Abstract
Rationale:
Pharmacotherapy for patients infected with human immunodeficiency virus (HIV) is complex and increases the potential for drug therapy problems (DTPs). We described the frequency and type of DTPs in a Nigerian cohort of HIV infected patients on antiretroviral therapy (ART), as well as the changes in HIV clinical outcomes after pharmacists’ intervention.
Methods:
A prospective 1-year descriptive study was conducted from July 2010 to June 2011, at the adult HIV clinic of Jos University Teaching Hospital, Nigeria. DTPs and the associated pharmacist-initiated interventions were documented. Chi-square and Wilcoxon signed ranks test was used as appropriate, to compare the main outcome measures of pre- and post-intervention levels of viral load and CD+ cell count.
Results:
A total of 64,839 prescriptions were dispensed to 9320 patients. Interventions were documented for 85 unique patients (incidence of 1.31 interventions/1000 prescriptions), of which 62 (73%) and 3 (3.5%) were on first- and second-line ART, respectively, while 20 (23.5%) were yet to commence ART. Reasons for pharmacist intervention included failure to initiate therapy for HIV or hepatitis B infection; therapeutic failure (25.9%); and drug toxicity (24.7%). After intervention, the percentage of patients with HIV ribonucleic acid level <400 copies/mL rose from 29.4% to 67.1% (P < 0.001), while median (interquartile range) CD4+ cell count increased from 200 (123–351) to 361 (221–470) cells/mm3 (P < 0.001).
Conclusion:
Pharmacist intervention resulted in clinically significant improvements in patients HIV virological and immunological outcomes. This highlights an important role for the pharmacist in the treatment and care of HIV-infected patients, in a multidisciplinary team.
Keywords: Antiretroviral therapy, drug therapy problem, pharmacist intervention
Introduction
Pharmacotherapy for patients infected with human immunodeficiency virus (HIV) is complex and presents unique challenges, for a number of reasons. Treatment of HIV typically requires the use of three or more antiretroviral (ARV) medications, and careful consideration must be given to the pharmacologic properties of each in order to optimize outcomes.[1] Therapeutic options and treatment guidelines are continually evolving, with approximately 30 new ARVs approved since 1987 and more in advance stages of the drug development pipeline.[2] In addition, ARVs are highly susceptible to drug-drug interactions. Furthermore, HIV-infected patients require additional education and counseling regarding their treatment, how to take their medications, the importance of adherence and ways to identify and cope with long-term consequences of therapy.[1] These complexities increase the potential for drug therapy problems (DTPs).
A DTP is any undesirable event that involves a patient's drug therapy, which actually or potentially interferes with achieving desired health outcomes, and requires clinical judgment to resolve.[3,4] Identification, prevention and resolution of DTPs is the bedrock of pharmaceutical care. Pharmacists play a key role in preventing and resolving DTPs by identifying actual or potential DTPs and make recommendations to the patient or other healthcare providers, as appropriate, to resolve such problems. This role is particularly important when providing pharmaceutical care to HIV-infected persons given the complex nature of drug therapy for HIV and other co-morbidities.[5,6]
Several studies provide evidence of the beneficial impact of HIV-specialized pharmacists on HIV treatment outcomes and treatment adherence among infected individuals.[7,8] In an observational cohort study of 1571 HIV-infected patients prescribed their initial antiretroviral therapy (ART) regimen in clinics either with or without a clinical pharmacist, patients exposed to a clinical pharmacist were twice more likely to achieve an HIV ribonucleic acid (RNA) level <500 copies/mL at 12 months (odds ratio = 2.01, 95% confidence interval: 0.92-4.3).[9] Similar findings were also noted by Scott et al., in that HIV pharmacist-led interventions, such as ARV regimen simplification and adherence counseling were associated with better drug therapy outcomes, with 96% of patients able to accomplish or maintain undetectable viral loads postintervention compared with 63% preintervention (P < 0.001).[10]
A review of published studies evaluating HIV pharmacists’ impacts on primary outcomes showed that over 68% of the studies were conducted in the US and other developed countries.[11] Literature is sparse on similar studies conducted in sub-Saharan Africa. An earlier published study conducted in Kenya focused on clinically significantly drug interactions and was not pharmacist initiated.[12] Furthermore, most of the published reports of clinical pharmacists’ activities were descriptive in nature and lacked corresponding data on improvements in patients’ clinical outcomes. The aim of the current study is to describe changes in clinical endpoints (viral load, CD4+ count and ARV toxicity-related laboratory evaluations) that occurred after intervention by a pharmacist among patients on ART in a large outpatient HIV treatment center in Nigeria.
Subjects and Methods
This was a prospective case series of HIV-infected adults (≥15 years old) for whom a pharmacist-initiated intervention was made to prevent or resolve a DTP. The study was conducted from July 2010 to June 2011, at the adult HIV clinic of Jos University Teaching Hospital, Nigeria (JUTH HIV clinic). The JUTH HIV clinic was first established in 2002 through the Federal Government of Nigeria National HIV treatment Program. Since 2004, the JUTH HIV clinic has received additional funding for patient treatment care, and support as well as capacity building as part of the Harvard President's Emergency Plan for AIDS Relief (PEPFAR)/AIDS Prevention Initiative in Nigeria (APIN) plus program. At the time of study, over 13,000 HIV-infected adults were enrolled in care, and approximately 9300 of these patients were on ART.
Antiretroviral therapy eligibility and laboratory monitoring schedule
Antiretroviral therapy eligibility criteria were based on National Adult ART Guidelines, which at the time of the study recommended ART for all HIV-infected adults with CD4 cell count <200 cells/mm3, as well as for those with CD4 cell count <350 cells/mm3 and clinical stage 3 or 4 conditions.[13] If eligible, patients were dispensed ART monthly, free of charge, in the JUTH HIV clinic. Per program protocol, patients underwent laboratory evaluations pre-ART initiation, and then at 12 weeks and subsequently every 24 weeks (approximately) after ART initiation. These included plasma HIV viral load, CD4 cell count, basic blood chemistry and complete blood counts.
Pharmacists’ dispensing and prescription evaluation procedures
At the time of ART initiation and each refill visit, clinic pharmacists provided patient education on ART and the importance of adherence, as well as screened prescriptions to identify potential and actual DTP, taking into consideration patient factors such as medication history, comorbidities, drug interactions and aberrant laboratory test results. At time of dispensing, the pharmacist documented prescription records into an electronic pharmacy record (FileMaker Pro, v10), which had been developed as part of the Harvard PEPFAR/APIN plus program. Identified DTPs were documented in the pharmacy database. Information related to the DTP that was documented routinely included, patient demographic data, type of DTP, laboratory parameters and actions taken to resolve the DTP. Only interventions accepted by the attending physician were documented. For the purpose of this study, interventions were graded as major or minor by two independent clinical pharmacists. Major interventions included identified DTPs that could have a direct impact on patient outcomes, such as therapeutic failure, management of comorbidities, or abnormal laboratory values that may be related to drug toxicity, while minor interventions included those specific to the written prescription, such as illegible handwriting and identity problems.
In order to evaluate changes in patients’ HIV virological and immunological outcome measures (HIV RNA level, CD4+ cell count) and common ARV-related toxicity parameters (alanine transaminase [ALT], serum creatinine [Cr], hemoglobin [Hb]), these data variables were collected at time points before and after pharmacist intervention. Preintervention was defined as the value at or within 12 months prior to the intervention; postintervention was defined as the value within 12 months after the intervention. All patients included in this study signed an informed consent as per the clinic protocol at the time of enrollment into the HIV treatment program.
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 20 (IBM Corp., Armonk, NY, USA). For the descriptive analysis, categorical variables were expressed as percentages and frequencies, while continuous variables were expressed as median (interquartile range [IQR]). Change in HIV clinical outcomes pre-pharmacist to postpharmacist intervention were evaluated at 95% significance level using Wilcoxon signed ranks test for continuous data or Pearson Chi-square or Fisher's exact analysis, as appropriate, for categorical variables.
Results
During the 1-year study period, four HIV-specialized pharmacists dispensed 64,839 prescriptions to 9320 unique HIV-infected patients. A total of 85 major, pharmacist-initiated interventions were made in 85 unique patients (0.9% of the patient population seen during the study period), which resulted in an incidence rate of 1.31 interventions/1000 prescriptions. Among those patients in whom prescription interventions were made, 56 (65.9%) were female, and the median (IQR) age was 35 (31–40) years, neither of which differed significantly from the overall patient population seen at the clinic. Furthermore, among the 85 patients in whom a pharmacist-led intervention was made, 65 (76.5%) were already taking ART (“ART-experienced”), of whom 62 (95.4%) and 3 (4.6%) were on first- and second-line ART, respectively. The remaining 20 patients (23.5%) were yet to commence ART (“ART-naïve”). ART-experienced patients had been on treatment for a median (IQR) duration of 39 (22-60) months at the time of intervention. There were 8 (9.3%) study patients lost to follow-up prior to the end of the study follow-up period.
The patterns of DTPs reported in the study are summarized in Table 1. The most common type of DTP, documented in 49.3% of major interventions, was an untreated indication, such as failure to initiate ART when eligible or suboptimal treatment for hepatitis B co-infection. This was followed by therapeutic failure and drug toxicity, which represented 25.9% and 22.9% of all documented DTPs, respectively.
Table 1.
Description of drug therapy problems
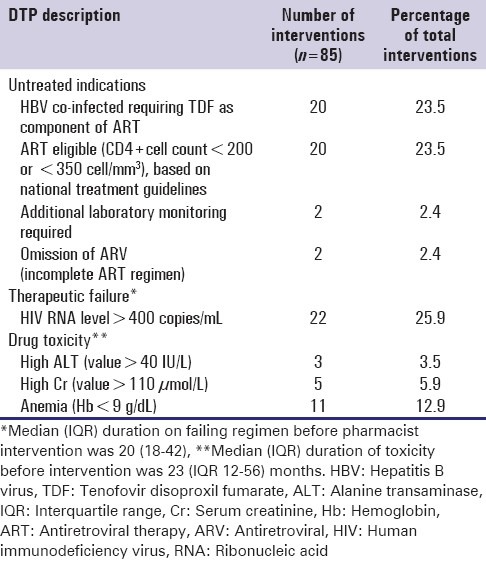
Pharmacist-led intervention frequently resulted in medication changes to resolve the DTPs, which included an ARV drug substitution to a safer first-line drug, a switch from first to second-line ART, or ART initiation in 33.8%, 23.5%, and 24.7% of patients, respectively. Other actions taken, as a result, of pharmacist intervention included the addition of a new drug (8.2%); drug discontinuation (2.4%); and dosage adjustment or and adherence education (1.2% each).
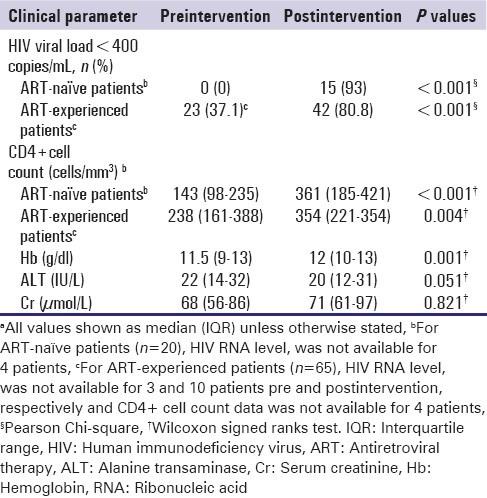
The HIV and ARV toxicity outcome measures pre- and post-intervention are summarized in Table 2. Overall, there was a significant improvement in virological and immunological outcomes from the time pre to postpharmacist intervention. The proportion of patients with an HIV RNA level <400 copies/mL increased from 29.4% to 67.1% (P < 0.001), while the median CD4+ cell count increased from 200 (123–351) cells/mm3 to 361 (221–470) cells/mm3 (P < 0.001). The changes were notable for ART-naïve and ART-experienced patients [Table 2]. Although statistically significant changes were observed in median Hb and ALT values from pre-intervention to post-intervention, the number of patients with abnormal Hb, ALT, and Cr values pre-intervention and post-intervention did not differ significantly (data not shown).
Table 2.
Clinical outcomes pre and post pharmacist interventiona
Discussion
To the best of our knowledge, this is the first report from a large outpatient HIV clinic in Nigeria to describe HIV treatment outcomes following pharmacist-led intervention. Although the number of major interventions in this study was few, the improvements in virologic and immunologic outcomes that occurred among these patients postpharmacist intervention were significant.
In this study, untreated therapeutic indication was the most commonly identified major DTP. Specifically, pharmacists initiated intervention resulted in the modification of ART in 20 HIV/hepatitis B virus (HBV) co-infected patients who were not receiving an optimized treatment regimen against HBV, which includes the use of two ARVs that are also active against HBV, such as tenofovir plus lamivudine or tenofovir plus emtricitabine.[14] Optimal HBV treatment reduces the risk of liver injury and mortality by optimizing suppression of HBV replication, as well as reduces the risk of selecting for HBV resistance.[15,16,17,18,19] Another common untreated indication warranting pharmacists intervention identified in our study was failure to initiate ART among patients eligible for HIV. Interventions resulting in the initiation of ART for eligible patients have profound patient and population level benefits, as the use of ART not only reduces HIV and nonHIV related morbidity and mortality among HIV-infected patients, most significantly among those with lower CD4 cell counts, but also significantly reduces the rate of HIV transmission to currently uninfected persons.[14] Highlighting the importance of earlier initiation of ART, since the time of this study, national and international guidelines have continued to evolve and now recommend ART for individuals with CD4 ≤350 cells/mm3,[20] or ≤500 cells/mm3,[14] respectively. As our study findings suggest, pharmacists play an important role in aiding the implementation of these ever-changing guidelines by identifying patients who are now eligible for ART.
Importantly, our study also identified that pharmacists play a key role in helping to identify patients that may be failing their current ART regimen. Prompt identification of ART failure is important, as maintaining a failing regimen may results in accumulation of resistant mutations, thereby limiting future treatment options.[21,22] Although we were unable to evaluate the impact on ARV resistance during this period, among the 22 patients in whom virological failure was detected by pharmacists, 81% achieved viral suppression postintervention, which included a switch to second-line ART and/or enhanced adherence counseling. Notably, we observed that patients were maintained on failing regimens for a median duration of >20 months before pharmacists’ intervention, which highlights an opportunity for earlier intervention with heightened awareness by the pharmacist and clinical team. Following identification of and intervention for these critical DTPs, virological and immunological improvements were observed among both ART-naïve and ART-experienced patients, which is comparable to many other studies evaluating the impact of pharmacists’ intervention on HIV outcomes.[11,20]
The impact of pharmacists’ intervention resulted in a reduction in ARV associated toxicity. We postulate that this was related to dosage modifications, drug substitutions and addition of new drugs recommended by HIV pharmacists.
The pharmacist's intervention rate in this study was surprisingly low. Previous studies have reported pharmacist-identified prescription error rates of 7-50% among HIV-infected patients receiving ART.[23,24] The low pharmacists intervention rate in this study may be attributed to many factors, including a high-patient burden and under-reporting of interventions. The high-patient burden in our clinic setting is a major barrier to the provision of optimal pharmaceutical care. Most published studies reporting high-medication error rates among patients on ART were carried out in developed countries with adequate capacity and pharmacists: Patient ratio of <50;[10,11] our pharmacists-patient ratio was nearly 50 times higher than this. This high patient burden may contribute to under-reporting of interventions, as there is often not adequate time during clinic to document each pharmacist intervention. Another possible contributor to under-reporting in our study is the fact that only interventions accepted by the attending physician were documented. Under-reporting of interventions by pharmacists is a known phenomenon that has been observed in other studies.[23,24] Boardman et al. analyzed pharmacists’ activities on the wards and found that under one-third (31%) of interventions were actually documented. The interventions that were documented tended to be those of highest clinical importance and those that were time-consuming to the pharmacist. Lack of time was the main reason interventions were not documented.[23] Given the low rate of documented major interventions in our study; we recognize that under-reporting is a possible limitation to the interpretation of our findings, which makes it difficult to determine the actual impact of pharmacists’ intervention. As suggested by Boardman et al., the interventions documented in our study were those of greatest clinical importance, and may, therefore, represent those most likely to result in favorable HIV clinical outcomes. Additionally, patients in our study were not randomized to receive specific pharmacist's intervention; hence, the impact of pharmacist intervention on treatment outcomes could not be fully evaluated.
Conclusion
Though the documented pharmacists’ intervention rate was low in this study, we were still able to describe several important DTPs among HIV-infected patients in our clinic in whom favorable HIV virological and immunological outcomes postpharmacist intervention was observed. This study provides further evidence that, with the growing number of HIV-infected individuals worldwide and the ever-increasing intricacies of HIV treatment options, clinical pharmacists educated in the pharmacotherapy of HIV are very useful resources and are essential members of the HIV multidisciplinary care team.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.ASHP statement on the pharmacist's role in the care of patients with HIV infection. Am J Health Syst Pharm. 2003;60:1998–2003. doi: 10.1093/ajhp/60.19.1998. [DOI] [PubMed] [Google Scholar]
- 2.Food and Drug Administration. Antiretroviral drugs approved by FDA for HIV. [Last cited on 2014 Feb 20]. Available from: http://www.fda.gov/ForConsumers/By Audience/ForPatientAdvocates/ucm076940.htm .
- 3.Strand LM, Morley PC, Cipolle RJ, Ramsey R, Lamsam GD. Drug-related problems: Their structure and function. DICP. 1990;24:1093–7. doi: 10.1177/106002809002401114. [DOI] [PubMed] [Google Scholar]
- 4.Cipolle RJ, Strand LM, Morley PC. New York, NY: McGraw-Hill; 2012. Pharmaceutical Care Practice: The Patient-Centered Approach to Medication Management Services. [Google Scholar]
- 5.Gallant JE, Adimora AA, Carmichael JK, Horberg M, Kitahata M, Quinlivan EB, et al. Essential components of effective HIV care: A policy paper of the HIV Medicine Association of the Infectious Diseases Society of America and the Ryan White Medical Providers Coalition. Clin Infect Dis. 2011;53:1043–50. doi: 10.1093/cid/cir689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oqua D, Agu KA, Isah MA, Onoh OU, Iyaji PG, Wutoh AK, et al. Improving pharmacy practice through public health programs: Experience from Global HIV/AIDS initiative Nigeria project. Springerplus. 2013;2:525. doi: 10.1186/2193-1801-2-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson KC, Hindman J, Johnson SC, Valuck RJ, Kiser JJ. Assessing the effectiveness of pharmacy-based adherence interventions on antiretroviral adherence in persons with HIV. AIDS Patient Care STDS. 2011;25:221–8. doi: 10.1089/apc.2010.0324. [DOI] [PubMed] [Google Scholar]
- 8.Ma A, Chen DM, Chau FM, Saberi P. Improving adherence and clinical outcomes through an HIV pharmacist's interventions. AIDS Care. 2010;22:1189–94. doi: 10.1080/09540121003668102. [DOI] [PubMed] [Google Scholar]
- 9.Horberg MA, Hurley LB, Silverberg MJ, Kinsman CJ, Quesenberry CP. Effect of clinical pharmacists on utilization of and clinical response to antiretroviral therapy. J Acquir Immune Defic Syndr. 2007;44:531–9. doi: 10.1097/QAI.0b013e318031d7cd. [DOI] [PubMed] [Google Scholar]
- 10.Scott JD, Abernathy KA, Diaz-Linares M, Graham KK, Lee JC. HIV clinical pharmacists - The US perspective. Farm Hosp. 2010;34:303–8. doi: 10.1016/j.farma.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Saberi P, Dong BJ, Johnson MO, Greenblatt RM, Cocohoba JM. The impact of HIV clinical pharmacists on HIV treatment outcomes: A systematic review. Patient Prefer Adherence. 2012;6:297–322. doi: 10.2147/PPA.S30244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kigen G, Kimaiyo S, Nyandiko W, Faragher B, Sang E, Jakait B, et al. Prevalence of potential drug-drug interactions involving antiretroviral drugs in a large Kenyan cohort. PLoS One. 2011;6:e16800. doi: 10.1371/journal.pone.0016800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abuja, Nigeria: 2007. [Last cited on 2014 Feb 20]. Federal Ministry of Health. National guidelines for HIV and AIDS treatment and care in adolescents and adults. Available from: http://www.who.int/hiv/amds/Nigeria_adult_2007.pdf . [Google Scholar]
- 14.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach. 2013. Jun, [Last cited 2014 Feb 20]. Available from: http://www.who.int/hiv/pub/guidelines/arv2013/download/en/ [PubMed]
- 15.Thio CL, Seaberg EC, Skolasky R, Jr, Phair J, Visscher B, Muñoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 16.Lok AS, McMahon BJ. Chronic hepatitis B: Update 2009. Hepatology. 2009;50:661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- 17.European Association for the Study of the Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167–85. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 18.European AIDS Clinical Society. Guidelines for treatment of HIV infected adults in Europe. Version 6. [Last cited on 2013 May 25]. Available from: http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/eacsguidelines-6.pdf .
- 19.Benhamou Y, Bochet M, Thibault V, Di Martino V, Caumes E, Bricaire F, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology. 1999;30:1302–6. doi: 10.1002/hep.510300525. [DOI] [PubMed] [Google Scholar]
- 20.Federal Ministry of Health. National guidelines for HIV and AIDS treatment and care in adolescents and adults. 2010. Oct, [Last cited on 2013 May 25]. Available from: http://www.who.int/hiv/pub/guidelines/nigeria_art.pdf?ua=1 .
- 21.Sigaloff KC, Hamers RL, Wallis CL, Kityo C, Siwale M, Ive P, et al. Unnecessary antiretroviral treatment switches and accumulation of HIV resistance mutations; two arguments for viral load monitoring in Africa. J Acquir Immune Defic Syndr. 2011;58:23–31. doi: 10.1097/QAI.0b013e318227fc34. [DOI] [PubMed] [Google Scholar]
- 22.Rawizza HE, Chaplin B, Meloni ST, Darin KM, Olaitan O, Scarsi KK, et al. Accumulation of protease mutations among patients failing second-line antiretroviral therapy and response to salvage therapy in Nigeria. PLoS One. 2013;8:e73582. doi: 10.1371/journal.pone.0073582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boardman H, Fitzpatrick R. Self reported clinical pharmacist interventions under-estimate their input to patient care. Pharm World Sci. 2001;23:55–9. doi: 10.1023/a:1011270507539. [DOI] [PubMed] [Google Scholar]
- 24.Yehia BR, Mehta JM, Ciuffetelli D, Moore RD, Pham PA, Metlay JP, et al. Antiretroviral medication errors remain high but are quickly corrected among hospitalized HIV-infected adults. Clin Infect Dis. 2012;55:593–9. doi: 10.1093/cid/cis491. [DOI] [PMC free article] [PubMed] [Google Scholar]


