Abstract
Objective
Marchiafava-Bignami disease (MBD) is a rare condition mainly associated with alcoholism, although it may be mimicked by several other disorders that cause corpus callosum lesions. Our objective was to identify helpful features for differential diagnosis and assess whether any treatment can be recommended.
Methods
We reviewed 122 reports containing data on 153 subjects with confirmed MBD that was associated with either alcoholism or malnutrition and 20 reports with data on 53 subjects with conditions mimicking MBD. All the cases had been verified ante mortem by brain imaging. Unconditional logistic regression was used to demonstrate factors that were associated with the outcome of MBD.
Results
The mimicking conditions were differentiated from MBD by the occurrence of solitary and rapidly disappearing splenial lesions; fewer signs and symptoms with exception of seizures, hemiparesis, and tetraparesis; nystagmus; and rapid, complete recovery. MBD occurred most frequently among alcoholics, but it was also reported in 11 non-alcoholics (7·2% of all the MBD cases). A better outcome was observed among those who were treated within two weeks after onset of symptoms with parenteral thiamine (p=0·033).
Conclusions
As thiamine deficiency is frequently associated with alcoholism, malnutrition and prolonged vomiting, we recommend prompt treatment of MBD with parenteral thiamine in such subjects. Recovery should be followed by repeated neuropsychological and MRI examinations, preferably using diffusion tensor imaging.
Keywords: Marchiafava-Bignami disease, outcome, treatment, thiamine, differential diagnosis
Introduction
Marchiafava-Bignami disease (MBD) was originally described as a rare, fatal disease affecting wine drinkers.1 Characterized by demyelination and necrosis of the corpus callosum, it has long been considered to be of either a toxic or nutritional etiology. Eighty-nine autopsy cases recorded between 1898 and 1959 were reviewed in 1961 by Ironside et al.,2 who concluded that there is both individual and racial susceptibility to this disease. Since that time, a growing number of cases with non-fatal outcomes have been reported, and other entities closely mimicking the disease have also been described.3 The development of modern brain imaging techniques has allowed early detection of lesions suggesting the MBD, even in the absence of typical clinical syndromes. Thus, the clinicians can now suspect the diagnosis of MBD even in the emergency room (ER) and can immediately consider appropriate treatment options. However, the several potentially treatable disorders including certain brain infections, epileptic states, and others 3 may demonstrate the neuroimaging abnormalities that closely resemble those seen in MBD. Therefore, ER physicians need to be well aware about these mimicking disorders and differentiate them from MBD.
Some non-alcoholics have also presented with phenotypic and radiological findings that are typical of MBD.4–6 The accuracy of the diagnosis and the outcome and efficacy of the treatment may be influenced by the disease stage of the subject. As MBD is a relatively rare condition, it is unpractical to conduct randomized trials with regard to its treatment. The only feasible study design that can reveal the effect of treatment is a review of published case reports, but this has not yet been done. To characterize the essential features for a correct diagnosis of MBD and to find efficient treatments, we reviewed published case reports starting with papers from 1981.
Material and Methods
Search strategy and selection criteria
We searched the PubMed, EMBASE, Scopus and LILACS databases from 1981 to January 2012 for papers containing the keyword “Marchiafava-Bignami” in either the title or the abstract. All papers published in European languages or in Japanese were considered. In addition, we reviewed the reference lists of both the selected papers and of the most extensive reviews to identify further reports. Three authors (MH, PS and MAL) reviewed the papers independently. We included 122/133 case report papers retrieved (see Appendix 1). Papers reporting MBD cases that were only verified by autopsy were excluded as were two papers with deficient details. Of the cases reported, 84 were from Europe (France: 41, Spain: 17, Germany: eight, Italy: four, Turkey: three, Finland: three, Switzerland: two, Poland: two, the Netherlands: one, Belgium: one, Austria: one, and Portugal: one), and 78 from outside Europe (Japan: 42, India: 11, USA: eight, Korea: five, China: four, Thailand: three, Mexico: two, Uruguay: two and Brazil: one). Cases that were reported in more than one paper were considered only once. A total of 153 MBD cases were analyzed, all of which had been examined and diagnosed using either head MRI or CT ante mortem. These cases were also associated with alcoholism, malnutrition, or both. In this review alcoholics include subjects described either to be chronic current alcoholics (n=82) or dependent on alcohol (n=6) and others (n=54) who were reported to be chronic excessive alcohol consumers with a long history of alcohol abuse or heavy drinking habits. Frequently the drinking pattern was reported to be continuous daily drinking. Binge drinking was never mentioned. The papers did not always define nutritional status. If reported, the used definitions included malnutrition, anorexia, cachexia or refusal of food intake for several days. In this paper we consider all these conditions to mean malnutrition. However, a single finding of low blood thiamine was not considered to indicate malnutrition, because many alcoholics with thiamine deficiency may be calorically well nourished.
In addition, we searched PubMed for reports describing the clinical characteristics and radiological findings of subjects with MRI-verified, reversible, callosal lesions mimicking MBD, which were not associated with alcoholism or malnutrition (keywords: corpus callosum, reversible lesion). This was done because we wanted to compare the MBD data with cases mimicking MBD. We gathered data on 53 such cases from 20/23 papers (see Appendix 2). We noted that the authors of the papers had considered some of their cases to have MBD, even though no association with alcoholism or malnutrition was observed.
Data extraction
Three authors systematically evaluated the retrieved papers in a blinded manner, and information was collected on a specifically designed form. The following data were extracted: signs and symptoms observed on admission and during the overt phase of the disease; time from symptom onset to the last clinical observation; outcome attributable to MBD (Glasgow Outcome Scale [GOS]); delay from the onset of symptoms to admission, acute (≤ two weeks) or sub-acute (>two weeks) categorization; location of the corpus callosum lesions that were observed at the first and second examinations post-admission and those observed during the chronic phase (>three months after admission); other MRI features; PET; SPECT; MRI spectroscopy; laboratory tests, including blood thiamine and cerebrospinal fluid, drug treatment (thiamine, other vitamins and steroids); drug doses, timing and mode of drug administration (parenteral or oral); the presence of Wernicke’s disease (diagnosed either clinically, by radiology or at autopsy); and diabetes. If data on given signs, symptoms, or other items of information were missing from the report, then the feature concerned was considered absent.
Statistical analysis
Categorical variables were compared using Fisher’s exact two-tailed test, the Pearson Chi-square test, while the continuous variables were compared between groups by means of the Mann-Whitney U-test or Student’s t-test.
Unconditional logistic regression was used to search for factors that were associated with a poor outcome (severe disability or worse outcome according to GOS) in subjects with MBD. Included in the analysis were subjects treated with thiamine, subjects that had data on outcome, the delay from the onset of symptoms to admission (categorised as acute ≤two weeks or sub-acute >two weeks), and the location of the corpus callosum lesions. The factors tested were age, sex, loss of consciousness, seizures, alcoholism, malnutrition, Wernicke’s disease, diabetes mellitus, delay from the onset of symptoms to admission, steroid treatment, location of the lesion in MRI, dichotomization to a solitary splenial lesion, and other types of lesions. The final adjusted multivariable model included age, gender, loss of consciousness, seizures, alcoholism, steroid treatment, location of the lesion, and delay from the onset of symptoms to admission. Two-tailed P values less than 0·05 were considered statistically significant.
Results
Demographic and clinical features
The demographic and clinical data of the 153 MBD cases and of the 53 selected cases of callosal lesion mimicking MBD in MRI are summarised in Table 1. The MBD subjects were significantly older (p<0·001) than MBD-mimics. However, both groups showed a wide age range (1–78 years for mimics versus 14–79 years for MBD). The groups also differed significantly for other variables except for presence of diabetes and treatment with steroids.
Table 1.
Demographic and clinical characteristics of subjects with MBD or a condition mimicking it
| Characteristic | MBD cases 153 |
MBD mimics 53† |
|---|---|---|
| Mean age at onset, years (95% CI) | 48·4 (46·6–50·3)‡ | 25·3 (20·4–30·3) |
| Men, n (%) | 123 (80·4)‡ | 28 (52·8) |
| Alcoholism (%) | 142 (92·8)‡ | 0 |
| Malnutrition* (%) | 64 (39·8)‡ | 0 |
| Wernicke’s disease (%) | 19 (12·4)‡ | 0 |
| Thiamine treatment (%) | 103 (67·3)‡ | 3 (5·7) |
| Steroid treatment (%) | 19 (12·4) | 10 (18·9) |
| Diabetes (%) | 10 (6·5) | 6 (11·3) |
Malnutrition or prolonged vomiting without alcohol consumption in 11 cases
Includes 23 subjects with a cerebral infection, 14 with epilepsy, 5 with hypoglycaemia and 11 with miscellaneous conditions (for details, see Appendix 2).
p<0·01
Signs and symptoms
The spectrum of clinical signs and symptoms that were observed upon admission and/or during the first week after admission differed between the MBD patients and the MBD mimics (Table 2). The MBD patients frequently showed an altered mental state, which included confusion, delirium, unconsciousness, impaired memory, and/or disorientation on admission. Impaired walking, dysarthria, mutism, signs of disconnection or split brain syndrome, pyramidal signs, primitive reflexes, rigidity, incontinence, sensory symptoms, and gaze palsy or diplopia were also more frequently found in the MBD cases than in the MBD mimics. On the other hand, hemi- or tetraparesis and nystagmus were almost as frequent, and seizures were more frequent among the mimics. CSF data were available for 62 MBD subjects and 25 mimics, which showed CSF proteins and cells to be increased in 5/62 (8·1%) and 0/62 of the MBD subjects and in 2/25 (8·0%) and 9/25 (36·0%) of the mimics. Elevated CSF cell content was always due to central nervous system infection.
Table 2.
Clinical symptoms and signs in MRI of subjects with MBD or a condition mimicking it
| MBD cases n = 153 |
MBD mimics n = 53 |
|
|---|---|---|
|
| ||
| Symptom/sign | n (%) | n (%) |
| Altered mental state | 123 (80·4)‡ | 13 (24·5) |
| Impaired walking | 104 (68·0)‡ | 5 (9·4) |
| Loss of consciousness | 80 (52·3)‡ | 9 (17·0) |
| Dysarthria | 62 (40·5)‡ | 5 (9·4) |
| Impaired memory | 59 (38·6)‡ | 2 (3·8) |
| Signs of disconnection | 55 (35·9)‡ | 1 (1·9) |
| Pyramidal sign | 37 (24·2)‡ | 1 (1·9) |
| Seizures | 32 (20·9) | 17 (32·1) |
| Primitive reflexes | 30 (19·6)† | 1 (1·9) |
| Mutism | 28 (18·3)* | 2 (3·8) |
| Rigidity | 25 (16·3)† | 0 |
| Hemiparesis or tetraparesis | 22 (14·4) | 7 (13·2) |
| Sensory symptoms | 21 (13·7)* | 1 (1.9) |
| Incontinence | 17 (11·1)† | 0 |
| Nystagmus | 15 (9·8) | 4 (7·5) |
| Ophtalmoplegia or gaze palsy | 15 (9·8)* | 0 |
| Facial palsy | 8 (5·2) | 1 (1·9) |
p < 0.001,
p < 0.01,
p < 0.05 for difference between cases and mimics
Lesions
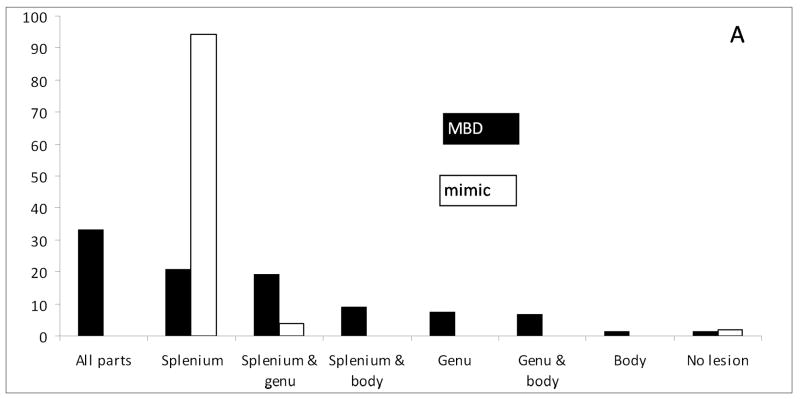
The lesions in the subjects with MBD were often located across the entire corpus callosum (splenium, body, and genu), irrespective of whether brain imaging was performed during the acute (Fig. 1) or the chronic phase of the disease (Appendix 3). Lesions that were seen on admission frequently persisted during follow-up. Only 17/61 (27·9%) of those visible in the acute phase had disappeared entirely, and 23/61 (37·7%) had disappeared partially in the follow-up MRIs. Lesions outside the splenium were very uncommon in the mimicking cases (2/53, 3·8%). The MBD mimic lesions usually disappeared within a week; residual MRI lesions were seen at follow-up in only 2/47 (4·3%) instances. Callosal atrophy (n=36) or necrosis (n=45) was frequently reported among the subjects with MBD, whereas there were no such findings among the mimics. Deep white matter lesions (p=0·007) and cortical lesions (p=0.003) were significantly more frequent in the subjects with MBD (60/121, 49·6% and 32/104, 30·8%) compared with the mimics (6/28, 21·4% and 1/27, 3·7%). Lesions that were enhanced by gadolinium were frequently reported in the subjects with MBD (16/90, 17·8%), but this was not found in the mimics.
Figure 1.
Percentage distribution of callosal lesions among the 120 MBD and 53 mimic cases examined with MRI within 2 weeks of onset of the disease.
Demographic features of alcoholic versus non-alcoholic MBD subjects
Since 11 subjects in this series (7·2%) were non-alcoholics and were reported to have developed MBD in the absence of alcohol abuse, we also looked at the characteristics of the MBD subjects with and without alcoholism. The non-alcoholic subjects with MBD were younger and were more frequently female (see Appendix 4). The non-alcoholics were always reported as suffering from malnutrition or prolonged vomiting, whereas the nutritional state was reported for only 66/142 alcoholics; 53 (80·3%) of whom were said to be suffering from malnutrition. The nutritional state of the alcoholics with MBD did not differ significantly from the non-alcoholics.
Outcome
The outcome was not reported in 15 MBD subjects. The remainder of MBD subjects showed a significant heterogeneity in outcome (p<0·001), which was influenced by alcoholism (see Appendix 5). More than half of the non-alcoholics (7/11, 63·6%) were reported to have recovered completely, whereas only one alcoholic in every ten (14/142, 9·9%) showed a good outcome. On the other hand, the proportion of those who died was about equal in both groups. The outcome for the MBD mimics was usually good, and most (45, 84·9%) reported being normal or having minimal disability after recovering from the acute phase of disease. Of 25 MBD subjects with a solitary lesion in the splenium, 3 (12%) had poor outcome (severe disability), while of 105 subjects with other lesions, 39 (37.1%) had poor outcome (19 with severe disability, 5 with vegetative state and 15 died) (p=0.016).
Effect of treatment on the outcome for MBD subjects
The available treatment outcomes for the MBD subjects who were taking thiamine and steroids are shown in Table 5. These data were available for 144/153 MBD subjects. There was a significant linear trend for better outcomes among those who were treated with thiamine (p=0·027), whereas treatment with steroids did not show any significant trend. The outcome of the subjects who were given thiamine in the acute phase of the disease was significantly better (p=0·026) than that of those treated in the chronic phase. Blood thiamine levels on admission were reported for 20/153 (13·1%) subjects with MBD and for 5/53 (9·4%) of those with a condition mimicking MBD. Levels that were below normal were observed in 16/20 (80·0%) of MBD cases and 1/5 (20·0%) MBD mimic cases respectively (p=0·023). Thiamine had been administered by infusion to 60/103 MBD subjects and orally to 3 MBD subjects, but these data were not available for 40 MBD subjects. The duration of thiamine treatment ranged from one to 105 days.
Several factors were tested as predictors for outcome in the MBD subjects who had been treated with thiamine. Those who were treated with thiamine within two weeks after the onset of symptoms had a significantly (p=0·033) lower rate of poor outcome (15/64, 23%) than those with delayed treatment (12/26, 46%). After adjustment for several confounding factors, delay from the onset of symptoms to admission, i.e. delayed treatment with thiamine, was the only significant risk factor for poor outcome (Table 6)
Discussion
The reported cases of MBD and MBD mimics clearly had different spectra of clinical features and radiological findings. MRI findings of acquired lesions of the corpus callosum have previously been presented.7 We also observed that the outcome among the subjects with MBD was extremely variable and seemed to be influenced by lesion location and perhaps by timing of thiamine treatment.
Subjects with MBD differ from those having an MBD mimic disorder in several aspects. First, MBD was observed to be associated with alcoholism, malnutrition, or Wernicke’s disease, as can be expected in view of our definition of MBD. With the exception of a few case reports,8,9 MBD has been diagnosed only in subjects experiencing either alcoholism or malnutrition, and its link with dietary deficiency in alcoholics is particularly strong, because heavy alcohol drinking is generally known to result in thiamine wastage even without caloric malnutrition. MBD mimics are usually not associated with these factors, but rather with cerebral infection, epilepsy, antiepileptic drug withdrawal, hypoglycaemia, high altitude sickness, and systemic lupus erythematosus.3.10 Secondly, the spectrum of symptoms and signs is different. Subjects with MBD often show severe signs and symptoms, including an altered mental state, impaired walking, deficient memory, dysarthria, pyramidal signs, and disconnection lasting for several weeks. They also show slow recovery, whereas the signs and symptoms of mimicking conditions are usually mild and disappear within a week. Delirious behaviour, disturbed consciousness, and seizures are common features of both MBD and the MBD mimics; however, seizures seem to occur even more frequently in the MBD mimics.
MBD shows no typical clinical presentations. Split brain syndrome11 has been reported as a characteristic feature, but it has lost its relevance as a diagnostic finding since callosal lesions are now detected by modern brain imaging, enabling a diagnosis in the ER setting among confused subjects. Signs of interhaemispheric disconnection may be difficult to detect without neuropsychological testing, particularly in subjects with a lower level of consciousness. In chronic MBD cases, a varying spectrum of disconnection signs may be observed depending on the location of the lesions in the corpus callosum.12–15 Although disconnection is a typical clinical feature of the disease, it may easily remain undetected if not sought out, and it may even be seen in a mimicking condition.9
Thirdly, the lesions show different locations and distributions in the corpus callosum. The lesions in the MBD mimics are usually located in the splenium, whereas single splenial lesions are seen only in one third of the MBD subjects. Other locations or distributions of lesions throughout the whole corpus callosum can frequently be detected. Large lesions located symmetrically at the midline of the splenium are no longer the only type of lesions associated with MBD.16 Some MBD lesions seem to be enhanced by gadolinium, but no lesion enhancement has been described in the MBD mimics. Solitary splenial lesions were associated with better outcome than lesions located in other parts or across the entire corpus callosum.
Positron emission tomography (PET), single photon emission computed tomography (SPECT), perfusion measures and magnetic resonance spectroscopy (MRS) have been used to characterise lesions in the corpus callosum that are related to functional brain damage. These examinations were performed only in a handful of the present cases, thus making analysis impossible. However, several studies employing [18F]-2-fluoro-2-deoxy-D-glucose PET and technetium99m hexylmethylpropylene aminoexime SPECT and/or N-isopropyl-p-[123I]iodoamphetamine SPECT have demonstrated brain hypometabolism and hypoperfusion.17–20 These findings reflect an advanced disease state with permanent brain damage and are seen only in MBD subjects with a poor19 or partial recovery.20 These findings do not occur in fully recovered subjects.21 Hypoperfusion has also been observed in MR perfusion examinations,22–23 except after complete recovery.24 MR spectroscopy has revealed an increase in choline/creatine, indicating demyelination during the early phase of the disease followed by a decrease in N-acetylaspartate/creatine, indicating axonal damage.20, 22–25 However, these observations do not provide any aetiological explanation for the MBD.
Studies using MRI diffusion-weighted imaging (DWI) have shown a low apparent diffusion coefficient, which has been interpreted as demonstrating the presence of cytotoxic oedema in the corpus callosum.22, 23, 27–29 DWI reveals the earliest signs of lesions26 and can identify more extensive callosal lesions in MBD27 than fluid-attenuated inversion recovery, Sometimes cytotoxic oedema has been observed simultaneously in other parts of the brain, and it may precede the development of callosal necrosis and predict a poor outcome.22, 27–28 This is not always the case, however, since complete recovery after thiamine therapy has also been observed.30–31 Diffusion tensor imaging (DTI) could be employed for studying the clinical correlates of MBD and the recovery process.32
The actual mechanisms leading to callosal damage in MBD and the clinical conditions that mimic it are still unclear. The aetiological reasons have been speculated upon in several papers3,10,33–36 without reaching any firm conclusions. Although MBD seems to carry prospects of a better outcome now than before,37 the prognosis is still not good, and alcoholic MBD seems to be associated with a less favourable outcome than non-alcoholic MBD. Since the outcome of MBD is variable, it is important to look for hints on how to treat the disease.
Corticosteroids may reduce brain oedema, suppress demyelination, stabilize the blood-brain barrier, and reduce inflammation. Although some papers have reported improvements after steroid treatment,38–41 we could not observe any positive net effect in our analyses. Many cases were treated with both steroids and multivitamins, and it was difficult to see which played the major role in recovery. No adverse effects of steroid treatment were reported, however.
We observed a significant trend for a better overall outcome in subjects who were treated with thiamine compared to those who remained untreated. Early thiamine treatment significantly reduced the risk of a poor outcome. Publication bias is possible and the categorization of the overall outcome into two groups reduced the power of our analysis. However, it is clear that many obvious treatment failures were also reported.22, 27, 28, 39, 42–44 The administration of vitamins, particularly thiamine, may have become a treatment option because MBD has been observed to be associated with Wernicke’s disease in 15 to 20% of subjects.45 We similarly observed a relatively high frequency of Wernicke’s disease among the present subjects reported to have MBD (12.4%). Subjects having either alcoholic46 or non-alcoholic Wernicke’s disease4 may show signs and symptoms of MBD. Finally, the use of DWI has demonstrated splenial lesions, which are typical of MBD in subjects with Wernicke’s disease.47 Accordingly, the lesions in the corpus callosum may be one manifestation of thiamine deficiency, although lesions in the mamillary bodies, the periaqueductal region, and the walls of the third ventricle are much more common.
It has been said that since the aetiology of MBD is unknown, no specific therapy can be recommended.48 However, our review revealed that many subjects had completely recovered after thiamine therapy.6, 30, 49–52 It may be that some treatment failures were attributable to a delay after symptom onset to hospital admission. This was supported by the observation that subjects treated in the acute phase with thiamine had a better outcome than those treated in the chronic phase. In any case, the effectiveness of steroids and other treatments has remained hypothetical. In contrast to previous suggestions, thiamine therapy has also resulted in good or total recovery in non-alcoholics,6, 21, 27, 41 and this finding indicates that this therapy is also effective for them. Accordingly, we consider thiamine therapy justified and recommendable as a first line of treatment for cases of MBD associated with alcoholism, malnutrition, or prolonged vomiting and even for MBD mimics in case their thiamine levels are below normal.
The major limitation of our study is the lack of complete data. Many of the case reports included incomplete data on the clinical status, the treatment, and the outcome of cases. Neuropsychological examinations were not always performed or reported. Despite our efforts to contact the authors, we were not able to remedy these defects. We only recorded cases that had been diagnosed as MBD. Since we did not gather the case reports on MBD mimics in a systematic manner, the results concerning them should be taken as no more than suggestive; however, we regard our sample as representative. There are a growing number of diseases and conditions that have transient lesions in the splenium of the corpus callosum associated with them, but the pathophysiology of these lesions remains unclear. The major strength of our study is that data have been gathered on almost all of the MRI-verified cases carrying a diagnosis of MBD. This became possible by also reviewing papers that were not published in English.
We would like to make two recommendations based on our review. First, the method of choice for imaging a subject with MBD is DWI, and recovery could best be monitored by means of DTI and fiber tracking.32 Second, due to the high frequency of thiamine deficiency among alcoholics (even if not suffering from caloric malnutrition) and malnourished people (including those who suffer from prolonged vomiting), prompt therapy with parenteral thiamine seems to be indicated. It is difficult to prove the efficacy of such therapy, whether combined with steroids or not in a placebo-controlled randomized trial, because the condition is very rare. The dose of thiamine should be the same as recommended for Wernicke’s disease,53 and the therapy should continue for as long as recovery is going on.
Supplementary Material
Table 3.
Outcome for 144 patients with MBD, by mode of treatment
| Treated with thiamine n=103 |
Not treated with thiamine n=41* |
Treated with steroids n=19 |
Not treated with steroids n=125* |
|
|---|---|---|---|---|
|
| ||||
| Outcome | n (%) | n (%) | n (%) | n (%) |
| Normal | 17 (16·5) | 4 (8·0) | 4 (21·1) | 17 (13·6) |
| Minimal disability | 24 (23·3) | 6 (12·0) | 1 (5·3) | 29 (23·2) |
| Moderate disability | 29 (28·2) | 12 (24·0) | 5 (26·3) | 36 (28·8) |
| Severe disability | 18 (17·5) | 7 (14·0) | 5 (26·3) | 20 (16·0) |
| Vegetative state | 3 (2·9) | 2 (4·0) | 1 (5·3) | 4 (3·2) |
| Dead | 12 (11·7) | 10 (20·0) | 3 (15·8) | 19 (15·2) |
Not treated or information on treatment not available
p=0.027 for the difference in linear trend between the thiamine and outcome groups.
Table 4.
Characteristics predicting a poor outcome among 90 subjects with MBD treated with thiamine
| Characteristic | Univariable OR (95% CI) | Multivariable OR (95% CI) |
|---|---|---|
| Age (per year) | 1·02 (0·98–1·06) | 1·02 (0·97–1·06) |
| Male sex | 0·82(0·29–2·32) | 0·51 (0·14–1·80) |
| Loss of consciousness | 1·29 (0·52–3·19) | 1·70 (0·62–4·70) |
| Seizures | 1·23 (0·43–3·49) | 1·28 (0·39–4·14) |
| Alcoholism | 2·74 (0·31–23·90) | 1·69 (0·12–22·90) |
| Steroid treatment | 1·71 (0·54–5·41) | 1·49 (0·39–5·65) |
| Lesion locations≠ | 4·62 (0·99–21·63) | 4·27 (0·76–24·07) |
| Delayed thiamine treatment | 2·80 (1·07–7·34)* | 2·91 (1·02–8·36)* |
A solitary splenial lesion is used as a reference category for the other locations. Subjects without a risk factor serve as a reference category for the independent factors.
p < 0.05
Acknowledgments
This study was funded by the Oulu University Hospital. In addition, ZKW is partially supported by the NIH/NINDS P50 NS072187, Mayo Clinic Center for Regenerative Medicine We would like to thank Ms. Kelly E. Viola from the Mayo Clinic for excellent editorial assistance.
Appendix 4 Demographic and clinical characteristics of the subjects with MBD
| Alcoholic 142 |
Non-alcoholic 11 |
|
|---|---|---|
|
| ||
| Characteristic | n (%) | n (%) |
| Mean age at onset, years (95% CI) | 49·4 (47·7–51·1) | 36·1 (23·2–48·9) |
| Men, n | 120 (84·5) ‡ | 3 (27·3) |
| Malnutrition | 53/66 (80·3) ≠ | 11 (100) |
| Diabetes mellitus | 10 (7·0) | 0 |
| Wernicke’s disease | 17 (12·0) | 2 (18·2) |
| Acute onset | 82 (57·7) | 7 (63·6) |
| Thiamine treatment | 96 (67·6) | 7 (63·6) |
| MR verification | 132 (93·0) | 10 (90·9) |
Mean difference in age = 13.3 (0.4–26.2) years, p=0.045
p<0.001 for sex difference
Information on nutritional state was not available for 76 alcoholics
Appendix 5. Outcome on the Glasgow Outcome Scale for subjects reported as having MBD
| Alcoholic n=142 |
Non-alcoholic n=11 |
All reported cases n=162* |
|
|---|---|---|---|
| Outcome | n (%) | n (%) | n (%) |
| Not reported | 8 (5·6) | 1 (9·1) | 15 (9·3) |
| Normal | 14 (9·9) | 7 (63·6) | 21 (13·0) |
| Minimal disability | 29 (20·4) | 1 (9·1) | 32 (19·7) |
| Moderate disability | 41 (28·9) | 0 | 41 (25·3) |
| Severe disability | 25 (17·6) | 0 | 25 (15·4) |
| Vegetative state | 5 (3·5) | 0 | 5 (3·1) |
| Dead | 20 (14·1) | 2 (18·2) | 22 (13·6) |
Includes 9 subjects with missing data on alcoholism and malnutrition
Footnotes
Conflicts of interest
We declare that we have no conflicts of interest.
Contributors
MH was responsible for the study design and obtained funding. MH PS and SJ planned and performed the analyses of the data, and wrote the first draft of the paper. MAL, SF and ZKW participated to data collection and interpretation of the data. All the authors critically revised the manuscript.
References
- 1.Marchiafava E, Bignami A. Sopra un’alterazione del corpo calloso osservata in soggetti alcoolisti. Riv Patol Nerv Ment. 1903;8:544–49. [Google Scholar]
- 2.Ironside R, Bosanquet FD, McMenemey WH. Central demyelination of the corpus callosum (Marchiafava-Bignami disease) Brain. 1961;84:212–30. doi: 10.1093/brain/84.2.212. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Monco JC, Cortina IE, Ferreira E, et al. Reversible splenial lesion syndrome (RESLES): what’s in a name? J Neuroimaging. 2011;21(2):e1–14. doi: 10.1111/j.1552-6569.2008.00279.x. [DOI] [PubMed] [Google Scholar]
- 4.Leong ASY. Marchiafava-Bignami disease in a non-alcoholic Indian male. Pathology. 1979;11:241–9. doi: 10.3109/00313027909061950. [DOI] [PubMed] [Google Scholar]
- 5.Kosaka K, Aoki M, Kawasaki N, et al. A non-alcoholic Japanese patient with Wernicke’s encephalopathy and Marchiafava-Bignami disease. Clin Neuropathol. 1984;3(6):231–6. [PubMed] [Google Scholar]
- 6.Hillbom M, Pyhtinen J, Pylvänen V, et al. Pregnant, vomiting, and coma. Lancet. 1999;353:1584. doi: 10.1016/S0140-6736(99)01410-5. [DOI] [PubMed] [Google Scholar]
- 7.Uchino A, Takase Y, Nomiyama K, et al. Acquired lesions of the corpus callosum: MR imaging. Eur Radiol. 2006;16:905–14. doi: 10.1007/s00330-005-0037-9. [DOI] [PubMed] [Google Scholar]
- 8.Murthy SB, Jawaid A, Bock JE, et al. Marchiafava-Bignami disease (MBD) in a non-alcoholic patient: a case report. Can J Neurol Sci. 2010;37:138–40. doi: 10.1017/s0317167100009823. [DOI] [PubMed] [Google Scholar]
- 9.Boutboul D, Lidove O, Aquilar C, et al. Marchiafava-Bignami disease complicating SC hemoglobin disease and Plasmodium Falciparum infection. Presse Med. 2010;39:990–3. doi: 10.1016/j.lpm.2010.02.052. [DOI] [PubMed] [Google Scholar]
- 10.Gallucci M, Limbucci N, Paonessa A, et al. Reversible focal splenial lesions. Neuroradiol. 2007;49:541–4. doi: 10.1007/s00234-007-0235-z. [DOI] [PubMed] [Google Scholar]
- 11.Berlucchi G. Frontal callosal disconnection syndromes. Cortex. 2012;48:36–45. doi: 10.1016/j.cortex.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Rosa A, Demiati M, Cartz L, et al. Marchiafava-Bignami disease, syndrome of interhemispheric disconnection, and right-handed agraphia in a left-hander. Arch Neurol. 1991;48:985–8. doi: 10.1001/archneur.1991.00530210118032. [DOI] [PubMed] [Google Scholar]
- 13.Kamaki M, Kawamura M, Moriya H, et al. Crossed homonymous hemianopia and crossed left hemispatial neglect in a case of Marchiafava-Bignami disease. J Neurol Neurosurg and Psych. 1993;56:1027–32. doi: 10.1136/jnnp.56.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalckreuth W, Zimmermann P, Preilowski B, et al. Incomplete split-brain syndrome in a patient with chronic Marchiafava-Bignami disease. Behav Brain Res. 1994;64:219–28. doi: 10.1016/0166-4328(94)90134-1. [DOI] [PubMed] [Google Scholar]
- 15.Berek K, Wagner M, Chemelli AP, et al. Hemispheric disconnection in Marchiafava-Bignami disease: clinical, neuropsychological and MRI findings. J Neurol Sci. 1994;123:2–5. doi: 10.1016/0022-510x(94)90195-3. [DOI] [PubMed] [Google Scholar]
- 16.Friese SA, Bitzer M, Freudenstein D, et al. Classification of acquired lesions of the corpus callosum with MRI. Neuroradiol. 2000;42:795–802. doi: 10.1007/s002340000430. [DOI] [PubMed] [Google Scholar]
- 17.Moriyama Y, Mimura M, Kato M, et al. A case of Marchiafava Bignami disease presenting with severe dysphoria. Seishin Ikagu. 2000;42:1181–6. [Google Scholar]
- 18.Ishii K, Ikejiri Y, Sasaki M, et al. Regional cerebral glucose metabolism and blood flow in a patient with Marchiafava-Bignami disease. Am J Neuroradiol. 1999;20:1249–51. [PMC free article] [PubMed] [Google Scholar]
- 19.Pappata S, Chabriat H, Levasseur M, et al. Marchiafava-Bignami disease with dementia: severe cerebral metabolic depression revealed by PET. J Neural Transm. 1994;8:131–7. doi: 10.1007/BF02250924. [DOI] [PubMed] [Google Scholar]
- 20.Nalini A, Kovoor JME, Dawn R, et al. Marchiafava-Bignami disease: two cases with magnetic resonance imaging and positron emission tomography scan findings. Neurol India. 2009;57:644–48. doi: 10.4103/0028-3886.57813. [DOI] [PubMed] [Google Scholar]
- 21.Maki N, Hokoishi K, Komori K, et al. A case of Marchiafava-Bignami disease caused by anorexia nervosa. No To Shinkei. 2001;53:669–71. [PubMed] [Google Scholar]
- 22.Tuntiyatorn L, Laothamatas J. Acute Marchiafava-Bignami disease with callosal, cortical and white matter involvement. Emerg Radiol. 2008;15:137–40. doi: 10.1007/s10140-007-0640-y. [DOI] [PubMed] [Google Scholar]
- 23.Lee SH, Kim SS, Kim SH, et al. Acute Marchiafava-Bignami disease with selective involvement of the precentral cortex and splenium. The Neurologist. 2011;17:213–7. doi: 10.1097/NRL.0b013e31821a25ae. [DOI] [PubMed] [Google Scholar]
- 24.Machado Á, Soares-Fernandes J, Ribeiro M, et al. Alcohol abuse and acute behavioural disturbances in a 24-year-old patient. J Clin Neurosci. 2009;16:811, 859. doi: 10.1016/j.jocn.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Gambini A, Falini A, Moiola L, et al. Marchiafava-Bignami disease: longitudinal MR imaging and MR spectroscopy study. Am J Neuroradiol. 2003;24:249–53. [PMC free article] [PubMed] [Google Scholar]
- 26.Inakagi T, Saito K. A case of Marchiafava-Bignami disease demonstrated by MR diffusion-weighted image. No To Shinkei. 2000;52:633–7. [PubMed] [Google Scholar]
- 27.Ménégon P, Sibon I, Pacgai C, et al. Marchiafava-Bignami disease: diffusion-weighted MRI in corpus callosum and cortical lesions. Neurology. 2005;65:475–7. doi: 10.1212/01.wnl.0000171348.55820.89. [DOI] [PubMed] [Google Scholar]
- 28.Ihn YK, Hwang SS, Park YH. Acute Marchiafava-Bignami disease: diffusion-weighted MRI in cortical and callosal involvement. Yonsei Med J. 2007;48:321–4. doi: 10.3349/ymj.2007.48.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hlaihel C, Gonnaud P-M, Champin S, et al. Diffusion-weighted magnetic resonance imaging in Marchiafava-Bignami disease: follow-up studies. Neuroradiology. 2005;47:520–4. doi: 10.1007/s00234-005-1368-6. [DOI] [PubMed] [Google Scholar]
- 30.Aggenlu L, Oner Y, Kocer B, et al. The value of diffusion-weighted imaging in the diagnosis of Marchiafava-Bignami disease: apropos of a case. J Neuroimaging. 2008;18:188–90. doi: 10.1111/j.1552-6569.2007.00202.x. [DOI] [PubMed] [Google Scholar]
- 31.Tung C-S, Wu S-L, Tsou J-C, et al. Marchiafava-Bignami disease with widespread lesions and complete recovery. Am J Neuroradiol. 2010;31:1506–7. doi: 10.3174/ajnr.A1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sair HI, Mohamed FB, Patel S, et al. Diffusion tensor imaging and fiber-tracking in Marchiafava-Bignami disease. J Neuroimaging. 2006;16:281–5. doi: 10.1111/j.1552-6569.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- 33.Prilipko O, Delavelle J, Lazeyras F, et al. Reversible cytotoxic edema in the splenium of the corpus callosum related to antiepileptic treatment: report of two cases and literature review. Epilepsia. 2005;46:1633–6. doi: 10.1111/j.1528-1167.2005.00256.x. [DOI] [PubMed] [Google Scholar]
- 34.Conti M, Salis A, Urigo C, et al. Transient focal lesion in the splenium of the corpus callosum: MR imaging with an attempt to clinical-physiopathological explanation and review of the literature. Radiol Med. 2007;112:921–35. doi: 10.1007/s11547-007-0197-9. [DOI] [PubMed] [Google Scholar]
- 35.Singh P, Gogoi D, Vyas S, et al. Transient splenial lesion: further experience with two cases. Indian J Radiol Imaging. 2010;20:254–7. doi: 10.4103/0971-3026.73531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malhotra HS, Garg RK, Vidhate MR, et al. Boomerang sign: clinical significance of transient lesion in splenium of corpus callosum. Ann Indian Acad Neurol. 2012;15:151–7. doi: 10.4103/0972-2327.95005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heinrich A, Runge U, Khaw AV. Clinicoradiologic subtypes of Marchiafava-Bignami disease. J Neurol. 2004;251:1050–9. doi: 10.1007/s00415-004-0566-1. [DOI] [PubMed] [Google Scholar]
- 38.Gerlach A, Oehm E, Wattchow J, et al. Use of high-dose cortisone in a patient with Marchiafava-Bignami disease. J Neurol. 2003;250:758–60. doi: 10.1007/s00415-003-1072-6. [DOI] [PubMed] [Google Scholar]
- 39.Kawarabuki K, Sakakibara T, Hirai M, et al. Marchiafava-Bignami disease: magnetic resonance imaging findings in corpus callosum and subcortical white matter. Eur J Radiol. 2003;48:175–7. doi: 10.1016/S0720-048X(02)00349-2. [DOI] [PubMed] [Google Scholar]
- 40.Nardone R, Venture A, Buffone E, et al. Transcranial magnetic stimulation shows impaired transcallosal inhibition in Marchiafava-Bignami syndrome. Eur J Neurol. 2006;13:749–53. doi: 10.1111/j.1468-1331.2006.01302.x. [DOI] [PubMed] [Google Scholar]
- 41.Tao H, Kitagawa N, Kako Y, et al. A case of anorexia nervosa with Marchiafava-Bignami disease that responded to high-dose intravenous corticosteroid administration. Psychiatry Res: Neuroimaging. 2007;156:181–4. doi: 10.1016/j.pscychresns.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Logak M, Fève A, Samson Y, et al. Contribution of positron emission tomography in a patient with Marchiafava-Bignami disease: laminar sclerosis of Morel? Rev Neurol. 1996;152:47–50. [PubMed] [Google Scholar]
- 43.Johkura K, Naito M, Naka T. Cortical involvement in Marchiafava-Bignami disease. Am J Neuroradiol. 2005;26:670–3. [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshizaki T, Hashimoto T, Fujimoto K, et al. Evolution of callosal and cortical lesions on MRI in Marchiafava-Bignami disease. Case Rep Neurol. 2010;2:19–23. doi: 10.1159/000296705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kohler CG, Ances BM, Coleman AR, et al. Marchiafava-Bignami disease: literature review and case report. Neuropsychiatry, Neuropsychol, and Behav Neurol. 2000;13:67–76. [PubMed] [Google Scholar]
- 46.Koeppen AH, Barron KD. Marchiafava-Bignami disease. Neurology. 1978;28:290–4. doi: 10.1212/wnl.28.3.290. [DOI] [PubMed] [Google Scholar]
- 47.Loh Y, Watson WD, Verma A, et al. Restricted diffusion of the splenium in acute Wernicke’s encephalopathy. J Neuroimaging. 2005;15:373–5. doi: 10.1177/1051228405279037. [DOI] [PubMed] [Google Scholar]
- 48.Haas L, Tjan D, Die JV, et al. Coma in an alcoholic: Marchiafava-Bignami disease. New Zeal Med J. 2006;119:1–6. [PubMed] [Google Scholar]
- 49.Tobita M, Mochizuki H, Takahashi S, et al. A case of Marchiafava-Bignami disease with complete recovery: Sequential imaging documenting improvement of callosal lesions. Tohoku J Exp Med. 1997;182:175–9. doi: 10.1620/tjem.182.175. [DOI] [PubMed] [Google Scholar]
- 50.Kinoshita Y, Yasukouchi H, Tsuru E, et al. Rapid improvement of callosal edema by thiamine administration in Marchiafava-Bignami disease: a case report. No To Shinkei. 2004;56:425–8. [PubMed] [Google Scholar]
- 51.Khandelwal A, Agarwal A, Inamdar A, et al. Marchiafava-Bignami disease: literature review and a case report. Indian J Psychiatry. 2009;51:S134. [Google Scholar]
- 52.Aggarwal A, Kandelwal A, Jiloha RC. A case of Marchiafava Bignami disease: complete recovery with thiamine. J Neuropsychiatry Clin Neurosci. 2011;23:E28. doi: 10.1176/jnp.23.2.jnpe28. [DOI] [PubMed] [Google Scholar]
- 53.Galvin R, Bråthen G, Ivashynka A, et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17:1408–18. doi: 10.1111/j.1468-1331.2010.03153.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.