Abstract
Cirrhotic cardiomyopathy is a cardiac condition observed in patients with cirrhotic regardless of the etiologies. It is characterized by the impaired systolic response to physical stress, diastolic dysfunction, and electrophysiological abnormalities, especially QT interval prolongation. Its pathophysiology and clinical significance has been a focus of various researchers for the past decades. The impairment of β-adrenergic receptor, the increase in endogenous cannabinoids, the presence of cardiosuppressants such as nitric oxide and inflammatory cytokines are the proposed mechanisms of systolic dysfunction. The activation of cardiac renin-angiotensin system and salt retention play the role in the development of cardiac hypertrophy and impaired diastolic function. QT interval prolongation, which is observed in 40-50 % of cirrhotic patients, occurs as a result of the derangement in membrane fluidity and ion channel defect. The increased recognition of this disease will prevent the complications of overt heart failure after procedures such as transjugular intrahepatic portosystemic shunt (TIPS) and liver transplantation. Better understandings of the pathogenesis and pathology of cirrhotic cardiomyopathy is crucial in developing more accurate diagnostic tools and specific treatments of this condition.
Keywords: Cirrhotic cardiomyopathy, Pathophysiology, Cardiac dysfunction
Introduction
It has been described for almost 60 years that cirrhosis was associated with hyperdynamic circulation characterized by increased cardiac output and reduced peripheral vascular resistance [1]. It was later found that liver dysfunction not only adversely affected the circulation but also the cardiac contractility. Multiple studies revealed impaired hemodynamic responses to physical (exercise) and pharmacological stress [2, 3]. These abnormalities were initially believed to be a direct toxic effect of alcohol; however, subsequent studies in human and animal models of nonalcoholic cirrhosis demonstrated similar manifestation of blunted cardiac contractility in response to stress and electrophysiological abnormality [4–6]. This disorder was formally termed as “cirrhotic cardiomyopathy.” In this review, we summarized the pathyphysiology and clinical significance of cirrhotic cardiomyopathy.
Definition and epidemiology
Cirrhotic cardiomyopathy is defined by chronic cardiac dysfunction typified by impaired cardiac contractility in response to stress and altered diastolic relaxation with electrophysiological abnormalities in cirrhotic patients with no known cardiac diseases [7–9]. At the 2005 World Congress of Gastroenterology at Montreal, a group of experts proposed diagnostic and supportive criteria for cirrhotic cardiomyopathy as follows: (1) systolic dysfunction: blunted increase in cardiac output on exercise, volume challenge or pharmacological stimuli or resting ejection fraction <55 %, (2) diastolic dysfunction: the ratio of early to late (atrial) phases of ventricular filling or E/A ratio <1.0 (age-corrected), prolonged deceleration time (>200 ms), or prolonged isovolumetric relaxation time (>80 ms), (3) supportive criteria: electrophysiological abnormalities, abnormal chronotropic response, electromechanical uncoupling/dyssynchrony, prolonged QTc interval, enlarged left atrium, increased myocardial mass, increased brain natriuretic peptide (BNP) and pro-BNP, or increased troponin I [7].
There is limited data regarding the actual prevalence of cirrhotic cardiomyopathy due to the fact that the disease usually remains silent with near normal cardiac function unless the patients are exposed to stress. It has been estimated that as many as 50 % of patients undergoing liver transplantation developed some signs of cardiac dysfunction [10] and about 7–21 % of patients died from heart failure in the post liver transplantation period [11]. Baik et al. [12] proposed that the majority of cirrhotic patients in Child-Pugh class B and C presented at least one feature of cirrhotic cardiomyopathy namely QTc prolongation and diastolic dysfunction. Moreover, they suggested that diagnostic tests for diastolic dysfunction (e.g. echocardiogram or dynamic cardiac MRI) might be a useful screening tool to detect cirrhotic cardiomyopathy since some element of diastolic dysfunction is present in virtually all patients with moderately advanced cirrhosis.
Clinical manifestation and pathophysiologic process
Systolic dysfunction
Systolic function is normal or increased at rest in the majority of cirrhotic patients with the presentation of hyperdynamic circulation characterized by high cardiac output and tachycardia (2). Physical or pharmacological stress usually unmasked underlying systolic dysfunction in these patients [3, 13, 14]. A study by Wong et al. [13] showed that the increase in cardiac output and ejection fraction in response to exercise were significantly lower in cirrhotics compared to controls. Moreover, Limas et al. [14] demonstrated that patients with alcoholic cirrhosis with ascites exhibited a decrease in left ventricular response to an increase in afterload by angiotensin infusion. The proposed mechanisms of decreased cardiac performance are reduced heart rate response to stress, impaired cardiac contractility and skeletal muscle wasting [15].
There have been some evidences suggesting that systolic dysfunction might contribute to the development of hepa-torenal syndrome (HRS) [16, 17]. It was believed that when the degree of splanchnic vasodilatation was so severe that the increase in cardiac output was not sufficient to maintain circulatory hemostasis, hypotension pursued, followed by stimulation of sympathetic nervous system (SNS), renin-angiotensin-aldosterone system (RAAS), salt and water retention and the development of ascites. HRS occurred as a consequence of intense vasoconstriction from the aforementioned process. A study by Ruiz-Del-Arbol et al. [16] demonstrated that plasma renin and cardiac output were the independent predictors of HRS and the probability of developing HRS was significantly higher in patients with cardiac output\6 L/min. Furthermore, in the event of infection such as spontaneous bacterial peritonitis (SBP), the release of tumor necrosis factor- α (TNF- α) might play a role in the decline in systolic function and precipitate HRS [18].
There are several possible mechanisms explaining the development of systolic dysfunction as illustrated in Fig. 1. The first mechanism is the decreased cardiac responsiveness (chronotropic and inotropic incompetence) through the defect in cardiac β-adrenergic receptor signaling. The impairment of β-adrenergic receptor signaling happens as a consequence of sympathetic over-activity and prolonged exposure to noradrenaline [15]. The low arterial blood pressure and reduced central blood volume are the main triggers of SNS via baroreceptor and volume-mediated receptor, respectively [19]. Sympathetic hyperactivity has been shown to cause direct myocyte damage and reduced β -adrenergic function [15, 20]. Various studies in human and animals demonstrated the desensitization of β-adrenergic receptor either by the down-regulation of β-adrenergic receptor (with subsequent decrease in receptor density) or the alteration of receptor function leading to decreased cAMP production [21–24].
Fig. 1.
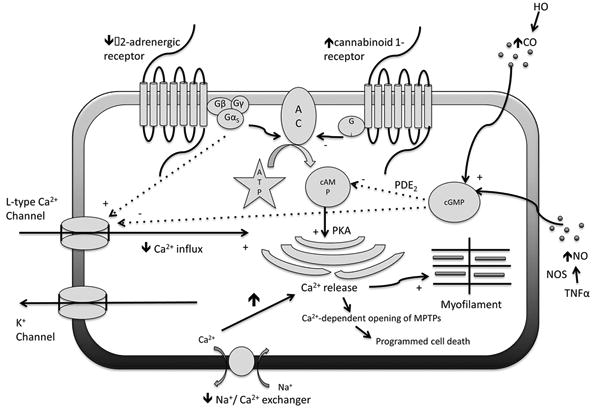
The pathophysiologic process of impaired cardiac contractility and cardiac responsiveness to stress. The defect in cardiac β-adrenergic receptor signaling, the increase in endocannabinoid activity, the negative effect of NO and CO, and myocyte apoptosis are the four possible mechanisms responsible for the cardiac dysfunction. Several intracellular signaling pathways are involved in this pathophysiological process as demonstrated above
Another mechanism that has been a topic of interest for many recent researches is the effect of endogenous cannabinoids (EC), most importantly anandamide (AEA), on cardiac function. Anandamide is synthesized from membrane phospholipid and released from the cell when needed. It exerts its effect through an orphan G protein-coupled receptor called Cannabinoid-1 or CB1 receptor [25]. A study by Gaskari et al. [26] showed the blunted response of ventricular papillary muscle isolated from bile duct ligated (BDL) rats to isoproterenol. This effect was restored by preincubation with CB1 antagonist (AM251). Their study also demonstrated that anandamide reuptake blockers (VDM11 and AM404) increased papillary muscle relaxation in BDL rats but not in controls. This experiment suggested that AEA caused cardiac hypo-responsiveness through CB1 receptor and that endocannabinoids system affected the cirrhotic heart through the locally increased AEA release but not the increased expression of CB1 receptor. A recent study in BDL mice demonstrated that the expression of cardiac AEA was induced by an inflammatory cytokine, tumor necrosis factor-alpha (TNF- α) and endocannabinoid reuptake inhibitor UCM707 escalated the effect of TNF- α on reducing cardiac contractility [27]. This investigation supported the hypothesis that inflammation might trigger the release of AEA, which subsequently depressed systolic function.
Other potential mechanism is the presence of cardiodepressant substances such as nitric oxide (NO) and carbon monoxide (CO). van Obbergh et al. [28] found that the impaired contractility of isolated hearts from BDL rats was recovered by the addition of nitric oxide synthase (NOS) inhibitor, NG monomethyl-L-arginine, while this effect was not observed in controls. Another study suggested that inflammatory cytokines such as TNF- α and interleukin (IL)-1b might play a role in the stimulation of inducible NOS (NOS2) and NO production [29]. NO exerts its negative inotropic effect through the stimulation of cyclic guanosine monophosphate (cGMP), which affects cardiac contraction in 3 different ways: (1) by hastening the degradation of cyclic adenosine monophosphate (cAMP) (the main mediator of β-adrenergic stimulation), (2) by activating protein kinase G, which inhibits the sarcolemmal L-type calcium channel, and (3) by inhibiting the calcium release from sarcoplasmic reticulum [30]. The enzyme heme oxygenase (HO) catalyzes the metabolism of heme to biliverdin and subsequently bilirubin creating CO in the process. CO, like NO, acts on cardiac myocytes through cGMP [31]. It has been shown in cirrhotic rats that CO and inducible (HO-1) isoform might play a role in suppression of cardiac contractility [32].
Recent studies also found that the Na+/Ca2+ exchanger plays an important role in the pathogenesis of cirrhotic cardiomyopathy. Na+/Ca2+ exchanger is essential in keeping the balance between Ca2+ influx and efflux, thus maintaining the steady-state intracellular free Ca2+ concentration. The abnormalities of the Na+/Ca2+ exchanger in cirrhotic patients result in the excess Ca2+ influx leading to cardiomyocyte apoptosis [10, 33].
As previously mentioned, cirrhotic patients have elevated catecholamine levels as a result of sympathetic overactivity. Recent studies showed the important relationship between sympathetic over-activity and the elevation of inflammatory cytokines such as Interleukin-8 (IL-8), Interleukin-6 (IL-6), Interleukin-1b (IL-1b), TNF- α, and notably transforming growth factor- β (TGF-β) [34]. It is important to note that TGF-β stimulates mitogen-activated protein kinases (MAPK), particularly the isoform MAPK/ P-38, and promotes cardiomyocyte apoptotic cell death. Activation of MAPK/P38- α by TGF- β highlights apoptosis as another potential mechanism of cardiomyopathy [10, 34].
Diastolic dysfunction
Diastolic dysfunction is characterized by abnormal left ventricular relaxation impeding blood flow through the ventricle, increasing left ventricular end-diastolic pressure, and increasing atrial contribution to late ventricular filling. These abnormalities represent as increased the E/A ratio and prolonged deceleration time on the 2-dimensional Doppler echocardiography. The recent guidelines of the American Society of Echocardiography have suggested that the measure of the early diastolic mitral annular velocity (e′) is a more accurate marker of diastolic dysfunction [35]. The presence of septal e′ <8 cm/s, lateral e′ <10 cm/s, and enlarged left atrium (LA ≥34 mL/m2) define left ventricular diastolic dysfunction. The degree of severity can also be graded according to average E/e′ ratio. LA volume and peak LA strain at the end of ventricular systole (PALS), determined by 3D echocardiography and speckle tracking echocardiography respectively, have been recently proposed as additional markers for diastolic dysfunction [36, 37].
The clinical significance of diastolic dysfunction was supported by the unexpected heart failure after transjugular intrahepatic portosystemic shunts (TIPS) [38]. A study by Huonker et al. [39] exhibited the increase in the left atrial diameter, the pulmonary capillary wedge pressure, and total pulmonary resistance after the TIPS, which reflected the presence of diastolic dysfunction in cirrhotics. The histopathology of diastolic dysfunction was demonstrated in the autopsy series of cirrhotic patients and patients with alcoholism without underlying heart diseases, which showed cardiomyocyte hypertrophy, altered pigmentation, interstitial fibrosis and myofiber vacuolization [40, 41]. The evidence supporting the pathogenesis of diastolic dysfunction is scarce; however, it is proposed that alteration in collagen configuration, sodium retention and activation of RAAS are potential mechanisms [31].
Glenn et al. [42] studied the role of titin and collagen in the pathogenesis of diastolic dysfunction in BDL rats. They found the increase in the stiffer collagen I, and the decrease in the more compliant collagen III in cirrhotic rats. Moreover, Protein kinase A (PKA), the important post-translational modulator of titin's action, was significantly reduced in cirrhotic rats. The fall in PKA levels can lead to the decreased phosphorylation of titin and thus the increase in passive tension. Also, Phosphorylation of troponin I and calcium dissociation from troponin C may reduce as a consequence of decreased PKA levels causing a rise in diastolic time. A study in patients with heart failure supported that relatively hypophosphorylation of the stiff N2B titin isoform might pay a role in elevating cardiomyocyte resting tension and diastolic stiffness [43].
There has been some data indicating that salt and water retention in cirrhosis may play a part in the development of diastolic dysfunction. Animal models have shown that high salt intake can lead to concentric left ventricular hypertrophy and elevated left ventricular filling pressure without a rise in blood pressure [44, 45]. Salt loading causes cardiac hypertrophy through the activation of cardiac aldo-sterone production independently of the circulating RAAS [45]. Other than an effect of aldosterone, cardiac angiotension II, acting via angiotensin-1 receptor, per se can induce cardiomyocyte hypertrophy and gene programming, and cardiac fibroblast proliferative and fibrosis [46]. Saltstimulated overexpression of transforming growth factor- β1 (TGF- β1) in the heart is another possible mechanism contributing to cardiac hypertrophy, intramyocardial fibrosis and fibrosis of intramyocardial arteries [47].
Decreased intestinal motility, increased intestinal permeability, alteration in local mucosal immune system contribute to the increase in bacterial translocation and endotoxemia in cirrhotic patients [48]. A recent study by Karagiannakis et al. [49] showed that the serum level of lipopolysaccharide-binding protein (LBP), a marker of an exposure to bacterial endotoxin, was independently associated with the presence of diastolic dysfunction. Furthermore, the LBP was positively correlated with the severity of diastolic dysfunction determined by average E/e′. They postulated that the bacterial endotoxin aggravated the splanchnic vasodilation and hyperdynamic circulation, which worsened the cardiac strain.
Electrophysiological abnormalities
Electrophysiological abnormalities observed in cirrhosis comprised prolonged QT interval and the defect in electromechanical coupling. The abnormalities have been linked to the defect in the sympathetic system and vagal impairment (autonomic dysfunction) [10]. The prevalence of QT interval prolongation is approximately 40–50 % [50, 51]. According to a study by Bernardi et al. [50], the prevalence of prolonged QT interval increased substantially from Child-Pugh class A to B; however, did not differ among different etiologies of cirrhosis. Although Bernardi et al. [50] showed that patients with prolonged QT interval had lower survival rate than the normal counterparts; this finding was not reproduced by a more recent study by Bal et al. [51]. Their results demonstrated that age, Child-Pugh scores and alcoholic cirrhosis were independent predictors of QT interval prolongation but its presence had no effect on mortality. Moreover, the same study showed that QT interval normalized in 55 % of patients post liver transplantation. Trevisani et al. [52] evaluated QT interval in non-cirrhotic portal hypertension and cirrhotic patients post TIPS and found that prolonged QT interval was also present in patients with non-cirrhotic portal hypertension. Other interesting result from that study was the worsening of QT interval after TIPS. Combining both findings suggested that porto-systemic shunting and the delivery of cardioactive substances from splanchnic to systemic circulation might be the possible pathogenesis of QT interval prolongation.
Electromechanical coupling is the process of converting an electrical stimulus to a mechanical response and its defect plays a part in the impairment of cardiac function. Bernardi et al. [53] assessed the cardiovascular responsiveness to exercise in cirrhotic patients and discovered the prolongation of pre-ejection period, electromechanical delay and pre-ejection period to left ventricular ejection time ratios in patients with cirrhosis. They also found that the decrease in these parameters in response to exercise was impaired in cirrhotics. These data indicated that the defect in electromechanical coupling might result in cardiac contractility dysfunction. The presence of electromechanical dyssynchrony was confirmed in the study by Henriksen et al. [54], which revealed that the relation between electrical (QT) and mechanical systole (systolic time) in cirrhotic patients with prolonged QT interval was lessened.
The derangement in plasma membrane fluidity and subsequent changes in membrane receptor and ion channel function are main contributors of electrophysiological abnormalities in cirrhotic cardiomyopathy. A study in BDL rats illustrated the increase in cholesterol-to-phospholipid ratio and membrane rigidity in cardiac plasma membrane of cirrhotic hearts [55]. Those changes led to the decline in β-adrenoreceptor density and b-adrenoreceptor-mediated cAMP production. Membrane fluidity and β-adrenorecep-tor function were restored after incubated with fluidizing agent. Bile acids itself also have the direct toxic effect on membrane fluidity and cardiac β-adrenoceptor density and affinity [56].
Plasma membrane changes may also cause ion channel defects leading to a marked prolongation of action potential and QT interval. A rat model of cirrhosis demonstrated decreases in K+ current (both Ca2+-independent transient outward K+ current and delayed rectifying K+ current), which led to prolonged repolarization phase and action potential [57]. According to that experiment, those changes were likely explained by a decrease in current density such as a reduced functional K+ channels. A study in rat ventricles showed that action potential prolongation resulted in a marked reduction in peak inward Ca2+-dependent membrane current during the plateau phase, a slower rate of decline in Ca2+ current and a longer relaxation time [58]. This phenomenon might cause a prolonged cardiac contraction and impaired relaxation [10].
Sympathetic over-activity might also play a role in QT prolongation. A study by Henriksen demonstrated that plasma noradrenaline level was positively correlated with prolonged QT in cirrhosis and a non-selective β-blocker could shorten the QT interval [54]. In the presence of ion channel alteration (as stated above), the influence of sympathetic activity on QT interval might be different from normal individuals. This hypothesis was supported by the increased risk of cardiac arrhythmia with elevated sympathetic activity in patients with type 1 and 5 long QT syndromes, who possessed mutant genes coding for delayed rectified potassium channels in the heart [59]. Moreover, a sudden rise in sympathetic tone could transiently prolong QTc interval as shown in a recent study in cirrhotic patients with acute gastrointestinal (GI) bleeding [60]. Trevisani et al. [60] measured QTc interval in 70 cirrhotic and 40 non-cirrhotic patients with acute GI bleeding and found that QTc increased significantly at the time of bleeding and returned to baseline in 6 weeks in cirrhotic patients but no changes were observed in controls. Interestingly, the investigators also demonstrated that QTc interval could predict bleeding-related mortality at 6 weeks. It was postulated that the acute surge in sympathetic tone at the time of bleeding and the release of cardiosuppressants during stress might contribute to the prolongation of QT interval.
Management Of Cirrhotic Cardiomyopathy
To date, there is no well-established guideline regarding the diagnosis or the treatment of cirrhotic cardiomyopathy. Since most patients remain asymptomatic in the resting condition, the treatment is initiated only when the symptoms of overt heart failure become apparent. Management of heart failure in cirrhotic patients at this point is similar to non-cirrhotic ones including salt and fluid restriction, diuretics and afterload reduction. However, afterload reduction, which is a main component of heart failure management, might be a challenge in cirrhotics, who already have baseline arterial hypotension. Furthermore, the use of cardiac glycosides, such as digitalis, might not be effective in increasing cardiac contraction in cirrhotic patients based on a study by Limas et al. [14]. The investigators demonstrated the persistent left ventricular systolic dysfunction and unchanged hemodynamics after the infusion of ouabain (short acting cardiac glycoside) in patients with alcoholic cirrhosis [14].
The benefit of β-blockers in cirrhotic heart failure is not as clear as noncirrhotic ones. A use of non-selective β-blocker has been shown to reduce prolonged QT interval toward normal values in patients with cirrhosis along with some beneficial effect in improving electromechanical uncoupling [61]. Nevertheless, the same study also showed the reduction in cardiac output in patients who received propranolol, which might pose a detrimental effect to the heart especially during stress such as infection [62]. Whether the decrease in QT interval will reduce clinically significant arrhythmia or the use of β-blockers will have any mortality benefits need to be further elucidated.
Pozzi et al. [63] conducted a study to evaluate the hemodynamic effect of a long-term treatment with K-Canrenoate (aldosterone antagonist) in Child A preas-citic cirrhotic patients. They found that K-Canrenoate significantly reduced hepatic venous pressure gradient, left ventricular wall thickness and left ventricular end-diastolic volume; however, the treatment failed to improve diastolic dysfunction. It was believed that ventricular wall thickness decreased as a result of anti-fibrotic effect of aldosterone antagonist. The authors proposed that the combination of β-blockers and aldosterone antagonists might have additive effects in improving cardiac function in cirrhotic patients. Further studies are warranted to evaluate this hypothesis.
Similar to other complications of cirrhosis, liver transplantation is a possible cure for cirrhotic cardiomyopathy. Sampathkumar et al. [64] reported a series of patients after liver transplantation and found 7 out of 754 patients who had normal pre-operative echocardiography developed overt heart failure after the operation. However, 86 % of those patients fully recovered in term of ejection fraction and did not recur at the median follow-up of 15 months. Reversibility of cardiac dysfunction after liver transplantation was also observed in a more recent study, which followed hemodynamic parameters of 40 cirrhotic patients pre- and post-transplantation. The results revealed the complete regression of ventricular wall thickness, and diastolic dysfunction, and the restoration of systolic response and exercise capacity under physical stress in all patients between 6 and 12 months post-transplantation [5].
Because of the current limitations and therapeutic options for patients with cirrhotic cardiomyopathy, there remains a critical need for newer, more effective agents for treating this condition. In the future, therapeutic options may focus on specific targets which are pathophysiologic-based, such as antagonists to CB1-receptor, TNF- α, and NOS inhibitor as a treatment of systolic dysfunction or angiotension II and TGF-b1 blockers as a treatment of diastolic dysfunction.
Conclusion
Cirrhotic cardiomyopathy is a recently recognized complication of cirrhosis. Although most patients remain asymptomatic, this condition can pose significant morbidity and mortality in the presence of stressful events such as infection, TIPS and liver transplantation. Due to its diagnostic difficulties, the increased awareness is important in preventing the complications of cirrhotic cardiomyopathy. Understanding the pathophysiologic process of systolic dysfunction, diastolic dysfunction and electrophysiological abnormalities in cirrhotic cardiomyopathy is crucial for the further development of more accurate diagnostic tools and specific treatment.
Acknowledgments
This study is supported by K08 AA016570 from the NIH/NIAAA, 1I01CX000361-01 from the Veterans Affairs Research and Administration, Indiana University Research Support Fund Grant, and W81XWH-12-1-0497 from United States Department of Defense (All to S.L.).
Abbreviations
- AC
Adenylate cyclase
- ATP
Adenosine triphosphate
- cAMP
Cyclic adenosine monophosphate
- cGMP
Cyclic guanosine monophosphate
- CO
Carbon monoxide
- HO
Heme oxygenase
- MPTPs
Mitochondrial permeability transition pores
- NO
Nitric oxide
- NOS
Nitric oxide synthase
- PDE2
Prostaglandin E2
- PKA
Protein kinase A
- TNF- α
Tumor necrosis factor- α
Footnotes
Compliance with ethical requirements and Conflict of interest All authors comply with the ethical policies in preparation of this manuscript. Maneerat Chayanupatkul and Suthat Liangpunsakul have nothing to disclose regarding conflicts of interest.
Contributor Information
Maneerat Chayanupatkul, Department of Medicine, Einstein Medical Center, Philadelphia, PA, USA.
Suthat Liangpunsakul, Email: sliangpu@iupui.edu, Division of Gastroenterology/Hepatology, Department of Medicine, Indiana University Hospital, 550 University Boulevard, UH 4100, Indianapolis, IN 46202-5149, USA; Roudebush Veterans Administration Medical Center, Indiana University, Indianapolis, IN, USA.
References
- 1.Kowalski H, Abelmann WH. The cardiac output at rest in Laennec's cirrhosis. J Clin Invest. 1953;32:1025–1033. doi: 10.1172/JCI102813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gould L, Shariff M, Zahir M, Di LM. Cardiac hemodynamics in alcoholic patients with chronic liver disease and a presystolic gallop. J Clin Invest. 1969;48:860–868. doi: 10.1172/JCI106044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelbaek H, Eriksen J, Brynjolf I, Raboel A, Lund JO, Munck O, et al. Cardiac performance in patients with asymptomatic alcoholic cirrhosis of the liver. Am J Cardiol. 1984;54:852–855. doi: 10.1016/s0002-9149(84)80220-9. [DOI] [PubMed] [Google Scholar]
- 4.Grose RD, Nolan J, Dillon JF, Errington M, Hannan WJ, Bou-chier IA, et al. Exercise-induced left ventricular dysfunction in alcoholic and non-alcoholic cirrhosis. J Hepatol. 1995;22:326–332. doi: 10.1016/0168-8278(95)80286-x. [DOI] [PubMed] [Google Scholar]
- 5.Torregrosa M, Aguade S, Dos L, Segura R, Gonzalez A, Evan-gelista A, et al. Cardiac alterations in cirrhosis: reversibility after liver transplantation. J Hepatol. 2005;42:68–74. doi: 10.1016/j.jhep.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Mohamed R, Forsey PR, Davies MK, Neuberger JM. Effect of liver transplantation on QT interval prolongation and autonomic dysfunction in end-stage liver disease. Hepatology. 1996;23:1128–1134. doi: 10.1002/hep.510230529. [DOI] [PubMed] [Google Scholar]
- 7.Moller S, Henriksen JH. Cardiovascular complications of cirrhosis. Gut. 2008;57:268–278. doi: 10.1136/gut.2006.112177. [DOI] [PubMed] [Google Scholar]
- 8.Moller S, Henriksen JH. Cirrhotic cardiomyopathy. J Hepatol. 2010;53:179–190. doi: 10.1016/j.jhep.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 9.Timoh T, Protano MA, Wagman G, Bloom M, Vittorio TJ. A perspective on cirrhotic cardiomyopathy. Transplant Proc. 2011;43:1649–1653. doi: 10.1016/j.transproceed.2011.01.188. [DOI] [PubMed] [Google Scholar]
- 10.Zardi EM, Abbate A, Zardi DM, Dobrina A, Margiotta D, Van Tassell BW, et al. Cirrhotic cardiomyopathy. J Am Coll Cardiol. 2010;56:539–549. doi: 10.1016/j.jacc.2009.12.075. [DOI] [PubMed] [Google Scholar]
- 11.Myers RP, Lee SS. Cirrhotic cardiomyopathy and liver transplantation. Liver Transpl. 2000:S44–S52. doi: 10.1002/lt.500060510. [DOI] [PubMed] [Google Scholar]
- 12.Baik SK, Fouad TR, Lee SS. Cirrhotic cardiomyopathy. Orphanet J Rare Dis. 2007;2:15. doi: 10.1186/1750-1172-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong F, Girgrah N, Graba J, Allidina Y, Liu P, Blendis L. The cardiac response to exercise in cirrhosis. Gut. 2001;49:268–275. doi: 10.1136/gut.49.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Limas CJ, Guiha NH, Lekagul O, Cohn JN. Impaired left ventricular function in alcoholic cirrhosis with ascites. Ineffectiveness of ouabain. Circulation. 1974;49:754–760. doi: 10.1161/01.cir.49.4.755. [DOI] [PubMed] [Google Scholar]
- 15.Moller S, Henriksen JH. Cirrhotic cardiomyopathy: a patho-physiological review of circulatory dysfunction in liver disease. Heart. 2002;87:9–15. doi: 10.1136/heart.87.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruiz-del-Arbol L, Monescillo A, Arocena C, Valer P, Gines P, Moreira V, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology. 2005;42:439–447. doi: 10.1002/hep.20766. [DOI] [PubMed] [Google Scholar]
- 17.Krag A, Bendtsen F, Henriksen JH, Moller S. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2010;59:105–110. doi: 10.1136/gut.2009.180570. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-del-Arbol L, Urman J, Fernandez J, Gonzalez M, Navasa M, Monescillo A, et al. Systemic, renal, and hepatic hemodynamic derangement in cirrhotic patients with spontaneous bacterial peritonitis. Hepatology. 2003;38:1210–1218. doi: 10.1053/jhep.2003.50447. [DOI] [PubMed] [Google Scholar]
- 19.Moller S, Henriksen JH. The systemic circulation in cirrhosis. In: Gines P, Arroyo V, Rodes J, Schrier RW, editors. Ascites and Renal Dysfunction in Liver Disease. Malden: Blackwell; 2005. pp. 139–155. [Google Scholar]
- 20.Brum PC, Kosek J, Patterson A, Bernstein D, Kobilka B. Abnormal cardiac function associated with sympathetic nervous system hyperactivity in mice. Am J Physiol Heart Circ Physiol. 2002;283:H1838–H1845. doi: 10.1152/ajpheart.01063.2001. [DOI] [PubMed] [Google Scholar]
- 21.Gerbes AL, Remien J, Jungst D, Sauerbruch T, Paumgartner G. Evidence for down-regulation of beta-2-adrenoceptors in cir-rhotic patients with severe ascites. Lancet. 1986;1:1409–1411. doi: 10.1016/s0140-6736(86)91556-4. [DOI] [PubMed] [Google Scholar]
- 22.Hausdorff WP, Caron MG, Lefkowitz RJ. Turning off the signal: desensitization of beta-adrenergic receptor function. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 23.Lee SS, Marty J, Mantz J, Samain E, Braillon A, Lebrec D. Desensitization of myocardial beta-adrenergic receptors in cir-rhotic rats. Hepatology. 1990;12:481–485. doi: 10.1002/hep.1840120306. [DOI] [PubMed] [Google Scholar]
- 24.Ma Z, Lee SS. Cirrhotic cardiomyopathy: getting to the heart of the matter. Hepatology. 1996;24:451–459. doi: 10.1002/hep.510240226. [DOI] [PubMed] [Google Scholar]
- 25.Baldassarre M, Giannone FA, Napoli L, Tovoli A, Ricci CS, Tufoni M, et al. The endocannabinoid system in advanced liver cirrhosis: pathophysiological implication and future perspectives. Liver Int. 2013;33:1298–1308. doi: 10.1111/liv.12263. [DOI] [PubMed] [Google Scholar]
- 26.Gaskari SA, Liu H, Moezi L, Li Y, Baik SK, Lee SS. Role of endocannabinoids in the pathogenesis of cirrhotic cardiomyopa-thy in bile duct-ligated rats. Br J Pharmacol. 2005;146:315–323. doi: 10.1038/sj.bjp.0706331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang YY, Liu H, Nam SW, Kunos G, Lee SS. Mechanisms of TNFalpha-induced cardiac dysfunction in cholestatic bile duct-ligated mice: interaction between TNFalpha and endocannabi-noids. J Hepatol. 2010;53:298–306. doi: 10.1016/j.jhep.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Obbergh L, Vallieres Y, Blaise G. Cardiac modifications occurring in the ascitic rat with biliary cirrhosis are nitric oxide related. J Hepatol. 1996;24:747–752. doi: 10.1016/s0168-8278(96)80272-8. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Ma Z, Lee SS. Contribution of nitric oxide to the patho-genesis of cirrhotic cardiomyopathy in bile duct-ligated rats. Gastroenterology. 2000;118:937–944. doi: 10.1016/s0016-5085(00)70180-6. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Estan J, Ortiz MC, Lee SS. Nitric oxide and renal and cardiac dysfunction in cirrhosis. Clin Sci (Lond) 2002;102:213–222. [PubMed] [Google Scholar]
- 31.Wong F. Cirrhotic cardiomyopathy. Hepatol Int. 2009;3:294–304. doi: 10.1007/s12072-008-9109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu H, Song D, Lee SS. Role of heme oxygenase-carbon monoxide pathway in pathogenesis of cirrhotic cardiomyopathy in the rat. Am J Physiol Gastrointest Liver Physiol. 2001;280:G68–G74. doi: 10.1152/ajpgi.2001.280.1.G68. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Zhang X, Kubo H, Harris DM, Mills GD, Moyer J, et al. Ca2+ influx-induced sarcoplasmic reticulum Ca2+ overload causes mitochondrial-dependent apoptosis in ventricular myocytes. Circ Res. 2005;97:1009–1017. doi: 10.1161/01.RES.0000189270.72915.D1. [DOI] [PubMed] [Google Scholar]
- 34.Zaky A, Lang JD. Cirrhosis-associated cardiomyopathy. J Anesth Clin Res. 2012;3:1–7. [Google Scholar]
- 35.Nagueh SF, Appleton CP, Gillebert TC, Marino PN, Oh JK, Smiseth OA, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. doi: 10.1016/j.echo.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 36.Sampaio F, Pimenta J, Bettencourt N, Fontes-Carvalho R, Silva AP, Valente J, et al. Systolic and diastolic dysfunction in cirrhosis: a tissue-Doppler and speckle tracking echocardiography study. Liver Int. 2013;33:1158–1165. doi: 10.1111/liv.12187. [DOI] [PubMed] [Google Scholar]
- 37.Wiese S, Hove JD, Bendtsen F, Moller S. Cirrhotic cardiomy-opathy: pathogenesis and clinical relevance. Nat Rev Gastroen-terol Hepatol. 2013;11(3):177–186. doi: 10.1038/nrgastro.2013.210. [DOI] [PubMed] [Google Scholar]
- 38.Braverman AC, Steiner MA, Picus D, White H. High-output congestive heart failure following transjugular intrahepatic portal-systemic shunting. Chest. 1995;107:1467–1469. doi: 10.1378/chest.107.5.1467. [DOI] [PubMed] [Google Scholar]
- 39.Huonker M, Schumacher YO, Ochs A, Sorichter S, Keul J, Rossle M. Cardiac function and haemodynamics in alcoholic cirrhosis and effects of the transjugular intrahepatic portosystemic stent shunt. Gut. 1999;44:743–748. doi: 10.1136/gut.44.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lunseth JH, Olmstead EG, Forks G, et al. A study of heart disease in one hundred and eight hospitalized patients dying with portal cirrhosis. AMA Arch Intern Med. 1958;102:405–413. doi: 10.1001/archinte.1958.00030010405009. [DOI] [PubMed] [Google Scholar]
- 41.Schenk EA, Cohen J. The heart in chronic alcoholism. Clinical and pathologic findings. Pathol Microbiol (Basel) 1970;35:96–104. doi: 10.1159/000162206. [DOI] [PubMed] [Google Scholar]
- 42.Glenn TK, Honar H, Liu H, ter Keurs HE, Lee SS. Role of cardiac myofilament proteins titin and collagen in the pathogen-esis of diastolic dysfunction in cirrhotic rats. J Hepatol. 2011;55:1249–1255. doi: 10.1016/j.jhep.2011.02.030. [DOI] [PubMed] [Google Scholar]
- 43.Borbely A, Falcao-Pires I, van Heerebeek L, Hamdani N, Edes I, Gavina C, et al. Hypophosphorylation of the Stiff N2B titin isoform raises cardiomyocyte resting tension in failing human myocardium. Circ Res. 2009;104:780–786. doi: 10.1161/CIRCRESAHA.108.193326. [DOI] [PubMed] [Google Scholar]
- 44.Fields NG, Yuan BX, Leenen FH. Sodium-induced cardiac hypertrophy. Cardiac sympathetic activity versus volume load. Circ Res. 1991;68:745–755. doi: 10.1161/01.res.68.3.745. [DOI] [PubMed] [Google Scholar]
- 45.Takeda Y, Yoneda T, Demura M, Miyamori I, Mabuchi H. Sodium-induced cardiac aldosterone synthesis causes cardiac hypertrophy. Endocrinology. 2000;141:1901–1904. doi: 10.1210/endo.141.5.7529. [DOI] [PubMed] [Google Scholar]
- 46.Kim S, Iwao H. Molecular and cellular mechanisms of angio-tensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. [PubMed] [Google Scholar]
- 47.Yu HC, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, Johnston CI. Salt induces myocardial and renal fibrosis in nor-motensive and hypertensive rats. Circulation. 1998;98:2621–2628. doi: 10.1161/01.cir.98.23.2621. [DOI] [PubMed] [Google Scholar]
- 48.Wiest R, Garcia-Tsao G. Bacterial translocation (BT) in cirrhosis. Hepatology. 2005;41:422–433. doi: 10.1002/hep.20632. [DOI] [PubMed] [Google Scholar]
- 49.Karagiannakis DS, Vlachogiannakos J, Anastasiadis G, Vafiadis-Zouboulis I, Ladas SD. Frequency and severity of cirrhotic car-diomyopathy and its possible relationship with bacterial endo-toxemia. Dig Dis Sci. 2013;58:3029–3036. doi: 10.1007/s10620-013-2693-y. [DOI] [PubMed] [Google Scholar]
- 50.Bernardi M, Calandra S, Colantoni A, Trevisani F, Raimondo ML, Sica G, et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998;27:28–34. doi: 10.1002/hep.510270106. [DOI] [PubMed] [Google Scholar]
- 51.Bal JS, Thuluvath PJ. Prolongation of QTc interval: relationship with etiology and severity of liver disease, mortality and liver transplantation. Liver Int. 2003;23:243–248. doi: 10.1034/j.1600-0676.2003.00833.x. [DOI] [PubMed] [Google Scholar]
- 52.Trevisani F, Merli M, Savelli F, Valeriano V, Zambruni A, Riggio O, et al. QT interval in patients with non-cirrhotic portal hypertension and in cirrhotic patients treated with transjugular intrahepatic porto-systemic shunt. J Hepatol. 2003;38:461–467. doi: 10.1016/s0168-8278(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 53.Bernardi M, Rubboli A, Trevisani F, Cancellieri C, Ligabue A, Baraldini M, et al. Reduced cardiovascular responsiveness to exercise-induced sympathoadrenergic stimulation in patients with cirrhosis. J Hepatol. 1991;12:207–216. doi: 10.1016/0168-8278(91)90940-d. [DOI] [PubMed] [Google Scholar]
- 54.Henriksen JH, Fuglsang S, Bendtsen F, Christensen E, Moller S. Dyssynchronous electrical and mechanical systole in patients with cirrhosis. J Hepatol. 2002;36:513–520. doi: 10.1016/s0168-8278(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 55.Ma Z, Meddings JB, Lee SS. Membrane physical properties determine cardiac beta-adrenergic receptor function in cirrhotic rats. Am J Physiol. 1994;267:G87–G93. doi: 10.1152/ajpgi.1994.267.1.G87. [DOI] [PubMed] [Google Scholar]
- 56.Gazawi H, Ljubuncic P, Cogan U, Hochgraff E, Ben-Shachar D, Bomzon A. The effects of bile acids on beta-adrenoceptors, fluidity, and the extent of lipid peroxidation in rat cardiac membranes. Biochem Pharmacol. 2000;59:1623–1628. doi: 10.1016/s0006-2952(00)00259-8. [DOI] [PubMed] [Google Scholar]
- 57.Ward CA, Ma Z, Lee SS, Giles WR. Potassium currents in atrial and ventricular myocytes from a rat model of cirrhosis. Am J Physiol. 1997;273:G537–G544. doi: 10.1152/ajpgi.1997.273.2.G537. [DOI] [PubMed] [Google Scholar]
- 58.Bouchard RA, Clark RB, Giles WR. Effects of action potential duration on excitation–contraction coupling in rat ventricular myocytes. Action potential voltage-clamp measurements Circ Res. 1995;76:790–801. doi: 10.1161/01.res.76.5.790. [DOI] [PubMed] [Google Scholar]
- 59.Kass RS, Moss AJ. Long QT syndrome: novel insights into the mechanisms of cardiac arrhythmias. J Clin Invest. 2003;112:810–815. doi: 10.1172/JCI19844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trevisani F, Di MA, Zambruni A, Biselli M, Santi V, Erroi V, et al. QT interval prolongation by acute gastrointestinal bleeding in patients with cirrhosis. Liver Int. 2012;32:1510–1515. doi: 10.1111/j.1478-3231.2012.02847.x. [DOI] [PubMed] [Google Scholar]
- 61.Henriksen JH, Bendtsen F, Hansen EF, Moller S. Acute non-selective beta-adrenergic blockade reduces prolonged frequency-adjusted Q-T interval (QTc) in patients with cirrhosis. J Hepatol. 2004;40:239–246. doi: 10.1016/j.jhep.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 62.Wong F, Salerno F. Beta-blockers in cirrhosis: friend and foe? Hepatology. 2010;52:811–813. doi: 10.1002/hep.23852. [DOI] [PubMed] [Google Scholar]
- 63.Pozzi M, Grassi G, Ratti L, Favini G, Dell’Oro R, Redaelli E, et al. Cardiac, neuroadrenergic, and portal hemodynamic effects of prolonged aldosterone blockade in postviral child A cirrhosis. Am J Gastroenterol. 2005;100:1110–1116. doi: 10.1111/j.1572-0241.2005.41060.x. [DOI] [PubMed] [Google Scholar]
- 64.Sampathkumar P, Lerman A, Kim BY, Narr BJ, Poterucha JJ, Torsher LC, et al. Post-liver transplantation myocardial dysfunction. Liver Transplant Surg. 1998;4:399–403. doi: 10.1002/lt.500040513. [DOI] [PubMed] [Google Scholar]


