Abstract
Background:
Chronic medical disorders are often complicated by cognitive impairments, making medical intervention that can alleviate cognitive disturbances desirable. Vinpocetine enhances cerebral utilization of oxygen and glucose and consequently improves cerebral functions including memory.
Aim:
This study assessed the efficacy of vinpocetine (Cognitol™) in improving memory and concentration in cognitively impaired patients.
Subjects and Methods:
A prospective analytical study of 56 cognitively impaired patients compared with age, sex and level of education matched 56 controls. Cognitive performance was assessed with the Short Blessed Test, which was pilot-tested. Baseline cognitive performances of the patients and controls were obtained and thereafter cognitive performances of the patients were assessed at 6 and 12 weeks after administration of vinpocetine at a dose of 5 mg twice-a-day. Comparative analysis of their performances at baseline was done using the Student t-test, while the improvement in patients’ performances and effect of disease variables on cognitive performances were analyzed with one-way analysis of variance and likelihood ratio analysis respectively.
Results:
The mean (standard deviation) [SD] ages of the cognitively impaired patients (56/112) and controls (56/112) were 49.5 (18.9) and 53.8 (15.8) years respectively (P = 0.19; 95% confidence interval [CI]: 2.2-10.8). The pilot study yielded an optimal cut-off error score of 6 with a sensitivity of 71.4%, specificity of 96.4% and accuracy of 83.9%. Patients performed significantly worse than the controls (P < 0.001; 95% CI 6.7-11.4). There were significant improvements in memory and concentration with vinpocetine therapy (P < 0.05). The clinical variables of the patients had no effect on the trend of cognitive performances.
Conclusions:
Vinpocetine was effective in improving memory and concentration of patients with epilepsy and dementia although the efficacy was minimal in demented patients.
Keywords: Concentration, Dementia, Epilepsy, Memory, Nigeria, Vinpocetine
Introduction
Chronic medical disorders are often complicated by cognitive impairments with attending unpleasant consequences, which include disruptions in an academic career, family life, adherence to medical therapy and managerial abilities.[1,2,3] Therefore medical intervention that can alleviate cognitive disturbances in the affected persons is desirable. Vinpocetine is a derivative of Vincamine, an alkaloid of common periwinkle plant (vinca minor), which has been shown to exert a brain neuro-protective effect by a combined action on cerebral circulation, brain metabolism and rheological properties of the blood. This boost in the cerebral metabolism thus enhances both oxygen and glucose utilization and consequently improving cerebral functions and protection even in conditions of hypoxia and ischemia.[4,5,6] Early experiments had shown an improvement of the cerebral circulation and oxygen utilization without changes in the systemic circulation. It has been shown that vinpocetine has cognition-enhancing effects resulting in improved abilities to retain and recall information. Thus, it has been part of the armamentarium for the management of memory disturbance.[7]
Several chronic neurological disorders have been shown to be accompanied by cognitive deteriorations.[8] This study assessed patients with epilepsy (PWE) and dementia, two medical disorders with established cognitive disturbances that have been extensively investigated in Nigerian patients. Epilepsy is a common neurological disorder with prevalence of between 5.6 and 37/1000 of the population in Nigeria,[9] a figure that is significantly higher than 4-6/1000 reported in the developed countries.[10] It is a highly stigmatized disorder that is often complicated by memory impairments, attention deficits and psychomotor retardation.[1] Similarly, cognitive deterioration is common in Nigerian Africans with dementia,[8,11,12,13,14]. Memory impairment, deterioration in abilities to cope with activities of daily living and personality changes are the hallmark of dementia diagnosis.[13]
Studies that evaluated cognitive performances of Nigerian patients with different medical disorders such as epilepsy, dementia, Parkinson's disease, stroke, HIV/AIDS and chronic renal failure have utilized various cognitive tools. These tools used in previous studies, which included Community Screening Instrument for Dementia,[14,15,16,17] Iron Psychology (acronym FePsy),[12,18] modified HIV dementia scale[19] and mini-mental state examination,[20] have been validated. Despite this, cognitive assessment is not routinely done in most sub-Saharan neurological clinics including Nigeria. This is probably due to lack of expertise and complexity of the validated cognitive tools in our environment. The Short Blessed Test (SBT) also referred to as the six item cognitive test (6ICT)[21] was chosen because it is a very simple test, which can be administered by non-professionals (i.e., non-neurologist or non-neuropsychologist) in an average time of 3-4 min. A pilot study was conducted to validate it before its use as a screening tool for this study. Doubtless to say, the simplicity of the tool will aid its applications in medical clinics by different cadres of health-care givers to monitor cognitive abilities of patients with chronic diseases.
The knowledge of the consequences and impact of cognitive dysfunctions on the quality-of-life of affected patients demand regular cognitive assessment to detect impairments and introduce prompt intervention to alleviate them. Several medications have been introduced for the treatment of memory impairments in patients with dementia.[22] Most of these medications address the symptoms of dementia by palliating the deficiency in the cholinergic innervation to the cerebral cortex.[23] An increasing number of clinical studies have demonstrated that cholinesterase inhibition can have modest, but detectable effects, such as improvement in cognitive performance as measured by selected tools such as the Alzheimer's Disease Assessment Scale-cognitive subscale.[24,25] Newer categories of drugs based on entirely different mechanisms of action have been developed. One example of these is memantine, an N-methyl-D-aspartate antagonist, which has been approved for treatment of memory disturbance in Alzheimer's disease.[25] Ethyl apovincaminate, also known as vinpocetine, is a vincamine derivative that has been used in the clinical practice for over 25 years for the treatment of cerebrovascular disorders and related symptoms.[26] Its effects on cerebral blood flow, brain metabolism, memory functions, and its neuroprotective actions have been confirmed in numerous animal experiments and human studies.[26,27] The objective of this study was to assess the efficacy of Vinpocetine (Cognitol) as a memory enhancer and its effect on concentration in PWE and dementia who were cognitively impaired.
Subjects and Methods
This was a prospective analytical study of 56 cognitively impaired patients compared with age, sex and level of education matched 56 controls. The study participants were consecutive patients with cognitive impairment recruited by purposive sampling at presentation to the neurology clinic of the University Teaching hospital Benin City between June and October 2012. The patients were diagnosed with either epilepsy or dementia, two neurological disorders, which have been previously shown to be associated with cognitive disturbances.[1,13] Cognitive assessment was done with the SBT.[21] The test was administered in simple English with instructions given to patients based on each task. All the study participants were educated. Controls were recruited from among staff members of the hospital and patients attending the general practice clinic for treatment of minor ailments like malaria. Control subjects had no history of chronic medical diseases. The cognitive performances of the patients and controls were obtained at baseline and the patients were followed-up for 12 weeks after administration of vinpocetine at a dose of 5 mg twice a day. The cognitive performances of the patients were then assessed at 6 and 12 weeks.
The exclusion criteria included subjects less than 18 years of age, patients with comorbidity (diabetes mellitus, hypertension and associated intracranial disorders, e.g., brain tumor and other metabolic diseases), those with central nervous system infections, those with an inconclusive diagnosis of epilepsy or dementia, major axis one psychiatric illness, presence of clinical signs of cardiac failure, alcohol intake above 120 g/week or 13 units/week, history of previous head injury with the loss of consciousness and patients on anti-cholinergic medications.
Ethical approval for the conduct of the study was obtained from the Hospital Ethics Committee. All study participants gave informed consent before the commencement of study (informed consent was obtained from close relations (next of kin) of six patients with Alzheimer's dementia). All clinical information and study results were treated with confidentiality.
Sample size determination
Using the command sampsi, in Stata 10SE software (StataCorp, Texas, USA), for sampling of proportions a sample size of 40 for the test participants and 40 for the controls was obtained using a power of 95% and hypothesized value of 0.5. This guided the recruitment of participants for the study ensuring that each arm has at least 40 participants.
Test instrument
The SBT also referred to as the 6ICT,[21] is a tool that comprises six questions, which assess concentration and memory. The sixth question on memory was modified to read a local name and address– Amen Omoruyi, 14, Lawani Street, Benin. The details of the other questions are in Reviewers. The maximum total score of the SBT is 28. The total score is calculated as a weighted sum of the total errors implying that the higher the score the more likely the presence of a dementing disorder with memory impairment. Normative values from Memory and Aging Project[28] recommended a score range of 0-4 as normal cognition; 5-9 as questionable impairment; and 10 and above as the presence of dementing illness. The original validation study showed a score of 6 or less as normal in 95% of subjects.[21]
Intervention
Each study participant received 10 mg vinpocetine a day in two divided doses for 3 months. Vinpocetine (Cognitol) is already being used as part of treatment for memory impairment in our neurology clinics so the participants received the drug as part of the prescription pattern. The baseline cognitive assessment was done before the eligible patients commenced their drug. The drug was provided at no cost to the study participants by the study investigator. Documentation of all adverse effects was made during and after the administration of the drug. The expectation however was that the drug carried “no risk” as it has been shown from previous studies to be safer than coffee.[5]
Statistical analysis
The data was analyzed with Stata version 10SE (StataCorp, Texas, USA). The demographic data was analyzed using descriptive statistics-frequency table and distribution and measures of variation-means, quantiles, standard deviations (SDs) and confidence intervals (CIs). The statistical differences in demographic data between controls and patients were assessed with Chi-square distribution analysis. The cognitive scores of the patients and controls on the SBT were compared for significant differences using the Student t-test. The patients’ performances were analyzed for improvement with one-way analysis of variance. The impact of the disease variables (i.e., seizure variables-duration, frequency and type of anti-epileptic drugs, and duration and severity of dementia) was assessed with likelihood ratio analysis. The cut-off score on the SBT was determined using the receiver operating characteristics (ROC).
Results
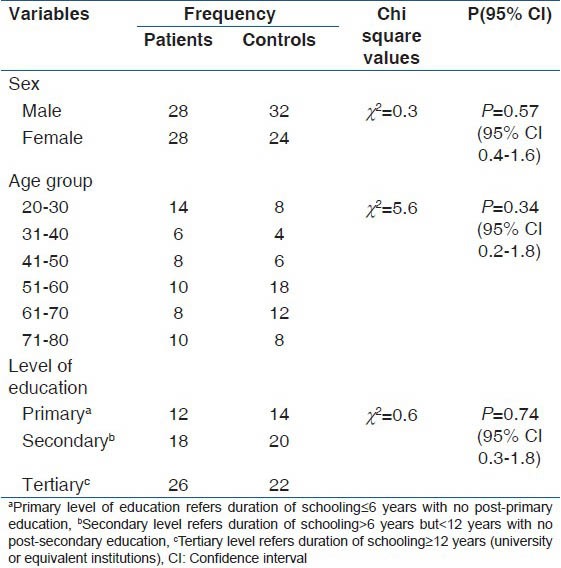
A total of 112 subjects participated in the study comprising 56 patients with cognitive impairments and 56 age, sex and level of education matched controls. The cognitively impaired patients were made up of 28 demented patients and 28 PWE. The mean age of the 56 patients was 49.5 (18.9) years (95% CI 44.4-54.5) with an age range of 22-79 years while the mean age of the 56 controls was 53.8 (15.8) years (95% CI: 49.6-58.0) with an age range of 22-76 years. There was no significant difference in the means of their ages (P = 0.23). Patients consisted of 28/56 (50%) males and 28/56 (50%) females while the controls were 35/62 (57.1%) males and 24/56 (42.9%) females. The details of demographic characteristics of the patients and controls are presented in Table 1.
Table 1.
Demographic data of study participants
Clinical characteristics of study participants
The PWE and dementia were recruited using the purposive sampling. All the PWE had generalized tonic-clonic seizures and were on carbamazepine (between 400 mg and 800 mg/day in two divided doses) for seizure control. They had detailed neurological evaluation at presentation and electro-encephalographic examination. The categorization of their seizure type was based on the International League Against Epilepsy classification.[29] The details of other seizure variables are presented in Table 2.
Table 2.
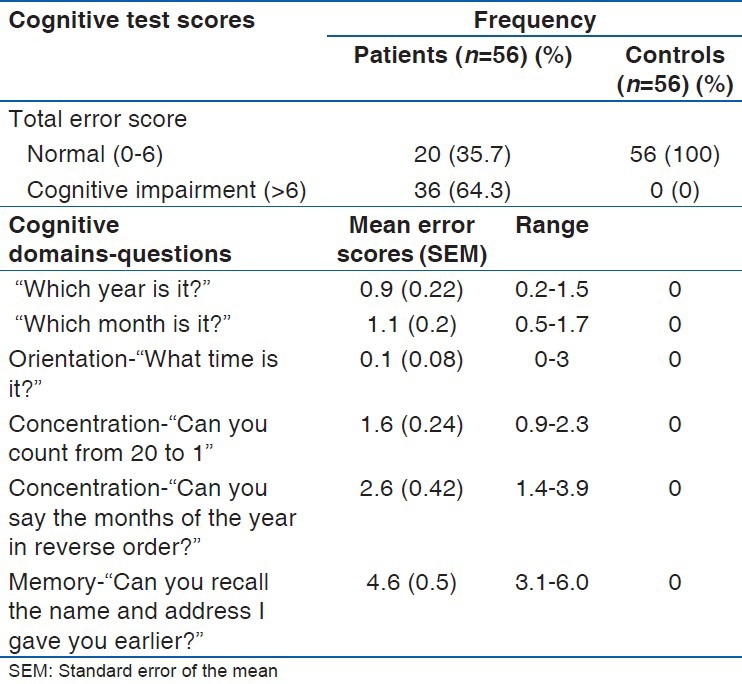
Mean error scores of study participants at baseline
Patients with dementia were categorized into two types: Those with Alzheimer's disease based on the clinical criteria of steadily progressive memory loss and those with vascular dementia based on the clinical criteria of the presence of vascular risk factors of hypertension and previous ischemic strokes and the step-ladder course of memory impairment. At the point of recruitment, none of these patients was on any medication. Details of other clinical parameters are presented in Table 2.
Determination of cut-off score on the SBT and validation assessment-pilot study
A total of 10 patients with confirmed cognitive impairment based on clinical history of memory impairment and problems coping with activities of daily living confirmed by close relations and Mini-Mental status examination scores of 20 or less was recruited for the pilot study. These patients’ cognitive functions were assessed with the 6-item short concentration-orientation-memory test and their performances compared with 10 normal subjects. Patients and controls were matched for age, sex and level of education.
The mean ages for patients and controls were 50.9 (18.6) years (95% CI: 37.6-64.2) and 51.5 (12.3) years (95% CI: 42.7-60.3) respectively, with no significant difference (P = 0.92; 95% CI: 14.2-15.4). Patients (n = 10) comprised five males (50%) and five females (50%), likewise the controls (n = 10). The level of the education distribution for both groups was also same: Two with primary education (20%), four with secondary (40%) and four with tertiary education (40%). The mean total error scores for the controls and patients were 1.7 (2.1) (95% CI: 0.2-3.2) with a range of 0-6 and 13.7 (8.5) (95% CI: 7.6-19.8) with a range of 4-28 respectively; with a statistically significant difference (P < 0.001; 95% CI 6.2-17.8). By implication, the total correct score followed the reverse pattern with mean scores of 26.3 (2.1) (95% CI: 24.8-27.8); range: 22-28 and 14.3 (8.5) (95% CI: 8.2-20.4); range: 0-24. The ROC curve was obtained for the total error scores to determine the cut-off score that corresponds to the optimal sensitivity and specificity values for the 6-item cognitive tool [Figure 1].
Figure 1.
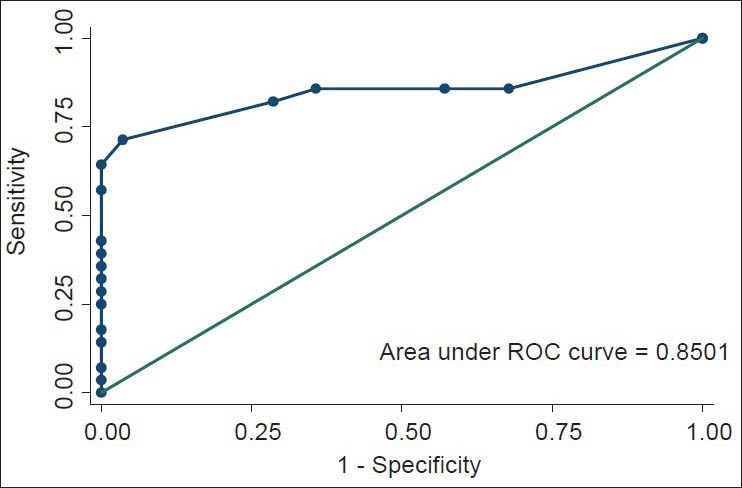
Receiver operating characteristic curve for total error scores
The optimal cut-off score of 6 was obtained with a sensitivity of 71.4% and specificity of 96.4%. The accuracy of the SBT was 83.9%. This cut-off score was same as that obtained by Katzman et al.[21] The area under the curve of 0.85 (SE 0.6) was significant with 95% CI: 0.7-0.97. The cut-off error score of 6 was used for this study.
Effect of Vinpocetine (Cognitol™) on cognitive performances of patients
The cognitive performances of the patients were compared with the performances of the controls using the 6-item SBT at baseline. Thereafter, the cognitive performances of the patients who were categorized into those with epilepsy and those with dementia were assessed at 6 and 12 weeks after administration of vinpocetine (Cognitol™). Table 2 showed the cognitive performances of the patients and controls at baseline and baseline scores by cognitive domains while Table 3 presented the cognitive scores of the patients at baseline, 6 and 12 weeks.
Table 3.
Mean cognitive error scores of patients by domains
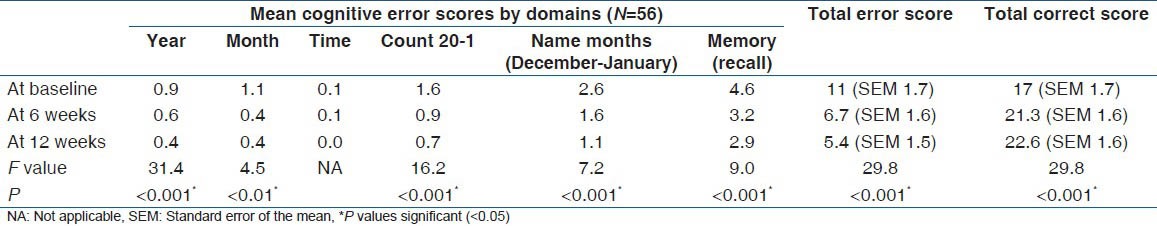
The mean total error scores for the controls and the patients were 1.96 (SD: 1.8) (95% CI: 1.5-2.4); range: 0-6 and 11 (SD: 8.8) (95% CI: 8.6-13.4); range: 0-28 respectively. The 75% percentile of the scores for the controls and patients were 4 and 18.5 respectively. The difference in their performances was statistically significant with t = 7.7 and a P < 0.001 (95% CI: 6.7-11.4) [Figure 2]. There were progressive improvements in cognitive performances of patients over a period of 12 weeks with vinpocetine (Cognitol) therapy in all cognitive domains tested as shown by steady reduction in the mean of error scores [Table 3].
Figure 2.
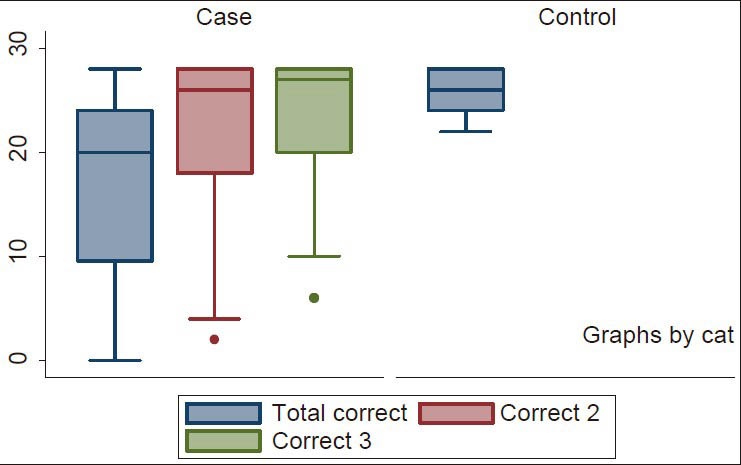
Box plot showing correct scores of study participants. Total correct 1: Mean correct scores at baseline; correct 2: Mean correct scores at 6 weeks; correct 3: Mean correct scores at 12 weeks
Selective improvement based on the severity of cognitive impairment
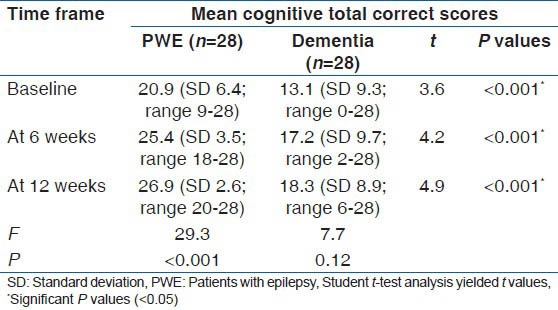
The sub-group analysis of the 56 patients revealed that the more severe the cognitive impairment the less the improvement observed with vinpocetine therapy. With the categorization of patients into PWE and dementia, the mean cognitive performance (as determined with total correct scores) of PWE was noted to be higher than those with dementia. The mean total correct scores for the PWE and dementia patients at baseline were 20.9 (SD: 6.4) (95% CI: 18.4-23.4); range: 9-28 and 13.1 (SD: 9.3) (95% CI: 9.5-16.7); range: 0-28 respectively. This was statistically significantly different (P < 0.001; 95% CI 3.5-12.1) implying that patients with dementia had worse cognitive abilities. This is an expected trend. Similarly, the total correct scores at 6 and 12 weeks followed the same trend as presented in Table 4.
Table 4.
Mean cognitive correct scores by patient's category
Despite the improvement in the cognitive performances of patients with dementia observed with 12 weeks therapy of vinpocetine, this did not reach statistical significance (P = 0.12; 95% CI: 0.8-11.2). On the other hand, however, the PWE showed sustained statistically significant improvement in cognitive performance during the 12 week therapy (P < 0.001; 95% CI: 3.1-8.9). The mean total error scores of the PWE gradually declined from the baseline score of 7.1 (SD: 6.4) (95% CI: 4.6-9.6); range 0-19 to 2.6 (SD: 3.5) (95% CI: 1.2-4.0); range: 0-10 at 6 weeks then to 1.1 (SD: 2.6) (95% CI: 0.1-2.1); range: 0-8 at 12 weeks. Contrariwise the initial decline from baseline error score of 14.9 (SD: 9.3) (95% CI: 11.3-18.5); range: 0-28 to 10.8 (SD: 9.7) (95% CI: 7.0-14.6); range: 0-26 at 6 weeks in patients with dementia, which was initially significant (P = 0.04; 95% CI: 9.2-0.9) was not sustained at 12 weeks when the mean error score was 9.7 (SD: 8.9) (95% CI: 6.3-13.2); range: 0-22 and the t value obtained when compared with the 6-week score yielded 0.6 and P value of 0.53 (95% CI: 4.9-7.2) [Figure 3].
Figure 3.
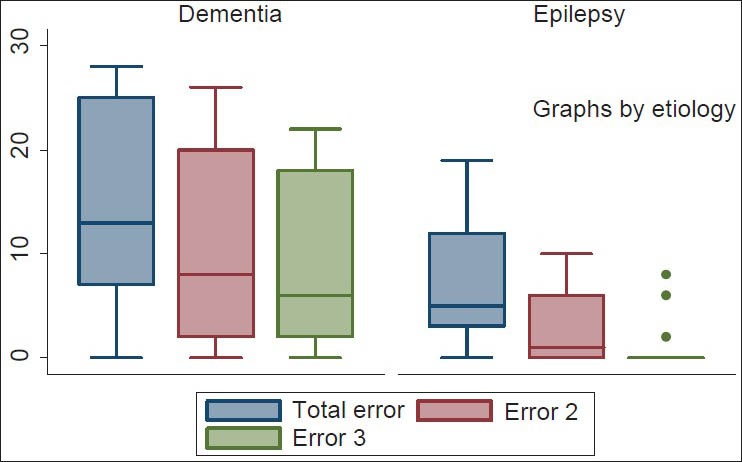
Box plot showing total error scores of patients with epilepsy and dementia. Total error 1: Mean error scores at baseline; error 2: Mean error scores at 6 weeks; error 3: Mean error scores at 12 weeks
The analysis of the various cognitive domains revealed that the more remarkable improvement in recall ability (memory) was observed in the first 6 weeks and among the PWE [Figure 4]. This was revealed in the likelihood ratios for lack of improvement (i.e., chances of committing errors on testing) in recall ability after 6 weeks of vinpocetine therapy among the PWE and the dementia patients, which were 7.3 (P < 0.01) and 20.7 (P = 0.01) respectively. The same trend was observed for concentration abilities [Figure 5].
Figure 4.
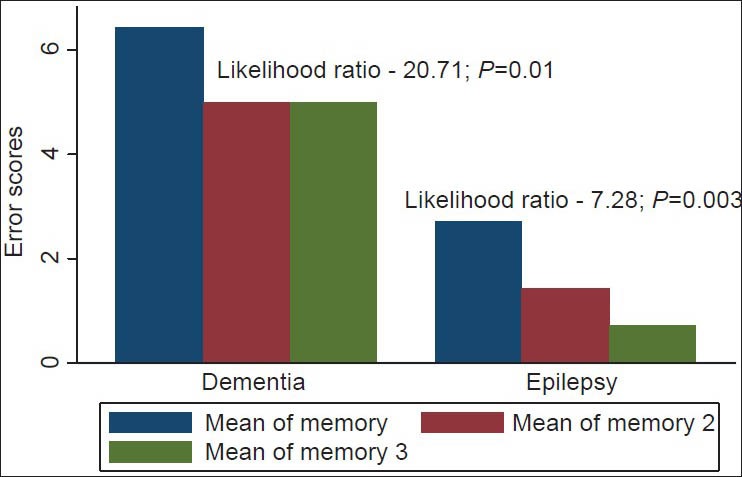
Bar chart showing memory error scores for patients with epilepsy and dementia. Mean of memory 1: Mean error scores at baseline; mean of memory 2: Mean error scores at 6 weeks; mean of memory 3: Mean error scores at 12 weeks
Figure 5.
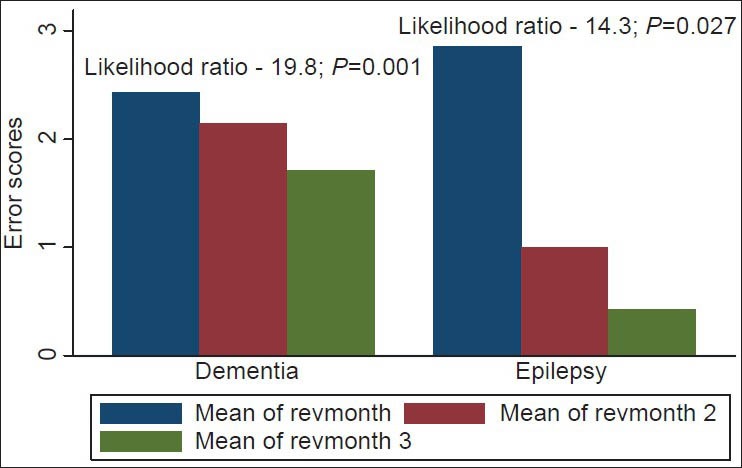
Bar chart showing concentration error scores for patients with epilepsy and dementia. Mean of revmonth 1: Mean error scores at baseline; mean of revmonth 2: Mean error scores at 6 weeks; mean of revmonth 3: Mean error scores at 12 weeks
Impact of disease variables on cognitive performances
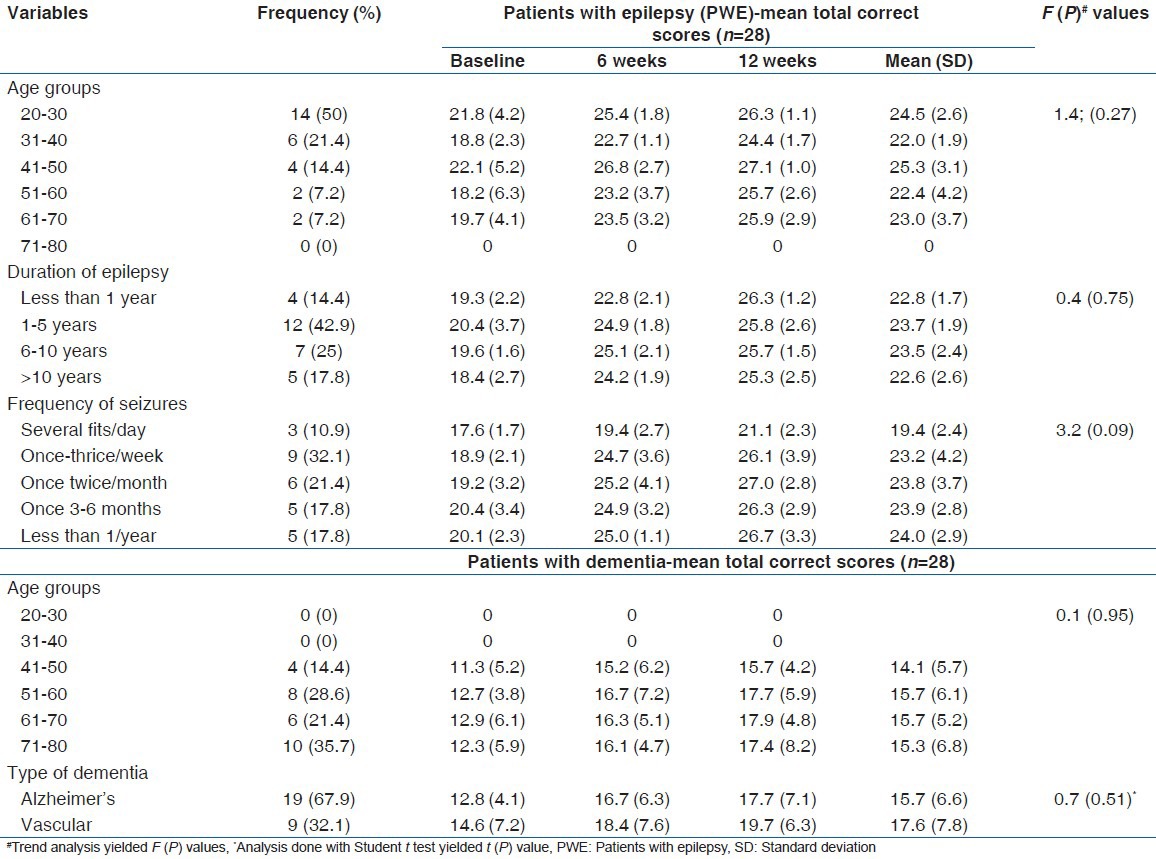
The analysis of the relative impact of clinical variables of the patients on their cognitive performances revealed that the age categories, duration of epilepsy and seizure frequency of the PWE had no significant impact on the trend of cognitive performance with vinpocetine therapy. Similarly, among patients with dementia, the type of dementia and their age categories had no influence on the trend of their performances. Details of analysis are presented on Table 5.
Table 5.
Clinical characteristics of patients and effects on cognitive performance
Adverse reactions
There was no significant adverse effect reported among the study participants. Two of the 56 patients (3.6%) reported transient period of drowsiness which lasted for 2-3 days after the commencement of the drug. Patients were reviewed on a two-weekly basis to assess for any adverse effect. Numerous clinical studies have demonstrated the safety of vinpocetine during long-term administration.[30,31] It has not been shown to change laboratory tests or produce allergic symptoms.[32] Furthermore, no serious drug-drug interactions have been reported.
Discussion
This prospective interventional study showed the beneficial effects of vinpocetine on the cognitive performances of patients with demonstrable disturbances in their cognitive abilities prior to administration of the drug. In addition, the pilot study demonstrated the utility of the SBT in routine cognitive assessment of patients with medical diseases that may be complicated by cognitive deterioration as shown by its acceptable accuracy of 83.9%. It is important to point out that the SBT can only suffice as a screening tool as it is limited in the scope of cognitive domains it can detect. It tests for concentration and memory. The cut-off error score of 6 obtained by the test designers was also obtained in this study,[21] implying that the cut-off value of six can be used for categorizing Nigerians into those who are and are not cognitively impaired.
Though the findings of this preliminary study revealed significant improvement in cognitive abilities of affected patients with vinpocetine treatment, the improvement observed with patients who had severe impairments was not statistically significant. It thus implied that vinpocetine needs to be commenced early once mild cognitive dysfunction is noted in any patient to achieve significant improvement in cognitive performance. Furthermore, it is not obvious from this study why the cognitive improvement plateau out after 6 weeks in patients with dementia. It may be that the drug needs to be continued for more than the 12 week-period of this study to achieve additional benefits. It is not certain if continuing vinpocetine would ensure sustain improvement over time. This requires further studies design to assess cognitive functioning in patients on this therapy for more than 12-24 months.
Previous studies that assessed the effect of vinpocetine on the cognitive abilities of patients with ischemic disturbances of cerebral circulation showed considerable improvement in memory functions in most of the patients, thus corroborating the findings of this study.[26,27,31,32,33] One of the studies used the Mini-Mental Status Questionnaire, Sandoz Clinical Assessment-Geriatric scale and Clinical Global Impression scale and showed that vinpocetine caused improvement of memory functions of patients with chronic cerebral dysfunction at a higher dose of 30 mg a day without any significant untoward effects.[31] In our study, a better improvement, though insignificant, in cognitive ability of patients with vascular dementia was observed compared to those with Alzheimer's dementia. This observation may be related to the role of underlying chronic cerebrovascular changes in the pathogenesis of the vascular type, which makes vinpocetine more effective considering its modes of action.
The probable mechanism by which vinpocetine achieved improvement in memory has been allied to its ability to boost the cerebral metabolism by increasing cerebral blood flow thus enhancing both oxygen and glucose utilization, increasing the rate of neuronal utilization of ATP and consequently improving cerebral functions. It is also believed that this drug maintains neuronal electrical conductivity and protect from damage caused by excessive intracellular release of calcium. It also inhibits abnormal platelet aggregation that may interfere with cerebral hemodynamics.[5,6,26,27,32,33]
The results of this preliminary study have shown the benefits of Vinpocetine (Cognitol™) in the treatment of cognitive impairments especially memory and sustained attention deficits in Nigerian Africans. Furthermore, it has also demonstrated the usefulness of the SBT for the screening of memory and sustained attention impairments in our patients.
Limitation of study
The use of the SBT for cognitive assessment is a limitation because of the restricted cognitive domains that it covers. However, the simplicity of the test and its utility in resource poor settings makes it valuable for screening. Furthermore, the 12 week duration of therapy precludes the assessment of the long-term effects of vinpocetine on cognition. Though the sampling technique has an appreciable power, studies with larger sample sizes involving patients with other chronic medical diseases are needed for further evaluation of cognitive benefits of vinpocetine.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Ogunrin O, Adamolekun B, Ogunniyi AO, Aldenkamp AP. Cognitive function in Nigerians with newly diagnosed epilepsy. Can J Neurol Sci. 2000;27:148–51. [PubMed] [Google Scholar]
- 2.Odiase FE, Ogunrin OA, Ogunniyi AA. Memory performance in HIV/AIDS – A prospective case control study. Can J Neurol Sci. 2007;34:154–9. doi: 10.1017/s0317167100005977. [DOI] [PubMed] [Google Scholar]
- 3.Tozzi V, Balestra P, Galgani S, Murri R, Bellagamba R, Narciso P, et al. Neurocognitive performance and quality of life in patients with HIV infection. AIDS Res Hum Retroviruses. 2003;19:643–52. doi: 10.1089/088922203322280856. [DOI] [PubMed] [Google Scholar]
- 4.Kiss B, Szpomy L. On the possible role of central monoaminergic systems in the central nervous systems actions of vinpocetine. Drug Dev Res. 1988;14:263–79. [Google Scholar]
- 5.Shibuya T, Sato K. Effects of vinpocetine on experimental brain ischemia, histological study of brain monoamines. Igaku No Ayumi. 1986;139:217–9. [Google Scholar]
- 6.Nicholson CD. Pharmacology of nootropics and metabolically active compounds in relation to their use in dementia. Psychopharmacology (Berl) 1990;101:147–59. doi: 10.1007/BF02244119. [DOI] [PubMed] [Google Scholar]
- 7.Biró K, Kárpáti E, Szporny L. Protective activity of ethyl apovincaminate on ischaemic anoxia of the brain. Arzneimittelforschung. 1976;26:1918–20. [PubMed] [Google Scholar]
- 8.Hachinski V, Iadecola C, Petersen RC, Breteler MM, Nyenhuis DL, Black SE, et al. National Institute of Neurological Disorders and Stroke - Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards. Stroke. 2006;37:2220–41. doi: 10.1161/01.STR.0000237236.88823.47. [DOI] [PubMed] [Google Scholar]
- 9.Osuntokun BO, Adeuja AO, Schoenberg BS, Bademosi O, Nottidge VA, Olumide AO, et al. Neurological disorders in Nigerian Africans: A community-based study. Acta Neurol Scand. 1987;75:13–21. doi: 10.1111/j.1600-0404.1987.tb07883.x. [DOI] [PubMed] [Google Scholar]
- 10.Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in Rochester, Minnesota: 1940-1980. Epilepsia. 1991;32:429–45. doi: 10.1111/j.1528-1157.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 11.Imarhiagbe F, Ogunrin O, Ogunniyi A. Cognitive performance of hypertensive elderly Nigerians: A case control study. Afr J Med Med Sci. 2005;34:269–73. [PubMed] [Google Scholar]
- 12.Ogunrin AO, Unuigbe EI, Azubuike C. Memory and perceptuo-motor performance in Nigerians with chronic renal impairment. Med Sci Monit. 2006;12:CR535–9. [PubMed] [Google Scholar]
- 13.Hendrie HC, Osuntokun BO, Hall KS, Ogunniyi AO, Hui SL, Unverzagt FW, et al. Prevalence of Alzheimer's disease and dementia in two communities: Nigerian Africans and African Americans. Am J Psychiatry. 1995;152:1485–92. doi: 10.1176/ajp.152.10.1485. [DOI] [PubMed] [Google Scholar]
- 14.Akinyemi RO, Okubadejo NN, Akinyemi JO, Owolabi MO, Owolabi LF, Ogunniyi A. Cognitive dysfunction in Nigerians with Parkinson's disease. Mov Disord. 2008;23:1378–83. doi: 10.1002/mds.22087. [DOI] [PubMed] [Google Scholar]
- 15.Ogunrin AO, Odiase FE, Ogunniyi A. Reaction time in patients with HIV/AIDS and correlation with CD4 count: A case-control study. Trans R Soc Trop Med Hyg. 2007;101:517–22. doi: 10.1016/j.trstmh.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Obiabo YO, Ogunrin OA, Ogun AS. Effects of highly active antiretroviral therapy on cognitive functions in severely immune-compromised HIV-seropositive patients. J Neurol Sci. 2012;313:115–22. doi: 10.1016/j.jns.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Sunmonu TA, Komolafe MA, Ogunrin AO, Ogunniyi A. Cognitive assessment in patients with epilepsy using the community screening interview for dementia. Epilepsy Behav. 2009;14:535–9. doi: 10.1016/j.yebeh.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 18.Ogunrin AO, Adamolekun B. Cognitive neuro-assessment in Nigerian Africans: Predictive validity of a computerized testing. Ann Biomed Sci. 2007;6:28–44. [Google Scholar]
- 19.Ogunrin OA, Eze UE, Alika F. Usefulness of the HIV dementia scale in Nigerian patients with HIV/AIDS. South Afr J HIV Med. 2009;10:38–43. [Google Scholar]
- 20.Imam I. Neurological manifestations of HIV infection in Nigerians. Afr J AIDS Res. 2007;6:187–92. doi: 10.2989/16085900709490413. [DOI] [PubMed] [Google Scholar]
- 21.Katzman R, Brown T, Fuld P, Peck A, Schechter R, Schimmel H. Validation of a short orientation-memory-concentration test of cognitive impairment. Am J Psychiatry. 1983;140:734–9. doi: 10.1176/ajp.140.6.734. [DOI] [PubMed] [Google Scholar]
- 22.Talesa VN. Acetylcholinesterase in Alzheimer's disease. Mech Ageing Dev. 2001;122:1961–9. doi: 10.1016/s0047-6374(01)00309-8. [DOI] [PubMed] [Google Scholar]
- 23.Tariot PN, Solomon PR, Morris JC, Kershaw P, Lilienfeld S, Ding C. A 5-month, randomized, placebo-controlled trial of galantamine in AD. The Galantamine USA-10 study group. Neurology. 2000;54:2269–76. doi: 10.1212/wnl.54.12.2269. [DOI] [PubMed] [Google Scholar]
- 24.Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer's disease. Am J Psychiatry. 1984;141:1356–64. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 25.Selkoe DJ. Treating Alzheimer's disease: A new era begins. Neurol Alert. 2000;18:81–2. [Google Scholar]
- 26.Krieglstein J, Rischke R. Vinpocetine increases the neuroprotective effect of adenosine in vitro. Eur J Pharmacol. 1991;205:7–10. doi: 10.1016/0014-2999(91)90762-f. [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki M. The effect of a cerebral vasodilator, vinpocetine, on cerebral vascular resistance evaluated by the Doppler ultrasonic technique in patients with cerebrovascular diseases. Angiology. 1995;46:53–8. doi: 10.1177/000331979504600107. [DOI] [PubMed] [Google Scholar]
- 28.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The consortium to establish a registry for Alzheimer's disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–65. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 29.Commission on Classification and Terminology. International league against epilepsy. Proposal for classification of epilepsies and epileptic syndromes. Epilepsia. 1985;26:268–78. [PubMed] [Google Scholar]
- 30.Feigin VL, Doronin BM, Popova TF, Gribatcheva EV, Tchervov DV. Vinpocetine treatment in acute ischaemic stroke: A pilot single-blind randomized clinical trial. Eur J Neurol. 2001;8:81–5. doi: 10.1046/j.1468-1331.2001.00181.x. [DOI] [PubMed] [Google Scholar]
- 31.Balestreri R, Fontana L, Astengo F. A double-blind placebo controlled evaluation of the safety and efficacy of vinpocetine in the treatment of patients with chronic vascular senile cerebral dysfunction. J Am Geriatr Soc. 1987;35:425–30. doi: 10.1111/j.1532-5415.1987.tb04664.x. [DOI] [PubMed] [Google Scholar]
- 32.Szobor A, Klein M. Ethyl apovincaminate therapy in neurovascular diseases. Arzneimittelforschung. 1976;26:1984–9. [PubMed] [Google Scholar]
- 33.Kiss B, Kárpáti E. Mechanism of action of vinpocetine. Acta Pharm Hung. 1996;66:213–24. [PubMed] [Google Scholar]


