Abstract
The NR2B subunit of N-methyl D-aspartate glutamate receptors influences pharmacological properties and confers greater sensitivity to the modulatory effects of ethanol. This study examined behavioral responses to acute ethanol in a conditional knockout mouse model that allowed for a delayed genetic deletion of the NR2B subunit to avoid mouse lethality. Mice lacking the NR2B gene (knockout) were produced by mating NR2B[f/f] mice with CAMKIIa-drive tTA transgenic mice and the tetO-CRE transgenic mice. Adult male and female offspring representing each of the resultant genotypes (knockout, CAM, CRE, and wild-type mice) were tested for open field locomotor activity following acute low and high dose ethanol challenge as well as loss of righting reflex. Findings indicate that male and female mice lacking the NR2B subunit exhibited greater overall activity in comparison to other genotypes during the baseline locomotor activity test. NR2B knockout mice exhibited an exaggerated stimulant response to 1.5 g/kg (ip) and an exaggerated depressant response to 3.0 g/kg (ip) ethanol challenge. Additionally, NR2B knockout mice slept longer following a high dose of ethanol (4.0 g/kg, ip). To evaluate pharmacokinetics, clearance rates of ethanol (1.5, 4.0 g/kg, ip) were measured and showed female NR2B knockouts had a faster rate of metabolism only at the higher ethanol dose. Western blot analyses confirmed significant reduction in NR2B expression in the forebrain of knockout mice. Collectively, these data indicate the NR2B subunit of the N-methyl D-aspartate glutamate receptor is involved in regulating low-dose stimulant effects of ethanol and the depressant/hypnotic effects of ethanol.
INTRODUCTION
The N-methyl D-aspartate (NMDA) glutamate receptor is a ligand-gated ion channel, which is thought to be arranged as a tetrameric complex containing two obligatory NR1 subunits that are co-assembled with subunits of the NR2 and/or NR3 variety, with subunit composition conferring unique receptor channel (ion-gating) properties (Stephenson et al., 2008; Traynelis et al., 2010; Yashiro and Philpot, 2008). Subunit composition of NMDA receptors has also been shown to determine sensitivity to ethanol, with receptors composed of either NR2A or NR2B subunits exhibiting enhanced ethanol sensitivity compared to receptors having the 2C or 2D subtypes (Blevins et al., 1997; Masood et al., 1994; Mirshahi and Woodward, 1995).
Several studies have shown that various NMDA receptor antagonists (e.g., dizocilpine, ketamine) mimic the discriminative stimulus effects of ethanol (Grant, 1999; Hodge et al., 2006; Krystal et al., 2003) and reduce ethanol self-administration as well as conditioned rewarding effects of ethanol (Boyce-Rustay and Cunningham, 2004; McMillen et al., 2004). Additionally, NMDA receptor antagonists have been reported to potentiate the low-dose stimulant effects of ethanol (Meyer and Phillips, 2003; Shen and Phillips, 1998), as well as ataxic/depressant and hypnotic actions of ethanol (Beleslin et al., 1997; Boyce-Rustay and Holmes, 2005; Palachick et al., 2008; Silveri and Spear, 2002). However, the relative contribution of specific NMDA receptor subunits in mediating these effects has not been fully elucidated.
Despite the relatively limited availability of subunit-specific experimental tools, studies have attempted to isolate the role of NR2A vs. NR2B subunits in behavioral actions of ethanol through pharmacological or genetic antagonism. For example, genetic deletion of the NR2A subunit did not alter ethanol consumption, low-dose locomotor stimulant, or high-dose depressant/hypnotic effects of ethanol, but NR2A knockout mice did exhibit enhanced ethanol-induced motor incoordination (Boyce-Rustay and Holmes, 2005; 2006). Unfortunately, it has been difficult to confirm these results because there are no pharmacological agents that exhibit NR2A subunit specificity. On the other hand, there are pharmacological agents that display relative selectivity for NR2B subunit containing NMDA receptors (e.g., ifenprodil, Ro 25-6981) and these NR2B “selective” antagonists have been reported to potentiate depressant/hypnotic effects of ethanol (Boyce-Rustay and Holmes, 2005; Malinowska et al., 1999; Palachick et al., 2008; Yaka et al., 2003). Since genetic deletion of the NR2B subunit is lethal (Kutsuwada et al., 1996), it has not been possible to test behavioral actions of ethanol in conventional NR2B knockout mice. However, a conditional NR2B knockout mouse was recently constructed in which deletion of the NR2B gene is developmentally delayed by expressing the CRE recombinase under the CAMKIIα promoter, thereby bypassing the postnatal lethality and enabling viable offspring (Brigman et al., 2010). The present study utilized a variation of these conditional NR2B knockout mice to examine sensitivity to the low-dose stimulant and high-dose depressant effects of ethanol on locomotor activity, as well as the depressant/hypnotic effects of ethanol.
MATERIALS AND METHODS
Generation of NR2B Deficient Mice
The targeting of the NR2B (GLuN2B) gene was described in detail in Brigman et al. (2010). Briefly, 3 loxP sites and a neomycin resistance gene cassette were inserted around exon 3 of the NR2B gene by homologous recombination in 129/SvEvTac embryonic stem cells. After production of a mouse line carrying the modified NR2B allele, these mice were crossed with E2a-CRE transgenic mice (Lakso, 1996) to eliminate the neomycin resistance gene cassette. As a result, the newly modified allele only contains 2 loxP sites flanking exon 3. This new mouse line was then crossed to CAMKIIα-tTA transgenic (Mayford et al., 1996) and to phCMV-tetO-CRE transgenic mice (Saam and Gordon, 1999) to create animals carrying one transgene and one copy of the floxed NR2B allele. Note that each transgenic line was back-crossed for more than 10 generations onto the C57BL/6J strain. Also note that from the chimeric mouse generated after embryonic stem cell injection to this stage, the modified NR2B allele was moved 5 generations (98.4%) onto the C57BL/6J strain. Finally, the mice were bred to homozygosity for the floxed NR2B allele, creating CAMKIIα-tTA-NR2B[f/f] and phCMV-tetO-CRE[f/f] breeders.
Twenty-four double-floxed (NR2B[f/f]) females and 12 double-floxed males were shipped from Dr. E. Delpire (Vanderbilt University) to MUSC. Six of the males also carried a CAMKIIα promoter driven tetracycline transactivator (CAMKIIα-tTA-NR2B[f/f]) while the remaining six males carried a human cytomegalovirus-tet-operator (tetO)-driven CRE recombinase transgene (phCMV-tetO-CRE-NR2B[f/f]). After quarantine and a period of acclimation to the vivarium at MUSC, interbreeding commenced between the NR2B[f/f] females and the two transgenic lines of males. Once sufficient numbers of male and female NR2B[f/f] mice carrying the CAMKIIα-tTA or phCMV-tetO-CRE transgenes were generated, interbreeding these mice (CAMKIIα-tTA-NR2B[f/f] × phCMV-tetO-CRE-NR2B[f/f]) produced four unique genotypes: NR2B[f/f]; CAMKIIα-tTA-NR2B[f/f]; phCMV-tetO-CRE-NR2B[f/f]; and CAMKIIα-tTA-phCMV-tetO-CRE-NR2B[f/f]. These genotypes are denoted in this report as wildtype (WT), CAM, CRE, and knockout (KO), respectively. Deletion of the NR2B gene is developmentally delayed (enabling viable offspring) because the tTA gene product is expressed in forebrain postnatally (~p14) based on the CAMKIIα promoter (Viberg et al, 2008), where it binds to the tetO to drive CRE recombinase. Thus, NR2B[f/f] mice harboring both the tTA-CAMKIIα and tetO-CRE transgenes will be rendered NR2B deficient in forebrain, with CAM, CRE, and WT littermates serving as controls.
Experimental Subjects
A total of 90 mice were used for behavioral testing, representing male and female mice of each of the genotypes (WT: 16 males, 10 females; CRE: 8 males, 3 females; CAM: 10 males, 11 females; KO: 13 males, 19 females). Tailsnips were processed to determine genotype by Western blot analyses (see below). Mice were acclimated for a minimum of 2 weeks prior to the start of the experiment because mice were transferred from the breeding colony to the experimental housing room and also because mice moved from multiple to individual housing conditions. All mice were individually housed so that ethanol and control mice did not interact. Mice were housed under a 12-hr light/dark cycle in a temperature- and humidity-controlled AAALAC-accredited animal facility. The animals had free access to food (Teklad rodent diet) and water throughout the experiments. Adequate measures were taken to minimize pain or discomfort. Experiments were carried out in accordance with the Guidelines of the National Institute of Health (NIH) regarding the care and use of animals (NIH Publication No. 80-23, revised, 1996) and were approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina.
Western Blot Analysis
To confirm the loss of NR2B-containing subunits of NMDA receptors in forebrain, we performed Western blot analysis on tissue punches from a number of forebrain regions, including dorsomedial prefrontal cortex (dmPFC), dorsal hippocampus (HPC), dorsolateral striatum (DLS), nucleus accumbens (NAc), amygdala (central and lateral aspects; AMY), and bed nucleus of the stria terminalis (BNST) from an independent group of male mice representing each of the genotypes (N= 4-7/genotype). Additionally, Western blot analyses were performed on tissue samples collected from midbrain (mesencephalic reticular formation; RF) and hindbrain (medulla/pons) structures to verify that expression of NR2B-containing subunits of NMDA receptors were not altered in brain regions lacking CAMKIIα expression. Brains were rapidly removed and placed in ice-cold saline before blocking 2 mm sections using a mouse brain block (ASI Instruments, Inc., Warren, MI). Tissue punches (2 mm; Ted Pella, Inc., Redding, CA) from dmPFC, HPC, DLS, NAc, AMY, BNST, RF, and medulla were obtained and immediately transferred into 2% lithium dodecyl sulfate (LDS), and lysates were generated by brief probe sonication. Aliquots of each sample were taken to determine protein concentration using the bicinchoninic acid assay (Pierce Biotechnology, Inc., Rockford, IL), and the remaining lysates were stored at −80° C until Western blot analysis.
An aliquot of each sample was diluted with NuPAGE 4X LDS sample loading buffer (Invitrogen Corp., Carlsbad, CA; pH 8.5) containing 500 mM dithiothreitol, and samples were denatured for 10 min at 70° C. Five μg of each sample was separated using the Bis-Tris (375 mM resolving buffer and 125 mM stacking buffer, pH 6.4; 7.5% acrylamide) discontinuous buffer system with MOPS electrophoresis buffer (50 mM MOPS, 50 mM Tris, 0.1% SDS, 1 mM EDTA, pH 7.7). Protein was then transferred to Immobilon-P PVDF membranes (Millipore, Bedford, MA) using a semi-dry transfer apparatus (Bio-Rad Laboratories, Hercules, CA). After transfer, blots were washed with phosphate-buffered saline containing 0.1% Tween 20 (PBST) and then blocked with PBST containing 5% nonfat dried milk (NFDM) for 1 h at room temperature with agitation. The membranes were then incubated overnight at 4°C with primary antibodies diluted in PBST containing 0.5% NFDM and washed in PBST prior to 1 h incubation at room temperature with horseradish peroxidase conjugated secondary antibodies diluted 1:1000 in PBST. Membranes received a final wash in PBST and the antigen-antibody complex was detected by enhanced chemiluminescence. Film autoradiograms were quantified by computer-assisted densitometry using Image J 1.42q (National Institutes of Health, MD). Antibodies used in these studies were NR1 (1:4000; BD Pharmingen, Franklin Lakes, NJ), NR2B (1:1000; NeuroMab, Antibodies, Inc. & UC Davis, Davis, CA), and NR2A (1:1000; Millipore).
Locomotor Activity Testing
At 18-24 weeks of age, mice were tested for locomotor activity in a novel open-field arena for 15 min immediately following a saline injection. The following week, investigation of ethanol-induced alterations in locomotor activity was conducted using a latin square design, with mice given saline, 1.5 g/kg ethanol and 3.0 g/kg ethanol. Mice were tested once per week at each dose, for a total of three weeks of testing. Animals were weighed and injected with the appropriate dose of ethanol or saline immediately prior to being placed in the testing chamber for a 15 min session. All injections were administered intraperitoneally (ip.) in a volume of 10 ml/kg.
Locomotor activity was measured in four acrylic open field arenas (40.5 × 40.5 × 30.5 cm) using Digiscan Activity Monitors (Omnitech Electronics, Inc., Columbus, OH) housed in sound-attenuating chambers. The monitors, equipped with 8 × 8 matrices of infrared sensors, were interfaced with Versamax software (Accuscan Instruments, Inc., Columbus, OH) and a PC computer. Animals of one sex were tested sequentially, and the chambers wiped with water and dried in between each animal. In between testing of the males and females, chambers were wiped down with an ethanol solution and allowed to completely dry. The primary measure of ambulatory behavior was distance moved (cm) collected in 1-min time bins over the 15 min test sessions.
Loss of Righting Reflex
Two weeks later, the hypnotic effects of a high dose of ethanol (4.0 g/kg, ip.) were investigated. Following ethanol administration, mice were placed in a supine position on a flat surface and monitored for loss of righting reflex (LORR). Inability of the mouse to right itself twice within a 30 sec period defined LORR. The latency (sec) until LORR was recorded for each animal. Recovery from LORR was recorded when a mouse demonstrated capability of placing all four limbs on the table surface twice within a 30 sec period. The duration (min) of the LORR (time between onset of LORR and regaining the righting reflex) was also recorded for each subject. For determination of blood ethanol concentration (BEC) at “waking”, blood samples were collected at the time animals regained their righting reflex. Blood samples (40 μl) were collected from the retro-orbital sinus using a heparinized capillary tube. Plasma was separated by centrifugation and the ethanol concentration measured using an Analox Instrument analyzer (Lunenburg, MA), as previously described (Griffin et al., 2009).
Pharmacokinetics
Potential differences in the pharmacokinetics of ethanol were assessed in a separate cohort of mice to determine if NR2B KO mice have similar ethanol clearance rates as control littermates. A total of 87 mice (10 months old) were used representing male and female mice of each of the genotypes (WT: 12 males, 12 females; CAM: 12 males, 3 females; CRE: 12 males, 12 females; KO: 12 males, 12 females). Half of the mice in each group were injected (ip.) with 4.0 g/kg, ethanol and blood samples were collected at 5, 60 and 240 min post ethanol injection while the remaining mice in each group were injected with 4.0 g/kg ethanol and blood samples were collected at 30, 120, and 480 min post ethanol injection. Blood samples were collected and analyzed in the same manner as described above. Two weeks later the same mice were injected with 1.5 g/kg ethanol to determine pharmacokinetics of ethanol at a lower dose of ethanol. Half of the mice in each group were injected (ip.) with 1.5 g/kg, ethanol and blood samples were collected at 5, 60 and 120 min post ethanol injection while the remaining mice in each group were injected with 1.5 g/kg ethanol and blood samples were collected at 30, 90, and 240 min post ethanol injection.
Data Analysis
For western blot analyses, differences in optical density for NR2B, NR1 and NR2A were separately analyzed by ANOVA, with Genotype (WT, CRE, CAM, KO) as the main factor. Baseline locomotor activity in a novel open field was analyzed by a 3-way ANOVA with Genotype (WT, CRE, CAM, KO) and Sex (male, female) as between-subject factors and Time (15 1-min bins) as a repeated measure. To determine the effects of ethanol on locomotor activity, distance traveled (cm) was analyzed by a 4-way ANOVA (Genotype × Sex × Dose × Time), with Dose (0, 1.5, 3.0 g/kg ethanol) included as an additional repeated measure. Difference scores were used to control for differences in baseline (saline) locomotor activity across the genotypes (i.e., distance moved following 1.5 or 3.0 g/kg ethanol – group mean for distance moved following saline), and analyzed similarly. Latency (sec) and duration (min) of LORR and BEC data were analyzed by 2-way ANOVA (Genotype × Sex). Since none of the locomotor activity or LORR measures significantly differed as a function of Sex, data were collapsed across this variable and analyzed accordingly. Post-hoc analyses were performed using Fisher’s Least Significant Difference test and group differences determined significant at the 0.05 alpha level.
To determine if differences in ethanol clearance rate existed between the different genotypes, BECs were analyzed as a three-level hierarchical linear model. This analysis allowed Time to be nested with Session and Dose to be nested with Subject, even though different mice were examined at different times following injection. Genotype, Sex and their interaction were tested for both intercept and slope values (the effect of either Genotype or Sex on Dose or slope appears as a “cross-level” interaction in this model). Time, as well as intercept was treated as a random variable in that slopes and intercepts were fit for individual subjects. Each parameter was assumed to be drawn from a normal population. Examination of BEC distributions verified that data were unreliable below 60 mg/dl and these values were not used in the final analysis (n = 24 from 120 min, 1.5 g/kg; n = 24 from 480 min, 4.0 g/kg).
RESULTS
Knockdown of NR2B containing NMDA receptors in forebrain structures
We determined the extent of knock-down of NR2B-containing NMDA receptors in multiple brain regions in adult WT, CRE, CAM, and KO mice. As expected, we observed a significant reduction in NR2B expression levels in all of the forebrain structures examined (dmPFC, HPC, DLS, NAc, AMY, and BNST) in the KO mice compared to WT, CRE and CAM littermate controls (Figures 1 and 2; Supplemental Table 1). While knock-down of NR2B was not complete, residual expression of NR2B in these regions is likely explained by NR2B expression in oligodendrocytes and interneurons (Chen and Reiner, 1996; Kuppenbender, 2000; Salter and Fern, 2005; Xi, 2009). Consistent with data from a recent report (Brigman, 2010), KO (NR2B-deficient) mice also showed a significant reduction in expression levels of NR1 in many of these same forebrain regions (Figures 1 and 2; Supplemental Table 1). In contrast, no significant changes in NR2A subunit expression were observed in any brain region for all of the genotypes (Figures 1 and 2; Supplemental Table 1). As expected, expression levels of NR2B in RF and medulla in KO mice were not altered in comparison to WT, CAM, and CRE mice (Figure 2; Supplemental Table 1).
Figure 1. Conditional knockdown of the NR2B subunit of the NMDA receptor in NR2B deficient (KO) mice.
A significant reduction in protein levels of NR2B was observed in (a) dorsomedial prefrontal cortex (dmPFC), (b) dorsal hippocampus (HPC), (c) dorsolateral striatum (DLS), and (d) nucleus accumbens (NAc) in NR2B deficient (KO) mice in comparison with wildtype (WT), CAM, and CRE mice (n = 4-7 males/genotype). NR1 expression levels in NR2B deficient mice were also significantly reduced in each of these regions. No significant changes in NR2A subunit expression levels were observed in any of the genotypes. * = differs from WT, CRE, CAM
Figure 2. Conditional knockdown of the NR2B subunit of the NMDA receptor in NR2B deficient (KO) mice.
A significant reduction in protein levels of NR2B was observed in (a) amygdala (AMY) and (b) bed nucleus of stria terminalis (BNST), but not in (c) mesencaphalic reticular formation (RF) or (d) medulla in NR2B deficient (KO) mice in comparison with wildtype (WT), CAM, and CRE mice (n = 4-7 males/genotype). NR1 expression levels in NR2B deficient mice were also significantly reduced in amygdala and BNST. No significant changes in NR2A subunit expression levels were observed in any of the genotypes. * = differs from WT, CRE, CAM
Locomotor activity in a novel open field
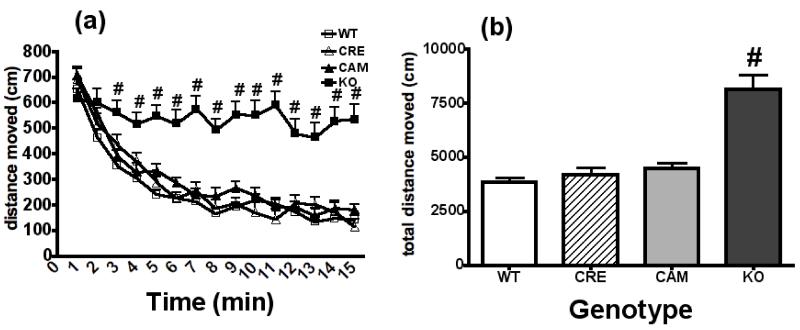
Locomotor activity in a novel open field following saline injection was measured in each of the four genotypes (WT, CRE, CAM, KO). Since analysis indicated no significant interaction of Sex with Genotype and Time [F(42,1148)= 0.84, p> 0.05], activity data are presented collapsed across Sex (Figure 3). Spontaneous locomotor activity gradually decreased over the course of the 15-min test period for WT, CRE, and CAM mice. In contrast, NR2B deficient (KO) mice maintained elevated activity levels throughout the entire testing session (Figure 3a). This impression is supported by a significant Genotype × Time interaction [F(42,1204)= 7.68, p< 0.01], and post-hoc analyses indicated that locomotor activity was greater in KO mice compared to control littermates starting at the third minute and for the duration of the 15 min test period (p< 0.05). Further, analysis of total distance moved (cm) for the entire 15 min session indicated that NR2B deficient (KO) mice were significantly more active in the novel open field in comparison to the control genotypes [F(3,86)= 19.98, p< 0.001] (Figure 3b).
Figure 3. Lack of locomotor habituation in NR2B deficient mice.
NR2B deficient (KO) mice had greater levels of locomotor activity in a novel open field. Distance moved (cm) is shown for WT, CRE, CAM and KO mice in (a) 1-min bins to depict time course of activity levels and (b) total distance moved for the entire 15-min session. # = significantly different from all other genotypes. Sample sizes were as follows WT = 26, CRE = 11, CAM = 21, KO = 32.
Ethanol-induced alterations in locomotor activity
Locomotor activity in an open field following an acute injection of ethanol (0, 1.5, or 3.0 g/kg) was measured in each of the four genotypes (WT, CRE, CAM, KO). Since analysis of these data did not reveal a significant interaction of Sex with Genotype, Dose, and Time [F(84,1932)= 0.63, p> 0.05], data are presented collapsed across Sex for each Genotype. Analysis of distance moved (cm) each minute of the 15 min test session revealed a significant Genotype × Dose × Time interaction [F(84,1932)= 1.49, p< 0.05] (Figure 4). Post-hoc analyses indicated that locomotor activity following saline administration was significantly greater in KO mice compared to WT mice starting at the second minute of testing (p< 0.05), and activity did not differ among saline-injected control littermates (WT, CRE, CAM). The low-dose ethanol challenge (1.5 g/kg) produced a modest stimulant response in WT, CRE and CAM mice, with elevated activity relative to saline occurring predominantly in the earlier portion of the testing period. In contrast, post-hoc analyses indicated that 1.5 g/kg ethanol produced an exaggerated locomotor stimulant response in KO mice compared to control littermates and this was evident from 2-13 min of the activity session (p< 0.05). The 3.0 g/kg ethanol challenge dose produced a slight depressant effect on locomotor activity relative to the saline condition in all control genotypes (WT, CRE, CAM) tested. This dose of ethanol also produced a reduction in locomotor activity in KO mice, but the effect was more robust when compared with the higher saline-induced level of activity (p< 0.05; Figure 4).
Figure 4. Dose response effects of Ethanol in WT and NR2B deficient mice.
All genotypes (WT, CRE, CAM, KO) show dose response effects of Ethanol on locomotor activity with 1.5 g/kg Ethanol stimulating activity and 3.0 g/kg Ethanol reducing activity. Distance moved (cm) is shown for (a) WT, (b) CRE, (c) CAM and (d) KO mice in 1-min bins to depict time course of activity levels. * = differs from saline; # = differs from WT. sample sizes were as follows: WT: 16 males, 10 females; CRE: 8 males, 3 females; CAM: 10 males, 11 females; KO: 13 males, 19 females.
Since NR2B deficient (KO) mice expressed higher levels of activity compared to control littermates following saline administration, data were expressed as a difference score for each subject (i.e., distance moved following 1.5 or 3.0 g/kg ethanol – group mean for distance moved following saline) to facilitate analysis of potential differences between genotypes in sensitivity to the low-dose stimulant and/or high-dose depressant effects of ethanol (Figure 5). Data in Figure 5 represent difference scores from baseline values shown in Figure 4. Analysis of ethanol-related locomotor activity difference scores indicated no significant interaction of Sex with Genotype and Dose [F(6,140)= 0.74, p> 0.05]. A significant Genotype × Dose interaction [F(6,140)= 4.00, p< 0.001] followed by post-hoc tests revealed that NR2B deficient (KO) mice exhibited significantly greater locomotor activity following 1.5 g/kg ethanol relative to saline, and this low-dose stimulant response was significantly greater in KO mice compared to control genotypes (p< 0.05). Likewise, while all genotypes exhibited reduced locomotor activity following 3.0 g/kg ethanol relative to the saline condition, this effect only reached significance for KO mice (p< 0.05). Thus, even when the significant higher level of baseline (saline) locomotor activity in KO mice is accounted for, NR2B deficient (KO) mice exhibited an exaggerated stimulant response to 1.5 g/kg ethanol (p< 0.05), as well an augmented reduction in activity following the 3.0 g/kg challenge in comparison to control littermates (p< 0.05; Figure 5).
Figure 5. NR2B deficient mice are more sensitive to the stimulating and sedating effects of Ethanol.
To control for baseline hyperactivity in NR2B deficient (KO) mice, data were expressed as difference scores (distance moved following 1.5 or 3.0 g/kg ethanol – group mean for distance moved following saline). Even when baseline hyperactivity is accounted for, NR2B deficient (KO) mice exhibited an exaggerated stimulant response to 1.5 g/kg ethanol, as well an augmented reduction in activity following the 3.0 g/kg challenge in comparison to control littermates * = differs from saline; ^ differs from 1.5 g/kg EtOH, # differs from WT, CRE, CAM. Same mice from Figure 4.
Although a quasi Latin square design was employed for the locomotor activity testing, we examined whether prior ethanol exposure in the locomotor test altered subsequent response to ethanol. To test for possible order effects of ethanol dose testing, analyses used test Session as an additional factor. As expected, we did not see any main effects of Session (at 1.5 g/kg EtOH: [F(1,47)= 0.04, p> 0.05]; at 3.0 g/kg EtOH: [F(1,47)= 2.19, p> 0.05]), or a significant interaction of Session with Genotype and Sex (at 1.5 g/kg EtOH: [F(1,47)= 0.15, p> 0.05]; at 3.0 g/kg EtOH: [F(1,47)= 0.14, p> 0.05]), suggesting that the different genotypes were not differentially susceptible to tolerance or sensitization induced by prior ethanol exposure.
Ethanol-induced loss of righting reflex
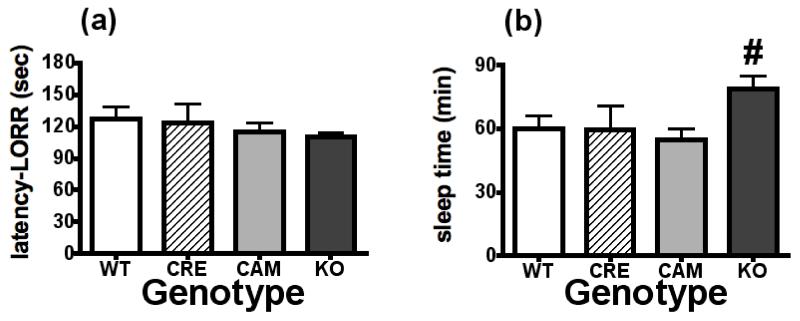
Analysis of LORR latency and sleep times in each of the four genotypes (WT, CRE, CAM, KO) revealed that, again, Sex did not interact with Genotype [LORR: F(3,61)= 0.34, p> 0.05; sleep time: F(3,64)= 0.84, p> 0.05]. Thus, LORR latency (panel A) and duration (sleep time) (panel B) data for each of the genotypes were collapsed across Sex (Figure 6). While there was no effect of Genotype on LORR latency [F(3,65)= 0.81, p> 0.05], NR2B deficient (KO) mice exhibited significantly longer LORR duration (sleep time) in comparison to the control genotypes following the 4.0 g/kg ethanol challenge [F(3,68)= 3.10, p< 0.05]. Upon waking, there were no differences in BECs between the 4 different genotypes [F(3,61)= 1.90, p> 0.05]. BEC values (mg/dl) for each of the groups were as follows (mean ± SEM): WT: males 389.3 ± 12.5, females 411.9 ± 18.5; CRE: males 383.2 ± 34.6, females 336.4 ± 46.7; CAM: males 386.5 ± 21.7, females 350.7 ± 22.1; KO: males 359.0 ± 20.4, females 400.6 ± 14.7.
Figure 6. NR2B deficient mice are more sensitive to the hypnotic effects of Ethanol.
(a) NR2B deficient (KO) mice were not different from the other genotypes in how long it took them to lose their righting reflex. (b) NR2B deficient (KO) mice did however have longer sleep times following 4.0 g/kg Ethanol. # = significantly different from WT, CRE, CAM. Sample sizes were as follows: WT = 21, CRE = 11, CAM = 19, KO = 26.
Ethanol pharmacokinetics
To further investigate the possibility that altered pharmacokinetics (ethanol metabolism and/or elimination) may contribute to the differences in LORR duration, BECs were analyzed as a three-level hierarchical linear model. The results of the preliminary model, showed that a) BECs declined linearly with time with no significant departure from linearity and b) individual mice varied systematically; i.e., the fit of a random model was superior to the fixed model (all mice within a Genotype and Sex shared the same slope). Thus, traditional analysis by repeated measure ANCOVA or regression were not appropriate given that all mice were not sampled at every time point (Raudenbush and Bryk, 2002).
The only overall effect observed was a Sex × Genotype × Dose interaction on variation among the slopes, (X2(3)= 16.8,p= 0.001). This resulted from the fact that the high dose female KO mice had a steeper slope (more rapid disappearance) than all other groups except for WT females and CRE females (all p’s< 0.003), though it approached significance in the latter two cases (p= 0.08). This was not the case for the male KO mice, nor did this effect appear at the low dose (Sex × Genotype, X2(3)= 1.44, p> 0.5). Also at the high dose, WT males had a modestly elevated intercept (p= 0.053), though this did not result in an overall main effect or interaction (Figure 7a-d). Finally, close inspection of peak BEC levels appeared to indicate a faster rate of ethanol absorption following the 1.5 g/kg dose. Further analysis of BEC levels 5 min following injection of 1.5 g/kg ethanol showed ethanol absorption did not differ statistically between NR2B KO mice and control littermates [F(3, 33)= 1.88, p> 0.05].
Figure 7. Female NR2B deficient mice have a more rapid clearance of high dose ethanol.
To determine if NR2B deficient (KO) mice metabolize ethanol at a different rate than control littermates, mice from each of the genotypes were injected with 1.5 and 4.0 g/kg ethanol (i.p.) and blood samples were taken at various times. Overall there were no differences in the clearance rate of ethanol between the genotypes. Only the female KO mice had a steeper slope (i.e., more rapid clearance rate of high dose ethanol) than control littermates. There were no differences in slope or intercept for any of the genotypes at the low ethanol dose. Sample sizes = 12/genotype except CAM females = 3.
DISCUSSION
Since genetic deletion of the NR2B subunit of NMDA receptors results in neonatal lethality (Kutsuwada et al., 1996), the conditional NR2B KO mice with developmentally-delayed knockdown of this subunit represent a unique experimental tool to investigate the role of NR2B containing NMDA receptors in mediating pharmacological and behavioral effects of ethanol in adult animals. In the present study, conditional NR2B KO mice along with control littermates were used to examine the biphasic (stimulant – depressant/hypnotic) pharmacological effects of ethanol. Results demonstrated a unique behavioral profile both prior to and following ethanol exposure among mice lacking NR2B-containing NMDA receptors in the forebrain. Analysis of Western blots verified significant reduction in NR2B protein expression in brain regions of these mice where CAMKIIα expression levels are high (Sola et al., 1999; Vallano, 1988). Baseline (saline) locomotor activity in an open field arena was significantly greater in NR2B deficient (KO) mice compared to other genotypes (control littermates: WT, CRE, CAM). NR2B deficient mice also differed in their locomotor response to both low-dose stimulating and high-dose sedating effects of ethanol. Specifically, even with a higher baseline level of activity, NR2B deficient (KO) mice exhibited an exaggerated locomotor stimulant response to 1.5 g/kg ethanol compared to that displayed by control littermates. Further, the locomotor depressant effects of 3.0 g/kg ethanol was more robust relative to baseline (saline) activity in KO mice compared to WT, CRE, and CAM controls. This apparent enhanced sensitivity to the depressant/hypnotic effects of ethanol in NR2B deficient (KO) mice was further supported by results demonstrating increased LORR duration following 4.0 g/kg ethanol challenge. BECs following 1.5 g/kg ethanol challenge did not significantly differ among the genotypes. Although female NR2B deficient (KO) mice displayed a more rapid clearance of 4.0 g/kg ethanol, this enhanced metabolism of ethanol is in opposition to the enhanced sensitivity findings found for locomotor activity (i.e., more rapid clearance would be associated with blunted sensitivity to ethanol) and further suggests that exaggerated sensitivity to ethanol in NR2B deficient mice is not due to differences in pharmacokinetics.
Knockdown of NR2B-containing NMDA receptors
Consistent with a previous report (Brigman et al., 2010), we confirmed a substantial, but not complete knock-down of NR2B-containing NMDA receptors in dmPFC and HPC in NR2B conditional knockout mice, and a significant reduction in expression levels of the obligatory NR1 subunit without a compensatory increase in NR2A in the mutant mice. In addition, the CAMKIIα-tTA and phCMV-tetO-CRE transgene combination drove deletion of NR2B also in DLS NAc, AMY, and BNST, which was not reported in the Brigman et al. (2010) study. Consistent with low expression patterns of CAMKIIα in brain stem structures (Sola et al., 1999; Vallano, 1988), we did not observe knock-down of NR2B in RF or medulla. Although we did not measure changes in surface expression or function of NMDA receptors, Brigman and colleagues demonstrated that isolated NMDA excitatory postsynaptic potentials were not different between WT and mutant mice, suggesting that the number of functional surface NMDA receptors was similar across genotypes (Brigman et al., 2010). This finding is not surprising given the large excess (~10-fold) of intracellular NR1 subunits awaiting assembly in endoplasmic reticulum (ER) that are rapidly degraded if not co-assembled with a NR2 subunit (Huh, 1999; Wenthold et al., 2003). It is thought that formation of functional NMDA receptors is the result of initial homodimerization of two NR1 subunits, followed by subsequent dimer dissociation and heterotetramerization with NR2 subunits (Farina et al., 2011). After post-translational modification, and association with scaffolding molecules (i.e., PSD-95, SAP102) and target motor proteins (i.e., KIF17), NMDA receptor complexes are then transported for delivery to the surface membrane (for reviews, see Stephenson, 2008; Wenthold et al., 2003). Over-expression of NR2A/NR2B subunits, but not NR1 subunits in cerebellar granular neurons increases surface expression of functional NMDA receptors (Prybylowski, 2002). Targeted knock-down of NR1 in CA1 pyramidal neurons caused an accumulation of NR2 subunits in the ER (Fukaya, 2003). Thus, it appears that a critical factor in NMDA receptor surface expression is the availability of NR2 subunits. The reduction in NR1 observed in NR2B conditional knockout mice is likely due to reduced availability of NR2B subunits leading to rapid degradation of a portion of NR1 in the ER. However, the large excess of NR1 in this pool would explain the lack of compensatory change in NR2A subunit expression observed in the present study and the lack of a functional change in surface NMDA receptors, as reported by Brigman et al. (2010).
Increased baseline (saline) locomotor activity in NR2B deficient mice
Results from this study demonstrate that baseline (saline) locomotor activity in an open field was significantly greater in NR2B deficient mice compared to control littermates. Although activity levels were similar for all genotypes during the first two minutes of testing, the groups substantially diverged as the test session progressed. Specifically, while locomotor activity systematically decreased over the 15 min test session in WT, CRE, and CAM mice, activity remained relatively stable in the KO mice, which resulted in an overall higher level of activity in the mutant mice compared to control genotypes. There was no significant difference in locomotor activity among WT, CRE, and CAM control littermates following saline injection. In one report, transgenic mice harboring the tTA-CAMKIIα transgenes with a C57BL/6J genetic background exhibited decreased locomotor activity in an open field compared to WT controls (McKinney et al., 2008). Whether saline administration in the present study accounts for the different outcomes in this mouse genotype is not clear at present.
It has been previously suggested that high activity levels that gradually decrease over the course of a testing session are a consequence of habituation to the novel environment (Gallitano-Mendel et al., 2007). Thus, given the temporal pattern of activity exhibited in KO mice, it would appear that mice lacking NR2B-containing NMDA receptors do not habituate to new surroundings as displayed by control (WT, CRE, CAM) mice. These data are particularly interesting in that NMDA receptors and, particularly, those containing the NR2B subunit have been implicated in various forms of learning/memory as well as the perception of novel environments/objects (Brigman et al., 2010, Rosenblum et al., 1997, Nunez-Jaramillo et al., 2008). For example, impaired performance on various learning/memory tasks (e.g., Morris water maze, discrete trial T-maze, trace fear conditioning) as well as impaired synaptic plasticity (e.g., LTP) in the hippocampus were recently reported in mice deficient in NR1/NR2B containing NMDA receptors (Brigman et al., 2010). It should be noted that since NR2B deficient mice did not show significantly higher activity levels within the first 5 min of the session, it is likely that these mice do not exhibit differences in initial response to novelty, as hyper-responsiveness to novel environments is generally indexed as greater initial levels of activity upon immediate exposure to a new environment (Gallitano-Mendel et al., 2007). Rather, these data suggest that NR2B deficient mice exhibit a deficit in capability to habituate to a novel environment.
NR2B deficient mice are more sensitive to the locomotor stimulant and depressant effects of ethanol
Ethanol-induced locomotor activity in an open field was measured to determine if NR2B deficient mice displayed a unique locomotor response profile to the low-dose stimulant (1.5 g/kg) and high-dose depressant (3.0 g/kg) effects of the drug. All mice, regardless of genotype, showed the expected dose-related effects of ethanol on locomotor activity. That is, all mice exhibited increases in activity following 1.5 g/kg ethanol challenge and decreased activity following administration of 3.0 g/kg ethanol compared to saline. These dose-related locomotor stimulant and depressant effects of ethanol were relatively modest in control (WT, CRE, CAM) mice. However, knockdown of NR2B-containing NMDA receptors in the forebrain, rendered mice more sensitive to both the stimulating and sedating effects of ethanol. Even after controlling for differences in basal (saline) activity levels, an exaggerated locomotor stimulant and depressant response to ethanol (1.5 and 3.0 g/kg, respectively) was evident in NR2B deficient mice as compared to control littermates (Figure 5). It should be noted that we (Becker, 1988) and others (Bejanian et al., 1993; Ginsburg and Lamb, 2008; Tambour et al., 2006) have commonly used these doses to assess the effects of ethanol of locomotor activity in C57BL/6J mice. Although NR2B KO mice had an exaggerated locomotor depressant response to high dose ethanol, these mice did not lose their righting reflex, as evidenced by the fact that these mice ambulated and registered activity counts. Further, potential effects of testing order were examined to determine if prior ethanol exposure in the locomotor test altered subsequent response to ethanol. There were no significant effects of prior ethanol exposure suggesting that the different genotypes were not differentially susceptible to tolerance or sensitization.
The augmented locomotor activity response to ethanol’s stimulant and depressant effects in NR2B deficient mice are congruent with similar outcomes reported by others following administration of subunit-nonselective NMDA receptor antagonists (e.g., Shen and Phillips, 1998). Further, results from the present study suggest a role for NR2B-containing NMDA receptors in mediating these behavioral effects of ethanol since similar effects were not observed in studies using NR2A knockout mice (Boyce-Rustay and Holmes, 2005; 2006). One factor to consider is the possibility that enhanced sensitivity to the locomotor effects of ethanol in NR2B KO mice was related to an interaction between ethanol and the apparent continued novelty of the testing (open field) environment. It is possible that NR2B KO mice would have eventually habituated to the open field had we used a shorter interval between activity tests or more saline-only exposures. However, all locomotor activity data were normalized to baseline (saline) activity levels and the NR2B KO mice continued to show greater sensitivity to ethanol. Together, these results suggest a possible shift to the left in sensitivity to the ethanol dose-response function for locomotor activity in NR2B deficient mice. However, additional doses of ethanol would need to be tested to confirm this possibility.
NR2B deficient mice are more sensitive to the depressant/hypnotic effects of ethanol
To further explore the role of NR2B-containing NMDA receptors in the depressant/hypnotic effects of ethanol, latency and duration of LORR following a 4.0 g/kg ethanol challenge were recorded in NR2B deficient mice and control littermates. While no differences among the genotypes were observed in latency to lose the righting reflex, NR2B deficient mice exhibited significantly greater duration of the LORR compared to control mice. These data are generally congruent with the locomotor depressant effects in demonstrating enhanced sensitivity to depressant/hypnotic effects of ethanol in NR2B deficient mice. These results are also in agreement with studies demonstrating NMDA receptor antagonists potentiate ethanol-induced LORR (Boyce and Holmes, 2005; Palachick et al., 2008)
Enhanced sensitivity to ethanol in NR2B deficient mice is not due to pharmacokinetics
To further investigate the possibly that altered pharmacokinetics (ethanol metabolism and/or elimination) may contribute to the apparent differences in LORR duration, BECs were evaluated at various times following 1.5 and 4.0 g/kg ethanol. Female NR2B deficient (KO) mice displayed a more rapid clearance of 4.0 g/kg ethanol, however, this enhanced metabolism of ethanol is in opposition to the enhanced sensitivity findings found for sleep duration (i.e., more rapid clearance would be associated with shorter sleep times). Furthermore, there were no effects of Sex in any of the behavioral measures making it unlikely that the apparent faster rate of metabolism in female KO mice accounts for differences in locomotor activity levels or LORR duration relative to control genotypes. Additionally, the rate of ethanol clearance following 1.5 g/kg ethanol was similar among all genotypes for males and females, despite large locomotor behavioral differences observed in KO mice after administration of this low ethanol dose. However, examination of peak BEC levels appeared to indicate a faster rate of ethanol absorption following the 1.5 g/kg dose. Further analysis of BEC levels 5 min following injection of 1.5 g/kg ethanol showed ethanol absorption did not differ statistically between NR2B KO mice and control littermates. Taken together, it is not likely that enhanced sensitivity to stimulant and depressant/hypnotic effects of ethanol exhibited by NR2B KO mice is due to altered pharmacokinetics.
Ethanol and the NR2B subunit of NMDA receptors
While NMDA glutamate receptors are a major target of ethanol in the CNS, and presence of the NR2B subunit is thought to convey enhanced sensitivity to ethanol-induced inhibition of NMDA receptor-mediated activity (Jin and Woodward, 2006; Nagy et al., 2004), few studies have employed pharmacological agents that target the NR2B subunit in examining the role of NR2B subunit-containing NMDA receptors in mediating biphasic effects of ethanol. Studies in rats have shown NR2B subunit-“selective” antagonists (e.g., ifenprodil) systemically administered to have little effect on spontaneous locomotor activity (Mikolajczak et al., 2003; Mikolajczak et al., 2002). Other studies in mice have shown ifenprodil to decrease (Boyce-Rustay and Cunningham, 2004) or have no effect on ethanol-stimulated locomotor activity (Broadbent et al., 2003). Additionally, ifenprodil was reported to enhance ethanol-induced LORR in rats (Mikolajczak et al., 2002). If NR2B knockouts are indeed super-sensitive to ethanol’s stimulant and depressant/hypnotic actions, then the ethanol phenotypes demonstrated here should be reproduced by co-administering ethanol and a NR2B-selective antagonist to WT mice. Interestingly, NR2A knockout mice were shown to exhibit similar ethanol-induced sleep times as WT control mice, but duration of LORR was potentiated by the NR2B-selective antagonist, Ro 25-6981, in both NR2A knockout and WT mice (Boyce-Rustay and Holmes, 2005). Collectively, these results suggest that pharmacological manipulation of NR2B containing NMDA receptors may alter some of the pharmacological effects of ethanol. Discrepancies in findings may be due to the extent of NR2B subunit blockade that may vary according to drug dose, treatment regimen, and off-target pharmacological activity (Traynelis et al., 2010), as well as method of subunit blockade (genetic knockdown vs. pharmacological blockade). Although viability of offspring was ensured by developmentally delaying NR2B knockdown, it is possible that unknown compensatory changes in the NR2B KO mice may have contributed to the behavioral results in the present study. Future studies will address this by inducing NR2B subunit knockdown at a time closer in proximity to behavioral testing (through the withdrawal of doxycycline-containing diet to activate the phCMV-tetO-driven CRE transgene).
Greater sensitivity to ethanol in NR2B deficient mice may likely be due to a disruption of normal neuronal network activity that typically mediates behavioral responses to ethanol. NR2B-containing NMDA receptors are coupled to specific signaling transduction pathways that mediate homeostatic responses. For example, ifenprodil, a selective antagonist for the NR2B subunit, reduces Ca2+ current through the NMDA receptor (Church et al, 1994). Alteration of typical Ca2+ efflux disrupts normal functioning of neuronal homeostatic processes including modified activation of Ca2+/calmodulin-dependent kinases, protein kinase C and transcription factors important for synaptic plasticity (Toscano et al, 2002). Therefore, one interpretation of the present data is that knockdown of the NR2B/NR1-containing NMDA receptors rendered mice more sensitive to ethanol by disrupting intrinsic homeostatic processes that mediate neuron excitability. Given that ethanol inhibits NMDA receptor function (Blevins et al, 1997), it is conceivable that any alteration of NMDA subunits would impair normal function of the receptor. NR2B subunits have been shown to be particularly sensitive to the inhibitory effects of ethanol on NMDA receptor function (Lovinger, 1995; Blevins et al, 1997). Substantial knockdown of NR2B subunits would certainly alter sensitivity of NMDA receptors and, consequently, alter excitability of neurons in these networks. It may be that knockdown of NR2B/NR1 containing NMDA receptors in the striatum and nucleus accumbens decreased excitability of medium-spiny GABAergic neurons and thereby disinhibited neuronal networks that led to a potentiation of ethanol-induced locomotor activity. It would be interesting to measure intrinsic excitability of striatal neurons in these NR2B deficient mice to determine if these neurons are more (or less) excited following acute exposure to ethanol. Future studies involving targeted manipulation of NR2B-containing NMDA receptors in specific brain regions will provide insights about the role of this subunit in these biphasic pharmacological effects of ethanol.
Conclusions
In summary, NR2B deficient mice are more sensitive to the stimulant and depressant/hypnotic effects of systemically administered ethanol. These data suggest that the NR2B subunit of NMDA receptors plays a significant role in mediating/modulating behavioral responsiveness to various pharmacological effects of ethanol. Future studies will characterize performance of NR2B KO mice on PFC- and hippocampal-dependent behaviors (e.g., cognitive and memory tasks). Understanding the role of NR2B subunit-containing NMDA receptors in mediating neurobehavioral actions of ethanol will enhance insight about the potential for these receptors to serve as targets for pharmacological treatments for alcohol-related problems.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by NIAAA grants AA13514 to E.D. and AA14095 to H.C.B. The authors would like to thank Dr. John J. Woodward for insightful comments during the preparation of this manuscript. A special thanks to Kay Fernandez, Josh Sanchez and Michelle Cunningham and Catherine Hindman for their technical assistance.
REFERENCES
- Becker HC. Effects of the imidazobenzodiazepine RO15-4513 on the stimulant and depressant actions of ethanol on spontaneous locomotor activity. Life Sci. 1988;43(7):643–50. doi: 10.1016/0024-3205(88)90069-0. [DOI] [PubMed] [Google Scholar]
- Bejanian M, Jones BL, Alkana RL. Low-level hyperbaric antagonism of ethanol-induced locomotor depression in C57BL/6J mice: dose response. Alcohol Clin Exp Res. 1993;17(5):935–9. doi: 10.1111/j.1530-0277.1993.tb05644.x. [DOI] [PubMed] [Google Scholar]
- Beleslin DB, Djokanović N, Jovanović Mićić D, Samardzić R. Opposite effects of GABAA and NMDA receptor antagonists on ethanol-induced behavioral sleep in rats. Alcohol. 1997;14(2):167–73. doi: 10.1016/s0741-8329(96)00140-1. [DOI] [PubMed] [Google Scholar]
- Blevins T, Mirshahi T, Chandler LJ, Woodward JJ. Effects of acute and chronic ethanol exposure on heteromeric N-methyl-D-aspartate receptors expressed in HEK 293 cells. J Neurochem. 1997;69:2345–54. doi: 10.1046/j.1471-4159.1997.69062345.x. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cunningham CL. The role of NMDA receptor binding sites in ethanol place conditioning. Behav Neurosci. 2004;118:822–34. doi: 10.1037/0735-7044.118.4.822. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Functional roles of NMDA receptor NR2A and NR2B subunits in the acute intoxicating effects of ethanol in mice. Synapse. 2005;56(4):222–5. doi: 10.1002/syn.20143. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Holmes A. Ethanol-related behaviors in mice lacking the NMDA receptor NR2A subunit. Psychopharmacology (Berl) 2006;187:455–66. doi: 10.1007/s00213-006-0448-6. [DOI] [PubMed] [Google Scholar]
- Brigman JL, Wright T, Talani G, Prasad-Mulcare S, Jinde S, Seabold GK, Mathur P, Davis MI, Bock R, Gustin RM, Colbran RJ, Alvarez VA, Nakazawa K, Delpire E, Lovinger DM, Holmes A. Loss of GluN2B-containing NMDA receptors in CA1 hippocampus and cortex impairs long-term depression, reduces dendritic spine density, and disrupts learning. J Neurosci. 2010;30(13):4590–600. doi: 10.1523/JNEUROSCI.0640-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbent J, Kampmueller KM, Koonse SA. Expression of behavioral sensitization to ethanol by DBA/2J mice: the role of NMDA and non-NMDA glutamate receptors. Psychopharmacology (Berl) 2003;167:225–34. doi: 10.1007/s00213-003-1404-3. [DOI] [PubMed] [Google Scholar]
- Chen Q, Reiner A. Cellular distribution of the NMDA receptor NR2A/2B subunits in the rat striatum. Brain Res. 1996;743(1-2):346–52. doi: 10.1016/s0006-8993(96)01098-0. [DOI] [PubMed] [Google Scholar]
- Church J, Fletcher EJ, Baxter K, MacDonald JF. Blockade by ifenprodil of high voltage-activated Ca2+ channels in rat and mouse cultured hippocampal pyramidal neurones: comparison with N-methyl-D-aspartate receptor antagonist actions. Br J Pharmacol. 1994;113(2):499–507. doi: 10.1111/j.1476-5381.1994.tb17017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya M, Kato A, Lovett C, Tonegawa S, Watanabe M. Retention of NMDA receptor NR2 subunits in the lumen of endoplasmic reticulum in targeted NR1 knockout mice. Proc Natl Acad Sci U S A. 2003;100(8):4855–60. doi: 10.1073/pnas.0830996100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallitano-Mendel A, Izumi Y, Tokuda K, Zorumski CF, Howell MP, Muglia LJ, Wozniak DF, Milbrandt J. The immediate early gene early growth response gene 3 mediates adaptation to stress and novelty. Neuroscience. 2007;148(3):633–43. doi: 10.1016/j.neuroscience.2007.05.050. Epub 2007 Aug 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg BC, Lamb RJ. Taurine and ethanol interactions: behavioral effects in mice. Eur J Pharmacol. 2008;578(2-3):228–37. doi: 10.1016/j.ejphar.2007.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA, Colombo G. Discriminative stimulus effects of ethanol: effect of training dose on the substitution of N-methyl-D-aspartate antagonists. J Pharmacol Exp Ther. 1993;264(3):1241–7. [PubMed] [Google Scholar]
- Hodge CW, Cox AA, Bratt AM, Camarini R, Iller K, Kelley SP, Mehmert KK, Nannini MA, Olive MF. The discriminative stimulus properties of self-administered ethanol are mediated by GABA(A) and NMDA receptors in rats. Psychopharmacology (Berl) 2001;154(1):13–22. doi: 10.1007/s002130000619. [DOI] [PubMed] [Google Scholar]
- Hoffman PL, Rabe CS, Moses F, Tabakoff B. N-methyl-D-aspartate receptors and ethanol: inhibition of calcium flux and cyclic GMP production. J Neurochem. 1989;52:1937–40. doi: 10.1111/j.1471-4159.1989.tb07280.x. [DOI] [PubMed] [Google Scholar]
- Huh KH, Wenthold RJ. Turnover analysis of glutamate receptors identifies a rapidly degraded pool of the N-methyl-D-aspartate receptor subunit, NR1, in cultured cerebellar granule cells. J Biol Chem. 1999;274(1):151–7. doi: 10.1074/jbc.274.1.151. [DOI] [PubMed] [Google Scholar]
- Jin C, Woodward JJ. Effects of 8 different NR1 splice variants on the ethanol inhibition of recombinant NMDA receptors. Alcohol Clin Exp Res. 2006;30(4):673–9. doi: 10.1111/j.1530-0277.2006.00079.x. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Petrakis IL, Limoncelli D, Webb E, Gueorgueva R, D’Souza DC, Boutros NN, Trevisan L, Charney DS. Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacology. 2003;28(11):2020–8. doi: 10.1038/sj.npp.1300252. [DOI] [PubMed] [Google Scholar]
- Küppenbender KD, Standaert DG, Feuerstein TJ, Penney JB, Jr, Young AB, Landwehrmeyer GB. Expression of NMDA receptor subunit mRNAs in neurochemically identified projection and interneurons in the human striatum. J Comp Neurol. 2000;419(4):407–21. doi: 10.1002/(sici)1096-9861(20000417)419:4<407::aid-cne1>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor epsilon 2 subunit mutant mice. Neuron. 1996;16(2):333–44. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- Lakso M, Pichel JG, Gorman JR, Sauer B, Okamoto Y, Lee E, Alt FW, Westphal H. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc. Natl. Acad. Sci. USA. 1996;93:5860–5685. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Developmental decrease in ethanol inhibition of N-methyl-D-aspartate receptors in rat neocortical neurons: relation to the actions of ifenprodil. J Pharmacol Exp Ther. 1995;274(1):164–72. [PubMed] [Google Scholar]
- Malinowska B, Napiórkowska-Pawlak D, Pawlak R, Buczko W, Göthert M. Ifenprodil influences changes in mouse behaviour related to acute and chronic ethanol administration. Eur J Pharmacol. 1999;377(1):13–9. doi: 10.1016/s0014-2999(99)00393-3. [DOI] [PubMed] [Google Scholar]
- Masood K, Wu C, Brauneis U, Weight FF. Differential ethanol sensitivity of recombinant N-methyl-D-aspartate receptor subunits. Mol Pharmacol. 1994;45:324–9. [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang YY, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- McKinney BC, Schneider JS, Schafer GL, Lowing JL, Mohan S, Zhao MX, Heng MY, Albin RL, Seasholtz AF, Akil H, Murphy GG. Decreased locomotor activity in mice expressing tTA under control of the CaMKII alpha promoter. Genes Brain Behav. 2008;7(2):203–13. doi: 10.1111/j.1601-183X.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- McMillen BA, Joyner PW, Parmar CA, Tyer WE, Williams HL. Effects of NMDA glutamate receptor antagonist drugs on the volitional consumption of ethanol by a genetic drinking rat. Brain Res Bull. 2004;64(3):279–84. doi: 10.1016/j.brainresbull.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Meyer PJ, Phillips TJ. Bivalent effects of MK-801 on ethanol-induced sensitization do not parallel its effects on ethanol-induced tolerance. Behav Neurosci. 2003;117(3):641–9. doi: 10.1037/0735-7044.117.3.641. [DOI] [PubMed] [Google Scholar]
- Mikolajczak P, Okulicz-Kozaryn I, Kaminska E, Niedopad L, Polanska A, Gebka J. Effects of acamprosate and some polyamine site ligands of NMDA receptor on short-term memory in rats. Eur J Pharmacol. 2002;444(1-2):83–96. doi: 10.1016/s0014-2999(02)01276-1. [DOI] [PubMed] [Google Scholar]
- Mikolajczak P, Okulicz-Kozaryn I, Kaminska E, Szulc M, Dyr W, Kostowski W. Lack of ifenprodil anxiolytic activity after its multiple treatment in chronically ethanol-treated rats. Alcohol Alcohol. 2003;38(4):310–5. doi: 10.1093/alcalc/agg078. [DOI] [PubMed] [Google Scholar]
- Mirshahi T, Woodward JJ. Ethanol sensitivity of heteromeric NMDA receptors: effects of subunit assembly, glycine and NMDAR1 Mg(2+)-insensitive mutants. Neuropharmacology. 1995;34:347–55. doi: 10.1016/0028-3908(94)00155-l. [DOI] [PubMed] [Google Scholar]
- Nagy J. The NR2B subtype of NMDA receptor: a potential target for the treatment of alcohol dependence. Curr Drug Targets CNS Neurol Disord. 2004;3:169–79. doi: 10.2174/1568007043337409. [DOI] [PubMed] [Google Scholar]
- Núñez-Jaramillo L, Jimenez B, Ramirez-Munguía N, Delint-Ramírez I, Luna-Illades C, Tapia R, Bermúdez-Rattoni F. Taste novelty induces intracellular redistribution of NR2A and NR2B subunits of NMDA receptor in the insular cortex. Brain Res. 2008;1215:116–22. doi: 10.1016/j.brainres.2008.03.032. [DOI] [PubMed] [Google Scholar]
- Palachick B, Chen YC, Enoch AJ, Karlsson RM, Mishina M, Holmes A. Role of major NMDA or AMPA receptor subunits in MK-801 potentiation of ethanol intoxication. Alcohol Clin Exp Res. 2008;32(8):1479–92. doi: 10.1111/j.1530-0277.2008.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prybylowski K, Fu Z, Losi G, Hawkins LM, Luo J, Chang K, Wenthold RJ, Vicini S. Relationship between availability of NMDA receptor subunits and their expression at the synapse. J Neurosci. 2002;22(20):8902–1. doi: 10.1523/JNEUROSCI.22-20-08902.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. Second Edition Sage Publications; Thousand Oaks: 2002. [Google Scholar]
- Rosenblum K, Berman DE, Hazvi S, Lamprecht R, Dudai Y. NMDA receptor and the tyrosine phosphorylation of its 2B subunit in taste learning in the rat insular cortex. J Neurosci. 1997;17(13):5129–35. doi: 10.1523/JNEUROSCI.17-13-05129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saam JR, Gordon JI. Inducible gene knockouts in the small intestinal and colonic epithelium. J. Biol. Chem. 1999;274:38071–38082. doi: 10.1074/jbc.274.53.38071. [DOI] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438(7071):1167–71. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Shen EH, Phillips TJ. MK-801 potentiates ethanol’s effects on locomotor activity in mice. Pharmacol Biochem Behav. 1998;59(1):135–43. doi: 10.1016/s0091-3057(97)00389-4. [DOI] [PubMed] [Google Scholar]
- Silveri MM, Spear LP. The effects of NMDA and GABAA pharmacological manipulations on ethanol sensitivity in immature and mature animals. Alcohol Clin Exp Res. 2002;26(4):449–56. [PubMed] [Google Scholar]
- Solà C, Tusell JM, Serratosa J. Comparative study of the distribution of calmodulin kinase II and calcineurin in the mouse brain. J Neurosci Res. 1999;57(5):651–62. [PubMed] [Google Scholar]
- Stephenson FA, Cousins SL, Kenny AV. Assembly and forward trafficking of NMDA receptors (Review) Mol Membr Biol. 2008;25:311–20. doi: 10.1080/09687680801971367. [DOI] [PubMed] [Google Scholar]
- Tambour S, Didone V, Tirelli E, Quertemont E. Locomotor effects of ethanol and acetaldehyde after peripheral and intraventricular injections in Swiss and C57BL/6J mice. Behav Brain Res. 2006;172(1):145–54. doi: 10.1016/j.bbr.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Toscano CD, Hashemzadeh-Gargari H, McGlothan JL, Guilarte TR. Developmental Pb2+ exposure alters NMDAR subtypes and reduces CREB phosphorylation in the rat brain. Brain Res Dev Brain Res. 2002;139(2):217–26. doi: 10.1016/s0165-3806(02)00569-2. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62(3):405–96. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallano ML. Identification and regional distribution of a type II calcium/calmodulin-dependent kinase in mouse brain. Biochem Pharmacol. 1988;37(12):2381–8. doi: 10.1016/0006-2952(88)90364-4. [DOI] [PubMed] [Google Scholar]
- Viberg H, Mundy W, Eriksson P. Neonatal exposure to decabrominated diphenyl ether (PBDE 209) results in changes in BDNF, CaMKII and GAP-43, biochemical substrates of neuronal survival, growth, and synaptogenesis. Neurotoxicology. 2008;29(1):152–9. doi: 10.1016/j.neuro.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Prybylowski K, Standley S, Sans N, Petralia RS. Trafficking of NMDA receptors. Annu Rev Pharmacol Toxicol. 2003;43:335–58. doi: 10.1146/annurev.pharmtox.43.100901.135803. [DOI] [PubMed] [Google Scholar]
- Xi D, Zhang W, Wang HX, Stradtman GG, Gao WJ. Dizocilpine (MK-801) induces distinct changes of N-methyl-D-aspartic acid receptor subunits in parvalbumin-containing interneurons in young adult rat prefrontal cortex. Int J Neuropsychopharmacol. 2009;12(10):1395–408. doi: 10.1017/S146114570900042X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaka R, Tang KC, Camarini R, Janak PH, Ron D. Fyn kinase and NR2B-containing NMDA receptors regulate acute ethanol sensitivity but not ethanol intake or conditioned reward. Alcohol Clin Exp Res. 2003;27(11):1736–42. doi: 10.1097/01.ALC.0000095924.87729.D8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–94. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.