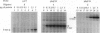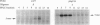Abstract
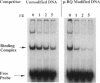
Benzene is a ubitiquous human environment mental carcinogen. One of the major metabolites is hydroquinone, which is oxidized in vivo to give p-benzoquinone (p-BQ). Both metabolites are toxic to human cells. p-BQ reacts with DNA to form benzetheno adducts with deoxycytidine, deoxyadenosine, and deoxyguanosine. In this study we have synthesized the exocyclic compounds 3-hydroxy-3-N4-benzetheno-2'-deoxycytidine (p-BQ-dCyd) and 9-hydroxy-1,N6-benzetheno-2'-deoxyadenosine (p-BQ-dAdo), respectively, by reacting deoxycytidine and deoxyadenosine with p-BQ. These were converted to the phosphoamidites, which were then used to prepare site-specific oligonucleotides with either the p-BQ-dCyd or p-BQ-dAdo adduct (pbqC or pbqA in sequences) at two different defined positions. These oligonucleotides were efficiently nicked 5' to the adduct by partially purified HeLa cell extracts--the pbqC-containing oligomer more rapidly than the pbqA-containing oligomer. In contrast to the enzyme binding to derivatives produced by the vinyl chloride metabolite chloroacetaldehyde, the oligonucleotides up to 60-mer containing p-BQ adducts did not bind measurably to the same enzyme preparation in a gel retardation assay. Furthermore, there was no competition for the binding observed between oligonucleotides containing 1,N6-etheno A deoxyadenosine (1,N6-etheno-dAdo; epsilon A in sequences) and these oligomers containing either of the p-BQ adducts, even at 120-fold excess. When highly purified fast protein liquid chromatography (FPLC) enzyme fractions were obtained, there appeared to be two closely eluting nicking activities. One of these enzymes bound and cleaved the epsilon A-containing deoxyoligonucleotide. The other enzyme cleaved the pbqA- and pbqC-containing deoxyoligonucleotides. One additional unexpected fact was that bulk p-BQ-treated salmon sperm DNA did compete effectively with the epsilon A-containing oligonucleotide for protein binding. This raises the possibility that such DNA contains other, as-yet-uncharacterized adducts that are recognized by the same enzyme that recognizes the etheno adducts. In summary, we describe a previously undescribed human DNA repair activity, possibly a glycosylase, that excises from DNA pbqC and pbqA, exocyclic adducts resulting from reaction of deoxycytidine and deoxyadenosine with the benzene metabolite, p-BQ. This glycosylase activity is not identical to the one previously reported from this laboratory as excising the four etheno bases from DNA.
Full text
PDF
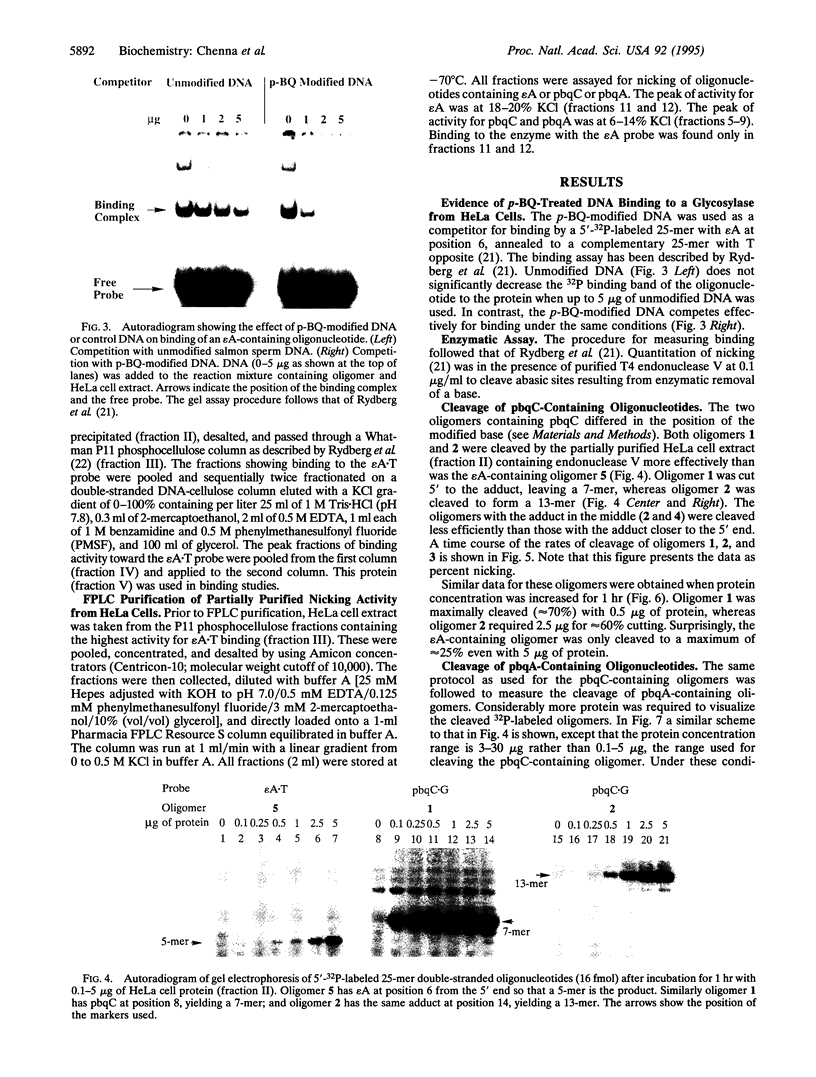
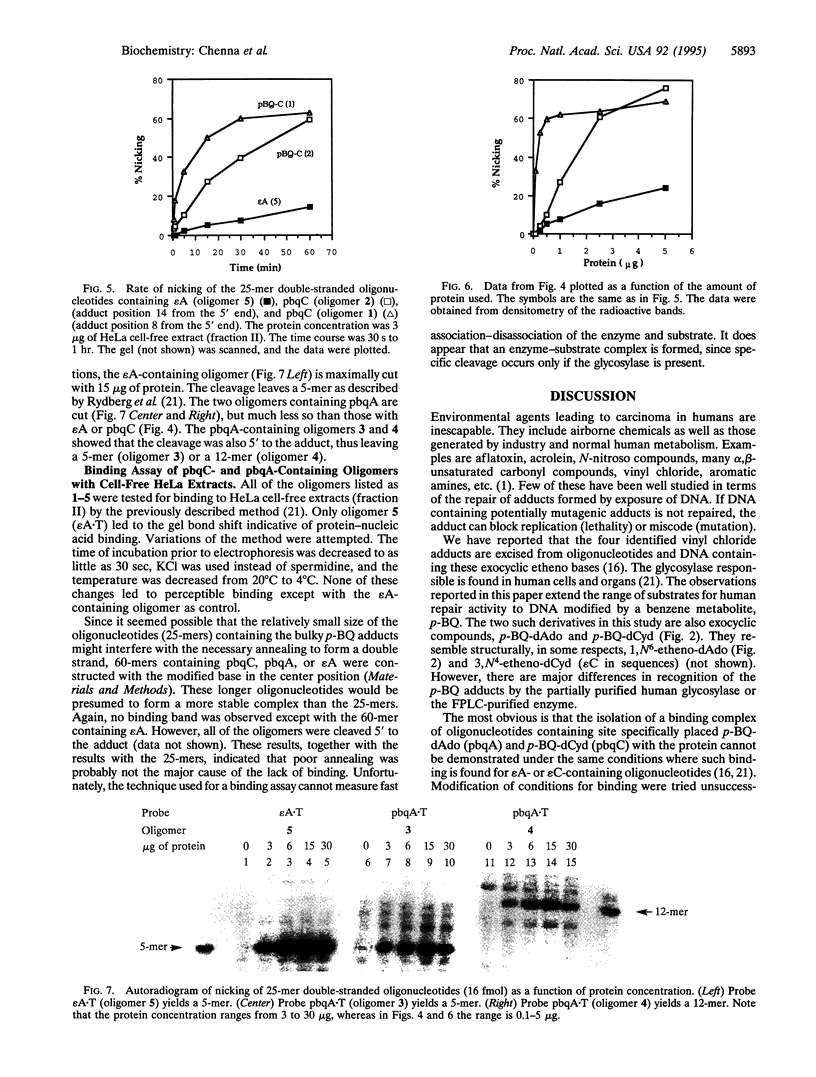

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartsch H., Barbin A., Marion M. J., Nair J., Guichard Y. Formation, detection, and role in carcinogenesis of ethenobases in DNA. Drug Metab Rev. 1994;26(1-2):349–371. doi: 10.3109/03602539409029802. [DOI] [PubMed] [Google Scholar]
- Basu A. K., Wood M. L., Niedernhofer L. J., Ramos L. A., Essigmann J. M. Mutagenic and genotoxic effects of three vinyl chloride-induced DNA lesions: 1,N6-ethenoadenine, 3,N4-ethenocytosine, and 4-amino-5-(imidazol-2-yl)imidazole. Biochemistry. 1993 Nov 30;32(47):12793–12801. doi: 10.1021/bi00210a031. [DOI] [PubMed] [Google Scholar]
- Bodell W. J., Levay G., Pongracz K. Investigation of benzene-DNA adducts and their detection in human bone marrow. Environ Health Perspect. 1993 Mar;99:241–244. doi: 10.1289/ehp.9399241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. C., Preston B. D., Cahill D. S., Dosanjh M. K., Singer B., Loeb L. A. The vinyl chloride DNA derivative N2,3-ethenoguanine produces G----A transitions in Escherichia coli. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9974–9978. doi: 10.1073/pnas.88.22.9974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosanjh M. K., Chenna A., Kim E., Fraenkel-Conrat H., Samson L., Singer B. All four known cyclic adducts formed in DNA by the vinyl chloride metabolite chloroacetaldehyde are released by a human DNA glycosylase. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1024–1028. doi: 10.1073/pnas.91.3.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. P., Kim D. H., Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991 Mar-Apr;4(2):168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., MacDonald T. L. Mechanisms of cytochrome P-450 catalysis. FASEB J. 1990 May;4(8):2453–2459. doi: 10.1096/fasebj.4.8.2185971. [DOI] [PubMed] [Google Scholar]
- Huff J. E., Haseman J. K., DeMarini D. M., Eustis S., Maronpot R. R., Peters A. C., Persing R. L., Chrisp C. E., Jacobs A. C. Multiple-site carcinogenicity of benzene in Fischer 344 rats and B6C3F1 mice. Environ Health Perspect. 1989 Jul;82:125–163. doi: 10.1289/ehp.8982125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jowa L., Winkle S., Kalf G., Witz G., Snyder R. Deoxyguanosine adducts formed from benzoquinone and hydroquinone. Adv Exp Med Biol. 1986;197:825–832. doi: 10.1007/978-1-4684-5134-4_78. [DOI] [PubMed] [Google Scholar]
- Jowa L., Witz G., Snyder R., Winkle S., Kalf G. F. Synthesis and characterization of deoxyguanosine-benzoquinone adducts. J Appl Toxicol. 1990 Feb;10(1):47–54. doi: 10.1002/jat.2550100109. [DOI] [PubMed] [Google Scholar]
- Kuśmierek J. T., Singer B. 1,N2-ethenodeoxyguanosine: properties and formation in chloroacetaldehyde-treated polynucleotides and DNA. Chem Res Toxicol. 1992 Sep-Oct;5(5):634–638. doi: 10.1021/tx00029a007. [DOI] [PubMed] [Google Scholar]
- Laib R. J. The role of cyclic base adducts in vinyl-chloride-induced carcinogenesis: studies on nucleic acid alkylation in vivo. IARC Sci Publ. 1986;(70):101–108. [PubMed] [Google Scholar]
- Misra R. R., Chiang S. Y., Swenberg J. A. A comparison of two ultrasensitive methods for measuring 1,N6-etheno-2'-deoxyadenosine and 3,N4-etheno-2'-deoxycytidine in cellular DNA. Carcinogenesis. 1994 Aug;15(8):1647–1652. doi: 10.1093/carcin/15.8.1647. [DOI] [PubMed] [Google Scholar]
- Pendlebury A., Frayling I. M., Santibanez Koref M. F., Margison G. P., Rafferty J. A. Evidence for the simultaneous expression of alternatively spliced alkylpurine N-glycosylase transcripts in human tissues and cells. Carcinogenesis. 1994 Dec;15(12):2957–2960. doi: 10.1093/carcin/15.12.2957. [DOI] [PubMed] [Google Scholar]
- Pongracz K., Bodell W. J. Detection of 3'-hydroxy-1,N6-benzetheno-2'-deoxyadenosine 3'-phosphate by 32P postlabeling of DNA reacted with p-benzoquinone. Chem Res Toxicol. 1991 Mar-Apr;4(2):199–202. doi: 10.1021/tx00020a012. [DOI] [PubMed] [Google Scholar]
- Pongracz K., Kaur S., Burlingame A. L., Bodell W. J. Detection of (3'-hydroxy)-3,N4-benzetheno-2'-deoxycytidine-3'-phosphate by 32P-postlabeling of DNA reacted with p-benzoquinone. Carcinogenesis. 1990 Sep;11(9):1469–1472. doi: 10.1093/carcin/11.9.1469. [DOI] [PubMed] [Google Scholar]
- Roy R., Brooks C., Mitra S. Purification and biochemical characterization of recombinant N-methylpurine-DNA glycosylase of the mouse. Biochemistry. 1994 Dec 20;33(50):15131–15140. doi: 10.1021/bi00254a024. [DOI] [PubMed] [Google Scholar]
- Rydberg B., Dosanjh M. K., Singer B. Human cells contain protein specifically binding to a single 1,N6-ethenoadenine in a DNA fragment. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6839–6842. doi: 10.1073/pnas.88.15.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydberg B., Qiu Z. H., Dosanjh M. K., Singer B. Partial purification of a human DNA glycosylase acting on the cyclic carcinogen adduct 1,N6-ethenodeoxyadenosine. Cancer Res. 1992 Mar 1;52(5):1377–1379. [PubMed] [Google Scholar]
- Singer B., Kuśmierek J. T., Folkman W., Chavez F., Dosanjh M. K. Evidence for the mutagenic potential of the vinyl chloride induced adduct, N2, 3-etheno-deoxyguanosine, using a site-directed kinetic assay. Carcinogenesis. 1991 Apr;12(4):745–747. doi: 10.1093/carcin/12.4.745. [DOI] [PubMed] [Google Scholar]
- Snyder R., Jowa L., Witz G., Kalf G., Rushmore T. Formation of reactive metabolites from benzene. Arch Toxicol. 1987;60(1-3):61–64. doi: 10.1007/BF00296948. [DOI] [PubMed] [Google Scholar]