Abstract
Background:
With the current World Health Organization (WHO) “Option B+” for prevention of mother to child transmission (PMTCT) of human immunodeficiency virus (HIV), virtual elimination of mother to child transmission (eMTCT) is highly achievable.
Aim:
The aim of this study is to determine the rate of MTCT of HIV from mothers who started highly active antiretroviral therapy (HAART) for life from diagnosis during pregnancy to the exposed babies who had daily nevirapine in the first 6 weeks of life.
Subjects and Methods:
HIV positive mothers and their exposed babies who enrolled for the hospital PMTCT protocol from January 1, 2009 to December 31, 2011 were studied. The babies were tested for HIV using deoxyribo nucleic acid polymerase chain reaction test at 6 weeks, and then HIV rapid tests at 18 months.
Results:
A total of 5,946 booked mothers had HIV testing and counseling (HTC) within the study period. Two hundred and twenty-three (223/5946, 3.7%) were positive, out of which 188 (188/223, 84.3%) enrolled for the PMTCT interventions while 35 (35/223, 15.7%) did not enroll. Three of the enrollees were lost to follow up and two were referred to another PMCT center. Of the remaining 183 enrolled HIV positive mothers, one gave birth to a set of twins, giving a total of 184 exposed babies. There were two cases of intrauterine fetal death of unknown fetal HIV status. None of the 182 remaining babies evaluated for HIV testing tested positive to HIV.
Conclusions:
With adequate suppression of maternal viral replication with HAART using the WHO Option B+, eMTCT of HIV is achievable in a developing country like Nigeria where infant breastfeeding is a norm.
Keywords: Developing country, Elimination of mother to child transmission, Highly active antiretroviral therapy, Human immunodeficiency virus
Introduction
Without prevention of mother to child transmission (PMTCT) interventions, the rate of maternal to child transmission (MTCT) of human immunodeficiency virus (HIV) ranged from 25% to 35%.[1] Transmission can occur in pregnancy, delivery, and during breastfeeding periods. High maternal viral loads, vaginal delivery, prolonged rupture of fetal membranes, low CD4 cell counts, and prolonged breastfeeding were implicated in MTCT of HIV in the era of inadequate maternal viral suppression with antiretroviral monotherapy.[2,3,4,5] Other risk factors include short duration of antiretroviral therapy (ART) before delivery and poor adherence to treatment.
Major breakthroughs in MTCT of HIV started in 1994 when 67% reduction in MTCT of HIV was achieved with antenatal, intra-partum and infant zidovudine (AZT) monotherapy and formula feeding in the acquired immunodeficiency syndrome (AIDS) Clinical Trials Group 076 study.[6] In 1999, 47% reduction was achieved with single-dose nevirapine (sdNVP) simpler regimens (HIVNET 012).[7] The addition of sdNVP to “Thai AZT Regimen” in 2003, dramatically reduced transmission to only 2-3% in non-breast feeding population with higher MTCT of HIV of about 5-6% in a breastfeeding population.[8]
Previously, elective caesarean delivery was often recommended in order to reduce the risk of MTCT when the viral load is detectable or >1000 copies/mL[9,10] However, with the current era of World Health Organization (WHO) Option B+ for highly active antiretroviral therapy (HAART) - based PMTCT interventions, the benefits of elective caesarean delivery, limiting the rupture of fetal membranes to <4 h duration, and limiting breast feeding to the first 6-12 months of life are doubtful in view of the possibility of maximal viral suppression achievable with the intervention.[11,12]
The current WHO Option B and B+ for HAART - based PMTCT interventions have shown that with adequate suppression of maternal viral replication, virtual elimination of maternal to child transmission (eMTCT) to <2% is achievable by 2015.[13,14] This option was introduced because replacement infant feeding in developing countries, became a big obstacle because of the associated social, cultural, economic, hygienic, and stigmatization problems.[15] The current WHO Option B and B+ for HAART - based PMTCT interventions whereby breastfeeding is allowed for up to 12 months with the mother on HAART and the infant on daily NVP in the first 6 weeks, is anticipated to have a positive impact on MTCT reduction especially in breastfeeding population like Nigeria.[16]
There is a paucity of data in Nigeria on the rate of MTCT of HIV following this new method of PMTCT intervention and there is no study in this regard from the study area. This study therefore aimed at determining the rate of MTCT of HIV from mothers who started HAART for life from diagnosis during pregnancy to the exposed babies who had daily NVP in the first 6 weeks of life.
Subjects and Methods
Setting
Enugu State University Teaching Hospital Parklane (ESUTHP) Enugu is a state owned health institution that evolved from Nursing Home in 1930 for the colonial masters to a teaching hospital status in June 2006. Pregnant women book for antenatal care in this institution only on Thursdays. The women and few of the accompanying husbands were given group HIV counseling in the antenatal hall and their questions answered by the PMTCT nurse counselor. Filled routine investigation forms (including HIV tests for women and their husbands) were given to them. The HIV tests were done the same day by laboratory scientists attached to the departmental side laboratory and the results released to the clients the same day through the PMTCT nurses. Individual counseling was only given to HIV positive mothers and their husbands the same day because of limited manpower and rooms for counseling. The HIV positive clients were given an appointment on the days the PMTCT coordinator runs antenatal clinic. The PMTCT clinic is integrated with the normal antenatal clinic to avoid stigmatization. Enugu state university teaching hospital parklane highly active antiretroviral therapy - based prevention of mother to child transmission protocol.
Inclusion criteria
These included booked HIV positive mothers, who had HAART during pregnancy, labor, and breastfeeding periods. Also included were their HIV exposed babies who had NVP or AZT prophylaxis in their first 6 weeks of life. Exclusion criteria: These involved all unbooked HIV positive women, and all the booked HIV positive women who commenced HAART for <4 months before delivery.
Enugu state university teaching hospital parklane highly active antiretroviral therapy - based prevention of mother to child transmission protocol
This ESUTHP HAART-based PMTCT protocol was designed and used in this institution since its activation to a comprehensive HIV/AIDS site in January 1, 2009. The protocol involves routine HIV testing and counseling on the Thursday antenatal booking clinic and also in the labor ward for unbooked mothers presenting in labor, but the mothers have right to opt out. For mothers who tested negative at booking, HIV retesting was scheduled at 28-36 weeks gestation. Partners and family HIV testing and counseling (HTC) were planned for mothers who tested positive at booking or at the 28-36 weeks gestation. Tuberculosis and opportunistic infections screenings together with co-trimoxazole prophylaxis for Pneumocystic carina pneumonia infection were also offered to HIV positive mothers. Other basic antenatal services like tetanus toxoid immunization, intermittent preventive treatment for malaria using sulfadoxine pyrimethamine, and routine drugs were given to women. In addition, they were encouraged to sleep under the insecticide treated nets. The HIV positive mothers were commenced on HAART after 14 weeks of gestation, which they continued on during labor, breastfeeding and also after cessation of breast feeding irrespective of CD4 count levels and clinical staging. The common first line HAART regimen used in the facility were a combination of AZT, lamuvidine (3TC) and NVP when CD4 count was <250 cells/mL; AZT, 3TC and efavirenz (EFV) (when gestational age is >14 weeks); and 3TC, EFV and tenofovir disoproxil fumarate. The HIV exposed babies were given daily NVP from birth till 6 weeks of age. Family planning services were provided for them after delivery and their neonates were offered routine infant immunization. Three trained PMTCT nurses routinely tracked and monitored the HIV positive mothers and their exposed babies through home visits and phone calls. The HIV exposed babies were also followed up on Wednesday clinic by the trained consultant pediatricians. Babies’ blood samples were taken to Nnamdi Azikiwe Teaching Hospital Nnewi, Anambra state for HIV deoxyribo nucleic acid polymerase chain reaction (PCR) test at 6 weeks. At 18 months of age, HIV testing was repeated using HIV rapid test at the ESUTHP, Enugu. Furthermore, the antenatal booking registers of the HIV positive mothers and that of the paired case notes of their exposed infants were reviewed and relevant data analyzed by simple proportions and percentages. The study was approved by the relevant ethical committee of the hospital.
Results
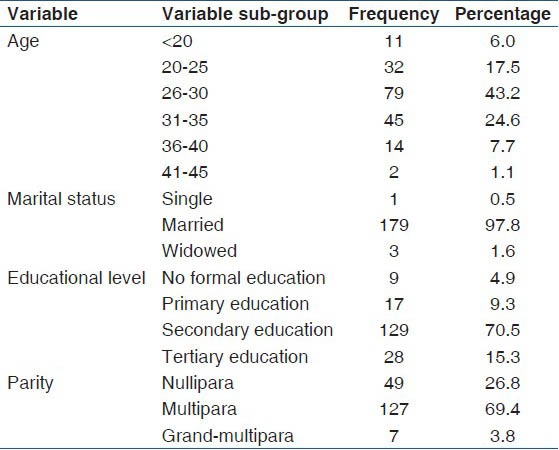
A total of 5946 booked mothers had HTC within the study period. Five thousand seven hundred and twenty-three (5723/5946, 96.3%) mothers tested negative to HIV. Two hundred and twenty-three (223/5946, 3.7%) mothers tested positive out of which 188 (188/223, 84.30%) enrolled for the PMTCT interventions while 35 (35/223, 15.70%) did not enroll because they were already receiving antiretroviral drugs in other facilities. Three of the enrollees were lost to follow-up and 2 were referred to another facility for PMTCT on request. The mean age of the patients was 28.8 (4.1), and most (91.3%, 167/183) were ≤35 years, married (97.8%, 179/183), had secondary education (70.5%, 129/183), and multiparous (69.4%, 127/183). Details of the socio-demographic characteristics of the patients were as shown in Table 1. Of the 183 HIV positive mothers recruited, one gave birth to a set of twins, giving a total of 184 exposed babies. Two HIV positive mothers with singleton gestations had unexplained intrauterine fetal death at term. The HIV status of the stillborn babies was unknown. A total of 166 (90.71%) mothers were delivered vaginally, while 9.3% (n = 3) had a cesarean delivery. Most of the babies (91.8%, 167/182) had exclusive breastfeeding while 5.5% (10/182) were on mixed breastfeeding and 2.7% (5/182) on breast milk substitute.
Table 1.
Sociodemographic characteristics of the patients
The HIV testing at 6 weeks for the 182 live births was negative. Four babies that tested negative to HIV testing at 6 weeks died before the age of 18 months following acute illnesses. Two died from acute diarrheal diseases, one from severe malaria, and one from undiagnosed cause. The HIV testing at 18 months for the remaining 178 babies was negative.
Discussion
A total of 182 out of 184 HIV exposed babies (98.9%) tested negative at 18 months. The HIV status of the remaining two exposed babies (1.1%) that died in utero was not evaluated but they were at risk of MTCT of HIV. The results of the present study confirmed that adhering to HAART in pregnancy, labor and during breastfeeding by HIV positive mothers can result in virtual eMTCT of <2% even in developing countries like Nigeria where breastfeeding and vaginal delivery are the norms. The findings of this study is similar to the MTCT rate of 0.0% and 1.1% reported in Ouagadougou, Burkina Faso and Maiduguri, Nigeria, respectively for HIV positive mothers on HAART.[17,18] In Orlu, Imo state, Nigeria, a MTCT rate of 13.6% was reported in unbooked HIV positive mothers who had no form of ART.[19]
Virtual eMTCT of HIV requires simpler, safe, and effective ART regimen like the WHO Option B+ which is a lifelong HAART regimen that can be administered by nurses at the community levels. The WHO Option B+ as currently practiced in the study center has the added advantages of reducing maternal morbidity and mortality, preventing the development of drug resistance, preventing new HIV transmission during breastfeeding and in discordant couples.[15] The overall good result reported in this study could be attributed to the adoption of the WHO Option B+ protocol in addition to the quality of HIV testing and counseling, and follow-up by the PMTCT nursing staff and treatment specialists in the center. HIV viral load and retroviral drug sensitive testing are very important components of ART and should also be made universally accessible as well as HAART to every HIV positive mothers. This will help in monitoring maternal response to ARV drugs and ultimately the suppression of maternal viral replication, which is the goal of any modern PMTCT intervention strategy.[14,15] Maternal HAART initiated during pregnancy and continued through breastfeeding periods and for life as done in this study has been proven to be a culturally acceptable and non-stigmatizing safe breastfeeding option in the developing countries where safe water, electricity and uninterrupted supplies of breast-milk substitutes are not always available.[15]
In view of the possibility of “window period” associated with HIV infection, re-testing for HIV at 28-36 weeks gestation was done for all the mothers who had tested negative at booking. The challenges of PMTCT observed in this report included the absence of facilities for measuring maternal viral load, which determines the extent of suppression of maternal viral replication, and also lack of the infant PCR diagnostic test, which necessitated referral to another center for early diagnosis of pediatric HIV infections.
The limitations of this study include the fact that it involved only the HIV positive women that booked for antenatal care in the tertiary institution. These women are likely to be wealthier and more educated than the general population and hence more likely to adhere to HAART and other PMTCT interventions. The sample size of 184 for the exposed babies seems small to make generalization to the entire population. The data on infant feeding methods was based on maternal recall, and hence we cannot exclude the possibility of recall bias.
Conclusion/Recommendation
With adequate provision of HAART to all HIV infected pregnant women for life, virtual elimination of MTCT and thus pediatric HIV infections is possible in a society where infant breast feeding and vaginal delivery are the norms. Government commitment and financial support are critical for future sustainability of the currently United States of American government-driven HIV/AIDS projects.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Geneva: 2012. [Last accessed on 2013 Feb 16]. UNAIDS: Global Report: UNAIDS Report on the Global AIDS Epidemic. Available from: http://www.unaids.org/en/media/unaids/contentassets/documents/epidemiology/2012/gr2012/20121120_unaids_global_report_2012_with_annexes_en.pdf . [Google Scholar]
- 2.European Collaborative Study. Risk factors for mother-to-child transmission of HIV-1. Lancet. 1992;339:1007–12. doi: 10.1016/0140-6736(92)90534-a. [DOI] [PubMed] [Google Scholar]
- 3.Tess BH, Rodrigues LC, Newell ML, Dunn DT, Lago TD. Breastfeeding, genetic, obstetric and other risk factors associated with mother-to-child transmission of HIV-1 in Sao Paulo State, Brazil. Sao Paulo Collaborative Study for Vertical Transmission of HIV-1. AIDS. 1998;12:513–20. doi: 10.1097/00002030-199805000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Renjifo B, Gilbert P, Chaplin B, Msamanga G, Mwakagile D, Fawzi W, et al. Preferential in-utero transmission of HIV-1 subtype C as compared to HIV-1 subtype A or D. AIDS. 2004;18:1629–36. doi: 10.1097/01.aids.0000131392.68597.34. [DOI] [PubMed] [Google Scholar]
- 5.Simpson BJ, Shapiro ED, Andiman WA. Prospective cohort study of children born to human immunodeficiency virus-infected mothers, 1985 through 1997: Trends in the risk of vertical transmission, mortality and acquired immunodeficiency syndrome indicator diseases in the era before highly active antiretroviral therapy. Pediatr Infect Dis J. 2000;19:618–24. doi: 10.1097/00006454-200007000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Connor EM, Sperling RS, Gelber R, Kiselev P, Scott G, O'sullivan MJ, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173–80. doi: 10.1056/NEJM199411033311801. [DOI] [PubMed] [Google Scholar]
- 7.Guay LA, Musoke P, Fleming T, Bagenda D, Allen M, Nakabiito C, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 8.Lallemant M, Jourdain G, Le Coeur S, Mary JY, Ngo-Giang-Huong N, Koetsawang S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351:217–28. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- 9.Boucher M, Cohen H, Gruslin A, Money D, Steben M, Wong T. Mode of delivery for pregnant women infected by the human immunodeficiency virus. J SOGC. 2001;23:348–50. [Google Scholar]
- 10.ACOG committee opinion. Scheduled cesarean delivery and the prevention of vertical transmission of HIV infection. Number 219, August 1999. Committee on Obstetric Practice. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet. 1999;66:305–6. [PubMed] [Google Scholar]
- 11.European Collaborative Study. Mother-to-child transmission of HIV infection in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;40:458–65. doi: 10.1086/427287. [DOI] [PubMed] [Google Scholar]
- 12.Mark S, Murphy KE, Read S, Bitnun A, Yudin MH. HIV mother-to-child transmission, mode of delivery, and duration of rupture of membranes: Experience in the current era. Infect Dis Obstet Gynecol. 2012;2012:267969. doi: 10.1155/2012/267969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Geneva: WHO; 2010. [Last accessed on 2013 Feb 16]. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. Recommendations for a Public Health Approach (2010 version) Available from: http://www.whqlibdoc.who.int/publications/2010/9789241599818_eng.pdf . [PubMed] [Google Scholar]
- 14.WHO. Geneva: WHO; 2012. [Last accessed on 2012 Jul 11]. Use of antiretroviral drugs for treating pregnant women and preventing HIV infection in infants: Programmatic update. Available from: http://www.who.int/hiv/pub/mtct/programmatic_update2012/en/index.html . [Google Scholar]
- 15.Kuhn L, Stein Z, Susser M. Preventing mother-to-child HIV transmission in the new millennium: The challenge of breast feeding. Paediatr Perinat Epidemiol. 2004;18:10–6. doi: 10.1111/j.1365-3016.2003.00528.x. [DOI] [PubMed] [Google Scholar]
- 16.de Vincenzi I Kesho Bora Study Group. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): A randomised controlled trial. Lancet Infect Dis. 2011;11:171–80. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 17.Kouanda S, Tougri H, Cisse M, Simpore J, Pietra V, Doulougou B, et al. Impact of maternal HAART on the prevention of mother-to-child transmission of HIV: Results of an 18-month follow-up study in Ouagadougou, Burkina Faso. AIDS Care. 2010;22:843–50. doi: 10.1080/09540120903499204. [DOI] [PubMed] [Google Scholar]
- 18.Chama CM, Bello M, Ajayi BA, Zarma S, Gashau W. The use of highly active antiretroviral therapy for the prevention of mother-to-child transmission of the human immunodeficiency virus in Nigeria. J Obstet Gynaecol. 2010;30:362–6. doi: 10.3109/01443611003672104. [DOI] [PubMed] [Google Scholar]
- 19.Okeudo C, Ezem B, Ojiyi E. Mother-to-child transmission rate of HIV at Orlu, South-Eastern Nigeria. Internet J Gynaecol Obstet. 2012;16:2. [Google Scholar]


