, 17.53] |
| 8 Birthweight < 10th centile | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 1.36 [1.10, 1.68] |
| 9 Birthweight < 3rd centile | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 1.66 [1.10, 2.51] |
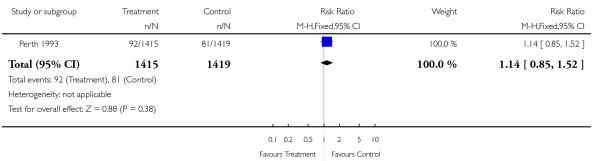
| 10 Low birthweight (< 2.5 kg) | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 1.14 [0.85, 1.52] |
| 11 Very low birthweight (< 1.5 kg) | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 1.27 [0.65, 2.49] |
| 12 Neonatal resuscitation | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 0.98 [0.92, 1.05] |
| 13 Neonatal ventilation | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 0.67 [0.41, 1.09] |
| 14 Admission to special care baby unit | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 0.95 [0.68, 1.31] |
| 15 Apgar score < 7 at 5 minutes | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 0.77 [0.46, 1.27] |
| 16 Neonatal intraventricular haemorrhage | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 0.80 [0.22, 2.98] |
| 17 Perinatal mortality | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 0.59 [0.30, 1.17] |
| 18 Stillbirths | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 0.84 [0.36, 1.93] |
| 19 Neonatal deaths | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 0.30 [0.08, 1.09] |
| 20 Neonatal death (excluding congenital abnormalities) | 1 | 2834 | Risk Ratio (M-H, Fixed, 95% CI) | 0.40 [0.08, 2.06] |
Analysis 1.1. Comparison 1 Routine ultrasound > 24 weeks vs no/concealed/selective ultrasound > 24 weeks, Outcome 1 Antenatal admission.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound > 24 weeks vs no/concealed/selective ultrasound > 24 weeks
Outcome: 1 Antenatal admission
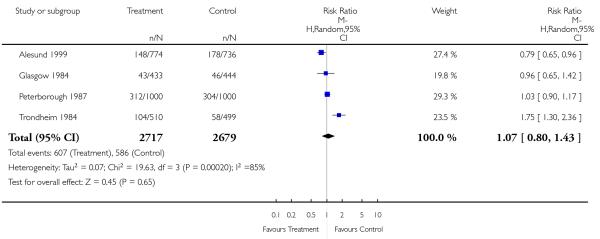 |
Analysis 1.2. Comparison 1 Routine ultrasound > 24 weeks vs no/concealed/selective ultrasound > 24 weeks, Outcome 2 Number of days in hospital (mean, standard deviation (SD)).
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound > 24 weeks vs no/concealed/selective ultrasound > 24 weeks
Outcome: 2 Number of days in hospital (mean, standard deviation (SD))
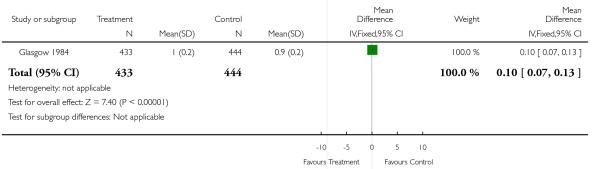 |
Analysis 1.3. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 3 CTG (cardiotocograph).
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 3 CTG (cardiotocograph)
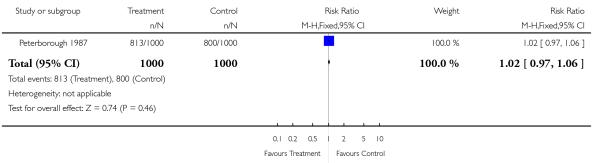 |
Analysis 1.4. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 4 Further ultrasound scan/s.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 4 Further ultrasound scan/s
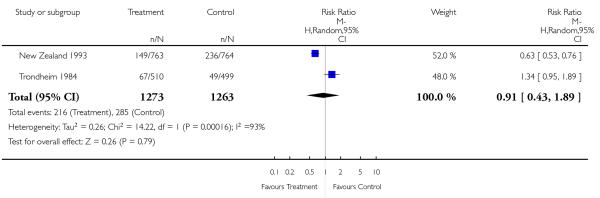 |
Analysis 1.5. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 5 Induction of labour.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 5 Induction of labour
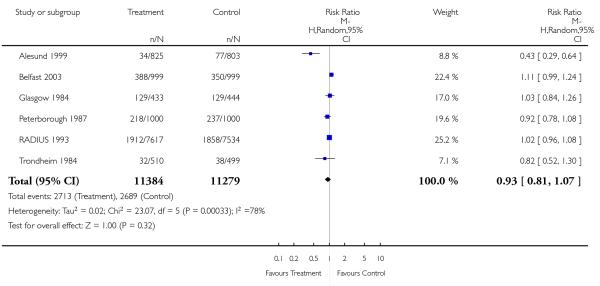 |
Analysis 1.6. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 6 Instrumental delivery.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 6 Instrumental delivery
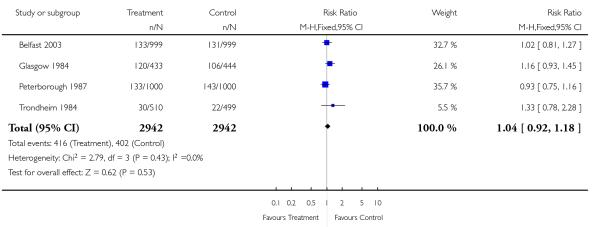 |
Analysis 1.7. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 7 Caesarean section.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 7 Caesarean section
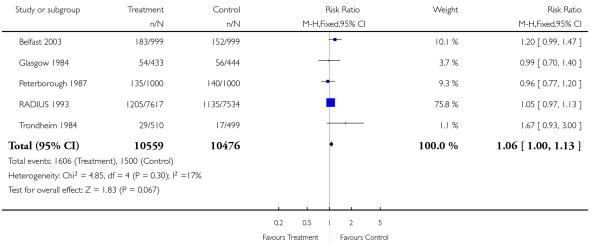 |
Analysis 1.8. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 8 Elective caesarean section.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 8 Elective caesarean section
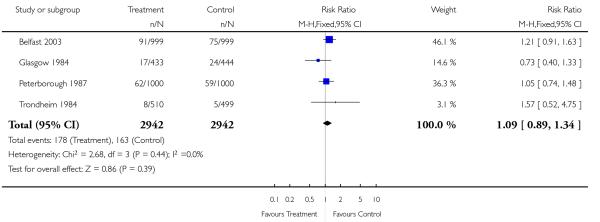 |
Analysis 1.9. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 9 Emergency caesarean section.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 9 Emergency caesarean section
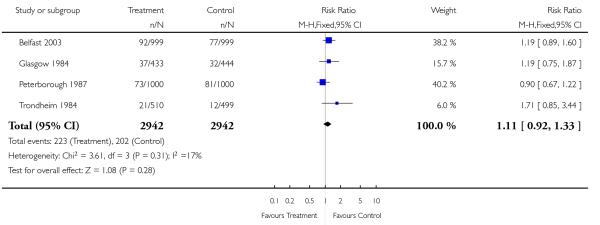 |
Analysis 1.10. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 10 Preterm delivery < 37 weeks’ gestation.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 10 Preterm delivery < 37 weeks’ gestation
 |
Analysis 1.11. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 11 Post-term delivery >42 weeks’ gestation.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 11 Post-term delivery >42 weeks’ gestation
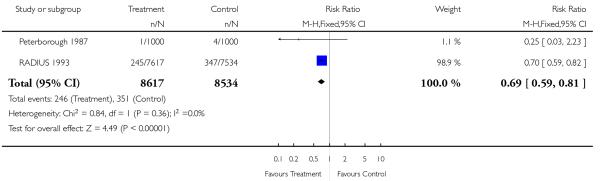 |
Analysis 1.12. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 12 Gestation at delivery (mean, SD).
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 12 Gestation at delivery (mean, SD)
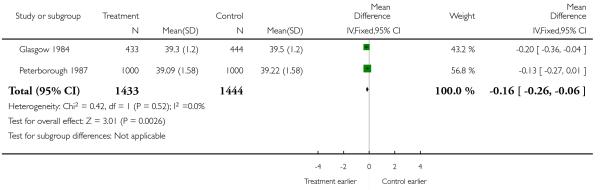 |
Analysis 1.13. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 13 Birthweight (mean, SD).
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 13 Birthweight (mean, SD)
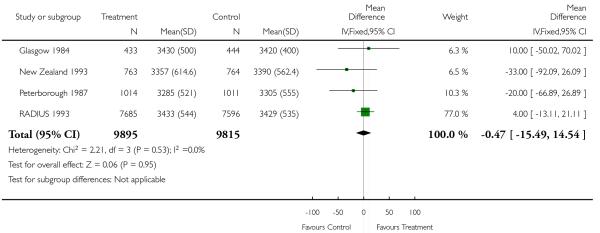 |
Analysis 1.14. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 14 Birthweight <10th centile.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 14 Birthweight <10th centile
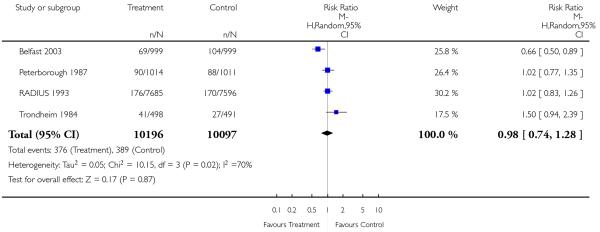 |
Analysis 1.15. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 15 Birthweight <5th centile.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 15 Birthweight <5th centile
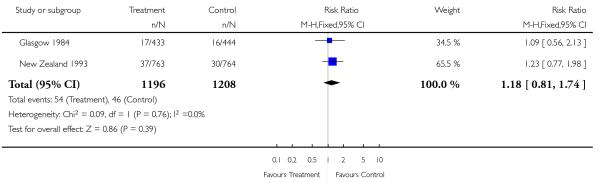 |
Analysis 1.16. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 16 Low birthweight <2.5 kg.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 16 Low birthweight <2.5 kg
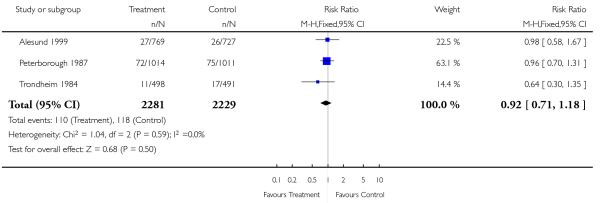 |
Analysis 1.17. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 17 Neonatal resuscitation.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 17 Neonatal resuscitation
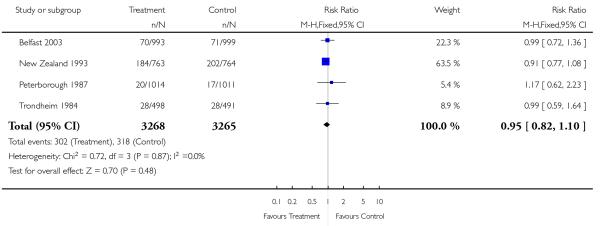 |
Analysis 1.18. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 18 Neonatal ventilation.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 18 Neonatal ventilation
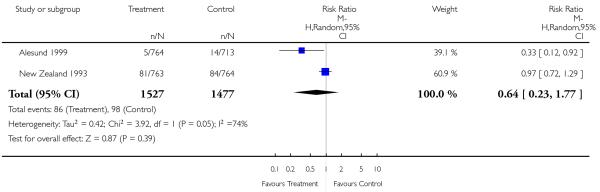 |
Analysis 1.19. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 19 Admission to special care baby unit.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 19 Admission to special care baby unit
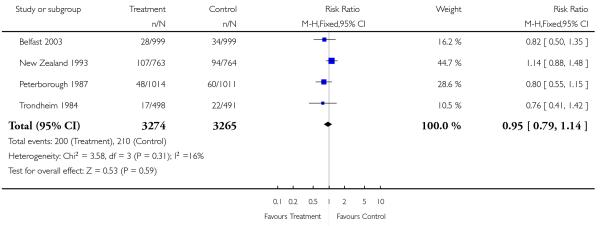 |
Analysis 1.20. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 20 Apgar score <7 at 5 minutes.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 20 Apgar score <7 at 5 minutes
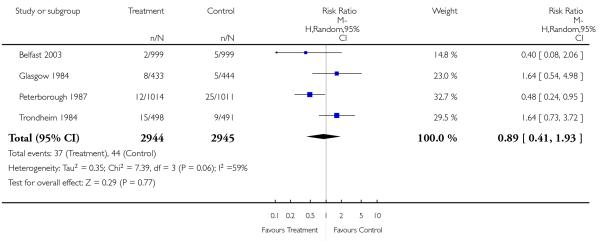 |
Analysis 1.21. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 21 Moderate neonatal morbidity.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 21 Moderate neonatal morbidity
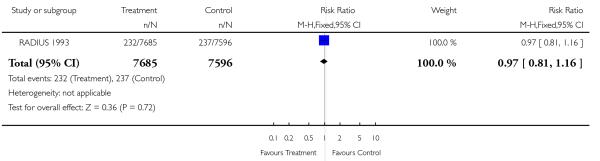 |
Analysis 1.22. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 22 Severe neonatal morbidity.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 22 Severe neonatal morbidity
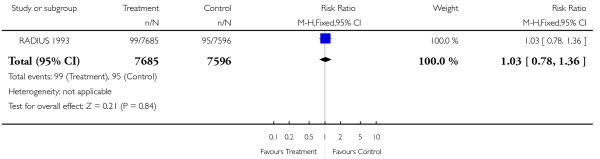 |
Analysis 1.23. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 23 Perinatal mortality.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 23 Perinatal mortality
 |
Analysis 1.24. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 24 Stillbirths.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 24 Stillbirths
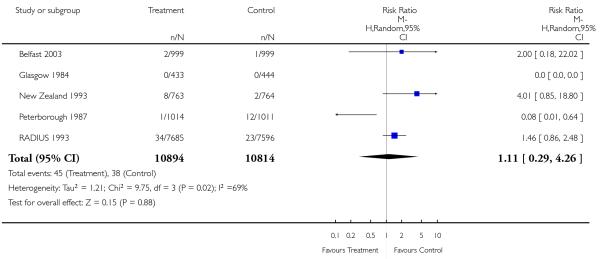 |
Analysis 1.25. CComparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 25 Neonatal deaths.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 25 Neonatal deaths
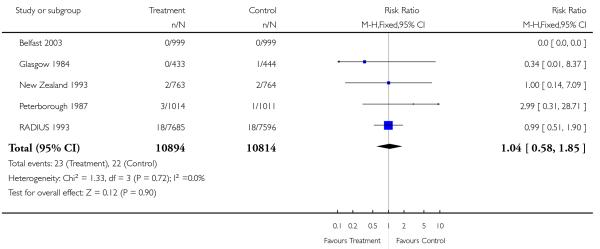 |
Analysis 1.26. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 26 Perinatal mortality (excluding congenital abnormalities).
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 26 Perinatal mortality (excluding congenital abnormalities)
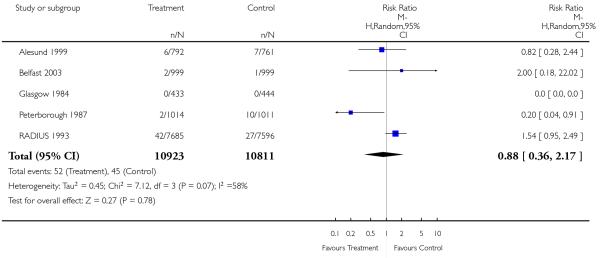 |
Analysis 1.27. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 27 Stillbirths (excluding congenital abnormalities).
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 27 Stillbirths (excluding congenital abnormalities)
 |
Analysis 1.28. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 28 Neonatal deaths (excluding congenital abnormalities).
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 28 Neonatal deaths (excluding congenital abnormalities)
 |
Analysis 1.29. Comparison 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks, Outcome 29 Perinatal mortality (twins).
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 1 Routine ultrasound >24 weeks vs no/concealed/selective ultrasound >24 weeks
Outcome: 29 Perinatal mortality (twins)
 |
Analysis 2.1. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 1 CTG (cardiograph).
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 1 CTG (cardiograph)
 |
Analysis 2.2. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 2 Induction of labour.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 2 Induction of labour
 |
Analysis 2.3. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 3 Caesarean section.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 3 Caesarean section
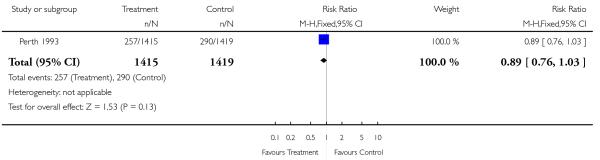 |
Analysis 2.4. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 4 Elective caesarean section.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 4 Elective caesarean section
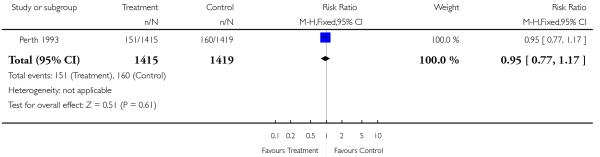 |
Analysis 2.5. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 5 Emergency caesarean section.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 5 Emergency caesarean section
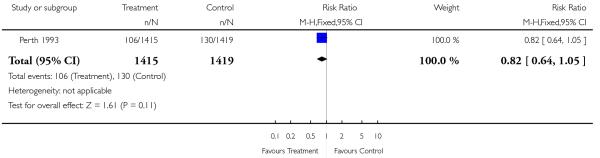 |
Analysis 2.6. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 6 Gestation at delivery (mean, SD).
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 6 Gestation at delivery (mean, SD)
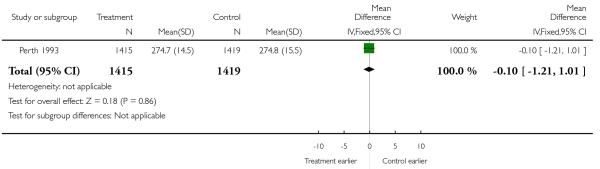 |
Analysis 2.7. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 7 Birthweight (mean, SD).
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 7 Birthweight (mean, SD)
 |
Analysis 2.8. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 8 Birthweight < 10th centile.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 8 Birthweight < 10th centile
 |
Analysis 2.9. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 9 Birthweight < 3rd centile.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 9 Birthweight < 3rd centile
 |
Analysis 2.10. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 10 Low birthweight (<2.5 kg).
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 10 Low birthweight (<2.5 kg)
 |
Analysis 2.11. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 11 Very low birthweight (< 1.5 kg).
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 11 Very low birthweight (< 1.5 kg)
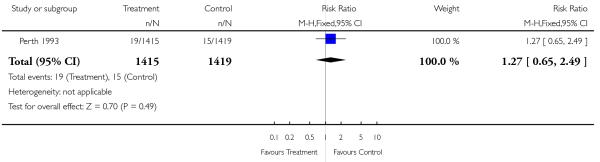 |
Analysis 2.12. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 12 Neonatal resuscitation.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 12 Neonatal resuscitation
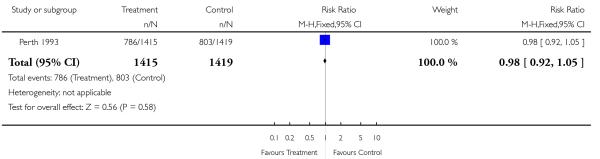 |
Analysis 2.13. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 13 Neonatal ventilation.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 13 Neonatal ventilation
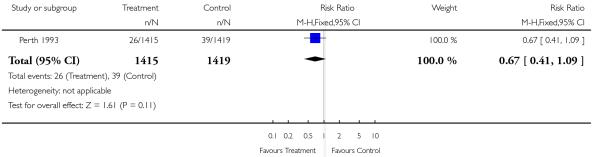 |
Analysis 2.14. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 14 Admission to special care baby unit.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation))
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 14 Admission to special care baby unit
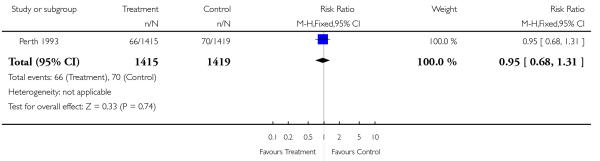 |
Analysis 2.15. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 15 Apgar score < 7 at 5 minutes.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 15 Apgar score < 7 at 5 minutes
 |
Analysis 2.16. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 16 Neonatal intraventricular haemorrhage.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 16 Neonatal intraventricular haemorrhage
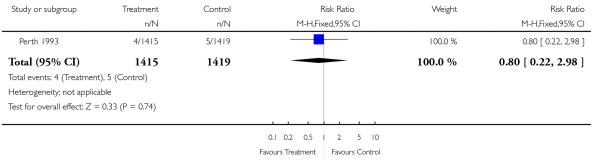 |
Analysis 2.17. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 17 Perinatal mortality.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 17 Perinatal mortality
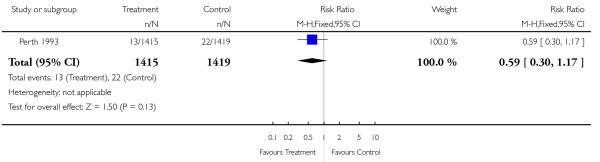 |
Analysis 2.18. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 18 Stillbirths.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 18 Stillbirths
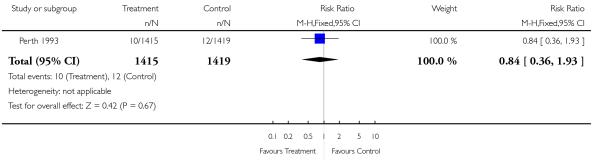 |
Analysis 2.19. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 19 Neonatal deaths.
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 19 Neonatal deaths
 |
Analysis 2.20. Comparison 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound, Outcome 20 Neonatal death (excluding congenital abnormalities).
Review: Routine ultrasound in late pregnancy (after 24 weeks’ gestation)
Comparison: 2 Serial ultrasound and Doppler ultrasound vs selective ultrasound
Outcome: 20 Neonatal death (excluding congenital abnormalities)
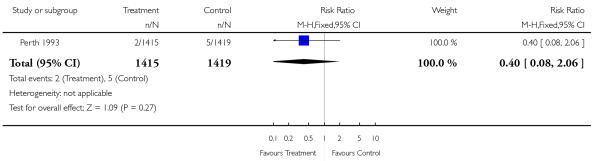 |
WHAT’S NEW
Last assessed as up-to-date: 27 May 2008.
| Date | Event | Description |
|---|---|---|
| 13 May 2009 | Amended | No changes - republished to fix technical problem. |
HISTORY
Protocol first published: Issue 2, 1999
Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 28 May 2008 | New search has been performed | We updated the search in February 2008 and identified an additional trial (Belfast 2003). We updated the methods, included risk of bias tables and updated the analyses. There are some small changes in the results but there are no substantial changes to the conclusions |
| 28 May 2008 | New citation required but conclusions have not changed | A new author joined the review team to prepare this update. |
| 26 February 2008 | Amended | Converted to new review format. |
| 5 February 2007 | Amended | Review withdrawn from publication. |
Footnotes
DECLARATIONS OF INTEREST: JP Neilson was principal investigator in a trial that was considered for inclusion.
References to studies included in this review
- Alesund 1999 {published and unpublished data} .Eik-Nes SH. Effects of routine two-stage ultrasound screening in pregnancy: the Alesund randomised controlled trial revisited. Personal communication. 1984 [Google Scholar]
- Eik-Nes SH, Okland O, Aure JC, Ulstein M. Ultrasound screening in pregnancy: a randomised controlled trial [letter] Lancet. 1984;1:1347. doi: 10.1016/s0140-6736(84)91834-8. [DOI] [PubMed] [Google Scholar]
- *; Eik-Nes SH, Salvesen KA, Okland O, Vatten LJ. Routine ultrasound fetal examination in pregnancy: the Alesund randomised controlled trial. Ultrasound in Obstetrics and Gynecology. 2000;15(6):473–8. doi: 10.1046/j.1469-0705.2000.00147.x. [DOI] [PubMed] [Google Scholar]
- Belfast 2003 {published data only} .McKenna D, Tharmaratnam S, Harper A, Dornan J. A randomised controlled trial using serial directed real time ultrasound to identify the at risk fetus in a low risk population. XVI FIGO World Congress of Obstetrics & Gynecology. Book 1; Washington DC, USA. 2000.Sep 3-8, 2000. p. 25. [Google Scholar]
- McKenna D, Tharmaratnam S, Harper A, Dornan J. A randomised controlled trial using serial directed real time ultrasound to identify the at-risk fetus in a low risk population [abstract] Prenatal and Neonatal Medicine. 2000;5(Suppl 2):151. [Google Scholar]
- *; McKenna D, Tharmaratnam S, Mahsud S, Bailie C, Harper A, Dornan J. A randomized trial using ultrasound to identify the high-risk fetus in a low-risk population. Obstetrics & Gynecology. 2003;101(4):626–32. doi: 10.1016/s0029-7844(02)03122-8. [DOI] [PubMed] [Google Scholar]
- Glasgow 1984 {published data only} .Neilson JP, Munjanja SP, Whitfield CR. Screening for small for dates fetuses: a controlled trial. BMJ. 1984;9:1179–82. doi: 10.1136/bmj.289.6453.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New Zealand 1993 {published data only} .Duff G. A randomised controlled trial in a hospital population of ultrasound measurement screening for the small for dates baby; Proceedings of 2nd International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; Hong Kong. 1993; Sep 7-10, 1993. p. 90. [DOI] [PubMed] [Google Scholar]
- *; Duff GB. A randomised controlled trial in a hospital population of ultrasound measurement screening for the small for dates baby. Australian and New Zealand Journal of Obstetrics and Gynaecology. 1993;33(4):374–8. doi: 10.1111/j.1479-828x.1993.tb02113.x. [DOI] [PubMed] [Google Scholar]
- Norway 1992 {published data only} .Salvesen KA. Routine ultrasonography in utero and development in childhood - a randomized controlled follow-up study. Acta Obstetricia et Gynecologica Scandinavica. 1995;74:166–7. [Google Scholar]
- Salvesen KA. Ultrasound and left-handedness: a sinister association? Ultrasound in Obstetrics & Gynecology. 2002;19(3):217–21. doi: 10.1046/j.1469-0705.2002.00659.x. [DOI] [PubMed] [Google Scholar]
- *; Salvesen KA, Bakketeig LS, Eik-nes SH, Undheim JO, Okland O. Routine ultrasonography in utero and school performance at age 8-9 years. Lancet. 1992;339(8785):85–9. doi: 10.1016/0140-6736(92)90998-i. [DOI] [PubMed] [Google Scholar]
- Salvesen KA, Eik-Nes SH. Ultrasound during pregnancy and birthweight, childhood malignancies and neurological development. Ultrasound in Medicine & Biology. 1999;25(7):1025–31. doi: 10.1016/s0301-5629(99)00051-4. [DOI] [PubMed] [Google Scholar]
- Salvesen KA, Eik-Nes SH. Ultrasound during pregnancy and subsequent childhood non-right handedness: a meta-analysis. Ultrasound in Obstetrics & Gynecology. 1999;13(4):241–6. doi: 10.1046/j.1469-0705.1999.13040241.x. [DOI] [PubMed] [Google Scholar]
- Salvesen KA, Jacobsen G, Vatten LJ, Eik-Nes SH, Bakketeig LS. Routine ultrasonography in utero and subsequent growth during childhood. Ultrasound in Obstetrics & Gynecology. 1993;3:6–10. doi: 10.1046/j.1469-0705.1993.03010006.x. [DOI] [PubMed] [Google Scholar]
- Salvesen KA, Vatten LJ, Eik-Nes SH, Hugdahl K, Bakketeig LS. Routine ultrasonography in utero and subsequent handedness and neurological development. BMJ. 1993;307(6897):159–64. doi: 10.1136/bmj.307.6897.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen KA, Vatten LJ, Jacobsen G, Eik-Nes SH, Okland O, Molne K, et al. Routine ultrasonography in utero and subsequent vision and hearing at primary school age. Ultrasound in Obstetrics & Gynecology. 1992;2:243–7. doi: 10.1046/j.1469-0705.1992.02040243.x. [DOI] [PubMed] [Google Scholar]
- Perth 1993 {published and unpublished data} .Evans S, Newnham J, MacDonald W, Hall C. Characterisation of the possible effect on birthweight following frequent prenatal ultrasound examinations. Early Human Development. 1996;45(3):203–14. doi: 10.1016/0378-3782(96)01728-8. [DOI] [PubMed] [Google Scholar]
- Harding K, Evans S, Newnham J. Screening for the small fetus: a study of the relative efficacies of ultrasound biometry and symphysiofundal height. Australian and New Zealand Journal of Obstetrics and Gynaecology. 1995;35:160–4. doi: 10.1111/j.1479-828x.1995.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Newnham J, MacDonald W, Gurrin L, Evans S, Landau L, Stanley F. The effect of frequent prenatal ultrasound on birthweight: follow up at one year of age; Proceedings of the 14th Annual Congress of the Australian Perinatal Society in conjunction with the New Zealand Perinatal Society; Adelaide, Australia. 1996.Mar 24-27, 1996. p. A26. [Google Scholar]
- Newnham JP, Doherty DA, Kendall GE, Zubrick SR, Landau LL, Stanley FJ. Effects of repeated prenatal ultrasound examinations on childhood outcome up tp 8 years of age: follow-up of a randomised controlled trial. Lancet. 2004;364:2038–44. doi: 10.1016/S0140-6736(04)17516-8. [DOI] [PubMed] [Google Scholar]
- *; Newnham JP, Evans SF, Michael CA, Stanley FJ, Landau LI. Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet. 1993;342:887–91. doi: 10.1016/0140-6736(93)91944-h. [DOI] [PubMed] [Google Scholar]
- Peterborough 1987 {published data only} .Proud J, Grant AM. Third trimester placental grading by ultrasonography as a test of fetal wellbeing. BMJ. 1987;294:1641–4. doi: 10.1136/bmj.294.6588.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADIUS 1993 {published data only} .Crane JP, LeFevre ML, Winborn RC, Evans JK, Ewigman BG, Bain RP, et al. A randomized trial of prenatal ultrasonographic screening: Impact on the detection, management and outcome of anomalous fetuses. American Journal of Obstetrics and Gynecology. 1994;171:392–9. doi: 10.1016/s0002-9378(94)70040-0. [DOI] [PubMed] [Google Scholar]
- *; Ewigman BG, Crane JP, Frigoletto FD, LeFevre ML, Bain RP, McNellis D, et al. Effect of prenatal ultrasound screening on perinatal outcome. New England Journal of Medicine. 1993;329:821–7. doi: 10.1056/NEJM199309163291201. [DOI] [PubMed] [Google Scholar]
- LeFevre ML, Bain RP, Ewigman BG, Frigoletto FD, Crane JP, McNellis D, et al. A randomised trial of prenatal ultrasonographic screening: impact on maternal management and outcome. American Journal of Obstetrics and Gynecology. 1993;169:483–9. doi: 10.1016/0002-9378(93)90605-i. [DOI] [PubMed] [Google Scholar]
- Trondheim 1984 {published data only} .Bakketeig LS, Jacobsen G, Brodtkorb CJ, Eriksen BC, Eik-Nes SH, Ulstein MK, et al. Randomised controlled trial of ultrasonographic screening in pregnancy. Lancet. 1984;2:207–10. doi: 10.1016/s0140-6736(84)90492-6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Belanger 1996 {published data only} .Belanger K, Hobbins JC, Muller JP, Howard S. Neurological testing in ultrasound exposed infants. American Journal of Obstetrics and Gynecology. 1996;174(1 Pt 2):413. [Google Scholar]
- Hendrix 2000 {published data only} .Hendrix NW, Grady CS, Chauhan SP. Clinical vs sonographic estimate of birth weight in term parturients. Journal of Reproductive Medicine. 2000;45(4):317–22. [PubMed] [Google Scholar]
- Morrison 1992 {published data only} .Morrison JC. Is shoulder dystocia predictable by a ponderal index obtained ultrasonographically? Personal communication. 1992 [Google Scholar]
- Ong 2001 {published data only} .Ong S. Third trimester placental grading by ultrasound and its impact on perinatal mortality. National Research Register. 2001 [Google Scholar]
- Owen 1994 {published data only} .Owen P, Donnet L, Ogston S, Christie A, Patel N, Howie P. A study of fetal growth velocity. British Journal of Obstetrics and Gynaecology. 1994;101:270. doi: 10.1111/j.1471-0528.1996.tb09516.x. [DOI] [PubMed] [Google Scholar]
- Secher 1986 {published data only} .Secher NJ, Hansen PK, Lenstrup C, Eriksen PS. Controlled trial of ultrasound screening for light for gestational age (LGA) infants in late pregnancy. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1986;23:307–13. doi: 10.1016/0028-2243(86)90165-6. [DOI] [PubMed] [Google Scholar]
- Secher 1987 {published data only} .Secher NJ, Hansen PK, Lenstrup C, Eriksen PS, Morsing G. A randomized study of fetal abdominal diameter and fetal weight estimation for detection of light-for-gestation infants in low-risk pregnancies. British Journal of Obstetrics and Gynaecology. 1987;94:105–9. doi: 10.1111/j.1471-0528.1987.tb02334.x. [DOI] [PubMed] [Google Scholar]
- Wladimiroff 1980 {published data only} .Wladimiroff JW, Laar J. Ultrasonic measurement of fetal body size. A randomized controlled trial. Acta Obstetricia et Gynecologica Scandinavica. 1980;59:177–9. doi: 10.3109/00016348009154637. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Ellwood 1997 {published data only} .Ellwood D, Peek M, Curren J. Predicting adverse pregnancy outcomes with ultrasound. A randomised controlled trial. Personal communication. 1997 [Google Scholar]
Additional references
- Abramowicz 2007 .Abramowicz JS, Sheiner E. In utero imaging of the placenta: Importance for diseases of pregnancy. Placenta. 2007;21(Suppl A):S14–S22. doi: 10.1016/j.placenta.2007.02.004. [DOI] [PubMed] [Google Scholar]
- ACOG 2004 .ACOG Committee on Ethics ACOG Committee Opinion. Number 297, August 2004. Nonmedical use of obstetric ultrasonography. Obstetrics & Gynecology. 2004;104(2):423–4. doi: 10.1097/00006250-200408000-00049. [DOI] [PubMed] [Google Scholar]
- Altman 1989 .Altman DG, Hytten F. Assessment of fetal size and fetal growth. In: Chalmers I, Enkin M, Keirse MJNC, editors. Effective care in pregnancy and childbirth. Oxford University Press; Oxford: 1989. pp. 411–8. [Google Scholar]
- Barker 1993 .Barker DJP, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–41. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- Brace 1989 .Brace RA, Wolf EJ. Characterisation of normal gestational changes in amniotic fluid volume. American Journal of Obstetrics and Gynecology. 1989;161:382–8. doi: 10.1016/0002-9378(89)90527-9. [DOI] [PubMed] [Google Scholar]
- Bricker 2007b .Bricker L, Neilson JP. Routine Doppler ultrasound in pregnancy. Cochrane Database of Systematic Reviews. 2007;(2) doi: 10.1002/14651858.CD001450.pub2. Art. No.: CD001450. DOI: 10.1002/14651858.CD001450.pub2. [DOI] [PubMed] [Google Scholar]
- Chitty 1995 .Chitty LS. Ultrasound screening for fetal abnormalities. Prenatal Diagnosis. 1995;15:1241–57. doi: 10.1002/pd.1970151306. [DOI] [PubMed] [Google Scholar]
- EFSUMB 1995 .Societies for Ultrasound in Medicine, Biology. European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) Watchdog Committee, 1994 Clinical Safety Statement (1995) European Journal of Ultrasound. 1995;2:77. [Google Scholar]
- Garcia 2002 .Garcia J, Bricker L, Henderson J, Martin M, Mugford M, Nielson J, et al. Women’s views of pregnancy ultrasound: a systematic review. Birth. 2002;29(4):225–50. doi: 10.1046/j.1523-536x.2002.00198.x. [DOI] [PubMed] [Google Scholar]
- Gonen 1996 .Gonen R, Spiegal D, Abend M. Is macrosomia predictable, and are shoulder dystocia and birth trauma preventable? Obstetrics & Gynecology. 1996;88(4):526–9. doi: 10.1016/0029-7844(96)00230-x. [DOI] [PubMed] [Google Scholar]
- Grannum 1979 .Grannum PA, Berkowitz RL, Hobbins JC. The ultrasonic changes in the maturing placenta and their relation to fetal pulmonary maturity. American Journal of Obstetrics and Gynecology. 1979;133:915–22. doi: 10.1016/0002-9378(79)90312-0. [DOI] [PubMed] [Google Scholar]
- Harding 1995 .Harding K, Evans S, Newnham J. Screening for the small fetus: a study of the relative efficacies of ultrasound biometry and symphysiofundal height. Australian and New Zealand Journal of Obstetrics and Gynaecology. 1995;35:160–4. doi: 10.1111/j.1479-828x.1995.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Henderson 2002 .Henderson J, Bricker L, Roberts T, Mugford M, Garcia J, Neilson J. British National Health Service’s and women’s costs of antenatal ultrasound screening and follow-up tests. Ultrasound in Obstetrics & Gynecology. 2002;20(2):154–62. doi: 10.1046/j.1469-0705.2002.00724.x. [DOI] [PubMed] [Google Scholar]
- Higgins 2008 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.0 [updated February 2008] The Cochrane Collaboration; 2008. Available from www.cochrane-handbook.org. [Google Scholar]
- Holmes 1996 .Holmes RP, Soothill PW. Intra-uterine growth retardation. Current Opinion in Obstetrics and Gynecology. 1996;8:148–54. [PubMed] [Google Scholar]
- Leeson 1997 .Leeson S, Aziz N. Customised fetal growth assessment. British Journal of Obstetrics and Gynaecology. 1997;104:648–51. doi: 10.1111/j.1471-0528.1997.tb11973.x. [DOI] [PubMed] [Google Scholar]
- Lindqvist 2005 .Lindqvist PG, Molin J. Does antenatal identification of small-for-gestational age fetuses significantly improve their outcome? Ultrasound in Obstetrics & Gynecology. 2005;25(3):258–64. doi: 10.1002/uog.1806. [DOI] [PubMed] [Google Scholar]
- Lurie 1995 .Lurie S, Yalel Y, Hagay ZJ. The evaluation of accelerated fetal growth. Current Opinion in Obstetrics and Gynecology. 1995;7(6):477–81. doi: 10.1097/00001703-199512000-00014. [DOI] [PubMed] [Google Scholar]
- Neilson 1989 .Neilson JP, Grant A. Ultrasound in pregnancy. In: Chalmers I, Enkin M, Keirse MJNC, editors. Effective care in pregnancy and childbirth. Oxford University Press; Oxford: 1989. pp. 419–39. [Google Scholar]
- Neilson 1998 .Neilson JP. Ultrasound for fetal assessment in early pregnancy (Cochrane Review) Cochrane Database of Systematic Reviews. 1998;(4) doi: 10.1002/14651858.CD000182. DOI: 10.1002/14651858.CD000182. [DOI] [PubMed] [Google Scholar]
- Newnham 1996 .Newnham J, MacDonald W, Gurrin L, Evans S, Landau L, Stanley F. The effect of frequent prenatal ultrasound on birthweight: follow up at one year of age; Proceedings of the 14th Annual Congress of the Australian Perinatal Society in conjunction with the New Zealand Perinatal Society; 1996; Adelaide, Australia. 1996.Mar 24-27, p. A26. [Google Scholar]
- Newnham 2004 .Newnham JP, Doherty DA, Kendall GE, Zubrick SR, Landau LL, Stanley FJ. Effects of repeated prenatal ultrasound examinations on childhood outcome up tp 8 years of age: follow-up of a randomised controlled trial. Lancet. 2004;364:2038–44. doi: 10.1016/S0140-6736(04)17516-8. [DOI] [PubMed] [Google Scholar]
- Nwosu 1993 .Nwosu EC, Walkinshaw S, Chia P, Manasse PR, Atlay RD. Undiagnosed breech. British Journal of Obstetrics and Gynaecology. 1993;100(6):531–5. doi: 10.1111/j.1471-0528.1993.tb15302.x. [DOI] [PubMed] [Google Scholar]
- RCOG 1997 .Royal College of Obstetricians and Gynaecologists . Report of RCOG working party on Ultrasound screening for fetal abnormalities. RCOG; London: 1997. [Google Scholar]
- RevMan 2008 .RevMan . Review Manager (RevMan). 5.0. The Nordic Cochrane Centre, The Cochrane Collaboration; Copenhagen: 2008. [Google Scholar]
- Rizos 1979 .Rizos N, Miskin M, Benzie RJ, Ford JA. Natural history of placenta praevia ascertained by diagnostic ultrasound. American Journal of Obstetrics and Gynecology. 1979;133:287–91. doi: 10.1016/0002-9378(79)90680-x. [DOI] [PubMed] [Google Scholar]
- Sheiner 2007 .Sheiner E, Shoham-Vardi I, Abramowicz JS. What do clinical users know regarding safety of ultrasound during pregnancy? Journal of Ultrasound in Medicine. 2007;26(3):319–25. doi: 10.7863/jum.2007.26.3.319. quiz 326-7. [DOI] [PubMed] [Google Scholar]
- Weeks 1995 .Weeks JW, Pitman T, Spinnato II-JA. Fetal macrosomia: does antenatal prediction affect delivery route and birth outcome? American Journal of Obstetrics and Gynecology. 1995;173(4):1215–9. doi: 10.1016/0002-9378(95)91356-4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Bricker 2007a .Bricker L, Neilson JP. Routine ultrasound in late pregnancy (after 24 weeks’ gestation) Cochrane Database of Systematic Reviews. 2007;(2) doi: 10.1002/14651858.CD001451.pub2. Art. No.: CD001451. DOI: 10.1002/14651858.CD001451.pub2. [DOI] [PubMed] [Google Scholar]
- Neilson 1995 .Neilson JP, Pregnancy and Childbirth Module . Routine fetal anthropometry in late pregnancy. [revised 12 May 1994] In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C, editors. The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM] 2. The Cochrane Collaboration; Oxford: Update Software 1995. [Google Scholar]
- * Indicates the major publication for the study


