Abstract
The increased prevalence of obesity has led to increased numbers of bariatric surgical procedures being performed annually. Postoperative metabolic improvements in glucose levels, blood pressure and lipids have led to recognition that surgery may prove to be a highly effective therapy for type 2 diabetes (T2D). A recent report evaluates durability of diabetes remission and metabolic improvements.
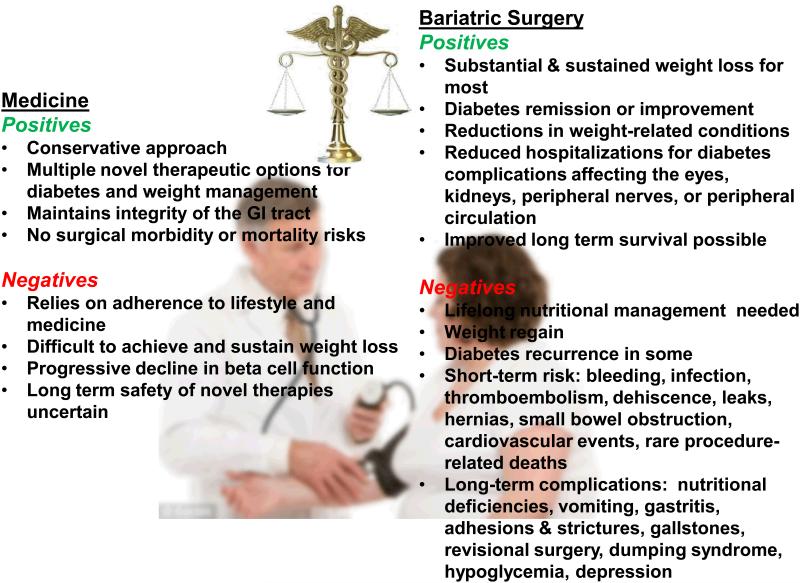
Lifestyle modification, weight loss, and medical therapies are the foundation of disease management for type 2 diabetes. However, weight loss is difficult to achieve and sustain, and progressive hyperglycemia requiring additional medication use is common. Emerging data over the past few years have demonstrated that bariatric surgery may provide an additional therapeutic option, particularly in individuals early in the course of T2D. Bariatric surgeries lead to substantial and sustained weight loss for most patients, with the magnitude varying according to the procedure performed. For example, the Swedish Obesity Subjects (SOS) study, a long-term, prospective, controlled trial, demonstrated mean weight loss in surgical patients of 23% after 2 years, which was sustained at 18% by 20 years 1. In comparison, matched controls receiving usual medical care had no significant weight change over this same interval. Furthermore, bariatric surgery is associated with improvements in obesity-related comorbidities including hypertension and dyslipidemia, and reduced incidence of myocardial infarction (29%), stroke (34%), and cancer in women (42%) 1. Up to 80% of individuals with T2D at the time of surgery may improve glycemic control or achieve disease remission, without use of medication. Moreover, those without T2D at the time of surgery have a 73% reduction in incident diabetes 1 and may have 30-40% reductions in overall mortality 1, 2. When performed at centers of excellence, these benefits are achieved with low operative mortality rates, ranging from 0.1 to 0.5% 3, with longer-term intestinal and nutritional complications varying by procedure.
Thus, bariatric surgery may represent a reasonable therapeutic approach for diabetes and weight management in patients with reasonable surgical risk who are otherwise unable to achieve or sustain health goals, a position supported by the International Diabetes Federation 4 and the American Diabetes Association 5. However, many consider bariatric surgery as a draconian last-resort step for diabetes management 6, in part due to surgical risks and also because long-term efficacy rates have been uncertain. The study of Brethauer and colleagues 7 adds to our knowledge about the durability of bariatric surgery on T2D remission. Clinical outcomes of 217 patients with T2D at the time of Roux-en-Y gastric bypass (RYGB, n=162), gastric band (n=32), and sleeve gastrectomy (n=23) were assessed after a median follow up of 6 years (range 5-9 years). On average, patients lost 55% of excess weight during this interval. In parallel, 24% of patients achieved complete remission, defined as normal measures of glycemia (A1c below 6%, fasting glucose below 100 mg/dl), and 26% achieved partial remission (A1c 6-6.4%, fasting glucose 100-125 mg/dl), sustained for at least one year in the absence of diabetes medications. An additional 34% had a reduction in HbA1c over 1%, but still required medication. Thus, diabetes improved in 84% of patients undergoing bariatric surgery. Moreover, patients were 3.6-fold and 1.4-fold more likely to achieve blood pressure and lipid goals, respectively, and realized a 7% decrease in Framingham 10-year cardiovascular risk scores - a 25% relative improvement from baseline. Furthermore, there may be regression in early diabetic nephropathy, as indicated by reduced serum creatinine and urinary albumin.
Limitations of this study 7 include the retrospective design with lack of randomization and medical comparison group, a mixture of surgical procedures (predominance of RYGB), loss of follow up of 20% of the index population, insufficient study size for characterization of cardiovascular and mortality outcomes, and absence of assessment of adverse health outcomes related to surgery.
Nevertheless, the findings of Brethauer and colleagues 7 add to a growing body of work 8 regarding the long-term efficacy and durability of bariatric surgery. The efficacy of bariatric surgery to improve diabetes is particularly notable for those with shorter duration of T2D who require only oral medication preoperatively, suggesting the importance of residual beta cell function for clinical response rates.
As T2D is widely recognized to be a progressive disorder, it should not be surprising that initial disease remission is not sustained for all patients, and diabetes may recur in about one third over 5 years 7, 8, with increased recurrenceover time. Fewer than 20% of those with initial remission remain in remission by 20 years 9. Brethauer and colleagues 7 and others demonstrate that weight regain is a major contributor to diabetes relapse in this setting. However, prolonged periods of glycemic control early in diabetes are associated with continued reduction in risk of long-term complications, including myocardial infarction and death 10. Recent data from the SOS study suggests this “metabolic memory” may also be observed in patients with T2D in response to bariatric surgery, as demonstrated by progressive reduction in relative riskfor hospitalizations for diabetes-related complications in the surgical compared to control patient groups 9.
Given the success of bariatric surgery for diabetes-related outcomes, key scientific questions remain to identify the mechanisms responsible for glycemic and metabolic improvement. While sustained weight loss contributes substantially to health benefits, emerging evidence demonstrates that weight-independent mechanisms may also contribute following certain bariatric procedures (reviewed in in (11)). For example, improvements in glycemia occur within days of RYGB 11, before significant weight loss occurs, and appear to be disproportionate to the caloric restriction occurring in the postoperative period. Additional mechanisms engaged by gastrointestinal manipulation may involve changes in metabolically active peptides, including reductions in ghrelin and other orexigenic hormones, increases in glucagon-like peptide-1 (GLP-1), peptide YY (PYY), oxyntomodulin, and cholecystokinin, and vagally-mediated neural network responses. Together these changes may alter appetite and satiety, modulate systemic metabolism, and improve insulin secretion by the β-cell. In addition, exclusion of the stomach and proximal intestine from alimentary flow and early delivery of digested nutrients to the jejunium and distal intestine, may contribute to increased plasma levels of bile acids, intestinal remodeling 12 and changes in the microbiome, all of which could potentially contribute to improved metabolism.
Studies that evaluate the longer term outcomes for patients with T2D undergoing bariatric surgery will continue to provide insights on ways to optimize health and help to guide clinical decision-making for both patients and providers.
Figure 1.
Acknowledgements
This work was supported by NIDDK R56-DK095451 (ABG) and P30-DK03836 (ABG and MEP). We also acknowledge support by the Joslin Diabetes Center and thank its philanthropic donors.
Contributor Information
Allison B. Goldfine, Joslin Diabetes Center, Boston, MA.
Mary Elizabeth Patti, Harvard Medical School, Boston, MA.
References:
- 1.Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219–34. doi: 10.1111/joim.12012. [DOI] [PubMed] [Google Scholar]
- 2.Adams TD, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–61. doi: 10.1056/NEJMoa066603. [DOI] [PubMed] [Google Scholar]
- 3.Vetter ML, Cardillo S, Rickels MR, Iqbal N. Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med. 2009;150:94–103. doi: 10.7326/0003-4819-150-2-200901200-00007. [DOI] [PubMed] [Google Scholar]
- 4.Dixon JB, Zimmet P, Alberti KG, Mbanya JC, Rubino F. Bariatric surgery for diabetes: the International Diabetes Federation takes a position. J Diabetes. 2011;3:261–4. doi: 10.1111/j.1753-0407.2011.00144.x. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinkney JH, Johnson AB, Gale EA. The big fat bariatric bandwagon. Diabetologia. 2010;53:1815–22. doi: 10.1007/s00125-010-1845-2. [DOI] [PubMed] [Google Scholar]
- 7.Brethauer SA, et al. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013;258:628–37. doi: 10.1097/SLA.0b013e3182a5034b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arterburn DE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23:93–102. doi: 10.1007/s11695-012-0802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sjöström L. Diabetes remission and prevention after usual care and bariatric surgery. Results over 15 years in the Swedish Obese Subjects trial; the 49th European Association for the Study of Diabetes (EASD); Barcelona, Spain. 2013. [Google Scholar]
- 10.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 11.Schauer PR, et al. Effect of laparoscopic Roux-en Y gastric bypass on type 2 diabetes mellitus. Ann Surg. 238:467–84. doi: 10.1097/01.sla.0000089851.41115.1b. discussion 84-5 (2003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saeidi N, et al. Reprogramming of intestinal glucose metabolism and glycemic control in rats after gastric bypass. Science. 2013;341:406–10. doi: 10.1126/science.1235103. [DOI] [PMC free article] [PubMed] [Google Scholar]