Abstract
Despite advances in medical therapy for chronic heart failure (HF), advanced HF carries a dismal prognosis. Options such as transplantation and durable mechanical circulatory support have greatly improved outcomes for these patients, but their introduction has introduced signifcant complexity to patient management. Although much of this management occurs at specialized heart transplant centers, it is the responsibility of the primary cardiologist of the patient with advanced HF to refer patients at the appropriate time and to help them navigate the difficult decisions related to the pursuit of advanced therapies. We present a unique pathway that incorporates guidelines, recent data, and expert opinion to help general cardiologists determine which patients should be referred for transplantation or durable mechanical circulatory support, and when they should be referred. Decision making on referral to the heart transplant center is also summarized.
Keywords: advanced heart failure, cardiac transplantation, mechanical circulatory support, critical pathway
Advances in medical therapy for chronic, ambulatory heart failure (HF) have improved survival but simultaneously increased the number of patients with refractory, advanced disease. These patients typically have limiting HF symptoms at rest, and thus require frequent hospitalizations. Advanced HF carries a dismal prognosis, with up to 75% of patients dying within 6 months despite optimal medical therapy.1–3 The gold standard for management of advanced HF is cardiac transplantation, with 1-year survival approaching 90% and 11-year survival of 50%;4 however, only ~2200 hearts are transplanted each year in the United States, far too few for the more than 100,000 patients who may meet criteria for cardiac transplantation.4 Because of the scarcity of donor organs, transplant eligibility is restricted to carefully selected candidates. As a result, many patients with advanced HF with advanced age and multiple comorbidities are not eligible for transplantation.
To overcome these challenges, alternate treatment options have been used, including extended criteria cardiac transplantation and chronic mechanical circulatory support (MCS). Some transplant programs have adopted a strategy to accept organs, after they have been turned down for all other recipients, for transplant into older recipients and those with comorbid conditions that would otherwise exclude them from standard list cardiac transplantation. Typically, these donor organs are from older donors, or are hearts with mild left ventricular hypertrophy, mildly reduced left ventricular function, or limited coronary artery disease. By matching these marginal allografts with extended criteria transplant recipients, the number of patients benefiting from transplantation can be increased.5,6 Although extended criteria programs have made organs available for more patients requiring transplantation, there still remains a shortage of available donor hearts. Fortunately, advances in MCS have allowed for the creation of durable left ventricular assist devices (LVADs) that can be implanted either as a bridge to heart transplantation or as chronic support (termed destination therapy) for advanced HF, creating an option that is not dependent on the availability of donor organs. Survival with newer continuous-flow LVADs may approach that of cardiac transplantation,3,7–11 significantly improving the prognosis for patients with advanced HF.
Statement of Purpose
These rapid technological and medical advances have allowed patients with advanced HF to live longer with better quality of life; however, they have also introduced considerable complexity to patient management. Much of this management is done at specialized heart transplant centers, yet the general cardiologist or primary care physician plays an important role in the decision-making process. The decision whether to pursue advanced HF therapies and which specific strategy to pursue is ultimately a personalized decision made by the patient and a multidisciplinary team, taking into account patient preferences and a variety of medical and psychosocial factors.12 The primary cardiologist of the patient with advanced HF acts as gatekeeper and guide to the process of advanced therapies. He or she is responsible for referring the patient to a heart transplant center at the appropriate time, and for helping navigate the difficult decisions related to advanced HF. This pathway attempts to incorporate expert opinion and recent guidelines to clarify the process of referral for and decision to pursue advanced HF therapies. In addition we develop a pathway to help general cardiologists determine which of their patients should be referred to an advanced HF center and how to prepare them for the process.
Description of Pathway
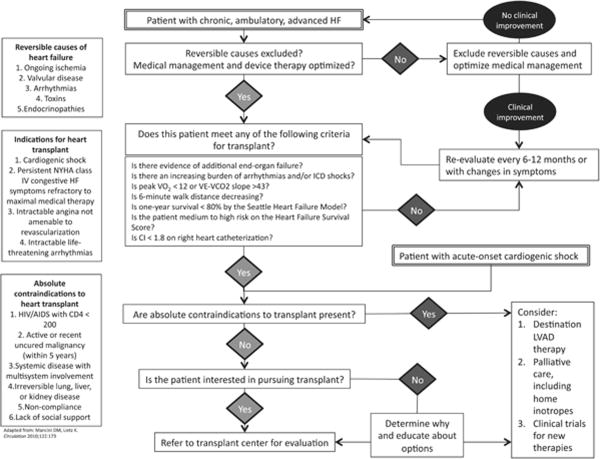
The initial step in determining whether a patient requires referral to a heart transplantation center is to exclude reversible causes of HF including ongoing ischemia, severe valvular disease, tachyar-rythmias, toxin exposure, and endocrinopathies that can be corrected without the need for temporary MCS. Following this, the cardiologist should ensure that the patient's medical therapy has been optimized. A complete discussion of the medical therapy of HF has been covered in multiple reviews,13 and the American College of Cariology (ACC)/American Heart Association (AHA) guidelines.14 In brief, optimal medical therapy for patients with HF and reduced ejection fraction typically includes an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker, beta-blocker, and aldosterone antagonist. In selected patients, treatment should include hydralazine, isosorbide dinitrate, an implantable cardioverter defibrillator, and/or cardiac resynchronization therapy. Importantly, intolerance of medical therapy because of hypotension or renal insufficiency is a poor prognostic marker and warrants referral for advanced therapies.15–18
Once it has been established that the patient's management has been optimized, the next step is to determine whether the patient would derive benefit from advanced therapies. Consensus guidelines,19,20 expert opinion,4 and recent data have identified key clinical characteristics that the clinician can use to help determine whether a patient is a suitable candidate for advanced HF therapies:
Evidence of end-organ failure. Signs of worsening organ failure related to poor perfusion due to HF (ie, renal or liver dysfunction) should prompt urgent referral before the development of permanent extracardiac organ dysfunction, even when the underlying cause may be reversible. Patients presenting with acute cardiogenic shock due to viral myocarditis, postpartum cardiomyopathy, postcardiotomy shock, or acute myocardial infarction are included in this group.
Increasing burden of arrhythmias and/or implantable cardioverter defibrillator shocks even in the absence of overt HF symptoms.
Cardiopulmonary exercise testing (CPET). This is generally regarded as the gold standard for assessing aerobic capacity in patients with HF. Both VO2 max (the maximum capacity of the body to transport and use oxygen during exercise) and VE-VCO2 slope (the rate of increase in ventilation per unit of CO2 production) have been shown to correlate with prognosis in HF.21,22 Traditionally, a VO2 max of less than 14 ml/kg/min has been considered the threshold for consideration of advanced HF therapies.21 In the current era of beta-blocker therapy and cardiac resynchronization therapy, patients with VO2 max less than 12 ml/kg/min have a survival benefit after cardiac transplantation.23 CPET testing has been incorporated into the International Society for Heart and Lung Transplantation (ISHLT) guidelines as listing criteria for cardiac transplantation.20
Decreasing 6-minute walk distance. Contemporary data have demonstrated that the prognostic value of 6-minute walk testing in advanced HF approaches that of CPET.24 Although 6-minute walk has not yet been incorporated into guidelines, it may prove to be an inexpensive, office-based test to help predict which patients will benefit from advanced therapies.
Risk models. The Heart Failure Survival Score uses clinical variables in combination with CPET to further stratify ambulatory patients into low-, medium-, and high-risk groups. This model's use in determining suitability for transplant has been endorsed by the ISHLT in patients considered borderline by VO2 max.20 The Seattle Heart Failure Model incorporates only clinical data, and is not included in the ISHLT guidelines, because it was published after the publication of guidelines.25 This model's capacity to predict 1-year survival is equivalent to the Heart Failure Survival Score,26 though it may underestimate risk in the sickest patients.27,28 Patients with 1-year survival less than 80% should be considered for transplantation.4
Cardiac index. In patients considered for cardiac transplantation, right heart catheterization should be performed. This allows for assessment of pulmonary vascular resistance and cardiac index. As discussed below, pulmonary vascular resistance is an important variable in determining appropriate advanced HF therapies. Although cardiac index is not included as an indication for transplantation or LVAD implantation in ISHLT20 or ACC/AHA guidelines,14 and it was not an inclusion criteria in the large trials comparing destination LVAD with medical therapy,9,29 retrospective data suggest that patients with normal VO2 max and cardiac index <1.8 L/min/m2 have outcomes inferior to patients with normal VO2 max and cardiac index >1.8 L/min/m2,30 suggesting that patients with low cardiac index may benefit from advanced therapies.
Quality of life indices. Multiple quality of life indices have been developed for patients with HF, and it has been demonstrated that scores improve with implantation of LVAD29 and transplantation.31 These indices are neither routinely used clinically nor incorporated into the guidelines, but they may correlate with mortality in the population of patients awaiting heart transplant,32 providing a patient-centered method of assessing appropriateness for advanced HF therapies.
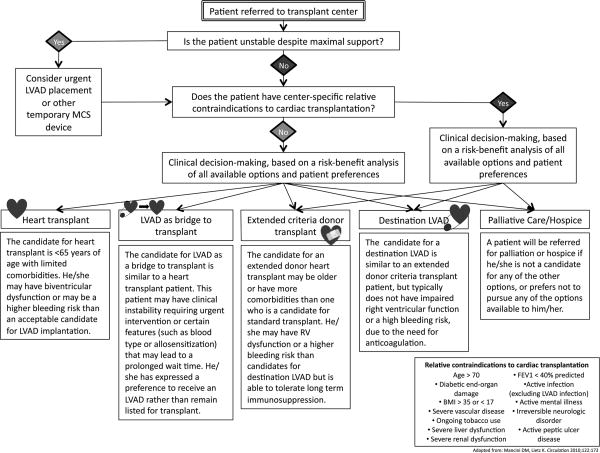
Once a patient has been considered a candidate for advanced therapies by meeting one or more of the above criteria, the clinician must next facilitate referral to an appropriate center. Some centers are able to provide evaluation for transplantation, destination LVAD therapy, and clinical trials for investigational therapies, whereas other centers may provide a more limited set of therapeutic options. Figures 1 and 2 help outline major branch points in this decision-making process, including patient preferences and absolute contraindications to heart transplant. Patients with absolute contraindications are generally those with comorbid conditions that would significantly limit posttransplant survival in the absence of cardiovascular disease, or those that will not be compliant with their posttransplant medical regimen due to poor psychosocial support or prior noncompliance (Fig. 1). Figure 2 lists additional relative contraindications to heart transplantation.4,20 These relative contraindications are center specific and may help determine options following referral. However, neither the existence of absolute nor relative contraindications should prevent referral of a patient to a cardiac transplantation program. If a patient meets listing criteria, then referral to a transplant center is recommended.14
FIGURE 1.
Pathway for the selection of patients for referral for advanced heart failure therapies. HIV, human immunodeficiency virus; ICD, implantable cardioverter-defibrillator; NYHA, New York Heart Association.
FIGURE 2.
Pathway for determining patient options for advanced heart failure therapies. FEV1, forced expiratory volume in one second; BMI, body mass index.
Assessment of the patient on arrival to the transplant center initially focuses on three main factors—clinical stability, candidacy for transplantation, and patient preferences. Frequently, candidacy for transplantation and patient preferences require time for consideration, so clinical stability and end-organ perfusion become primary considerations. If there is evidence of progressive end-organ hypo-perfusion, patients are rapidly evaluated for MCS. This concept reinforces the importance of early referral for patients to advanced HF regardless of perceived contraindications to transplantation.
Under ideal circumstances and with evidence of clinical stability, patients and providers are able to proceed with a risk–benefit analysis of all available advanced HF therapies. This risk–benefit analysis is complex and patient specific, taking into account both patient preferences and center-specific relative contraindications that may impair posttransplant survival. For example, many centers will only consider obese patients for extended criteria donor transplantation or destination MCS because obese heart transplant recipients have twice the 5-year mortality compared to patients with body mass index <30 kg/m2.33
Another example is right ventricular function and degree of right-sided HF symptoms when considering univentricular support. Currently available durable ventricular assist device technologies offer only left ventricular support.34 Those individuals with impaired right ventricular function before LVAD implantation have worse outcomes.35 Several risk models have been developed that use clinical variables before LVAD implantation to predict risk of right ventricular failure after implantation.36 Although LVAD implantation is not absolutely contraindicated in patients at high risk for right ventricular failure, these patients are at higher risk of perioperative complications and may have a better outcome with transplantation than LVAD.
The ability to tolerate anticoagulation is also taken into account when considering advanced therapies. With newer continuous-flow LVADs, anticoagulation is recommended to prevent thrombus formation within the device. For a variety of reasons—anticoagulation, acquired von Willenbrand factor deficiency, gastrointestinal arterio-venous malformations, and impaired platelet aggregation—more than 50% of patients have bleeding events that require blood transfusion after continuous-flow LVAD implantation.37 Some of these bleeding events can be treated with endoscopic procedures, but cardiac transplantation may ultimately be used to rescue patients failing LVAD therapy due to bleeding. Patients known to be at higher risk for bleeding are less likely to have optimal outcomes with LVAD therapy although higher mortality has not been seen with bleeding events.38
Although these are among the more significant factors used to determine the ultimate disposition of patients referred for heart transplantation, the above text is by no means exhaustive. Descriptions of the ideal patients for cardiac transplantation, extended-donor cardiac transplantation, destination LVAD, and LVAD as a bridge to transplant can be found in Figure 2. We suggest that all treatment strategies available at the referral center should be evaluated for the patient in a multidisciplinary fashion to provide timely therapy that will optimize survival and quality of life. This should include palliative measures or hospice care in situations where the prognosis remains very poor despite advanced therapies or where surgical risk is preclusive.
Summary
Currently, more than 100,000 patients have advanced HF meeting criteria for cardiac transplantation. Mortality in these patients remains high, and recent clinical trials of novel medical therapies have largely failed to show benefit in patients with advanced HF. By contrast, advances in LVAD technology have greatly expanded the options available to patients with advanced HF.
This rapidly changing field requires specialization and patients benefit from early referral to a specialty center. Unfortunately, many patients are referred to heart transplant centers too late or not at all. This pathway attempts to provide a simplified rubric for determining which patients are eligible for advanced HF therapies, so that patients, general cardiologists, and advanced HF specialists can work together in determining the optimal treatment plan.
Acknowledgments
This article was prepared without external funding.
Footnotes
Disclosures: Robert Mentz has served a consultancy for Heartware, Inc., and has received travel expenses from Thoratec Corp. Chetan Patel serves as a CME reviewer for MDConsult and theheart.org, and has received travel expenses from Thoratec Corp. and Heartware, Inc. For the remaining authors, none were declared.
References
- 1.Hershberger RE, Nauman D, walker TL, et al. Care processes and clinical outcomes of continuous outpatient support with inotropes (COSI) in patients with refractory endstage heart failure. J Card Fail. 2003;9:180–187. doi: 10.1054/jcaf.2003.24. [DOI] [PubMed] [Google Scholar]
- 2.Rogers JG, Butler J, Lansman SL, et al. INTrEPID Investigators. Chronic mechanical circulatory support for inotrope-dependent heart failure patients who are not transplant candidates: results of the INTrEPID Trial. J Am Coll Cardiol. 2007;50:741–747. doi: 10.1016/j.jacc.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 3.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 4.Mancini D, Lietz K. Selection of cardiac transplantation candidates in 2010. Circulation. 2010;122:173–183. doi: 10.1161/CIRCULATIONAHA.109.858076. [DOI] [PubMed] [Google Scholar]
- 5.Felker GM, Milano CA, Yager JE, et al. Outcomes with an alternate list strategy for heart transplantation. J Heart Lung Transplant. 2005;24:1781–1786. doi: 10.1016/j.healun.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 6.Forni A, Luciani GB, Chiominto B, et al. Results with expanded donor acceptance criteria in heart transplantation. Transplant Proc. 2011;43:953–959. doi: 10.1016/j.transproceed.2011.01.117. [DOI] [PubMed] [Google Scholar]
- 7.Miller LW, Pagani FD, Russell SD, et al. HeartMate II Clinical Investigators. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 8.Kirklin JK, Naftel DC, Kormos RL, et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant. 2010;29:1–10. doi: 10.1016/j.healun.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slaughter MS, Rogers JG, Milano CA, et al. HeartMate II Investigators. advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 10.Patel CB, Alexander KM, Rogers JG. Mechanical circulatory support for advanced heart failure. Curr Treat Options Cardiovasc Med. 2010;12:549–565. doi: 10.1007/s11936-010-0093-6. [DOI] [PubMed] [Google Scholar]
- 11.Daneshmand MA, Rajagopal K, Lima B, et al. Left ventricular assist device destination therapy versus extended criteria cardiac transplant. Ann Thorac Surg. 2010;89:1205–1209. doi: 10.1016/j.athoracsur.2009.12.058. discussion 1210. [DOI] [PubMed] [Google Scholar]
- 12.Allen LA, Stevenson LW, Grady KL, et al. Decision making in advanced heart failure: a scientific statement from the American Heart Association. Circulation. 2012;125:1928–1952. doi: 10.1161/CIR.0b013e31824f2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coronel SM, Krantz MJ. Medical therapy for symptomatic heart failure: a contemporary treatment algorithm. Crit Pathw Cardiol. 2007;6:15–17. doi: 10.1097/01.hpc.0000256145.90640.85. [DOI] [PubMed] [Google Scholar]
- 14.Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration with the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1–e90. doi: 10.1016/j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Lee TT, Chen J, Cohen DJ, et al. The association between blood pressure and mortality in patients with heart failure. Am Heart J. 2006;151:76–83. doi: 10.1016/j.ahj.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Ather S, Chan W, Chillar A, et al. Association of systolic blood pressure with mortality in patients with heart failure with reduced ejection fraction: a complex relationship. Am Heart J. 2011;161:567–573. doi: 10.1016/j.ahj.2010.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ambrosy AP, Vaduganathan M, Mentz RJ, et al. Clinical profile and prognostic value of low systolic blood pressure in patients hospitalized for heart failure with reduced ejection fraction: insights from the Efficacy of Vasopressin Antagonism in Heart Failure: Outcome Study with Tolvaptan (EVEREST) trial. Am Heart J. 2013;165:216–225. doi: 10.1016/j.ahj.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Damman K, Navis G, Voors AA, et al. Worsening renal function and prognosis in heart failure: systematic review and meta-analysis. J Card Fail. 2007;13:599–608. doi: 10.1016/j.cardfail.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Feldman D, Pamboukian SV, Teuteberg JJ, et al. International Society for Heart and Lung Transplantation. The 2013 International Society for Heart and Lung Transplantation Guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Mehra MR, Kobashigawa J, Starling R, et al. Listing criteria for heart transplantation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates–2006. J Heart Lung Transplant. 2006;25:1024–1042. doi: 10.1016/j.healun.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Mancini DM, Eisen H, Kussmaul W, et al. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 22.Poggio R, Arazi HC, Giorgi M, et al. Prediction of severe cardiovascular events by VE/VCO2 slope versus peak VO2 in systolic heart failure: a meta-analysis of the published literature. Am Heart J. 2010;160:1004–1014. doi: 10.1016/j.ahj.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 23.Peterson LR, Schechtman KB, Ewald GA, et al. Timing of cardiac transplantation in patients with heart failure receiving beta-adrenergic blockers. J Heart Lung Transplant. 2003;22:1141–1148. doi: 10.1016/s1053-2498(02)01225-1. [DOI] [PubMed] [Google Scholar]
- 24.Forman DE, Fleg JL, Kitzman DW, et al. 6-min walk test provides prognostic utility comparable to cardiopulmonary exercise testing in ambulatory outpatients with systolic heart failure. J Am Coll Cardiol. 2012;60:2653–2661. doi: 10.1016/j.jacc.2012.08.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy WC, Mozaffarian D, Linker DT, et al. The Seattle Heart Failure Model: prediction of survival in heart failure. Circulation. 2006;113:1424–1433. doi: 10.1161/CIRCULATIONAHA.105.584102. [DOI] [PubMed] [Google Scholar]
- 26.Goda A, Williams P, Mancini D, et al. Selecting patients for heart transplantation: comparison of the Heart Failure Survival Score (HFSS) and the Seattle heart failure model (SHFM) J Heart Lung Transplant. 2011;30:1236–1243. doi: 10.1016/j.healun.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 27.Regoli F, Scopigni F, Leyva F, et al. Collaborative Study Group. Validation of Seattle Heart Failure Model for mortality risk prediction in patients treated with cardiac resynchronization therapy. Eur J Heart Fail. 2013;15:211–220. doi: 10.1093/eurjhf/hfs162. [DOI] [PubMed] [Google Scholar]
- 28.Benbarkat H, Addetia K, Eisenberg MJ, et al. Application of the Seattle heart failure model in patients >80 years of age enrolled in a tertiary care heart failure clinic. Am J Cardiol. 2012;110:1663–1666. doi: 10.1016/j.amjcard.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 29.Rogers JG, Aaronson KD, Boyle AJ, et al. HeartMate II Investigators. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 30.Methvin A, Georgiopoulou VV, Kalogeropoulos AP, et al. Usefulness of cardiac index and peak exercise oxygen consumption for determining priority for cardiac transplantation. Am J Cardiol. 2010;105:1353–1355. doi: 10.1016/j.amjcard.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 31.Ortega T, Díaz-Molina B, Montoliu MA, et al. Research Network on Transplantation. The utility of a specific measure for heart transplant patients: reliability and validity of the Kansas City Cardiomyopathy Questionnaire. Transplantation. 2008;86:804–810. doi: 10.1097/TP.0b013e318183eda4. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan MD, Levy WC, Russo JE, et al. Summary health status measures in advanced heart failure: relationship to clinical variables and outcome. J Card Fail. 2007;13:560–568. doi: 10.1016/j.cardfail.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Lietz K, John R, Burke EA, et al. Pretransplant cachexia and morbid obesity are predictors of increased mortality after heart transplantation. Transplantation. 2001;72:277–283. doi: 10.1097/00007890-200107270-00020. [DOI] [PubMed] [Google Scholar]
- 34.Patel CB, Rogers JG. Durable mechanical circulatory support devices. Prog Cardiovasc Dis. 2011;54:132–143. doi: 10.1016/j.pcad.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 35.Kormos RL, Teuteberg JJ, Pagani FD, et al. HeartMate II Clinical Investigators. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010;139:1316–1324. doi: 10.1016/j.jtcvs.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Matthews JC, Koelling TM, Pagani FD, et al. The right ventricular failure risk score a pre-operative tool for assessing the risk of right ventricular failure in left ventricular assist device candidates. J Am Coll Cardiol. 2008;51:2163–2172. doi: 10.1016/j.jacc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saurez J, Patel CB, Felker GM, et al. Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circ Heart Fail. 2011;4:779–784. doi: 10.1161/CIRCHEARTFAILURE.111.962613. [DOI] [PubMed] [Google Scholar]
- 38.Morgan JA, Paone G, Nemeh HW, et al. Gastrointestinal bleeding with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2012;31:715–718. doi: 10.1016/j.healun.2012.02.015. [DOI] [PubMed] [Google Scholar]