Abstract
The ventral bed nucleus of the stria terminalis (vBNST) has been implicated in stress-induced cocaine use. Here we demonstrate that, in the vBNST, corticotropin releasing factor (CRF) is expressed in neurons that innervate the ventral tegmental area (VTA), a site where the CRF receptor antagonist antalarmin prevents the reinstatement of cocaine seeking by a stressor, intermittent footshock, following intravenous self-administration in rats. The vBNST receives dense noradrenergic innervation and expresses β adrenergic receptors (ARs). Footshock-induced reinstatement was prevented by bilateral intra-vBNST injection of the β-2 AR antagonist, ICI-118,551, but not the β-1 AR antagonist, betaxolol. Moreover, bilateral intra-vBNST injection of the β-2 AR agonist, clenbuterol, but not the β-1 agonist, dobutamine, reinstated cocaine seeking, suggesting that activation of vBNST β-2 AR is both necessary for stress-induced reinstatement and sufficient to induce cocaine seeking. The contribution of a β-2 AR-regulated vBNST-to-VTA pathway that releases CRF was investigated using a disconnection approach. Injection of ICI-118,551 into the vBNST in one hemisphere and antalarmin into the VTA of the contralateral hemisphere prevented footshock-induced reinstatement, whereas ipsilateral manipulations failed to attenuate stress-induced cocaine seeking, suggesting that β-2 AR regulate vBNST efferents that release CRF into the VTA, activating CRF receptors, and promoting cocaine use. Last, reinstatement by clenbuterol delivered bilaterally into the vBNST was prevented by bilateral vBNST pretreatment with antalarmin, indicating that β-2 AR-mediated actions in the vBNST also require local CRF receptor activation. Understanding the processes through which stress induces cocaine seeking should guide the development of new treatments for addiction.
Keywords: BNST, cocaine, CRF, relapse, stress, VTA
Introduction
In many cocaine addicts, relapse to drug use is triggered by the onset of craving in response to episodes of stress. Due to the uncontrollable and often unavoidable nature of stress, its contribution to relapse is particularly problematic when managing cocaine addiction.
Using rodent reinstatement models, preclinical researchers have begun to define the neurocircuitry that underlies stress-induced relapse. One prominent pathway that has been implicated consists of a dopaminergic projection from the ventral tegmental area (VTA) to the medial prefrontal cortex, where dopamine D1 receptor activation increases the activity of a glutamatergic pathway to the nucleus accumbens that is critical for cocaine seeking (Capriles et al., 2003; McFarland et al., 2004). During stress, the VTA is regulated by the neuropeptide corticotropin releasing factor (CRF; Wang et al., 2005), in part via CRF-R1 receptors (Blacktop et al., 2011), to evoke cocaine seeking.
The VTA receives inputs from brain regions involved in integration of the stress response, including those comprising the “extended amygdala”: the nucleus accumbens shell, central amygdala, and bed nucleus of the stria terminalis (BNST; Phillipson, 1979). Among these structures, the BNST, which serves as an interface between stress and motivational/reward systems, appears to be particularly important for stress-induced cocaine seeking, in that its inactivation prevents stress-induced reinstatement in rodents (McFarland et al., 2004; Briand et al., 2010). A subgroup of neurons projecting from the BNST to the VTA express CRF (Rodaros et al., 2007) and therefore may be responsible for stress-induced increases in VTA CRF and CRF-dependent cocaine seeking. However, CRF projections to the VTA from the ventral BNST (vBNST), the subregion implicated in stress-induced cocaine seeking (McFarland et al., 2004), have not been described. The BNST, particularly the vBNST, receives dense noradrenergic innervation (Phelix et al., 1992) and expresses β adrenergic receptors (ARs; Cecchi et al., 2007), which have been reported to regulate efferent projections (Nobis et al., 2011), including those that innervate the VTA (Dumont and Williams, 2004; Silberman et al., 2013). Both stress-induced reinstatement (Erb and Stewart, 1999; Erb et al., 2001) and noradrenergic regulation of BNST efferents to the VTA (Silberman et al., 2013) require BNST CRF receptor activation, suggesting that norepinephrine in the BNST promotes local CRF release, thereby activating a second CRF-releasing pathway to the VTA.
Considering the important role for noradrenergic signaling in stress-induced reinstatement in rodents (Erb et al., 2000; Mantsch et al., 2010) and craving in human cocaine-dependent populations (Jobes et al., 2011), AR regulation of a vBNST-to-VTA CRF-releasing pathway is a likely mechanism through which stress promotes cocaine seeking. We have found that β-2 AR activation is both necessary and sufficient for stress-induced reinstatement in mouse models (Vranjkovic et al., 2012). Moreover, it has been demonstrated that a mixture of β-AR antagonists, administered into the vBNST, prevents stress-induced reinstatement (Leri et al., 2002). In this study, we demonstrate direct CRF neuronal projections from the vBNST to the VTA and examine the relationship between stress-induced β-2 AR activation in the vBNST and CRF-R1 receptor-dependent actions in the VTA that lead to cocaine seeking.
Materials and Methods
Subjects
Male Sprague Dawley rats (Harlan Laboratories) ∼90-d-old at the time of delivery (325 g) were used. Rats were housed singly in a temperature- and humidity-controlled, Association for Assessment and Accreditation of Laboratory Animal Care accredited facility under a 12 h light/dark cycle (lights off at 07:00 h) and had access to food and water at all times, except during the food training periods during which they were kept at 90% of their free-feeding body weight. All procedures were performed in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Drugs
Cocaine HCl was acquired from the National Institute on Drug Abuse through its drug supply program. The selective β-1 AR antagonist betaxolol HCl, the selective β-2 AR antagonist (-)-(isopropylamino)-1-[(7-methyl-4-indanyl)oxy]butan-2-ol (ICI-118,551) HCl, the selective β-1 AR agonist dobutamine HCl, the selective β-2 AR agonist clenbuterol, and the axonal transport inhibitor colchicine HCl were purchased from Sigma-Aldrich. Cocaine was dissolved in bacteriostatic saline. Other drugs were dissolved in sterile water. Colchicine was delivered intracerebroventricularly in a volume of 2 μl over a 5 min period. Intracranial drug injections occurred in a volume of 0.2 μl/side over a 1 min period.
Catheter and cannula implantation
For intravenous self-administration (SA) and reinstatement testing, rats were implanted with catheters into the jugular vein under ketamine HCl (100 mg/kg i.p.) and xylazine (2 mg/kg, i.p.) anesthesia, as described previously (Blacktop et al., 2011), along with 11 mm 26-gauge guide cannulae aimed at the vBNST (0.6 mm posterior to bregma; 3.5 mm lateral to midline; 6.9 mm ventral to the skull surface at a 15° angle), and/or the VTA (5.6 mm posterior to bregma; 2.2 mm lateral to midline; 6.9 ventral to the skull surface at a 12° angle) for intracranial injections. The tips of the guide cannulae were aimed 0.5 mm above the target injection sites.
Cocaine self-administration
Following a 2 week recovery from surgery, rats were trained to self-administer cocaine (1.0 mg/kg/inf, i.v.) by pressing a response lever under an FR1 schedule in a computer-interfaced operant conditioning chamber (Med Associates) during daily 2 h sessions. During these sessions, the active (front) lever was extended into the chamber and the corresponding stimulus light was illuminated. Pressing this lever resulted in a cocaine infusion (200 μl over 5 s), followed by a 25 s time-out period during which the stimulus light was extinguished but the lever remained extended. Responding on a second, inactive lever was recorded but had no programmed consequences. Once stable SA under the FR1 schedule was observed (>15 infusions), the requirements for SA were gradually increased until rats displayed stable responding under ta FR4 schedule (<10% variation from the mean over 3 sessions), at which time daily access was increased to 6 h. Rat were permitted to self-administer daily under these conditions for 14 consecutive days after which they underwent extinction training.
Extinction training and reinstatement testing
Extinction sessions were identical to SA conditions except that (1) the sessions were 2 h in duration and (2) the cocaine solution was replaced with saline. Daily extinction training sessions were conducted until rats displayed <15 cocaine lever responses for consecutive sessions (range, 6–10 d) at which time rats were tested for reinstatement. Reinstatement sessions were identical to extinction sessions except that they were preceded by exposure to shock and/or intracranial drug delivery. Shocks (0.5 mA, 0.5 s duration) were delivered on average every 40 s (range, 10–70 s) through the grid floors of the chambers over a 15 min period that immediately preceded the 2 h reinstatement test session (Blacktop et al., 2011; Graf et al., 2011). During the shock session, the levers were retracted and the stimulus lights were off, but a houselight in the chamber was illuminated. Reinstatement was defined as an increase in cocaine lever responding relative to the prior extinction session. When rats were tested multiple times for reinstatement, test sessions were separated by additional extinction session and were required to display <15 cocaine lever responses before retesting.
Effects on food-reinforced lever pressing
In most cases where significant reductions in shock-induced reinstatement were observed, the effects of the same manipulations on lever pressing reinforced by food delivery (45 mg sucrose-sweetened food pellets; BioServ) were also examined in separate rats to confirm that reduced reinstatement was not attributable to motor impairment that interfered with the ability of the rats to press the lever. Following surgical intracranial implantation of guide cannulae and recovery, these rats were maintained at 90% of their free-feeding body weights and trained to self-administer food pellets by pressing a lever under a FR4 schedule. Effects on food-reinforced responding were tested after stable patterns of lever pressing were observed (<10% variation from the mean over 3 sessions).
Histological confirmation of injection sites
The accuracy of cannula implantation was confirmed postmortem in each rat after cardiac perfusion with 60 ml 0.15% NaCl followed by 60 ml of 2.5% buffered neutral formalin under sodium pentobarbital anesthesia (55 mg/kg). Brains were removed and stored in 2.5% buffered formalin before vibratome sectioning (40 μm), slide mounting, and staining with cresyl violet for examination using a light microscope. Rats with injection sites outside of the VTA or the vBNST were excluded from data analysis.
Experiment 1: effects of intra-BNST β AR antagonists on stress-induced reinstatement
We initially examined the role of β-1 and β-2 AR activation in the vBNST in stress-induced reinstatement by testing rats for shock-induced reinstatement following bilateral intra-vBNST injection of the β-1 AR selective antagonist, betaxolol (1 nmol/307 ng per side), the β-2 AR antagonist selective antagonist, ICI-118,551 (1 nmol/277 ng per side) or vehicle (sterile water). Concentrations were selected based on selectivity for human β receptor subtypes (Baker, 2005). Moreover, the antagonist concentrations were previously used to selectively target brain β ARs (Delfs et al., 2000; Leri et al., 2002; Cecchi et al., 2007; LaLumiere et al., 2010). Rats received vBNST injections 15 min before shock. Nine total rats were used for this experiment. Five rats were tested for shock-induced reinstatement following pretreatment with vehicle, betaxolol, and ICI-118,551; two rats were tested following only vehicle and betaxolol; and two rats were tested for following only vehicle and ICI-118,551. An additional group of rats (n = 6) was tested for the effects of ICI-118,551 and vehicle on food-reinforced lever pressing.
Experiment 2: reinstatement by intra-vBNST injection of β-AR agonists
To further examine the role of vBNST β-1 and β-2 ARs in stress-induced cocaine seeking, a separate group of rats was tested for the ability of bilateral intra-vBNST injection of the β-1 AR selective agonist dobutamine (1 nmol/ 301 ng per side) or the β-2 AR selective agonist clenbuterol (36 pmol/10 ng per side) to induce reinstatement. Concentrations were again chosen based on selectivity for human β receptor subtypes (Baker, 2010). Further, the clenbuterol dose has been previously used to selectively target brain β-2 ARs (Roozendaal et al., 2008; LaLumiere et al., 2010). Ten total rats were used for this experiment. Seven rats were tested for reinstatement in response to dobutamine, clenbuterol, and vehicle. An additional three rats were tested only with clenbuterol and vehicle.
Experiment 3: effect of VTA CRF receptor antagonism on stress-induced reinstatement
To confirm our earlier finding that shock-induced reinstatement requires CRF receptor activation in the VTA (Blacktop et al., 2011), we also tested rats for the effects of intra-VTA pretreatment with the CRF-R1 receptor antagonist, antalarmin (Webster et al., 1996; 1.32 nmol/500 ng/side). We (Blacktop et al., 2011) and others (Lowery-Gionta et al., 2012) have used this antalarmin dose to investigate CRF-R1 receptor-dependent contributions to behavior. Six rats were used for this experiment. Each of them was tested for the effects of both antalarmin and vehicle on shock-induced reinstatement
Experiment 4: identification of CRF-positive neurons in the vBNST that project to the VTA
Retrograde tracing using CTb.
We used cholera toxin B subunit (CTb) as a retrograde tracer to identify neurons in the vBNST with direct efferent projections to the VTA. Rats were anesthetized with ketamine and xylazine and placed in a stereotaxic frame. Heat-sterilized Hamilton syringes were used to pressure inject 0.2 μl biotinylated CTb (List Biological Laboratories) unilaterally into the VTA over a 10 min period (posterior to bregma −5.6 mm, lateral −2.6 mm, ventral −8.6 mm; Paxinos and Watson, 2006). Syringes were left in place for 20 min after injections and slowly removed from the brain over a 10 min period.
Colchicine treatment.
Because it has been reported that inhibition of axonal transport using pretreatment with the microtubule polymerization inhibitor colchicine is necessary for detection of CRF-immunoreactive cell bodies within the vBNST (Sakanaka et al., 1986) anesthetized rats received injections of colchicine (1.25 μmol/500 μg in 2 μl) into a lateral ventricle (posterior to bregma −1.0 mm, lateral 1.4 mm, ventral −5.3 mm) 10 d after injection with CTb, thus avoiding interference with retrograde transport of CTb.
Tissue collection and preparation.
Twenty-four hours after the colchicine injections, rats were anesthetized with sodium pentobarbital (55 mg/kg, i.p.) and perfused transcardially with 30 ml of 0.9% NaCl, followed by 60 ml of cold (4°C) 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.0. Brains were quickly removed and immersed in fixative for 24 h at 4°C. Brains were washed twice in 0.1 m sodium phosphate buffer for 12 h each, after which they were cryoprotected in 30% sucrose in 0.1 m-phosphate buffer for 3 d at 4°C. Brains were rapidly frozen and serial coronal sections (30 μm) were cut on a cryostat. Sections were stored at −20°C until immunofluorescence procedures were conducted.
Fluorescence immunocytochemistry.
Immunofluorescence for CTb and CRF was conducted on free-floating 30 μm sections. Sections were rinsed three times for 10 min in 0.05 m KPBS and once for 10 min in 0.1 m glycine in 0.05 KPBS, followed by blocking in 3% normal donkey serum in 0.4% Triton KPBS for 20 min. Sections were then incubated for 48 h at 4°C with rabbit anti-CRF (supplied by Dr Wylie Vale, The Salk Institute) diluted 1:8000 in 0.1% Triton KPBS with 3% normal donkey serum. Sections were then rinsed 3× 10 min in 0.05 m KPBS, followed by incubation with an AlexaFluor 488-conjugated donkey anti-rabbit secondary antibody (Catalog #A11008; 1:500; Life Technologies) in 0.05 m KPBS for 2 h at room temperature, and rinsed three times for 10 min in 0.05 m KPBS. For detection of biotinylated CTb, sections were incubated for 24 h at 4°C in goat anti-biotin polyclonal antibody (Catalog # SP 3000; Vector Laboratories) diluted 1:30,000 in 0.1% Triton KPBS and 3% normal donkey serum. After rinsing, sections were incubated 2 h with AlexaFluor 598-conjugated donkey anti-goat IgG (Catalog #A-110581; 1:500; Life Technologies) and washed three times for 10 min in 0.05 m KPBS. Sections were briefly rinsed in distilled water and mounted onto gel-coated SuperFrost Plus slides and coverslipped using Vectashield mounting medium with DAPI (Catalog #H-1200; Vector Laboratories). Photomicrographs were acquired using a Retiga 2000R digital camera (QImaging) on a Nikon 80i microscope using NIS Elements software (Nikon Instruments).
Experiment 5: effects of vBNST and VTA disconnection on stress-induced cocaine seeking
A disconnection approach was used to determine whether a β 2-AR activated, CRF-dependent pathway between the vBNST and the VTA is required for stress-induced cocaine seeking. At the time of catheterization, separate groups of rats were implanted with two cannulae aimed unilaterally at the vBNST and VTA in either the contralateral or the ipsilateral hemisphere(s) of the brain. The effect of pathway disconnection was examined in six rats by injection of the β-2 AR antagonist ICI 118,551 (1 nmol/277 ng) into the vBNST in one hemisphere and the CRF-R1 receptor antagonist antalarmin (1.32 nmol/500 ng) into the VTA of the other before testing for shock-induced reinstatement. For comparison, the same rats were tested for shock-induced reinstatement following injection of vehicle into each region. As a control to ensure that reductions in reinstatement were attributable to disconnection, a second groups of rats (n = 7) received a unilateral injection of ICI 118,551 into the vBNST in one hemisphere and a unilateral injection of antalarmin into the VTA in the same hemisphere before testing for shock-induced reinstatement. These rats were also tested for the effects of ipsilateral vehicle injections. For this experiment, the hemispheres into which cannulae were implanted were randomized across rats in each group such that in the contralateral treatment group, half of the rats received ICI 118,551 injections into the left vBNST and antalarmin injections into the right VTA, whereas the other half received ICI 118,551 injections into the right vBNST and antalarmin injections into the left VTA. Likewise, half of the rats in the ipsilateral treatment group received drug injections into sites in the right hemisphere, whereas the remaining rats received injections into sites in the left hemisphere. To examine the potential contribution of nonspecific behavioral suppression to the effects on reinstatement, an addition group of rats (n = 6) was tested for the effects of contralateral drug and vehicle injections on food-reinforced lever pressing.
Experiment 6: role of vBNST CRF-R1 receptors in cocaine seeking induced by vBNST β-2 AR activation
It has been reported that CRF-R1 antagonist injections directly into the vBNST can also block stress-induced cocaine seeking (Erb and Stewart, 1999; Erb et al., 2001). To determine whether β-2 ARs in the vBNST regulate efferent pathways to the VTA to produce cocaine seeking via a mechanism that also involves local CRF actions, we tested for the ability of intra-BNST pretreatment with antalarmin to block reinstatement in response to local delivery of the beta2 AR agonist clenbuterol. Reinstatement in response to bilateral intra-BNST delivery of clenbuterol (36 pmol/10 ng per side) was examined following bilateral intra-BNST pretreatment with antalarmin (1.32 nmol/500 ng/side) or vehicle (10 min pretreatment) in counterbalanced sequence in six rats. To test the hypothesis that CRF-R1 receptor activation in the vBNST is downstream from β-2 AR activation in the sequence of events that mediate stress-induced cocaine seeking, a separate group of six rats was tested for reinstatement in response to bilateral intra-vBNST CRF (63 pmol/300 ng/side; Erb and Stewart, 1999) following bilateral intra-vBNST pretreatment with the selective β-2 AR antagonist, ICI-118,551 (1 nmol/277 ng per side) or vehicle (10 min pretreatment).
Statistical analyses
Statistical analyses were conducted using Predictive Analytics SoftWare statistics software (SPSS). Statistical significance was determined using ANOVA followed, when appropriate, by further analyses of main effects using ANOVA and/or post hoc testing using Bonferroni-corrected t tests.
Results
Cocaine SA and extinction in rats used for the various reinstatement experiments are shown in Table 1 and did not differ across experiments. In general, each group of rats displayed escalation, as previously demonstrated under these conditions, and showed reduced cocaine seeking across extinction testing, which consisted of an average of 8.24 (±0.28) sessions before extinction criteria were met. The injection sites for rats used for each experiment are depicted in Figure 1. A total of 11 rats were excluded from analyses due to cannula misplacement. In all cases, drug effects were not observed with inaccurate injections.
Table 1.
Cocaine SA and extinction
| Experiment no. (n) | Cocaine SA |
Extinction |
||
|---|---|---|---|---|
| SA day 1 | SA day 14 | First Ext | Last Ext | |
| 1 (9) | 77.22 ± 7.71 | 92.78 ± 8.19 | 66.33 ± 11.50 | 9.89 ± 2.35 |
| 2 (10) | 68.57 ± 8.36 | 89.38 ± 7.07 | 104.20 ± 24.21 | 9.70 ± 1.14 |
| 3 (6) | 75.10 ± 5.86 | 90.21 ± 7.26 | 70.20 ± 29.14 | 11.60 ± 1.47 |
| 5 (13) | 71.82 ± 4.73 | 85.74 ± 4.01 | 68.09 ± 12.83 | 12.00 ± 2.39 |
| 6 (12) | 74.00 ± 4.82 | 82.00 ± 3.44 | 129.62 ± 16.09 | 8.38 ± 1.21 |
Data represent cocaine SA (infusions/6 h session ± SE) on days 1 and 14 of SA testing and cocaine lever responding (responses/2 h session ± SE) on the first day of extinction training (First Ext) and the last day of extinction training prior to reinstatement testing (Last Ext) in rats from each of the reinstatement experiments.
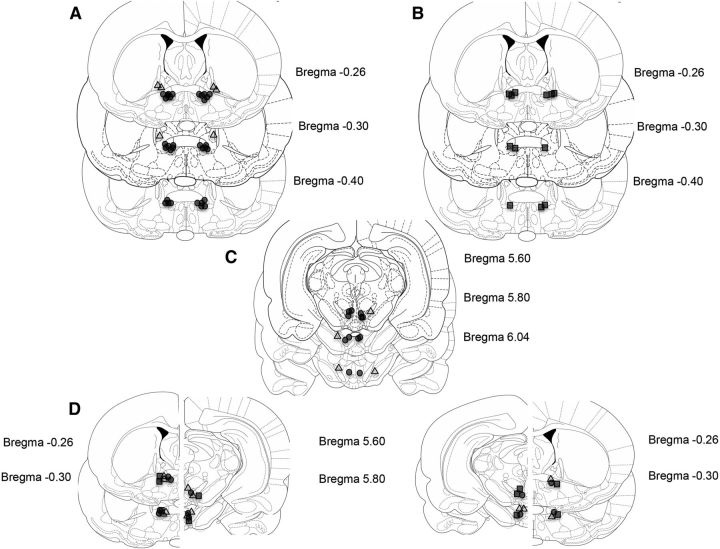
Figure 1.
Intracranial injection sites. Representative atlas diagrams (Paxinos and Watson, 2006) of injection sites within the vBNST and the VTA. A, Rats from Experiments 1, 2 and 5; vBNST hits are depicted as circles and misses as triangles. B, Food control rats for Experiment 1; hits are depicted as squares and misses as triangles. C, Rats from Experiment 3; VTA hits are depicted as circles and misses as triangles. D, Rats used for the Experiment 4; contralateral hits are depicted as circles, ipsilateral hits are depicted as squares and food-controls are depicted as triangles.
Experiment 1: effects of intra-BNST β AR antagonists on stress-induced reinstatement
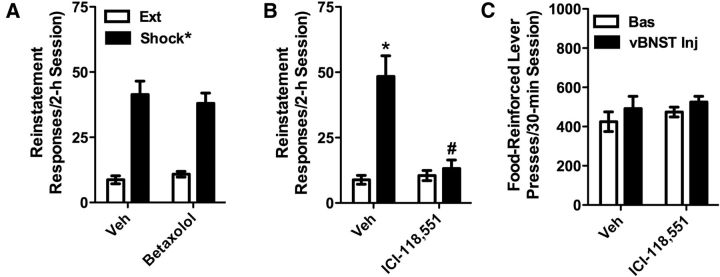
The effect of bilateral intra-vBNST injection of the β-1 AR antagonist betaxolol (1 nmol/307 ng per side) on shock-induced reinstatement was examined in seven rats and is shown in Figure 2A. Two-way repeated measures (reinstatement × betaxolol) ANOVA showed a significant overall reinstatement effect (F(1,6) = 65.437; p < 0.001) but no main effect of betaxolol pretreatment or interaction between betaxolol treatment and reinstatement. Shock produced robust reinstatement regardless of whether rats were pretreated with betaxolol or vehicle. The effects of bilateral intra-vBNST injection of the β-2 AR antagonist ICI-118,551 (1 nmol/277 ng per side) pretreatment on shock-induced reinstatement were also examined in seven rats and are shown in Figure 2B. Two-way repeated-measures ANOVA showed a significant reinstatement effect (F(1,6) = 52.475; p < 0.001) and a significant main effect of ICI-118,551 pretreatment (F(1,6) = 10.224; p < 0.05), as well as a significant reinstatement × ICI-118,551 pretreatment interaction (F(1,6) = 20.213; p < 0.01). Shock reinstated cocaine seeking in vehicle but not ICI-118,551 pretreated rats (p < 0.01 vs extinction; Ext) and reinstatement was reduced in ICI-118,551 pretreated versus vehicle pretreated rats (p < 0.01). To assess potential motor impairment resulting from intra-vBNST ICI-118,551 delivery that may have nonspecifically interfered with reinstatement responding, a separate group of six rats was tested for effects of intra-vBNST ICI-118,551 on sucrose pellet-reinforced responding. ICI-118,551 pretreatment failed to alter lever pressing under these conditions (Fig. 2C). In three of the rats that were tested, guide cannulae were misplaced such that they received injections into areas that comprise the dorsal BNST. Notably, in these rats, shock-induced reinstatement was observed in both vehicle-pretreated (Ext: 8.67 ± 1.76 responses vs shock: 32.33 ± 2.03) and ICI-118,551-pretreated (Ext: 12.67 ± 1.76 responses vs shock: 28.33 ± 4.81) rats. Effects of shock were specific to the previously active lever as shock failed to increase responding on a previously inactive lever in vehicle-pretreated rats (Ext: 1.37 ± 0.51 responses vs shock: 1.02 ± 0.44). Neither betaxolol nor ICI-118,551 affected inactive lever responding during reinstatement testing (vehicle: 1.02 ± 0.44 responses; betaxolol: 1.00 ± 0.33; ICI-118,551: 0.75 ± 0.31).
Figure 2.
Footshock-induced reinstatement of cocaine seeking is blocked by intra-vBNST injection of the β-2 AR antagonist, ICI-118,551, but not the β-1 AR antagonist, betaxolol. Data represent the effects of bilateral intra-vBNST injections of betaxolol (1 nmol/307 ng per side; A; n = 7) or ICI-118,551 (1 nmol/277 ng per side; B; n = 7) or vehicle on reinstatement (responses/2 h session ± SE) by electric footshock. Significant reinstatement was observed following intra-vBNST pretreatment with vehicle or betaxolol (*p < 0.05 vs Ext), but not ICI-118,551. Likewise, responding following ICI-118,551, but not betaxolol, was significantly reduced compared with vehicle pretreatment (#p < 0.05 vs vehicle). By contrast, intra-vBNST ICI-118,551 failed to altered food-reinforced lever pressing (C; n = 6).
Experiment 2: reinstatement by intra-vBNST injection of β-AR agonists
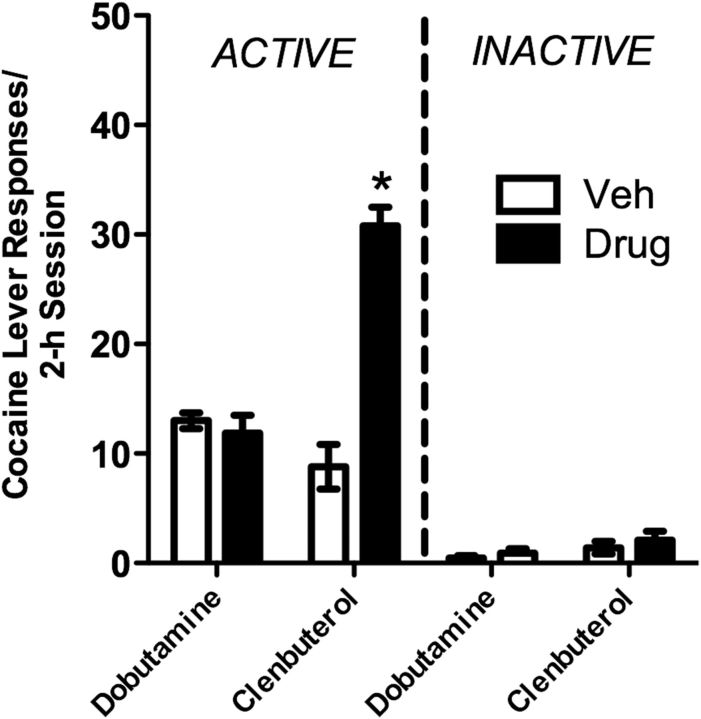
Following SA and extinction, bilateral intra-vBNST delivery of clenbuterol (36 pmol/10 ng per side) but not dobutamine (1 nmol/ 301 ng per side), reinstated cocaine seeking (Fig. 3). A paired t test showed that, compared with vehicle, pretreatment with clenbuterol significantly increased previously active lever pressing (t(9) = 11.156; p < 0.001). Neither dobutamine (0.88 ± 0.22 responses) nor clenbuterol (0.83 ± 0.55 responses) increased responding on a previously inactive lever relative to vehicle pretreatment (0.63 ± 0.18) responses. Guide cannulae were misplaced in three rats such that they received injections into sites within the dorsal BNST. In contrast to rats that received intra-vBNST injections, neither clenbuterol (Ext: 8.67 ± 1.99 vs clenbuterol: 5.33 ± 5.95) nor dobutamine (Ext: 10.00 ± 2.07 vs dobutamine: 9.33 ± 7.60) produced reinstatement in these rats.
Figure 3.
Reinstatement of extinguished cocaine seeking by bilateral injection of the β-2 AR selective agonist, clenbuterol, but not the β-1 AR selective agonist, dobutamine, into the vBNST. Data represent responding on the cocaine lever (responses/2 h session ± SE; active) or a previously inactive lever. Intra-vBNST clenbuterol (36 pmol/10 ng per side; n = 10), but not dobutamine (1 nmol/301 ng per side; n = 7) reinstated extinguished cocaine seeking (*p < 0.05 vs vehicle). Neither dobutamine nor clenbuterol increased “inactive” lever responding.
Experiment 3: effect of VTA CRF receptor antagonism on stress-induced reinstatement
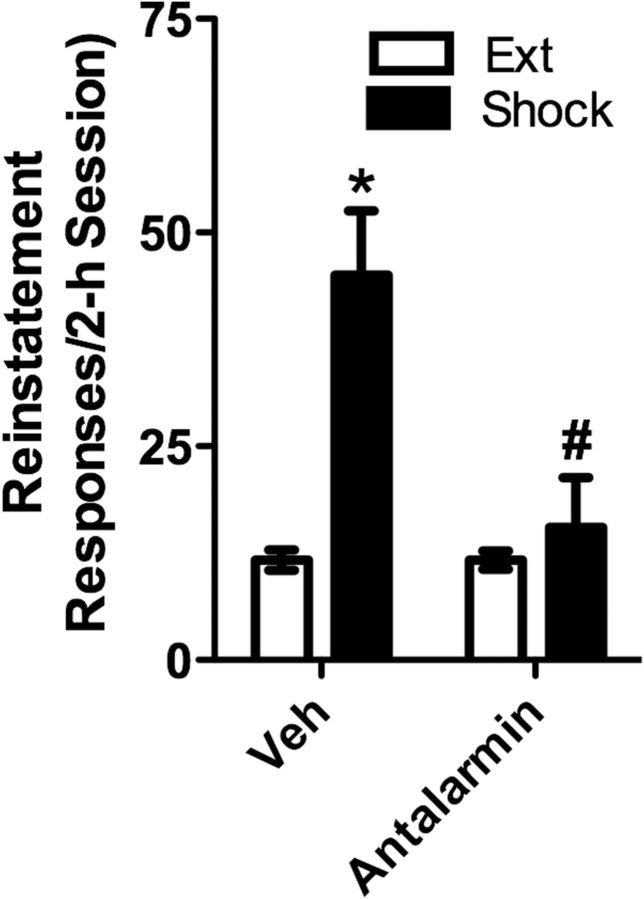
As previously reported (Blacktop et al., 2011), bilateral injection of the CRF-R1 receptor selective antagonist, antalarmin (1.32 nmol/500 ng per side) into the VTA prevented reinstatement. The effect of bilateral intra-VTA antalarmin pretreatment on shock-induced reinstatement was examined in six rats as shown in Figure 4. Two-way repeated-measures ANOVA showed a significant reinstatement effect (F(1,5) = 12.971; p < 0.05) and a significant main effect of antalarmin pretreatment (F(1,5) = 9.175; p < 0.05), as well as a significant reinstatement × antalarmin pretreatment interaction (F(1,5) = 8.461; p < 0.05). As previously reported, shock reinstated cocaine seeking in vehicle but not antalarmin pretreated rats (p < 0.05 vs Ext) and reinstatement was reduced in antalarmin pretreated versus vehicle pretreated rats (p < 0.05). As we have already demonstrated that intra-VTA antalarmin delivery does not alter food-reinforced lever pressing (Blacktop et al., 2011), we did not test for these effects in this study. In two of the rats that were tested, guide cannulae were misplaced such that they received injections outside of the VTA. Notably, in these rats, shock-induced reinstatement was observed in both vehicle-pretreated (Ext: 13 and 12 responses vs shock: 35 and 29 responses) and antalarmin-pretreated (Ext: 5 and 12 responses vs shock: 39 and 41 responses) rats. Effects of shock were specific to the previously active lever as shock failed to increase responding on a previously inactive lever in vehicle-pretreated rats (Ext: 1.12 ± 0.29 responses vs shock: 0.89 ± 0.65). Antalarmin did not affect inactive lever responding during reinstatement testing (vehicle: 0.89 ± 0.65 responses; antalarmin: 0.81 ± 0.52).
Figure 4.
Bilateral injection of the CRF receptor antagonist, antalarmin, into the VTA prevents shock-induced reinstatement of cocaine seeking. Data represent the effects of bilateral intra-vBNST delivery of antalarmin (1.32 nmol/500 ng per side; Fig. 2A; n = 6) or vehicle on reinstatement (responses/2 h session ± SE) following electric footshock. Significant reinstatement was observed after intra-vBNST pretreatment with vehicle (*p < 0.05 vs Ext) but not antalarmin. Likewise, responding following antalarmin was significantly reduced compared with vehicle pretreatment (#p < 0.05).
Experiment 4: identification of CRF-positive neurons in the vBNST that project to the VTA
To determine whether CRF-positive neurons within the vBNST project to the VTA, we injected CTb unilaterally into the VTA and looked for the presence of CTb/CRF double-labeled perikarya in the vBNST in four rats. Representative photomicrographs from one rat are shown in Figure 5. CTb-immunoreactive (CTb-ir) perikarya were observed in the vBNST ipsilateral, to the VTA injection site (average of 45 CTb-ir perikarya/ipsilateral vBNST). By contrast, and consistent with a previous report (Dong and Swanson, 2006), CTb immunoreactivity in the vBNST contralateral to the VTA injection site was minimal (average of four immunoreactive cells/contralateral vBNST). CRF-like-immunoreactive (CRF-ir) perikarya and fibers were observed in the vBNST (Fig. 5). Under higher-magnification, three cell types were observed in the vBNST: CTb-ir/CRF-immunonegative, CTB-ir/CRF-ir, and CTb-immunonegative/CRF-ir cells. Notably, most CRF-positive cells in the ipsilateral vBNST were also CTb-immunoreactive (∼80%). By contrast, dual-labeled CRF- and CTb-positive cells were not observed in the contralateral vBNST.
Figure 5.
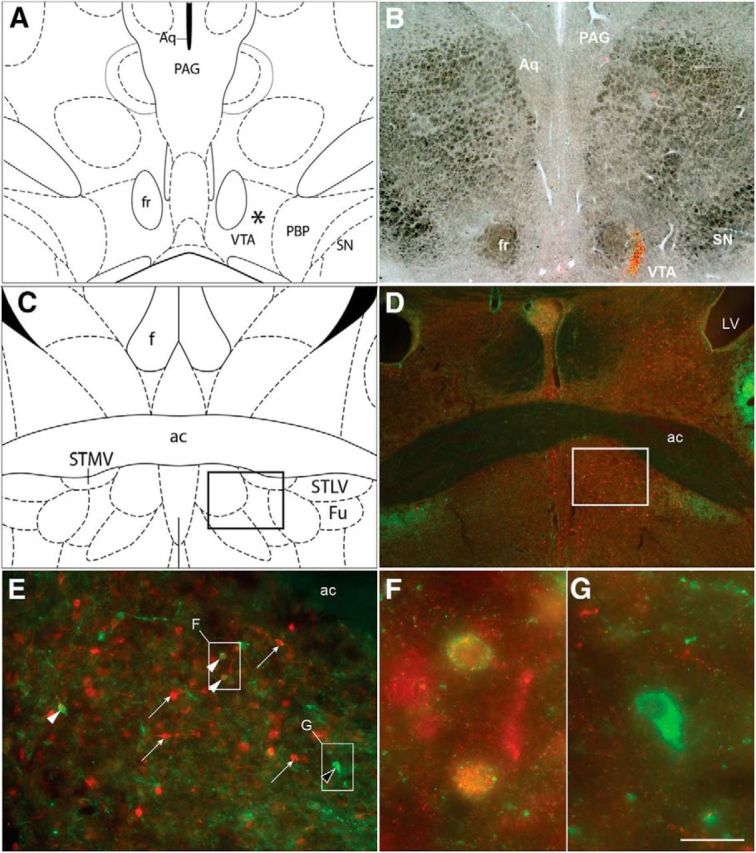
CRF neurons within the vBNST project to the VTA. A, Line drawing (adapted from Paxinos and Watson, 2006) of a coronal section containing the area of the VTA targeted for CTb microinjections and depicted in B. B, Representative photomicrograph depicting the VTA in brightfield, and CTb immunofluorescence in red. C, Line drawing of a coronal section containing the vBNST used for immunostaining of CRF and CTb. D–G, Representative photomicrographs depicting immunostaining for CRF (green) and CTb (red) in the BNST ipsilateral to the VTA injection site. Rectangle in D indicates area shown at higher-magnification in E. E, White arrowheads indicate CRF-ir/CTb-ir (VTA-projecting) neurons; white arrows indicate CTb-ir/CRF-immunonegative neurons; black arrowhead indicates a CRF-ir/CTb-immunonegative cell. Rectangles in E represent areas shown at higher-magnification in indicated panels. F, CRF-ir/CTb-ir neurons in vBNST. G, CRF-positive/CTb-immunonegative cell in BnST. Scale bars: B, D, 500 μm; E, 100 μm; F, G, 33 μm. ac, Anterior commissure; Aq, cerebral aqueduct; f, fornix; fr, fasciculus retroflexus; Fu, bed nucleus of the stria terminalis, fusiform part; PAG, periaqueductal gray; PBP, parabrachial pigmented nucleus of the VTA; SN, substantia nigra; STLV, bed nucleus of the stria terminalis, lateral division, ventral part; STMV, bed nucleus of the stria terminalis, medial division, ventral part.
Experiment 5: effects of vBNST and VTA disconnection on stress-induced cocaine seeking
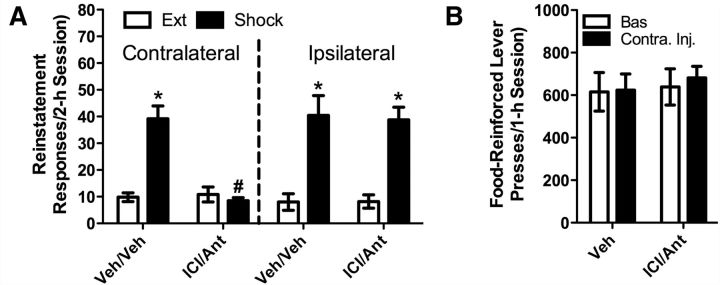
To determine the role of a β-2 AR regulated pathway originating in the vBNST that releases CRF into the VTA in stress-induced cocaine seeking, we used a disconnection approach in which we delivered ICI-118,551 (1 nmol/277 ng) into the vBNST of one hemisphere and antalarmin (1.32 nmol/500 ng) into the contralateral VTA (n = 6 rats). To confirm that any effect on reinstatement was attributable to pathway disconnection, a second group of rats (n = 5) received ICI-118,551 into the vBNST of one hemisphere and antalarmin into the ipsilateral VTA. Contralateral but not ipsilateral antagonist delivery prevented shock-induced reinstatement (Fig. 6A), suggesting that, during stress, β-2 AR in the vBNST regulate one or more pathways that release CRF into the VTA to induce cocaine seeking. A three-way ANOVA showed a significant interaction between reinstatement test condition (shock vs extinction; repeated measure), antagonist pretreatment (ICI-118,551/antalarmin vs vehicle/vehicle treatment; repeated measure), and injection site (contralateral vs ipsilateral; between-subjects; F(1,9) = 7.169; p < 0.05). Further analysis revealed a significant interaction between antagonist treatment and shock-induced reinstatement only in rats that received contralateral antagonist injections (F(1,5) = 25.851; p < 0.01). Post hoc testing showed that significant shock-induced reinstatement was observed following vBNST/VTA vehicle (contralaterally or ipsilaterally) and following ipsilateral intra-vBNST ICI-118,551 and intra-VTA antalarmin pretreatments (p < 0.05 for each comparison) but not following contralateral antagonist delivery. Furthermore, shock-induced reinstatement was significantly reduced following contralateral antagonist injections relative to either vehicle pretreatment or ipsilateral antagonist delivery (p < 0.05 for each comparison). To assess potential motor impairment resulting from the disconnection approach that may have nonspecifically interfered with reinstatement responding, a separate group of six rats were tested for effects on sucrose pellet-reinforced responding. Contralateral intra-vBNST ICI-118,551 and intra-VTA antalarmin delivery failed to alter lever pressing under these conditions (Fig. 6B). Effects of shock were specific to the previously active lever as shock failed to increase responding on a previously inactive lever in vehicle/vehicle-pretreated rats (Ext: 0.88 ± 0.35 responses vs shock: 1.11 ± 0.27). Neither ipsilateral nor contralateral manipulations affected inactive lever responding during reinstatement testing (vehicle/vehicle: 1.11 ± 0.27 responses; ipsilateral: 0.97 ± 0.66; contralateral: 0.93 ± 0.66).
Figure 6.
Disconnection of a β-2 AR-regulated vBNST-to-VTA CRF-releasing pathway prevents stress-induced cocaine seeking. To determine the role of a β-2 AR regulated pathway originating in the vBNST that releases CRF into the VTA in stress-induced cocaine seeking, we used a disconnection approach in which we delivered ICI-118,551 into the vBNST of one hemisphere and antalarmin into the contralateral VTA (n = 6 rats) and tested for shock-induced cocaine seeking. To confirm that any effect on reinstatement was attributable to pathway disconnection, a second group of rats (n = 5) received an ICI-118,551 injection into the vBNST of one hemisphere and an antalarmin injection into the ipsilateral VTA. Significant shock-induced reinstatement was observed following vBNST/VTA vehicle (contralaterally or ipsilaterally) and following ipsilateral intra-vBNST ICI-118,551/intra-VTA antalarmin pretreatment (*p < 0.05 vs Ext) but not following contralateral antagonist delivery (A). Furthermore, shock-induced reinstatement was significantly reduced following contralateral antagonist injections relative to either vehicle pretreatment or ipsilateral antagonist delivery (#p < 0.05 for each comparison). By contrast, contralateral antagonist injections failed to altered food-reinforced lever pressing (B; n = 6).
Experiment 6: role of vBNST CRF-R1 receptors in cocaine seeking induced by vBNST β-2 AR activation
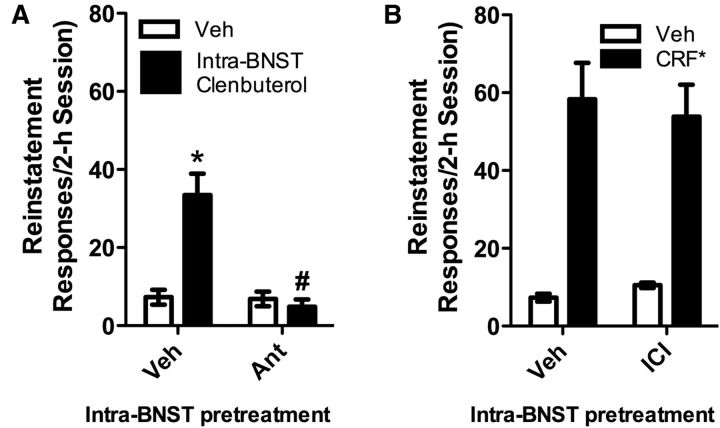
Because it has been reported that CRF receptors in the BNST are also required for stress-induced cocaine seeking (Erb and Stewart, 1999; Erb et al., 2001), and because BNST β ARs have been proposed to modulate efferent projections to the VTA via regulation of local CRF actions (Silberman et al., 2013), we hypothesized that reinstatement induced by vBNST β-2 AR activation requires local CRF receptor activation. To test this hypothesis we delivered antalarmin (1.32 nmol/500 ng per side) bilaterally into the vBNST before bilateral vBNST injection of clenbuterol (36 pmol/10 ng per side). As shown in Figure 7, intra-vBNST antalarmin delivery prevented intra-vBNST clenbuterol-induced cocaine seeking (n = 7). Two-way repeated-measures ANOVA showed a significant reinstatement effect (F(1,6) = 25.910; p < 0.01) and a significant main effect of antalarmin pretreatment (F(1,6) = 29.883; p < 0.01), as well as a significant reinstatement × antalarmin pretreatment interaction (F(1,6) = 35.968; p < 0.01). Clenbuterol reinstated cocaine seeking in vehicle but not antalarmin-pretreated rats (p < 0.01 vs Ext) and reinstatement was reduced in antalarmin- versus vehicle-pretreated rats (p < 0.01). By contrast, bilateral intra-vBNST pretreatment with the β-2 AR antagonist, ICI-118,551 (1 nmol/277 ng per side) failed to attenuate reinstatement in response to intra-vBNST CRF delivery (63 pmol/300 ng per side). Two-way repeated-measures ANOVA showed an overall effect of CRF (F(1,5) = 46.925) but no overall effect of ICI-118,551 pretreatment or no CRF × ICI-118,551 interaction. Altogether, these findings indicate that CRF receptor activation is likely downstream from β-2 AR activation in the sequence of events responsible for stress-induced reinstatement. Effects of intra-BNST clenbuterol (2.14 ± 0.67 responses vs 1.38 ± 0.73 responses during extinction) and CRF (0.66 ± 0.42 vs 0.00 ± 0.00 responses during extinction) on previously inactive lever responding were not observed. Likewise, neither intra-BNST antalarmin (antalarmin/clenbuterol: 1.16 ± 0.16 responses) nor intra-BNST ICI-118,551 (ICI/CRF: 2.5 ± 1.93 responses) significantly altered inactive lever pressing during reinstatement.
Figure 7.
Reinstatement of cocaine seeking by intra-vBNST clenbuterol injection requires activation of vBNST CRF-R1 receptors. Data represent the effects of A: bilateral intra-vBNST pretreatment with antalarmin (1.32 nmol/500 ng per side) or vehicle on reinstatement (responses/2 h session ± SE) in response to bilateral intra-vBNST clenbuterol injection (36 pmol/10 ng per side), or B: bilateral intra-vBNST pretreatment with ICI-118,551 (1 nmol/277 ng per side) or vehicle on reinstatement in response to bilateral intra-vBNST CRF injection (63 pmol/300 ng per side). Significant clenbuterol-induced reinstatement was observed in vehicle but not antalarmin pretreated rats (*p < 0.05 vs Ext). Responding following antalarmin was significantly reduced compared with vehicle pretreatment (#p < 0.05). By contrast, significant CRF-induced reinstatement was observed in both vehicle and ICI-118,551 pretreated rats (*p < 0.05 vs Ext) and did not significantly differ between the two pretreatment conditions.
Discussion
Our results extend earlier findings that the reinstatement of cocaine seeking by a stressor, electric footshock, requires CRF actions in the VTA (Wang et al., 2005; Blacktop et al., 2011) and vBNST β AR activation (Leri et al., 2002) by showing that vBNST β-2 AR activation is both necessary and sufficient for stress-induced reinstatement. Moreover, using a disconnection approach in which we block β-2 ARs in the vBNST of one hemisphere and VTA CRF-R1 receptors in the contralateral hemisphere, we demonstrate that vBNST β-2 ARs regulate a CRF-releasing pathway to the VTA that is necessary for stress-induced cocaine seeking. As VTA CTb delivery labels CRF-positive cells in the ipsilateral vBNST, it is likely that this pathway consists (at least partly) of vBNST CRF neurons that project directly to the VTA. Consistent with reports that CRF actions in the BNST are required for stressor-induced cocaine seeking (Erb and Stewart, 1999; Erb et al., 2001), we demonstrate that vBNST CRF-R1 activation is necessary for reinstatement induced by local delivery of the β-2 AR agonist, clenbuterol, suggesting that β-2 AR-mediated activation of this BNST-to-VTA pathway also requires local CRF receptor activation.
Findings from laboratory studies in human cocaine addicts (Jobes et al., 2011) and preclinical experiments in rodents (Erb et al., 2000; Mantsch et al., 2010) point to a role for noradrenergic signaling in the stress-induced relapse to cocaine use. Specifically, our studies in mice suggest that β-2 ARs are critical for stress-induced cocaine seeking (Vranjkovic et al., 2012). As a target for ascending noradrenergic projections (Ricardo and Koh, 1978; Weller and Smith, 1982; Woulfe et al., 1990) and a key site for integration of stress and reward networks (Flavin and Winder, 2013; Stamatakis et al., 2014), the BNST is a likely location at which stress-induced increases in norepinephrine regulate drug use. Although shock-induced increases in norepinephrine have not been reported, a variety of distinct stressors, including immobilization (Pacak et al., 1995), visceral pain (Deyama et al., 2009), and exposure to a shock-conditioned stimulus (Onaka and Yagi, 1998), fox odor (Fendt et al., 2005), or an aversive tastant (Park et al., 2012), have been demonstrated to increase noradrenergic neurotransmission in the BNST. In particular, the vBNST receives very dense noradrenergic innervation (Woulfe et al., 1988; Georges and Aston-Jones, 2001; Egli et al., 2005). Moreover, delivery of a mixture of β-1 and β-2 AR antagonists into the vBNST has been reported to prevent shock-induced reinstatement in rats (Leri et al., 2002). Our data extend these findings by demonstrating that β-2 but not β-1 AR activation in the vBNST is necessary for shock-induced cocaine seeking and that intra-vBNST injection of a β-2 AR- but not a β-1 AR-selective agonist is sufficient to reinstate.
Beta-2 ARs are expressed throughout the BNST (Cecchi et al., 2007). Whereas in the dorsal BNST β AR activation can have either excitatory or inhibitor effects on synaptic transmission, β AR effects in the vBNST are predominantly inhibitory (Egli et al., 2005) and include inhibition of neurons that project directly to the VTA (Dumont and Williams, 2004). In the dorsal BNST, β-AR regulation of neuronal activity requires CRF-R1 activation (Nobis et al., 2011). Although the involvement of CRF in norepinephrine-dependent effects in the vBNST has not been established, our finding that local antalarmin pretreatment blocks reinstatement by intra-vBNST clenbuterol delivery supports a role for local CRF actions in the regulation of cocaine seeking by norepinephrine and β-2 AR activation. Consistent with this possibility, previous studies demonstrated that (1) noradrenergic terminals synapse on the dendrites of CRF-positive neurons in the vBNST (Phelix et al., 1994), (2) β-2 AR-mediated reinstatement requires CRF-R1 activation (McReynolds et al., 2014), and (3) the β-2 AR antagonist, ICI-118,551, blocks stress-induced increases of BNST CRF mRNA (McReynolds et al., 2014). Overall, the findings are consistent with reports that cocaine seeking induced by central norepinephrine delivery is blocked by CRF receptor antagonism, whereas suppression of noradrenergic function using the α-2 AR agonist, clonidine, does not affect reinstatement in response to central CRF delivery (Brown et al., 2009).
Notably, the magnitude of cocaine seeking in response to intra-vBNST clenbuterol was lower than that observed following either shock or CRF. This could be attributed to (1) secondary effects of BNST β-2 AR activation that are not engaged by shock or CRF and offset responding, (2) unique attributes of clenbuterol itself (e.g., pharmacokinetics, ligand-specific receptor desensitization), or (3) the requirement for additional stress-responsive mediators to fully engage the processes that lead to cocaine seeking.
Our findings suggest that the vBNST influences cocaine seeking via regulation of the VTA. Consistent with this possibility, stress-induced reinstatement in mice is associated with activation of VTA-projecting BNST neurons (Briand et al., 2010) and a role for a vBNST-VTA pathway in the expression of cocaine seeking has been reported (Sartor and Aston-Jones, 2012). In light of these findings, it is surprising that vBNST β-ARs have been found to exert inhibitory effects on neurons that project to the VTA, likely via stimulation of local GABA release (Dumont and Williams, 2004). Both GABAergic and glutamatergic projections from the BNST to the VTA have been identified (Georges and Aston-Jones, 2001, 2002; Kudo et al., 2012; Jennings et al., 2013). Moreover, CRF-positive terminals within the VTA coexpress either the glutamatergic neuronal marker, vesicular glutamate transporter 2, or the GABAergic neuronal marker, glutamic acid decarboxylase, and make contacts that have morphological characteristics of either excitatory or inhibitory synapses, suggesting that CRF can be coreleased into the VTA along with either GABA or glutamate (Tagliaferro and Morales, 2008). Further investigation is needed to (1) confirm the neurochemical phenotype of VTA-projecting vBNST CRF-positive neurons, (2) clarify the mechanism through which β-2 ARs regulate these neurons and determine how this regulation might change following cocaine exposure, and (3) examine the likelihood that β-2 AR in the vBNST may also regulate CRF and/or CRF actions in the VTA via parallel multisynaptic pathways. Consistent with the last possibility, BNST efferents can influence the VTA through multisynaptic pathways that include regions, such as the lateral habenula and rostromedial tegmental area (Dong and Swanson, 2006; Kaufling et al., 2009; Lammel et al., 2012).
It has been found that (1) shock elevates VTA CRF levels (Wang et al., 2005), (2) intra-VTA CRF delivery is sufficient to induce cocaine seeking (Wang et al., 2005; Blacktop et al., 2011), and (3) VTA CRF receptor activation is required for stress-induced reinstatement (Wang et al., 2005; Blacktop et al., 2011) in rats. Our demonstration that intra-VTA antalarmin delivery prevents shock-induced reinstatement is consistent with these reports. As the antalarmin dose used may also block CRF-R2 receptors, our findings are not definitive regarding which CRF receptor subtype is involved. However, we have reported that, in rats tested under identical conditions, intra-VTA delivery of a CRF-R2-selective antagonist does not prevent shock-induced reinstatement, whereas intra-VTA injection of a CRF-R1 but not a CRF-R2-selective agonist is sufficient to induce cocaine seeking (Blacktop et al., 2011).
CRF actions in the VTA are complex and involve both excitatory and inhibitory effects that vary depending on the receptor and site (Ungless et al., 2003; Riegel and Williams, 2008; Wanat et al., 2008; Beckstead et al., 2009; Hahn et al., 2009; Wanat et al., 2013). Likely VTA targets for CRF include mesocortical DA cells, which are activated during stress (Deutch et al., 1991). Elevated medial prefrontal cortex DA, via activation of DA receptors, is necessary for stress-induced reinstatement (Capriles et al., 2003; McFarland et al., 2004). We demonstrate that the mechanisms through which vBNST β-2 ARs regulate this CRF projection to the VTA requires local CRF actions, likely at CRF-R1. This finding is consistent with reports that (1) depolarization of BNST neurons by the nonselective β-AR agonist, isoproterenol, is CRF-dependent (Nobis et al., 2011), (2) CRF in the BNST depolarizes neurons that project to the VTA (Silberman et al., 2013), and (3) stress-induced increases in CRF mRNA in the BNST in mice are β-2 AR-dependent (McReynolds et al., 2014). Moreover it is consistent with reports that intra-BNST antalarmin delivery prevents stress-induced cocaine seeking (Erb and Stewart, 1999; Erb et al., 2001). Although the requirement for vBNST CRF is evident, the source of this CRF has not been identified and could include intrinsic cell populations within the BNST and/or CRF-releasing projections originating in other regions (e.g., the central amygdala). A clearer mechanistic understanding awaits determination of BNST β-2 AR receptor localization as it relates to that of CRF and CRF-R1.
Although it is likely that the mechanisms through which stress promotes relapse are not identical across drug classes, both CRF-R1 receptor activation (Shaham et al., 1997; Lê et al., 2000; Bruijnzeel et al., 2009) and central noradrenergic signaling (Erb et al., 2000; Shaham et al., 2000; Lê et al., 2005; Yamada and Bruijnzeel, 2011) are necessary for stress-induced reinstatement of heroin-, nicotine-, and alcohol-seeking in rats. Moreover, in the case of heroin, stress-induced drug seeking requires CRF (Wang et al., 2006) and noradrenergic (Wang et al., 2001) signaling within the BNST. Notably, similar processes in the BNST may be engaged during withdrawal from ethanol (Francesconi et al., 2009; Huang et al., 2010; Silberman et al., 2013) and opioids (Delfs et al., 2000; Fuentealba et al., 2000), suggesting that this pathway to the VTA, or parallel pathways originating in other BNST subregions may also contribute to withdrawal-related drug seeking.
To summarize, we report that, during stress, norepinephrine released into the vBNST activates β-2 ARs, which via a CRF-dependent process, activate neurons that release CRF into the VTA, thereby leading to cocaine seeking. Identification of the processes responsible for stress-induced cocaine seeking should guide the development of new treatment approaches aimed at managing relapse, particularly in individuals whose use is stress-related.
Footnotes
This work was supported by National Institute on Drug Abuse Grant DA15758 to J.R.M.
The authors declare no competing financial interests.
References
- Baker JG. The selectivity of β-adrenoceptor antagonists at the human β1, β2 and β3 adrenoceptors. Br J Pharmacol. 2005;144:317–322. doi: 10.1038/sj.bjp.0706048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker JG. The selectivity of β-adrenoceptor agonists at human β1-, β2- and β3-adrenoceptors. Br J Pharmacol. 2010;160:1048–1061. doi: 10.1111/j.1476-5381.2010.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckstead MJ, Gantz SC, Ford CP, Stenzel-Poore MP, Phillips PE, Mark GP, Williams JT. CRF enhancement of GIRK channel-mediated transmission in dopamine neurons. Neuropsychopharmacology. 2009;34:1926–1935. doi: 10.1038/npp.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacktop JM, Seubert C, Baker DA, Ferda N, Lee G, Graf EN, Mantsch JR. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J Neurosci. 2011;31:11396–11403. doi: 10.1523/JNEUROSCI.1393-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briand LA, Vassoler FM, Pierce RC, Valentino RJ, Blendy JA. Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein. J Neurosci. 2010;30:16149–16159. doi: 10.1523/JNEUROSCI.2827-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZJ, Tribe E, D'souza NA, Erb S. Interaction between noradrenaline and corticotrophin-releasing factor in the reinstatement of cocaine seeking in the rat. Psychopharmacology. 2009;203:121–130. doi: 10.1007/s00213-008-1376-4. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Prado M, Isaac S. Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry. 2009;66:110–117. doi: 10.1016/j.biopsych.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capriles N, Rodaros D, Sorge RE, Stewart J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2003;168:66–74. doi: 10.1007/s00213-002-1283-z. [DOI] [PubMed] [Google Scholar]
- Cecchi M, Capriles N, Watson SJ, Akil H. β1 Adrenergic receptors in the bed nucleus of stria terminalis mediate differential responses to opiate withdrawal. Neuropsychopharmacology. 2007;32:589–599. doi: 10.1038/sj.npp.1301140. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature. 2000;403:430–434. doi: 10.1038/35000212. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Lee MC, Gillham MH, Cameron DA, Goldstein M, Iadarola MJ. Stress selectively increases fos protein in dopamine neurons innervating the prefrontal cortex. Cereb Cortex. 1991;1:273–292. doi: 10.1093/cercor/1.4.273. [DOI] [PubMed] [Google Scholar]
- Deyama S, Katayama T, Kondoh N, Nakagawa T, Kaneko S, Yamaguchi T, Yoshioka M, Minami M. Role of enhanced noradrenergic transmission within the ventral bed nucleus of the stria terminalis in visceral pain-induced aversion in rats. Behav Brain Res. 2009;197:279–283. doi: 10.1016/j.bbr.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, dorsomedial nucleus: implications for cerebral hemisphere integration of neuroendocrine, autonomic, and drinking responses. J Comp Neurol. 2006;494:75–107. doi: 10.1002/cne.20790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont EC, Williams JT. Noradrenaline triggers GABAA inhibition of bed nucleus of the stria terminalis neurons projecting to the ventral tegmental area. J Neurosci. 2004;24:8198–8204. doi: 10.1523/JNEUROSCI.0425-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli RE, Kash TL, Choo K, Savchenko V, Matthews RT, Blakely RD, Winder DG. Norepinephrine modulates glutamatergic transmission in the bed nucleus of the stria terminalis. Neuropsychopharmacology. 2005;30:657–668. doi: 10.1038/sj.npp.1300639. [DOI] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotropin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenergic receptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Fendt M, Siegl S, Steiniger-Brach B. Noradrenaline transmission within the ventral bed nucleus of the stria terminalis is critical for fear behavior induced by trimethylthiazoline, a component of fox odor. J Neurosci. 2005;25:5998–6004. doi: 10.1523/JNEUROSCI.1028-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavin SA, Winder DG. Noradrenergic control of the bed nucleus of the stria terminalis in stress and reward. Neuropharmacology. 2013;70:324–330. doi: 10.1016/j.neuropharm.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francesconi W, Berton F, Repunte-Canonigo V, Hagihara K, Thurbon D, Lekic D, Specio SE, Greenwell TN, Chen SA, Rice KC, Richardson HN, O'Dell LE, Zorrilla EP, Morales M, Koob GF, Sanna PP. Protracted withdrawal from alcohol and drugs of abuse impairs long-term potentiation of intrinsic excitability in the juxtacapsular bed nucleus of the stria terminalis. J Neurosci. 2009;29:5389–5401. doi: 10.1523/JNEUROSCI.5129-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba JA, Forray MI, Gysling K. Chronic morphine treatment and withdrawal increase extracellular levels of norepinephrine in the rat bed nucleus of the stria terminalis. J Neurochem. 2000;75:741–748. doi: 10.1046/j.1471-4159.2000.0750741.x. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci. 2001;21:RC160. doi: 10.1523/JNEUROSCI.21-16-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf EN, Hoks MA, Baumgardner J, Sierra J, Vranjkovic O, Bohr C, Baker DA, Mantsch JR. Adrenal activity during repeated long-access cocaine self-administration is required for later CRF-induced and CRF-dependent stressor-induced reinstatement in rats. Neuropsychopharmacology. 2011;36:1444–1454. doi: 10.1038/npp.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Hopf FW, Bonci A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J Neurosci. 2009;29:6535–6544. doi: 10.1523/JNEUROSCI.4773-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MM, Overstreet DH, Knapp DJ, Angel R, Wills TA, Navarro M, Rivier J, Vale W, Breese GR. Corticotropin-releasing factor (CRF) sensitization of ethanol withdrawal-induced anxiety-like behavior is brain site specific and mediated by CRF-1 receptors: relation to stress-induced sensitization. J Pharmacol Exp Ther. 2010;332:298–307. doi: 10.1124/jpet.109.159186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings JH, Sparta DR, Stamatakis AM, Ung RL, Pleil KE, Kash TL, Stuber GD. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobes ML, Ghitza UE, Epstein DH, Phillips KA, Heishman SJ, Preston KL. Clonidine blocks stress-induced craving in cocaine users. Psychopharmacology. 2011;218:83–88. doi: 10.1007/s00213-011-2230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufling J, Veinante P, Pawlowski SA, Freund-Mercier MJ, Barrot M. Afferents to the GABAergic tail of the ventral tegmental area in the rat. J Comp Neurol. 2009;513:597–621. doi: 10.1002/cne.21983. [DOI] [PubMed] [Google Scholar]
- Kudo T, Uchigashima M, Miyazaki T, Konno K, Yamasaki M, Yanagawa Y, Minami M, Watanabe M. Three types of neurochemical projection from the bed nucleus of the stria terminalis to the ventral tegmental area in adult mice. J Neurosci. 2012;32:18035–18046. doi: 10.1523/JNEUROSCI.4057-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Niehoff KE, Kalivas PW. The infralimbic cortex regulates the consolidation of extinction after cocaine self-administration. Learn Mem. 2010;17:168–175. doi: 10.1101/lm.1576810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Ran C, Huang KW, Betley MJ, Tye KM, Deisseroth K, Malenka RC. Input-specific control of reward and aversion in the ventral tegmental area. Nature. 2012;491:212–217. doi: 10.1038/nature11527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Harding S, Juzytsch W, Watchus J, Shalev U, Shaham Y. The role of corticotrophin-releasing factor in stress-induced relapse to alcohol-seeking behavior in rats. Psychopharmacol. 2000;150:317–324. doi: 10.1007/s002130000411. [DOI] [PubMed] [Google Scholar]
- Lê AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology. 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery-Gionta EG, Navarro M, Li C, Pleil KE, Rinker JA, Cox BR, Sprow GM, Kash TL, Thiele TE. Corticotropin releasing factor signaling in the central amygdala is recruited during binge-like ethanol consumption in C57BL/6J mice. J Neurosci. 2012;32:3405–3413. doi: 10.1523/JNEUROSCI.6256-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantsch JR, Weyer A, Vranjkovic O, Beyer CE, Baker DA, Caretta H. Involvement of noradrenergic neurotransmission in the stress- but not cocaine-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: role for beta-2 adrenergic receptors. Neuropsychopharmacology. 2010;35:2165–2178. doi: 10.1038/npp.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland K, Davidge SB, Lapish CC, Kalivas PW. Limbic and motor circuitry underlying footshock-induced reinstatement of cocaine-seeking behavior. J Neurosci. 2004;24:1551–1560. doi: 10.1523/JNEUROSCI.4177-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds JR, Vranjkovic O, Thao M, Baker DA, Makky K, Lim Y, Mantsch JR. Beta-2 adrenergic receptors mediate stress-evoked reinstatement of cocaine-induced conditioned place preference and increases in CRF mRNA in the bed nucleus of the stria terminalis in mice. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3535-0. Advance online publication. Retrieved July 28, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobis WP, Kash TL, Silberman Y, Winder DG. β-Adrenergic receptors enhance excitatory transmission in the bed nucleus of the stria terminalis through a corticotrophin-releasing factor receptor-dependent and cocaine-regulated mechanism. Biol Psychiatry. 2011;69:1083–1090. doi: 10.1016/j.biopsych.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onaka T, Yagi K. Role of noradrenergic projections to the bed nucleus of the stria terminalis in neuroendocrine and behavioral responses to fear-related stimuli in rats. Brain Res. 1998;788:287–293. doi: 10.1016/S0006-8993(98)00012-2. [DOI] [PubMed] [Google Scholar]
- Pacak K, McCarty R, Palkovits M, Kopin IJ, Goldstein DS. Effects of immobilization on in vivo release of norepinephrine in the bed nucleus of the stria terminalis in conscious rats. Brain Res. 1995;688:242–246. doi: 10.1016/0006-8993(95)00566-9. [DOI] [PubMed] [Google Scholar]
- Park J, Wheeler RA, Fontillas K, Keithley RB, Carelli RM, Wightman RM. Catecholamines in the bed nucleus of the stria terminalis reciprocally respond to reward and aversion. Biol Psychiatry. 2012;71:327–334. doi: 10.1016/j.biopsych.2011.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 6. San Diego: Academic; 2006. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Monoamine innervation of bed nucleus of stria terminalis: an electron microscopic investigation. Brain Res Bull. 1992;28:949–965. doi: 10.1016/0361-9230(92)90218-M. [DOI] [PubMed] [Google Scholar]
- Phelix CF, Liposits Z, Paull WK. Catecholamine-CRF synaptic interaction in a septal bed nucleus: afferents of neurons in the bed nucleus of the stria terminalis. Brain Res Bull. 1994;33:109–119. doi: 10.1016/0361-9230(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Phillipson OT. Afferent projections to the ventral tegmental area of Tsai and interfascicular nucleus: a horseradish peroxidase study in the rat. J Comp Neurol. 1979;187:117–143. doi: 10.1002/cne.901870108. [DOI] [PubMed] [Google Scholar]
- Ricardo JA, Koh ET. Anatomical evidence of direct projections from the nucleus of the solitary tract to the hypothalamus, amygdala, and other forebrain structures in the rat. Brain Res. 1978;153:1–26. doi: 10.1016/0006-8993(78)91125-3. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Williams JT. CRF facilitates calcium release from intracellular stores in midbrain dopamine neurons. Neuron. 2008;57:559–570. doi: 10.1016/j.neuron.2007.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodaros D, Caruana DA, Amir S, Stewart J. Corticotropin-releasing factor projections from limbic forebrain and paraventricular nucleus of the hypothalamus to the region of the ventral tegmental area. Neuroscience. 2007;150:8–13. doi: 10.1016/j.neuroscience.2007.09.043. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Schelling G, McGaugh JL. Corticotropin-releasing factor in the basolateral amygdala enhances memory consolidation via an interaction with the β-adrenoceptor–cAMP pathway: dependence on glucocorticoid receptor activation. J Neurosci. 2008;28:6642–6651. doi: 10.1523/JNEUROSCI.1336-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanaka M, Shibasaki T, Lederis K. Distribution and efferent projections of corticotropin-releasing factor-like immunoreactivity in the rat amygdaloid complex. Brain Res. 1986;382:213–238. doi: 10.1016/0006-8993(86)91332-6. [DOI] [PubMed] [Google Scholar]
- Sartor GC, Aston-Jones G. Regulation of the ventral tegmental area by the bed nucleus of the stria terminalis is required for expression of cocaine preference. Eur J Neurosci. 2012;36:3549–3558. doi: 10.1111/j.1460-9568.2012.08277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Funk D, Erb S, Brown TJ, Walker CD, Stewart J. Corticotropin-releasing factor, but not corticosterone, is involved in stress-induced relapse to heroin-seeking in rats. J Neurosci. 1997;17:2605–2614. doi: 10.1523/JNEUROSCI.17-07-02605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Highfield D, Delfs J, Leung S, Stewart J. Clonidine blocks stress-induced reinstatement of heroin seeking in rats: an effect independent of locus coeruleus noradrenergic neurons. Eur J Neurosci. 2000;12:292–302. doi: 10.1046/j.1460-9568.2000.00899.x. [DOI] [PubMed] [Google Scholar]
- Silberman Y, Matthews RT, Winder DG. A corticotropin releasing factor pathway for ethanol regulation of the ventral tegmental area in the bed nucleus of the stria terminalis. J Neurosci. 2013;33:950–960. doi: 10.1523/JNEUROSCI.2949-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, Stuber GD. Amygdala and bed nucleus of the stria terminalis circuitry: implications for addiction-related behaviors. Neuropharmacology. 2014;76:320–328. doi: 10.1016/j.neuropharm.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliaferro P, Morales M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J Comp Neurol. 2008;506:616–626. doi: 10.1002/cne.21576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Singh V, Crowder TL, Yaka R, Ron D, Bonci A. Corticotropin-releasing factor requires CRF binding protein to potentiate NMDA receptors via CRF receptor 2 in dopamine neurons. Neuron. 2003;39:401–407. doi: 10.1016/S0896-6273(03)00461-6. [DOI] [PubMed] [Google Scholar]
- Vranjkovic O, Hang S, Baker DA, Mantsch JR. β-Adrenergic receptor mediation of stress-induced reinstatement of extinguished cocaine-induced conditioned place preference in mice: roles for β1 and β2 adrenergic receptors. J Pharmacol Exp Ther. 2012;342:541–551. doi: 10.1124/jpet.112.193615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Hopf FW, Stuber GD, Phillips PE, Bonci A. Corticotropin-releasing factor increases mouse ventral tegmental area dopamine neuron firing through a protein kinase C-dependent enhancement of Ih. J Physiol. 2008;586:2157–2170. doi: 10.1113/jphysiol.2007.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat MJ, Bonci A, Phillips PE. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat Neurosci. 2013;16:383–385. doi: 10.1038/nn.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Shaham Y, Zitzman D, Azari S, Wise RA, You ZB. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J Neurosci. 2005;25:5389–5396. doi: 10.1523/JNEUROSCI.0955-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Fang Q, Liu Z, Lu L. Region-specific effects of brain corticotropin-releasing factor receptor type 1 blockade on footshock-stress- or drug-priming-induced reinstatement of morphine conditioned place preference in rats. Psychopharmacology. 2006;185:19–28. doi: 10.1007/s00213-005-0262-6. [DOI] [PubMed] [Google Scholar]
- Wang X, Cen X, Lu L. Noradrenaline in the bed nucleus of the stria terminalis is critical for stress-induced reactivation of morphine-conditioned place preference in rats. Eur J Pharmacol. 2001;432:153–161. doi: 10.1016/S0014-2999(01)01487-X. [DOI] [PubMed] [Google Scholar]
- Webster EL, Lewis DB, Torpy DJ, Zachman EK, Rice KC, Chrousos GP. In vivo and in vitro characterization of antalarmin, a nonpeptide corticotropin-releasing hormone (CRH) receptor antagonist: suppression of pituitary ACTH release and peripheral inflammation. Endocrinology. 1996;137:5747–5750. doi: 10.1210/endo.137.12.8940412. [DOI] [PubMed] [Google Scholar]
- Weller KL, Smith DA. Afferent connections to the bed nucleus of the stria terminalis. Brain Res. 1982;232:255–270. doi: 10.1016/0006-8993(82)90272-4. [DOI] [PubMed] [Google Scholar]
- Woulfe JM, Hrycyshyn AW, Flumerfelt BA. Collateral axonal projections from the A1 noradrenergic cell group to the paraventricular nucleus and bed nucleus of the stria terminalis in the rat. Exp Neurol. 1988;102:121–124. doi: 10.1016/0014-4886(88)90084-2. [DOI] [PubMed] [Google Scholar]
- Woulfe JM, Flumerfelt BA, Hrycyshyn AW. Efferent connections of the A1 noradrenergic cell group: a DBH immunohistochemical and PHA-L anterograde tracing study. Exp Neurol. 1990;109:308–322. doi: 10.1016/S0014-4886(05)80022-6. [DOI] [PubMed] [Google Scholar]
- Yamada H, Bruijnzeel AW. Stimulation of α2-adrenergic receptors in the central nucleus of the amygdala attenuates stress-induced reinstatement of nicotine seeking in rats. Neuropharmacology. 2011;60:303–311. doi: 10.1016/j.neuropharm.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]