Abstract
Adaptation of reaching movements to a novel dynamic environment is associated with changes in neuronal activity in the primary motor cortex (M1), suggesting that M1 neurons are part of the internal model. Here, we investigated whether such changes in neuronal activity, resulting from motor adaptation, were also accompanied by changes in human corticospinal excitability, which reflects M1 activity at a macroscopic level. Participants moved a cursor on a display using the right wrist joint from the starting position toward one of eight equally spaced peripheral targets. Motor-evoked potentials (MEPs) were elicited from the wrist muscles by transcranial magnetic stimulation delivered over the left M1 before and after adaptation to a clockwise velocity-dependent force field. We found that the MEP elicited even during the preparatory period exhibited a directional tuning property, and that the preferred direction shifted clockwise after adaptation to the force field. In a subsequent experiment, participants simultaneously adapted an identical wrist movement to two opposing force fields, each of which was associated with unimanual or bimanual contexts, and the MEP during the preparatory period was flexibly modulated, depending on the context. In contrast, such modulation of the MEP was not observed when participants tried to adapt to two opposing force fields that were each associated with a target color. These results suggest that the internal model formed in the M1 is retrieved flexibly even during the preparatory period, and that the MEP could be a very useful probe for evaluating the formation and retrieval of motor memory.
Introduction
Performing accurate movements under a wide variety of environments is crucial for daily life. The brain accomplishes this by flexible formation and retrieval of internal models of the dynamics of the body and its environment (Shadmehr et al., 2010). To examine the directional tuning pattern of muscle activity that was characterized by the preferred direction (PD), a previous human study (Thoroughman and Shadmehr, 1999) used electromyography (EMG) while participants performed reaching movements. The authors demonstrated that the adaptation of reaching movements to a velocity-dependent rotational force field was accompanied by the rotation of the PD in the same direction. Considering the direct linkage of primary motor cortex (M1) neurons with the motor neurons of muscles, the PDs of M1 neurons (Georgopoulos et al., 1982) should also rotate in the direction of the force field. Previous studies using nonhuman primates have demonstrated that the adaptation of a reaching movement to a rotational force field changed the directional tuning properties of M1 neurons during the execution period (Gandolfo et al., 2000; Li et al., 2001; Arce et al., 2010a,b) as well as the preparatory period (Mandelblat-Cerf et al., 2011). These results suggest that M1 neurons are part of the internal model.
However, not all the recorded neurons in the M1 exhibited the same PD rotation; a considerable number exhibited the opposite rotation. Furthermore, other properties, such as the firing frequency and tuning width, also changed in a complex manner (Li et al., 2001; Arce et al., 2010b). Therefore, we questioned whether modification of the activity in each M1 neuron associated with force-field adaptation would affect more macroscopic neuronal activity in the M1. Transcranial magnetic stimulation (TMS) might be a useful tool to investigate this issue. Orban de Xivry et al. (2013) reported that the motor-evoked potentials (MEPs) evoked at the onset of reaching movements toward two targets changed after adaptation to a force field. In the present study, we investigated the MEP changes associated with motor adaptation more closely. We examined how they were tuned with the direction of a wrist movement, how this directional tuning property was modified after adaptation to a force field, and whether these adaptation-dependent changes could be observed during the preparatory period (Experiment 1). We found that even during the preparatory period, the MEP exhibited a directional tuning pattern and that, after adaptation, the PD rotated in the same direction as the force field.
Recent studies have reported that distinct internal models can be simultaneously formed and retrieved, depending on behavioral contexts (Nozaki et al., 2006; Howard et al., 2008, 2010; Nozaki and Scott, 2009; Yokoi et al., 2011, 2014; Hirashima and Nozaki, 2012). Based on the results of Experiment 1, the MEP was expected to be modulated, depending on the behavioral context. We examined this prediction by having participants adapt to two conflicting force fields: a clockwise (CW) and counter-clockwise (CCW) force field applied with unimanual and bimanual movement, respectively (Experiment 2). We also examined the modulation of MEPs when participants could not adapt to two conflicting force fields that varied depending on the target color (Experiment 3).
Materials and Methods
Participants
Eight healthy volunteers [all men, aged 27.6 ± 2.8 years (mean ± SD)] participated in Experiment 1. Twelve healthy participants (9 men and 3 women, aged 22.3 ± 1.9 years) participated in Experiment 2. Thirteen healthy participants (10 men and 3 women, aged 23.2 ± 1.3 years) participated in Experiment 3. All participants were right-handed, as assessed by Oldfield's Edinburgh Handedness Inventory (Oldfield, 1971). All participants gave informed consent before the experiments, and all experiments were approved by the ethics committee of the Graduate School of Education, The University of Tokyo.
Apparatus and general experimental procedure
The participants were comfortably seated in a chair facing a computer display placed vertically in front of them (Fig. 1A). The forearm of the participant was placed horizontally on a wooden board and maintained in a semisupinated (palm vertical) position using fabricated plates. The handle of a robotic manipulandum (Phantom Premium 1.5HF, Geomagic) was tightly fixed to the participant's hand by tape (the handle was placed between the third and fourth fingers). A partition was placed above the participants' forearm to prevent participants from seeing their forearms and hands. Participants were required to manipulate the robotic device using wrist movement to move a visual cursor on the computer display. The position of the visual cursor corresponded to the wrist movement. In the case of the right wrist, the cursor's up-and-down and right–left motions corresponded to radial–ulnar and extension–flexion movements around the wrist joint, respectively (Fig. 1B).
Figure 1.
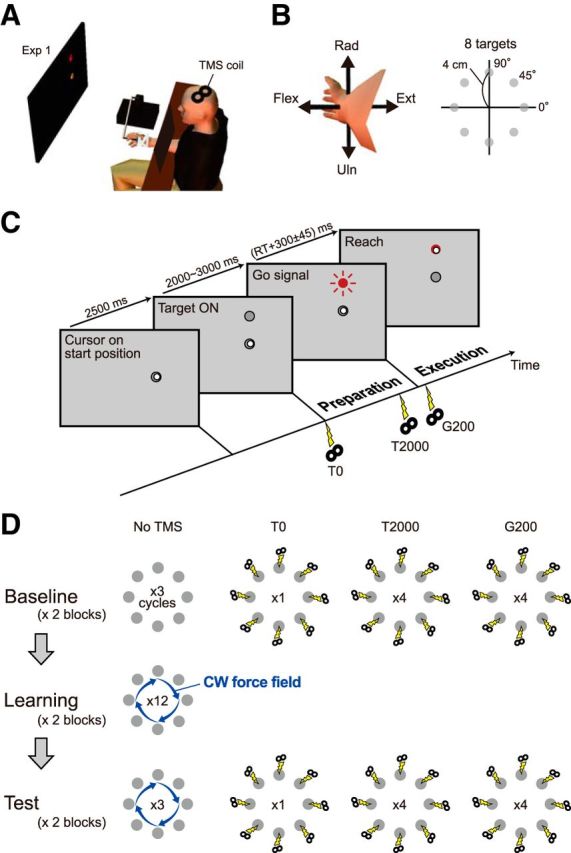
Experimental setup and procedure for Experiment 1. A, Participants manipulated the cursor on the display by moving the handle of the manipulandum with their wrists. The coil of the TMS was placed on the left M1. B, The forearm was in a semisupinated position. A target appeared in one of eight equally spaced positions. The radial–ulnar and extension–flexion movements around the wrist joint corresponded to up–down and right–left motions of the cursor, respectively. C, Trial flow for the delayed reaching task in Experiment 1. TMS was applied at target appearance (T0), 2 s after target appearance (T2000), or 0.2 s after the Go signal (G200). D, Task procedure for Experiment 1. During the learning and test sessions, a velocity-dependent CW curl force field was applied to the handle.
We used a delayed reaching task paradigm: a gray target appeared on the display and, after a variable waiting time (2–3 s), the target color turned red, signaling the participant to initiate the wrist movement to move the cursor toward the target (Fig. 1C). After the trial, the robotic handle was automatically returned to the starting position by a spring-like force implemented by the robotic device. The required movement time was 300 ± 45 ms, and a message of caution (“Slow” or “Fast”) was presented if the participant's movement was out of this range. The movement of the tip of the robotic handle from the starting position to the target position was set to 4 cm. The position of the handle was recorded at a 500 Hz sampling frequency.
EMG
Surface EMG signals were recorded from the wrist muscles, including the flexor carpi radialis (FCR), flexor carpi ulnaris (FCU), extensor carpi radialis (ECR), and extensor carpi ulnaris (ECU) muscles. The EMG signals were amplified and bandpass filtered between 20 and 450 Hz (Bagnoli 8, Delsys) and were digitized at 2 kHz using a data logger system (WE7000, Yokogawa). At the end of the experiments, the participants performed maximum voluntary contraction (MVC) to determine the maximal EMG level, which was used to normalize the EMG signal for each participant.
TMS
Single-pulse TMS was delivered on the left M1 with a Magstim 200 stimulator (Magstim) through a 70 mm figure-eight coil. The resting motor threshold (rMT) was determined as the minimum stimulus intensity that produced an MEP of ∼50 μV from one of the four muscles in 50% of the trials. The stimulation intensity was set at 120% above rMT. If the MEPs were not clearly visible for all four muscles, then 130% above rMT was applied, which was enough to elicit MEPs from all four muscles (the number of participants requiring 130% was two, one, and two, for Experiments 1, 2, and 3, respectively). The stimulus intensity was 53.6 ± 15.3% of the machine stimulus output in Experiment 1, 52.8 ± 8.2% in Experiment 2, and 53.0 ± 10.5% in Experiment 3. We used the Brainsight frameless stereotaxy system (Rogue Research), which allowed us to stimulate the same position in the left M1 with the same coil orientation throughout the experiments.
Experiment 1: MEP modulation with motor learning
The aim of Experiment 1 was to investigate how corticospinal excitability before movement onset was modulated by the direction of movement, and how the tuning property was modified after motor adaptation to a force field. Figure 1C shows the task flow when a target appeared at the “up” direction. A gray target appeared for 2.5 s in one of eight different directions (0–315° with 45° separation; 0° indicates wrist extension; Fig. 1B) after the cursor was placed at the start position, and then turned red after 2–3 s to indicate a “go” signal. Figure 1D shows the details of the experimental protocol. In the baseline session, participants performed the wrist movements toward a target without any force field applied. TMS was applied to the left M1 for some of the trials at the appearance of the visual target (T0), at 2000 ms after target appearance (T2000), or at 200 ms after the go signal (G200). The baseline session consisted of two blocks; each block consisted of 96 trials (eight directions × three trials without TMS, eight directions × one trial with TMS at T0, eight directions × four trials with TMS at T2000, and eight directions × four trials with TMS at G200). One cycle consisted of eight trials (i.e., reaching toward eight targets), the order of which was randomized in each cycle. In the learning session, participants were trained to perform the movements in the presence of a CW velocity-dependent force field; f = Bv, where f is a force vector (fx, fy)t, v is a vector of the handle's velocity in the frontal plane (vx, vy)t and B is a viscosity matrix [0 10; −10 0] N · s/m. The learning session consisted of 192 trials (eight directions × 12 cycles × two blocks) followed by a test session. The experimental protocol of the test session was the same as those of the baseline session (i.e., two blocks of 96 trials, etc.), except that the force field was present.
Data analysis of Experiment 1
Learning performance.
We measured motor performance in the trials without TMS, calculating the maximum lateral deviation of the handle's position from a line between the start point and target.
EMG signal processing.
To evaluate the MEP, we quantified its peak-to-peak amplitude (we also calculated the area of the MEP, but the results were similar). To evaluate muscle activity during the early part of the movement, we calculated the integrated EMG (iEMG) using the EMG signals from −100 ms before movement onset, and 0 ms at movement onset, which was full-wave rectified and then low-pass filtered (cutoff frequency, 50 Hz). The EMG signal was normalized to the MVC level for each muscle of each participant. In the present study, we focused on the MEP modulation before execution of the movement. To confirm the absence of any muscle activity when evoking MEP, background EMG activity was quantified as the mean rectified EMG activity during the 100 ms before TMS application. If a background EMG of >25 μV was observed (<1.25% of the EMG level for the MVC), then the data were discarded from the analysis. The percentage of discarded data was 6% in Experiment 1, 3.7% in Experiment 2, and 5.3% in Experiment 3.
Calculation of PD.
The iEMG and the amplitude of the MEP at T2000 and G200 were averaged for each movement direction of each participant, and then the averaged values across participants were calculated for each movement direction. The PD of each muscle was calculated as the direction of the resultant vector (i.e., PD vector), which was obtained as a vectorial summation of the radial vectors toward the eight directions.
Experiment 2: motor learning by unimanual and bimanual movements
As shown later in the Results section, in Experiment 1, we observed a rotation of the PD of the MEP in the CW direction after adaptation to the CW force field. Thus, it would be reasonable to assume that the PD would rotate CCW after adaptation to the CCW force field. On the basis of this idea, when the movement direction was up (i.e., 90°), the MEP amplitude in FCR should be larger after adaptation to the CW force than after adaptation to the CCW force (Fig. 2B, left). Conversely, the MEP amplitude of ECR should be larger after adaptation to the CCW force than after adaptation to the CW force (Fig. 2B, right). Experiment 2 was designed to examine our hypothesis that, when the participants simultaneously adapted to CW and CCW force fields in association with unimanual and bimanual behavioral contexts, respectively (Nozaki et al., 2006), the corticospinal excitability was modulated depending on the context even before movement execution.
Figure 2.
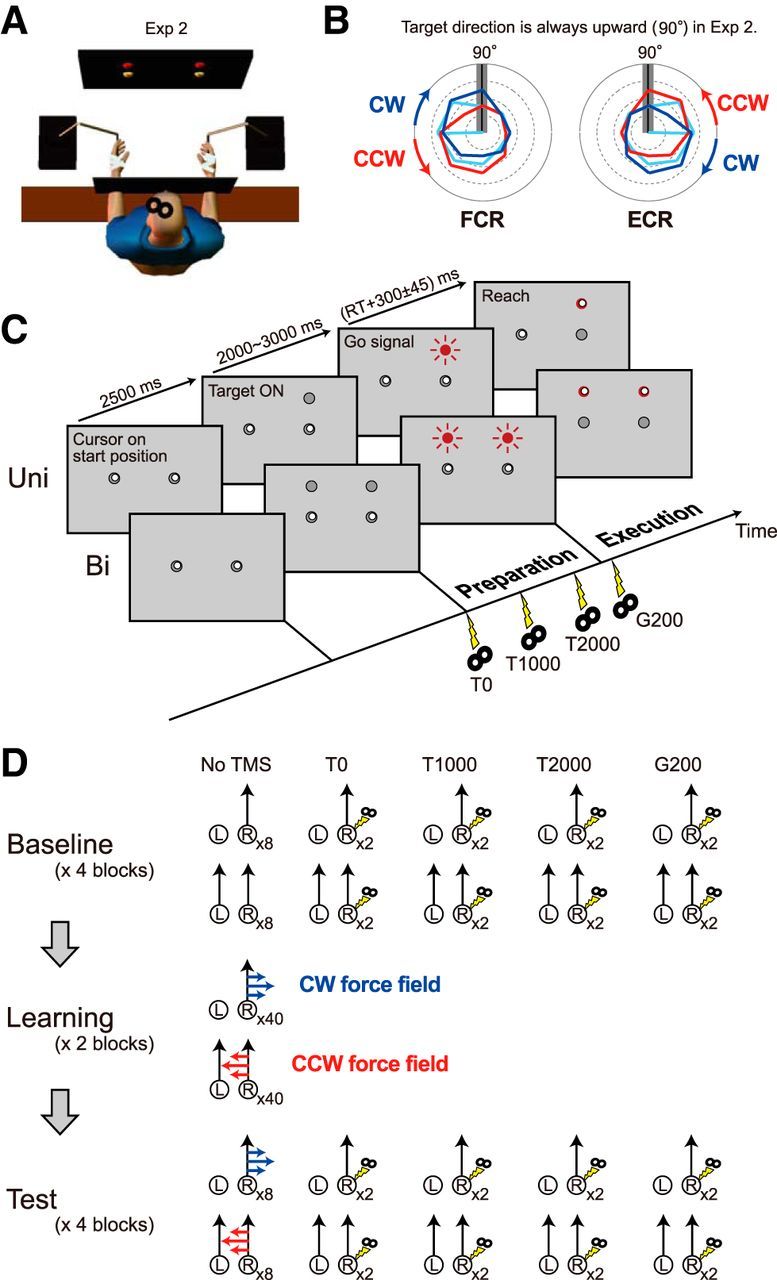
Experimental setup and procedure for Experiment 2. A, Participants alternated unimanual and bimanual movements. B, Based on the results of Experiment 1, tuning curves of MEP (cyan lines) in polar plot were predicted to rotate CW and CCW after motor adaptation to the CW force field (blue lines) and to the CCW force field (red lines), respectively. This possible rotation could increase or decrease the MEP for the FCR or ECR, respectively, for the upward (90°) direction (gray bar). C, Trial flow for Experiment 2. TMS was applied at target appearance (T0); 1 s (T1000) and 2 s (T2000) after target appearance; and 0.2 s after the go signal (G200). D, Task procedure for Experiment 2. One or two targets alternately appeared only at the upward (90°) position. During the learning and test sessions, CW and CCW force fields were imposed on unimanual and bimanual movement, respectively.
In Experiment 2, the target appeared only at the 90° position. The timing of target appearance was the same as in Experiment 1. Participants performed alternately either unimanual movement when a target appeared for the right hand or bimanual movement when targets appeared for both hands (Fig. 2A,C). In the learning session, we applied a velocity-dependent CW or CCW force field during a unimanual or bimanual movement, respectively.
TMS was applied either at target appearance (T0), at 1000 ms after target appearance (T1000), at 2000 ms after target appearance (T2000), or at 200 ms after the go signal (G200; Fig. 2C). The task procedure was similar to that in Experiment 1; it included a baseline session, a learning session, and a test session (Fig. 2D). The baseline session consisted of four blocks; each block consisted of 32 trials (eight trials of no stimulation, and two trials for each of T0, T1000, T2000, and G200 in each unimanual or bimanual movement). The learning session consisted of two blocks that consisted of 80 trials (40 trials for each context), and the second block included eight catch trials (i.e., null force field) in each context. The procedures for the test session were the same as those of the baseline session, except that the force field was present.
Experiment 3: motor learning by target color cues
As shown later in the Results section, we observed modulation of corticospinal excitability from the preparatory period in Experiments 1 and 2, and this macroscopic modification would contribute to the formation and retrieval of the appropriate internal model in novel environments. However, there is some debate about the link between the modulation of corticospinal excitability and formation of an internal model. Factors that are not directly related to the formation of an internal model, such as muscle cocontraction (Franklin et al., 2003) and feedback gains (Franklin et al., 2012), increase in the early phase of the learning period and may be responsible for the modulation of corticospinal excitability seen in Experiments 1 and 2. To investigate these issues, we conducted a further control experiment (Experiment 3), in which participants tried to adapt to CW and CCW force fields in association with target colors that were presented as contextual cues. According to previous studies (Gandolfo et al., 1996; Osu et al., 2004; Shadmehr et al., 2005), it is difficult to adapt identical movements to conflicting force fields simultaneously based on visual information. The experimental protocol in Experiment 3 was the same as those in Experiment 2, except that cyan and magenta target colors were used as contextual cues for CW and CCW fields, respectively.
Data analysis of Experiments 2 and 3
To evaluate the degree of motor learning, we calculated the maximum lateral deviation of movement trajectory during the catch trials. The MEP amplitude, iEMG, and background EMG activity were calculated in the same manner as in Experiment 1. The ECR and FCR are muscles that act as agonists for the movement toward the target at the 90° position (Hoffman and Strick, 1993). These two muscles should contribute to the adaptation to CW and CCW force fields, because MEP changes after force-field adaptation are observed for the muscles involved in the particular task (Orban de Xivry et al., 2013). Thus, we focused our analysis only on these two muscles (i.e., ECR and FCR) in Experiments 2 and 3.
Statistical analysis
In Experiment 1, a one-way repeated-measures ANOVA and Tukey's honest significant difference (HSD) test were used to evaluate the differences in the maximum lateral deviation of the trajectory among baseline (six cycles), early-learning (first six cycles in the learning session), late-learning (last six cycles in the learning session), and test (six cycles) sessions. The change in the amplitude of MEP at T0 from baseline to test session was evaluated using a paired t test.
A bootstrap technique (Thoroughman and Shadmehr, 1999) was used to estimate the confidence interval of the magnitude and angle of the PD vectors of MEP and iEMG. This confidence interval was then used to evaluate the following: whether the significant directional modulation was observed, whether the amplitude was significantly changed from T2000 to G200, and whether the angle was changed after the adaptation to the force field.
For each participant, we obtained the averaged MEP and iEMG for each movement direction. Consequently, eight sets (i.e., for eight participants) of MEPs and iEMG data vectors that consisted of eight elements (i.e., eight directions) were obtained. To test the presence of significant directional modulation, the MEP or iEMG data for each participant were shuffled with respect to movement direction. The PD vector was then calculated from this shuffled data. This process was repeated 10,000 times and the distribution of the amplitude of the PD vector was sorted by rank and used to compare the amplitude of the actual data to estimate the confidence interval of the amplitude. Then, we calculated the probability (i.e., p value) that the actual amplitude could be observed in the distribution.
The bootstrap technique was also used to test whether the PD (i.e., angle) changed significantly between baseline and the test session. We created a dataset by resampling eight vectors with replacements from the original eight vectors, and then the PD vector was calculated for this resampled dataset. By repeating this process 10,000 times, the distribution of angles of the PD vectors was obtained. The distribution of baseline PDs was then sorted by rank and compared with the PDs for the test session to determine the probability (i.e., p value) that the PD for the test could be observed in the distribution. Similarly, to test whether the amplitude was significantly changed from T2000 to G200, we obtained the distribution of the amplitude for bootstrap samples at T2000, and compared it with the actual amplitude at G200.
In Experiments 2 and 3, a two-way repeated-measures ANOVA with behavioral context (unimanual and bimanual movement in Experiment 2; target colors in Experiment 3) and session (baseline and test sessions) as factors was used to evaluate context-dependent and learning-dependent changes in the maximal lateral deviation of the trajectory for baseline and catch trials, and in the iEMG for each muscle. A three-way repeated-measures ANOVA with behavioral context, session, and timing of TMS (T0, T1000, T2000, and G200) as factors was used to evaluate context-dependent and learning-dependent changes in MEPs for each muscle. The threshold for all statistical analyses was set at 0.05.
Results
Experiment 1: modulation of corticospinal excitability with movement directions
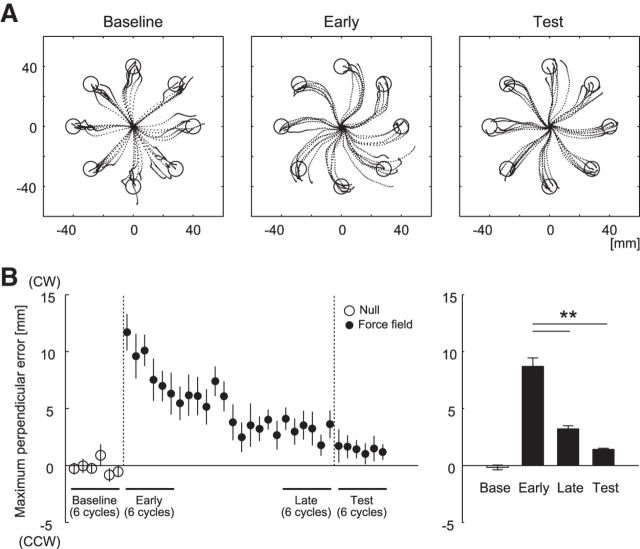
Figure 3 shows typical examples of movement trajectories (Fig. 3A) and the maximum lateral deviation averaged over one cycle (Fig. 3B, left). One-way repeated-measures ANOVA revealed the significant main effect of learning in the deviation (F(2,14) = 53.3, p < 0.0001). Tukey's HSD post hoc analyses revealed that the maximum lateral deviation of the last six cycles in the learning session, and of the six cycles in the test session, was significantly smaller than that of the early six cycles in the learning session (p's < 0.001; Fig. 3B, right). These results indicate that participants adapted to the force field during the learning session, and that adaptation was not destroyed in the test session during which the TMS was applied.
Figure 3.
Learning performance in Experiment 1. A, Movement trajectories of a typical participant (the data with TMS was excluded) during the baseline, early-learning (the first 6 cycles in the learning session), and test sessions. B, Cycle-dependent changes in the maximum lateral deviation of the handle from the target direction (left). The averaged values of six cycles for the baseline, early-learning, late-learning, and test sessions (right). A positive value indicates CW direction. The values are presented as mean ± SEM. **p < 0.001.
Figure 4A shows a typical example of MEP evoked from the FCR at T2000 for the eight movement directions. Since T2000 occurred much earlier than the movement onset, background EMG activity was negligible. Nevertheless, the MEP exhibited modulation with movement direction: it was maximal and minimal, for flexion and extension of the wrist, respectively (Fig. 4A, left). Significant directional modulations of the MEP were observed in the data averaged across participants for T2000 (p's < 0.05) in both baseline (cyan) and test (blue) sessions, except for the ECU in baseline and the FCU in test sessions (Fig. 4B). For the MEP at G200, although the G200 was ∼300 ms earlier than the movement onset (479 ± 5 ms), significant directional modulations of the MEP were observed in all cases (p's < 0.005; Fig. 4B). The iEMG of each muscle in both baseline and test sessions also exhibited significant directional modulation (p's < 0.001; Fig. 4B); for example, the PD of the FCR pointed in the radial-flexion direction. It should be noted that the PD of the MEP at T2000 and G200 appears to be similar to that of the iEMG (Fig. 4B).
Figure 4.
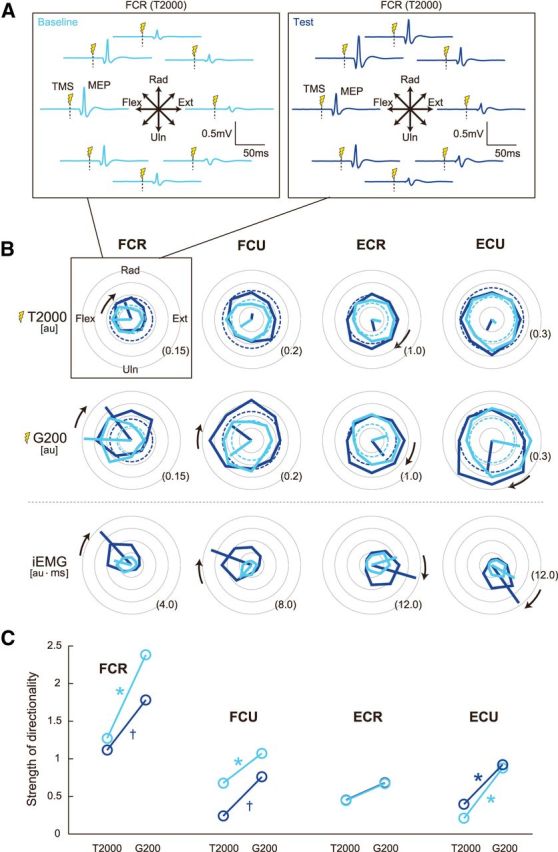
Directional tuning of muscle activity and corticospinal excitability. A, MEP traces, evoked at T2000, obtained from the FCR muscle of a typical participant. Up–down and right–left plot corresponds to the radial–ulnar and extension–flexion movements, respectively. Cyan lines are baseline and blue lines are test. TMS was delivered at the vertical dotted lines. B, Tuning curves shown in polar coordinates. The MEP amplitude at T2000 and G200, and iEMG for the baseline (cyan) and test (blue) sessions. The broken circles displayed in the panels of MEP amplitudes indicate the MEP obtained at T0. The MEP at T0 was assumed to be independent of movement direction. The thick lines are PD vectors. CW black arrows indicate that the PDs were significantly rotated after adaptation (p's < 0.05). The values written at the outer circles indicate the value normalized by the level of the MVC. C, The magnitude of the PD vector of MEP at T2000 and G200 for each muscle is plotted to evaluate the strength of the directional modulation. The magnitude of PD vector is normalized by the MEP amplitude averaged across eight movement directions. *p < 0.05 and †p < 0.10 from T2000 to G200.
In the analyses described above, MEP data that were accompanied by background EMGs greater than a threshold (25 μV) were discarded. However, it is still possible that small changes in background EMG and movement direction may influence the directional modulation of MEPs. To examine this issue, we calculated the correlation coefficients between background EMG and MEPs for eight movement directions. We did not observe any significant correlations for any muscles, during both baseline and test sessions, indicating that the directional modulation of MEPs was not caused by simple differences in background EMG.
The strength of directional modulation (i.e., the magnitude of the PD vector) from T2000 to G200 significantly increased in the FCR, FCU, and ECU (p's < 0.05) during baseline, and in the ECU (p = 0.02) during the test sessions. Increases in the FCR and FCU approached significance during the test sessions (p's < 0.1; Fig. 4C), indicating that clearer task-relevant activity was initiated in the M1 as the onset of movement approached.
Experiment 1: modulation of corticospinal excitability with motor adaptation
The amplitudes of MEP at T0 were not significantly changed from baseline to the test sessions for all four muscles, although increasing tendencies were observed during the test sessions (Fig. 4B, broken circles). We investigated how the PDs of the iEMGs and MEPs rotated with motor adaptation. The bootstrap technique indicated that the PD of iEMG significantly rotated in the CW direction for the FCR, FCU, ECR, and ECU (p's < 0.001; Fig. 4B, CW black arrows indicate significant rotation). In addition to such learning-dependent rotation of the PD, the amplitude of the PD vector for the iEMG was significantly increased for the FCR (p < 0.005), FCU (p < 0.001), ECR (p < 0.001), and ECU (p < 0.001; Fig. 4B). As for the MEP, the PD at T2000 significantly rotated in the CW direction for the FCR (p = 0.03) and ECR (p < 0.001) from the baseline to the test sessions. We could not evaluate the changes in PD for ECU and FCU, because these muscles did not show significant directional modulation during the baseline (ECU) and test (FCU) periods. The PD at G200 also rotated significantly in the CW direction for the FCR (p < 0.01), FCU (p = 0.02), ECR (p < 0.01), and ECU (p < 0.001). Thus, after motor adaptation, the PD of the MEP rotated in a manner consistent with the rotation of the PD of the iEMG.
Experiment 2: corticospinal excitability modulation depending on behavioral context
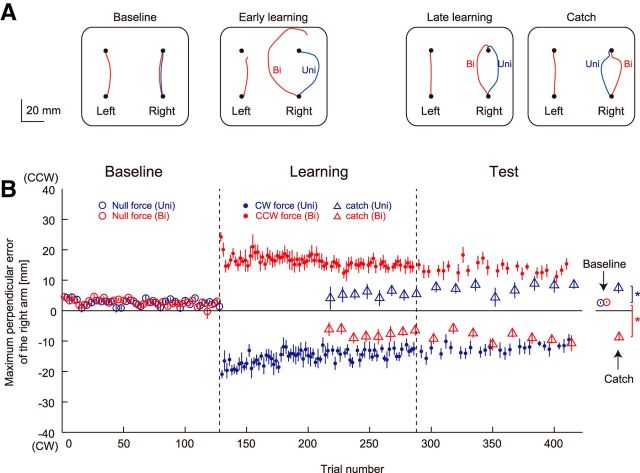
Figure 5A shows the movement trajectories for one participant for baseline, early-learning, late-learning, and catch trials. In the early-learning period (the first eight trials in the learning session), the trajectory deviated in the direction of the force field for both unimanual and bimanual movements. In the late-learning period (the last eight trials in the test session), however, the maximum lateral deviations decreased. Furthermore, the maximum lateral deviations for the catch trial in the test session were in the opposite direction of the force fields: 7.46 ± 6.28 mm for unimanual movement (Fig. 5B, blue triangle) and −8.72 ± 4.18 mm for bimanual movement (Fig. 5B, red triangle). A two-way repeated-measures ANOVA revealed a significant interaction between behavioral context and session in the maximum lateral deviations for catch trials (F(1,11) = 36.73, p < 0.001). There were also simple main effects between baseline and test sessions for unimanual (F(1,11) = 6.86, p = 0.024) and bimanual movement (F(1,11) = 104.12, p < 0.001). These results indicate that participants actually adapted to two conflicting force fields simultaneously.
Figure 5.
Learning performance in Experiment 2. A, Movement trajectories of a participant during the baseline, early-learning (first 8 trials in the learning session), and late-learning (last 8 trials in the learning session) sessions, and during catch trials (no force field) in the test session. Unimanual and bimanual movements are represented by blue and red lines, respectively. B, Changes in the maximum lateral deviation of the right arm across participants during unimanual (blue) and bimanual movement (red). Open and filled circles represent the data for null-force and force-field trials, respectively. Triangles represent the aftereffect observed in the catch trials. A positive value indicates CCW direction. The values are presented as mean ± SEM. *p < 0.05.
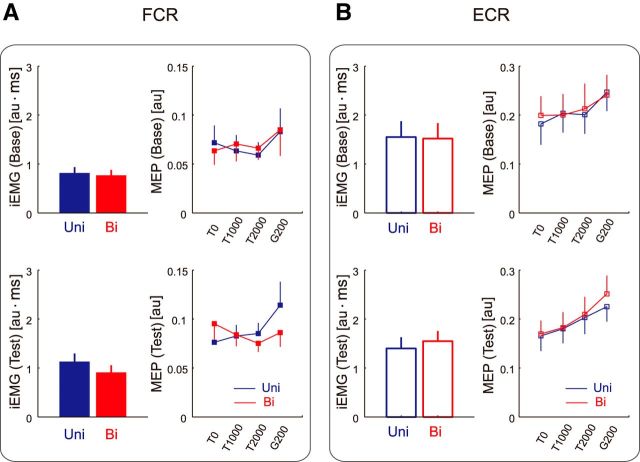
Figure 6A,B shows the iEMG and MEP evoked at each timing (i.e., T0, T1000, T2000, and G200) for the FCR and ECR. Two-way repeated-measures ANOVAs revealed significant interactions between behavioral context and session in the iEMG for the FCR (F(1,11) = 5.87, p = 0.034) and ECR (F(1,11) = 7.71, p = 0.018). A significant simple main effect between baseline and test sessions was also observed for the FCR (F(1,11) = 10.49, p = 0.008 for unimanual movement; F(1,11) = 5.14, p = 0.045 for bimanual movement). Thus, the iEMG for both FCR and ECR increased with training, but the degree of increase was dependent on the behavioral context (i.e., the iEMG for FCR and ECR increased specifically for unimanual and bimanual movement, respectively).
Figure 6.
Context-dependent changes in iEMG and MEP for the FCR and ECR in Experiment 2. A, B, iEMG and MEP at T0, T1000, T2000, and G200 for the FCR (A) and ECR (B). Blue and red lines indicate that the data are for unimanual and bimanual movements, respectively. The values are presented as mean ± SEM (the EMG data were normalized to the level of the MVC).
As with the MEPs, a three-way repeated-measures ANOVA revealed no significant context-dependent effects for the ECR, but significant interactions between behavioral context, session, and TMS timing for the FCR (F(3,33) = 3.39, p = 0.029). We also observed a significant simple interaction between behavioral context and timing of TMS during the test sessions (F(3,33) = 4.86, p = 0.007). These results indicate that FCR MEPs were modulated differently with the behavioral context through learning to the two conflicting force fields. Notably, we also found a significant simple interaction between behavioral context and session for the FCR (F(1,11) = 5.41, p = 0.040 at T0; F(1,11) = 6.92, p = 0.023 at T2000). Thus, as we predicted, training induced a greater increase in the MEP of FCR at T2000 during unimanual movement, but the increase in the MEP for FCR at T0 was greater during bimanual movement. The increase in the MEP at T0 was counterintuitive, but this was possible considering that participants alternated between unimanual and bimanual movements in Experiment 2; we speculated that the unimanual movement training influenced corticospinal excitability before the next bimanual movement was initiated.
As in Experiment 1, we excluded the MEP data when the background EMG was greater than a threshold. However, it was still possible that differences in small background EMG influenced the results. To examine this issue, we applied the same three-way repeated-measures ANOVA to the background EMG data. We did not observe any context-dependent effects for the background EMG, indicating that there was no significant impact of background EMG on the context-dependent effects observed in the MEPs.
Experiment 3: modulation of corticospinal excitability depending on color context
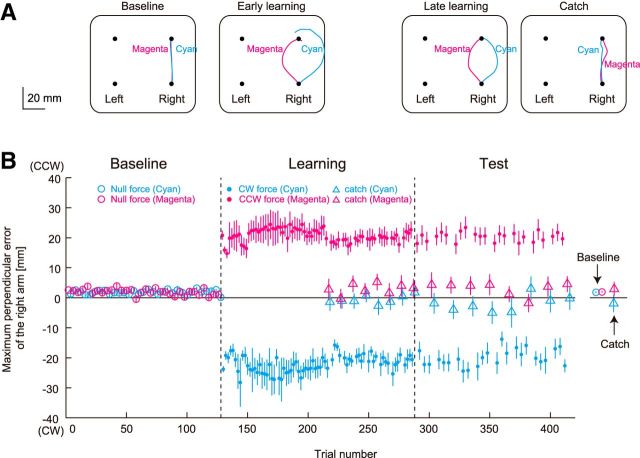
Figure 7A shows the movement trajectories of one participant in the same format as Figure 5A. In the early-learning and late-learning periods, trajectories deviated in the direction of the force field for both cyan and magenta targets, and the maximum lateral deviations for the catch trial in the test session did not oppose the direction of the force fields: −1.91 ± 10.3 mm for the cyan target (Fig. 7B, cyan triangle) and 2.92 ± 8.47 mm for the magenta target (Fig. 7B, magenta triangle). A two-way repeated-measures ANOVA for the maximum lateral deviations for catch trials revealed no significant context-dependent or learning-dependent effects. These results indicate that participants were unable to adapt to the two conflicting force fields.
Figure 7.
Learning performance in Experiment 3. A, Movement trajectories of a participant during the baseline, early-learning (first 8 trials in the learning session), and late-learning (last 8 trials in the test session) sessions, and during catch trials in the test session. Cyan (CW) and magenta (CCW) target color cues are represented by cyan and magenta lines, respectively. B, Changes in the maximum lateral deviation of the right arm across participants for unimanual reaching movements toward cyan and magenta targets. Open and filled circles represent the data for null-force and force-field trials, respectively. Triangles represent the aftereffect observed in the catch trials. A positive value indicates CCW direction. The values are presented as mean ± SEM.
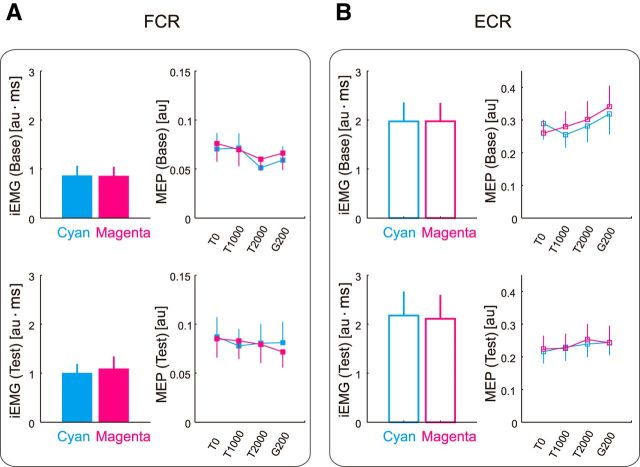
Figure 8A,B shows the iEMG and MEP evoked at each timing (i.e., T0, T1000, T2000, and G200) for the FCR and ECR in Experiment 3. Two-way repeated-measures ANOVAs revealed no significant context-dependent effects for iEMG in either muscle. However, we observed a significant main effect for iEMG of FCR between baseline and test sessions (F(1,12) = 6.52, p = 0.025). A three-way repeated-measures ANOVA revealed that there was no significant context-dependent effect on MEPs in either muscles (thus, the increase in iEMG for FCR from baseline to test session was not reflected in the MEP). Therefore, in accordance with the behavioral data (Fig. 7), the iEMG and MEP results indicate that participants were unable to vary their motor commands following the contextual cues provided by the target color.
Figure 8.
Context-dependent changes in iEMG and MEP for the FCR and ECR in Experiment 3. A, B, iEMG and MEP at T0, T1000, T2000, and G200 for the FCR (A) and ECR (B). Cyan and magenta lines indicate that the data are for cyan and magenta target, respectively. The values are presented as mean ± SEM (the EMG data were normalized to the level of the MVC).
Discussion
We found that the MEP amplitude was tuned with the wrist movement direction even during the preparatory period and that the directional tuning curve rotated after adapting to a rotational velocity-dependent force field (Experiment 1). Experiment 2 showed that participants could adapt an identical wrist movement to two conflicting force fields when each of them was associated with either a unimanual or bimanual movement. Furthermore, the MEP of agonist muscles during the preparatory period was also modulated depending on the behavioral context. In contrast, Experiment 3 demonstrated that MEP modulation was not observed when the participants could not adapt to two force fields associated with target colors as contextual cues. Together, the modulation of corticospinal excitability from the preparatory period onwards may represent a macroscopic modification of M1 activity, which contributes to the formation of a novel internal model and the retrieval of an appropriate internal model to make voluntary movements in novel environments.
Modulation of corticospinal excitability with direction of movement
In accordance with previous studies (Hoffman and Strick, 1999; Thoroughman and Shadmehr, 1999; de Rugy and Carroll, 2010), the iEMG was tuned with the direction of movement (Fig. 4B). In particular, the results were in agreement with those by Hoffman and Strick (1999) who examined muscle activities during wrist movements: the FCR, FCU, ECR, and ECU are most active for radial-flexion, flexion, extension, and ulnar-extension movement, respectively.
First, we examined whether such a directional tuning property could be observed in MEPs. It has been shown that MEPs are more easily evoked in muscles that are more distal, and that is why the finger muscles are often used in TMS studies (Devanne et al., 2006). However, the anatomical configuration of finger muscles is complex, and finger muscles are not suitable for investigating directional tuning. Based on these considerations, we decided to examine the wrist movement, instead of finger movements or reaching movements involving the elbow and shoulder joints. In accordance with the results for iEMG, modulation with movement direction was observed in the MEP evoked at T2000 and G200 (Fig. 4B). The PD of the MEP at G200 and T2000 was similar to that of the iEMG for each muscle (Fig. 4B). Furthermore, the strength of directional modulation (i.e., the magnitude of the PD vector) increased with the shift from T2000 to G200 (Fig. 4C).
These results were similar to those of previous studies that have found that the activity of a single neuron in the M1 exhibited clearer directional tuning with the approach of motor execution (Georgopoulos et al., 1988; Cisek et al., 2003). Previous studies have already shown that corticospinal excitability during flexion/extension movement of a finger is modulated even before movement onset according to whether the muscle is an agonist or antagonist (Sommer et al., 2001; Z'Graggen et al., 2009). Our observations have extended these results and reveal that functionally appropriate and precise modulation occurred, depending on the direction of movement.
Modification of corticospinal excitability associated with motor learning
After the adaptation to the CW force field, the PD of iEMG rotated in the CW direction (Fig. 4B), which was consistent with the finding of a previous study that used reaching movements of the shoulder and elbow joints (Thoroughman and Shadmehr, 1999). The results of the current study indicate that such learning-dependent modification of the PD can be observed for the MEP not only immediately before movement onset (G200), but also during the earlier preparatory period (T2000; Fig. 4). Neuronal activity in the M1 has been shown to be modified after adaptation to a rotational force field during movement (Gandolfo et al., 2000; Li et al., 2001; Arce et al., 2010a,b). For example, Mandelblat-Cerf et al. (2011) reported that learning-dependent changes occurred even before movement, and suggested that these changes reflected adjustments to the motor plan for the novel environment by a population of neurons. Our results further indicate that, even if the degree of the PD changes are different from neuron to neuron (Li et al., 2001; Arce et al., 2010b), the macroscopic modifications to the directional tuning pattern in the M1 are observable during the preparatory period.
Several previous TMS studies have examined how short-term training with repetitive movements can change corticospinal excitability (i.e., use-dependent facilitation; Classen et al., 1998). Recently, Orban de Xivry et al. (2013) reported that the adaptations of reaching movements to a force field that was abruptly introduced were associated with an increase in the MEP only for the muscles involved in compensation for the force field. The present results are novel because we have revealed that the directional tuning properties of corticospinal excitability were modulated by adaptation to a novel dynamic environment even during the preparatory period.
Modulation of corticospinal excitability depending on contextual cues
Experiment 2 showed that participants could adapt an identical wrist movement to two conflicting force fields (CW and CCW) if each force field was associated with either a unimanual or bimanual movement, respectively (Fig. 5). This result replicated the findings of previous studies that have examined adaptation of reaching movements of the upper limb to two conflicting force fields associated with unimanual and bimanual movements (Nozaki et al., 2006; Nozaki and Scott, 2009). In contrast, Experiment 3 showed that the adaptation was impossible when the force fields were associated with contextual cues in the form of target color (Gandolfo et al., 1996; Osu et al., 2004; Shadmehr et al., 2005). The present study suggests that the formation and recall of distinct internal models depending on unimanual and bimanual movements, but not color cues, are not confined only to reaching movements using shoulder and elbow joints, but also occur in wrist movements.
In accordance with the prediction from Experiment 1 (Fig. 2B), after sufficient training with the movement in a radial direction, the iEMG of the FCR was greater with the CW force field during unimanual movement than with the CCW force field during bimanual movement. The opposite trend was observed for the iEMG of the ECR (Fig. 6A). Considering that the changes in the PD were similar between MEP and iEMG (Experiment 1; Fig. 4B), we could predict that the MEP was also larger for the unimanual movement for the FCR and for the bimanual movement for the ECR. We observed context-dependent changes in MEP of the FCR following training that were partially consistent with these predictions. Furthermore, at T2000, there was a significant interaction between behavioral context and session, evidence that switching between distinct motor memories occurs much earlier than movement onset. These changes in MEP were unlikely to result from increments of cocontraction or feedback gain associated with the difficulty of the task, because the changes in MEP were not observed in Experiment 3 and changes in small background EMG did not explain the context-dependent effects observed.
Previous studies have demonstrated that participants face considerable difficulty in adapting the same movement to conflicting force fields simultaneously depending on cognitive contextual cues (Gandolfo et al., 1996; Shadmehr et al., 2005), especially when the two force fields are alternately presented (Osu et al., 2004). In contrast, recent studies have provided evidence that in particular contexts, humans can adapt simultaneously to distinct dynamics. These contexts include whether the opposite arm is stationary or moving (Nozaki et al., 2006), the kinematics of the opposite arm (Howard et al., 2010; Yokoi et al., 2011, 2014), or whether the movement plans are adapted for the same movement (Hirashima and Nozaki, 2012). However, it is largely unknown why simultaneous adaptation is possible in some cases (Cothros et al., 2009; Addou et al., 2011; Howard et al., 2012) and impossible in other cases (Karniel and Mussa-Ivaldi, 2002; Shadmehr et al., 2005). The present study demonstrated that the formation and retrieval of distinct motor memories could be evaluated using the changes in the MEP between baseline and test sessions. However, to obtain more precise information, future studies will be needed to examine the MEP changes during the learning phases, and to compare them between conditions under which participants can or cannot adapt to two conflicting force fields.
In conclusion, we have demonstrated that corticospinal excitability and its directional tuning patterns are systematically modulated by motor adaptation. These results are consistent with the view that portions of motor memories are stored in the M1 (Gandolfo et al., 2000; Li et al., 2001; Arce et al., 2010a,b; Orban de Xivry et al., 2013). Furthermore, the results indicate that motor memories stored in the M1 are appropriately retrieved before movement onset, depending on behavioral contexts. Such flexible modulation of corticospinal excitability with movement direction and motor learning may play a crucial role in the appropriate execution of voluntary movements in a wide variety of dynamic environments. Studying the macroscopic modulation of corticospinal excitability will shed light on how the human nervous system acquires a new internal model to make appropriate goal-directed movements.
Footnotes
This work was supported by the NEXT Program (Funding Program for Next Generation World-Leading Researchers; #LS034) and KAKENHI (Grants in Aid for Scientific Research; #20670008, #26242062, #26250322 to D.N.; #22700590 to H.K.). We thank all the participants in this study. We thank K. Abe for assistance with the experiments.
The authors declare no competing financial interests.
References
- Addou T, Krouchev N, Kalaska JF. Colored context cues can facilitate the ability to learn and to switch between multiple dynamical force fields. J Neurophysiol. 2011;106:163–183. doi: 10.1152/jn.00869.2010. [DOI] [PubMed] [Google Scholar]
- Arce F, Novick I, Mandelblat-Cerf Y, Israel Z, Ghez C, Vaadia E. Combined adaptiveness of specific motor cortical ensembles underlies learning. J Neurosci. 2010a;30:5415–5425. doi: 10.1523/JNEUROSCI.0076-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce F, Novick I, Mandelblat-Cerf Y, Vaadia E. Neuronal correlates of memory formation in motor cortex after adaptation to force field. J Neurosci. 2010b;30:9189–9198. doi: 10.1523/JNEUROSCI.1603-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisek P, Crammond DJ, Kalaska JF. Neural activity in primary motor and dorsal premotor cortex in reaching tasks with the contralateral versus ipsilateral arm. J Neurophysiol. 2003;89:922–942. doi: 10.1152/jn.00607.2002. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Cothros N, Wong J, Gribble PL. Visual cues signaling object grasp reduce interference in motor learning. J Neurophysiol. 2009;102:2112–2120. doi: 10.1152/jn.00493.2009. [DOI] [PubMed] [Google Scholar]
- de Rugy A, Carroll TJ. Changes in muscle directional tuning parallel feedforward adaptation to a visuomotor rotation. Exp Brain Res. 2010;203:701–709. doi: 10.1007/s00221-010-2280-9. [DOI] [PubMed] [Google Scholar]
- Devanne H, Cassim F, Ethier C, Brizzi L, Thevenon A, Capaday C. The comparable size and overlapping nature of upper limb distal and proximal muscle representations in the human motor cortex. Eur J Neurosci. 2006;23:2467–2476. doi: 10.1111/j.1460-9568.2006.04760.x. [DOI] [PubMed] [Google Scholar]
- Franklin DW, Osu R, Burdet E, Kawato M, Milner TE. Adaptation to stable and unstable dynamics achieved by combined impedance control and inverse dynamics model. J Neurophysiol. 2003;90:3270–3282. doi: 10.1152/jn.01112.2002. [DOI] [PubMed] [Google Scholar]
- Franklin S, Wolpert DM, Franklin DW. Visuomotor feedback gains upregulate during the learning of novel dynamics. J Neurophysiol. 2012;108:467–478. doi: 10.1152/jn.01123.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfo F, Mussa-Ivaldi FA, Bizzi E. Motor learning by field approximation. Proc Natl Acad Sci U S A. 1996;93:3843–3846. doi: 10.1073/pnas.93.9.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfo F, Li C, Benda BJ, Schioppa CP, Bizzi E. Cortical correlates of learning in monkeys adapting to a new dynamical environment. Proc Natl Acad Sci U S A. 2000;97:2259–2263. doi: 10.1073/pnas.040567097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT. On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci. 1982;2:1527–1537. doi: 10.1523/JNEUROSCI.02-11-01527.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos AP, Kettner RE, Schwartz AB. Primate motor cortex and free arm movements to visual targets in three-dimensional space. II. Coding of the direction of movement by a neuronal population. J Neurosci. 1988;8:2928–2937. doi: 10.1523/JNEUROSCI.08-08-02928.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima M, Nozaki D. Distinct motor plans form and retrieve distinct motor memories for physically identical movements. Curr Biol. 2012;22:432–436. doi: 10.1016/j.cub.2012.01.042. [DOI] [PubMed] [Google Scholar]
- Hoffman DS, Strick PL. Step-tracking movements of the wrist. III. Influence of changes in load on patterns of muscle activity. J Neurosci. 1993;13:5212–5227. doi: 10.1523/JNEUROSCI.13-12-05212.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DS, Strick PL. Step-tracking movements of the wrist. IV. Muscle activity associated with movements in different directions. J Neurophysiol. 1999;81:319–333. doi: 10.1152/jn.1999.81.1.319. [DOI] [PubMed] [Google Scholar]
- Howard IS, Ingram JN, Wolpert DM. Composition and decomposition in bimanual dynamic learning. J Neurosci. 2008;28:10531–10540. doi: 10.1523/JNEUROSCI.3473-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard IS, Ingram JN, Wolpert DM. Context-dependent partitioning of motor learning in bimanual movements. J Neurophysiol. 2010;104:2082–2091. doi: 10.1152/jn.00299.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard IS, Ingram JN, Franklin DW, Wolpert DM. Gone in 0.6 seconds: the encoding of motor memories depends on recent sensorimotor states. J Neurosci. 2012;32:12756–12768. doi: 10.1523/JNEUROSCI.5909-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karniel A, Mussa-Ivaldi FA. Does the motor control system use multiple models and context switching to cope with a variable environment? Exp Brain Res. 2002;143:520–524. doi: 10.1007/s00221-002-1054-4. [DOI] [PubMed] [Google Scholar]
- Li CS, Padoa-Schioppa C, Bizzi E. Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron. 2001;30:593–607. doi: 10.1016/S0896-6273(01)00301-4. [DOI] [PubMed] [Google Scholar]
- Mandelblat-Cerf Y, Novick I, Paz R, Link Y, Freeman S, Vaadia E. The neuronal basis of long-term sensorimotor learning. J Neurosci. 2011;31:300–313. doi: 10.1523/JNEUROSCI.4055-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki D, Scott SH. Multi-compartment model can explain partial transfer of learning within the same limb between unimanual and bimanual reaching. Exp Brain Res. 2009;194:451–463. doi: 10.1007/s00221-009-1720-x. [DOI] [PubMed] [Google Scholar]
- Nozaki D, Kurtzer I, Scott SH. Limited transfer of learning between unimanual and bimanual skills within the same limb. Nat Neurosci. 2006;9:1364–1366. doi: 10.1038/nn1785. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry JJ, Ahmadi-Pajouh MA, Harran MD, Salimpour Y, Shadmehr R. Changes in croticospinal excitability during reach adaptation in force fields. J Neurophysiol. 2013;109:124–136. doi: 10.1152/jn.00785.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osu R, Hirai S, Yoshioka T, Kawato M. Random presentation enables subjects to adapt to two opposing forces on the hand. Nat Neurosci. 2004;7:111–112. doi: 10.1038/nn1184. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Donchin O, Hwang EJ, Hemminger SE, Rao AK. Learning dynamics of reaching. In: Riehle A, Vaadia E, editors. Motor cortex in voluntary movements: a distributed system for distributed functions. Boca Raton, FL: CRC; 2005. pp. 297–328. [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Sommer M, Classen J, Cohen LG, Hallett M. Time course of determination of movement direction in the reaction time task in humans. J Neurophysiol. 2001;86:1195–1201. doi: 10.1152/jn.2001.86.3.1195. [DOI] [PubMed] [Google Scholar]
- Thoroughman KA, Shadmehr R. Electromyographic correlates of learning an internal model of reaching movements. J Neurosci. 1999;19:8573–8588. doi: 10.1523/JNEUROSCI.19-19-08573.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi A, Hirashima M, Nozaki D. Gain field encoding of the kinematics of both arms in the internal model enables flexible bimanual action. J Neurosci. 2011;31:17058–17068. doi: 10.1523/JNEUROSCI.2982-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi A, Hirashima M, Nozaki D. Lateralized sensitivity of motor memories to the kinematics of the opposite arm reveals functional specialization during bimanual actions. J Neurosci. 2014;34:9141–9151. doi: 10.1523/JNEUROSCI.2694-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Z'Graggen WJ, Conforto AB, Wiest R, Remonda L, Hess CW, Kaelin-Lang A. Mapping of direction and muscle representation in the human primary motor cortex controlling thumb movements. J Physiol. 2009;587:1977–1987. doi: 10.1113/jphysiol.2009.171066. [DOI] [PMC free article] [PubMed] [Google Scholar]