Abstract
In collaboration with the Ukrainian Research Center for Radiation Medicine, the U.S. National Cancer Institute initiated a cohort study of children and adolescents exposed to Chornobyl fallout in Ukraine to better understand the long-term health effects of exposure to radioactive iodines. All 13,204 cohort members were subjected to at least one direct thyroid measurement between 30 April and 30 June 1986 and resided at the time of the accident in the northern part of Kyiv, Zhytomyr, or Chernihiv Oblasts, which were the most contaminated territories of Ukraine as a result of radioactive fallout from the Chornobyl accident. Thyroid doses for the cohort members, which had been estimated following the first round of interviews, were re-evaluated following the second round of interviews. The revised thyroid doses range from 0.35 mGy to 42 Gy, with 95 percent of the doses between 1 mGy and 4.2 Gy, an arithmetic mean of 0.65 Gy, and a geometric mean of 0.19 Gy. These means are 70% of the previous estimates, mainly because of the use of country-specific thyroid masses. Many of the individual thyroid dose estimates show substantial differences because of the use of an improved questionnaire for the second round of interviews. Limitations of the current set of thyroid dose estimates are discussed. For the epidemiologic study, the most notable improvement is a revised assessment of the uncertainties, as shared and unshared uncertainties in the parameter values were considered in the calculation of the 1,000 stochastic estimates of thyroid dose for each cohort member. This procedure makes it possible to perform a more realistic risk analysis.
Keywords: Chornobyl, thyroid, dose reconstruction
INTRODUCTION
There are two main research components in epidemiological studies of health effects resulting from radiation exposure. The first one is the careful investigation and estimation of the causal factor, which is the radiation dose. The second is the analysis of the observed health effects induced by the radiation dose. Problems associated with dosimetric support of epidemiological studies resulting from nuclear radiation accidents are often more difficult than those related to the detection and analysis of radiation-induced effects. Dosimetric problems are usually more complex because they involve the retrospective assessment of radiation exposure, which occurred under difficult accident conditions, and the development of accident-specific ecological and metabolic models. When the epidemiologic study calls for the estimation of radiation doses to specific individuals, it is necessary to perform personal interviews in order to collect detailed information on residential history and on lifestyle and dietary habits. The availability of results of dosimetric measurements taken directly at the time of the radiation accident or soon afterwards is also extremely important for the dose reconstruction
A striking example of the complexity of retrospective dose estimations is the reconstruction of the thyroid dose due to the intake of radioiodines after the Chornobyl accident. Because the atmospheric release of radioactive materials took place over a period of several days under changing meteorological conditions, the temporal and spatial deposition on the ground of radioiodines and other radionuclides was extremely heterogeneous. In addition, the estimation of the radiation exposures has to account for a wide variety of behaviors and diets as well as for large-scale relocations of people after the accident. Also, analysis of direct thyroid measurements is very complex because several types of radiation devices and methods are used to conduct the direct thyroid measurements, especially in the first weeks after an accident, and because of the loss of information that is inevitable under accident conditions.
The description of different aspects of the estimation of the thyroid doses received by residents of Ukraine has been reported in a number of publications (Likhtarev et al. 1993a, 1993b, 1995b, 2002b, 2003b, 2006; Likhtarev and Chumak 1994; Likhtarov et al. 2005). As new knowledge and data became available, it was necessary to revise all components and stages of the dose estimation system. Such revisions are typically performed in large dose reconstruction studies, such as that of the A-bomb survivors (Milton and Shohoji 1968; RERF 1986, 2005).
One of the most comprehensive individual thyroid dose reconstructions for Ukrainian subjects was conducted within the framework of the Ukrainian-American study of thyroid cancer and other thyroid diseases in Ukraine following the Chornobyl accident (Tronko et al. 2006). In this study the thyroid dose reconstruction system is based on five components:
direct thyroid measurements that are available for all cohort members;
personal interviews of all cohort members on individual dietary and lifestyle habits;
estimates of 131I deposition on the ground in each location of residence of the cohort members;
a radioecological model used to assess the temporal variation of 131I in the thyroid, both before and after the time of the direct thyroid measurement; and
measurements of thyroid volume and mass that were performed on Ukrainian children and adolescents during the 1990s.
The first estimates of individual thyroid dose for all members of the Ukrainian-American cohort were obtained in 2002; along with a description of the corresponding thyroid dose reconstruction system, called TD-02, the first dose estimates were published by Likhtarev et al. (2006). In the conclusions of that paper, it is written that: “Although these estimates of thyroid dose and of their uncertainties will be used at this stage of the epidemiological study, all aspects of the dose estimation process are being re-evaluated, so that more accurate and precise thyroid dose estimates resulting from 131I intakes could be derived in a few years.”
The following activities have been carried out since 2002 to improve the thyroid dose estimates:
A substantial effort was made to interview all cohort members a second time, so that detailed information on personal history (relocation from the contaminated territory and consumption of contaminated foods) could be clarified. For that purpose, extended and more convenient forms of inquiries were developed and the interviews were administered by trained interviewers. Four elaborated dosimetry questionnaires were developed: 1) for the cohort members who were older than 10 y at the time of the accident; 2) for the parents of the cohort members who were 10 y old and younger at the time of the accident; 3) for the mothers of breast-fed cohort members; 4) for the cohort members or their parents who had been evacuated from the town of Prypiat’. In addition, treatment of missing or fuzzy responses was improved.
A new mesoscale model of atmospheric transport and ground deposition on the Ukrainian territory of the radioactive materials released during the Chornobyl accident was developed (Talerko 2005a). This model was calibrated using the results of reliable and detailed measurements of 137Cs ground deposition performed over the entire territory of Ukraine by the Ukraine State Hydrometeorology Service (De Cort and Tsaturov 1996). Daily (and hourly for the town of Prypiat’) 131I and 137Cs ground deposition densities were calculated using the model for all locations of residence of the cohort members.
Parameter values of the iodine and cesium ecological transport models were adjusted to the Ukrainian situation as the result of a thorough analysis of the available data on radionuclide transport in the environment that were published after the Chornobyl accident. The characteristics of the distributions of the parameter values were re-evaluated.
A vastly improved Monte-Carlo procedure of evaluation of the uncertainties in the individual thyroid dose estimates was developed. In this improved procedure, a distinction is made between the shared and the unshared parameters, and the most extreme values of the parameters are censored.
The contribution of the incorporated radiocesiums to the signal read by the detectors sed for the direct thyroid measurements has been estimated; this contribution had not been taken into consideration in the previous estimation of the thyroid doses.
The thyroid doses resulting from intakes of short-lived radionuclides have been estimated for all cohort members.
Oblast-specific thyroid masses were established on the basis of autopsy measurements (Likhtarov et al., in press) and measurements of thyroid volume performed in the 1990s by the Sasakawa Memorial Health Foundation among children and adolescents of Kyiv and Zhytomyr Oblasts (SMHF 1997).
Therefore, with the exception of the direct thyroid measurements, all components of the thyroid dose reconstruction system, TD-02, that had been previously used were thoroughly analyzed. This led to the development of an improved thyroid dose reconstruction system, called TD-10, which takes into account all findings of the analysis. These TD-10 thyroid dose estimates are to be used in the epidemiologic analyses conducted within the framework of the Ukrainian-American project.
The purpose of this publication is: (1) to describe the improvements in the thyroid dose reconstruction system that have been made since 2002, (2) to analyze the thyroid doses obtained in TD-10 and compare with those of TD-02, and (3) to discuss the limitations of TD-10 thyroid dose estimates.
MATERIALS AND METHODS
Structure of the study cohort
The Ukrainian cohort consists of 13,204 individuals who were selected among the persons who satisfied the following criteria: (1) they resided in April-May 1986 in the northern part of one of three oblasts of Ukraine: Kyiv, Zhytomyr or Chernihiv, which were the most contaminated territories of Ukraine with radioactive fallout from the Chornobyl accident; (2) they were born before the Chornobyl accident; (3) they were less than 19 y old at the time of the Chornobyl accident; and (4) their thyroid activity had been measured between 26 April and 30 June 1986. All cohort members or their relatives (for the children who were less than 10 y old at the time of accident) were interviewed once and nearly all were interviewed twice between 1998 and 2006. Compared with 13,215 cohort members reported by Tronko et al. (2006), 11 subjects were excluded as they did not satisfy criteria (1) and (2) mentioned above. The numbers of boys and girls in the Ukrainian cohort were approximately equal; the numbers of each in ages 0–14 y ranged from 340–490; and the numbers who were 15 y old or older were smaller, about 200 and 220, respectively.
The 13,204 cohort members of the Ukrainian-American study (Stezhko et al 2004; Tronko et al. 2006) have been classified into five groups: (1) the subjects who were evacuated from Prypiat’ (Prypiat’ evacuees), (2) the subjects who were breast-fed at the time of the accident, (3) the subjects who consumed stable iodine for prophylactic reasons, (4) the subjects who, for some reason, were administered the first but not the second round of interviews, and (5) all other subjects. Some of the subjects in groups 1 to 4 belong to more than one group (Table 1).
Table 1.
Distribution of the 13,204 Ukrainian cohort members into the different groups and information on the numbers of subjects of groups 1 to 4 who belong to one or more groups.
| Group
|
Number of subjects | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
| ||||
| Prypyat’ evacuees (750 subjects) | Breast-fed children (452 subjects) | Iodine prophylaxis (3,866 subjects) | 1-st interview only (1,455 subjects) | |
| Noa | No | No | No | 7,638 |
| No | No | No | Yes | 1,067 |
| No | No | Yes | No | 3,115 |
| No | No | Yes | Yes | 205 |
| No | Yes | No | No | 352 |
| No | Yes | No | Yes | 24 |
| No | Yes | Yes | No | 51 |
| No | Yes | Yes | Yes | 2 |
| Yes | No | No | No | 163 |
| Yes | No | No | Yes | 77 |
| Yes | No | Yes | No | 409 |
| Yes | No | Yes | Yes | 78 |
| Yes | Yes | No | No | 15 |
| Yes | Yes | No | Yes | 2 |
| Yes | Yes | Yes | No | 6 |
| Yes | Yes | Yes | Yes | 0 |
| Total | 13,204 | |||
“No” and “Yes” indicate that the subject does not or does belong to the group under consideration, respectively.
For this second round of dose calculation, environmental models have been improved, site-specific parameter values, along with their distributions have been developed, and additional personal information has been obtained in the second round of interviews. The most substantial changes in the thyroid dose reconstruction system have been made for the group of Prypiat’ evacuees and for the group of breast-fed children.
Estimation of the thyroid doses and of their uncertainties: general information
General information on the estimation of the thyroid doses and of their uncertainties will be given in this section, while the following sections and the two appendixes will address specific issues.
Estimation of the thyroid doses
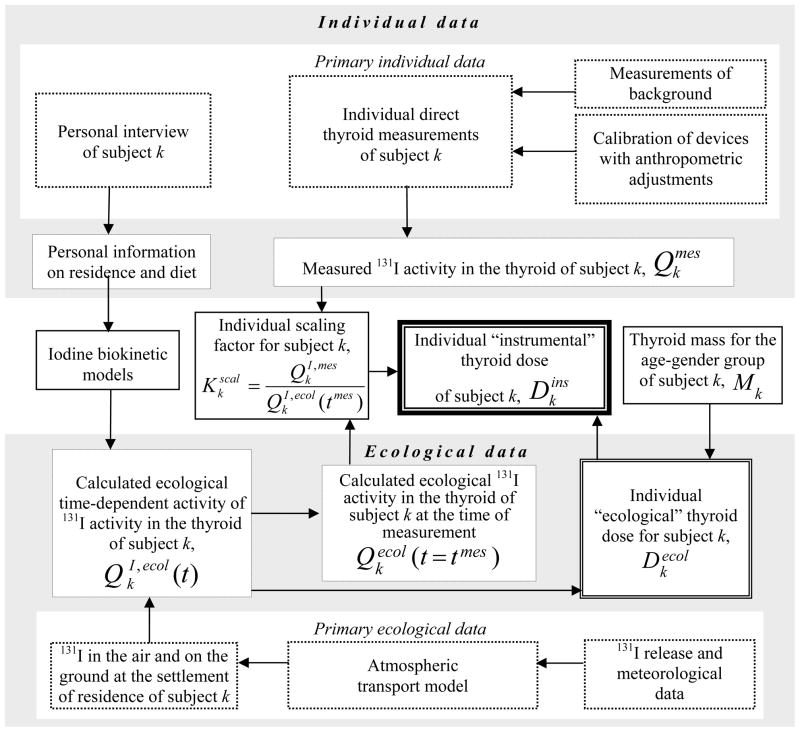
The general structure of the TD-10 thyroid dose reconstruction system is presented in a schematic manner in Fig. 1. Throughout the text as well as in this figure, two types of thyroid dose are mentioned: the “instrumental” thyroid dose, which is derived from the direct thyroid measurement that was performed on the subject under consideration, and the “ecological” thyroid dose, which is based on the use of ecological models. As can be seen from the figure, the individual dose estimates are based on individual data (the top two rows of boxes) and on ecological data (the bottom two rows of boxes). Within these two groups of boxes, the primary data and the results of their processing are separated. The middle row shows some of the most important boxes: the biokinetic models, the thyroid mass, the individual scaling factor, and, finally, the individual instrumental thyroid dose derived from the subject-specific calculated values of ecological dose and scaling factor (Likhtarov et al. 2005, Likhtarev et al. 2006). For subject k, the individual instrumental dose is expressed as:
| (1) |
where is the scaling factor (unitless); is the individual “ecological” dose (mGy).
Fig. 1.
General scheme of the TD-10 thyroid dose reconstruction system.
The ecological dose (mGy) is calculated as:
| (2) |
where: is the energy of 131I decay absorbed in the thyroid (MeV per decay); Cu is a unit conversion coefficient, equal to 13.82 (Bq kBq−1 g kg−1 J MeV−1 s d−1 mGy Gy−1); Mk is the thyroid mass (g); is the ecological time-integrated activity of the 131I activity content in the thyroid during the time period from 0 to T=66 days after the accident for subject k (kBq d). is calculated using the ecological model of iodine transport and the iodine biokinetic model as:
| (3) |
where is the 131I activity in the thyroid of subject k that varies with time (kBq). Appendix 1 provides details of the ecological model used in TD-10. In that ecological model, many parameter values originate from international studies or studies that were not made in Ukraine. Although a substantial effort was made to use as many Ukrainian-specific values as possible, ecological studies devoted to the investigation of the environmental behavior of 131I were not conducted in Ukraine after the Chernobyl accident.
The scaling factor is the ratio of the measured ( ) and modeled ( ) 131I thyroid activity at the time of measurement, tmes, for an individual k. The direct thyroid measurements, which had been performed on all cohort members within a few weeks after the accident, were made by placing a gamma-ray detector against the neck at the level of the thyroid of the subject. The reading of the device, which varied according to the type of device, was in number of counts, number of counts per second, μR per hour, or percent of a standard radioactive source. The thyroid activity for the measured subject k, which was determined using the device-specific calibration factor (Fig. 1), includes contributions of 131I and of cesium radioisotopes (134Cs, 136Cs, and 137Cs). The 131I activity in the thyroid was obtained as:
| (4) |
where is the relative contribution of the cesium radioisotopes to the reading of the device.
In comparison to the TD-02 dose reconstruction system, the changes made in TD-10 with respect to the ecological variation with time of the 131I activity and, consequently, to the ecological time-integrated activity , are mainly related to improvements in the ecological transport model: the use of updated 131I ground deposition densities, and the use of an improved questionnaire for the second round of interviews. Other improvements include: (1) taking into account the radiocesium contribution to the detected signal in order to correct the value of the measured activity used to calculate the scaling factor , and (2) the use of substantially revised age-dependent values of the thyroid mass Mk. A minor difference is the age at exposure that is the age of the subject at the time of the accident (26 April 1986) instead of the age at the time of the direct thyroid measurement as it was used in TD-02.
Estimation of the uncertainties in the thyroid doses
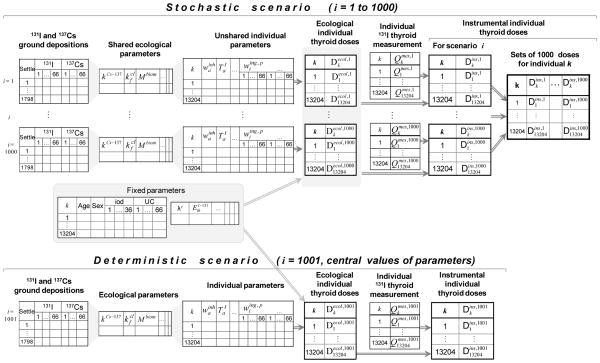
A procedure of Monte-Carlo simulation was developed and applied to estimate the thyroid dose uncertainties. The sequence and stages of this Monte-Carlo simulation in TD-10 are shown in Fig. 2. Preparatory work consisted of assigning central values and distributions to each variable parameter based on the analysis of data collected within the framework of the study, literature data, or expert judgment. Three types of distribution were used: uniform, triangular, and censored lognormal. The parameters were classified into three categories:
Fig. 2.
Representation of the Monte-Carlo simulation system developed in TD-10 for the estimation of the ecological and instrumental thyroid doses and of their uncertainties.
Twenty-four fixed parameters (“constants”), such as the radioactive decay constant of 131I, which are assumed to be known without any uncertainty (Table A1.1 in Appendix 1). In addition, dates of intake of stable iodine tablets during first 36 days after the accident (until 31 May 1986) and relocation of cohort member during first 66 days after the accident (until 30 June 1986) are also assumed to be known without uncertainty;
Twenty-nine subject independent (“shared”) parameters, such as those that characterize the variation with time of the environmental contamination of the ground, air, and foodstuffs. The central estimates and distributions assigned to the shared parameters are presented in Table A1.2;
Twenty-two subject dependent (“unshared”) parameters are mainly related to the metabolism and individual consumptions of milk, milk products and leafy vegetables by the study subjects during first 66 days after the accident (until 30 June 1986). Table A1.3 gives the list of unshared parameters and their characteristics. Detailed information on breathing rate and biological half-times of elimination of iodine from the thyroid and cesium from human body is given in Table A1.4.
For each variable parameter, 1,000 values were simulated. Each of the 1,000 sets of the shared (subject independent) parameters, together with the set of fixed parameters, define a “scenario of exposure” of the cohort members (see Fig. 2). The Monte-Carlo procedure used in TD-10 consists of placing the 13,204 subjects in each of the 1,000 scenarios of exposure and to obtain 1,000 realizations of doses, changing the subject-dependent (unshared) values for each subject from one scenario of exposure to another. In addition, a 1001st realization was calculated, in which all parameters were assigned their central value. Hence, for each of the 13,204 cohort members, 1,001 values of thyroid dose have been generated.
The Monte-Carlo procedure illustrated in Fig. 2 was applied to obtain the simulated individual thyroid dose estimates, but it was preceded by a more specific Monte-Carlo procedure to prepare the simulated values of daily deposition of 131I and 137Cs needed for each of the 1,001 scenarios of exposure and for each of the 1,798 Ukrainian settlements where any of the 13,204 cohort members resided at one time or another during the first 66 days after the accident (see Appendix 2). In this preliminary Monte-Carlo procedure, simulation was applied only to the cumulative values of 131I and 137Cs deposition in the settlements. The daily depositions of 131I and 137Cs were then inferred from the cumulative depositions in such a way that the relative variations with time of the daily deposition densities of 131I and 137Cs in a given settlement were conserved in all scenarios of exposure; the absolute values, however, changed from one scenario of exposure to another as they were scaled to the cumulative values of 131I and 137Cs deposition in the settlement. Therefore, in this preliminary Monte-Carlo procedure, 1,001 sets of simulated daily radionuclide deposition densities of 131I and 137Cs in each of the settlements of residence of the cohort members were generated prior to the implementation of the standard Monte-Carlo procedure illustrated in Fig. 2.
131I and 137Cs daily ground deposition densities
A model of atmospheric transport called LEDI (Lagrangian-Eulerian Diffusion model) was used for the reconstruction of the radioactive fallout of 137Cs and 131I in Ukraine during the first 12 days after the Chornobyl accident (Talerko 2005b). The model LEDI takes into account advection and turbulent diffusion, as well as spatial and temporal changes in the meteorological conditions, and calculates the dry and wet deposition of the radionuclides (essentially 131I and 137Cs) released during the accident, accounting for their radioactive decay during the atmospheric transport:
the release rates of 137Cs and 131I were estimated on an hourly basis; because of the hot temperature of the releases, the initial plume rose by convection over the destroyed reactor. The characteristics of the initial plume (maximal height and initial vertical distribution of the released activity) were considered to vary as a function of time during the period of substantial releases from 26 April to 5 May 1986. The release activity within any given hour was implemented by means of separate puffs originating from various heights (up to 1,200 m above ground) according to the distribution of the activity along the initial vertical plume;
the atmospheric transport of the activity contained in each puff took into account the meteorological conditions, which were based on measurement data of the State Committee for Meteorology of the former USSR;
the air concentrations and the daily activities of 137Cs and 131I that were deposited on the ground at given locations were estimated as sums of the contributions of all puffs with different release times and different initial heights.
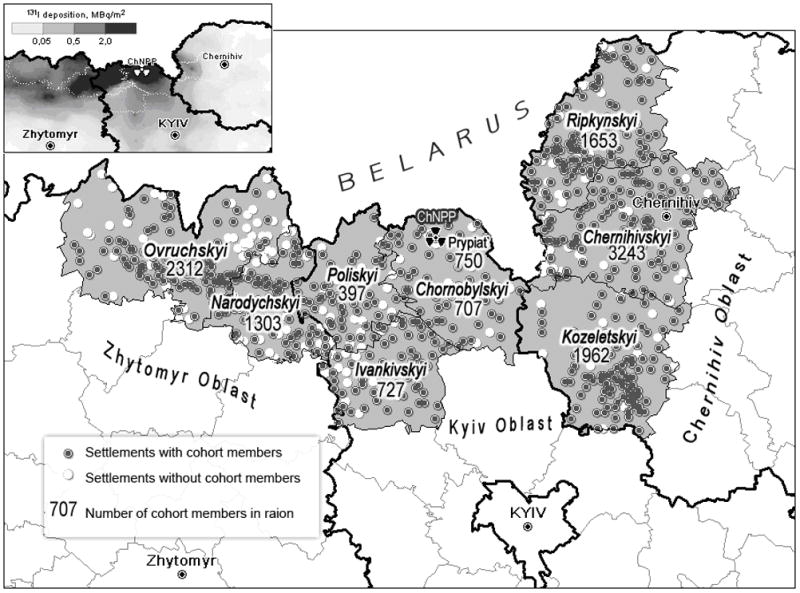
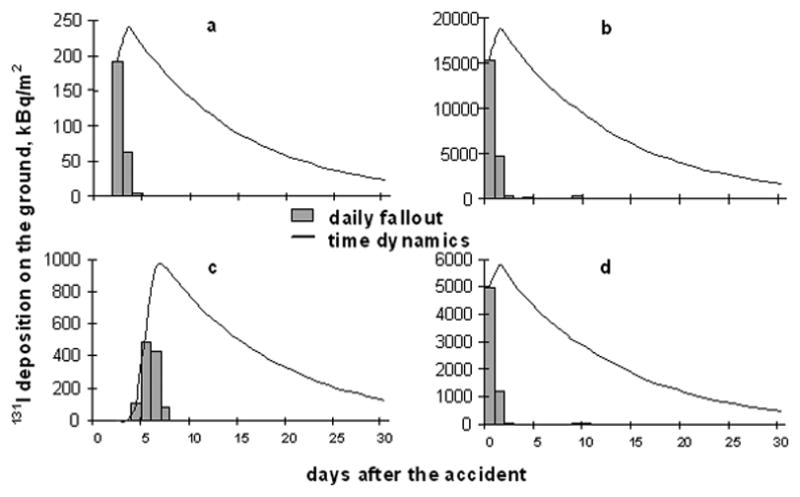
The 137Cs and 131I and daily averaged activity concentrations in near-surface air as well as the cumulative and daily deposition densities have been calculated for 12,716 Ukrainian settlements, including 4,336 settlements in the 3 most contaminated Kyiv, Zhytomyr and Chernihiv Oblasts. For the purposes of this study, the levels of 131I and 137Cs cumulative and daily ground deposition in all 1,798 locations (settlements) where the cohort members resided some time during the first two months after the Chornobyl accident were estimated. The spatial pattern of cumulative 131I ground deposition densities in the northern part of Ukraine estimated using the atmospheric transport model LEDI is shown in Fig. 3. The figure also shows the distribution of the numbers of cohort members over the raions of residence at the time of the accident. Fig. 4 presents examples of daily ground deposition of 131I and of corresponding time dynamics of ground contamination for four settlements where cohort members resided during the first two months after the accident. It is clear that almost all of the 131I ground deposition occurred within a few days, but that the timing was different in the four locations. Table 2 gives the averages and ranges of cumulative 131I ground deposition, , among the settlements of the raions where the cohort members resided at the time of the accident.
Fig. 3.
Cumulative 131I ground deposition density (MBq m−2) in the northern part of Ukraine where the cohort members resided at the time of the accident (map in the upper left part of the Figure) and distribution of the cohort members over the eight most contaminated raions of Kyiv, Zhytomyr and Chernihiv Oblasts. At the time of the accident, 150 members of the cohort resided outside those eight raions.
Fig. 4.
Examples of daily fallout of 131I and of the time dynamics of the total deposition on the ground estimated by means of the atmospheric transport model LEDI for several locations (Talerko et al. 2005b): Chernihiv-city (a), Poliske, Kyiv Oblast (b), Novi Petrivci, Kyiv Oblast (c), Narodychi, Zhytomyr Oblast (d).
Table 2.
Averages and ranges of cumulative 131I ground deposition density, , among settlements of the raions where cohort members resided at the time of the accident.
| Oblast | Raion | Number of settlements where subjects resided at the time of the accident | Cumulative 131I ground deposition
, MBq·m−2
|
|
|---|---|---|---|---|
| Range | Average | |||
| Zhytomyr | Narodychy | 60 | 1.1 – 28 | 4.9 |
| Ovruch | 114 | 0.58 – 9.7 | 2.3 | |
|
| ||||
| Kyiv | Ivankiv | 58 | 0.33 – 3.1 | 1.3 |
| Poliske | 45 | 0.21 – 115 | 8.6 | |
| Chornobyl | 49 | 0.67 – 115 | 15 | |
|
| ||||
| Chernihiv | Kozelets | 98 | 0.14 – 1.1 | 0.30 |
| Ripky | 102 | 0.10 – 4.7 | 0.52 | |
| Chernihiv | 106 | 0.09 – 9.5 | 0.47 | |
| Chernihiv-city | 1 | - | 0.26 | |
|
| ||||
| Other oblasts and raions of Ukrainea | 12 | 0.01 – 0.21 | 0.07 | |
Fifteen of the cohort subjects were not in Ukraine at the time of the accident. They moved to Ukraine in May 1986.
Correction of the measured signal due to radiocesium in the body
A detailed description of the procedures used to perform the direct thyroid measurements and to evaluate the uncertainties associated with the location of the detector relative to the thyroid was provided by Likhtarev et al. (1993b; 1995). As a result of the accident, the environment was contaminated not only by radioiodines but also by a number of other radionuclides. Some of the most important radionuclides that contributed to the internal doses resulting from the consumption of contaminated foodstuffs were the cesium radioisotopes 134Cs, 136Cs, and 137Cs. Because the ingested radiocesiums were distributed uniformly in the muscles of the human body, gamma rays from these radionuclides contributed to the signal measured near the thyroid. In order to subtract that contribution from the measured signal, the variation with time of the radiocesium body burden was estimated.
The radiocesium concentration in the human body (and thyroid) was calculated using similar models and equations as those for iodine, the main difference being that the parameter values are specific for radiocesium. Sums of two exponentials were used for the description of the transfer of cesium into cow’s milk, the metabolism of cesium in the human body, and the transfer of cesium into mother’s milk. The parameter values of the environmental transfer model used for radiocesium in the current thyroid dose estimation system are given in Tables A1.1–A1.3.
The relative contribution of the radioisotopes of cesium (137Cs, 134Cs, 136Cs) to the signal registered near the neck, , was calculated as:
| (5) |
where: Q131(t), Q137(t), Q134(t), Q136(t) are the contents of 131I in the thyroid and the contents of 137Cs, 134Cs, 136Cs in the body, respectively, expressed in MBq; P131, P137, P134, P136 are empirical calibration factors for 131I, 137Cs, 134Cs and 136Cs, respectively, expressed in MBq per number of counts, number of counts per hour, μR per hour, or percent of a standard radioactive source, according to the type of device.
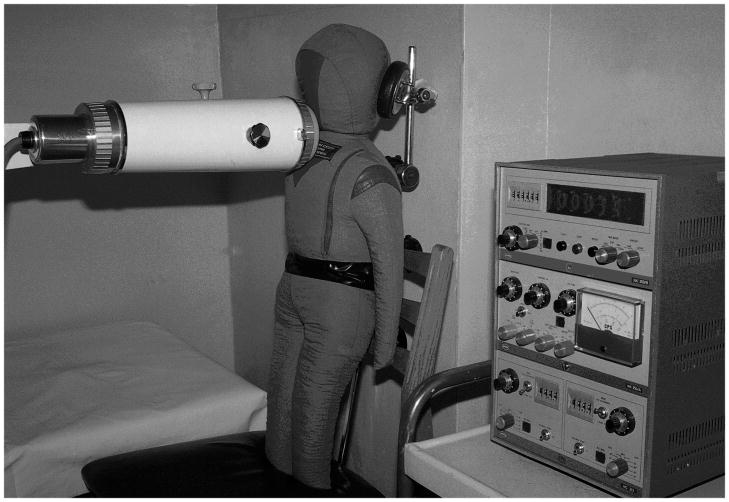
The calibration factors P131, P137, P134, and P136, were empirically established for all types of devices used to perform the direct thyroid measurements (Fig. 5), using standard anthropomorphic phantoms (Likhtarev et al 1991; Perevoznikov et al. 1994). The calibration factors of the devices vary according to the type of device and to the age-dependent anthropometric characteristics of the subject.
Fig. 5.
Procedure of empirical estimation of the radiocesium contribution (calibration factor) to the signal registered near the neck using an anthropomorphic phantom contaminated with 137Cs (the example shown is for the spectrometer NK-350)
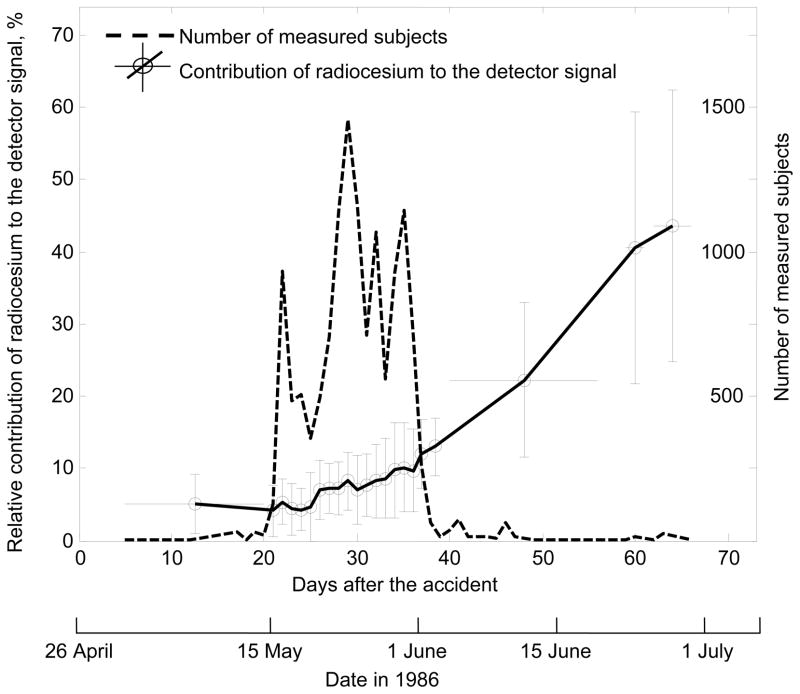
The values of depend substantially on the time elapsed since the accident when the direct thyroid measurement was performed, because of the different values of effective half time of residence of 131I in thyroid and the radiocesiums in the body. The variation with time after the accident of the relative contribution (average and ranges) of the incorporated radiocesiums to the detector’s signal is presented in Fig. 6, where the numbers of cohort members measured at different times after the accident are also shown. The average value of the contribution of the incorporated radiocesiums to the detector signal, , is 5% when the direct thyroid measurements were performed less than 20 days after the accident and to gradually increased (up to 15–40%) with time after the accident (Chepurny 2007). About 94% of the cohort had their direct thyroid measurements performed between 16 and 31 May 1986, i.e., between 20 and 38 days after the accident. For that group, the average relative contribution of radiocesium to the detected signal is 7.6% with a 95% confidence interval from 1.7 to 19%.
Fig. 6.
Variation with time after the accident of the relative contribution of the radiocesiums to the detector’s signal (left vertical axis) and of the number of measured cohort members (right vertical axis).
Calculation of 131I concentrations in specific foodstuffs
The consumption of the following foodstuffs is considered in the TD-10 dose reconstruction system: locally produced cow’s milk (private milk), milk purchased from a store (shop milk), goat’s milk, mother’s breast milk, milk products, and leafy vegetables. In comparison to the TD-02 dose reconstruction system, the calculations of the 131I concentrations in shop milk and in breast milk were considerably modified as indicated below.
Shop milk and milk products
The calculation of the 131I concentration in milk from private cows was carried out using equation (A1.11) in Appendix 1. However, cow’s milk sold in shops came from milk-processing factories that received the private milk and milk from collective farms produced in a number of neighboring settlements with different levels of radioiodine deposition. The milk-processing factory/factories of the raion mixed the private milk delivered from the settlements of that raion. In the calculation procedure, it was assumed that the milk processed in the factory/factories of a given raion (which was then supplied to the grocery shops of the raion) was derived from the private milk of all settlements of that raion and that the processing time was one day. The city of Kyiv had a large population and a correspondingly large demand for milk. Based on discussions with officials, it was assumed that milk was delivered to the milk factories of Kyiv from the eight raions that surround the city. The concentration of 131I in shop milk was taken to be the average of the private milk concentrations over all settlements in the eight raions, corrected for one day of radioactive decay.
An important factor influencing the radioactivity content of 131I in shop milk was the radiological control that was introduced at the milk-processing factories on 6 May 1986. A permissible level of 3,700 Bq L−1 was imposed for the total activity concentration of 131I+137Cs+134Cs+136Cs in milk or milk products. If the calculated average value of the total concentration of 131I+137Cs+134Cs+136Cs in shop milk or milk products exceeded the permissible level, the total concentration of those radionuclides was taken to be equal to 3,700 Bq L−1.
Breast milk
The cohort includes 452 members who consumed breast milk in April-June 1986. The estimation of the 131I concentrations in breast milk consisted of two steps:
In the first step, the inhalation and ingestion intakes of 131I by the breast-feeding mother were based on the environmental transfer model and on the consumption rates of foodstuffs provided during the administration of the separate questionnaire for nursing mothers.
In the second step, the transfer of 131I from intakes by the mother to concentration in breast milk was evaluated.
Equations (A1.13)–(A1.16) in Appendix 1 were used for the calculations of the concentration of 131I in the breast milk of the mothers of the cohort members.
Contribution of the short-lived isotopes of iodine to thyroid exposure
Among the short-lived radioiodines, only 133I (half-life 20.8 h) and 132I produced as the decay of 132Te (half-lives of 2.3 h and 78.2 h, respectively), can contribute substantially to the thyroid dose from inhalation, and, to a much lower extent, to the dose from ingestion. Since the thyroidal activities of 132I and 133I had decayed to negligible levels at the time of the direct thyroid measurements, the thyroid doses due to the intakes of those two radionuclides could only be reconstructed by means of radioecological modeling.
The ecological thyroid dose from 133I was calculated in the same way as the dose from 131I, taking into account specific parameters for 133I: energy absorbed in thyroid, , activity ratio, KI–133/I–131, and radioactive decay constant, λr (see Tables A1.1, A1.2).
For both inhalation and ingestion, the thyroid doses due to intakes of 132I are much smaller than those due to the intakes of 132Te, which is the precursor of 132I, because the half-life of 132Te is much longer than that of 132I and the two radionuclides are in radioactive equilibrium in the environment. Therefore, the thyroid doses from 132Te-132I were based on the intakes of 132Te. The dose inhalation resulting from the intake of 132Te was calculated in the same way as the inhalation dose from 131I, with modifications due to an adjustment of 1.5 for the ratio of the released activities of 132Te and 131I, specific radioactive decay constant of 132Te (see Tables A1.1, A1.2) and the use of appropriate thyroid dose coefficients from ICRP (1994, 1995).
With regard to the dose from ingestion, the milk pathway for 132Te-132I was considered to be negligible because the transfer factor of 132Te from fodder to cow’s milk is much lower than that for 131I and also because tellurium, contrary to iodine, does not concentrate in the thyroid. However, the thyroid dose from consumption of leafy vegetables, which is usually greater than that from milk consumption because of the direct transfer of 132Te from deposition to intake, was considered. The intake of 132Te with leafy vegetables was calculated in the same way as the intake of 31I, using the same values (see Table A1.2) for the deposition velocity (600 m d−1), the short and long weathering half-times (7 and 28 days, respectively) and the same fraction of activity susceptible to weathering (0.5). Finally, ecological thyroid doses for 132Te-132I from the consumption of leafy vegetables were derived from the calculated intakes, using appropriate thyroid dose coefficients from ICRP Publications (ICRP 1993).
The individual “instrumental” thyroid dose (mGy) of cohort subject k due to internal exposure by short-lived radioiodines was then estimated as:
| (6) |
where: is the scaling factor calculated for 131I, that is, the ratio of the measured, , and modeled, , 131I thyroid activity at the time of measurement, tmes for individual k; is the ecological thyroid dose of cohort subject k due to 133I (mGy); is the ecological thyroid dose of cohort subject k due to 132I produced by decay of 132Te (mGy).
Estimation of the radiation doses for the Prypiat’ evacuees
The cohort included 750 subjects who were evacuated from the town of Prypiat’ on 26–28 April 1986. Separate calculations were made to assess their exposures to radiation prior to and following their evacuation.
Prior to the evacuation
For the time period of approximately 36 hours between the beginning of the accidental releases and the evacuation, inhalation of radioactivity was the only exposure pathway resulting in doses from internal irradiation. A specific questionnaire was developed for the cohort subjects who had been evacuated; they were asked to provide information on (1) their location, either indoors or outdoors, on an hourly basis in the town, which had been divided into 14 sectors (Likhtarev and Chumak 1994) and (2) their activities at that time. If the subject was indoors, he or she was asked whether the small ventilator window widely found in Ukrainian residences was open.
The hourly average radionuclide concentrations in air in each of the 14 sectors of the town were estimated by means of a short-range atmospheric transport model for the time-interval from 2 am on 26 April to 12 pm on 28 April 1986 (Talerko 2005b). The 131I activity (kBq) in the thyroid of subject k due to inhalation in Prypiat’, , was calculated using equations (A1.17)–(A1.18) in Appendix 1.
Following the evacuation
After evacuation most of the Prypiat’ evacuees spent some time in contaminated areas of northern Ukraine where they consumed radioiodine contaminated milk and/or milk products. The corresponding intakes of radioiodines were calculated in the same way as for all other cohort subjects who had not been evacuated.
Thyroid mass
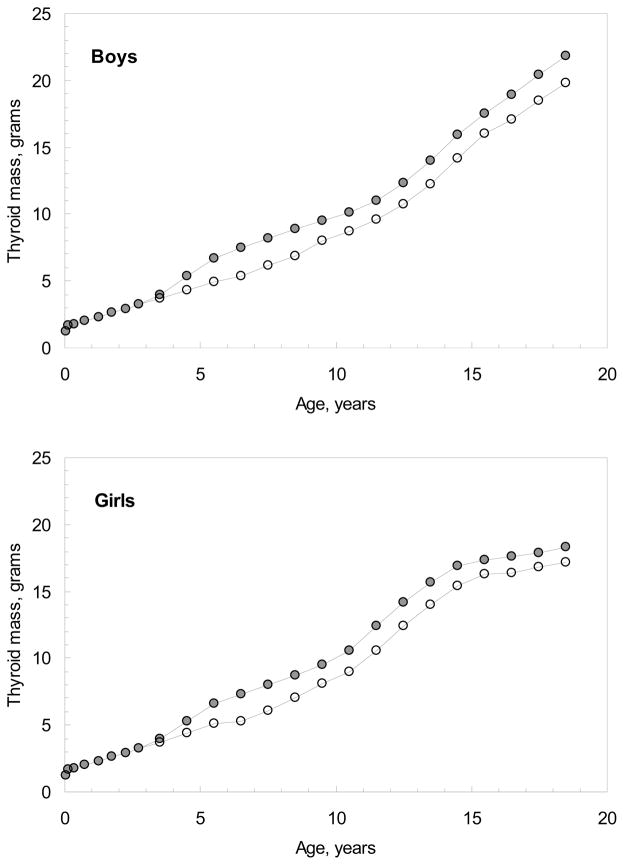
In the current study, the results of ultrasound measurements of thyroid volume that were performed within the framework of the SMHF Program (SMHF 1997) for age-gender groups of 5–16 year olds of Kyiv and Zhytomyr Oblasts were used. The thyroid masses measured in Kyiv Oblast were assumed to be appropriate for children from Chernihiv Oblast, which borders the eastern side of Kyiv Oblast (Fig. 3). Direct autopsy measurements of thyroid mass for young boys and girls less than 3 years old were also made to supplement the results of the SMHF (Likhtarov et al. 2013). The age-gender-dependent thyroid masses used in the TD-10 dose reconstruction system are shown in Fig. 7. For all age- and gender-groups censored lognormal distributions were adopted for the thyroid mass.
Fig. 7.
Age- dependent thyroid mass for boys and girls of Kyiv and Chernihiv Oblasts (filled circles) and Zhytomyr Oblast (open circles).
Fig. 7 illustrates some differences in thyroid masses measured in Kyiv Oblast and those measured in Zhytomyr Oblast, which lies to the west of Kyiv Oblast (Fig. 3). This result is in conflict with expectations because the ecological condition and life-style of the populations residing in the northern raions of Kyiv, Zhytomyr, and Chernihiv Oblasts are rather similar.
Personal interviews
Two rounds of interviews of the cohort members were conducted during the time period from 1998 to 2006. The first interviews were used to estimate the thyroid doses that were previously published (Likhtarev et al. 2006). On the basis of experience acquired during the first round of interviews, improved questionnaires were prepared for the second round of interviews. Similar questionnaire forms were prepared for the four categories of cohort members:
the “main questionnaire” was used to interview the cohort members who were born between 1968 and 1976;
a slightly modified main questionnaire for cohort subjects born between 1977 and the time of the accident in 1986. The main questionnaire was simply edited to address the questions to proxies of the subjects, who were the mothers or other relatives of the subjects, as the subjects themselves were deemed unable to reliably recall their whereabouts and dietary habits when they were less than 10 years old at the time of the accident;
breast-fed children (whose mothers were interviewed using the main questionnaire and a specific addendum); and
the Prypiat’ evacuees, also interviewed using the main questionnaire and a detailed extension.
These questionnaires were improved in comparison to the questionnaire that was used for the first round of interviews because they included:
clearer and more relevant forms of the questions related to the dates and settlements of relocation, as well as to the consumption of foodstuffs;
visual aids (glasses, cups, plates) to help recall the consumption rates of foodstuffs;
additional questions on: (1) the frequency and type of intake of stable iodine for prophylactic reasons; (2) the height and weight of the subject in 1986, and, when appropriate, the whereabouts and life style of the residents of Prypiat’.
Each of the four questionnaires contained primary and secondary questions. Following a positive response on a primary question (for instance, “Did you drink milk?”) the respondent was asked secondary questions, such as: “When did you drink milk?”, “What kind of milk?”, “How much?”, “How often?” etc. Altogether, the main questionnaire included 17 primary questions and 81 secondary questions, the extension of the questionnaire for the breast feeding mothers included 7 primary and 39 secondary questions, while the questionnaire for the Prypiat’ evacuees included 15 primary and 91 secondary questions.
In all questionnaires, the primary questions clarified the following subject-specific information:
personal data: name, address, date of birth:
daily whereabouts during the first two months after the accident (until the end of June 1986);
types of consumed foodstuffs;
daily consumption rates of milk, milk products and leafy vegetables during April-June 1986;
whether and, if yes, when, what kind, and how much stable iodine was taken for prophylactic purposes.
After manual completion of the questionnaire forms during the interviews, the processing consisted of:
visual checking of the degree of completeness and consistency of the responses;
transfer of the hand-written information into electronic form suitable for computer calculations, including double entry to eliminate mistakes;
conversion of the non-numerical responses into numerical indices, e.g., names and types of settlements, dates, time intervals, type of iodine prophylaxis, types of consumed foodstuffs, frequency of consumption, etc. The resulting numbers and numerical indices for each subject formed his or her “numerical matrix”;
-
treatment of illogical responses - for example:
date of departure from a settlement preceding the date of arrival in that settlement;
inconsistencies in addresses given for the time period of two months following the accident; or
unrealistically high consumption rates of foodstuffs (e.g., 5–10 L d−1 of milk);
-
treatment of the missing responses - mainly concerning the information on the consumption of foodstuffs:
unknown source of consumed milk (private or shop);
unknown day of beginning or end of the consumption of milk, milk products or leafy vegetables;
unknown consumption rates of foodstuffs;
unknown frequency of consumption;
unknown unit of consumption (e.g., liters or grams).
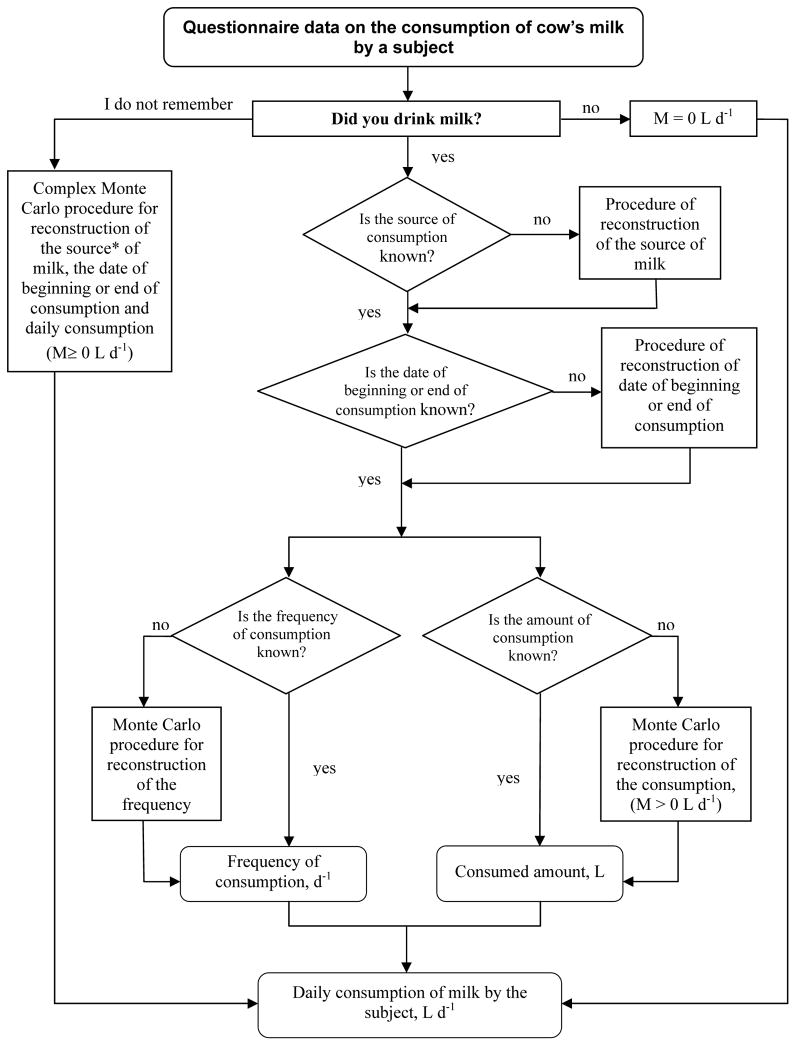
The estimated or reconstructed replacements for illogical or missing answers were then used to correct the numerical matrices of the subjects. As an example, the procedure of reconstruction of the origin, frequency, and consumption rate of cow’s milk is given in Fig. 8. In that procedure, the missing value (origin, frequency, or consumption rate) was replaced with a random value taken from the distribution of responses provided by the other subjects in the same age-gender group.
Fig. 8.
Scheme of assignment of the daily consumption rate of milk according to the questionnaires used in TD-10.
The reconstruction or correction of the illogical or missing answers was necessary for 4,877 questionnaires. For 3,184 questionnaires the reconstruction of the answers was related to the time of relocation from the settlements of origin. The information on the consumption of milk, milk products, and leafy vegetables was reconstructed or corrected for 1,327, 480, and 481 questionnaires, respectively. For 904 questionnaires the information on iodine prophylaxis was clarified. For some questionnaires more than one procedure was required to complete the set of answers.
For a variety of reasons, the second interview was not administered on 1,455 subjects, including 554 from Kyiv Oblast, 438 from Zhytomyr Oblast, 452 from Chernihiv Oblast, and 11 from other oblasts of Ukraine. In those cases, the thyroid dose calculations were performed using the results of the first interviews. The procedure consisted in filling in the appropriate questionnaire for the second interview using the responses from the first round of interviews and, for the missing information, using random values taken from the distributions of responses provided by the other subjects in the same age-gender group during the second round of interviews.
RESULTS AND DISCUSSION
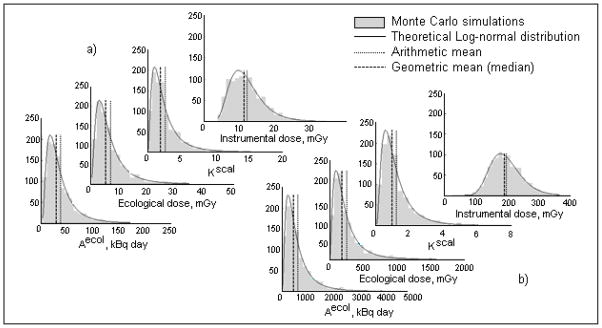
Instrumental and ecological thyroid doses were estimated for the 13,204 Ukrainian cohort members. Examples of results of Monte-Carlo simulations showing the distributions of individual ecological and instrumental doses and of related quantities are presented in Fig. 9. Of particular significance for the TD-10 system are the 1,001 values of instrumental thyroid dose calculated for each individual k.
Fig. 9.
Illustrations of distributions of individual ecological and instrumental doses and of related quantities obtained from the Monte-Carlo simulations: a) example of a subject with “simple” behavior (without relocation), b) example of a subject with multiple relocations during May-June of 1986.
Thyroid dose estimation TD-10
For reasons of clarity, only the arithmetic means of the distributions of the 1,000 stochastic values of the individual instrumental thyroid doses are reported here and are simply referred to as “individual thyroid doses”. For the entire cohort, the thyroid doses ranged from 0.35 mGy to 42 Gy, with an arithmetic mean of 0.65 Gy and a geometric mean of 0.19 Gy. The distribution of the thyroid doses is shown in Table 3.
Table 3.
Distribution of the instrumental thyroid doses TD-10 for the 13,204 Ukrainian cohort members.
| Dose interval (Gy) | Entire cohort | Kyiv Oblasta | Zhytomyr Oblast | Chernihiv Oblast | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N | % of entire cohort | N | % of oblast’s cohort | N | % of oblast’s cohort | N | % of oblast’s cohort | |
| <0.02 | 804 | 6.1 | 147 | 5.6 | 51 | 1.4 | 601 | 8.7 |
| 0.02 – 0.05 | 1,582 | 12.0 | 314 | 11.9 | 74 | 2.0 | 1,191 | 17.3 |
| 0.05 – 0.1 | 2,069 | 15.7 | 367 | 13.9 | 260 | 7.1 | 1,441 | 20.9 |
| 0.1 – 0.2 | 2,275 | 17.2 | 439 | 16.7 | 479 | 13.2 | 1,350 | 19.6 |
| 0.2 – 0.5 | 2,836 | 21.4 | 611 | 23.2 | 933 | 25.6 | 1,282 | 18.6 |
| 0.5 – 1 | 1,628 | 12.3 | 394 | 15.0 | 661 | 18.1 | 573 | 8.3 |
| 1 – 5 | 1,767 | 13.4 | 326 | 12.4 | 1,004 | 27.6 | 431 | 6.2 |
| 5 – 10 | 170 | 1.3 | 26 | 1.0 | 124 | 3.4 | 20 | 0.3 |
| >10 | 73 | 0.6 | 8 | 0.3 | 58 | 1.6 | 6 | 0.1 |
| All | 13,204b | 100.0 | 2,632 | 100.0 | 3,644 | 100.0 | 6,895 | 100.0 |
including Kyiv city (14 subjects)
33 subjects did not reside in any of the 3 oblasts on April 26, 1986
The analysis of the 1,000 stochastic estimates of the thyroid dose for each cohort member showed that the distributions of the individual doses for given individuals were approximately lognormal and could therefore be conveniently characterized by their geometric standard deviations (GSD). The GSDs obtained for the 13,204 cohort members varied from 1.26 to 10.6, with an arithmetic mean of 1.55 and a geometric mean of 1.47. The distribution of the number of subjects according to GSD interval is presented in Table 4. The GSDs of 96% of the dose estimates, which averaged 0.67 Gy, were less than 2.0. The largest GSDs were associated with very small doses and were due primarily to measurement uncertainties.
Table 4.
Distribution of the GSD attached to the distributions of the estimates of instrumental thyroid dose for the 13,204 Ukrainian cohort members.
| GSD interval | N | % | Mean dose, Gy |
|---|---|---|---|
| 1.26 – 1.5 | 7,982 | 60.5 | 0.66 |
| 1.5 – 2 | 4,711 | 35.7 | 0.68 |
| 2 – 3 | 294 | 2.2 | 0.26 |
| >3 | 217 | 1.6 | 0.017 |
Uncertainties in thyroid doses estimated in this study are lower than reported by other investigators. Kopecky et al (2004) reported that GSDs in doses estimated for 3,191 in-area participants of the Hanford Thyroid Disease Study vary from 1.56 to 5.42 with mean GSD of 2.18. Simon et al. (2006) reported that in thyroid doses reconstructed for 3,122 subjects of the Utah Thyroid Cohort Study GSDs varies from about 1.5 to 8.5 with majority of values lie in range from 2.0 to 3.0. In a case-control study of thyroid cancer in 198 Russian children exposed following the Chernobyl accident, the GSDs of the thyroid dose varies from 1.8 to 3.5 with a median 2.2 (Kopecky et al. 2006). In a case-control study of thyroid cancer in 1,615 Belarusian and Russian children exposed following the Chernobyl accident, the GSDs of the thyroid dose varies from 1.7 to 4.0 with a median of 2.2 (Drozdovitch et al. 2010).
All subjects of our unique cohort had measurement of exposure rate against their neck that was used to derive the 131I activity in the thyroid and, consequently, thyroid dose. Likhtarev et al. (2003) showed that from 70 to 99% of the uncertainty in the doses derived from direct thyroid measurements are arisen from the following two main sources: variability of thyroid mass and errors in the estimates of 131I thyroidal activity. Distribution of thyroid masses for given age- and gender-specific group is characterized by the GSD varies from 1.32 to 1.45 (Likhtarov et al. 2013). A coefficient of variation in the range of 25–40% for 131I activity measured in the thyroid in Ukrainian population was estimated by Likhtarev et al. (1995). Therefore, our dose estimates, which were derived from direct thyroid measurements, are less uncertain than those calculated in the studies where only the modeling was used.
In the TD-10 dose reconstruction system, only the overall uncertainties are provided for the individual instrumental thyroid dose estimates. A separate estimation of the Berkson and classic errors, which would lead to a more realistic radiation risk analysis (Kukush et al. 2011), has not been implemented.
Thyroid exposure of Prypiat’ evacuees
Of particular interest are the thyroid doses received by the Prypiat’ evacuees: they range from 5.7 mGy to 25 Gy, with an arithmetic mean of 0.96 Gy. The relatively low thyroid doses received by the Prypiat’ evacuees reflect the effectiveness of their evacuation, which prevented them from receiving not only ingestion doses from the consumption of contaminated foodstuffs, but also higher inhalation doses during the days with substantial releases of radioactive materials. The distribution of the number of Prypiat’ evacuees according to the percentage of thyroid dose received before evacuation is given in Table 5. About half of the Prypiat’ evacuees received a higher thyroid dose after evacuation than before evacuation. For 23% of Prypiat’ evacuees (173 cohort subjects), the pre-evacuation doses were less than 10% of the entire thyroid doses. At the other end of the spectrum, for 13% of Prypiat’ evacuees (96 cohort subjects) the pre-evacuation doses were more than 90% of the entire thyroid doses received in April-June 1986.
Table 5.
Distribution of cohort’s Prypiat’ evacuees over the fraction of instrumental thyroid dose caused by the staying in Prypiat’-town before evacuation.
| Percentage of instrumental thyroid dose received before evacuation | N | Percentage of Prypiat’ evacuees |
|---|---|---|
| 0–10 | 173 | 23 |
| 10–20 | 79 | 10.5 |
| 20–30 | 66 | 8.8 |
| 30–40 | 47 | 6.3 |
| 40–50 | 50 | 6.7 |
| 50–60 | 46 | 6.1 |
| 60–70 | 56 | 7.4 |
| 70–80 | 70 | 9.3 |
| 80–90 | 67 | 8.9 |
| 90–100 | 96 | 13 |
Contribution of the short-lived radioiodines to the thyroid dose
The most important contribution to the thyroid dose arises from intakes of 131I. Estimates of thyroid dose resulting from intakes of shorter-lived radioiodines (132I and 133I) were also made. Our calculations, as well as literature data (Balonov et al. 2003; UNSCEAR 2000), show that the contribution of intakes of shorter-lived radioiodines to the thyroid doses is relatively small, being on the order of 5 to 10% on average (Table 6). The highest contributions (ranging from 0.90% to 79%, with an average of 25%) were obtained for the Prypiat’ evacuees, who (1) resided in the proximity of the Chornobyl reactor at the time of the accident; (2) took stable iodine pills to reduce the thyroid uptake of radioiodines (mainly 131I); and (3) moved to low-contaminated areas in the first days following the accident in order to prevent them from consuming highly-contaminated milk, milk products, or leafy vegetables. Although all these factors, except the first, resulted in higher contributions to the overall thyroid dose from the intakes of the shorter-lived radioiodines, they also resulted in a reduction of the overall thyroid dose.
Table 6.
Average contribution of the shorter-lived radioiodines to the instrumental thyroid dose.
| Place of residence on 26 April 1986 | N | Average contribution of the short-lived radioiodines to the instrumental thyroid dose, percent
|
||
|---|---|---|---|---|
| for all subjects | for those who did not take stable iodine | for those who took stable iodine | ||
| outside of the 30-km zone | 11,766 | 4.9 | 4.5 | 6.3 |
| 30-km zone, excluding Prypiat’ | 688 | 10 | 6.1 | 13 |
| Prypiat’ | 750 | 25 | 14 | 30 |
| All locations (entire cohort) | 13,204 | 6.3 | 4.8 | 10 |
Comparison of the instrumental thyroid dose estimates of TD-10 and TD-02
Median ratios of TD-10 (Dins,2010) and TD-02 (Dins,2002) individual instrumental thyroid dose estimates according to age at the time of the accident for the entire cohort and for different subgroups are presented in Table 7. Table 7 shows that for the entire cohort of 13,204 subjects, with or without iodine prophylaxis, the median TD-10 thyroid dose estimates are uniformly lower than the median estimates found using the TD-02 methodology. This is due mainly to the use of region-specific thyroid masses in TD-10 as well as to modifications in the model of ecological transport. The median ratios of TD-10 to TD-02 doses were estimated to be 0.72 and 0.71 for boys and girls, respectively (not shown in Table 7). For a fraction of subjects in each age group, however, the ratio of the two dose estimates was greater than one. With respect to the Pripyat’ evacuees who ingested stable iodine, most median dose ratios were close to or greater than one and a quarter of dose ratios were ~1.5 or higher for seven age groups. Ratios of doses for the Pripyat’ evacuees who did not resort to iodine prophylaxis were generally lower.
Table 7.
Median ratios of TD-10 (Dins,2010) and TD-02 (Dins,2002) individual instrumental thyroid dose estimates according to age at the time of the accident for the entire cohort and for different subgroups.
| Age, years | Entire cohort | Subjects with iodine prophylaxis | Prypiat’ evacuees
|
|||||
|---|---|---|---|---|---|---|---|---|
| with iodine prophylaxis | without iodine prophylaxis | |||||||
|
| ||||||||
|
Dins,2010/Dins,2002
| ||||||||
| median | 25th–75th percentiles | median | 25th–75th percentiles | median | 25th–75th percentiles | median | 25th–75th percentiles | |
| 0 | 0.70 | 0.55 – 0.89 | 0.78 | 0.55 – 1.0 | 0.49 | 0.31 – 0.59 | 0.54 | 0.38 – 0.78 |
| 1 | 0.75 | 0.63 – 0.92 | 0.86 | 0.65 – 1.1 | 0.71 | 0.44 – 1.0 | 0.77 | 0.45 – 1.0 |
| 2 | 0.75 | 0.63 – 0.90 | 0.80 | 0.67 – 1.0 | 0.77 | 0.63 – 0.91 | 0.74 | 0.60 – 0.88 |
| 3 | 0.69 | 0.58 – 0.83 | 0.79 | 0.64 – 1.0 | 0.83 | 0.59 – 1.2 | 0.83 | 0.64 – 1.0 |
| 4 | 0.61 | 0.52 – 0.77 | 0.68 | 0.56 – 0.93 | 0.94 | 0.58 – 1.3 | 0.70 | 0.59 – 0.94 |
| 5 | 0.59 | 0.48 – 0.77 | 0.69 | 0.52 – 0.94 | 1.0 | 0.64 – 1.4 | 0.78 | 0.63 – 0.90 |
| 6 | 0.65 | 0.53 – 0.80 | 0.71 | 0.58 – 0.92 | 1.0 | 0.86 – 1.3 | 0.83 | 0.65 – 1.1 |
| 7 | 0.70 | 0.59 – 0.85 | 0.74 | 0.60 – 0.93 | 0.90 | 0.74 – 1.1 | 0.87 | 0.68 – 1.1 |
| 8 | 0.76 | 0.65 – 0.90 | 0.82 | 0.70 – 1.1 | 1.1 | 0.90 – 1.4 | 0.84 | 0.73 – 0.92 |
| 9 | 0.78 | 0.68 – 0.93 | 0.90 | 0.72 – 1.1 | 1.3 | 0.76 – 1.5 | 0.89 | 0.58 – 1.0 |
| 10 | 0.80 | 0.69 – 1.0 | 0.90 | 0.74 – 1.2 | 1.2 | 0.84 – 1.5 | 0.84 | 0.77 – 1.0 |
| 11 | 0.80 | 0.70 – 1.0 | 0.87 | 0.73 – 1.1 | 1.0 | 0.76 – 1.4 | 0.83 | 0.79 – 1.2 |
| 12 | 0.76 | 0.67 – 0.93 | 0.86 | 0.72 – 1.1 | 1.1 | 0.84 – 1.8 | 0.89 | 0.46 – 1.1 |
| 13 | 0.75 | 0.64 – 0.93 | 0.87 | 0.67 – 1.2 | 1.2 | 0.69 – 1.8 | 1.1 | 0.80 – 1.3 |
| 14 | 0.75 | 0.64 – 0.92 | 0.89 | 0.73 – 1.2 | 1.2 | 0.94 – 1.7 | 0.62 | 0.56 – 0.66 |
| 15 | 0.74 | 0.64 – 0.93 | 0.85 | 0.68 – 1.1 | 1.2 | 1.0 – 1.7 | 0.83 | 0.71 – 1.0 |
| >15 | 0.82 | 0.71 – 1.0 | 0.90 | 0.75 – 1.1 | 1.0 | 0.74 – 1.6 | 0.63 | 0.55 – 0.77 |
| Totals | 0.74 | 0.61 – 0.90 | 0.82 | 0.66 – 1.1 | 1.0 | 0.74 – 1.4 | 0.83 | 0.63 – 1.1 |
Analysis of specific components of TD-10
Ecological time-integrated thyroid activity,
The main improvements in TD-10 are related to the “ecological” component of the thyroid dose. Examples of ratios of time-integrated thyroid activities obtained in TD-10 ( ) and in TD-02 ( ) are given in Table 8 for the subjects of all settlements with at least 20 cohort members aged 8–12 y who stayed in their settlements of residence during the first two weeks after the accident (from 26 April to 11 May 1986), that is, when most of the thyroid dose was delivered. The ratios of cumulative 131I ground deposition used in TD-10 ( ) and TD-02 ( ) are also shown in Table 8.
Table 8.
Weighted average ratiosa of time-integrated ecological thyroid activity estimated in TD-10 (Aecol,2010) and in TD-02 (Aecol,2002) for the cohort subjects of different raions of Kyiv, Zhytomyr, and Chernihiv Oblasts, who stayed in their settlements from 26 April to 11 May 1986 and weighted average ratiosa of cumulative 131I ground deposition used in TD-10 ( ) and TD-02 ( ).
| Oblast | Raion | Ratios of time-integrated ecological thyroid activity, Aecol,2010/Aecol,2002
|
Ratio of cumulative 131I depositions | |
|---|---|---|---|---|
| Median | 25th – 75th percentiles | |||
| Zhytomyr | Narodychy | 5.6 | 3.2–10 | 4.1 |
| Ovruch | 7.1 | 4.0–12 | 2.5 | |
|
| ||||
| Kyiv | Ivankiv | 5.3 | 2.7–9.4 | 2.4 |
| Polisske | 5.0 | 1.6–8.9 | 7.7 | |
|
| ||||
| Chernihiv | Kozelets | 1.2 | 0.64–2.2 | 3.4 |
| Ripky | 1.9 | 1.0–3.8 | 1.1 | |
| Chernihiv | 0.70 | 0.36–1.6 | 1.3 | |
| Chernihiv-city | 0.84 | 0.49–1.4 | 0.54 | |
The average ratios were weighted according to number of subjects in each settlement or raion.
As is clear from Table 8, the ecological thyroid doses calculated in TD-10 for all eight considered raions of the three oblasts are different from those of TD-02. The increases or decreases in ecological thyroid dose from one dose reconstruction system to another are relatively well correlated with the corresponding modifications in the cumulative 131I ground deposition values.
Ecological time-integrated activity per unit of 131I deposition
Because the model of ecological transport of 131I and 137Cs is usually developed for a unit ground deposition of the radionuclide considered, rather close values of ecological time-integrated thyroid activity per unit of 131I cumulative ground deposition should be expected for the subjects of different areas, as the inter-individual variability is mainly due to different dietary habits and to the possible use of iodine prophylaxis.
Table 9 gives median values of time-integrated ecological activity per unit of 131I cumulative ground deposition ( ), where is the cumulative 131I ground deposition, for the cohort subjects who did not change their settlement of residence during the first 15 days after the accident. As expected, the values of Ãecol,2010 for the eight raions of Zhytomyr, Kyiv, and Chernihiv Oblasts that are considered are in relatively good agreement.
Table 9.
Median values of time-integrated ecological activity per unit of 131I cumulative ground deposition Ãecol,2010 for different raions of Kyiv, Zhytomyr, and Chernihiv Oblasts.
| Oblast | Raion | Time-integrated ecological activity per unit of 131I deposition, Ãecol,2010, MBq·d/(MBq·m−2)
|
|||||
|---|---|---|---|---|---|---|---|
| Boys | Girls | ||||||
|
| |||||||
| N | Median | 25th–75th percentiles | N | Median | 25th–75th percentiles | ||
| Zhytomyr | Narodychy | 427 | 0.84 | 0.45 – 1.4 | 465 | 0.51 | 0.32 – 0.87 |
| Ovruch | 934 | 0.79 | 0.46 – 1.3 | 918 | 0.53 | 0.31 – 0.84 | |
|
| |||||||
| Kyiv | Ivankiv | 187 | 0.64 | 0.39 – 1.3 | 167 | 0.42 | 0.23 – 0.76 |
| Poliske | 79 | 0.80 | 0.50 – 1.3 | 63 | 0.56 | 0.37 – 1.0 | |
| Chornobyl | 30 | 0.51 | 0.23 – 0.83 | 31 | 0.34 | 0.22 – 0.60 | |
|
| |||||||
| Chernihiv | Kozelets | 628 | 0.71 | 0.42 – 1.2 | 628 | 0.56 | 0.33 – 0.92 |
| Ripky | 458 | 0.79 | 0.48 – 1.3 | 452 | 0.59 | 0.34 – 1.0 | |
| Chernihiv | 867 | 0.77 | 0.42 – 1.3 | 821 | 0.53 | 0.30 – 0.91 | |
|
| |||||||
| Total | 3,630 | 0.77 | 0.44 – 1.3 | 3,582 | 0.54 | 0.32 – 0.90 | |
Scaling factor
In Table 10, median values of the scaling factor (Kscal) calculated in TD-10 are shown for the cohort subjects of rural settlements who did not change residence from 26 April to 11 May 1986. Because the individual scaling factor characterizes the ratio of the instrumental ( ) and ecological ( ) dose estimates for subject k, the results presented in Table 10 indicate that, on average, the ecological dose overestimates the instrumental dose for the subjects of the Kozelets Raion in Chernihiv Oblast and of all raions of Kyiv and Zhytomyr Oblasts. However, the median value of ecological dose is much higher than the instrumental dose for the subjects of the Chernihiv Raion of Chernihiv Oblast, while the median values of ecological and instrumental dose are about the same in the Ripky Raion of Chernihiv Oblast.
Table 10.
Median values of scaling factor Kscal for the cohort subjects of rural settlements who did not change their residence location from the time of the accident to 11 May 1986.
| Oblast | Raion | N | Scaling factor, Kscal
|
|
|---|---|---|---|---|
| Median | 25th – 75th percentiles | |||
| Zhytomyr | Narodychy | 892 | 0.74 | 0.29 – 1.7 |
| Ovruch | 1,852 | 0.41 | 0.19 – 0.90 | |
| Kyiv | Ivankiv | 354 | 0.36 | 0.16 – 0.86 |
| Poliske | 142 | 0.35 | 0.12 – 0.77 | |
| Chornobyl | 61 | 0.30 | 0.10 – 0.48 | |
| Chernihiv | Kozelets | 1,256 | 0.65 | 0.29 – 1.6 |
| Ripky | 910 | 0.96 | 0.47 – 2.2 | |
| Chernihiv | 1,638 | 1.9 | 0.74 – 5.0 | |
|
| ||||
| All locations | 7,212 | 0.74 | 0.29 – 2.0 | |
In Table 11 the information on the scaling factor Kscal is expanded to include all members of the cohort as well as different groups of the cohort from Kyiv, Zhytomyr and Chernihiv Oblasts, without any restriction on possible changes in the residence location. The results presented in Table 11 are in agreement with those of Table 10 as they show higher values of median scaling factors in Chernihiv Oblast than in Kyiv and Zhytomyr Oblasts. This is also true for the groups of cohort members who were breast fed or took iodine for prophylactic purposes.
Table 11.
Median scaling factor Kscal for different groups of the cohort from Kyiv, Zhytomyr, and Chernihiv Oblasts.
| Subgroup of cohort | Scaling factor Kscal
|
|||
|---|---|---|---|---|
| Three oblasts | Zhytomyr Oblast | Kyiv Oblast | Chernihiv Oblast | |
| Entire cohort | 0.81 | 0.57 | 0.43a | 1.1 |
| Iodine prophylaxis | 1.1 | 0.81 | 0.50a | 1.7 |
| Breast-fed | 1.1 | 0.69 | 1.0a | 1.5 |
| Prypiat’ evacuees with iodine prophylaxis | - | - | 0.84 | - |
| Prypiat’ evacuees without iodine prophylaxis | - | - | 0.79 | - |
Excluding the evacuees from Prypiat’
Instrumental time-integrated thyroid activity per unit of 131I ground deposition
In order to analyze the reasons for the differences in the median values of the scaling factor Kscal that are presented in Tables 10 and 11, the median values of instrumental individual time-integrated thyroid activity per unit of 131I cumulative ground deposition have been calculated for the subjects of the eight raions of Kyiv, Zhytomyr and Chernihiv Oblasts, where majority cohort members resided at the time of the accident. The median values of normalized instrumental individual time-integrated thyroid activity are presented in Table 12 for the subjects of rural settlements who did not change their location of residence at least up to 11 May 1986, during the time period when most of the thyroid dose was delivered.
Table 12.
Median values of normalized instrumental time-integrated activity Ãinst per unit of cumulative 131I deposition averaged for the subjects of the rural settlements of different raions of Kyiv, Zhytomyr, and Chernihiv Oblasts, who did not change their settlement of residence from 26 April to 11 May 1986, MBq d/(MBq m−2).
| Oblast | Raion | N |
Ãins, MBq d/(MBq m−2)
|
|
|---|---|---|---|---|
| Median | 25th – 75th percentiles | |||
| Zhytomyr | Narodychy | 892 | 0.70 | 0.31–1.32 |
| Ovruch | 1852 | 0.38 | 0.21–0.70 | |
|
| ||||
| Kyiv | Ivankiv | 354 | 0.28 | 0.12–0.53 |
| Poliske | 142 | 0.31 | 0.13–0.71 | |
| Chornobyl | 61 | 0.14 | 0.060–0.34 | |
|
| ||||
| Chernihiv | Kozelets | 1256 | 0.58 | 0.31–1.1 |
| Ripky | 910 | 0.92 | 0.53–1.9 | |
| Chernihiv | 1688 | 1.6 | 0.73–3.8 | |
Table 12 demonstrates that the median values of normalized instrumental time-integrated thyroid activity Ãins for the subjects of the raions of Zhytomyr and Kyiv Oblasts are 2–4 times lower than those for the subjects of the Ripky and Chernihiv Raions of Chernihiv Oblast. The normalized instrumental time-integrated thyroid activity for the Kozelets Raion of Chernihiv Oblast is also rather different (2–3 times lower) from the corresponding values obtained for the neighboring raions of Ripky and Chernihiv in that oblast.
The results of Table 12 do not confirm the expectation that the normalized instrumental time-integrated thyroid activity for all raions and oblasts should have similar values, as was found for the normalized ecological activity (Table 9). The reasons for the apparent inconsistencies presented in Table 12 deserve to be carefully investigated. From equations (1) and (4), the normalized instrumental time-integrated thyroid activity Ãins can be expressed as:
| (7) |
where is the calculated ecological thyroid activity at the time of measurement per unit of cumulative 131I ground deposition (kBq per kBq m−2).
As indicated above, the values shown in Table 9 of the normalized time-integrated thyroid activity for different raions of three oblasts are rather close. Hence from eq. (7), it follows that the reasons for region-dependent values of Ãins are due to inconsistent values of directly measured thyroid activity and/or of cumulative 131I ground depositions .
The direct thyroid measurements in 1986 were made in difficult accidental conditions by a number of dosimetric teams, by different (spectrometric and non-spectrometric) devices, both in and out of Kyiv, Zhytomyr and Chernihiv Oblasts (Likhtarev et al. 1993b, 1995); therefore, it is quite understandable that the methodology used for the measurements, the available description of the conditions of these measurements, the ways in which the thyroid activity was derived from the result of the direct thyroid measurement deserve additional careful consideration.
Regarding the values of cumulative 131I ground deposition, , it would be desirable to use the available high quality local measurements of 137Cs deposition to analyze the deposition patterns that have been obtained using the mesoscale model LEDI (Talerko, 2005b). Such so- called method “of local effective depositions” with the involvement of local data has been rather effectively used in (Pitkevich et al. 1993).
Limitations of the TD-10 set of thyroid dose estimates
Although the TD-10 set of thyroid dose estimates represents a vast improvement over the previous set, called TD-02, that had been obtained in 2002 and published in 2006 (Likhtarev et al. 2006), it nevertheless presents limitations that are likely due to the scarcity of measurements (131I ground deposition densities), to the fact that measurements were conducted under difficult conditions within a few weeks after the accident (direct thyroid measurements), or to the fact that other measurements were not performed for the strict purpose of dose reconstruction (thyroid volumes). Possibly for those reasons, apparent inconsistencies have been observed and are noted in the above sections. These apparent inconsistencies include:
notable differences between the measured values of thyroid mass for the children of Kyiv and Zhytomyr Oblast, which are in conflict with the measured urinary concentrations of stable iodine in those oblasts and with the expectation that the thyroid masses should be roughly the same in those oblasts; and
notable differences between the estimated instrumental doses normalized to the 131I ground deposition density for the subjects of Kyiv and Zhytomyr Oblasts on one hand and the subjects of some of the raions of Chernihiv Oblast on the other. These differences are presumably due to unexpected values of the 131I ground deposition densities in various locations (which were not measured and had to be estimated) and/or to errors in the direct thyroid measurements or in their interpretation, again in some locations.
CONCLUSIONS
In collaboration with the Ukrainian Research Center for Radiation Medicine, the U.S. National Cancer Institute initiated a cohort study of children and adolescents exposed to Chornobyl fallout in Ukraine to better understand the long-term health effects of exposure to radioactive iodines. All cohort members were subjected to at least one direct thyroid measurement between 30 April and 30 June 1986 and resided at the time of the accident in the northern part of Kyiv, Zhytomyr, or Chernihiv Oblasts, which were the most contaminated territories of Ukraine as a result of radioactive fallout from the Chornobyl accident.
Thyroid doses for the cohort members, which had been estimated following the first round of interviews (Likhtarev et al. 2006), were re-evaluated following the second round of interviews. We realize that there are some limitations in dose estimates as shown in the ‘Results and Discussion’ section. However, the thyroid doses presented in this paper for the 13,204 cohort members have been improved substantially compared to TD-02 due to the following:
clearer and more specific questionnaires;
the use of a new mesoscale model to calculate the ground deposition of radioiodines and radiocesiums;
a re-evaluation of the parameter values used in the ecological model;
the estimation of the contribution of the radiocesiums to the signal read by the detectors used to perform the direct thyroid measurements;
thyroid mass values that are based on measurements performed on Ukrainian citizens;
the inclusion of the thyroid doses resulting from intakes of shorter-lived radioiodines (132I and 133I); and
a more refined evaluation of the uncertainties.
The revised thyroid doses range from 0.35 mGy to 42 Gy, with 95% of the doses included between 1 mGy and 4.2 Gy, with an arithmetic mean of 0.65 Gy and a geometric mean of 0.19 Gy. These means are, on average, 70% of the previous estimates reported by Likhtarev et al. (2006), mainly because of the use of country-specific thyroid masses. However, many of the individual thyroid dose estimates show substantial differences, partly because of the use of an improved questionnaire for the second round of interviews.
The new set of thyroid dose estimates represents a vast improvement over the previous set though we acknowledge some limitations in the measurement data of ground deposition densities and direct thyroid measurements, as discussed. For the purposes of the epidemiologic study, the most notable improvement is a revised assessment of the uncertainties, as shared and unshared uncertainties in the parameter values have been taken into consideration in the calculation of the 1,000 stochastic estimates of thyroid dose for each cohort member. This procedure will make it possible to perform a more realistic risk analysis.
Acknowledgments
The authors are grateful to all of the subjects who participated in the study. Special thanks are to the staff of V.P. Komisarenko Institute of Endocrinology and Metabolism of the National Academy of Medical Sciences of Ukraine (Kyiv, Ukraine) who conducted personal interviews. The authors thank Cindy Clark, NIH Library Writing Center, for manuscript editing assistance. The research was supported by the Intramural Research Program of the U.S. National Cancer Institute, National Institutes of Health within the framework of the Ukraine-U.S. Study of Thyroid Cancer and Other Diseases Following the Chornobyl Accident.
APPENDIX 1. MODEL OF ECOLOGICAL TRANSPORT OF 131I USED IN THE THYROID DOSE RECONSTRUCTION SYSTEM
The ecological 131I thyroid dose for subject k is calculated on the basis of the variation with time of the 131I ground deposition in the settlements where subject k resided during the first two months after the accident:
| (A1.1) |
where: Mk is the thyroid mass for the subject k (g); is the energy of 131I decay absorbed by the thyroid (MeV per decay); Cu is a unit conversion coefficient, equal to 13.82 (Bq kBq−1 g kg−1 J MeV−1 s d−1 mGy Gy−1); is the variation with time of the 131I activity in the thyroid of subject k, calculated from the time-dependent 131I ground deposition in the settlements where subject k resided, using ecological transport models of 131I in the environment and of 131I behavior in the body, and taking into account the daily consumption rates of milk, milk products, and leafy vegetables reported in the personal interview (kBq).
For reasons of clarity, the equations presented in this Appendix are related to a single deposition, , of 131I in a given settlement s on the day of the accident (day 0) . In fact, many cohort members changed their location of residence during the first two months following the accident and the 131I deposition in their locations of residence occurred over several days. In the calculation of the thyroid dose estimates for the cohort members, the values of take into account the contributions from all settlements of residence of subject k and of all days of deposition of 131I in those settlements.
The arithmetic means and distributions of most of the parameters and input data used in the equations presented in this Appendix are listed in Tables A1.1 through A1.4. Separate databases, not included in this paper, include: (1) the daily deposition densities of 131I and 137Cs in all settlements of residence of all cohort members, (2) the locations of residence of the cohort members, (3) the schedule of intake of iodine tablets, when appropriate, as well as data on the subsequent decrease in thyroid uptake, and (4) consumption rates of milk, milk products, and leafy vegetables, as reported by the cohort members during their personal interviews.
It is also worth noting that only deposition densities of 131I and 137Cs over entire days are provided in the databases of deposition. In order to simplify the equations, it is assumed here that the depositions were instantaneous and occurred at 12:01 am.
The activity of 131I in the thyroid for the person k arises from two pathways: inhalation and ingestion :
| (A1.2) |
The variation with time of involves several processes: intake of 131I by inhalation and ingestion; uptake of 131I by the thyroid; elimination of 131I from the thyroid, which is characterized by the age-dependent biological elimination constant and radioactive decay of 131I in the thyroid, which is characterized by the radioactive decay constant λr,I–131 (d−1).
The activity of 131I in the thyroid from the inhalation pathway due to inhalation from a single deposition, of 131I, , is calculated as follows:
| (A1.3) |
with initial conditions:
| (A1.4) |
where is the time of deposition and is the 131I activity taken up by the thyroid as a result of the inhalation pathway; where is the biological half-time of iodine excretion from the thyroid (d); is the 131I ground deposition in the settlement s in the first day after the accident (kBq m−2); vI,soil is the dry deposition velocity for 131I (m d−1). Because wet deposition occurred rarely in Ukraine, the value of the wet deposition velocity is highly uncertain, and the contribution of inhalation to the thyroid dose is usually small, only dry deposition has been assumed in the calculation of the dose from inhalation; is the breathing rate for a cohort member of age a (m3 d−1); BI,inh is the transfer coefficient from lungs to blood for 131I (unitless); BI,th is the uptake of 131I from blood to the thyroid (unitless); is the factor of relative decrease in 131I thyroid uptake after intake of stable iodine tablets for prophylactic reasons, unitless.
The activity of 131I in the thyroid of subject k due to ingestion of contaminated foodstuffs (with a diet of type d) is calculated from the equation:
| (A1.5) |
with the initial condition , where is the beginning of diet d, is the 131I activity taken up by the thyroid the due to ingestion intake. The type of diet d relates to a combination of foodstuffs (milk, milk products, leafy vegetables) and of their consumption rates; is the ingestion intake of 131I by subject k with diet d, (Bq d−1); BI,ing is the absorption factor from the gastrointestinal tract by blood for 131I (unitless).
The ingestion intake of 131I, due to the consumption of the contaminated foodstuffs is calculated as:
| (A1.6) |
where CI,p is the 131I concentration in foodstuff p (kBq kg−1 or kBq L−1); is the storage time for foodstuff p for the settlement of type st (st = rur for rural settlements, urb – for urban settlements) (d); KI,cul,p is the factor of relative decrease in the activity of 131I in foodstuff p due to culinary processing (unitless); is the consumption rate of foodstuff p by cohort member k in diet d (kg d−1).
The concentration of 131I in pasture soil due to the single deposition in settlement s is calculated using the following equation:
| (A1.7) |
with the initial condition:
| (A1.8) |
where ; t0 is the time of deposition; is the mass interception factor (wet mass) for 131I, m2 kg−1; , where KI,gr is the fraction of deposited activity initially retained by pasture grass and Mbiom is the yield of pasture grass on the day of maximum fallout (or close to April 28, 1986) (kg m−2); Msoil is the surface density of the top 1 mm of soil (kg m−2). It is assumed that only the upper millimeter of soil is contaminated and that the soil density of that superficial layer is 900 (kg m−3).
The concentration of 131I in grass and leafy vegetables due to a single deposition is described by a two-exponential (N=2) function:
| (A1.9) |
with the following initial condition at the time of deposition t0:
| (A1.10) |
where and are the short (N = 1) and long (N = 2) weathering half-times of removal of 131I from the vegetation surface (d); are the fractions of 131I activity removed from the vegetation with the short (N =1) and long (N =2) half-times (unitless).
The variation with time of the 131I concentration in cow’s milk in the settlement s is calculated as:
| (A1.11) |
with the initial condition ;
where t0 is the beginning of the consumption of contaminated fodder by the cow. It was assumed that in 1986 the pasture season had begun on 26 April 1986 (the day of the accident) over the entire territory of Ukraine. and TI,cow is the biological half-time of 131I elimination from milk (d); is the 131I concentration in pasture grass (kBq kg−1); is the 131I concentration in pasture soil (kBq kg−1); TFI is the transfer factor of 131I from fodder to cow’s milk (d L−1); Igr is the consumption rate of fresh grass by cow (kg d−1); Isoil is the consumption rate of soil by cow (kg d−1).
The 131I concentration in goat’s milk, is described by the following mathematical form:
| (A1.12) |
where: is the ratio of iodine concentrations in goat milk and in private cow milk (unitless).
The concentration of 131I in breast milk of the mother of subject k, , due to inhalation ( ) and ingestion ( ) of 131I by the mother is calculated as:
| (A1.13) |
The differential equation describing the 131I concentration in mother’s milk due to inhalation following a single deposition is:
| (A1.14) |
with the initial condition:
| (A1.15) |
where is the time of deposition; is the effective constant of iodine excretion into breast milk (d−1); is the transfer coefficient of 131I from blood to breast milk (d L−1); wm,inh is the mother’s breathing rate (m3 d−1); BI,inh is the iodine absorption factor from gastrointestinal tract by blood (unitless); vI,soil is the dry deposition velocity for 131I (m d−1).
The differential equation describing the 131I concentration ( , kBq L−1) in breast milk due to the ingestion of contaminated foodstuffs by the mother is:
| (A1.16) |
with the initial condition , where is the beginning of consumption and is the daily ingestion of 131I by the mother of subject k, kBq d−1 (see eqn A1.6).
The activity of 131I in the thyroid from inhalation, of subject k prior to evacuation from Prypiat’ is calculated from the hourly concentrations of 131I in air. For the first hour of inhalation of 131I-contaminated air:
| (A1.17) |
with the initial condition:
| (A1.18) |
where h is the number of hours in a day (h d−1); is the time after the accident when subject k started to inhale the radioiodine activity (h); is the time-integrated 131I concentration in air over one hour in sector j (kBq h m−3); kf is the ratio of radioiodine concentrations in indoor and outdoor air; the indoor value is associated with the status of the small ventilator window (closed or open) (unitless).
Table A1.1.
List and values of the parameters considered as fixed in the thyroid dose reconstruction system.
| Notation | Unit | Parameter values | Description | Reference | |
|---|---|---|---|---|---|
| λr | d−1 | Radioactive decay constants of | (ICRP 1983) | ||
| 8.62×10−2 | 131I | ||||
| 0.21 | 132Te | ||||
| 0.8 | 133I | ||||
| 6.33×10−5 | 137Cs | ||||
| 9.21×10−4 | 134Cs | ||||
| 5.29×10−2 | 136Cs | ||||
|
|
MeV | 0.20 | Energy absorbed in thyroid per 131I radioactive decay | (Cristy and Eckerman 1987) | |
|
|
MeV | 0.57 | Energy absorbed in thyroid per 132I radioactive decay | (Cristy and Eckerman 1987) | |
|
|
MeV | 0.43 | Energy absorbed in thyroid per 133I radioactive decay | (Cristy and Eckerman 1987) | |
| Br, ing | unitless | 1 | Absorption fraction from gastrointestinal tract to blood (I, Cs) | (ICRP 1989; 1993) | |
| BCs, body | unitless | 1 | Fraction of radiocesium transferred from blood to soft tissues | (ICRP 1989; 1993) | |
| Kr,cul,milk | unitless | 1 | Factor of relative decrease in the activity of I and Cs in private milk due to culinary processing | expert judgment | |
| Kr,cul,sm | unitless | 1 | Factor of relative decrease in the activity of I and Cs in shop milk due to culinary processing | expert judgment | |
|
|
d | Delay between production and consumption of product p for urban settlements: | expert judgment; (Muller and Prohl 1993) | ||
| 0 | private milk | ||||
| 1 | shop milk | ||||
| 0 | goat milk | ||||
| 3 | milk products | ||||
| 1 | green vegetables | ||||
|
|
d | Delay between production and consumption of product p for rural settlements: | expert judgment; (Muller and Prohl 1993) | ||
| 0 | private milk | ||||
| 1 | shop milk | ||||
| 0 | goat milk | ||||
| 3 | milk products | ||||
| 0 | green vegetables | ||||
|
|
- | database | Factor of relative decrease in 131I thyroid uptake after intake of stable iodine tablets for prophylactic reasons | expert judgment; (Il’in et al. 1972) | |
| Iod | - | database | Schedule of intake of stable iodine tablets | questionnaire information | |
| UC | - | database | Information on relocation of cohort members | questionnaire information |
Table A1.2.
List, central estimates and types of distributions of the subject independent (“shared”) parameters in the thyroid dose reconstruction system.
| Notation | Unit | Name of parameter | Central estimate (AM) | Distribution type and parameters | Reference | |
|---|---|---|---|---|---|---|
|
|
kBq m−2 | Daily deposition density of 137Cs in settlement s on day n in time interval 26 April – 6 May, 1986 | Database | CLNa See Appendix 2 |
(Izrael et al. 1990; Talerko 2005a) | |
|
|
kBq m−2 | Daily deposition density of 131I in settlement s on day n in time interval 26 April – 6 May, 1986 | Database | CLN See Appendix 2 |
(Izrael et al. 1990; Talerko 2005b) | |
| kCs–137 | unitless | Normalized factor of 137Cs deposition variability | 1.0 | CLN(0.95, 1.4, 0.5, 2.0)b | Appendix 2 | |
| kI–131/Cs–137 | unitless | Normalized factor of correlation of the 131I to 137Cs deposition | 1.0 | CLN(0.92, 1.5, 0.45, 2.3) | Appendix 2 | |
| KCs–134/Cs–137 | unitless | Activity ratio of 134Cs and 137Cs in the releases from the reactor | 0.55 | CLN(0.55,1.1, 0.5, 0.6) | (Likhtarev et al. 2002; Muck et al. 2002) | |
| KCs–136/Cs–137 | unitless | Activity ratio of 136Cs and 137Cs in the releases from the reactor | 0.23 | CLN(0.23,1.1, 0.15, 0.27) | (Likhtarev et al. 2002; Muck et al. 2002) | |
| KTe–132/I –131 | unitless | Activity ratio of 132I and 131I (also 132Te and 131I) in the releases from the reactor | 1.5 | CLN(1.45, 1.3, 0.9, 2.5) | (Likhtarev et al. 2002) | |
| KI–133/I–131 | unitless | Activity ratio of 133I and 131I in the releases from the reactor | 1.6 | CLN(1.6, 1.1, 1.5, 1.7) | (Likhtarev et al. 2002; Muck et al. 2002) | |
| νI, soil | m d −1 | Deposition velocity of iodine on the ground (dry conditions) | 600 | CLN(540, 1.6, 210, 1380) | (Izrael et al. 1990; Talerko 2005a) | |
| νCs, soil | m d −1 | Deposition velocity of cesium on the ground (dry conditions) | 430 | CLN(380, 1.6, 85, 850) | (Izrael et al. 1990; Talerko 2005b) | |
|
|
unitless | Ratio of air activity of radionuclide in indoors with closed casement ventilator and outdoors | 0.025 | U(0.0, 0.05)c | (Chumak, 2008)d | |
|
|
unitless | Activity ratio of I or Cs concentration in air: indoors (with open casement ventilator) to outdoors | 0.3 | U(0.1, 0.5) | (Chumak, 2008) | |
|
|
m2 kg−1 | Mass interception factor of I or Cs by vegetation (wet mass) | 0.25 | TR(0.1, 0.2, 0.45)e | (IAEA 1996; Prister 2008) | |
| Msoil | kg m−2 | Surface density of top 1 mm of soil | 0.9 | TR(0.3, 1.0, 1.4) | (Nosk et al. 1994; Prohl et al. 2005) | |
| Mbiom | kg m−2 | Grass yield (wet mass) | 0.75 | TR(0.5, 0.75, 1.0) | (IAEA 1996; Muller and Prohl 1993) | |
|
|
d | Short weathering half-time of removal of iodine from the grass surface | 7.0 | CLN(6.9, 1.2, 4.5, 9.5) | (Prister 2008) | |
|
|
d | Long weathering half-time of removal of iodine from the grass surface | 28.0 | CLN(27.5, 1.2, 12, 37) | (Prister 2008) | |
|
|
unitless | Fraction of iodine contamination on grass that is removed with the short weathering half-time | 0.5 | U(0.3, 0.7) | (Prister 2008) | |
|
|
d | Short weathering half-time of removal of cesium from the grass surface | 3.0 | CLN(2.7, 1.6, 1.0, 6.5) | (Barkhudarov et al. 1989; Prister 2008) | |
|
|
d | Long weathering half-time of removal of cesium from the grass surface | 50 | CLN(44, 1.6, 10, 100) | (Barkhudarov et al. 1989; Prister 2008) | |
|
|
unitless | Fraction of cesium contamination on grass that is removed with the short weathering half-time | 0.7 | U(0.6, 0.8) | (Barkhudarov et al. 1989; Prister 2008) | |
| Igr | kg d −1 | Daily consumption of fresh grass by cow | 45 | TR(30, 45, 60) | (Barkhudarov et al. 1989; IAEA 1995) | |
| Isoil | kg d −1 | Daily consumption of soil by cow | 0.55 | TR(0.4, 0.55, 0.7) | (Prohl et al. 2005) | |
| TI,cow | d | Biological half-time of iodine in milk in cow’s body | 1.1 | CLN(1.0, 1.4, 0.5, 2.0) | (Prister 2008; Prister et al. 1988) | |
|
|
d | Short biological half-time of cesium in milk in cow’s body | 1.5 | CLN(1.5, 1.2, 1.0, 2.1) | (Muller and Prohl 1988, 1993; Prister 1988) | |
|
|
d | Long biological half-time of cesium in milk in cow’s body | 15 | CLN(14.8, 1.2, 10.3, 21.3) | (Barkhudarov et al. 1989; Muller and Prohl 1988, 1993) | |
|
|
unitless | Fraction of Cs activity with short biological half-time in milk in cow | 0.8 | U(0.7, 0.9) | (Muller and Prohl 1988, 1993; Prister et al. 1988) | |
| TFI | d L−1 | Transfer factor for iodine from daily intake by cow to concentration in cow’s milk | 0.01 | CLN(0.0065, 2.5, 0.001, 0.04) | (Hoffman 1978; Prister et al. 1988) | |
| TFCs | d L−1 | Transfer factor for cesium from daily intake by cow to concentration in cow’s milk | 0.008 | CLN(0.0055, 2.4, 0.001, 0.03) | (Coughtrey 1990; Prister et al. 1988) | |
|
|
unitless | Ratio of iodine concentrations in goat milk and in private cow milk | 9 | TR(2, 10, 15) | (Hoffman 1978; Prister et al. 1988) | |
|
|
unitless | Ratio of cesium concentrations in goat milk and in private cow milk | 1.4 | TR(0.2, 1.0, 3) | (Prister et al. 1988; USNRC 2003) |
censored lognormal distribution
CLN(GM, GSD, min, max): censored lognormal distribution with the following parameters: geometric mean (GM), geometric standard deviation (GSD), minimal value (min), maximal value (max)
U(min, max): uniform distribution with the following parameters: minimal value (min), maximal value (max)
V. Chumak, personal communication. 53 Melnikova Str, Kyiv, Ukraine, 2008.
TR(min, mode, max): triangular distribution with the following parameters: minimal value (min), mode of distribution (mode), maximal value (max)
Table A1.3.
List, central estimates and types of distributions of the subject dependent (“unshared”) parameters in thyroid dose reconstruction system.
| Notation | Unit | Name of parameter | Central estimate (AM) | Distribution values | Reference | |
|---|---|---|---|---|---|---|
|
|
m3 d −1 | Breathing rate | Table 5 | CLN(GMa, 1.4, 0.5×GM, 2×GM) | (ICRP 1994; NRSU 2003) | |
|
|
d | Biological half-time of iodine excretion from the thyroid | Table 5 | CLN(GM, 1.4, 0.5×GM, 2×GM) | (ICRP 1989, 1993) | |
|
|
d | Short biological half-time of cesium excretion from the body | Table 5 | CLN(GM, 1.4, 0.5×GM, 2×GM) | (ICRP 1989, 1993) | |
|
|
d | Long biological half-time of cesium excretion from the body | Table 5 | CLN(GM, 1.4, 0.5×GM, 2×GM) | (ICRP 1989, 1993) | |
|
|
unitless | Fraction of cesium that is excreted from the body with the long biological half-time | Table 5 | U(AM−0.05, AM +0.05) | (ICRP 1989, 1993) | |
| Ma | g | Thyroid mass | Database | CLN(GM, 1.6, 0.4×GM, 2.5×GM) | (SMHF, 1997) | |
|
|
kBq | Result of thyroid activity measurement | Database | CN(AM, STD, 0, AM+2×STD)b | Database information | |
| Kr,cul,mp | unitless | Decrease factor in the activity in milk products due to culinary processing | 0.7 | U(0.5, 0.9) | (IAEA 1994; Muller and Prohl 1993) | |
| Kr,cul, gr | unitless | Decrease factor in the activity in leafy vegetables due to culinary processing | 0.8 | U(0.6, 1.0) | (IAEA 1994; Muller and Prohl 1993) | |
| Br,inh | unitless | Transfer factor of I or Cs from lungs to blood | 0.61 | TR(0.40, 0.58, 0.85) | (ICRP 1994) | |
| BI,th | unitless | Transfer factor of iodine from blood to thyroid | 0.3 | TR(0.15, 0.25, 0.50) | (ICRP 1989, 1993) | |
|
|
kg d−1 | Consumption rate of private milk, commercial milk, goat milk, milk products, mother’s milk and leafy vegetables | Database | TR(0.75×AM, AM, 1.25×AM) | Questionnaire information | |
|
|
d L−1 | Effective transfer coefficient that describes the excretion of iodine into human milk | 0.4 | CLN(0.37, 1.4, 0.25, 0.89) | (Simon et al. 2002) | |
| TCCs | d L−1 | Transfer coefficient that describes the excretion of cesium into human milk | 0.3 | TR(0.15, 0.30, 0.45) | (UNSCEAR 2000) | |
|
|
d | Effective half time of iodine excretion into human milk | 0.58 | CLN(0.5, 1.7, 0.21, 1.33) | (Simon et al. 2002) | |
|
|
d | Short half time of cesium excretion into human milk | 2 | CLN(2.0, 1.2, 1.4, 2.9) | (UNSCEAR 2000) | |
|
|
d | Long half time of cesium excretion into human milk | 75 | CLN(74, 1.2, 52, 108) | (UNSCEAR 2000) | |
|
|
unitless | Fraction of short half-time cesium excretion into human milk | 0.1 | U(0.05, 0.15) | (UNSCEAR 2000) |
(derived from (Carroll et al 2006))
CN(AM, SD, min, max): censored normal distribution with the following parameters: arithmetic mean (AM), standard deviation (SD), minimal value (min), maximal value (max)
Table A1.4.
Central estimates and ranges of values used in the thyroid dose reconstruction system for the age-dependent breathing rate, and for thyroid iodine and body cesium excretion parameters.
| Age a | Breathing rate (m3 d−1), | Biological half-time of excretion of iodine from thyroid (d), | Short biological half-time of excretion of cesium from human body (d), | Long biological half-time of excretion of cesium from human body, (d) N | Fraction of cesium activity excreted from the body with the long biological half-time, | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM | min | max | AM | min | max | AM | min | max | AM | min | max | AM | min | max | |
| 0 | 2.9 | 1.4 | 5.4 | 15 | 7.1 | 28 | 16 | 7.56 | 30 | 16 | 7.56 | 30 | 0 | 0 | 0.05 |
| 1 | 5.6 | 2.7 | 10.6 | 20 | 9.4 | 38 | 13 | 6.14 | 25 | 16 | 7.56 | 30 | 0 | 0 | 0.05 |
| 2 | 6.5 | 3.1 | 12.3 | 22 | 10.4 | 42 | 11 | 5.2 | 21 | 17 | 8.03 | 32 | 0.14 | 0.09 | 0.19 |
| 3 | 7.4 | 3.5 | 14.0 | 25 | 11.8 | 47 | 10 | 4.72 | 19 | 22 | 10.4 | 42 | 0.28 | 0.23 | 0.33 |
| 4 | 8.3 | 3.9 | 15.6 | 28 | 13.2 | 53 | 10 | 4.72 | 19 | 26 | 12.3 | 49 | 0.41 | 0.36 | 0.46 |
| 5 | 9.3 | 4.4 | 17.5 | 30 | 14.2 | 57 | 9 | 4.25 | 17 | 30 | 14.2 | 57 | 0.55 | 0.50 | 0.60 |
| 6 | 10.4 | 4.9 | 19.6 | 38 | 18.0 | 72 | 8 | 3.78 | 15 | 34 | 16.1 | 64 | 0.58 | 0.53 | 0.63 |
| 7 | 11.5 | 5.4 | 21.7 | 46 | 21.7 | 87 | 8 | 3.78 | 15 | 38 | 18 | 72 | 0.61 | 0.56 | 0.66 |
| 8 | 12.6 | 5.9 | 23.7 | 54 | 25.5 | 102 | 7 | 3.31 | 13 | 42 | 19.8 | 79 | 0.64 | 0.59 | 0.69 |
| 9 | 13.6 | 6.4 | 25.8 | 62 | 29.3 | 117 | 6 | 2.83 | 11 | 46 | 21.7 | 87 | 0.67 | 0.62 | 0.72 |
| 10 | 14.8 | 7.0 | 28.0 | 70 | 33.1 | 132 | 6 | 2.83 | 11 | 50 | 23.6 | 94 | 0.70 | 0.65 | 0.75 |
| 11 | 16.0 | 7.5 | 30.2 | 72 | 34.0 | 136 | 5 | 2.36 | 9 | 59 | 27.9 | 112 | 0.73 | 0.68 | 0.78 |
| 12 | 17.2 | 8.1 | 32.4 | 74 | 35.0 | 140 | 4 | 1.89 | 8 | 67 | 31.7 | 127 | 0.77 | 0.72 | 0.82 |
| 13 | 18.3 | 8.7 | 34.7 | 76 | 35.9 | 144 | 4 | 1.89 | 8 | 76 | 35.9 | 144 | 0.80 | 0.75 | 0.85 |
| 14 | 19.5 | 9.2 | 36.9 | 78 | 36.9 | 147 | 3 | 1.42 | 6 | 84 | 39.7 | 159 | 0.84 | 0.79 | 0.89 |
| 15 | 20.3 | 9.6 | 38.4 | 80 | 37.8 | 151 | 2 | 0.94 | 4 | 93 | 43.9 | 176 | 0.87 | 0.82 | 0.92 |
| 16 | 20.7 | 9.8 | 39.2 | 82 | 38.7 | 155 | 2 | 0.94 | 4 | 96 | 45.4 | 181 | 0.88 | 0.83 | 0.93 |
| 17 | 21.2 | 10.0 | 40.0 | 84 | 39.7 | 159 | 2 | 0.94 | 4 | 100 | 47.2 | 189 | 0.88 | 0.83 | 0.93 |
| 18 | 21.6 | 10.2 | 40.8 | 87 | 41.1 | 164 | 2 | 0.94 | 4 | 103 | 48.7 | 195 | 0.89 | 0.84 | 0.94 |
APPENDIX 2. MONTE-CARLO PROCEDURE USED FOR THE TREATMENT OF 131I AND 137Cs DAILY DEPOSITION DENSITIES
The atmospheric transport model (Talerko 2005a; Talerko 2005b) was developed to estimate the daily deposition densities of 131I and 137Cs ( and , respectively) on the ground during the time period from 26 April to 6 May 1986 for all settlements of Ukraine including those where any of the cohort members resided during the first two months following the accident. For the simulation of 1,001 exposure situations (Fig. 2) in the stochastic procedure, normalized factors of 137Cs and 131I deposition variation (kCs–137 and kI–131) were introduced. The factors kCs–137 and kI –131 characterize the relative variation of daily deposition simultaneously in all locations of deposition. It was assumed that the factors kCs–137 and kI –131 are lognormally distributed with AM=1, GSDkI –131 = 1.7 and GSDkCs–137 = 1.4, respectively. Censored lognormal distributions have been used for kCs–137 and kI–131
Therefore in the i-th exposure situation (scenario), the daily depositions both for any day and settlement and are:
| (A2.1) |
| (A2.2) |
where and are the values of factors kCs–137 and kI–131 randomly specified for scenario i in the Monte-Carlo procedure.
The introduction of normalized factors of 137Cs and 131I deposition variation kCs–137 and kI–131 ensure that the ratio of daily 131I and 137Cs deposition in days 0, 1, …, n in a settlement and between settlements are not changed within any scenario of exposure i.
The factors and are correlated as:
| (A2.3) |
In eqn (A2.3), it is assumed that:
- kCs–137 is a censored lognormal distribution with an arithmetic mean equal to 1 and the following characteristics:
(A2.4) - is the normalized factor of 131I and 137Cs correlation, which is a censored lognormal distribution with an arithmetic mean equal to 1 and the following characteristics:
(A2.5)
References
- Balonov M, Kaidanovsky G, Zvonova I, Kovtun A, Bouville A, Luckyanov N, Voillequé P. Contributions of short-lived radioiodines to thyroid doses received by evacuees from the Chornobyl area estimated using early in-vivo measurements. Radiat Prot Dosim. 2003;105:593–599. doi: 10.1093/oxfordjournals.rpd.a006309. [DOI] [PubMed] [Google Scholar]
- Barkhudarov RM, Gordeev KI, Dibobes IK, Likhtarev IA, Margulis UY, Osanov DP, Pavlovsky OA, Prister BS, Savkin MN, Shamov VP. Methodological principles for calculating levels of external and internal exposure of the population used in taking strategic decisions. In: Romanenko AE, editor. Medical aspects of the Chernobyl accident. Vienna: International Atomic Energy Agency; 1989. pp. 171–182. IAEA-TECDOC-516. [Google Scholar]
- Carrroll RJ, Ruppert D, Stefanski LA, Crainiceanu CM. Measurement Error in Nonlinear Models: A Modern Perspective. 2. Chapman & Hall/CRC; Boca Raton: 2006. [Google Scholar]
- Chepurny M. Accounting for the effect of incorporated cesium isotopes on the activity measured in thyroid. Proceedings of International Scientific and Practical conference: Epidemiology of medical consequences of the Chernobyl accident; Kyiv. October 9–10, 2007; Kyiv: 2007. pp. 23–24. (in Russian) [Google Scholar]
- Coughtrey PJ. Commission of the European Communities report EUR 12608 EN. Luxembourg: Radioactivity Transfer to Animal Products. 1990
- Cristy M, Eckerman KF. Oak Ridge National Laboratory report ORNL/TM-8381/V1–7. Oak Ridge: 1987. Specific Absorbed Fraction of Energy at Various Ages from Internal Photon Sources. [Google Scholar]
- De Cort M, Tsaturov YS. Commission of the European Communities report EUR 16542 EN. Luxembourg: Atlas on caesium contamination of Europe after the Chernobyl nuclear plant accident. 1996
- Drozdovitch V, Khrouch V, Maceika E, Zvonova I, Vlasov O, Bratilova A, Gavrilin Y, Goulko G, Hoshi M, Kesminiene A, Shinkarev S, Tenet V, Cardis E, Bouville A. Reconstruction of radiation doses in a case-control study of thyroid cancer following the Chernobyl accident. Health Phys. 2010;99(1):1–16. doi: 10.1097/HP.0b013e3181c910dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman FO. A review of measured values of the milk transfer coefficient (fm) for iodine. Health Phys. 1978;35:413–416. [PubMed] [Google Scholar]
- International Atomic Energy Agency. IAEA Technical Reports Series 364. Vienna: International Atomic Energy Agency; 1994. Handbook of parameter values for the prediction of radionuclide transfer in temperate environments. [Google Scholar]
- International Atomic Energy Agency. First report of the VAMP Multiple Pathways Assessment Working Group. Vienna: International Atomic Energy Agency; 1995. Validation of models using Chornobyl fallout data from the Central Bohemia region of the Czech Republic/Scenario CB. IAEA-TECDOC-795. [Google Scholar]
- International Atomic Energy Agency. Second report of the VAMP terrestrial working group. Vienna: International Atomic Energy Agency; 1996. Modelling of radionuclide interception and loss processes in vegetation and of transfer in semi-natural ecosystems. IAEA-TECDOC-857. [Google Scholar]
- International Commission on Radiological Protection. Radionuclide transformations: energy and intensity of emissions. ICRP Publication 38. Annals of the ICRP. 1983:11–13. [PubMed] [Google Scholar]
- International Commission on Radiological Protection. Age-dependent doses to members of the public from intake of radionuclides: Part 1. ICRP Publication 56. Annals of the ICRP. 1989;20(2) [PubMed] [Google Scholar]
- International Commission on Radiological Protection. Age-dependent doses to members of the public from intake of radionuclides: Part 2. Ingestion dose coefficients. ICRP Publication 67. Annals of the ICRP. 1993;23(3–4) [PubMed] [Google Scholar]
- International Commission on Radiological Protection. Human respiratory tract model for radiological protection. ICRP Publication 66. Annals of the ICRP. 1994;24(1–3) [PubMed] [Google Scholar]
- International Commission on Radiological Protection. Age-dependent doses to members of the public from intake of radionuclides: Part 4. Inhalation dose coefficients. ICRP Publication 71. Ann ICRP. 1995;25(3/4) [PubMed] [Google Scholar]
- Il’in LA, Arhangel’skaya GV, Konstantinov YO, Likhtarev IA. Radioactive iodine in the problem of radiation safety. Moscow: Atomizdat; 1972. [Google Scholar]
- Izrael Yu A, Vakulovsky SM, Vetrov VA, Petrov VN, Rovinsky FYa, Stukin ED. Chornobyl: Radioactive contamination of the environment. Leningrad: Gidrometeoszdat; 1990. [Google Scholar]
- Kopecky KJ, Davis S, Hamilton TE, Saporito MS, Onstad LE. Estimation of thyroid radiation doses for the Hanford thyroid disease study: results and implications for statistical power of the epidemiological analyses. Health Phys. 2004;87(1):15–32. doi: 10.1097/00004032-200407000-00003. [DOI] [PubMed] [Google Scholar]
- Kopecky KJ, Stepanenko V, Rivkind N, Voillequé P, Onstad L, Shakhtarin V, Parshkov E, Kulikov S, Lushnikov E, Abrosimov A, Troshin V, Romanova G, Doroschenko V, Proshin A, Tsyb A, Davis S. Childhood thyroid cancer, radiation dose from Chernobyl, and dose uncertainties in Bryansk Oblast, Russia: A population-based case-control study. Radiat Res. 2006;166:367–374. doi: 10.1667/RR3596.1. [DOI] [PubMed] [Google Scholar]
- Kukush A, Shklyar S, Masiuk S, Likhtarov I, Kovgan L, Carroll R, Bouville A. Methods for estimation of radiation risk in epidemiological studies accounting for classical and Berkson errors in doses. Int J Biostat. 2011;7(1):Article 15. doi: 10.2202/1557-4679.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtarev IA, Repin VS, Parkhomenko VI, Likhtareva TM, Litvinets LA, Perevoznikov ON. USSR patent No. 1808214. Moscow: Human Phantom. 1991 Mar 21;1991
- Likhtarev IA, Shandala NK, Gulko GM, Kairo IA, Chepurnoy NI. Exposure doses to thyroid of the Ukrainian population after the Chernobyl accident. Health Phys. 1993a;64:594–599. doi: 10.1097/00004032-199306000-00003. [DOI] [PubMed] [Google Scholar]
- Likhtarev IA, Gulko GM, Kairo IA, Sobolev BG, Chepurnoy NI, Cheban AK, Nikonov DA, Djachkov IA, Prohl G, Henrichs K. Reliability and accuracy of the 131I thyroid activity measurements performed in the Ukraine after the Chornobyl accident in 1986. Munich: Institut fur Strahlenschutz; 1993b. [Google Scholar]
- Likhtarev I, Chumak V. Retrospective reconstruction of individual and collective external gamma doses of population evacuated after the Chernobyl accident. Health Phys. 1994;66:643–652. doi: 10.1097/00004032-199406000-00004. [DOI] [PubMed] [Google Scholar]
- Likhtarev IA, Gulko GM, Sobolev BG, Kairo IA, Prohl G, Roth P, Henrichs K. Evaluation of the 131I thyroid-monitoring measurements performed in Ukraine during May and June of 1986. Health Phys. 1995;69(1): 6–15. doi: 10.1097/00004032-199507000-00002. [DOI] [PubMed] [Google Scholar]
- Likhtarev IA, Kovgan LN, Jacob P, Anspaugh LR. Chornobyl accident: retrospective and prospective estimates of external dose of the population of Ukraine. Health Phys. 2002;82(3):290–303. doi: 10.1097/00004032-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Likhtarev I, Minenko V, Khrouch V, Bouville A. Uncertainties in thyroid dose reconstruction after Chornobyl. Radiat Prot Dosim. 2003;105:601–608. doi: 10.1093/oxfordjournals.rpd.a006310. [DOI] [PubMed] [Google Scholar]
- Likhtarev I, Bouville A, Kovgan L, Luckyanov N, Voilleque P, Chepurny M. Questionnaire- and measurement-based individual thyroid doses in Ukraine resulting from the Chornobyl nuclear reactor accident. Radiat Res. 2006;166:271–286. doi: 10.1667/RR3545.1. [DOI] [PubMed] [Google Scholar]
- Likhtarov I, Kovgan L, Vavilov S, Chepurny M, Bouville A, Luckyanov N, Jacob P, Voillequé P. Post-Chernobyl thyroid cancers in Ukraine. Part 1. Estimation of thyroid doses. Radiat Res. 2005;163:125–136. doi: 10.1667/rr3291. [DOI] [PubMed] [Google Scholar]
- Likhtarov I, Kovgan L, Masiuk S, Chepurny M, Ivanova O, Gerasymenko V, Boyko Z, Voillequé P, Antipkin Y, Lutsenko S, Oleynik V, Kravchenko V, Tronko M. Estimating thyroid masses for children, infants and fetuses in Ukraine exposed to 131I from the Chornobyl accident. Health Phys. 2013;104:78–86. doi: 10.1097/HP.0b013e31826e188e. [DOI] [PubMed] [Google Scholar]
- Milton RC, Shohoji T. ABCC report TR 1-68. Hiroshima: Radiation Effects Research Foundation; 1968. Tentative 1965 Radiation Dose (T65D) Estimation for Atomic Bomb Survivors. [Google Scholar]
- Muck K, Prohl G, Likhtarev I, Kovgan L, Meckbach R, Golikov V. A consistent radionuclide vector after the Chornobyl accident. Health Phys. 2002;82:141–156. doi: 10.1097/00004032-200202000-00002. [DOI] [PubMed] [Google Scholar]
- Muller H, Pröhl G. Cesium transport in food chains: comparison of model predictions and observations. In: Desmet G, editor. Reliability of radioactive transfer models. New York: Elsevier; 1988. pp. 104–112. [Google Scholar]
- Muller H, Pröhl G. Ecosys-87: A dynamic model for assessing radiological consequences of nuclear accidents. Health Phys. 1993;64:232–252. doi: 10.1097/00004032-199303000-00002. [DOI] [PubMed] [Google Scholar]
- Nosk BS, Prister BS, Loboda MV. Agrochemical and agroecological state of soils in Ukraine. Kiev: Urozhay; 1994. (in Ukrainian) [Google Scholar]
- Norms of Radiation Safety of Ukraine (NRSU-97) Kyiv: 2003. (in Ukrainian) [Google Scholar]
- Perevoznikov ON, Likhtarev IA, Litvinets LA, Yakovleva GN. Experience, problems and results of mass implementation of Whole Body Counters at post Chernobyl period. Assessment of the Health and Environmental Impact from Radiation Doses due to Released Radionuclides: Proceedings of the International Workshop at Chiba; January 18–20, 1994; Chiba, Japan. 1994. pp. 129–139. [Google Scholar]
- Pitkevich VA, Shershakov VM, Duba VV, Chekin S, Yu, Ivanov VK, Vakulovski SM, Mahonko KP, Volokitin AA, Tsaturov YuS, Tsyb AF. Reconstruction of composition of the Chernobyl radionuclide fallout in the territories of Russia. Radiation and Risk. 1993;3:62–93. (in Russian) [Google Scholar]
- Prister BS, Loschilov NA, Nemetc OF, Poiarkov VA. Bases of agricultural radiology. Kiev: Urozhay; 1988. (in Russian) [Google Scholar]
- Prister BS. Problems of agricultural radioecology and radiobiology during contamination of environment by young mixture of fission products. Chernobyl. 2008 (in Russian) [Google Scholar]
- Pröhl G, Olyslaegers G, Kanyar B, Pinedo P, Bergstrom U, Mobbs S, Eged K, Katona T, Simon I, Hallberg UB, Chen Q, Kowe, Zeevaert T. Development and comparison of five site-specific biosphere models for safety assessment of radioactive waste disposal. J Radiological Protection. 2005;25:343–373. doi: 10.1088/0952-4746/25/4/001. [DOI] [PubMed] [Google Scholar]
- Radiation Effects Research Foundation. Dosimetry system 1986 (DS86). US-Japan joint reassessment of atomic bomb radiation dosimetry in Hiroshima and Nagasaki. In: Roesch William C., editor. Final Report. Vol. 1. Hiroshima: Radiation Effects Research Foundation; 1986. [Google Scholar]
- Radiation Effects Research Foundation. Reassessment of the Atomic Bomb Radiation Dosimetry for Hiroshima and Nagasaki. Dosimetry System 2002 (DS02) In: Young Robert W, Kerr George D., editors. Report of the joint US-Japan Working Group. Hiroshima: 2005. [Google Scholar]
- Simon SL, Luckyanov N, Bouville A, VanMiddlesworth L, Weinstock RM. Transfer of I-131 into human breast milk and transfer coefficients for radiological dose assessments. Health Phys. 2002;82:796–806. doi: 10.1097/00004032-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Shibata Y, editors. Sasakawa Memorial Health Foundation. Chornobyl: A Decade. Amsterdam: Elsevier; 1997. [Google Scholar]
- Simon SL, Anspaugh LR, Hoffman FO, Scholl AE, Stone MB, Thomas BA, Lyon JL. 2004 update of dosimetry for the Utah Thyroid Cohort Study. Radiat Res. 2006;165: 208–222. doi: 10.1667/rr3483.1. [DOI] [PubMed] [Google Scholar]
- Stezhko VA, Buglova EE, Danilova LI, Drozd VM, Krysenko NA, Lesnikova NR, Minenko VF, Ostapenko VA, Petrenko SV, Polyanskaya ON, Rzheutski VA, Tronko MD, Bobylyova OO, Bogdanova TI, Ephstein OV, Kairo IA, Kostin OV, Likhtarev IA, Markov VV, Oliynyk VA, Shpak VM, Tereshchenko VP, Zamotayeva GA, Beebe GW, Bouville AC, Brill AB, Burch JD, Fink DJ, Greenebaum E, Howe GR, Luckyanov NK, Masnyk IJ, McConnell RJ, Robbins J, Thomas TL, Voillequé PG, Zablotska LB Chornobyl Thyroid Diseases Study Group of Belarus, Ukraine, and the USA 2004. A cohort study of thyroid cancer and other thyroid diseases following the Chornobyl accident: objectives, design, and methods. Radiat Res. 2004;161:481–492. doi: 10.1667/3148. [DOI] [PubMed] [Google Scholar]
- Talerko N. Mesoscale modelling of radioactive contamination formation in Ukraine caused by the Chornobyl accident. J Environ Radioact. 2005a;78:311–329. doi: 10.1016/j.jenvrad.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Talerko N. Reconstruction of 131I radioactive contamination in Ukraine caused by the Chornobyl accident using atmospheric transport modeling. J Environ Radioact. 2005b;84:343–362. doi: 10.1016/j.jenvrad.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Tronko MD, Howe GR, Bogdanova TI, Bouville AC, Epstein OV, Brill AB, Likhtarev IA, Fink DJ, Markov VV, Greenebaum E, Olijnyk VA, Masnyk IJ, Shpak VM, McConnell RJ, Tereshchenko VP, Robbins J, Zvinchuk OV, Zablotska LB, Hatch M, Luckyanov NK, Ron E, Thomas TL, Voillequé PG, Beebe GW. A cohort study of thyroid cancer and other thyroid diseases after the Chornobyl accident: thyroid cancer in Ukraine detected during first screening. JNCI. 2006;98:896–903. doi: 10.1093/jnci/djj244. [DOI] [PubMed] [Google Scholar]
- United Nations Scientific Committee on the Effects of Atomic Radiation. United Nations sales publication E.00.IX.3. New York: United Nations; 2000. Sources and Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation. [Google Scholar]
- U.S. Nuclear Regulatory Commission. US Nuclear Regulatory Commission Office of Nuclear Regulatory Research report NUREG/CR-6825, PNNL-14321. Washington, DC: 2003. Literature review and assessment of plant and animal transfer factors used in performance assessment modeling. [Google Scholar]