Abstract
Background:
Saliva represents an increasingly useful tool of diagnosis. Several factors such as salivary flow rates (SFRs) (unstimulated and stimulated) (U and S), pH, buffering capacity and consistency can be altered due to several disease processes or medications prescribed for various diseases. Alterations of SFRs, pH, buffering capacity and various ion concentrations can influence the pathogenesis of some of the oral diseases.
Aim:
Evaluation of the effect of diuretics on oral health status with regard to SFRs (U and S), pH, buffering capacity, total protein content, various ion concentrations and oral mucosal lesions.
Subjects and Methods:
A total of 100 patients were categorized into test group and control group based on usage of diuretics. Unstimulated and stimulated saliva were collected and evaluated for flow rates. Salivary pH was measured using pH meter. Buffering capacity was measured using Aranha's technique. Salivary Na+, K+ and Cl− concentrations were measured using electrolyte analyzer CORNLEY ACCULYTE-3P in ion-selective electrode method. Salivary total protein content was measured by spectrophotometric method. Dental Caries and periodontal status were measured by using decayed, missing, filled teeth index and Russell's periodontal index respectively. Oral mucosal examination was carried out to identify the mucosal lesions.
Results:
The obtained results were subjected to statistical analysis using Statistical package for social sciences software (SPSS), version 16, IBM Company by Chi-square test and unpaired t-test. Highly significant P for alterations of SFR/U (P < 0.001), SFR/S (P < 0.001), pH (P < 0.001), Na+ concentration (P < 0.001), buffering capacity (P < 0.001) and moderate significance for Cl− concentration (P < 0.01) were found. Alterations of total protein (P = 0.14) and K+ (P = 0.65) concentrations were not statistically significant. High prevalence was found for caries (P < 0.01), periodontal status (P < 0.001) and mucosal lesions (P < 0.01).
Conclusion:
Our study shows that diuretic medication significantly reduces SFRs (xerostomia) and alters salivary composition which may have an impact on the incidence of dental caries, periodontal diseases and mucosal lesion formation.
Keywords: Buffering capacity, Diuretics, pH, Saliva, Salivary flow rate, Xerostomia
Introduction
Saliva plays a pivotal role in the maintenance of oral health by exhibiting multiple host defense functions which include homeostatic processes, lubrication, antimicrobial activity and control of demineralization/remineralization of teeth.
Saliva is composed of variety of electrolytes including sodium, potassium, calcium, magnesium, bicarbonate, phosphates, immunoglobulins, proteins, enzymes, mucins, nitrogenous products such as urea and ammonia. Bicarbonates, phosphates and urea modulate pH (5.3-7.8) and buffering capacity of saliva. Macromolecule proteins and mucins serve to cleanse, aggregate and/or attach oral microorganisms and contribute to dental plaque metabolism. Calcium, phosphate and proteins work together as antisolubility factor which modulate demineralization and remineralization. Immunoglobulins, proteins and enzymes provide antimicrobial action to the saliva.
On average, unstimulated flow rate is 0.3 ml/min with an average total for 16 h of unstimulated flow (during waking hours) being 300 ml. Salivary flow rate (SFR) during sleep is nearly zero. Stimulated flow rate is, at maximum 7 ml/min. Stimulated saliva is reported to contribute as much as 80-90% of the average daily salivary production.[1]
There is a widespread understanding that pharmacotherapy is related to dryness of the mouth causing xerostomia, hyposalivation and altered saliva composition. There is also evidence that the prevalence of dry mouth is correlated to the number of daily drugs taken rather than to certain groups of drugs. However, there are studies supporting that some group of drugs are more xerogenic than others. Further, clinical studies have also established relationship between pharmacotherapy and dental caries.[1,2]
Diuretics are widely used in the treatment of the number of diseases such as hypertension, congestive cardiac failure and cirrhosis of liver, renal diseases such as nephrotic syndrome, chronic renal failure and acute glomerulonephritis. Diuretics act by enhancing urinary output thereby reducing the volume of circulatory fluid and reduce the work load of heart and kidney. Xerostomia is one of the commonly mentioned side-effects of diuretic medications.[3]
Diuretic agents and psychotropics were most commonly used medications inducing xerostomia in one study of elderly patients and were almost equally potent in reducing mean SFR. Thiazides may cause dry mouth, but there appear to be few reports showing a relationship between diuretic use and dry mouth. Subjectively, xerostomia was experienced 10 times more frequently after ingestion of furosemide than placebo.[4] Although a wide range of drugs can give rise to oral dryness. The degree of oral dryness and duration of the dryness depends on the drug taken and dosage of the drug.
Although many studies had been conducted to evaluate the effect of the diuretics on oral dryness, none of the studies had evaluated the effects of diuretics on all parameters of saliva such as flow rate, composition of ions, proteins, pH and buffering capacity, prevalence of caries, gingival diseases, periodontal diseases and mucosal lesions. Hence, the aim of our present study was to evaluate the effect of diuretics on SFR, composition of saliva, oral health status, prevalence of caries and periodontal diseases in diuretic drug users.
Subjects and Methods
Subject selection
The study was conducted for 1 year on patients selected from Department of General Medicine, Department of Cardiology and Department of Nephrology in Narayana Medical College and Hospital, Nellore. The purpose of the study and its medical implications were explained to the patient and an informed written consent was obtained. Before conducting the study, ethical clearance had been obtained from the ethical clearance committee of Narayana Dental College and Hospital, Nellore. The subjects were divided into the control group and test groups. A total of 50 healthy individuals (23 males, 27 females) were included in the control group, 50 individuals (27 males, 23 females) who were on diuretic medication included in the test group. The diuretic medication used by each patient and duration of the same was also confirmed through physicians who had examined the subjects prior to the study.
Inclusion criteria
Adult conscious, co-operative patients on diuretic medications for hypertension, congestive cardiac failure and chronic renal failure were included in the test group
Age and gender matched healthy conscious, co-operative adults willing to participate in the study as the control group.
Exclusion criteria
Patients who were using antihistamines, antisialogogues, sympathomimetic drugs, psychiatric medications
Presence of systemic conditions that could influence the salivary gland physiology such as diabetes mellitus, individuals with history of radiotherapy in the head and neck region and history of chemotherapeutic treatment in the last 3 months.
DMFT index,[5] oral hygiene index simplified (OHI-S),[6] plaque index,[7] Russell's periodontal index[8] were recorded and oral mucosal examination was carried out to identify the lesions.
Saliva collection
Saliva sample collection was performed between 9 a.m. and 10 a.m. For unstimulated saliva collection, subjects were instructed to restrain from food for 1 h prior to the investigation. Subjects were asked to rinse their mouth with plain water. Subjects were instructed to bend their heads forward after an initial swallow, to allow saliva to collect in the mouth. Later the subjects were asked to spit the saliva into a test tube once per min for 5 min and the flow rate was recorded as ml/5 min.
Stimulated saliva was collected immediately after collection of unstimulated saliva. During this procedure, subjects were instructed to chew 2 g preweighed paraffin wax. After chewing paraffin wax for 5 min, the subjects were asked to spit the saliva along with wax into the test tube. SFR was recorded as ml/5 min. Saliva was stored at −20°C in freezer.
Salivary analysis
Salivary analysis was carried out in the Department of Biochemistry, Narayana Medical College and Hospital, Nellore. After collection, saliva samples were centrifuged for 5 min with 3600g using table top centrifuge REMI-8C. After centrifugation of samples, total volume was measured. Salivary pH was measured using pH meter. Buffering capacity was measured using 0.1 N lactic acid. The volume of 0.1 N lactic acid required to lower the salivary pH to its critical pH (5.5) was measured. Sodium, potassium, chloride levels were measured by using electrolyte analyzer CORNLEY ACCULYTE-3P in ion-selective electrode method. Total protein content is measured using chemistry analyzer HUMALYZER 3000 in method of photometry having a wavelength of 620 nm. The obtained data was subjected to statistical analysis for scientific validation and appropriate interpretation. Statistical analysis was performed with the Statistical package for social sciences software (SPSS), version 16, IBM company. Statistical significance was defined as P < 0.05. Categorical variables were compared by the Chi-square test and continuous variables by the Student's t-test. Unadjusted and adjusted risk ratios were calculated with 95% confidence intervals.
Results
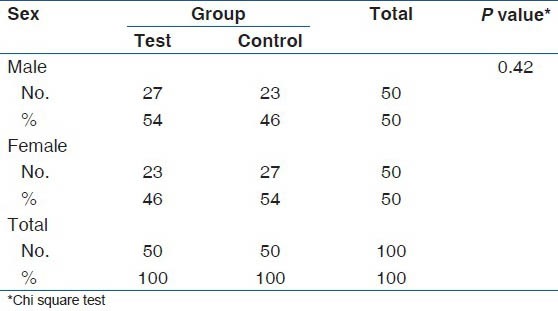
Mean age of the test group was 46.3 (2.7) years and mean age of the control group was 43.9 (2.4) years. When the age distribution was compared with test and control groups there was no significant difference (P = 0.11) as shown in Table 1. When the gender wise distribution was compared with test and control groups there was no significant difference (P 0.42) as shown in Table 2.
Table 1.
Age wise distribution of test and control groups
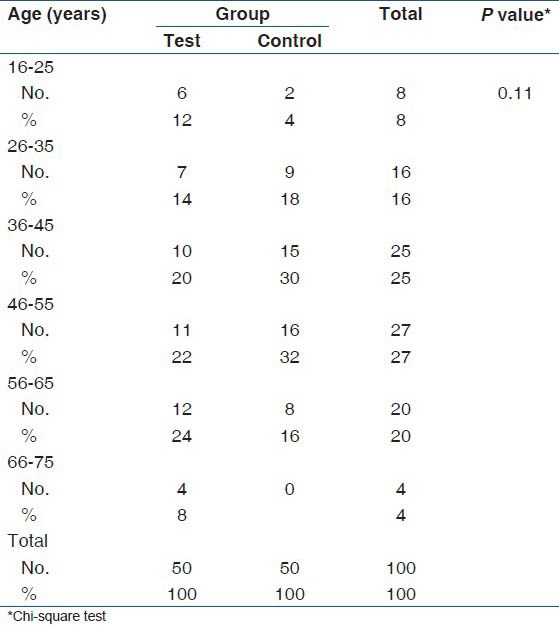
Table 2.
Gender wise distribution of test and control groups
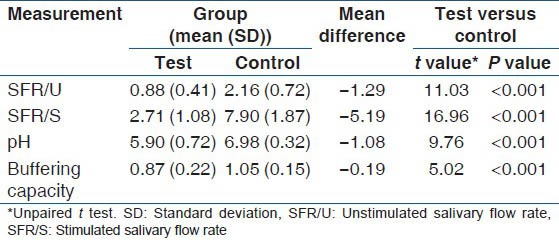
Unpaired t-test showed a high degree of significance (P ˂ 0.001) when SFR/U, SFR/S, salivary pH and buffering capacity were compared between test group and control group as shown in Table 3.
Table 3.
Comparison of SFR/U, SFR/S, pH and buffering capacity between study and control groups
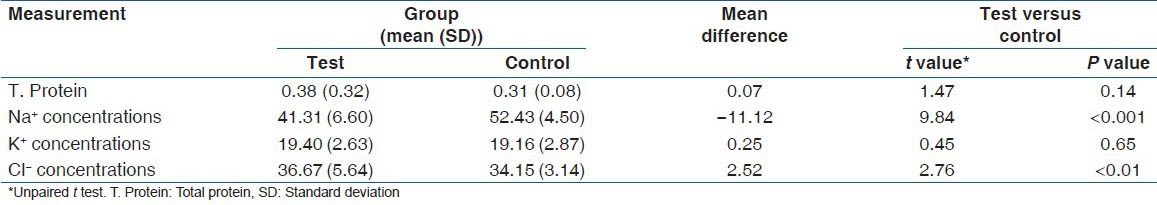
Unpaired t-test showed high significance (P ˂ 0.001) when Na+ concentration was compared and moderate significance (P < 0.01) when Cl− concentration was compared between test group and control group. No statistically significant difference was found when total protein content (P = 0.14) and K+ concentration (P = 0.65) were compared between the control and the test group as shown in Table 4.
Table 4.
Comparison of T. Protein, Na+, K+ and Cl- concentrations between test and control groups
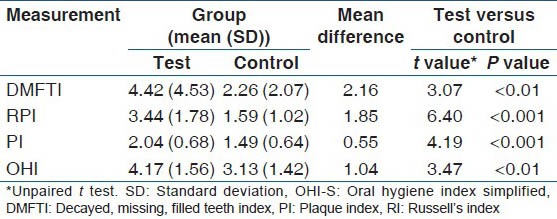
Unpaired t-test showed high significance when Russell's periodontal index (P ˂ 0.001), plaque index (P ˂ 0.001) and moderate significance were found when DMFT index (P < 0.01) and OHI (P < 0.01) were compared between control and test groups as shown in Table 5.
Table 5.
Comparison of DMFTI, RI, PI, OHI-S indices between test and control groups
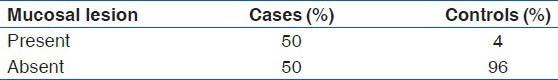
Mucosal lesions were present in 50% (25/50) of the test group and in only 6% (3/50) of the control group with a high degree of statistical significance in Chi-square test (P < 0.001) as shown in Table 6.
Table 6.
Comparison of mucosal lesion presence between study and control groups
In the test group, depapillation of tongue (glossitis) was present in 19 subjects, pseudomembranous candidiasis in four subjects, aphthous ulcers in one subject and herpes labialis in one subject, whereas in the control group only three subjects had mucosal lesions. Two subjects had reticular lichen planus and one subject had aphthous stomatitis.
The result of the present study have demonstrated alterations in various salivary parameters, higher prevalence of dry mouth, dental caries and periodontal disease in patients taking diuretics.
Discussion
In our present study age and gender did not show a significant difference when compared the control group and test group. High significance was observed when SFR/U, SFR/S were compared between test group and control groups.
In the present study, all subjects in the test group were using diuretics from a minimum of 2 weeks to 3 months continuously. Medication induced xerostomia is an important aspect since it can be indicative of systemic disease and can have negative effects on the oral cavity and the quality of life. It is commonly associated with reduction in saliva secretion (xerostomia) from salivary glands leading to taste disturbance, bad breath and painful ulcers and affects oral functions such as chewing, speech and swallowing.[9,10]
Studies have demonstrated that significantly reduced unstimulated and stimulated saliva was measured in subjects on systemic medication which included diuretics.[2,11] This is in accordance with our present study.
One study has reported that in resting whole saliva the output of both sodium and chloride tended to decrease especially during treatment with bendroflumethiazide while in submandibular-sublingual secretion the output of all the electrolytes was decreased, especially for potassium and chloride and during treatment with furosemide. This is because of the blockade of the electrolyte cotransport system by diuretics.[2] Potassium and total protein outputs were not affected since potassium sparing diuretics were used in our present study.
Studies have reported a significant correlation between stimulated saliva secretion and buffering capacity and pH as well as between pH and buffering capacity.[12] Similar correlation was found in our study.
Our present study showed higher caries prevalence and periodontal diseases in the test group when compared with control.
To the best of our present knowledge, this is the first study conducted to evaluate the effect of diuretics on various parameters of saliva, prevalence of oral mucosal lesions and assessment of oral health status by measuring DMFT,[5] PI,[6] OHI-S[7] and RPI.[8]
One study has reported that caries prevalence was more in persons who reported with dryness of mouth.[13] High counts of salivary lactobacilli have been related to xerostomia.[14] In a study salivary lactobacillus level correlated significantly with the total number of dry mouth complaints and the SFR/U.[15] High levels of lactobacilli may refer to active caries lesion progression and or reduced salivary gland function.[14] Immunoglobulins, proteins and enzymes provide antimicrobial action to the saliva. Due to xerostomia and reduced calcium and phosphate ions high prevalence of caries was found in our present study. Studies have reported that alterations in the physicochemical properties of saliva such as decreased SFR, pH, buffering capacity, significantly increased caries incidence.[13,16,17,18] Similar results were observed in our present study.
High degree of statistical significance for Russel's periodontal index, plaque index and mucosal lesions was observed when compared the control and test groups High prevalence of periodontitis could be due to decreased cleansing activity and reduced microbial activity by saliva in the test group. High prevalence of mucosal lesions was attributed to decreased cleansing activity of saliva.
Conclusion
In this study, we conclude that
Patients using diuretics have decreased SFR, pH, buffering capacity and Na+ and Cl− concentration, while the K+ concentration and total protein were unaltered in comparison to the control group
Patients on diuretic medication have a higher prevalence of xerostomia, periodontitis, dental caries and mucosal lesions when compared with that in the control group individuals.
In the present study, all patients in the study group showed xerostomia. It is not practically possible to stop the medication, so the physicians while prescribing xerostomia-inducing medications like diuretics should also focus attention on the management of drug induced xerostomia.
Acknowledgment
The present study was self-funded. We acknowledge Dr. Ramalingam, In-charge, Central Laboratory, Narayana Medical College and Hospital, Nellore for his valuable help throughout the study.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.de Almeida Pdel V, Grégio AM, Machado MA, de Lima AA, Azevedo LR. Saliva composition and functions: A comprehensive review. J Contemp Dent Pract. 2008;9:72–80. [PubMed] [Google Scholar]
- 2.Nederfors T, Nauntofte B, Twetman S. Effects of furosemide and bendroflumethiazide on saliva flow rate and composition. Arch Oral Biol. 2004;49:507–13. doi: 10.1016/j.archoralbio.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Greger R. New insights into the molecular mechanism of the action of diuretics. Nephrol Dial Transplant. 1999;14:536–40. doi: 10.1093/ndt/14.3.536. [DOI] [PubMed] [Google Scholar]
- 4.Scully C, Bagan JV. Adverse drug reactions in the orofacial region. Crit Rev Oral Biol Med. 2004;15:221–39. doi: 10.1177/154411130401500405. [DOI] [PubMed] [Google Scholar]
- 5.Klein HT, Palmer CE, Knutson J. Studies on dental caries. Caries experience and variation in the time of eruption of the permanent teeth. J Dent Res. 1938;2:20–3. [Google Scholar]
- 6.Greene JC, Vermillion JR. The simplified oral hygiene index. J Am Dent Assoc. 1964;68:7–13. doi: 10.14219/jada.archive.1964.0034. [DOI] [PubMed] [Google Scholar]
- 7.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 8.Russell AL. A system of classification and scoring for prevalence surveys of periodontal disease. J Dent Res. 1956;35:350–9. doi: 10.1177/00220345560350030401. [DOI] [PubMed] [Google Scholar]
- 9.Nederfors T, Isaksson R, Mörnstad H, Dahlöf C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population-Relation to age, sex and pharmacotherapy. Community Dent Oral Epidemiol. 1997;25:211–6. doi: 10.1111/j.1600-0528.1997.tb00928.x. [DOI] [PubMed] [Google Scholar]
- 10.Habbab KM, Moles DR, Porter SR. Potential oral manifestations of cardiovascular drugs. Oral Dis. 2010;16:769–73. doi: 10.1111/j.1601-0825.2010.01686.x. [DOI] [PubMed] [Google Scholar]
- 11.Samnieng P, Ueno M, Shinada K, Zaitsu T, Wright FA, Kawaguchi Y. Association of hyposalivation with oral function, nutrition and oral health in community-dwelling elderly Thai. Community Dent Health. 2012;29:117–23. [PubMed] [Google Scholar]
- 12.Dreizen S, Reed AI, Niedermeier W, Spies TD. Sodium and potassium as constituents of human salivary buffers. J Dent Res. 1953;32:497–503. doi: 10.1177/00220345530320040801. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadi E, Fallahi S, Alaeddini M, Hasani Tabatabaei M. Severe dental caries as the first presenting clinical feature in primary Sjögren's syndrome. Caspian J Intern Med. 2013;4:731–4. [PMC free article] [PubMed] [Google Scholar]
- 14.Nonzee V, Manopatanakul S, Khovidhunkit SO. Xerostomia, hyposalivation and oral microbiota in patients using antihypertensive medications. J Med Assoc Thai. 2012;95:96–104. [PubMed] [Google Scholar]
- 15.Ismail AI, Hasson H. Fluoride supplements, dental caries and fluorosis: A systematic review. J Am Dent Assoc. 2008;139:1457–68. doi: 10.14219/jada.archive.2008.0071. [DOI] [PubMed] [Google Scholar]
- 16.Preethi BP, Anand P, Reshma D. Evaluation of flow rate, pH, buffering capacity, calcium, total protein and total antioxidant levels of saliva in caries free and caries active children-An in vivo study. Biomed Res. 2010;21:289–94. doi: 10.1007/s12291-010-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DePaola DP. Saliva: The precious body fluid. J Am Dent Assoc. 2008;139(Suppl):5S–6. doi: 10.14219/jada.archive.2008.0348. [DOI] [PubMed] [Google Scholar]
- 18.Kuriakose S, Sundaresan C, Mathai V, Khosla E, Gaffoor FM. A comparative study of salivary buffering capacity, flow rate, resting pH, and salivary Immunoglobulin A in children with rampant caries and caries-resistant children. J Indian Soc Pedod Prev Dent. 2013;31:69–73. doi: 10.4103/0970-4388.115697. [DOI] [PubMed] [Google Scholar]


