Abstract
Aggressive behavior problems (ABP) are frequent yet poorly understood in children with Autism Spectrum Disorders (ASD) and are likely to co-vary significantly with comorbid problems. We examined the prevalence and sociodemographic correlates of ABP in a clinical sample of children with ASD (N = 400; 2–16.9 years). We also investigated whether children with ABP experience more intensive medical interventions, greater impairments in behavioral functioning, and more severe comorbid problems than children with ASD who do not have ABP. One in four children with ASD had Child Behavior Checklist scores on the Aggressive Behavior scale in the clinical range (T-scores ≥ 70). Sociodemographic factors (age, gender, parent education, race, ethnicity) were unrelated to ABP status. The presence of ABP was significantly associated with increased use of psychotropic drugs and melatonin, lower cognitive functioning, lower ASD severity, and greater comorbid sleep, internalizing, and attention problems. In multivariate models, sleep, internalizing, and attention problems were most strongly associated with ABP. These comorbid problems may hold promise as targets for treatment to decrease aggressive behavior and proactively identify high-risk profiles for prevention.
Keywords: autism spectrum disorders, psychotropic drugs, aggression, sleep, internalizing problems, attention problems
1. Introduction
Aggressive behaviors in children with Autism Spectrum Disorder (ASD) are the primary cause of residential placement (Mandell, 2008) and are associated with greater functional impairment and more intensive medical interventions (Lecavalier, 2006; Tureck, Matson, Turygin, & Macmillan, 2013; Witwer & Lecavalier, 2005). Additionally, aggressive behaviors in children with ASD are a frequent source of parental concern (Mazurek, Kanne, & Wodka, 2013) and are known to increase family stress, financial strain, and demands on caregivers (Hodgetts, Nicholas, & Zwaigenbaum, 2013; Lecavalier, Leone, & Wiltz, 2006). While aggressive behavior in ASD is important due to the detrimental effects on caregiving, it may also be a risk factor for later poor outcomes. For instance, in the general population, aggressive behavior in childhood is linked to other maladaptive behaviors including delinquency/conduct problems, emotional dysregulation, low peer acceptance, and peer rejection (Card, Stucky, Sawalani, & Little, 2008). However, despite the clinical significance of aggressive behaviors in ASD, the prevalence and correlates of these behaviors are poorly understood.
Previous research suggests that aggressive behaviors are more common among children with ASD than in other populations (Bronsard, Botbol, & Tordjman, 2010; Farmer & Aman, 2011; Mayes et al., 2012; McClintock, Hall, & Oliver, 2003). However, prevalence estimates of aggressive behaviors in children with ASD vary widely, ranging from 8–68% (see Table 1). This variation is likely due to differences in the definitions of aggressive behaviors, the measures used, and the sample ascertainment methods. Estimates are considerably higher when based on non-standardized measures of parent-reported aggressive behavior. For example, Kanne and Mazurek (2011) estimated the prevalence of current aggressive behavior as 56%, based on parent ratings of mild to severe physical aggression on a single item on the Autism Diagnostic Interview-Revised. In a recent study using a large sample (N = 1584) from the Autism Treatment Network (ATN), the prevalence of aggressive behavior was 53.7%, based on a yes or no response from parents about whether aggressive behaviors were a current concern (Mazurek et al., 2013). However, these estimates are difficult to evaluate, particularly when samples encompass children within a wide age range, because it is not known how parents of children without ASD at different ages would respond.
Table 1.
Selected previous studies on aggressive behaviors in children with ASD
| Study | Sample | ASD n |
Age (yrs) | Measure | Prevalence in ASD | Factors associated with increased aggression |
|---|---|---|---|---|---|---|
| Lecavalier (2006) |
Research | 326 | 3 – 21 (M = 9.6) |
Nisonger Child Behavior Rating Form |
23% (conduct problems, including aggression) |
|
| Dominick et al. (2007) |
Research | 67 | 4 – 14 (M = 7.6) |
Atypical Behavior Patterns Questionnaire |
32.7% | ↓ cognitive (NVIQ, VIQ, FSIQ), receptive and expressive language ↑ repetitive behaviors |
| Hartley et al. (2008) |
Referred | 169 | 1.5 – 5.8 (M = 3.5) |
CBCL Aggressive Behavior |
22.5% (T-scores ≥ 70) |
↓ MSEL Visual Reception, MSEL Expressive Language, adaptive behavior Not associated: age, sex, race/ethnicity, ADOS total score |
| Sikora et al. (2008) |
Referred | 147 | 3 – 5.9 (M = 4.5) |
CBCL Aggressive Behavior |
NA |
Not associated: age, sex, cognitive (MSEL Early Learning Composite) |
| Murphy et al. (2009) |
Research | 157 | 3 – 14.2 (M = 8.5) |
Behavior Problems Inventory |
53.5% (aggressive or destructive behavior) |
Not associated: age, sex, cognitive |
| Bronsard et al. (2010) |
Research | 74 | M = 11.6 |
Other-injurious Behavior Scale (Parent & caregiver report) |
34% (parent report) 58% (caregiver report) |
|
| Farmer & Aman (2011) |
Special Ed | 121 | 3 – 20 (M = 4.0) |
C-SHARP (item level) | 30.6 – 44.1% (pinches, bites, scratches others) |
↑ Autism versus PDD-NOS/Asperger’s Not associated: age, sex |
| Georgiades et al. (2011) |
Pathways | 335 | 2 – 4 (M = 3.3) |
CBCL Aggressive Behavior |
7.8% (T-scores ≥ 70) |
|
| Jang et al. (2011) |
Referred | 84 | 2.4 – 18 (M = 7.9) |
ASD-Behavior Problems for Children |
38.1% (aggression towards others) |
↑ ASD severity |
| Kanne & Mazurek (2011) |
Registry (Simons Simplex) |
1038 | 4 – 17 (M = 9.1) |
4 ADI-R items | 68% (directed to caregivers ever) 35.4% (definite aggression) |
↓ age ↑ repetitive behaviors, family income, parent-reported social and communicative symptoms (SRS) Not associated: sex, adaptive behavior, receptive vocabulary, caregiver education, ADOS severity scores |
| Mayes et al. (2011) |
Referred | 1,609 | 6 – 16 (M = 8.4) |
Pediatric Behavior Scale |
16.6% | |
| McTiernan et al. (2011) |
Research | 174 | 3 – 14 (M = 8.0) |
Behavior Problems Inventory |
56.3% (aggressive or destructive behavior) |
↓ IQ |
| Medeiros et al. (2011) |
Referred | 221 | 1.5 – 3 (M = 2.2) |
Baby and Infant Screen for Children with Autism Traits |
78.5% (aggression/destructi on) |
↑ total developmental quotient, communication, motor |
| Maskey et al. (2012) |
Registry | 863 | 2 – 18 | Parent questionnaire | 21.8% (≥ 3x/wk) |
Not associated: age, sex, language level, school type |
| Mazurek et al. (2013) |
Registry | 1584 | 2 – 17 (M = 5.9) |
Current parent concern (yes/no) |
53.7% | ↓ age, adaptive communication and socialization (VABS-II), caregiver education ↑ sleep difficulties, sensory problems, GI problems, self-injury Not associated: sex, race, IQ, language |
| Farmer et al. (2014) |
Referred | 414 | 1 – 21 (M = 7) |
C-SHARP, CBCL Aggressive Behavior |
19% (CBCL T- scores ≥ 70) |
↓ age, adaptive behavior, adaptive communication (VABS-II) Not associated: sex |
ABC, Adaptive Behavior Composite; ADI-R, Autism Diagnostic Interview-Revised; ADOS, Autism Diagnostic Observation Schedule; ASD-BPC; ASD Behavior Problems for Children; CBCL, Child Behavior Checklist; C-SHARP, Children’s Scale for Hostility and Aggression; FSIQ, Full Scale IQ; MSEL, Mullen Scales of Early Learning; NVIQ, Nonverbal IQ; PPVT, Peabody Picture Vocabulary Test; SRS, Social Responsiveness Scale; VABS-II, Vineland Adaptive Behavior Scales; VIQ, Verbal IQ.
In contrast, studies that have used validated measures of aggression tend to report lower prevalence estimates (see Table 1). For example, two previous studies measured aggressive behaviors using the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2000; 2001), a standardized measure with satisfactory national norms and with demonstrated reliability and validity in both clinical and non-clinical populations. In these studies, aggressive behavior problems (ABP), defined as CBCL Aggressive Behavior T-scores in the clinical range (≥ 70), were present in 8–23% of children with ASD (Georgiades et al., 2011; Hartley, Sikora, & McCoy, 2008). However, both studies included only young children, limiting the generalizability of the findings and the ability to examine age trends. Therefore, clarification is needed to identify accurate rates of aggressive behavior problems in populations with ASD, to determine whether these rates vary systematically with age, and to better understand the factors associated with increased risk of such behaviors.
In the general population, the developmental course and correlates of aggressive behaviors have been well studied (Broidy et al., 2003; Nagin & Tremblay, 2001; National Institute of Child Health & Human Development [NICHD] Early Child Care Research Network, 2004; Tremblay et al., 2004). Instrumental physical aggressive behaviors reliably peak at about 24 months of age and decline thereafter (Nagin & Tremblay, 1999; NICHD Early Child Care Research Network, 2004). Family variables such as low family income, low parent education levels, maternal antisocial behavior, maternal depression, and maternal early onset of childbearing account for significant variability in aggressive behaviors in typically developing children (Gross, Shaw, & Moilanen, 2008; Nagin & Tremblay, 2001; Tremblay et al., 2004). Additionally, higher rates of aggressive behaviors are associated with male sex (Lansford et al., 2006; NICHD Early Child Care Research Network, 2004), early language delays (Dionne, Tremblay, Boivin, Laplante, & Pérusse, 2003; Séguin, Parent, Tremblay, & Zelazo, 2009; Van Daal, Verhoeven, & Van Balkom, 2007), lower intellectual functioning (Tremblay, 2000), and higher levels of hyperactivity (Nagin & Tremblay, 2001). In most population samples, there are few children with significant aggressive behaviors who do not also exhibit clinically significant inattention/hyperactivity (Jester et al., 2005; Nagin & Tremblay, 2001).
Yet few of the factors associated with aggressive behaviors in typically developing populations have been consistently associated with aggressive behaviors in children with ASD. For example, the association between aggressive behavior and age is not clear. Higher levels of aggressive behaviors (primarily physical) have been found in younger children in some studies (Kanne & Mazurek, 2011; Mazurek et al., 2013), but not in others (Farmer & Aman, 2011; Hartley et al., 2008; Maskey, Warnell, Parr, Le Couteur, & McConachie, 2013; Murphy et al., 2005; Sikora, Hall, Hartley, Gerrard-Morris, & Cagle, 2008). Gender has consistently not been associated with aggressive behavior in children with ASD as in typical populations (Farmer & Aman, 2011; Hartley et al., 2008; Kanne & Mazurek, 2011; Kozlowski, Matson, & Rieske, 2012; Mazurek et al., 2013; Murphy, Healy, & Leader, 2009; Sikora et al., 2008). In terms of family demographics, higher levels of aggressive behaviors in children with ASD have been linked to both lower parent education levels (Mazurek et al., 2013) and higher family incomes (Kanne & Mazurek, 2011), leaving some question as to how aggression relates to family socio-economic status. Finally, similar to findings in typically developing children, increased aggressive behaviors have been found among children with ASD with impaired cognitive functioning (Dominick, Davis, Lainhart, Tager-Flusberg, & Folstein, 2007), language (Dominick et al., 2007; Hartley et al., 2008), and adaptive skills (Hartley et al., 2008; Mazurek et al., 2013), though negative findings have also been reported (Kanne & Mazurek, 2011; Maskey et al., 2013; Mazurek et al., 2013; Murphy et al., 2009).
Aggressive behaviors may also be influenced by the severity of a child’s ASD symptoms (Jang, Dixon, Tarbox, & Granpeesheh, 2011). In one study, aggressive children (based on parent report) had more severe parent-reported (but not clinician-observed) social and communicative deficits (Kanne & Mazurek, 2011). Aggressive behaviors have also been linked to increased repetitive, stereotyped, and ritualistic behaviors as well as resistance to change in children with ASD (Dominick et al., 2007; Kanne & Mazurek, 2011).
In addition to core ASD symptoms, having ASD increases the risk of a number of comorbid problems that are known to increase challenging behavior in this population (Matson & Kuhn, 2001; Matson et al., 2011; Matson, Neal, & Fodstad, 2010). Several of these comorbid problems have been associated with increased aggression among atypically and typically developing children. In children with ASD, increased aggressive behavior has been concurrently associated with greater sleep difficulties (Goldman, Richdale, Clemons, & Malow, 2012; Mayes & Calhoun, 2009; Mazurek et al., 2013), internalizing symptoms (Cervantes, Matson, Tureck, & Adams, 2013; Kim, Szatmari, Bryson, Streiner, & Wilson, 2000), and hyperactivity and attention deficits (Yerys et al., 2009). However, studies linking psychiatric comorbidities to aggressive behaviors have either focused only on toddlers with ASD (Cervantes et al., 2013) or have been limited by relatively small research samples due to their study designs and aims (Kim et al., 2000; Yerys et al., 2009). No previous studies have examined comorbid sleep and behavioral/emotional problems in the same sample. These comorbid problems are likely to co-vary significantly with aggressive behaviors in children with ASD.
A better understanding of the correlates of ABP in children with ASD would provide insight into the pathophysiology of aggressive behaviors in ASD and would also have direct clinical implications. For example, clinicians could use the study’s results to proactively counsel families of children with ASD who are at high risk for ABP. In addition, identifying modulating factors for some comorbid conditions, such as sleep or behavioral/emotional problems, could have a positive impact on children’s aggressive behavioral symptoms and family stress.
The first aim of the current study was to examine the prevalence of ABP using the CBCL, a well-validated measure, in a large clinical sample of children with confirmed diagnoses of ASD. The second aim was to examine whether correlates associated with increased aggressive behaviors in typical populations would be similarly associated with the presence of ABP in children with ASD (child age, gender, parent education, race/ethnicity). Our final aim was to examine differences between children with and without ABP to determine whether those with ABP receive more intensive medical interventions (complementary/alternative medicines, psychotropic medications), demonstrate more severe impairments in behavioral functioning (ASD symptoms, adaptive skills, intellectual and language levels), and experience more severe comorbid problems (sleep, internalizing, and attention).
2. Method
2.1. Participants
The current study included 400 children enrolled in the Autism Speaks ATN at Oregon Health and Science University (OHSU). The ATN, a collaboration among 17 academic health centers in the United States and Canada, was established to develop a model of comprehensive medical care for children and adolescents with ASD. The ATN participant registry includes children ages 2 to 18 years with a confirmed ASD diagnosis according to the Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; DSM-IV-TR; American Psychiatric Association, 2000) criteria and supported by administration of the Autism Diagnostic Observation Schedule (ADOS; Lord et al., 2000). Eligible families are invited to participate in the registry. Participation involves written consent and the collection of clinical data that are regarded as routine standard of care of ASD, such as medication usage, health, and behavior assessments. Registry protocols are approved by the Institutional Review Boards at each site, and trained study coordinators enter the data. Data from the current sample was obtained at the time of study conception and analysis, and thus included all children enrolled in the ATN Registry (based on the date of consent) at the OHSU site from May 2008 to September 2012.
2.2. Measures
2.2.1. Sociodemographics
Sociodemographic characteristics included child gender, age, race/ethnicity, and parent(s) education levels, based on parent report. Race was categorized as White, Black/African American, Asian, Native American or Alaskan Native, Native Hawaiian or Pacific Islander, or mixed race. Because of the small numbers in some categories, these were collapsed to White and All Other Races for analyses. Ethnicity was categorized as either Hispanic/Latino origin or Not Hispanic/Latino. Parent education level was classified as the maximum educational level of the child’s primary or secondary parent. For analyses, these were grouped as follows: high school graduate or less, some college, or college graduate or higher (see Table 2).
Table 2.
Sociodemographic Characteristics for Total Sample and by Aggressive Behavior Problem Status
| Total Sample (N = 400) |
ABP- vs. ABP+ |
|||||
|---|---|---|---|---|---|---|
| n | % | % ABP− (n = 300) |
% ABP+ (n = 100) |
χ2 | p | |
| Male | 332 | 83.0 | 84.3 | 79.0 | 1.16 | .28 |
| Race | 0.03 | .86 | ||||
| White | 315 | 78.8 | 85.5 | 84.0 | ||
| All Other Races | 55 | 13.7 | 14.5 | 16.0 | ||
| No data | 30 | 7.5 | ||||
| Ethnicity | 0.70 | .40 | ||||
| Hispanic or Latino origin | 48 | 12.0 | 13.9 | 9.8 | ||
| Not of Hispanic or Latino origin | 325 | 81.3 | 86.1 | 90.2 | ||
| No data | 27 | 6.7 | ||||
| Caregiver(s)’ highest level of education | 2.51 | .28 | ||||
| High school or less | 95 | 23.8 | 26.0 | 34.8 | ||
| Some college | 164 | 41.0 | 50.4 | 45.0 | ||
| College graduate or more | 76 | 19.0 | 23.6 | 20.2 | ||
| No data | 65 | 16.2 | ||||
Note: All percentages are column percentages.
2.2.2. Aggressive behavior problems: Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2000; 2001)
The presence of ABP was determined from CBCL Aggressive Behavior Scale T-scores. The CBCL is a well-validated parent questionnaire that measures behavioral and emotional problems in multiple domains, and is frequently used to assess behavioral/emotional problems in the general population and in ASD (Deprey & Ozonoff, 2009). There are two versions: preschool (ages 2–5 years) and school age (ages 6–18 years). Parents are asked to rate their children’s behaviors in the past six months on a 3-point Likert-scale (0 = not true; 1 = somewhat/sometimes true; 2 = very often true). Mean test-retest reliabilities of r = .85 and r = .88 across an eight day period have been reported for the preschool and school age forms, respectively (Achenbach & Rescorla, 2000; 2001).
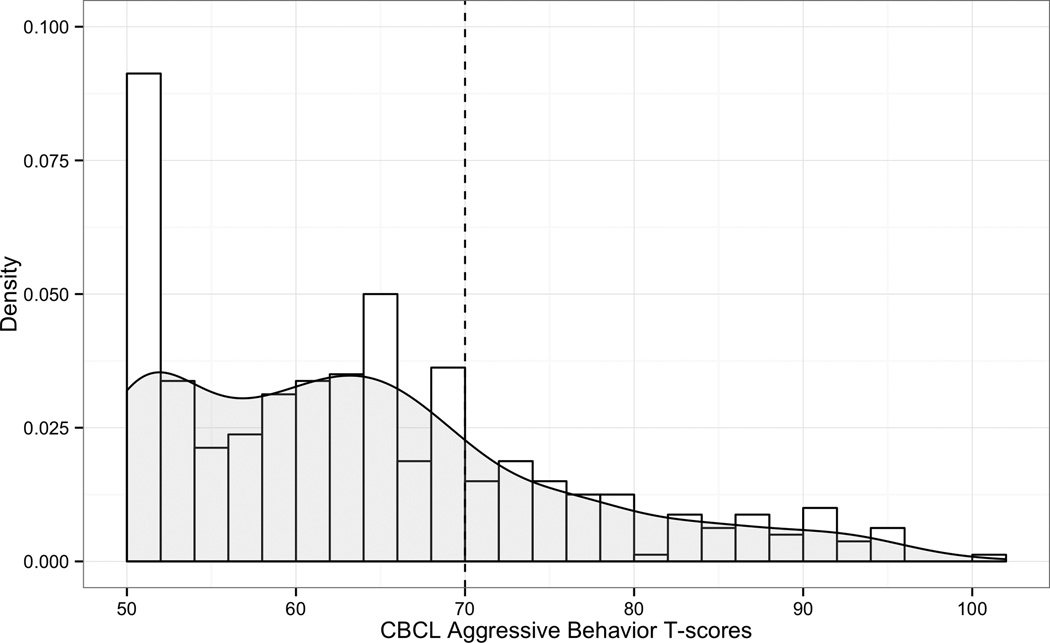
The CBCL Aggressive Behavior scale contains 19 items on the preschool form and 18 on the school age form. Items include: “gets in many fights,” “hits others,” “physically attacks people,” and “temper tantrums or hot temper” (Achenbach & Rescorla, 2000; 2001). Thus, this measure incorporates both physical and verbal forms of aggressive behavior. Raw total scores and T-scores (M = 50, SD = 10) are available for each scale. The clinical range of aggressive behavior on the CBCL is defined by T-scores ≥ 70, which corresponds to a score above the 98th percentile (Achenbach & Rescorla, 2000; 2001). The T-scores are truncated with a minimum score of 50, resulting in a substantially positively skewed distribution of scores (see Figure 1). To address this issue, we split the sample into two groups based on their CBCL Aggressive Behavior T-scores: (a) those with scores within the clinical range (≥ 70) (ABP+; n = 100); and (b) those with scores below the clinical range (< 70) (ABP-; n = 300).
Figure 1.
Histogram of CBCL Aggressive Behavior T-scores with kernel density plot overlaid (in gray).
2.2.3. Medical treatments
2.2.3.1. Complementary and alternative medications/treatments (CAM) and melatonin
Parents completed a medical history questionnaire at registry entry that includes questions about the following CAM: chiropractic care, dietary supplements (amino acids, high dosing vitamin B6 and magnesium, essential fatty acids, probiotics, digestive enzymes, glutathione), or dietary interventions (gluten-free, casein-free, no processed sugars). Because of the infrequent rate of endorsement of CAM, the variables were collapsed into a primary outcome measure of any CAM use. Current use of melatonin is also recorded.
2.2.3.2. Prescribed psychotropic medications
At registry entry, ATN clinicians record each child’s prescribed medications. We categorized medications as stimulants, selective serotonin reuptake inhibitors (SSRIs), α-adrenergic agents, anticonvulsants, antihistamines, and atypical neuroleptics. Due to the relatively small number of children receiving each type of medication, the variables were collapsed into a primary outcome measure of any psychotropic drugs prescribed.
2.2.4. Behavioral functioning
2.2.4.1. ASD severity: Autism Diagnostic Observation Schedule (Lord et al., 2000)
The ADOS is a standardized observational assessment that is organized into four modules based on the child’s spoken language level (Lord et al., 2000). The ADOS was scored according to the revised algorithms (Gotham, Risi, Pickles, & Lord, 2007). Calibrated severity scores (CSS) were calculated as an indicator of total ASD severity (Total CSS; Gotham, Pickles, & Lord, 2009) and severity of social affect symptoms and restricted and repetitive behavioral symptoms (RRB; Hus, Gotham, & Lord, 2012). Social Affect CSSs reflect the severity of the child’s social communication impairments, whereas RRB CSSs reflect the severity of symptoms such as hand flapping, sensory examination of materials or excessive references to particular topic. Children who received Module 4 (n = 11) were excluded from these analyses as CSSs are defined only for ADOS Modules 1–3.
2.2.4.2. Adaptive skills: Vineland Adaptive Behavior Scale (2nd ed.; VABS-II; Sparrow, Balla, & Cicchetti, 2005)
The VABS-II assesses functional skills used in everyday life in three primary domains: Communication, Socialization, and Daily Living Skills. The VABS-II also provides an Adaptive Behavior Composite as an estimate of overall adaptive functioning. Domain scores and the composite are standardized (M = 100, SD = 15). Test–retest reliability for the VABS-II has been established: subdomain reliability coefficients are excellent with most values exceeding 0.85 (Sparrow et al., 2005).
2.2.4.3. Cognitive functioning
Cognitive abilities were assessed using a range of measures. The majority of children were administered the Mullen Scales of Early Learning (n = 227; MSEL; Mullen, 1995). The MSEL Early Learning Composite (ELC) Standard Score was used as an estimate of IQ. Remaining participants were administered either the full Stanford-Binet Scales of Intelligence (n = 98; 5th ed.; SB-5; Roid, 2003), the abbreviated SB-5 battery (n = 3), or the Wechsler Preschool and Primary Scales of Intelligence (n = 1; 3rd ed.; Wechsler, 2003). Standardized norm-referenced IQ scores were therefore available for 329 participants. Preliminary analysis revealed that SB-5 IQ scores were normally distributed, whereas MSEL ELC scores significantly deviated from normal. Inspection of the data revealed that about half of the children who received the MSEL (n = 114) were assigned the lowest ELC score possible (49), creating a distribution with significant positive skew. To address this issue, we imputed IQ scores for these children using the regression relationship of ELC scores to VABS-II Adaptive Behavior Composite scores for children with ELCs greater than 49. However, the distributions of IQ scores (range, skewness, kurtosis, etc.) continued to differ substantially between the MSEL and SB-5. Therefore, for group comparison analyses, we created IQ categories as follows: average to above average IQ (≥ 85), below average range (70–84), and intellectual disability range (< 70).
Separate scores for Performance IQ (PIQ) and Verbal IQ (VIQ) were either available or could be estimated for a subset of participants (n = 323). The SB-5 provides standard scores for both PIQ and VIQ (n = 98). Unlike the SB-5, the MSEL does not provide separate standard scores for PIQ and VIQ. To estimate PIQ and VIQ, we calculated ratio IQs (i.e., developmental quotients) using MSEL age-equivalent scores, which have demonstrated convergent validity with other IQ measures (Bishop, Guthrie, Coffing, & Lord, 2011). This approach was selected in favor of analyzing MSEL domain T-scores because a significant number of participants (42%; n = 142) received the minimum possible score of 20 on at least one MSEL domain, resulting in significantly positively skewed distributions. We created three categories for group comparisons of PIQ (SB-5 PIQ and MSEL ratio PIQ) and VIQ (SB-5 VIQ and MSEL ratio VIQ): average to above average (≥ 85), below average range (70–84), and intellectual disability range (< 70).
2.2.4.4. Language functioning
The ADOS provides an item for the child’s overall level of non-echoed spoken language as scored by the clinician. Scores for this item vary by module. We created a measure of spoken language functioning according to the following criteria: phrase to fluent speech (Module 2: scores of 0 or 1; Module 3 or 4); some words/mostly single words (Module 2: score of 2 or Module 1: scores of 0, 1 or 2); and few to no words (Module 1: score of 3 or 4). Although Module 2 allows for a score of 3 (single words only), this score was never used in the current sample.
2.2.5. Comorbidities
2.2.5.1. Sleep problems: Children’s Sleep Habits Questionnaire (CSHQ; Owens, Spirito, & McGuinn, 2000)
Sleep problems were measured using the abbreviated version of the CSHQ, a validated parental questionnaire describing sleep behaviors in children ages 2–10 years (Owens et al., 2000). The CSHQ includes 39 items and is rated over the previous week by parents to screen for the most common sleep problems. The majority of sleep questions are answered on a 3-point scale (e.g., 1 = rarely; 2 = sometimes; 3 = usually). The CSHQ contains items related to eight sleep domains: (a) bedtime resistance; (b) sleep onset latency; (c) sleep duration; (d) anxiety around sleep; (e) night awakenings; (f) sleep disordered breathing; (g) parasomnias; and (h) morning waking/daytime sleepiness. The total sleep disturbance score is the sum of scores across 33 items. This score served as a continuous measure of child sleep difficulties.
2.2.5.2. Internalizing and attention problems: CBCL
The CBCL Internalizing Problems scale includes anxiety/depression, somatic complaints, and withdrawal (Achenbach & Rescorla, 2000; 2001). The CBCL Attention Problems scale includes 20 items such as “can’t concentrate,” “can’t pay attention for long,” and “can’t sit still, restless, or hyperactive” (Achenbach & Rescorla, 2000; 2001). It thus includes features of both inattention and hyperactivity; the two core domains associated with ADHD.
2.2.6. Parent concerns about aggressive behavior
The ATN Parent Questionnaire is completed at registry entry by parents and queries 15 specific concerns about their child’s behavior. One of these questions asks parents to indicate (“Yes” or “No”) whether aggressive behaviors are a current concern. Specifically, the question asks whether their child “intentionally hits, bites others, etc.” Responses to this question have been used in a previous study to identify the presence of physical aggression in children with ASD (Mazurek et al., 2013). We were interested in whether parent concerns about physical aggression aligned with ABP according to the CBCL.
2.3. Statistical analyses
Prior to analysis, we screened for skewness, kurtosis, and outliers. To examine categorical variables associated with ABP, we conducted chi-square tests. To examine differences between children with and without ABP on continuous variables, Mann-Whitney U tests were used due to non-normal distributions. Age was analyzed as a categorical variable due to a non-normal distribution with significant positive skew (mean age = 5.4; median age = 4.3). For all significant results, Cohen’s ds are reported as measures of effect size (.2 is a small effect; .5 a medium effect; .8 a large effect; Cohen, 1988). Lastly, we used stepwise logistic regression to examine the joint effects of cross-sectional predictors of the presence of ABP.
2.3.1. Missing data
Complete data for all 33 CSHQ items that contribute to the total sleep disturbance score was available for 261 participants. Participants with 50% or more missing CSHQ items (n = 42) were excluded from analyses. Following Bryman and Cramer (2001), for participants with less than 50% of CSHQ items missing (n = 97), we imputed missing data using mean responses for non-missing items (following reverse scoring for those items that are reverse scored). Although mean imputation is a debated method (see Gelman & Hill, 2007), we adopted it for several reasons. First, analysis of missing data patterns indicated that the assumption of missing completely at random (MCAR; Little, 1988) was met as there was no association between missingness and other observed variables, χ2 = 5.57, p = .85. This was confirmed in a series of logistic regression analyses. For example, whether complete data was available did not predict the presence of ABP, odds ratio (OR) = 1.00, p = .90. Likewise, the total number of missing CSHQ items did not predict ABP, OR = 0.99, p = .98. Second, for the majority of participants (75%), one or two CSHQ items were missing. We also compared results of analyses at different thresholds for imputation and found the same pattern of results regardless of whether only complete data was analyzed (0% imputed) or whether we imputed scores for children with 5%, 10%, 20%, 30%, 40%, and 50% of items missing. Thus, we opted to use 50% as our criteria for imputation in order to preserve generalizability of the analysis sample and statistical power.
Nevertheless, given the limitations of mean imputation, we also performed complete case analyses which are unbiased when data is MCAR (Gelman & Hill, 2007). For group comparisons with the CSHQ total sleep disturbance score as the dependent variable, results of analyses using only complete data are reported in the text. For the logistic regression analysis, results of two additional logistic regression analyses are reported in the text: one in which the total number of missing CSHQ items was included as a covariate and one in which only complete cases are analyzed.
3. Results
3.1. Prevalence and age trends of ABP
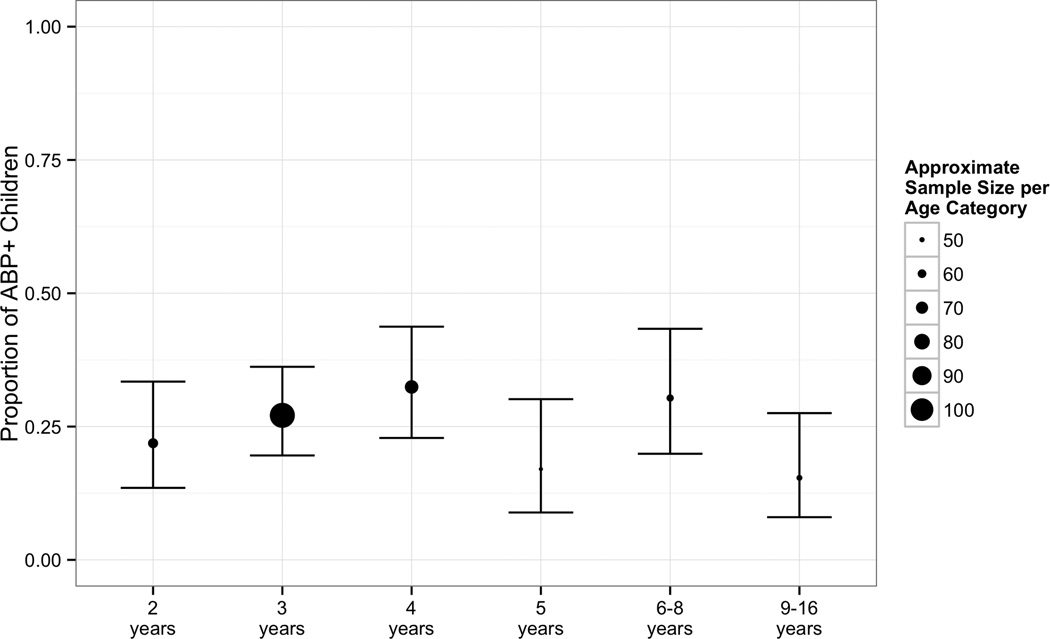
Prevalence of ABP according to the CBCL was 25%. Prevalence did not vary significantly as a function of age according to categorical (χ2 = 7.78, p = .17; see Figure 2) and continuous analyses (Kendall’s τ = .02, p = .54). A chi-square test also showed no association between ABP and CBCL form (preschool versus school-age, χ2 = 0.15, p = .69), indicating that the prevalence of ABP did not differ based on the version of the CBCL that was completed.
Figure 2.
Proportion of children with ABP by age with 95% confidence intervals.
3.2. Correlates of ABP
The presence of ABP was not associated with any sociodemographic measures (ps > .20, see Table 2). Children with ABP were significantly more likely to be prescribed psychotropic medications and melatonin (see Table 3). Children with ABP also had significantly less severe overall and social affect symptoms (see Table 3). There was a significant association between ABP and overall cognitive level (IQ). Follow-up comparisons revealed that a significantly larger proportion of children with ABP had FSIQ scores less than 85 (88.1% vs. 76.3%, χ2 = 4.59, p = .03, d = .24), compared to those without ABP. No significant associations were found between ABP with PIQ (χ2 = 3.35, p = .19) or VIQ (χ2 = 4.19, p = .12).
Table 3.
Medical Treatments, ASD Severity, Behavioral Functioning, and Comorbid Problems by Aggressive Behavior Problem Status
| %(n) or M(SD) |
|||||
|---|---|---|---|---|---|
| ABP- (n = 300) |
ABP+ (n = 100) |
χ2 or z | p | Cohen’s d | |
| Medical Treatments | |||||
| CAM use | 24.3 (73) | 31.0 (31) | 1.40 | .24 | |
| Melatonin use | 20.0 (60) | 31.0 (31) | 4.56 | .03 | .21 |
| Psychotropic medications | 29.0 (87) | 43.0 (43) | 6.08 | .01 | .25 |
| ASD severity (ADOS CSS) | |||||
| Totala | 7.35 (2.0) | 6.82 (1.9) | −2.37 | .02 | .24 |
| Social Affecta | 7.04 (2.1) | 6.48 (2.2) | −2.20 | .03 | .24 |
| RRBa | 7.82 (1.8) | 7.55 (1.6) | −1.78 | .08 | .18 |
| Adaptive Skillsb (VABS-II) | |||||
| Adaptive Behavior Compositeb | 67.31 (13.5) | 66.58 (12.9) | −0.50 | .62 | |
| Communicationb | 69.48 (15.9) | 69.58 (14.6) | 0.08 | .93 | |
| Socializationb | 67.62 (10.3) | 67.19 (8.1) | −0.01 | .99 | |
| Daily Living Skillsb | 72.87 (13.9) | 71.73 (13.9) | −1.07 | .29 | |
| Cognitive Functioning (IQ) | 6.44 | .04 | .28 | ||
| Average to above average range | 23.7 (58) | 11.9 (10) | |||
| Below average range | 11.4 (28) | 17.9 (15) | |||
| Intellectual disability range | 64.9 (159) | 70.2 (59) | |||
| No data n = 71 | |||||
| Language Functioning | 6.09 | .05 | .25 | ||
| Phrase to fluent speech | 39.2 (111) | 41.7 (40) | |||
| Some words | 25.4 (72) | 35.4 (34) | |||
| Few to no words | 35.3 (100) | 22.9 (22) | |||
| No data n = 21 | |||||
| Total Sleep Disturbance (CSHQ) | 47.50 (8.6) | 53.33 (9.9) | 4.98 | < .001 | .54 |
| Internalizing Problemsc (CBCL) | 63.21 (8.4) | 71.09 (6.9) | 7.49 | < .001 | .85 |
| Anxiety Problemsc | 57.48 (7.9) | 63.86 (10.4) | 5.55 | < .001 | .58 |
| Withdrawnc | 69.68 (9.9) | 75.98 (9.9) | 4.82 | < .001 | .49 |
| Somatic Complaintsc | 59.37 (7.8) | 63.00 (9.0) | 3.58 | < .001 | .37 |
| Attention Problemsc (CBCL) | 64.28 (8.4) | 72.31 (7.4) | 7.93 | < .001 | .87 |
ADOS CSS, Autism Diagnostic Observation Schedule calibrated severity score; CAM, Complementary and alternative medicines/treatment; CBCL, Child Behavior Checklist; CSHQ, Child Sleep Habits Questionnaire; IQ, intelligence quotient; VABS-II, Vineland Adaptive Behavior Scales.
Note: All percentages are column percentages. For continuous measures (ADOS CSSs, VABS-II, CSHQ, CBCL), the z test statistic from the Mann-Whitney U test is reported.
CSSs range from 1–10; no data was available for n = 36, 37, 37, respectively.
Standard scores (M = 100, SD = 15); no data was available for n = 17, 14, 13, 14, respectively.
T-scores (M = 50, SD = 10); complete data was available for all CBCL measures with the exception of Internalizing Problems (no data n = 1).
In terms of language levels, a significantly larger proportion of children with ABP had language at or above the level of some words (77.1% vs. 64.7%, χ2 = 4.51, p = .03, d = .22), compared to those without ABP. The presence of ABP was strongly associated with more sleep difficulties, internalizing problems, and attention problems (see Table 3). Follow-up analyses using only CSHQ total sleep disturbance scores based on complete data (n = 261) also revealed significantly more sleep difficulties among children with ABP compared to those without ABP (p < .0001).
3.3. Multivariate associations with ABP
We used multivariate logistic regression models to examine the joint effects of the following predictors: any psychotropic drug, melatonin use, ADOS total CSS, cognitive functioning, language functioning, CSHQ imputed total sleep disturbance, CBCL Internalizing Problems, and CBCL Attention Problems. The dependent variable in this analysis was the presence of ABP (ABP+ = 1, ABP- = 0). Considering their general developmental significance, age and gender were included in the initial model, although they never contributed to the model and were excluded from the final model to maintain statistical power at a satisfactory level. Other sociodemographic variables (race, ethnicity, parent education) were excluded from this analysis due to high levels of missing data and because they were not associated with ABP in bivariate analyses. A backward stepwise logistic regression was performed (entry criteria, p = .10; removal criteria, p = .05). This statistical method is appropriate for predictors that may have high collinearity because all are assessed simultaneously and those that account for the largest amount of variability among correlated predictors remain in the model. Backward selection is robust to a large number of initial predictors (van Belle, Fisher, Heagerty, & Lumley, 2004). It is also preferable over forward selection because it is less likely to exclude predictors involved in suppressor effects and runs a lower risk of making Type II errors (Field, Miles, & Field, 2012).
The final model predicting the presence of ABP is shown in Table 4 and included three continuous predictors: sleep, internalizing, and attention problems. The odds ratios for the final model indicate that for each one-unit increase in CSHQ total sleep disturbance scores, the odds of ABP+ are 1.04 times greater than the odds of ABP-. Similarly, with each one-unit increase in CBCL internalizing and attention problems T-scores, the odds of ABP+ are 1.12 and 1.11, respectively, times greater than the odds of ABP-. Fit indices also indicated that the model was a satisfactory fit.
Table 4.
Cross-sectional predictors of aggressive behavior problems (N = 357; 0 = ABP- [n = 265]; 1 = ABP+ [n = 92])
| Predictor | B (SE) | Wald χ2 | p | Adjusted Odds Ratio [95% Confidence Interval] |
|---|---|---|---|---|
| CBCL Internalizing Problems T-scorea | 0.12 (.02) | 26.77 | < .001 | 1.12 [1.08; 1.18] |
| CBCL Attention Problems T-scorea | 0.10 (.02) | 26.69 | < .001 | 1.11 [1.06; 1.15] |
| CSHQ Total Sleep Disturbanceb | 0.04 (.02) | 6.53 | .011 | 1.04 [1.01; 1.07] |
Note: R2 = .27 (Cox-Snell), .39 (Nagelkerke). Hosmer-Lemeshow C = 5.63, df = 8, p = .69. Independent variables not retained in the final model included any psychotropic drug, melatonin use, ADOS total calibrated severity scores, cognitive functioning, and language functioning.
These odds ratios represent the increase in the odds of aggression for each increase of 1 unit in the predictor CBCL T-score.
This odds ratio represents the increase in the odds of aggression for each increase of 1 unit in the predictor CSHQ score.
In a separate analysis in which the total number of CSHQ missing items was included as a predictor, the results were essentially unchanged; the total number of CSHQ missing items was not selected in the final model, p = .70. In the subset of the sample with complete CSHQ data (n = 261), all three predictors were significant: sleep problems, adjusted OR = 1.04, p = .04; internalizing problems, adjusted OR = 1.16, p < .001; and attention problems, adjusted OR = 1.11, p < .001. Thus, the adjusted ORs for sleep and attention problems were unchanged relative to the models using imputed CSHQ data, whereas the internalizing problems OR increased 3.6%.
3.4. Parent concerns about aggressive behavior
Physical aggressive behavior was one of the most frequent (50%) parental concerns at registry enrollment. Parents rated these behaviors as a concern for 83% of children with ABP, and for 39% without ABP. Of the children with ABP according to the preschool CBCL, 83.3% of parents noted this as a concern. Similarly, of the children with ABP according to the school age CBCL, 81.8% of parents noted this as a concern. Regardless of the CBCL form that was administered, approximately 16–18% of parents who had children with ABP did not note aggressive behaviors as a concern when asked.
4. Discussion
Aggressive behavior problems were present in 25% of children with ASD. This estimate is considerably lower than several recent estimates of over 50% (Bronsard et al., 2010; Kanne & Mazurek, 2011; Mazurek et al., 2013; McTiernan, Leader, Healy, & Mannion, 2011; Medeiros, Kozlowski, Beighley, Rojahn, & Matson, 2012; Murphy et al., 2009). Inconsistent ASD diagnostic criteria, definitions of aggressive behaviors, and the use of measures that are not validated in typical or ASD populations make comparisons across studies difficult. On the other hand, this prevalence estimate is at the higher end of the range of previous studies that used the CBCL to define ABP (Farmer et al., 2014; Georgiades et al., 2011; Hartley et al., 2008). For example, Georgiades and colleagues (2011) found a prevalence of 7.8% among children with ASD using the CBCL (ages 2–4 years). Similarly, using the Pediatric Behavior Scale (8 items: mean, threatens, fights, physically aggressive, destructive, lies, steals, self-injurious behavior), Mayes and colleagues (2012) found that 16.6% of children with autistic disorder (ages 6–16 years) displayed aggressive behaviors. The discrepancy between our prevalence estimate and these two previous studies is not due to differences in age ranges. Indeed, restricting our sample to only children ages 2–4 (n = 245), we found a prevalence of 27% (n = 67) using the same measure and criterion as Georgiades et al. (2011). Likewise, examining only those children with any ASD ages 6 to 16 in the current sample (n = 108), the prevalence of ABP was 23% (n = 25). Thus, in the current sample, it is likely that additional factors other than age impacted the presence of aggressive behaviors in ASD. For example, aggressive behaviors may be less prevalent in children with autistic disorder as opposed to any ASD, which could be consistent with our finding that the presence of ABP is associated with less severe ASD severity. Aggressive behaviors may also be less common in research samples than in clinic-referred samples of children with ASD.
Sociodemographic correlates of aggressive behavior in typical populations such as gender, parent education, race, and ethnicity tended to be weaker or absent in children with ASD, pointing to the possibility of a different mechanism and significance of ABP in ASD than in typical populations. However, these results should be interpreted with caution because of relatively high proportions of missing data in the ATN dataset for several sociodemographic measures.
In the current sample of children with ASD, those with ABP did differ from those without ABP in several important ways. Consistent with previous research (Tureck et al., 2013), children with ABP were significantly more likely to be prescribed psychotropic medications and to take melatonin. In terms of severity of ASD symptoms, children with ABP had significantly less severe ASD symptoms overall and in the social affect domain compared to those without ABP, based on clinician observation during the ADOS. This result is difficult to interpret given that both ADOS and CBCL scores reflect quantitatively and qualitatively more severe symptoms. It is possible that children with ASD who have relatively stronger social communication abilities are more able to direct their aggression toward others, where it is most likely to be noticed and therefore reported by parents. Alternatively, these children could engage in more aggressive behavior because they have more opportunities for peer or sibling contact, compared to children with ASD who have more impaired social communication abilities. A similar finding has been reported in the general population; for example, the presence of other young siblings in the household is associated with increased odds of being highly aggressive by more than a factor of four (Tremblay et al., 2004). Future studies that differentiate between frequency and severity of children’s aggressive behaviors and include measures of peer/sibling contact are needed to clarify these findings in ASD populations.
Our finding of less severe ASD symptoms in children with ABP contrasts with evidence that children with ASD who demonstrate aggressive behavior have more severe parent-reported social and communicative deficits (Kanne & Mazurek, 2011) based on the Social Responsiveness Scale (SRS; Constantino & Gruber, 2005). As those authors note, the ADOS may measure core symptoms specific to an ASD diagnosis, whereas the SRS may reflect broader ASD-related functioning that is more accurately measured via parent report (Kanne & Mazurek, 2011). On the other hand, several recent studies suggest that parent-reported measures of ASD symptoms such as the SRS may be less accurate when children exhibit behavioral difficulties such as aggression (Charman et al., 2007; Hus, Bishop, Gotham, Huerta, & Lord, 2013). The co-occurrence of aggressive behavior problems and ASD may lead to elevated overall parent concerns and therefore more parent-reported ASD-related symptoms, which may not be surprising given that aggressive behavior is linked to higher levels of caregiver stress in ASD populations (Lecavalier et al., 2006). Studies that examine factors associated with increased parental stress in ASD populations, and how this may impact the severity and types of parent-reported versus clinician-observed child behaviors, are necessary to better understand these effects.
In contrast to some previous studies (Farmer et al., 2014; Hartley et al., 2008; Mazurek et al., 2013), children with ABP were not more impaired in adaptive functioning than those without ABP. The lack of differences between children with and without ABP on the VABS-II Socialization scale may appear inconsistent with the negative association between ABP and ASD social affect symptoms since the two measures reflect similar constructs. However, several studies have failed to demonstrate reliable associations between ASD symptoms and adaptive skills (Kanne et al., 2010; Klin et al., 2007). These studies suggest that lower levels of ASD symptoms do not translate to higher levels of adaptive functioning (Kanne et al., 2010; Klin et al., 2007). Similarly, our findings suggest lower levels of aggressive behaviors do not necessarily imply better adaptive skills. Nevertheless, it is possible that aggressive behaviors that are developmentally less appropriate (e.g., physical aggression in adolescence) are associated with more severe impairments in adaptive skills. Future studies that differentiate between forms of aggressive behaviors may reveal such associations.
Aggressive behaviors were marginally more common among children with some words, but not in those with few to no words or with phrase to fluent speech. Children with ASD who have limited language abilities may experience more frustration trying to communicate with others, and therefore may be more likely to react aggressively. Alternatively, the CBCL measures several verbally aggressive behaviors (i.e., “teasing,” “arguing”), which could lead to higher levels of aggressive behaviors in children with some language abilities. However, ABP was not more common in children with phrase to fluent speech, indicating that verbal aggression items on the CBCL may not fully account for the elevated rates of aggressive behaviors in children with some words.
A significantly higher proportion of children with ABP had IQ scores falling below 85 compared to those without ABP (88.1% versus 76.3%, respectively). Increased aggression among ASD children with lower IQ has been reported in several studies (Dominick et al., 2007; McTiernan et al., 2011). In a recent longitudinal study,Estes et al. (2007) found that children with verbal or nonverbal IQ impairment at age six were more likely to demonstrate externalizing behaviors at age nine, suggesting that cognitive functioning may play a causal role in the ontogeny of behavior problems including aggression. Future studies with larger samples and consistent IQ measures over time are needed to clarify the direction of this effect.
Future understanding of aggressive behaviors in ASD must account for the heterogeneity of such behaviors, and future studies should consider employing measures of specific types of aggressive behaviors. For example, aggression researchers often differentiate between direct (aimed at inflicting physical harm) and indirect (aimed at harming social relations such as peer rejection or exclusion) aggressive behaviors in the general population (Card et al., 2008). Similarly, in a recent factor analysis using items from the Dutch version of the CBCL, the researchers were able to differentiate between direct (e.g., physical aggression, bullying, property destruction) and relational (e.g., argumentative, disobedient) aggressive behaviors among typically developing children (Ligthart, Bartels, Hoekstra, Hudziak, & Boomsma, 2005). Unfortunately, we did not have access to item-level scores on the CBCL. Analysis of such scores would allow researchers to better examine age and gender trends in the development of meaningful types of aggressive behaviors in children with ASD.
The current study is unique to the literature on aggressive behaviors in children with ASD because of the use of a large clinical sample of children with confirmed diagnoses of ASD, and the inclusion of multiple measures of comorbid problems. This study also has numerous limitations. The current sample was primarily young (mean age: < 6 years), white, and Non-Latino, limiting the generalizability of our results. All analyses were cross-sectional, and the directions of effects are therefore unclear. The CBCL as a measure of aggressive behaviors also has several limitations, especially when trying to measure such behaviors across age ranges and versions (preschool and school-age). Additionally, the CBCL Aggressive Behavior scale measures a relatively broad constellation of behaviors including items related to physical aggression, verbal aggression, mood changes, temper tantrums, and property destruction. The number and content of these items also differ by version, which may account in part for some of the differences in prevalence and correlates of ABP between this study and previous studies. It is possible that children who are physically aggressive but do not display other symptoms may not reach the clinical cut-off on this CBCL subscale. As this was a secondary data analysis, we were limited by the data available within the ATN dataset, including the CBCL as well as additional measures that may have been problematic for other reasons. In order to maximize statistical power, measures of cognitive functioning were derived from several different assessments. Floor effects were a particular problem for scores derived from the MSEL, which we attempted to address by analyzing IQ as a categorical variable. This likely reduced our statistical power than if we had been able to analyze these measures as continuous. Similarly, our measure of language functioning was also categorical, and was not derived from a formal language assessment administered by a speech/language professional.
Several of our measures were also limited by missing data, particularly the CSHQ measure of total sleep difficulties. We opted to use mean imputation for this measure, which has known limitations such as underestimation of the standard deviation and biasing correlations with other variables toward zero (Gelman & Hill, 2007). Imputed sleep difficulty scores were strongly associated with ABP in both univariate and multivariate tests. These results were also consistent with those based on the subset of children with compete CSHQ data only, which are unbiased when data is MCAR (Gelman & Hill, 2007). Nevertheless, the cross-sectional predictors of ABP identified in the current study warrant caution in interpretation and require replication with samples in which data collection procedures minimize incomplete data.
5. Conclusions
Consistent with previous research demonstrating that comorbid problems increase challenging behavior in children with ASD (Matson et al., 2010; 2011; Matson & Kuhn, 2001), the severity of comorbid sleep, internalizing, and attention problems significantly predicted the presence of concurrent ABP. Identifying modulating factors on aggressive behaviors could help to elucidate the developmental origins of such behaviors in ASD, identify targets for preventative interventions, and allow for proactive counseling for families and children with high-risk profiles on these variables. Importantly, regardless of the child’s age, approximately one out of five families with a child with ABP may not voice concerns about physical aggression to clinicians when asked. It may be that these children’s aggressive behaviors were more verbal than physical in nature. However, it is also possible that the behaviors are present but relatively less concerning for parents compared with the other challenges that these children face. Finally, parents may be reluctant to label their child as “aggressive” (Farmer & Aman, 2011). In clinical settings, it may be beneficial to administer questionnaires with known psychometric properties and normative data such as the CBCL to provide parents the opportunity to rate challenging behaviors that the clinician can then use to facilitate open discussions with families.
Given the bivariate associations between ABP and sleep problems as well as lower cognitive functioning, providers should consider proactively counseling families of children with these problems about management of aggressive behaviors. Conversely, in aggressive children with ASD, providers should look seriously at modifiable factors such as sleep, internalizing, and attention problems as a possible way of improving ABP. Longitudinal studies are needed to clarify the direction of the associations between comorbid problems and ABP. There is encouraging evidence that interventions designed to treat sleep apnea in ASD, for instance, can have positive effects on problem behaviors (Malow et al., 2006). Thus, treatment of comorbid problems has the potential to ameliorate the severity of aggressive behaviors for children with ASD.
HIGHLIGHTS.
Aggressive behavior problems (ABP) were present in 25% of children with ASD
ABP were predicted by comorbid sleep, internalizing, and attention problems
Attention to comorbid problems may aid in clinical treatment and counseling of ABP
Acknowledgements
This research was conducted using data collected as part of the Autism Treatment Network (ATN). The ATN is funded by Autism Speaks and a cooperative agreement (UA3 MC 11054) from the Health Resources and Services Administration to Massachusetts General Hospital. Dr. Zuckerman’s effort was funded by K23MH095828 from the U.S. National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare that they have no competing interests.
References
- Achenbach TM, Rescorla L. Manual for the ASEBA preschool forms & profiles: An integrated system of multi-informant assessment. Burlington, VT: ASEBA; 2000. [Google Scholar]
- Achenbach TM, Rescorla L. Manual for the ASEBA school-age forms & profiles: An integrated system of multi-informant assessment. Burlington, VT: ASEBA; 2001. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed.; text rev. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Bishop SL, Guthrie W, Coffing M, Lord C. Convergent validity of the Mullen Scales of Early Learning and the Differential Ability Scales in children with autism spectrum disorders. American Journal on Intellectual and Developmental Disabilities. 2011;116(5):331–343. doi: 10.1352/1944-7558-116.5.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broidy LM, Nagin DS, Tremblay RE, Bates JE, Brame B, Dodge KA, Vitaro F. Developmental trajectories of childhood disruptive behaviors and adolescent delinquency: A six-site, cross-national study. Developmental Psychology. 2003;39(2):222–245. doi: 10.1037//0012-1649.39.2.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronsard G, Botbol M, Tordjman S. Aggression in low functioning children and adolescents with autistic disorder. PLoS ONE. 2010;5(12):e14358. doi: 10.1371/journal.pone.0014358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryman A, Cramer D. Quantitative data analysis with SPSS release 10 for Windows. New York: Routledge; 2001. [Google Scholar]
- Card NA, Stucky BD, Sawalani GM, Little TD. Direct and indirect aggression during childhood and adolescence: A meta-analytic review of gender differences, intercorrelations, and relations to maladjustment. Child Development. 2008;79(5):1185–1229. doi: 10.1111/j.1467-8624.2008.01184.x. [DOI] [PubMed] [Google Scholar]
- Cervantes P, Matson JL, Tureck K, Adams HL. The relationship of comorbid anxiety symptom severity and challenging behaviors in infants and toddlers with autism spectrum disorder. Research in Autism Spectrum Disorders. 2013;7(12):1528–1534. [Google Scholar]
- Charman T, Baird G, Simonoff E, Loucas T, Chandler S, Meldrum D, Pickles A. Efficacy of three screening instruments in the identification of autistic-spectrum disorders. British Journal of Psychiatry. 2007;191:554–559. doi: 10.1192/bjp.bp.107.040196. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- Constantino JN, Gruber C. The Social Responsiveness Scale. Los Angeles, CA: Western Psychological Services; 2005. [Google Scholar]
- Deprey L, Ozonoff S. Assessment of comorbid psychiatric conditions in autism spectrum disorders. In: Goldstein S, Naglieri J, Ozonoff S, editors. Assessment of autism spectrum disorders. New York, NY: The Guilford Press; 2009. pp. 290–317. [Google Scholar]
- Dionne G, Tremblay R, Boivin M, Laplante D, Pérusse D. Physical aggression and expressive vocabulary in 19-month-old twins. Developmental Psychology. 2003;39(2):261–273. doi: 10.1037//0012-1649.39.2.261. [DOI] [PubMed] [Google Scholar]
- Dominick KC, Davis NO, Lainhart J, Tager-Flusberg H, Folstein S. Atypical behaviors in children with autism and children with a history of language impairment. Research in Developmental Disabilities. 2007;28(2):145–162. doi: 10.1016/j.ridd.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Estes AM, Dawson G, Sterling L, Munson J. Level of intellectual functioning predicts patterns of associated symptoms in school-age children with autism spectrum disorder. American Journal on Intellectual and Developmental Disabilities. 2007;112(6):439–449. doi: 10.1352/0895-8017(2007)112[439:LOIFPP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Farmer CA, Aman MG. Aggressive behavior in a sample of children with autism spectrum disorders. Research in Autism Spectrum Disorders. 2011;5(1):317–323. [Google Scholar]
- Farmer C, Butter E, Mazurek MO, Cowan C, Lainhart J, Cook EH, Aman M. Aggression in children with autism spectrum disorders and a clinic-referred comparison group. Autism. 2014 doi: 10.1177/1362361313518995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A, Miles J, Field Z. Discovering statistics using R. Thousand Oaks, CA: SAGE; 2012. [Google Scholar]
- Gelman A, Hill J. Data analysis using regression and multilevel hierarchical models. UK: Cambridge University Press; 2007. Missing-data imputation; pp. 529–543. [Google Scholar]
- Georgiades S, Szatmari P, Duku E, Zwaigenbaum L, Bryson S, Roberts W Pathways in ASD Study Team. Phenotypic overlap between core diagnostic features and emotional/behavioral problems in preschool children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2011;41(10):1321–1329. doi: 10.1007/s10803-010-1158-9. [DOI] [PubMed] [Google Scholar]
- Goldman SE, Richdale AL, Clemons T, Malow BA. Parental sleep concerns in autism spectrum disorders: Variations from childhood to adolescence. Journal of Autism and Developmental Disorders. 2012;42(4):531–538. doi: 10.1007/s10803-011-1270-5. [DOI] [PubMed] [Google Scholar]
- Gotham K, Pickles A, Lord C. Standardizing ADOS scores for a measure of severity in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2009;39(5):693–705. doi: 10.1007/s10803-008-0674-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotham K, Risi S, Pickles A, Lord C. The Autism Diagnostic Observation Schedule: Revised algorithms for improved diagnostic validity. Journal of Autism and Developmental Disorders. 2007;37(4):613–627. doi: 10.1007/s10803-006-0280-1. [DOI] [PubMed] [Google Scholar]
- Gross HE, Shaw DS, Moilanen KL. Reciprocal associations between boys' externalizing problems and mothers' depressive symptoms. Journal of Abnormal Child Psychology. 2008;36(5):693–709. doi: 10.1007/s10802-008-9224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, Sikora DM, McCoy R. Prevalence and risk factors of maladaptive behaviour in young children with autistic disorder. Journal of Intellectual Disability Research. 2008;52(10):819–829. doi: 10.1111/j.1365-2788.2008.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts S, Nicholas D, Zwaigenbaum L. Home sweet home? Families' experiences with aggression in children with autism spectrum disorders. Focus on Autism and Other Developmental Disabilities. 2013:1–9. [Google Scholar]
- Hus V, Bishop S, Gotham K, Huerta M, Lord C. Factors influencing scores on the Social Responsiveness Scale. Journal of Child Psychology and Psychiatry. 2013;54(2):216–224. doi: 10.1111/j.1469-7610.2012.02589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hus V, Gotham K, Lord C. Standardizing ADOS domain scores: Separating severity of social affect and restricted and repetitive behaviors. Journal of Autism and Developmental Disorders. 2012 doi: 10.1007/s10803-012-1719-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J, Dixon DR, Tarbox J, Granpeesheh D. Symptom severity and challenging behavior in children with ASD. Research in Autism Spectrum Disorders. 2011;5(3):1028–1032. [Google Scholar]
- Jester JM, Nigg JT, Adams K, Fitzgerald HE, Puttler LI, Wong MM, Zucker RA. Inattention/hyperactivity and aggression from early childhood to adolescence: Heterogeneity of trajectories and differential influence of family environment characteristics. Development and Psychopathology. 2005;17(1):99–125.. doi: 10.1017/50954579405050066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne SM, Mazurek MO. Aggression in children and adolescents with ASD: Prevalence and risk factors. Journal of Autism and Developmental Disorders. 2011;41(7):926–937. doi: 10.1007/s10803-010-1118-4. [DOI] [PubMed] [Google Scholar]
- Kanne SM, Gerber AJ, Quirmbach LM, Sparrow SS, Cicchetti DV, Saulnier CA. The role of adaptive behavior in autism spectrum disorders: Implications for functional outcome. Journal of Autism and Developmental Disorders. 2010;41(8):1007–1018. doi: 10.1007/s10803-010-1126-4. [DOI] [PubMed] [Google Scholar]
- Kim JA, Szatmari P, Bryson SE, Streiner DL, Wilson FJ. The prevalence of anxiety and mood problems among children with autism and Asperger syndrome. Autism. 2000;4(2):117–132. [Google Scholar]
- Klin A, Saulnier CA, Sparrow SS, Cicchetti DV, Volkmar FR, Lord C. Social and communication abilities and disabilities in higher functioning individuals with autism spectrum disorders: The Vineland and the ADOS. Journal of Autism and Developmental Disorders. 2007;37(4):748–759. doi: 10.1007/s10803-006-0229-4. [DOI] [PubMed] [Google Scholar]
- Kozlowski AM, Matson JL, Rieske RD. Gender effects on challenging behaviors in children with autism spectrum disorders. Research in Autism Spectrum Disorders. 2012;6(2):958–964. [Google Scholar]
- Lansford JE, Malone PS, Stevens KI, Dodge KA, Bates JE, Pettit GS. Developmental trajectories of externalizing and internalizing behaviors: Factors underlying resilience in physically abused children. Development and Psychopathology. 2006;18(1):35–55. doi: 10.1017/S0954579406060032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecavalier L. Behavioral and emotional problems in young people with pervasive developmental disorders: Relative prevalence, effects of subject characteristics, and empirical classification. Journal of Autism and Developmental Disorders. 2006;36(8):1101–1114. doi: 10.1007/s10803-006-0147-5. [DOI] [PubMed] [Google Scholar]
- Lecavalier L, Leone S, Wiltz J. The impact of behaviour problems on caregiver stress in young people with autism spectrum disorders. Journal of Intellectual Disability Research. 2006;50(Pt 3):172–183. doi: 10.1111/j.1365-2788.2005.00732.x. [DOI] [PubMed] [Google Scholar]
- Ligthart L, Bartels M, Hoekstra RA, Hudziak JJ, Boomsma DI. Genetic contributions to subtypes of aggression. Twin Research and Human Genetics. 2005;8(5):483–491. doi: 10.1375/183242705774310169. [DOI] [PubMed] [Google Scholar]
- Little R. A test of missing completely at random for multivariate data with missing values. Journal of the American Statistical Association. 1988;83:1198–1202. [Google Scholar]
- Little R, Rubin DB. Statistical analysis with missing data. New York: John Wiley & Sons; 1987. [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, Rutter M. The Autism Diagnostic Observation Schedule—Generic: A standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000 [PubMed] [Google Scholar]
- Malow BA, Marzec ML, McGrew SG, Wang L, Henderson LM, Stone WL. Characterizing sleep in children with autism spectrum disorders: A multidimensional approach. Sleep. 2006;29(12):1563–1571.. doi: 10.1093/sleep/29.12.1563. [DOI] [PubMed] [Google Scholar]
- Mandell DS. Psychiatric hospitalization among children with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2008;38(6):1059–1065. doi: 10.1007/s10803-007-0481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskey M, Warnell F, Parr JR, Le Couteur A, McConachie H. Emotional and behavioural problems in children with autism spectrum disorder. Journal of Autism and Developmental Disorders. 2013;43(4):851–859. doi: 10.1007/s10803-012-1622-9. [DOI] [PubMed] [Google Scholar]
- Matson JL, Kuhn DE. Identifying feeding problems in mentally retarded persons: Development and reliability of the screening tool of feeding problems (STEP) Research in Developmental Disabilities. 2001 doi: 10.1016/s0891-4222(01)00065-8. [DOI] [PubMed] [Google Scholar]
- Matson JL, Mahan SS, Fodstad JC, Worley JA, Neal DD, Sipes MM. Effects of symptoms of co-morbid psychopathology on challenging behaviours among infants and toddlers with Autistic Disorder and PDD-NOS as assessed with the Baby and Infant Screen for Children with aUtIsm Traits (BISCUIT) Developmental Neurorehabilitation. 2011;14(3):129–139. doi: 10.3109/17518423.2011.557029. [DOI] [PubMed] [Google Scholar]
- Matson JL, Neal D, Fodstad JC. The relation of social behaviours and challenging behaviours in infants and toddlers with autism spectrum disorders. Developmental Neurorehabilitation. 2010;13(3):164–169. doi: 10.3109/17518420903270683. [DOI] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL. Variables related to sleep problems in children with autism. Research in Autism Spectrum Disorders. 2009;3(4):931–941. [Google Scholar]
- Mayes SD, Calhoun SL, Aggarwal R, Baker C, Mathapati S, Anderson R, Petersen C. Explosive, oppositional, and aggressive behavior in children with autism compared to other clinical disorders and typical children. Research in Autism Spectrum Disorders. 2012;6:1–10. [Google Scholar]
- Mazurek MO, Kanne SM, Wodka EL. Physical aggression in children and adolescents with autism spectrum disorders. Research in Autism Spectrum Disorders. 2013:455–465. [Google Scholar]
- McClintock K, Hall S, Oliver C. Risk markers associated with challenging behaviours in people with intellectual disabilities: A meta-analytic study. Journal of Intellectual Disability Research. 2003;47(Pt 6):405–416. doi: 10.1046/j.1365-2788.2003.00517.x. [DOI] [PubMed] [Google Scholar]
- McTiernan A, Leader G, Healy O, Mannion A. Analysis of risk factors and early predictors of challenging behavior for children with autism spectrum disorder. Research in Autism Spectrum Disorders. 2011;5(3):1215–1222. [Google Scholar]
- Medeiros K, Kozlowski AM, Beighley JS, Rojahn J, Matson JL. The effects of developmental quotient and diagnostic criteria on challenging behaviors in toddlers with developmental disabilities. Research in Developmental Disabilities. 2012;33(4):1110–1116. doi: 10.1016/j.ridd.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service; 1995. [Google Scholar]
- Murphy GH, Beadle-Brown J, Wing L, Gould J, Shah A, Holmes N. Chronicity of challenging behaviours in people with severe intellectual disabilities and/or autism: A total population sample. Journal of Autism and Developmental Disorders. 2005;35(4):405–418. doi: 10.1007/s10803-005-5030-2. [DOI] [PubMed] [Google Scholar]
- Murphy O, Healy O, Leader G. Risk factors for challenging behaviors among 157 children with autism spectrum disorder in Ireland. Research in Autism Spectrum Disorders. 2009;3(2):474–482. [Google Scholar]
- Nagin DS, Tremblay RE. Parental and early childhood predictors of persistent physical aggression in boys from kindergarten to high school. Archives of General Psychiatry. 2001;58(4):389–394. doi: 10.1001/archpsyc.58.4.389. [DOI] [PubMed] [Google Scholar]
- Nagin D, Tremblay RE. Trajectories of boys' physical aggression, opposition, and hyperactivity on the path to physically violent and nonviolent juvenile delinquency. Child Development. 1999;70(5):1181–1196. doi: 10.1111/1467-8624.00086. [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. Trajectories of physical aggression from toddlerhood to middle childhood: Predictors, correlates, and outcomes. Monographs of the Society for Research in Child Development. 2004;69(4):vii–129. doi: 10.1111/j.0037-976x.2004.00312.x. [DOI] [PubMed] [Google Scholar]
- Owens JA, Spirito A, McGuinn M. The Children's Sleep Habits Questionnaire: Psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23(8):1043–1051. [PubMed] [Google Scholar]
- Roid G. Stanford-Binet Intelligence Scales. 5th ed. Rolling Meadows, IL: Riverside Publishing; 2003. [Google Scholar]
- Séguin JR, Parent S, Tremblay RE, Zelazo PD. Different neurocognitive functions regulating physical aggression and hyperactivity in early childhood. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2009;50(6):679–687. doi: 10.1111/j.1469-7610.2008.02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora DM, Hall TA, Hartley SL, Gerrard-Morris AE, Cagle SS. Does parent report of behavior differ across ADOS-G classifications: Analysis of scores from the CBCL and GARS. Journal of Autism and Developmental Disorders. 2008;38(3):440–448. doi: 10.1007/s10803-007-0407-z. [DOI] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales. 2nd ed. Circle Pines, MN: American Guidance Service; 2005. [Google Scholar]
- Tremblay RE. The development of aggressive behaviour during childhood: What have we learned in the past century? International Journal of Behavioral Development. 2000;24(2):129–141. [Google Scholar]
- Tremblay RE, Nagin DS, Séguin JR, Zoccolillo M, Zelazo PD, Boivin M, et al. Physical aggression during early childhood: Trajectories and predictors. Pediatrics. 2004;114(1):e43–e50. doi: 10.1542/peds.114.1.e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tureck K, Matson JL, Turygin N, Macmillan K. Rates of psychotropic medication use in children with ASD compared to presence and severity of problem behaviors. Research in Autism Spectrum Disorders. 2013;7(11):1377–1382. [Google Scholar]
- van Belle G, Fisher LD, Heagerty PJ, Lumley T. Biostatistics. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- Van Daal J, Verhoeven L, Van Balkom H. Behaviour problems in children with language impairment. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48(11):1139–1147. doi: 10.1111/j.1469-7610.2007.01790.x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4th ed. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Witwer A, Lecavalier L. Treatment incidence and patterns in children and adolescents with autism spectrum disorders. Journal of Child & Adolescent Psychopharmacology. 2005;15(4):671–681. doi: 10.1089/cap.2005.15.671. [DOI] [PubMed] [Google Scholar]
- Yerys BE, Wallace GL, Sokoloff JL, Shook DA, James JD, Kenworthy L. Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Research. 2009;2(6):322–333. doi: 10.1002/aur.103. [DOI] [PMC free article] [PubMed] [Google Scholar]