Abstract
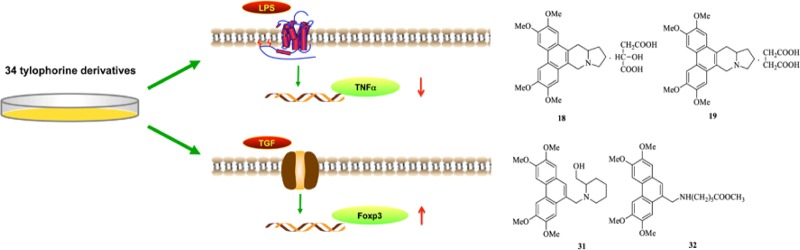
We have previously demonstrated that DCB-3503, a tylophorine analogue, has an anti-inflammatory property in murine models for autoimmune diseases. However, its mechanism remains unknown. Here, we have synthesized 34 derivatives of DCB-3503 and investigated their effects on T cells differentiation and TNF-α production. Six derivatives (4, 9, 13, 19, 31, and 32) could significantly promote the expression of Foxp3. Among these, the IC50 of 31 and 32 was about 500 μM. Eight analogues (1, 2, 4, 9, 12, 18, 19, and 21) showed anti-TNF-α effect in Raw 264.7 cells and murine splenocytes, of which 18 and 19 were most significant. Moreover, 31 and 18 showed a better activity and cell survival ratio when compared with DCB-3503 at various concentrations. In summary, we have demonstrated the anti-inflammatory characteristics of 34 novel tylophorine derivatives and discussed their structure–activity relationship in order to explore their therapeutic potentials for inflammatory diseases.
Keywords: Tylophorines derivatives, anti-inflammatory activity, Foxp3, TNF-α
Tylophorines are isolated from the roots of Tylophora atrofolliculata, which has been used as a traditional chinese medicine for the treatment of allergic and inflammatory diseases. Among several tylophorine analogs, DCB-3503 has a wide range of pharmacological activities as an antitumor,1−5 anti-inflammatory, and antiautoimmune disease agent in murine models.6−8 Recently, DCB-3503 has been shown to inhibit nuclear factor-kappa B-mediated transcription in vitro.9,10 Thus, it can be potentially used for treating inflammatory diseases. However, the role of DCB-3503 in regulatory T cells (Tregs) differentiation or production of inflammatory cytokines still remains unknown.
Peripheral tolerance is controlled and maintained by a subset of Tregs. Failure of such tolerance can lead to many autoimmune diseases.11 Forkhead box P3 (Foxp3) is the key transcription factor that determines the differentiation and function of Tregs.12 Besides thymus-derived naturally occurring Tregs (nTregs), naïve T cells can be induced to express Foxp3 upon T-cell receptor (TCR) stimulation in the presence of transforming growth factor-beta (TGF-β), and these inducible T regulatory cells (iTregs) exert similar suppressive functions as nTregs.13 Tumor necrosis factor-alpha (TNF-α) is a pleiotropic inflammatory cytokine that plays a central role in acute and chronic inflammatory diseases, such as rheumatoid arthritis (RA), Crohn’s disease, and bacterial septic shock.14−16 Blockade of the effect of this cytokine by anti-TNF-α antibody or a fusion of two soluble TNF receptors with a human immunoglobulin molecule has been used for the treatment of RA and inflammatory bowel disease.17−21 Treg cells dysfunction and TNF-α production represent two major therapeutic targets during the progress of autoimmune diseases. Therefore, to search for small molecules that can modulate expression of Foxp3 or suppress production of TNF-α may provide a strategy for the treatment of autoimmune diseases.
In this study, we have synthesized 34 novel DCB-3503 derivatives and analyzed their activity for the induction of Foxp3 expression within cultured T cells and inhibition of TNF-α production by murine splenocytes as well as Raw cells upon lipopolysaccharide (LPS) activation. Our results revealed that 4 (DCB-3503), 9, 13, 19, and two of C9-substituted phenanthrene-based tylophorine derivatives (PBTs) 31 and 32 can significantly promote Foxp3 expression in vitro. Eight analogues (1, 2, 4, 9, 12, 18, 19, and 21) can significant inhibit toward TNF-α in Raw 264.7 cells and primary murine splenocytes. Biological results revealed that the structure of PBTs is crucial for the differentiation of Tregs and that salt derivatives of tylophorine may sustain inhibition of TNF-α by improving the water solubility of DCB-3503. Moreover, the activity and cytotoxicity of compounds 31 and 18 were analyzed in comparison with DCB-3503 at various concentrations, and the results suggested that they may be superior to DCB-3503 in terms of therapeutic usage.
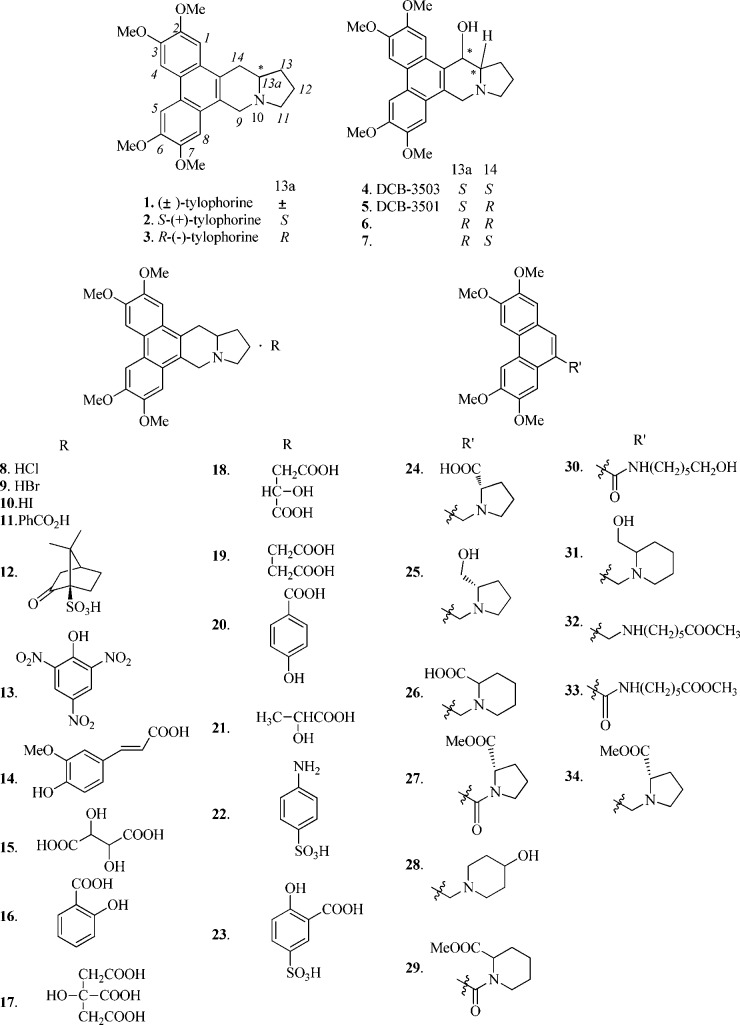
Structures of tested tylophorine derivatives are shown in Figure 1. Synthesis of 1,22 2–3,23 4–7,24 the salt derivatives of (±)-tylophorine 8–23,23 and C9-substituted phenanthrene-based tylophorine derivatives (PBTs) 24–31, 33, and 3425 were accomplished with procedures described in the literature.
Figure 1.
Chemical structures of tylophorine derivatives 1–34.
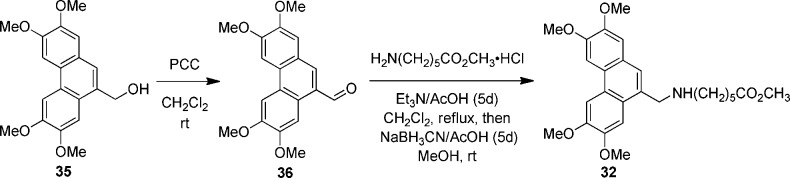
Oxidation of alcohol 35 with pyri-dinium chlorochromate gave rise to aldehyde 36, which was coupled with methyl 6-aminohexanoate hydro-chloride to generate compound 32 (Scheme 1).
Scheme 1. Synthesis of Compound 32.
Compounds 4, 9, 13, 19, 31, and 32 Significantly Promote Foxp3 Expression
To evaluate the effect of these tylophorine derivatives on the induction of Foxp3, Foxp3-green fluorescent protein (GFP) reporter mice (in which the GFP level can predict the expression of Foxp3 mRNA)26 were used to detect Foxp3 expression via acquisition of GFP+ cells. For compounds 1–3, (±)-tylophorine (1) and S-(+)-tylophorine (2) showed inhibitory effects on Foxp3 expression, whereas R-(−)-tylophorine (3) showed a slightly promoting effect. All 14-hydroxy tylophorine analogues (4–7) promoted Foxp3 expression, and 4 (DCB-3503) showed a better activity (39%) than its isomers (5–7). For the salt derivatives of (±)-tylophorine (8–23), various effects on Foxp3 expression were observed, of which hydrobromide (9), picrate (13), and succinate (19) significantly promoted Foxp3 expression (40%, 47%, and 48%, respectively). All PBTs (24–34) could enhance the Foxp3 expression except for 25 and 26, among which 31 and 32 were more significant (41% and 47%, respectively). Results of all derivatives are listed in Table 1. A representative FACS dot plot of three independent experiments is shown in Figure S1, Supporting Information.
Table 1. Foxp3 Promoting Activity of Compounds 1–34a.
| increased percentage of iTregs marked by Foxp3
(%) | |||||
|---|---|---|---|---|---|
| compd | 100 nM | compd | 100 nM | compd | 1 μM |
| 1 | –34 ± 4 | 13 | 47 ± 8 | 24 | 2 ± 3 |
| 2 | –44 ± 2 | 14 | 0.8 ± 4 | 25 | 0.2 ± 2 |
| 3 | 4 ± 6 | 15 | 32 ± 5 | 26 | 0.7 ± 3 |
| 4 | 39 ± 9 | 16 | –4 ± 11 | 27 | 5 ± 2 |
| 5 | 29 ± 8 | 17 | 0.1 ± 4 | 28 | 5 ± 2 |
| 6 | 12 ± 5 | 18 | 4 ± 2 | 29 | 7 ± 2 |
| 7 | 5 ± 2 | 19 | 48 ± 9 | 30 | 7 ± 2 |
| 8 | 9 ± 1 | 20 | –5 ± 1 | 31 | 41 ± 1 |
| 9 | 40 ± 12 | 21 | 7 ± 3 | 32 | 47 ± 2 |
| 10 | –12 ± 8 | 22 | –3 ± 1 | 33 | 16 ± 5 |
| 11 | –1 ± 2 | 23 | 29 ± 6 | 34 | 19 ± 3 |
| 12 | –2 ± 6 | ||||
Values are the mean ± SD of at least three independent experiments carried out in duplicate. Bold values denote compounds with >35% increase of iTregs marked by Foxp3.
Eleven Compounds Have Suppressed TNF-α Production in Raw 264.7 Cells
To analyze the anti-inflammatory effects of all synthesized compounds, Raw 264.7 cells (murine macrophage cell line) stimulated by LPS were used in a TNF-α detection assay. For compounds 1–3, (±)-tylophorine (1) (half-maximal inhibitory concentration (IC50) = 125 nM) and S-(+)-tylophorine (2) (78 nM) showed greater inhibitory effects compared with R-(−)-tylophorine (3) (430 nM). Among 14-hydroxytylophorine analogues (4–7), compounds 4 (IC50 = 36 nM) and 6 (238 nM) showed greater inhibition than their isomers. For salt derivatives (±)-tylophorine (8–23), compounds 8, 9, 12, 16, 17, 18, 19, and 21 showed significantly inhibitory effects (IC50 values = 52, 93, 27, 56, 61, 33, 56, and 100 nM, respectively). However, PBTs 24–34 only weakly inhibited TNF-α expression. Thus, 1, 2, 8, 9, 12, 16, 17, 18, 19, and 21 had comparable inhibitory effects as that of 4 (DCB-3503). Most of these compounds were salt derivatives, suggesting that the structure of salt derivatives can maintain the inhibitory effects by improving water solubility. Activity data for tylophorine derivatives are shown in Table 2.
Table 2. TNF-α Inhibitory Activitya.
| IC50s in Raw 264.7 | IC50s in murine splenocytes |
||
|---|---|---|---|
| compd | 6 h | 4 h | 24 h |
| 1 | 125 ± 6 nM | 35 ± 15 nM | 871 ± 10 nM |
| 2 | 78 ± 12 nM | 55 ± 15 nM | 1134 ± 17 nM |
| 3 | 430 ± 63 nM | 208 ± 14 nM | 1215 ± 20 nM |
| 4 | 36 ± 12 nM | 20 ± 14 nM | 97 ± 12 nM |
| 5 | 350 ± 16 nM | 370 ± 15 nM | 1292 ± 25 nM |
| 6 | 238 ± 27 nM | 133 ± 11 nM | 783 ± 32 nM |
| 7 | 817 ± 36 nM | 610 ± 43 nM | 965 ± 45 nM |
| 8 | 52 ± 16 nM | 300 ± 53 nM | 673 ± 52 nM |
| 9 | 93 ± 13 nM | 118 ± 15 nM | 628 ± 44 nM |
| 10 | 226 ± 12 nM | 100 ± 10 nM | 589 ± 35 nM |
| 11 | 181 ± 19 nM | 319 ± 12 nM | 739 ± 32 nM |
| 12 | 27 ± 12 nM | 30 ± 9 nM | 338 ± 23 nM |
| 13 | 162 ± 17 nM | 242 ± 38 nM | 312 ± 13 nM |
| 14 | 135 ± 13 nM | 213 ± 25 nM | 329 ± 23 nM |
| 15 | 122 ± 12 nM | 226 ± 38 nM | 368 ± 43 nM |
| 16 | 56 ± 17 nM | 252 ± 26 nM | 295 ± 16 nM |
| 17 | 61 ± 19 nM | 223 ± 26 nM | 631 ± 42 nM |
| 18 | 33 ± 4 nM | 13 ± 2 nM | 100 ± 11 nM |
| 19 | 56 ± 7 nM | 18 ± 4 nM | 72 ± 8 nM |
| 20 | 511 ± 6 nM | 760 ± 3 nM | 972 ± 22 nM |
| 21 | 100 ± 10 nM | 76 ± 8 nM | 918 ± 23 nM |
| 22 | 248 ± 17 nM | 215 ± 9 nM | 193 ± 14 nM |
| 23 | 283 ± 15 nM | 210 ± 11 nM | 160 ± 18 nM |
| 24 | 200 ± 13 μM | 234 ± 13 μM | 231 ± 12 μM |
| 25 | 307 ± 6 μM | 265 ± 13 μM | 304 ± 31 μM |
| 26 | 354 ± 11 μM | 292 ± 14 μM | 389 ± 24 μM |
| 27 | 217 ± 15 μM | 211 ± 11 μM | 363 ± 33 μM |
| 28 | 231 ± 12 μM | 265 ± 10 μM | 273 ± 42 μM |
| 29 | 227 ± 12 μM | 301 ± 15 μM | 329 ± 26 μM |
| 30 | 236 ± 10 μM | 251 ± 21 μM | 317 ± 46 μM |
| 31 | 41 ± 6 μM | 38 ± 18 μM | 92 ± 12 μM |
| 32 | 227 ± 11 μM | 115 ± 14 μM | 213 ± 11 μM |
| 33 | 241 ± 17 μM | 156 ± 16 μM | 257 ± 17 μM |
| 34 | 216 ± 14 μM | 153 ± 13 μM | 275 ± 15 μM |
Values are the mean ± SD of at least three independent experiments carried out in duplicate.
Eight Compounds Inhibited TNF-α Expression in Primary Murine Splenocytes
To further evaluate the effects of these compounds on LPS-triggered TNF-α production, splenocytes from BALB/c mice were cultured with LPS (100 ng/mL) in the presence of these compounds for 4 and 24 h, and the amounts of TNF-α in the supernatants were measured by ELISA.
(±)-Tylophorine (1), S-(+)-tylophorine (2), and the salt derivatives of (±)-tylophorine 9, 10, 12, and 21 showed significant inhibition (IC50 values (in nM) = 35, 55, 118, 100, 30, and 76, respectively) at 4 h but not at 24 h (Table 2). However, (±)- tylophorine malate (18) and (±)-tylophorine succinate (19) exhibited long-term inhibitory effects on TNF-α production at 4 h (IC50 = 13 and 18 nM, respectively) and 24 h (100 and 72 nM, respectively), which was consistent with 4 (DCB-3503) (20 and 97 nM at 4 and 24 h, respectively). Among 14-hydroxytylophorine compounds (4–7), 4 and 6 showed greater inhibition (IC50 = 20 and 133 nM, respectively) than 5 and 7 (370 and 610 nM, respectively), which was similar to their effects in Raw cells. The above findings suggested that C14-hydroxy and C13a-hydrogen were more active when they were in the trans configuration. The similar inhibitory effects of 1, 2, 4, 9, 12, 18, 19, and 21 shown in murine splenocytes as well as Raw cells suggested their potent anti-inflammatory effects as TNF-α inhibitors. All the C9-substituted PBTs 24–34 did not display any inhibition effect, suggesting that the indolizidine ring is important for the anti-TNF-α activity.
Cytotoxicity of Derivatives 1–34
Among compounds that can promote Foxp3 expression, the IC50s of 9 and 13 were 42 and 23 nM compared with 4 (DCB-3503; 53 nM), suggesting that the cytotoxicity of 9 and 13 was much greater than DCB-3503. The IC50 of derivative 19 was 317 nM, which suggested a lower cytotoxicity than 4 (DCB-3503; 53 nM). The IC50 of PBTs 31 and 32 was about 500 μM, whereas most cells died when they were cultured with 4 (DCB-3503) at 1 μM, which suggested that 31 and 32 had greater capacity for promoting Tregs differentiation.
For compounds 1, 2, 4, 9, 12, 18, 19, and 21 that can suppress TNF-α production, the IC50 of compounds 9 and 12 were 42 and 33 nM, which indicated greater cytotoxicity than that of DCB-3503. The IC50 of compounds 1, 2, and 21 was 261, 274, and 228 nM, respectively, and their cytotoxicity was similar. The IC50 of 18 and 19 was 963 and 317 nM respectively, whereas that of 4 (DCB-3503) was 53 nM (Table 3). Thus, derivatives 18 and 19 had greater potential as TNF-α inhibitors. Inhibitory effects toward TNF-α of these derivatives were inconsistent with their cytotoxicity, suggesting that the inhibitory effects were not due to their cytotoxicity. Moreover, the cytotoxicity of analogues 1–23 was greater than those of PBTs 24–34, suggesting that the structure of PBTs could reduce the toxicity of DCB-3503.
Table 3. Comparison of Cytotoxicity Properties of Derivatives 1–34a.
| compd | IC50 (nM) | compd | IC50 (nM) | compd | IC50 (uM) |
|---|---|---|---|---|---|
| 1 | 261 ± 8 | 13 | 23 ± 7 | 24 | 418 ± 32 |
| 2 | 274 ± 32 | 14 | 93 ± 39 | 25 | 422 ± 42 |
| 3 | 823 ± 31 | 15 | 14 ± 9 | 26 | 357 ± 65 |
| 4 | 53 ± 39 | 16 | 13 ± 7 | 27 | 320 ± 45 |
| 5 | 1057 ± 44 | 17 | 167 ± 49 | 28 | 132 ± 26 |
| 6 | 242 ± 16 | 18 | 963 ± 32 | 29 | 116 ± 39 |
| 7 | 1164 ± 35 | 19 | 317 ± 25 | 30 | 305 ± 34 |
| 8 | 105 ± 41 | 20 | 651 ± 21 | 31 | 513 ± 33 |
| 9 | 42 ± 14 | 21 | 228 ± 19 | 32 | 515 ± 22 |
| 10 | 53 ± 14 | 22 | 175 ± 23 | 33 | 423 ± 24 |
| 11 | 432 ± 45 | 23 | 160 ± 15 | 34 | 469 ± 22 |
| 12 | 33 ± 4 |
Values are the mean ± SD of at least three independent experiments carried out in duplicate.
Biological activity and cytotoxicity of 31 and 18
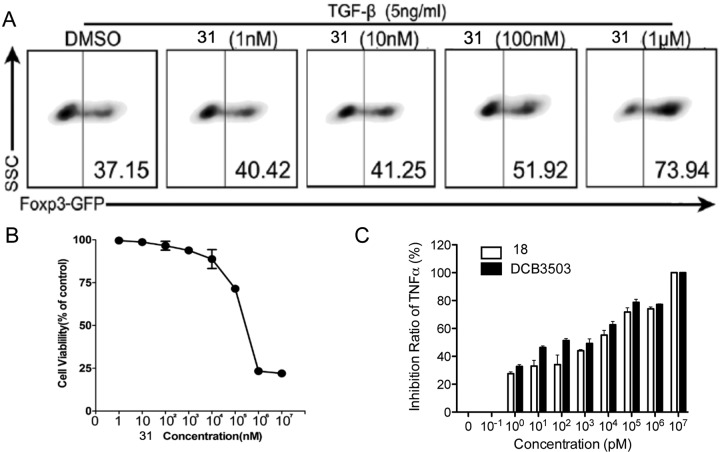
On the basis of the preliminary screening data, we have synthesized the PBT 31 and salt derivative of (±)-tylophorine 18 to further investigate their activity. The promotion ratio of analogue 31 on foxp3 expression has increased along with the increased concentration and reached 100% at 1 μM (Figure 2A). However, the highest promoting ratio of 4 (DCB-3503) on Foxp3 expression was approximately 40% at 100 nM, which was probably due to its cytotoxicity at higher concentrations. The IC50 of 31 was about 500 μM (Figure 2B) compared with 53 nM of 4 (DCB-3503), suggesting that the cytotoxicity of 31 was much lower than that of DCB-3503. Inhibition curves of 18 and 4 (DCB-3503) were similar (Figure 2C), but the cell survival of 18 was better than that of DCB-3503 at the same concentration (IC50 of 18 = 963 nM). Moreover, 18 could be dissolved in water but DCB-3503 was dissolved in dimethyl sulfoxide, suggesting that the salt analogue of DCB-3503 could improve its efficacy by enhancing its water solubility.
Figure 2.
Compound 31 has significantly promoted Foxp3 expression and showed slight cytotoxicity. Compound 18 exhibit similar inhibitory activity to that of DCB-3503. (A) Naive CD4+ T cells were sorted from B6 Foxp3-GFP mice and cultured with different concentrations of 31 in the presence of TGF-β (5 ng/mL) for 3 days. Cells were harvested, and the percentage of Foxp3 marked by GFP was analyzed by flow cytometry. One typical staining of three independent experiments was shown. (B) The 50% cytotoxic concentration (CC50) of compound 31. Raw 264.7 cells were cultured with different concentrations of compound 31 for 48 h. Vehicle-treated cells served as control. Values were representative of three experiments. (C) Splenocytes from BALB/c mice were mixed with different doses of compound 18 for 2 h, and vehicle and compound 4 (DCB-3503) served as control, followed by culturing with 50 ng/mL LPS for 24 h. TNF-α levels in supernatants were measured by ELISA. Data shown are combined from three experiments.
The anti-inflammatory mechanism of 31 and 18 demonstrated by our previous studies indicated that 31 may enhance Foxp3 expression through inhibition of the AKT/mTOR pathway and enhancement of demethylation of the promoter region by inhibition of the ERK pathway and DNMT1 expression.27 Compound 18 acts on the stability of TNF-α mRNA by decreasing phospho-p38, and 18 reduced differentiation of Th17 cells by attenuating interleukin-6 production28 (Figure S2, Supporting Information). Further studies are required to explore other anti-inflammatory mechanisms and direct targets of these tylophorine derivatives.
This is the first report to use Foxp3-GFP mice and macrophage cells to evaluate the anti-inflammatory activity of DCB-3503 derivatives. Among compounds that can promote Foxp3 expression (4, 9, 13, 19, 31, and 32), the cytotoxicity of 9 and 13 should be attenuated. Compounds 4 and 19 have better activity and cell viability. Compounds 31 and 32 show the highest activity and cell survival, suggesting that the structure of PBTs is important for the promotion of Foxp3 expression. Among compounds that can suppress TNF-α (1, 2, 4, 9, 12, 18, 19, and 21), the inhibitory effect of 9 and 12 may have been due to their cytotoxicity, whereas compounds 18 and 19 showed significant activity without cytotoxicity, suggesting that the salt derivatives of tylophorine are more effective for the inhibition of TNF-α. Interestingly, 4, 9, and 19 displayed both effects on promotion of Foxp3 and suppression of TNF-α expression, indicating their synergistic therapeutic use against inflammatory diseases.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 31300743) and the Natural Science Foundation of Chongqing city (cstc2013jcyjA1587).
Glossary
Abbreviations
- TNF-α
tumor necrosis factor-alpha
- LPS
lipopolysaccharide
- Foxp3
fork-head box P3
- DSS
dextran sulfate sodium
- TCR
T-cell receptor
- TGF-β
transforming growth factor-beta
- iTregs
inducible T regulatory cells
- nTregs
natural T regulatory cells
- IL
interleukin
- ELISA
enzyme-linked immuno-sorbent assay
- GFP
green fluorescent protein
Supporting Information Available
Experimental and synthetic methods and spectral characterization of compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
The manuscript was written through the contributions of all listed authors. All authors have approved the final version of this manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Donaldson G. R.; Atkinson M. R.; Murray A. W. Inhibition of protein synthesis in Ehrlich ascites-tumour cells by the phenanthrene alkaloids tylophorine, tylocrebrine and cryptopleurine. Biochem. Biophys. Res. Commun. 1968, 311104–109. [DOI] [PubMed] [Google Scholar]
- Rao K. N.; Venkatachalam S. R. Inhibition of dihydrofolate reductase and cell growth activity by the phenanthroindolizidine alkaloids pergularinine and tylophorinidine: the in vitro cytotoxicity of these plant alkaloids and their potential as antimicrobial and anticancer agents. Toxicol. In Vitro 2000, 14153–59. [DOI] [PubMed] [Google Scholar]
- Komatsu H.; Watanabe M.; Ohyama M.; Enya T.; Koyama K.; Kanazawa T.; Kawahara N.; Sugimura T.; Wakabayashi K. Phenanthroindolizidine alkaloids as cytotoxic substances in a Danaid butterfly, Ideopsis similis, against human cancer cells. J. Med. Chem. 2001, 44111833–1836. [DOI] [PubMed] [Google Scholar]
- Staerk D.; Lykkeberg A. K.; Christensen J.; Budnik B. A.; Abe F.; Jaroszewski J. W. In vitro cytotoxic activity of phenanthroindolizidine alkaloids from Cynanchum vincetoxicum and Tylophora tanakae against drug-sensitive and multidrug-resistant cancer cells. J. Nat. Prod. 2002, 6591299–1302. [DOI] [PubMed] [Google Scholar]
- Gao W.; Lam W.; Zhong S.; Kaczmarek C.; Baker D. C.; Cheng Y. C. Novel mode of action of tylophorine analogs as antitumor compounds. Cancer Res. 2004, 642678–688. [DOI] [PubMed] [Google Scholar]
- Choi J. Y.; Gao W.; Odegard J.; Shiah H. S.; Kashgarian M.; McNiff J. M.; Baker D. C.; Cheng Y. C.; Craft J. Abrogation of skin disease in LUPUS-prone MRL/FASlpr mice by means of a novel tylophorine analog. Arthritis Rheum. 2006, 54103277–3283. [DOI] [PubMed] [Google Scholar]
- Yang C. W.; Chen W. L.; Wu P. L.; Tseng H. Y.; Lee S. J. Anti-inflammatory mechanisms of phenanthroindolizidine alkaloids. Mol. Pharmacol. 2006, 693749–758. [DOI] [PubMed] [Google Scholar]
- You X.; Pan M.; Gao W.; Shiah H. S.; Tao J.; Zhang D.; Koumpouras F.; Wang S.; Zhao H.; Madri J. A.; Baker D.; Cheng Y. C.; Yin Z. Effects of a novel tylophorine analog on collagen-induced arthritis through inhibition of the innate immune response. Arthritis Rheum. 2006, 543877–886. [DOI] [PubMed] [Google Scholar]
- Shiah H. S.; Gao W.; Baker D. C.; Cheng Y. C. Inhibition of cell growth and nuclear factor-kappaB activity in pancreatic cancer cell lines by a tylophorine analogue, DCB-3503. Mol. Cancer Ther 2006, 5102484–2493. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Gao W.; Svitkin Y. V.; Chen A. P.; Cheng Y. C. DCB-3503, a tylophorine analog, inhibits protein synthesis through a novel mechanism. PLoS One 2010, 57e11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005, 64345–352. [DOI] [PubMed] [Google Scholar]
- Hori S.; Nomura T.; Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003, 29956091057–1061. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S.; Yamaguchi T.; Nomura T.; Ono M. Regulatory T cells and immune tolerance. Cell 2008, 1335775–787. [DOI] [PubMed] [Google Scholar]
- Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat. Rev. Immunol. 2002, 25364–371. [DOI] [PubMed] [Google Scholar]
- Plevy S. E.; Landers C. J.; Prehn J.; Carramanzana N. M.; Deem R. L.; Shealy D.; Targan S. R. A role for TNF-alpha and mucosal T helper-1 cytokines in the pathogenesis of Crohn’s disease. J. Immunol. 1997, 159126276–6282. [PubMed] [Google Scholar]
- Tracey K. J.; Fong Y.; Hesse D. G.; Manogue K. R.; Lee A. T.; Kuo G. C.; Lowry S. F.; Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature 1987, 3306149662–664. [DOI] [PubMed] [Google Scholar]
- Wooley P. H.; Dutcher J.; Widmer M. B.; Gillis S. Influence of a recombinant human soluble tumor necrosis factor receptor FC fusion protein on type II collagen-induced arthritis in mice. J. Immunol. 1993, 151116602–6607. [PubMed] [Google Scholar]
- Geiler J.; Buch M.; McDermott M. F. Anti-TNF treatment in rheumatoid arthritis. Curr. Pharm. Des. 2011, 17293141–3154. [DOI] [PubMed] [Google Scholar]
- Sandborn W. J.; Hanauer S. B. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflammatory Bowel Dis. 1999, 52119–133. [DOI] [PubMed] [Google Scholar]
- Rutgeerts P.; Sandborn W. J.; Feagan B. G.; Reinisch W.; Olson A.; Johanns J.; Travers S.; Rachmilewitz D.; Hanauer S. B.; Lichtenstein G. R.; de Villiers W. J.; Present D.; Sands B. E.; Colombel J. F. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353232462–2476. [DOI] [PubMed] [Google Scholar]
- Taxonera C.; Estelles J.; Fernandez-Blanco I.; Merino O.; Marin-Jimenez I.; Barreiro-de Acosta M.; Saro C.; Garcia-Sanchez V.; Gento E.; Bastida G.; Gisbert J. P.; Vera I.; Martinez-Montiel P.; Garcia-Moran S.; Sanchez M. C.; Mendoza J. L. Adalimumab induction and maintenance therapy for patients with ulcerative colitis previously treated with infliximab. Aliment. Pharmacol. Ther. 2011, 333340–348. [DOI] [PubMed] [Google Scholar]
- Su C. R.; Damu A. G.; Chiang P. C.; Bastow K. F.; Morris-Natschke S. L.; Lee K. H.; Wu T. S. Total synthesis of phenanthroindolizidine alkaloids (±)-antofine, (±)-deoxypergularinine, and their dehydro congeners and evaluation of their cytotoxic activity. Bioorg. Med. Chem. 2008, 16116233–6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.; Su B.; Wang Z.; Wu M.; Li Z.; Hu Y.; Fan Z.; Mi N.; Wang Q. Synthesis and antiviral activities of phenanthroindolizidine alkaloids and their derivatives. J. Agric. Food Chem. 2010, 5852703–2709. [DOI] [PubMed] [Google Scholar]
- Wang Z.; Wu M.; Wang Y.; Li Z.; Wang L.; Han G.; Chen F.; Liu Y.; Wang K.; Zhang A.; Meng L.; Wang Q. Synthesis and SAR studies of phenanthroindolizidine and phenanthroquinolizidine alkaloids as potent anti-tumor agents. Eur. J. Med. Chem. 2012, 51, 250–258. [DOI] [PubMed] [Google Scholar]
- Wang K.; Hu Y.; Liu Y.; Mi N.; Fan Z.; Wang Q. Design, synthesis, and antiviral evaluation of phenanthrene-based tylophorine derivatives as potential antiviral agents. J. Agric. Food Chem. 2010, 58, 12337–12342. [DOI] [PubMed] [Google Scholar]
- Fontenot J. D.; Rasmussen J. P.; Williams L. M.; Dooley J. L.; Farr A. G.; Rudensky A. Y. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 2005, 223329–341. [DOI] [PubMed] [Google Scholar]
- Meng X.; Zhang Y.; Jia Z.; Huo X.; He X.; Tian G.; Wu M.; Wang Z.; Zhou X.; Xiong S.; Gao X.; Wu Z.; Han J.; Zhao L.; Wang P.; Hong Z.; Wang Q.; Yin Z. A novel tylophorine analog W-8 up-regulates forkhead boxP3 expression and ameliorates murine colitis. J. Leukocyte Biol. 2013, 93183–93. [DOI] [PubMed] [Google Scholar]
- Wen T.; Li Y.; Wu M.; Sun X.; Bao X.; Lin Y.; Hao J.; Han L.; Cao G.; Wang Z.; Liu Y.; Wu Z.; Hong Z.; Wang P.; Zhao L.; Li Z.; Wang Q.; Yin Z. Therapeutic effects of a novel tylophorine analog, NK-007, on collagen-induced arthritis through suppressing tumor necrosis factor alpha production and Th17 cell differentiation. Arthritis Rheum. 2012, 6492896–2906. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.