Abstract
Xerostomia is a common complaint of nearly half of the elderly population and about one-fifth of younger adults. It causes several signs and symptoms, and compromise oral functions and health-related quality-of-life. Multiple reasons are proposed to describe the etiology of xerostomia such as local factors, psychogenic factors, and systemic diseases. In order to manage xerostomia effectively, identification of the main causality is mandatory. The aim of this review was to present systemic diseases leading to xerostomia with their mechanisms of action. We used various general search engines and specialized databases such as Google, Google Scholar, Yahoo, PubMed, PubMed Central, MedLine Plus, Medknow, EBSCO, ScienceDirect, Scopus, WebMD, EMBASE, and authorized textbooks to find relevant topics by means of Medical Subject Headings keywords such as “xerostomia,” “hyposalivations,” “mouth dryness,” “disease,” and “systemic.” We appraised 97 English-language articles published over the last 40 years in both medical and dental journals including reviews, meta-analysis, original papers, and case reports. Upon compilation of relevant data, it was concluded that autoimmune diseases most frequently involve salivary glands and cause xerostomia followed by diabetes mellitus, renal failure, and graft-versus-host disease. Moreover, the underlying mechanisms of systemic disease-related xerostomia are: autoimmunity, infiltration of immunocompetent cells, granuloma formation, fibrosis and dehydration, deposition of proteinaceous substances, bacterial infection, and side-effects of medications.
Keywords: Disease, Hyposalivations, Mouth dryness, Systemic, Xerostomia
Introduction
Xerostomia is the subjective complaint of oral dryness, while salivary gland hypofunction is an objective matter characterized by reduced salivary flow.[1] These two terms are often incorrectly used interchangeably.
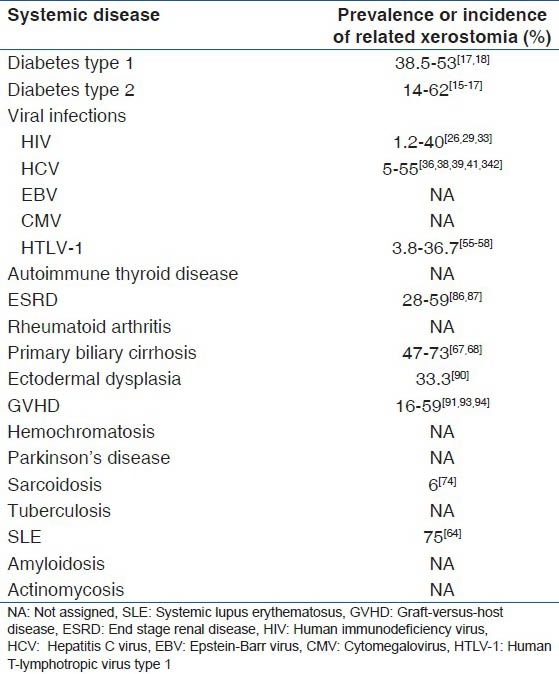
Xerostomia is a frequent annoying condition. It is estimated that 12-47% of the elderly and 10-19.3% of people in their early 30's have been suffering from dry mouth.[2,3,4] The symptoms of xerostomia are as follows: Cracked peeled atrophic lips, glossitis, progressive cervical, or cusp tip caries even with optimum oral hygiene, candidiasis, and pale corrugated dry buccal mucosa. The size, texture and tenderness of salivary glands should be assessed. Xerostomia can lead to dysphagia, dysgeusia, oral pain, dental caries, oral infection, periodontal disease, and finally can affect the health-related quality-of-life.[5,6,7,8] Malnutrition and psychosocial problems could be associated with dry mouth as well.[8] The basis of xerostomia is the alteration in both quantitative and qualitative function of salivary glands.[6] There are multiple causes with various mechanisms of xerostomia such as systemic diseases, anticholinergic effects of many drugs, psychological conditions, alcohol, head and neck radiation therapy, and physiological changes, but xerostomia-related systemic diseases have not been addressed as much as they worth it.[9] In order to better understanding, diseases which cause xerostomia, the underlying mechanisms and the incidence or prevalence of dry mouth due to systemic conditions are summarized in Tables 1 and 2. Multiple methods have been described to manage xerostomia. Saliva substitutes, topical stimulants, and parasympathetic agonists such as pilocarpine and cevimeline are approved medications to treat xerostomia.[10] Early detection of these diseases may aid to timely treatment of xerostomia. Some of these systemic conditions are so severe that distract the attention of health care workers away from the complications such as xerostomia, which might cause additional discomfort for the patient.
Table 1.
Mechanisms of xerostomia due to systemic diseases

Table 2.
Incidence or prevalence of xerostomia due to systemic diseases
The aim of this study was to describe systemic diseases leading to xerostomia to provide physicians and dentists with an update and comprehensive source for their clinical practice.
Methods of Literature Search
We used various general search engines such as Google, Google Scholar, and Yahoo as well as bibliographic databases such as PubMed, PubMed Central, Medline Plus, Medknow, EBSCO, ScienceDirect, Scopus, WebMD, EMBASE, and three authorized textbooks to find relevant topics by means of medical subject headings keywords such as “xerostomia,” “hyposalivations,” “mouth dryness,” “disease,” and “systemic.” The search was accomplished in 2013 and limited to English-language articles published over the last 40 years in both medical and dental journals. Totally, 258 articles were identified. After provisional assessment of the titles and abstracts by two reviewers, 106 articles were found to be relevant to the topic, and out of them 97 were available for us including 20 reviews and meta-analysis, 59 original papers, and 18 case reports regarding systemic disease resulting to xerostomia. Our review included articles published between 1997-2013, in the years of 1974, 1980, 1983, 1987, 1989, and 1990.
Results
After compilation of information from relevant articles and updated textbooks, we categorized systemic diseases resulting in xerostomia to endocrine diseases, viral infections, bacterial infections, autoimmune diseases, granulomatous diseases, storage diseases, and some other unclassified diseases as follows. Meanwhile, the underlying mechanisms of xerostomia due to systemic diseases, and the incidence or prevalence of xerostomia in each disease were summarized in Tables 1 and 2.
Endocrine diseases
Diabetes mellitus
Diabetes mellitus is an endocrine disease characterized by the deficit in production of insulin with consequent alteration of metabolism and balance of glucose concentration. According to its etiology, it is classified as Type 1 and 2.[11] Type 1 diabetes mellitus is a metabolic dysfunction characterized by hyperglycemia resulting from definite shortage of insulin secretion caused by autoimmune illness and genetic factors.[12] Type 2 diabetes mellitus (formerly known as non-insulin-dependent diabetes mellitus) is the most common form of disease featured by hyperglycemia, insulin resistance, and relative insulin deficiency. Type 2 diabetes results from interaction between genetic, environmental and behavioral risk factors.[13] It is estimated that there will be 380 million persons with diabetes mellitus in 2025.[14]
Patients with uncontrolled diabetes often report dry mouth, which is believed to be due to polyuria, dehydration, and autonomic dehydration.[11] The prevalence of xerostomia was reported in 14-62% of diabetes mellitus 2 cases,[15,16,17] and it was found in 38.5% and 53% of children and adolescents subjects with diabetes mellitus 1, respectively.[17,18]
Thyroid disease
Autoimmune thyroid disease, including Graves’ disease and Hashimoto's thyroiditis, is one of the most common immune-mediated conditions.[19] Autoimmune thyroid disease is characterized by the presence of serum antibodies against thyroid-specific or thyroid-restricted antigens like the thyroid stimulating hormone receptor, thyroperoxidase, and thyroglobulin).[20] The prevalence of autoimmune thyroid disease in the general population varies among countries. Prevalence has been estimated as 5-15% in women and 1-5% in men. In other words, autoimmune thyroid disease can be regarded as the most common autoimmune endocrine disease.[21] A considerable number of patients with primary Sjögren's syndrome (pSS) along with thyroid disease diagnosed by laboratory data and clinical presentation were reported.[22] The coexistence of Sjögren's syndrome and thyroiditis is frequent suggesting a common genetic or environmental predisposing factor with similar pathogenic mechanisms. pSS was reported to be 10 times more frequent in patients with autoimmune thyroid disease, while autoimmune thyroiditis was 9 times more frequent in pSS. Therefore, Sjögren's syndrome should be studied in patients with thyroid disease and vice versa.[23]
Viral infections
Human immunodeficiency virus
Human immunodeficiency virus (HIV) infection is one of the most devastating infections in modern times. Oral manifestations of HIV infection occur in approximately 30-80% of patients.[24] Oral lesions are among the early signs of HIV infection and can predict progression to acute immunodeficiency syndrome. The more common HIV-related lesions include oral candidiasis, herpes simplex infection, oral Kaposi's sarcoma, oral hairy leukoplakia, parotid gland enlargement, periodontal diseases (linear gingival erythema and necrotizing ulcerative periodontitis), human papilloma virus-associated warts, and ulcerative conditions including herpes simplex virus lesions, recurrent aphthous ulcers, neutropenic ulcers, and xerostomia.[25,26] Xerostomia is due to the side effects of HIV medications (e.g., didanosine) or the proliferation of CD8 + cells in the major salivary glands.[26] Parotid hypertrophy as a compensative reaction has been also found more commonly in HIV-positive children.[27,28] Xerostomia has been estimated as 1.2-40% in HIV-positive patients.[26,29,30,31,32,33] Although the role of HIV on xerostomia is relatively clear, there is a controversy about xerostomia prevalence as demonstrated by Sontakke et al., Pinheiro et al., and Nittayananta et al.[32,33,34] There is another conflict about xerostomia-related HIV medications in the literature, for example Lin claimed that xerostomia was not compounded by medications.[35] In contrast, some researchers believe that taking anti-HIV drugs is effective on the prevalence of xerostomia.[26,32]
Hepatitis C virus
The hepatitis C virus (HCV) is a linear, single-stranded RNA virus of the Flaviviridae family that was first identified in 1989.[36] HCV infection is a major health problem among the general population, and its extrahepatic manifestations have also been reported like Sicca syndrome.[37] Several autoimmune and immune complex-mediated disorders have been proposed to be related to HCV infection such as essential mixed cryoglobulinemia, which is frequently associated with Sjögren's syndrome. The association between HCV and Sjögren's syndrome may be related to the following reasons: (1) close association between HCV infection and mixed cryoglobulinemia, (2) the salivary gland tropism of HCV.[38] However, some studies did not find any relationship between xerostomia and the presence of HCV infection.[39,40] Xerostomia has been found among 5-55% of HCV-infected patients.[36,38,39,41,42] Xerostomia is also an adverse event during ribavirin-interferon therapy.[43,44]
Epstein-Barr virus
Epstein-Barr virus (EBV) is a human herpes virus that establishes long-term latent infection in B-lymphocytes named EBV infectious mononucleosis (EBV-IM).[45] EBV-IM is a common infection that affects 25-30% of adolescents and adults up to 30 years of age.[46] Association between EBV and autoimmune diseases, such as rheumatoid arthritis (RA), systemic lupus erythematosus, multiple sclerosis, autoimmune thyroiditis, inflammatory bowel diseases, insulin-dependent diabetes mellitus, systemic sclerosis, myasthenia gravis, autoimmune liver diseases, and Sjögren's syndrome have been suggested.[47] It is proposed that the initiating event in pSS is an infection with EBV, and the autoimmune exocrinopathy that progresses to keratoconjunctivitis Sicca and xerostomia is sequelae to this process. It is noted that during EBV infection, there are multiple copies of the EBV-encoded small RNAs available to bind to the La ribonucleoprotein and when infection occurs in subjects who are genetically predisposed to autoimmunity and have an impaired T-cell-mediated response to EBV, there is a loss of immunological tolerance to La with production of anti-La (SS-B). Thus the inflammatory process in exocrine glands, which culminates in the Sicca syndrome is due to the combined effects of chronic EBV infection and autoimmunity. The mean titer of anti-EBV nuclear-antigen antibodies was significantly higher in Sjögren's syndrome patients than in normal people.[48]
Cytomegalovirus
Cytomegalovirus (CMV) is a common infection with a seroprevalence among adolescents ranging from 47% to 89%.[49] The persistence of CMV with alteration of cell surface expression in certain tissues may initiate the tissue destruction that leads to the clinical manifestations of Sjögren's syndrome. Ductal cells of salivary and lacrimal glands are immunologically attacked due to CMV antigenic expression. The destruction of these ducts leads to xerostomia.[50] However, no relationship between xerostomia and anti-CMV antibodies was noted.[51]
Human T-lymphotropic virus type 1
Human T-lymphotropic virus Type 1 (HTLV-1) is known to cause HTLV-associated myelopathy (HAM)/tropical spastic paraparesis and adult T-cell leukemia.[52] It is estimated that 15-20 million persons are currently infected with HTLV-1 worldwide.[53]
Retroviruses such as HTLV-1 and HIV infect immunocompetent cells, resulting in the destruction or overstimulation of T-cells, and act as potential triggers for autoimmune disease.[54]
Previous studies reported a high prevalence rate of anti-HTLV-1 antibodies in the peripheral blood in 3.8-36.7% of patients with Sjögren's Syndrome.[55,56,57,58]
Bacterial infections
Actinomycosis
Actinomycosis is an anaerobic bacterial infection affecting men more frequently between the ages of 30-60 years. Almost half of actinomycosis cases occur in the cervicofacial region, and salivary glands may be involved as well. The organism can colonize inside the ducts of both submandibular and parotid glands and leads to abscess formation in the submandibular and masseter spaces, respectively.[59,60,61]
Autoimmune diseases
Rheumatoid arthritis
Rheumatoid arthritis is a systemic disease of connective tissue origin, which affects 1% of the world population. Women have a 3-fold higher incidence than do men. RA frequently presents with extra-articular features such as hematologic, neurologic, and cardiovascular involvement concomitant with dysfunction of lacrimal and salivary glands. Zalewska et al. showed impairment of salivary immunity system of the oral cavity in xerostomic patients with RA.[62] Secondary Sjögren's syndrome is associated with xerostomia and occurs with autoimmune diseases most frequently with RA.[63]
Systemic lupus erythematosus
Systemic lupus erythematosus (SLE) is an inflammatory connective tissue disease with characteristic autoantibodies. SLE is much more common in women than men. It may occur at any age, but appears most often in people between the ages of 10 and 50. More than 75% of patients with SLE are affected with xerostomia. Coexistence of Sjögren's syndrome and SLE has been found in 1/3 of SLE patients. SLE has been shown to be associated with a decreased unstimulated salivary flow rate.[64]
Primary biliary cirrhosis
Primary biliary cirrhosis (PBC) is a cholestatic autoimmune disease predominantly of middle-aged women with progressive destruction of interlobular bile ducts.[65] The most autoimmune disease in PBC patients is Sjögren's syndrome[66] whose symptoms have been observed in 47-73% of patients[67,68] Xerostomia as well as dysphagia seems to be associated with PBC.[69]
Scleroderma
Progressive systemic sclerosis or scleroderma is a chronic sclerotic disease with deposition of extracellular matrix throughout connective tissue and vascular abnormalities, which leads to tissue hypoxia.[70] Fibrosis of capillaries, excretory ducts and acini of salivary and lacrimal glands are associated with xerostomia as oral manifestations of scleroderma.[71] Lymphocytic infiltration has been observed among 15% of patients with systemic sclerosis, which is a sign of secondary Sjögren's syndrome.[72]
Granulomatous diseases
Sarcoidosis
Sarcoidosis is a systemic inflammatory disease with unknown etiology characterized by the presence of noncaseating granulomas that can affect any organ (mostly lungs and lymph nodes).[73] Coexistence of parotid and submandibular gland swelling and xerostomia has been reported in sarcoidosis patients.[74,75,76] Mansour et al., identified five patients representing both clinical and histological features of Sjögren's syndrome and sarcoidosis, suggesting inclusion of sarcoidosis as diagnostic criteria for Sjögren's syndrome.[74]
The salivary glands could be affected by sarcoidosis as well, which was reported in 6% of the cases. Parotid salivary gland enlargement was also detected in 6% of the patients.[74] Parotid gland enlargement in patients presenting with Sicca symptoms is believed to be of clinical significance. Such finding might be more likely associated with sarcoidosis, especially in patients presenting with negative serologic profiles.[74]
Tuberculosis
Tuberculosis (TB) is a chronic bacterial infection, caused by Mycobacterium TB leading to formation of granulomas in infected tissues. The lungs are most commonly affected, but other tissues, including the salivary glands, may be involved. Patients with TB may experience xerostomia and/or salivary gland swelling, with granuloma or cyst formation within the affected glands. Salivary gland enlargement usually presents as part of a characteristic symptom complex, however salivary gland changes have been reported in the absence of systemic symptoms.[77]
Granulomatous diseases such as sarcoidosis and TB may cause salivary gland hypofunction and lead to xerostomia.[78]
Storage diseases
Hemochromatosis
Hemochromatosis is defined as a pathological condition with iron overload in vital organs with a hereditary/primary cause.[79] Organs commonly affected by hemochromatosis are liver, heart, and endocrine glands. Iron deposition in salivary glands causes hyposalivation. Patients with normal ferritin level had normal salivary flow rate, whereas those with high levels of ferritin showed decreased stimulatory salivary flow rate.[79,80]
Amyloidosis
Amyloidosis is characterized by deposition of an extracellular protein-like material called amyloid. Amyloidosis causes various effects on different organs with a variety of extensions. In addition, amyloidosis may be associated with multiple myeloma or chronic infections. Amyloidosis may be accompanied with oral involvement in the form of macroglossia (10-40%), oral amyloid nodules, and dry mouth due to amyloid infiltration and destruction of salivary glands.[81] A case of pSS manifested as localized cutaneous nodular amyloidosis has been reported.[82] Meanwhile, a relationship between amyloidosis and xerostomia has been documented.[82,83,84]
Others
End-stage renal disease
End-stage renal disease (ESRD) represents a clinical state or condition with irreversible loss of the endogenous renal function to a degree, which is sufficient to render the patient permanently dependent upon renal replacement therapy in the form of dialysis or kidney transplantation. ESRD leads to accumulation of certain toxic elements, which affects normal functions of the body, and may have significant complications including cardiovascular disease, immune deficiency, anemia, renal function impairment, and bone disease.[85] Xerostomia was found in 28-59% of ESRD patients due to inability of kidneys to reabsorb sodium and the resultant polyuria.[86,87]
Ectodermal dysplasia
Ectodermal dysplasia is a hereditary disease causing anomalies in tissues of ectodermal origin. The significance of this disease lies in severe hypodontia, and an accompanying hypoplasia of the alveolar process. The clinical condition is aggravated by a significant xerostomia as a result of salivary gland aplasia or hypoplasia.[88,89] However, in some patients with ectodermal dysplasia with the presence of salivary glands, hyposalivation have been reported. In a study of 39 patients with ectodermal dysplasia, salivary flow rate was decreased in 13 (33.3%) patients.[90]
Hematopoietic stem cell transplantation and chronic graft-versus-host disease
Chronic graft-versus-host disease (cGVHD) is a multi-organ involvement that occurs post hematopoietic stem cell transplantation (HSCT), with the mouth being one of the most frequently affected sites.[91] The pathogenesis of GVHD is based on donor graft T-lymphocytes that recognize antigenic disparities between donor and recipient and the dysregulation of a broad panel of cytokines. GVHD occurs in 40-70% of patients treated by bone marrow and peripheral blood stem cell transplantation.[92] Oral manifestations are common in patients diagnosed with chronic graft-versus-host-disease.[91] Hull et al. mentioned xerostomia as the most common oral symptom in patients with history of HSCT with the majority of patients (53%) having clinical markers of oral cGVHD.[93] Noce et al. reported that 59.1% of patients diagnosed with cGVHD had salivary gland dysfunction.[91] Boer et al. showed a decrease in salivary flow rate (16% of patients) and a relation between hyposalivation intensity and elapsed time after HSCT.[94] There is similarity in oral clinical manifestations of GVHD and Sjögren's Syndrome because of the same autoimmune nature, but differences have also been found.[95] The suggested pathophysiological mechanisms of xerostomia and hyposalivation observed in GVHD are lymphocytic infiltration, parenchymal destruction, and fibrosis within salivary gland tissue.[95]
Parkinson's disease
Parkinson's disease (PD) is a relatively common, progressive, debilitating, and neurological disorder. Cardinal symptoms are resting tremor, bradykinesia, akinesia, restricted mobility, and postural instability. Levodopa (L-DOPA) has been used as a primary drug for over 30 years. L-DOPA is converted into dopamine in the dopaminergic neurons by DOPA decarboxylase enzyme. Proulx et al. have reported that patients with PD produce less saliva than normal. Factors influencing the production of saliva include the use of levodopa and female gender.[96] Hyposialorrhea is an early autonomic manifestation of PD.[97]
Conclusion
Salivary glands are involved due to many systemic diseases with the resultant complication of xerostomia. Autoimmune diseases, diabetes mellitus, ESRD, and GVHD are frequently associated with salivary hypofunction. The underlying mechanism of xerostomia differs in terms of disease. Autoimmunity accounts for xerostomia related to SLE, RA, PBC, thyroid disease, and some viral infections. Some conditions affect salivary glands through infiltration of immunocompetent cells or granuloma formation such as HIV infection, GVHD, sarcoidosis, and TB. Polyuria and dehydration is responsible for dry mouth associated with diabetes and end-stage renal failure, while GVHD and scleroderma cause xerostomia because of fibrosis. Deposition of proteinaceous substances and bacterial infection are also mentioned as alternative mechanisms for xerostomia. Identification of the main reason of xerostomia helps attain timely diagnosis and more appropriate treatment plan.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Thomson WM, Chalmers JM, Spencer AJ, Williams SM. The Xerostomia Inventory: A multi-item approach to measuring dry mouth. Community dent health. 1999;16:12–7. [PubMed] [Google Scholar]
- 2.Thomson WM. Issues in the epidemiological investigation of dry mouth. Gerodontology. 2005;22:65–76. doi: 10.1111/j.1741-2358.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- 3.Murray Thomson W, Poulton R, Mark Broadbent J, Al-Kubaisy S. Xerostomia and medications among 32-year-olds. Acta Odontol Scand. 2006;64:249–54. doi: 10.1080/00016350600633243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guggenheimer J, Moore PA. Xerostomia etiology, recognition and treatment. JADA. 2003;134:61–9. doi: 10.14219/jada.archive.2003.0018. [DOI] [PubMed] [Google Scholar]
- 5.Fox PC. Xerostomia: Recognition and management. Dent Assist. 2008;77:44–8. [PubMed] [Google Scholar]
- 6.Grötz K, Genitsariotis S, Vehling D, Al-Nawas B. Long-term oral Candida colonization, mucositis and salivary function after head and neck radiotherapy. Support Care Cancer. 2003;11:717–21. doi: 10.1007/s00520-003-0506-0. [DOI] [PubMed] [Google Scholar]
- 7.Momm F, Volegova-Neher NJ, Schulte-Mönting J, Guttenberger R. Different saliva substitutes for treatment of xerostomia following radiotherapy. Strahlenther Onkol. 2005;181:231–6. doi: 10.1007/s00066-005-1333-7. [DOI] [PubMed] [Google Scholar]
- 8.Turner M, Jahangiri L, Ship JA. Hyposalivation, xerostomia and the complete denture. A systematic review. JADA. 2008;139:146–50. doi: 10.14219/jada.archive.2008.0129. [DOI] [PubMed] [Google Scholar]
- 9.Daniels TE. Evaluation, differential diagnosis, and treatment of xerostomia. J Rheumatol Suppl. 2000;61:6. [PubMed] [Google Scholar]
- 10.Rayman S, Dincer E, Almas K. Xerostomia: Diagnosis and management in dental practice. N Y State Dent J. 2010;76:24–7. [PubMed] [Google Scholar]
- 11.Vasconcelos ACU, Soares MSM, Almeida PC, Soares TC. Comparative study of the concentration of salivary and blood glucose in type 2 diabetic patients. J Oral Sci. 2010;52:293–8. doi: 10.2334/josnusd.52.293. [DOI] [PubMed] [Google Scholar]
- 12.Bakianian Vaziri P, Vahedi M, Mortazavi H, Abdollahzadeh Sh, Hajilooi M. Evaluation of salivary glucose, IgA and flow rate in diabetic patients: A case-control study. J Dent (Tehran) 2010;7:13–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Olokoba AB, Obateru OA, Olokoba LB. Type 2 diabetes mellitus: a review of current trends. Oman Med J. 2012;27:269. doi: 10.5001/omj.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16:S27. doi: 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bajaj S, Prasad S, Gupta A, Singh VB. Oral manifestations in type-2 diabetes and related complications. Indian J Endocrinol Metab. 2012;16:777–9. doi: 10.4103/2230-8210.100673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khovidhunkit S-oP, Suwantuntula T, Thaweboon S, Mitrirattanakul S, Chomkhakhai U, Khovidhunkit W. Xerostomia, hyposalivation, and oral microbiota in type 2 diabetic patients: A preliminary study. J Med Assoc Thai. 2009;92:1220–8. [PubMed] [Google Scholar]
- 17.Busato IMS, Ignácio SA, Brancher JA, Grégio AMT, Machado MÂN, Azevedo-Alanis LR. Impact of xerostomia on the quality of life of adolescents with type 1 diabetes mellitus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:376–82. doi: 10.1016/j.tripleo.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Costa CC, Resende GB, Souza JM, Tavares SS, Almeida IC. Study of the oral manifestations in diabetic children and their correlation variables. Arq Bras Endocrinol Metabol. 2004;48:374–8. doi: 10.1590/s0004-27302004000300007. [DOI] [PubMed] [Google Scholar]
- 19.Cooper JD, Simmonds MJ, Walker NM, Burren O, Brand OJ, Guo H, et al. Seven newly identified loci for autoimmune thyroid disease. Hum Mol Genet. 2012;21:5202–8. doi: 10.1093/hmg/dds357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hutfless S, Matos P, Talor MV, Caturegli P, Rose NR. Significance of prediagnostic thyroid antibodies in women with autoimmune thyroid disease. J Clin Endocrinol Metab. 2011;96:E1466–71. doi: 10.1210/jc.2011-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cárdenas Roldán J, Amaya-Amaya J, Castellanos-de la Hoz J, Giraldo-Villamil J, Montoya-Ortiz G, Cruz-Tapias P, et al. Autoimmune thyroid disease in rheumatoid arthritis: A global perspective. Arthritis 2012. 2012:864907. doi: 10.1155/2012/864907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karsh J, Pavlidis N, Weintraub BD, Moutsopoulos HM. Thyroid disease in Sjögren's syndrome. Arthritis Rheum. 1980;23:1326–9. doi: 10.1002/art.1780231118. [DOI] [PubMed] [Google Scholar]
- 23.D’Arbonneau F1, Ansart S, Le Berre R, Dueymes M, Youinou P, Pennec YL. Thyroid dysfunction in primary Sjögren's syndrome: A long-term followup study. Arthritis Rheum. 2003;49:804–9. doi: 10.1002/art.11460. [DOI] [PubMed] [Google Scholar]
- 24.Okoje V, Obiechina A, Aken’Ova Y. Orofacial lesions in 126 newly diagnosed HIV/AIDS patients seen at the University College Hospital, Ibadan. Afr J Med Med Sci. 2006;35:97–101. [PubMed] [Google Scholar]
- 25.Nokta M. Oral manifestations associated with HIV infection. Curr HIV/AIDS Rep. 2008;5:5–12. doi: 10.1007/s11904-008-0002-8. [DOI] [PubMed] [Google Scholar]
- 26.Reznik D. Oral manifestations of HIV disease. Top HIV Med. 2005;13:143–8. [PubMed] [Google Scholar]
- 27.Expósito-Delgado A, Vallejo-Bolaños E, Martos-Cobo E. Oral manifestations of HIV infection in infants: A review article. Med Oral Patol Oral Cir Bucal. 2004;9:415. [PubMed] [Google Scholar]
- 28.Pinto A, De Rossi SS. Salivary gland disease in pediatric HIV patients: an update. J Dent Child (Chic) 2004;71:33–7. [PubMed] [Google Scholar]
- 29.Sharma G, Pai K, Suhas S, Ramapuram J, Doshi D, Anup N. Oral manifestations in HIV/AIDS infected patients from India. Oral Dis. 2006;12:537–42. doi: 10.1111/j.1601-0825.2006.01232.x. [DOI] [PubMed] [Google Scholar]
- 30.Taiwo O, Okeke E, Jalo P, Danfillo I. Oral manifestation of HIV/AIDS in Plateau state indigenes, Nigeria. West Afr J Med. 2006;25:32–7. doi: 10.4314/wajm.v25i1.28242. [DOI] [PubMed] [Google Scholar]
- 31.Freed JR, Marcus M, Freed BA, Der-Martirosian C, Maida CA, YOUNAI FS, et al. Oral health findings for HIV-infected adult medical patients from the HIV Cost and Services Utilization Study. JADA. 2005;136:1396–405. doi: 10.14219/jada.archive.2005.0053. [DOI] [PubMed] [Google Scholar]
- 32.Sontakke SA, Umarji H, Karjodkar F. Comparison of oral manifestations with CD4 count in HIV-infected patients. Indian J Dent Res. 2011;22:732. doi: 10.4103/0970-9290.93470. [DOI] [PubMed] [Google Scholar]
- 33.Pinheiro A, Marcenes W, Zakrzewska J, Robinson P. Dental and oral lesions in HIV infected patients: a study in Brazil. Int Dent J. 2004;54:131–7. doi: 10.1111/j.1875-595x.2004.tb00268.x. [DOI] [PubMed] [Google Scholar]
- 34.Nittayananta W, Chanowanna N, Jealae S, Nauntofte B, Stoltze K. Hyposalivation, xerostomia and oral health status of HIV-infected subjects in Thailand before HAART era. J Oral Pathol Med. 2010;39:28–34. doi: 10.1111/j.1600-0714.2009.00826.x. [DOI] [PubMed] [Google Scholar]
- 35.Lin A, Johnson D, Stephan K, Yeh C-K. Alteration in salivary function in early HIV infection. J Dent Res. 2003;82:719–24. doi: 10.1177/154405910308200912. [DOI] [PubMed] [Google Scholar]
- 36.Ramos-Casals M, Trejo O, García-Carrasco M, Font J. Therapeutic management of extrahepatic manifestations in patients with chronic hepatitis C virus infection. Rheumatology (Oxford) 2003;42:818–28. doi: 10.1093/rheumatology/keg299. [DOI] [PubMed] [Google Scholar]
- 37.Prunoiu C, Georgescu EF, Georgescu M, Simionescu C. Sjogren's syndrome associated with chronic hepatitis C-the benefit of the antiviral treatment. Rom J Morphol Embryol. 2008;49:557–62. [PubMed] [Google Scholar]
- 38.Grossmann SdMC, Teixeira R, de Oliveira GC, Gleber-Netto FO, Araújo FMG, Araújo FM, et al. Xerostomia, hyposalivation and sialadenitis in patients with chronic hepatitis C are not associated with the detection of HCV RNA in saliva or salivary glands. J Clin Pathol. 2010;63:1002–7. doi: 10.1136/jcp.2010.080036. [DOI] [PubMed] [Google Scholar]
- 39.de Mattos Camargo Grossmann S, Teixeira R, de Oliveira GC, do Carmo MAV. Detection of HCV RNA in saliva does not correlate with salivary flow or xerostomia in patients with chronic hepatitis C. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:851–6. doi: 10.1016/j.tripleo.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Carrozzo M, Gandolfo S. Oral diseases possibly associated with hepatitis C virus. Crit Rev Oral Biol Med. 2003;14:115–27. doi: 10.1177/154411130301400205. [DOI] [PubMed] [Google Scholar]
- 41.Nawito Z, Amin A, El-Fadl SA, El Einen KA. Sicca complex among Egyptian patients with chronic hepatitis C virus infection. Clin Rheumatol. 2011;30:1299–304. doi: 10.1007/s10067-011-1746-x. [DOI] [PubMed] [Google Scholar]
- 42.Giordano N, Amendola A, Papakostas P, Cipolli F, Agate VM, Battisti E, et al. Immune and autoimmune disorders in HCV chronic liver disease: Personal experience and commentary on literature. New Microbiol. 2005;28:311–7. [PubMed] [Google Scholar]
- 43.Nagao Y, Hashimoto K, Sata M. Candidiasis and other oral mucosal lesions during and after interferon therapy for HCV-related chronic liver diseases. BMC Gastroenterol. 2012;12:155. doi: 10.1186/1471-230X-12-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aghemo A, Rumi MG, Monico S, Banderali M, Russo A, Ottaviani F, et al. Ribavirin impairs salivary gland function during combination treatment with pegylated interferon alfa-2a in hepatitis C patients. Hepat Mon. 2011;11:918–24. doi: 10.5812/kowsar.1735143X.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arvey A, Tempera I, Lieberman PM. Interpreting the Epstein-Barr Virus [EBV] Epigenome Using High-Throughput Data. Viruses. 2013;5:1042–54. doi: 10.3390/v5041042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hallee TJ, Evans AS, Niederman JC, Brooks CM, Voegtly JH. Infectious mononucleosis at the United States Military Academy. A prospective study of a single class over four years. Yale J Biol Med. 1974;47:182–95. [PMC free article] [PubMed] [Google Scholar]
- 47.Lossius A, Johansen JN, Torkildsen Ø, Vartdal F, Holmøy T. Epstein-Barr Virus in Systemic Lupus Erythematosus, Rheumatoid Arthritis and Multiple Sclerosis—Association and Causation. Viruses. 2012;4:3701–30. doi: 10.3390/v4123701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fox R, Chilton T, Scott S, Benton L, Howell F, Vaughan J. Potential role of Epstein-Barr virus in Sjögren's syndrome. Rheum Dis Clin North Am. 1987;13:275–92. [PubMed] [Google Scholar]
- 49.Stadler LP, Bernstein DI, Callahan ST, Ferreira J, Simone GAG, Edwards KM, et al. Seroprevalence of cytomegalovirus [CMV] and risk factors for infection in adolescent males. Clin Infect Dis. 2010;51:e76–81. doi: 10.1086/656918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Burns J. Persistent Cytomegalovirus infection-The etiology of Sjogren's Syndrome. Med Hypotheses. 1983;10:451–60. doi: 10.1016/0306-9877(83)90011-7. [DOI] [PubMed] [Google Scholar]
- 51.Scully C. Sjögren's syndrome: no demonstrable association by serology of secondary Sjögren's syndrome with cytomegalovirus. J Oral Pathol Med. 1990;19:43–4. doi: 10.1111/j.1600-0714.1990.tb00780.x. [DOI] [PubMed] [Google Scholar]
- 52.Poetker SK, Porto AF, Giozza SP, Muniz AL, Caskey MF, Carvalho EM, et al. Clinical manifestations in individuals with recent diagnosis of HTLV type I infection. J Clin Virol. 2011;51:54–8. doi: 10.1016/j.jcv.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Proietti FA, Carneiro-Proietti ABF, Catalan-Soares BC, Murphy EL. Global epidemiology of HTLV-I infection and associated diseases. Oncogene. 2005;24:6058–68. doi: 10.1038/sj.onc.1208968. [DOI] [PubMed] [Google Scholar]
- 54.Manns MP, Rambusch EG. Autoimmunity and extrahepatic manifestations in hepatitis C virus infection. J Hepatol. 1999;31(Suppl 1):39–42. doi: 10.1016/s0168-8278(99)80372-9. [DOI] [PubMed] [Google Scholar]
- 55.Obermayer-Straub P, Manns MP. Hepatitis C and D, retroviruses and autoimmune manifestations. J Autoimmun. 2001;16:275–85. doi: 10.1006/jaut.2000.0488. [DOI] [PubMed] [Google Scholar]
- 56.Lee SJ, Lee JS, Shin MG, Tanaka Y, Park DJ, Kim TJ, et al. Detection of HTLV-1 in the labial salivary glands of patients with Sjogren's syndrome: A distinct clinical subgroup? J Rheumatol. 2012;39:809–15. doi: 10.3899/jrheum.111075. [DOI] [PubMed] [Google Scholar]
- 57.Hida A, Kawabe Y, Kawakami A, Migita K, Tominaga M, Nakamura H, et al. HTLV-I associated Sjögren's syndrome is aetiologically distinct from anti-centromere antibodies positive Sjögren's syndrome. Ann Rheum Dis. 1999;58:320–2. doi: 10.1136/ard.58.5.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ohyama Y, Nakamura S, Hara H, Shinohara M, Sasaki M, Ikebe-Hiroki A, et al. Accumulation of human T lymphotropic virus type I-infected T cells in the salivary glands of patients with human T lymphotropic virus type I-associated Sjogren's syndrome Arthritis Rheum. 1998;41:1972–8. doi: 10.1002/1529-0131(199811)41:11<1972::AID-ART12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 59.Neville BW, Damm DD, Allen CM, Bouqout JE. St Louis: Saunders Elsevier; 2009. Oral and maxillofacial pathology; p. 203. [Google Scholar]
- 60.Mamais C, Dias A, Walker J, Vydianath SR. Parotid actinomycosis mimicking metastatic lymphadenopathy. West Indian Med J. 2011;60:349–50. [PubMed] [Google Scholar]
- 61.Sittitrai P, Srivanitchapoom C, Pattarasakulchai T, Lekawanavijit S. Actinomycosis presenting as a parotid tumor. Auris Nasus Laryn×. 2012;39:241–3. doi: 10.1016/j.anl.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 62.Zalewska A, Knaś M, Waszkiewicz N, Waszkiel D, Sierakowski S, Zwierz K. Rheumatoid arthritis patients with xerostomia have reduced production of key salivary constituents. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:483–90. doi: 10.1016/j.oooo.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 63.Anurag Gupta B, Epstein JB, Sroussi H. Hyposalivation in elderly patients. J Can Dent Assoc. 2006;72:841–6. [PubMed] [Google Scholar]
- 64.Jensen J, Bergem H, Gilboe IM, Husby G, Axell T. Oral and ocular sicca symptoms and findings are prevalent in systemic lupus erythematosus. J Oral Pathol Med. 1999;28:317–22. doi: 10.1111/j.1600-0714.1999.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 65.Anurag Gupta B, Epstein JB, Sroussi H. Hyposalivation in elderly patients. J Can Dent Assoc. 2006;72:841–6. [PubMed] [Google Scholar]
- 66.Karp JK, Akpek EK, Anders RA. Autoimmune hepatitis in patients with primary Sjögren's syndrome: A series of two-hundred and two patients. Int J Clin Exp Pathol. 2010;25(3):582–6. [PMC free article] [PubMed] [Google Scholar]
- 67.Kumagi T, Heathcote EJ. Primary biliary cirrhosis. Orphanet J Rare Dis. 2008;23(3):1. doi: 10.1186/1750-1172-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Selmi C, Meroni PL, Gershwin ME. Primary biliary cirrhosis and Sjögren's syndrome: autoimmune epithelitis. J Autoimmun. 2012;39:34–42. doi: 10.1016/j.jaut.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mang F-W, Michieletti P, O’Rourke K, Cauch-Dudek K, Diamant N, Bookman A, et al. Primary biliary cirrhosis, sicca complex, and dysphagia. Dysphagia. 1997;12:167–70. doi: 10.1007/PL00009532. [DOI] [PubMed] [Google Scholar]
- 70.Cazal C, Sobral APV, Neves R, Freire-Filho F, Cardoso A, da Silveira M. Oral complaints in progressive systemic sclerosis: Two cases report. Med Oral Patol Oral Cir Bucal. 2008;13:E114–8. [PubMed] [Google Scholar]
- 71.Albilia JB, Lam DK, Blanas N, Clokie CM, Sándor GK. Small mouths… Big problems? A review of scleroderma and its oral health implications. J Can Dent Assoc. 2007;73:831–6. [PubMed] [Google Scholar]
- 72.Janin A, Gosselin B, Gosset D, Hatron P, Sauvezie B. Histological criteria of Sjögren's syndrome in scleroderma. Clin Exp Rheumatol. 1989;7:167. [PubMed] [Google Scholar]
- 73.Sekhri V, Sanal S, DeLorenzo LJ, Aronow WS, Maguire GP. Cardiac sarcoidosis: A comprehensive review. Arch Med Sci. 2011;7:546–54. doi: 10.5114/aoms.2011.24118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mansour M, Al-Hashimi I, Wright J. Coexistence of Sjögren's syndrome and sarcoidosis: A report of five cases. J Oral Pathol Med. 2007;36:337–41. doi: 10.1111/j.1600-0714.2007.00530.x. [DOI] [PubMed] [Google Scholar]
- 75.Surattanont F, Mandel L, Wolinsky B. Bilateral parotid swelling caused by sarcoidosis. JADA. 2002;133:738–41. doi: 10.14219/jada.archive.2002.0270. [DOI] [PubMed] [Google Scholar]
- 76.Poate T, Sharma R, Moutasim KA, Escudier M, Warnakulasuriya S. Orofacial presentations of sarcoidosis: A case series and review of the literature. Br Dent J. 2008;205:437–42. doi: 10.1038/sj.bdj.2008.892. [DOI] [PubMed] [Google Scholar]
- 77.Babazade F, Mortazavi H, Jalalian H. Parotid tuberculosis: A forgotten suspicion (a case report and literature review) Int J Dermatol. 2012;51:588–91. doi: 10.1111/j.1365-4632.2011.05014.x. [DOI] [PubMed] [Google Scholar]
- 78.Greenberg MS, Glick M, Ship JA. Ontario: BC Decker Inc; 2008. Burket's Oral Medicine; pp. 213–14. [Google Scholar]
- 79.Klopfleisch R, Olias P. The Pathology of comparative animal models of human haemochromatosis. J Comp Pathol. 2012;147:460–78. doi: 10.1016/j.jcpa.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 80.Sánchez-Pablo M-A, González-García V, del Castillo-Rueda A. Study of total stimulated saliva flow and hyperpigmentation in the oral mucosa of patients diagnosed with hereditary hemochromatosis. Series of 25 cases. Med Oral Patol Oral Cir Bucal. 2012;1(17):e45–9. doi: 10.4317/medoral.17206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wey SJ, Chen YM, Lai PJ, Chen DY. Primary sjögren syndrome manifested as localized cutaneous nodular amyloidosis. J Clin Rheumatol. 2011;17:368–70. doi: 10.1097/RHU.0b013e31823209ba. [DOI] [PubMed] [Google Scholar]
- 82.Elfatoiki FZ, Funck Brentano E, Blanc F, Clerici T, Saiag P. Nodular cutaneous amyloidosis associated with Sjögren's syndrome. Ann Dermatol Venereol. 2013;140:378–81. doi: 10.1016/j.annder.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 83.Juusela P, Tanskanen M, Nieminen A, Kari K, Suominen L, Uitto VJ, Kiuru-Enari S. Xerostomia in hereditary gelsolin amyloidosis. Amyloid. 2013;20:39–44. doi: 10.3109/13506129.2013.764284. [DOI] [PubMed] [Google Scholar]
- 84.Sowa T, Komatsu T, Fujinaga T, Kato T. A case of solitary pulmonary nodular amyloidosis with Sjögren's syndrome. Ann Thorac Cardiovasc Surg. 2013;19:247–9. doi: 10.5761/atcs.cr.12.01890. [DOI] [PubMed] [Google Scholar]
- 85.M SP, Rajan PM, Santhi S, Jothimalar Blood Lead in End-Stage Renal Disease Patients who were on Maintainence Haemodialysis. J Clin Diagn Res. 2012;6:1633–5. doi: 10.7860/JCDR/2012/4865.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Postorino M, Catalano C, Martorano C, Cutrupi S, Marino C, Cozzupoli P, et al. Salivary and lacrimal secretion is reduced in patients with ESRD. Am J Kidney Dis. 2003;42:722–8. doi: 10.1016/s0272-6386(03)00908-9. [DOI] [PubMed] [Google Scholar]
- 87.Dirschnabel AJ, Martins AS, Dantas S, Ribas MO, Grégio A, Alanis L, et al. Clinical oral findings in dialysis and kidney-transplant patients. Quintessence Int. 2011;42:127–33. [PubMed] [Google Scholar]
- 88.Lexner MO, Bardow A, Hertz JM, Almer L, Nauntofte B, Kreiborg S. Whole saliva in X-linked hypohidrotic ectodermal dysplasia. Int J Paediatr Dent. 2007;17:155–62. doi: 10.1111/j.1365-263X.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 89.Präger TM, Finke C, Miethke R-R. Dental findings in patients with ectodermal dysplasia. J Orofac Orthop. 2006;67:347–55. doi: 10.1007/s00056-006-0619-4. [DOI] [PubMed] [Google Scholar]
- 90.Nordgarden H, Jensen J, Storhaug K. Oligodontia is associated with extra-oral ectodermal symptoms and low whole salivary flow rates. Oral Dis. 2001;7:226–32. [PubMed] [Google Scholar]
- 91.Noce C, Gomes A, Copello A, Barbosa R, Sant’anna S, Moreira M, et al. Oral involvement of chronic graft-versus-host disease in hematopoietic stem cell transplant recipients. Gen Dent. 2011;59:458–62. quiz 463-4. [PubMed] [Google Scholar]
- 92.Nagler RM, Nagler A. Salivary gland involvement in graft-versus-host disease: The underlying mechanism and implicated treatment. Isr Med Assoc J. 2004;6:167–72. [PubMed] [Google Scholar]
- 93.Hull K, Kerridge I, Schifter M. Long-term oral complications of allogeneic haematopoietic SCT. Bone Marrow Transplant. 2012;47:265–70. doi: 10.1038/bmt.2011.63. [DOI] [PubMed] [Google Scholar]
- 94.Boer C, Correa M, Miranda E, de Souza C. Taste disorders and oral evaluation in patients undergoing allogeneic hematopoietic SCT. Bone Marrow Transplant. 2010;45:705–11. doi: 10.1038/bmt.2009.237. [DOI] [PubMed] [Google Scholar]
- 95.de la Rosa-Garcia E, Bologna-Molina R, Vega-González M, de Jesus T. Graft-versus-host disease, an eight case report and literature review. Med Oral Patol Oral Cir Bucal. 2006;11:E486–92. [PubMed] [Google Scholar]
- 96.Proulx M, De Courval FP, Wiseman MA, Panisset M. Salivary production in Parkinson's disease. Mov Disord. 2005;20:204–7. doi: 10.1002/mds.20189. [DOI] [PubMed] [Google Scholar]
- 97.Cersósimo M, Tumilasci O, Raina G, Benarroch E, Cardoso E, Micheli F, et al. Hyposialorrhea as an early manifestation of Parkinson disease. Auton Neurosci. 2009;150:150–1. doi: 10.1016/j.autneu.2009.04.004. [DOI] [PubMed] [Google Scholar]


