Abstract
Background
Clozapine is widely used for people with schizophrenia. Although agranulocytosis, weight gain, and cardiac problems are serious problems associated with its use, hypersalivation, sometimes of a gross and socially unacceptable quantity, is also common (30‐80%).
Objectives
To determine the clinical effects of pharmacological interventions for clozapine‐induced hypersalivation.
Search methods
We searched the Cochrane Schizophrenia Group Trials Register (March 2007), inspected references of all identified studies for further trials, contacted relevant pharmaceutical companies, drug approval agencies and authors of trials.
Selection criteria
We included randomised controlled trials comparing pharmacological interventions, at any dose and by any route of administration, for clozapine‐induced hypersalivation.
Data collection and analysis
We extracted data independently. For dichotomous data (homogenous) we calculated relative risk (RR) with 95% confidence intervals (CI) and numbers needed to treat (NNT) on an intention‐to‐treat basis. We calculated weighted mean difference (WMD) for continuous data.
Main results
Of the 15 trials identified, 14 were conducted in China and 14 in hospitals. The quality of reporting was poor with no studies clearly describing allocation concealment and much data were missing or unusable. All results are vulnerable to considerable bias. Most frequently the primary outcome was the diameter of the wet patch on the pillow. Antimuscarinics (astemizole, diphenhydramine, propantheline, doxepin) were the most commonly evaluated drugs. For the outcome of 'no clinically important improvement' astemizole and diphenhydramine were more effective than placebo (astemizole: n=97, 2 RCTs, RR 0.61 CI 0.47 to 0.81 NNT 3 CI 2 to 5; diphenhydramine: n=131, 2 RCTs, RR 0.43 CI 0.31 to 0.58, NNT 2 CI 1.5 to 2.5), but the doses of astemizole used were those that can cause toxicity. Data involving propantheline were heterogeneous (I2= 86.6%), but both studies showed benefit over placebo. Adverse effects were poorly recorded.
Of the other interventions, oryzanol (rice bran oil and rice embryo oil extract) showed benefit over the antimuscarinic doxepin in terms of 'no clinically important change' (n=104, 1 RCT, RR 0.45 CI 0.27 to 0.75, NNT 4 CI 2 to 7). The Chinese medicine suo quo wan (comprises spicebush root, Chinese yam and bitter cardamom) showed benefit over doxepin (n=70, 1 RCT, RR 'no clinically important change' 0.31 CI 0.16 to 0.59, NNT 3 CI 1.5 to 3.7).
Authors' conclusions
There are currently insufficient data to confidently inform clinical practice. The limitations of these studies are plentiful and the risk of bias is high. These trials, however, are invaluable guides for current and future study design. Well conducted randomised trials are possible. Some may be underway. Current practice outside of well designed randomised trials should be clearly justified.
Keywords: Antipsychotic Agents; Antipsychotic Agents/adverse effects; Clozapine; Clozapine/adverse effects; Drugs, Chinese Herbal; Drugs, Chinese Herbal/therapeutic use; Muscarinic Antagonists; Muscarinic Antagonists/therapeutic use; Phenylpropionates; Phenylpropionates/therapeutic use; Randomized Controlled Trials as Topic; Sialorrhea; Sialorrhea/chemically induced; Sialorrhea/drug therapy
Plain language summary
Interventions for people with schizophrenia who have too much saliva due to clozapine treatment
Clozapine is an antipsychotic medication used in the treatment of schizophrenia, a mental health problem that can cause symptoms such as hallucinations and delusions and social withdrawal. Clozapine may be useful in those for whom other medications have not worked very well. One of the common side‐effects of clozapine is having too much saliva in the mouth (hypersalivation). This can be embarrassing in public and problematic, especially at night. This review is about ways of reducing this problem and includes 15 trials containing 964 people, most of which were done in hospitals in China. Treatments included medications that had previously been useful for this problem or were thought to work in theory. The medications used were from a group of drugs called antimuscarinics, traditional Chinese medicines or others. The trials were short (all four weeks or less). From these trials the antimuscarinics; astemizole, diphenhydramine and propantheline, were shown to be better than placebo at reducing hypersalivation. Another medication called oryzanol and a Chinese traditional medicine called Suo quo wan were found to have benefit over doxepin, an antimuscarinic. However, because of the shortness of the trials, poor reporting and the limitations of design, it is difficult to draw any firm conclusions from these results. (Plain language summary prepared for this review by Janey Antoniou of RETHINK, UK, www.rethink.org)
Background
Clozapine is an antipsychotic drug that was first manufactured in 1959 and introduced into clinical practice in the 1970s. Clozapine's chemical profile and clinical effects differ from those of traditional drugs used to treat schizophrenia. In particular it causes less movement disorders than traditional antipsychotics such as haloperidol. Clozapine has been shown to be more effective in the treatment of schizophrenia than typical antipsychotics (Wahlbeck 1999). However due to a reversible but potentially fatal side effect, loss of granulocytic white cells (agranulocytosis), in many countries it is now reserved for those who are unresponsive to other antipsychotics. In addition to agranulocytosis, the optimal use of clozapine may also be compromised by other adverse effects including weight gain, cardiac problems and hypersalivation or sialorrhoea.
Clozapine‐induced hypersalivation occurs quite frequently; reported incidences range from 30% (Rogers 2000, Davydov 2000) to 80% (Ben‐Aryeh 1996, Schmauss 1989). Observation shows that clozapine‐induced hypersalivation can wear off with time; however, it can be severe and persistent and is often particularly problematic at night. The consequences of hypersalivation can be embarrassing and in some cases life threatening. Excessive drooling can lead to wet pillows and clothing and to speech difficulties that can be embarrassing and uncomfortable. Some people experience a choking sensation and aspiration of excess saliva may occur (Young 1998) with the risk of aspiration pneumonia (Hinkes 1996). Hypersalivation has also been associated with cases of parotid gland swelling and inflammation (Brodkin 1996, Robinson 1995).
Clozapine‐induced hypersalivation seems to be problematic in the early stages of treatment and is probably dose related (Taylor 2007). Various pharmacological approaches have been used to try and alleviate this problem; the evidence for their use is mainly in the form of case reports and small open studies with only one trial (Kreinin 2005). It is difficult to compare different treatments as there is often little information about the participants studied and no standard measurements or outcomes are used. To the best of our knowledge there are no drug treatments licensed for this indication.
Technical background The pathophysiology of hypersalivation is unclear; several possible mechanisms have been suggested. Clozapine has been shown to be a potent agonist at muscarinic M4 receptors (Zorn 1994); stimulation of M4 receptors causes an increase in salivation. Clozapine is also an alpha2 adrenoceptor antagonist; blockade of alpha2 receptors would be expected to increase salivation (Corrigan 1995). However, two studies of hypersalivation did not detect any significant differences in the composition or flow rate of saliva in people taking clozapine compared to controls (Ben‐Aryeh 1996, Rabinowitz 1996). Other explanations include an alteration in circadian rhythm with increased salivation at night (Ben‐Aryeh 1996) and interference with normal swallowing causing pooling of saliva (Rabinowitz 1996).
Pharmacological treatments are generally either anticholinergic, with the aim of blocking muscarinic receptors, or alpha 2 agonists, to reduce sympathetic stimulation of the salivary glands. Reinstein 1999 found in a non‐randomised trial that Terazosin (an alpha1 receptor antagonist) and Benzatropine (an antimuscarinic agent) in combination was more successful at controlling hypersalivation than either drug alone.
Objectives
To determine the clinical effects of pharmacological interventions for clozapine‐induced hypersalivation compared with placebo or no treatment.
Methods
Criteria for considering studies for this review
Types of studies
We included all relevant randomised controlled trials. We included trials that were described as double‐blind, but that did not mention whether the study was randomised, in a sensitivity analysis. If there was no substantive difference within primary outcomes (see 'Types of outcome measures') when these studies were added, then we included them in the final analysis. If there was a substantive difference, we used only clearly randomised trials and described the results of the sensitivity analysis in the text. We excluded quasi‐randomised studies, such as those allocating by using alternate days of the week.
Types of participants
We included people being treated with clozapine; irrespective of age, gender and diagnosis, with clozapine‐induced hypersalivation however identified (including recipient, carer and clinician).
Types of interventions
All pharmacological interventions, at any dose and by any route of administration, for clozapine‐induced hypersalivation compared with control or no treatment.
We planned to subdivide interventions into drug type: 1. Antimuscarinic drugs (for example, hyoscine, benzatropine) 2. Alpha adrenoceptor agonist drugs (for example, clonidine, lofexidine) 3. Alpha adrenoceptor antagonist drugs (for example, terazosin, yohimbine) 3. Others
Types of outcome measures
1. Measurement of salivation 1.1 Cure 1.2 No clinically important change in hypersalivation (as defined by individual studies)* 1.3 Average endpoint hypersalivation score 1.4 Average change in hypersalivation scores
2. Global state 2.1 Relapse 2.2 No clinically important change in global state (as defined by individual studies) 2.3 Average endpoint global state score 2.4 Average change in global state scores 2.5 Use of other medications
3. Service outcomes 3.1 Hospitalisation 3.2 Time to hospitalisation
4. Mental state (with particular reference to the positive and negative symptoms of schizophrenia) 4.1 No clinically important change in general mental state 4.2 Average endpoint general mental state score 4.3 Average change in general mental state scores 4.4 No clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia, depression, mania) 4.5 Average endpoint specific symptom score 4.6 Average change in specific symptom scores
5. General functioning. 5.1 No clinically important change in general functioning 5.2 Average endpoint general functioning score 5.3 Average change in general functioning scores 5.4 No clinically important change in specific aspects of functioning, such as social or life skills 5.5 Average endpoint specific aspects of functioning, such as social or life skills 5.6 Average change in specific aspects of functioning, such as social or life skills
6. Behaviour 6.1 No clinically important change in general behaviour 6.2 Average endpoint general behaviour score 6.3 Average change in general behaviour scores 6.4 No clinically important change in specific aspects of behaviour 6.5 Average endpoint specific aspects of behaviour 6.6 Average change in specific aspects of behaviour
7. Adverse effects ‐ general and specific 7.1 Clinically important general adverse effects 7.2 Average endpoint general adverse effect score 7.3 Average change in general adverse effect scores 7.4 Clinically important specific adverse effects 7.5 Average endpoint specific adverse effects 7.6 Average change in specific adverse effects 7.7 Sudden and unexpected death
8. Engagement with services
9. Satisfaction with treatment 9.1 Leaving the studies early 9.2 Recipient of care not satisfied with treatment 9.3 Recipient of care average satisfaction score 9.4 Recipient of care average change in satisfaction scores 9.5 Carer not satisfied with treatment 9.6 Carer average satisfaction score 9.7 Carer average change in satisfaction scores
10. Quality of life (recipient or informal carers or professional carers) 10.1 No clinically important change in quality of life 10.2 Average endpoint quality of life score 10.3 Average change in quality of life scores 10.4 No clinically important change in specific aspects of quality of life 10.5 Average endpoint specific aspects of quality of life 10.6 Average change in specific aspects of quality of life
11. Economic outcomes 11.1 Direct costs 11.2 Indirect costs
* We chose No clinically important change in hypersalivation (as defined by individual studies) as the primary outcome measure. We divided outcomes in to short term (less than three months), medium term (3‐12 months) and long term (over one year)
Search methods for identification of studies
Search strategy for identification of studies
1. Electronic searches 1.1 Update search We searched The Cochrane Schizophrenia Group Trials Register (March 2007) using the phrase: [(*hypersaliv* or *drool* or *saliva* or *ptyalism* or *sialism* or *sailorr*) and(*clozapin* OR *clozaril* OR *denzapin* OR *zaponex*) in Title, Abstract and Index fields of REFERENCE and (*drool* or * saliva* or *ptyalism* or *sialism* or *sailorr* or *sialosis*) in Outcomes field of STUDY]
This register is compiled by systematic searches of major databases, hand searches and conference proceedings (see Group Module).
1.2 Previous electronic search/s We searched the Cochrane Schizophrenia Group's Trials Register (May 2005) using the phrase: [(*hypersaliv* or *drool* or * saliva* or *ptyalism* or *sialism* or *sailorr*) and(*clozapin* OR *clozaril* OR *denzapin* OR *zaponex*) in REFERENCE and (*drool* or * saliva* or *ptyalism* or *sialism* or *sailorr* or *sialosis*) in STUDY]
Data collection and analysis
[For definitions of terms used in this, and other sections, please refer to the Glossary]
1. Selection of studies We (CC, VU, RJSS,HYL,JX and LD) independently inspected all reports of identified studies. It was usually possible to resolve any disagreement by consensus. However, where doubt remained we acquired the full article. We independently decided whether these met the review criteria. No blinding to the names of authors, institutions and journal of publication took place. Again, we resolved any disagreements by consensus. When this proved impossible, we sought further information and, in the interim, added these trials to the list of those 'Awaiting assessment'.
2. Assessment of methodological quality We allocated trials to three quality categories, as described in the Cochrane Collaboration guidelines (Higgins 2005). We only included trials in Category A or B in the review.
3. Data management 3.1 Data extraction We independently extracted data and resolved disagreement by discussion. When this was not possible we sought further information from trial authors.
3.2 Intention to treat analysis We analysed data on an intention‐to‐treat basis where possible and assumed that those who had not been accounted for had the less positive outcome. This rule did not include the outcome of 'death'. We tested this assumption with a sensitivity analysis. For continuous data it is impossible to manage the data in this way therefore 'completer' data were presented. Where possible, we would have hoped to convert continuous scores to dichotomous data.
If, for a given outcome, more than 50% of the total numbers randomised were not accounted for, we did not present results as such data are impossible to interpret with authority. If, however, more than 50% of those in one arm of a study were lost, but the total loss is less than 50%, we would have made this explicit in the relevant 'Risk of bias' table.
3.3 Crossover studies ‐ this paragraph was omitted from the first version of the protocol This area of research commonly uses cross over studies where one person is randomly allocated the treatment only to be crossed over to receive the comparison after certain designated time period. Often a period of drug free 'washout' is used between the interventions to try and ensure that no carry‐over effects of the first intervention remain before commencing the second treatment. The statistical methods for including crossover studies in meta‐analyses have developed considerably (Curtin 2002a, Curtin 2002b, Curtin 2002c, Elbourne 2002). From the statistical perspective it is now feasible to include data in meta‐analyses from two period crossover studies, although difficulties remain (Elbourne 2002).
For schizophrenia, however major difficulties remain. Carry over, when the effect of treatment number one would carry over into the second treatment period is difficult to predict and is not solely the function of how long the treatment intervention stays in the body (Fleiss 1984). Although a washout period may be employed often effects of treatments in schizophrenia are surprisingly slow to take effect and exposure to one treatment even weeks after all but minute traces are still to be found in the body can still have an effect. A second major difficulty is that the condition which is being investigated within the crossover study should be stable (Fleiss 1984). Schizophrenia is not usually very stable and hypersalivation is not (Taylor 2007).
We have only included data from crossover trials from before the period of first crossover because of a carry‐over effect that is impossible to predict and because hypersalivation is, in itself, not stable,
4. Data analysis 4.1 Binary data When summation was appropriate, with binary outcomes such as improved/not improved, we calculated the relative risk (RR) statistic with a 95% confidence interval (CI) and used a random effects model. In addition, as a measure of efficiency, we estimated the number needed to treat (NNT) or the number needed to harm (NNH) from the pooled totals.
4.2 Continuous data 4.2.1 Normally distributed data Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to all data before inclusion: (a) standard deviations and means were reported in the paper or were obtainable from the authors, (b) when a scale started from the finite number zero, the standard deviation, when multiplied by two, was less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, (Altman 1996), (c) if a scale started from a positive value (such as PANSS which can have values from 30 to 210) the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2SD>(S‐Smin), where S is the mean score and Smin is the minimum score. Endpoint scores on scales often have a finite start and end point and these rules can be applied to them. When continuous data are presented on a scale which includes a possibility of negative values (such as change on a scale), it is difficult to tell whether data are non‐normally distributed (skewed) or not. Skewed data are presented in the 'Other data' tables rather than included in the analysis.
For change data (endpoint minus baseline), the situation is even more problematic. In the absence of individual patient data it is impossible to know if data are skewed, though this is likely. After consulting the ALLSTAT electronic statistics mailing list, we presented change data in order to summarize available information. In doing this, it was assumed either that data were not skewed or that the analyses could cope with the unknown degree of skew. Again, without individual patient data it is impossible to test this assumption. Where both change and endpoint data were available for the same outcome category, we present only endpoint data. We acknowledge that by doing this, much of the published change data could have been excluded, but argue that endpoint data is more clinically relevant and that if change data were to be presented along with endpoint data, it would be given undeserved equal prominence. We contacted authors of studies that only reported change for endpoint figures.
4.2.2 Summary statistic For continuous outcomes we estimated a weighted mean difference (WMD) between groups. Again this was based on the random effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity. We did not consider continuous data presented without use of summary statistics (i.e. mean, SD, SE, median, interquartile range), although we noted the existence of these data in the text.
4.2.3 Valid Scales Many rating scales are available to measure outcomes in mental health trials (Marshall 2000). These scales vary in quality and many are poorly validated. It is generally accepted that measuring instruments should have the properties of reliability (the extent to which a test effectively measures anything at all) and validity (the extent to which a test measures that which it is supposed to measure). Before publication of an instrument, most scientific journals insist that its reliability and validity be demonstrated to the satisfaction of referees. As a minimum standard, data were excluded from unpublished rating scales. In addition, the rating scale should be either: (i) a self report, or (ii) completed by an independent rater or relative. More stringent standards for instruments may be set in future editions of this review.
Continuous data may be presented from different scales, rating the same outcome. In this event, we presented all data without summation and inspected the general direction of effect.
4.2.4 Conversion to a common metric To facilitate comparison between trials, we converted variables (such as days in hospital) that could be reported in different metrics (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
4.3 Cluster trials Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intra class correlation in clustered studies, leading to a 'unit of analysis' error (Divine 1992) whereby p values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997, Gulliford 1999).
If clustering had not been accounted for in primary studies, we would have presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. We would have sought to contact first authors of studies to obtain intra class correlation co‐efficients of their clustered data and to adjust for this by using accepted methods (Gulliford 1999). Should clustering have been incorporated into the analysis of primary studies, we would have presented these data as if from a non‐cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a 'design effect'. This is calculated using the mean number of participants per cluster (m) and the intra‐class correlation co‐efficient (ICC) Design effect = 1+(m‐1)*ICC (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
5. Investigation for heterogeneity Firstly, we considered all the included studies within any comparison to judge clinical heterogeneity. We then visually inspected the graphs to investigate the possibility of statistical heterogeneity and supplemented this using, primarily, the I‐squared statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I‐squared estimate was greater than or equal to 75%, we interpreted this as indicating the presence of high levels of heterogeneity (Higgins 2003). If inconsistency had been high, data would not have been summated, but we would have presented them separately and investigated reasons for heterogeneity.
6. Addressing publication bias We entered data from all identified and selected trials into a funnel graph (trial effect against trial size) in an attempt to investigate the likelihood of overt publication bias (Egger 1997).
7. General Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for the intervention.
Results
Description of studies
1. Excluded studies It was clear from most of the reports identified by our search that the full texts would not be relevant and we could see no purpose being served by adding all these references into the excluded list. Of the studies for which we did retrieve full texts three were excluded from the review; Zhai 1992 was a prospective cohort study rather than a trial and Xu 1997 did not state method of allocation. Ya‐Mei 2001 was a randomised placebo‐controlled double blind crossover trial that investigated the effect of pirenzepine (an antimuscarinic) in clozapine induced hypersalivation. It had 20 participants, who were inpatients diagnosed with schizophrenia using DSM‐IV criteria, treated solely on clozapine for a minimum of six months and had complained of sialorrhoea (average wet surface of more than 10cm). Age range of participants was 26 ‐ 41yrs, but gender was not specified. The authors excluded people with co‐morbid organic mental disorder and mental retardation. Concealment of allocation was not stated by the authors and blinding method was not described. The main outcome measure was the diameter of nocturnal saliva wetted tissue surface. This study was excluded because it was not possible to determine whether the reported data was pre‐crossover, thus rendering it unusable.
2. Studies awaiting assessment We have found a conference proceeding (Yao 1994), however there is insufficient information to evaluate if it is eligible for the current review. We are attempting to correspond with the author.
3. Ongoing studies We know of one trial going on in Kumar 2008. The aim is to randomise 70 participants with schizophrenia or schizoaffective disorder to modafinil or placebo. The primary outcomes are daytime sleepiness (scores on Epworth Sleepiness Scale) and nocturnal hypersalivation (scores on Nocturnal Hypersalivation Rating Scale). Modafinil is a novel alertness producing agent with as yet unclear mechanism of action. The investigators planned the trial after modafinil was given to five patients to combat sedation with clozapine; dramatic improvements in associated clozapine‐induced hyper‐salivation as well as beneficial effects on weight gain were observed clinically.
Another study is a proposed trial in Li 2008 and is currently in the planning stages. We were not able to obtain much information regarding this. However, it is proposed that participants will be randomly allocated to tabellae belladonnae compositae or placebo. The main component of tabellae belladonnae compositae are belladonna, atropinum hyoscyamine and hyoscine. It has atropine like effects but is less strong than atropine. The method of measuring hypersalivation is described but not named and measures the diameter of the wet patch caused by hypersalivation on the pillow.
4. Included studies Fifteen randomised controlled studies fulfilled the inclusion criteria and presented data that could be used for at least one of the main comparisons.
4.1 Methods The quality of the included studies will be commented upon below. This section refers only to the general design, setting and duration of the included studies.
4.1.1 Design All but Kreinin 2005 were parallel group design; Kreinin 2005 used crossover methods. In the original protocol for this review we had omitted a section within the Methods on managing data from crossover trials. We have amended this (see Methods section 3.3) but only after seeing the data. Kreinin 2005 does not report results pre‐crossover. We have therefore not used most outcomes, with the exception of leaving the study early.
4.1.2 Setting All but Kreinin 2005 were based in the Peoples Republic of China . At least 14 of the 15 studies were undertaken within hospitals. Zhou 1996 did not report clearly the setting in which their trial took place.
4.1.3 Duration In the Methods of this review we had pre‐defined a short‐term trial as one of less than three months duration. All trials in this review fall into that category. The duration of all clearly reported trials ranged from one to four weeks, with three lasting ten days, three lasting two weeks and six lasting four weeks. Zhou 1996 did not clearly state the duration but did seem to be longer than two weeks. Finally, Kreinin 2005, the one crossover study had two three week arms for each person with a one week washout period between the crossover. 4.2 Participants 4.2.1 Diagnosis All participants were diagnosed with schizophrenia; ten studies did not indicate diagnostic standard used. Kreinin 2005 used the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV); Fan 1996 and Zhou 1996 the Chinese Classification of Mental Disorders, 2nd edition (CCMD‐2); and finally Lu 1998 and Ren 2001 the revised edition of the same criteria (CCMD‐2‐R). All participants but those in Fan 1996 had also been on clozapine prior to the start of the trials (please see History). Fan 1996 was a prevention study where clozapine was started together with the intervention at the beginning of the trial.
In all trials but Fan 1996 participants also had clozapine‐induced hypersalivation. Kreinin 2005 used the Nocturnal Hypersalivation Rating Scale (NHRS) (Spivak 1997) and all participants scored two or above (mild, hypersalivation wakes the patient once during the night). The others used other (non‐validated) tools. Li 1993 included all participants who scored two or above (noticeably increased hypersalivation); Lu 1998 and Yang 1997 both included participants scored two or above on a different scale (hypersalivation during sleep, wet surface diameter 10‐20cm). Wang 1998 included participants scored one or above and included occasional hypersalivation during sleep, wet pillow surface <10cm. All other studies (Gong 1998; Kang 1993; Li 2004; Qian 1996; Ren 2001; Yao 1994; Yuan 2000; Zhou 1996; Lin 1999) stated that participants had hypersalivation but the severity of which was not described.
4.2.2 Size This review currently includes trials with a total of 924 participants. The number of participants per trial ranged from 16 (Qian 1996) to 138 (Gong 1998) with only three studies including over 100 people (Gong 1998, Ren 2001, Yang 1997).
4.2.3 Age and sex The age of participants ranged from 15‐68 years. The sex of participants was not stated in Gong 1998 and Yao 1994 but for those for whom we know the sex, 421/786 (53.56%) were men and 365/786 (46.44%) women. Six studies included both men and women, four included men only and two only women.
4.2.4 History Seven of the 15 studies did not report duration of schizophrenia but for those reported, duration ranged from three months to 40 years.
4.3 Interventions 4.3.1 General comments None of the studies described whether standard care was used in addition to the intervention or the placebo. No trials evaluated either alpha adrenoceptor agonist drugs (for example, clonidine, lofexidine) or alpha adrenoceptor antagonist drugs (for example, terazosin, yohimbine).
4.3.2 Placebo Eight studies compared an intervention with a placebo. Gong 1998 and Lin 1999 used vitamin B1 capsules, and Li 1993 vitamin C capsules as placebo. Kang 1993 and Li 2004 used starch as placebo, and Qian 1996 used flour with rice vinegar. Kreinin 2005 and Lu 1998 did not specify what the placebo was consisted with. Fan 1996 compared the intervention with no treatment.
4.3.3 Antimuscarinic drugs Astemizole (hismanal) (Gong 1998, Li 1993, Wang 1998, Yao 1994), diphenhydramine (Gong 1998, Lu 1998, Yang 1997) and propantheline (also known as probanthine) (Gong 1998, Lin 1999, Yang 1997, Yao 1994, Zhou 1996), are all antihistamines. The standard dose of astemizole is 10 mg/day. The doses astemizole were used at were 10 mg/day (Li 1993; Yao 1994), 10‐20 mg/day (Gong 1998), and 10 mg to possibly 30 mg (exact highest dose not specified but likely 30 mg) (Wang 1998). The standard dose of diphenhydramine is 25‐50 mg three or four times/day up to a maximum of 300 mg/day, and diphenhydramine was used at 50 mg/day (Lu 1998; Yang 1997) and 100‐200 mg/day(Gong 1998). The standard dose of propantheline is 75 mg in divided doses with a maximum dose of 120 mg/day. The doses propantheline were used at were 30 mg/day (Lin 1999; Yang 1997; Zhou 1996), 30 mg bd (Yao 1994) and 60‐120 mg/day (Gong 1998). In general, toxicity occurs after ingestion of three to five times of the standard dose. The high doses of astemizole (30 mg, Wang 1998) used can cause toxicity.
Doxepin (Ren 2001; Wang 1998; Yuan 2000; Zhou 1996) is a tricyclic antidepressant with antimuscarinic properties. Its standard starting dose is 75 mg/day with a maximum of 300 mg/day in divided dose. The doses doxepin were used at 25 mg/day (Zhou 1996), 25‐75 mg/day (Wang 1998), and 25‐50 mg tds (Ren 2001; Yuan 2000).
4.3.4 Other 4.3.4.1 Traditional Chinese medicines 4.3.4.1.1 Huang yuan san (Fan 1996) This intervention comprises of 1. sheng da huang (raw Rhubarb); and 2. sodium sulphate (Anhydrate). In Chinese Medicine, hypersalivation is regarded as a "phlegm‐rheum" disorder and the classical formulation for treating "phlegm‐rheum" disorder is using sheng da huang. However, there is no reference in Chinese Medicine regarding treatment of hypersalivation secondary to chemicals. In total 31 people received this treatment in trials included in this review.
4.3.4.1.2 Suo quan wan (Kang 1993 and Yuan 2000) This comprises of 1. wu yao (spicebush root <radix linderae strychnifoliae>); 2. shan yao (Chinese yam <radix dioscoreae oppositae>); and 3. yi zhi ren (bitter cardamom <fructus alpiniae oxyphyllae>). In Chinese Medicine, energy in the spleen is linked with the mouth and the major function of the kidneys is the control of fluid. It is viewed that if there is spleen and kidney insufficiency, the body cannot reabsorb fluid and there is dysfunction of the flow of fluid, which leads to salivation. Active ingredients in suo quan wan are known to be protective for the spleen and kidney and has been used to reduce diuresis and salivation. In total 59 people received this treatment in trials included in this review.
4.3.4.1.3 Wu dan san (Qian 1996) Wu dan san comprises of 1. Wu zhu yu (Medicinal Evodia Fruit <Fructus evodiae>); 2. Dan nan xing (Arsaema Cum bile); and 3. Rice vinegar. In total eight people received this treatment in trials included in this review.
4.3.4.2 Adjunctive antipsychotic drugs Kreinin 2005 added amisulpride to the standard clozapine treatment. The same authors had observed a beneficial effect on clozapine‐induced hypersalivation from sulpiride augmentation in a previous observational study. The rationale was that retrospective studies and case series had suggested amisulpride augmentation to improve the efficacy of clozapine, and due to its similarity to sulpiride, may also reduce clozapine‐induced hypersalivation. Recommended dose ranges are 400‐800 mg/day for acute psychotic episodes and 50‐300 mg/day for predominantly negative symptoms. In Kreinin 2005 it was used at a dose of 400 mg per day. Nine people received this treatment.
4.3.4.3 Rice bran oil derivatives Oryzanol (Li 2004) or oryzanolum (Ren 2001) is a substance extracted from rice bran oil and rice embryo oil. It has an antimuscarinic effect on the autonomic nervous system. In total 92 people received this treatment in trials included in this review. It was given at a dose of 30‐60 mg/day. It is unclear what would be standard dose.
4.4 Outcomes 4.4.1 Measurement of salivation All studies measured hypersalivation however, none of these appear to be validated other than Kreinin 2005 which used NHRS but data were unusable due to being a crossover trial.
Three studies (Li 1993, Lu 1998, and Yao 1994) reported both a curative effect and an endpoint hypersalivation score, Kang 1993 reported the change in hypersalivation scores before and after intervention, two reported the endpoint hypersalivation score alone (Fan 1996 and Li 2004), and seven reported the curative effect alone (Gong 1998, Lin 1999, Qian 1996, Ren 2001, Wang 1998, Yang 1997, Yuan 2000, and Zhou 1996). Eleven of the studies (Gong 1998, Li 1993, Lin 1999, Lu 1998, Qian 1996, Ren 2001, Wang 1998, Yang 1997, Yao 1994, Yuan 2000, and Zhou 1996) used a categorical score of curative effect. This was generally categorised as cured, markedly improved, improved, or no effect. Again for most studies (9/15) this involved a change in the diameter of wet pillow. This was either measured by an improvement of the diameter of the wet area of pillow (e.g. by proportion or by measurement) or by a change in the continuous hypersalivation score, which was scored by diameter. For the two other studies (Li 1993 and Yao 1994), curative effect was judged by an improvement in a hypersalivation scale.
Most of these tools quantify hypersalivation by measuring the diameter of pillow surface affected, for example ‐ from Zhou 1996:
score 1: hypersalivation during sleep, wet pillow surface diameter <10 cm score 2: mild hypersalivation whilst awake, wet pillow surface diameter during sleep 10‐20 cm score 3: hypersalivation whilst awake, wet pillow surface diameter during sleep >20 cm
Another method of quantifying hypersalivation was volume of saliva (Fan 1996) although it is not clear how this was measured. Li 1993 and Yao 1994 also reported a scale, which included the criteria of how noticeable the hypersalivation was. A wet pillow, but not the diameter affected, was included. The most severe score was defined as 'drooling on standing'. Only three studies (Li 2004; Lin 1999; Qian 1996) referenced another study (Yung 1993, not eligible for this review) in which the same measure had been used. This tool quantifies hypersalivation by the diameter of wet patch on the pillow surface and included mild hypersalivation and moist tongue surface as less severe scores, and obvious drooling whilst awake as the most severe score.
In summary, whether a hypersalivation score or curative effect was reported, diameter of wet pillow was used to judge hypersalivation in nine of the 11 studies.
4.4.2 Adverse effects Only three studies used validated scales (TESS) to monitor adverse effects (Li 1993, Wang 1998, and Yao 1994), and only one of these (Wang 1998) reported the results of the TESS score. All other studies but Li 2004 reported monitoring adverse effects, although it is unclear how they did this and whether scales or checklists were used or not.
4.5 Missing outcomes Although all studies measured the extent of salivation, most did not report validated outcomes of adverse effects. In addition, only Kreinin 2005 measured the effect on global state and psychotic symptoms, however these results could not be used as there were no data pre‐crossover. Although Wang 1998 described using BPRS to monitor mental state, no data were provided. No studies monitored service outcomes, general functioning, behaviour, engagement with services, quality of life, satisfaction with care for any of the people involved, or economic outcomes.
Risk of bias in included studies
1. Randomisation For 13 of the studies allocation was stated as being randomised but there were few further details and only one trial described how the allocation sequence was generated. Li 2004 described how allocation was undertaken using a toss of a coin. For two studies randomisation was not stated, but these trials were double blind (Qian 1996, Ren 2001) . The protocol for this review states that studies that do not mention randomisation but do mention blinding should be included subject to a sensitivity analysis. However, Qian 1996 compares wu dan san with placebo both applied to acupuncture points and Ren 2001 compares doxepin with oryzanol. These are unique studies and therefore we cannot perform a sensitivity analysis. These studies do not mention randomisation therefore we cannot make any assumptions as to whether they were in fact randomised or not.
No study described how the allocation sequence was concealed from those giving the treatment. There was no attempt to conceal the allocation in Lu 1998. 2. Blindness This was described as double‐blind for nine of the studies, but was either unclear or not stated for the other six (Fan 1996, Kang 1993, Kreinin 2005, Li 2004, Wang 1998, and Yuan 2000). None of the nine studies reported testing of the blinding. Blinding is recognised as being of importance in minimising observation bias. Therefore lack of blinding would be expected to reduce the methodological quality of these studies. It could also be expected that testing of this blinding would be a priority for those which did describe blinding.
3. Loss to follow up There was no loss to follow up in any of the included studies.
4. Data reporting Overall much of the data we found could not be used because of poor reporting. Findings were often presented as graphs, in percentiles or just reported as p‐values or chi‐square values. Studies often reported that there were no significant differences between the groups in the text rather than presenting the actual data. This is of little use to a reviewer.
5. Overall Two of the 15 studies (Li 1993, Yao 1994) were published before the first CONSORT (Consolidated Standards of Reporting Trials) statement (Begg 1996). Three were published in 1996 (Fan 1996; Qian 1996; Zhou 1996). The other ten studies were produced in the post‐CONSORT era. The CONSORT statement gives recommendations for how to report randomised trials using a checklist system. CONSORT is associated with improved reporting of randomised trials. None of the 15 trials included in this review used CONSORT.
Effects of interventions
1. The Search The search identified 157 citations from 67 studies. Of these we were only able to include 15 studies in the review. Fourteen were in Chinese which were extracted and translated into English, and one in English (Kreinin 2005). Many studies were multiply reported in different media.
2. Comparison 1: ANTIMUSCARINIC: 1. ASTEMIZOLE versus CONTROL Four studies compared astemizole with controls (10‐28 days duration, total N= 260 people). The dose of astemizole used in these studies was 10 mg/day (Yao 1994, Li 1993), 10‐20 mg/day (Gong 1998), and in Wang 1998 the starting dose was 10 mg/day which was then increased (details of increase not reported). The dose of clozapine was not stated in any of the studies other than Wang 1998 which stated that dosage ranged from 50‐500 mg.
Within these studies were comparisons of astemizole with diphenhydramine (an antimuscarinic), doxepin (an antimuscarinic), propantheline (an antimuscarinic) and placebo.
2.1 Hypersalivation 2.1.1 Hypersalivation: 1. No effect/not cured/not markedly improved Astemizole showed benefit over placebo, significantly less people on astemizole had no clinically important improvement compared with placebo (n=97, 2 RCTs, RR 0.61 CI 0.47 to 0.81 NNT 3 CI 2 to 5).
There was no significant difference between astemizole compared with diphenhydramine regarding clinically important improvement (n=68, 1 RCT, RR 1.78 CI 0.98 to 3.21, NNH 10 CI 3 to 6). Significantly more people on astemizole showed no clinically important improvement compared with propantheline (n=120, 2 RCTs, RR 2.46 CI 1.63 to 3.72, NNH 3 CI 2 to 4) and compared with doxepin (n=50, 1 RCT, RR 1.64 CI 1.14 to 2.37, NNH 3 CI 2 to 7).
2.1.2 Hypersalivation: 2. Average endpoint score (Scale: mixed clinical criteria, high score= bad, skewed data) Two studies reported average endpoint hypersalivation score (Li 1993, Yao 1994). Data comparing astemizole with placebo and propantheline were skewed but the average endpoint hypersalivation score was significantly lower in astemizole (10‐20 mg/day) than placebo (n=22, 1 RCT, WMD ‐1.00 CI ‐1.86 to ‐0.14). However, the score in astemizole (10 mg/day) was significantly higher than propantheline (30 mg bd) (n=50, 1 RCT, WMD 0.72 CI 0.17 to 1.27).
2.1.3 Hypersalivation :3. Change in hypersalivation scores (Scale: mixed clinical criteria, high score= good) One small trial found significant improvement in hypersalivation scores in astemizole compared with placebo (n=22, 1 RCT, MD 0.90 CI 0.33 to 1.47, p=0.002). There was significantly less improvement in hypersalivation scores in astemizole compared with propantheline (n=50, 1 RCT, MD ‐0.64 CI ‐1.14 to ‐0.14, p=0.01). These data are likely to be skewed.
2.2 Adverse effects: specific symptoms 2.2.1 Adverse effects: 1. Cardiac‐ tachycardia One small trial reported usable data for tachycardia as an adverse effect and there was no significant difference between astemizole (8%) and propantheline (0%) (n=50, RR 5.00 CI 0.25 to 99.2).
2.2.2 Adverse effects: 2. Gastric‐ constipation One trial reported constipation as an adverse effect. There were no significant differences between astemizole (˜19%) and placebo (˜18%) (n=75, 1 RCT, RR 1.08 CI 0.42 to 2.79); diphenhydramine (˜16%) (n=68, 1 RCT, RR 1.24 CI 0.44 to 3.54); or propantheline (˜32%) (n=67, 1 RCT, RR 0.60 CI 0.26 to 1.39).
2.2.3 Adverse effects: 3. Average endpoint score (TESS score: high score= bad) There were no significant differences in the endpoint average TESS scores between astemizole and placebo (n= 22, 1 RCT, WMD ‐0.37 CI ‐1.73 to 0.99).
2.3 Leaving the study early No data were reported.
3. Comparison 2: ANTIMUSCARINIC: 2. PROPANTHELINE vs CONTROL Five studies compared propantheline with a control (10‐28 days duration, total N= 400 people). The dose of propantheline used in these studies was 30 mg/day (Lin 1999, Yang 1997, Zhou 1996), 60 mg/day (Yao 1994), and 60 to 120 mg/day (Gong 1998). The dose of clozapine was stated in all studies apart from Yao 1994 and Gong 1998.
Within these studies were comparisons of propantheline with astemizole (an antimuscarinic), diphenhydramine (an antimuscarinic), doxepin (an antimascurinic) and placebo.
3.1 Hypersalivation 3.1.1 Hypersalivation: 1. No effect/ not cured/ not markedly improved Two studies compared propantheline with placebo. However these studies displayed a significant level of statistical heterogeneity (I‐squared=86.6%). Significantly less people on propantheline had no clinically important change in hypersalivation compared with those taking placebo (n=70, 1 RCT, RR 0.47 CI 0.3 to 0.72). In Lin 1999 the same effect was evidenced but less marked (n=32, RR 0.82 CI 0.63 to 1.06).
Compared with astemizole (10‐20 mg/day), less people randomised to propantheline (60‐120 mg/day) experienced no clinically important change in hypersalivation (n=117, 2 RCTs, RR 0.56 CI 0.35 to 0.89, NNT 5 CI 2 to 15).
Propantheline had no significant advantage compared with diphenhydramine or doxepin. The difference between propantheline (30‐120 mg/day) and diphenhydramine (50‐200 mg/day) was not significant (n=163, 2 RCTs, RR 1.15 CI 0.88 to 1.50). There was no significant difference between propantheline (30mg/day) and doxepin (25mg/day) (n=80, 1 RCT, RR 0.91 CI 0.44 to 1.90).
3.1.2 Hypersalivation:2. Average endpoint hypersalivation score (Scale: mixed clinical criteria, high score= bad, skewed data) The endpoint score was significantly lower in propantheline (30 mg bd) than in astemizole (10 mg/day) (n=50, 1 RCT, WMD ‐0.72 CI ‐1.27 to ‐0.17), however the data were skewed.
3.1.3 Hypersalivation:3. Average change in hypersalivation scores (Scale: mixed clinical criteria, high score= good) There was significantly more improvement in hypersalivation score in propantheline compared to astemizole (n=50, 1 RCT, WMD 0.64 CI 0.14 to 1.14, p=0.01). These data are likely to be skewed.
3.2 Adverse effects: Specific symptoms 3.2.1 Adverse effects: 1. Cardiac ‐ abnormal ECG There was no significant difference in participants with abnormal ECG between propantheline (10%) compared with doxepin (˜13%) (Zhou 1996) (n=80, 1 RCT, RR 0.80 CI 0.23 to 2.76) or those experiencing tachycardia between propantheline and astemizole (Yao 1994) (n=50, 1 RCT, RR 0.20 CI 0.01 to 3.97).
3.2.2 Adverse effects: 2. Gastric ‐ constipation In terms of constipation, there were no significant differences between propantheline (˜21%) and placebo (˜13%) (n=102, 2 RCTs, RR 1.80 CI 0.77 to 4.18), propantheline (˜32%) and diphenhydramine (˜16%) (n=63, 1 RCT, RR 2.06 CI 0.80‐5.36), propantheline (˜25%) and doxepin (˜28%) (n=80, 1 RCT, RR 0.91 CI 0.44‐1.90) or propantheline (32%) and astemizole (19%) (n=67, 1 RCT, RR 1.66 CI 0.72 to 3.84).
3.2.3 Adverse effects: 3. Hepatic ‐ abnormal liver function There was no significant difference between propantheline (˜8%) and doxepin (˜10%) (n=80, 1 RCT, RR 0.75 CI 0.18 to 3.14).
3.2.4 Adverse effects: 4. Movement disorders‐ extrapyrimidal There was no significant difference between propantheline (˜3%) and doxepin (˜5%) (n=80, 1 RCT, RR 0.50 CI 0.05 to 5.30).
3.3 Leaving the study early No data were reported.
4. Comparison 3: ANTIMUSCARINIC: 3. DIPHENHYDRAMINE vs CONTROL Three studies compared diphenhydramine with a control (10‐28 days duration, total N= 198 people). Doses of diphenhydramine used were 50 mg/day (Lu 1998, Yang 1997) and 100‐200 mg/day in Gong 1998. The doses of clozapine were stated in all studies other than Gong 1998.
Within these studies diphenhydramine was compared with astemizole (an antimuscarinic), propantheline (an antimuscarinic) and placebo.
4.1 Hypersalivation 4.1.1 Hypersalivation: 1. No effect/ not cured/ not markedly improved Diphenhydramine showed benefit compared with placebo. Less people on diphenhydramine (50‐200 mg/day) experienced no clinically important change in hypersalivation compared with those on placebo (n=131, 2 RCTs, RR 0.43 CI 0.31 to 0.58, NNT 2 CI 1.5 to 2.5).
There were no significance differences between diphenhydramine and either astemizole (n=68, 1 RCT, RR 0.70 CI 0.37‐1.32) or propantheline (n=183, 2 RCTs, RR 0.87 CI 0.67‐1.13).
4.1.2 Skewed data: Hypersalivation: 2. Average endpoint hypersalivation score (Scale: wet pillow diameter, high score= bad) Lower average endpoint hypersalivation scores were observed in participants on diphenhydramine 50 mg/day than those on placebo (n=60, 1 RCT, RR ‐1.62 CI ‐2.10 to ‐1.14), however the results were skewed.
4.1.3 Hypersalivation: 3. Average change in hypersalivation scores No data were reported.
4.2 Adverse effects: Specific symptoms 4.2.1 Adverse effects: 1. Gastric‐ constipation There was no significant difference for the frequency of constipation between diphenhydramine (˜26%) and placebo (˜23%) (n=131, 2 RCTs, RR 1.08 CI 0.59 to 1.95), diphenhydramine (˜16%) and astemizole (˜19%) (n=68, 1 RCT, RR 0.80 CI 0.28 to 2.28) or between diphenhydramine (˜16%) and propantheline (˜32%) (n=63, 1 RCT, RR 0.48 CI 0.19 to 1.26).
4.3 Leaving the study early No data were reported.
5. Comparison 4: ANTIMUSCARINIC: 4. DOXEPIN vs CONTROL Four studies compared doxepin with controls (7‐28 days duration, total N= 304). The dose of doxepin used was 25mg/day (Zhou 1996), 25 mg/day increasing in 25 mg/day increments up to 75 mg/day (Wang 1998), and 75‐150 mg/day (Ren 2001, Yuan 2000). The dose of clozapine used was stated in all of these studies.
Within these studies doxepin was compared with propantheline (an antimuscarinic), astemizole (an antimuscarinic), oryzanol (a rice bran oil derivative) and suo quan wan (a traditional Chinese medicine).
5.1 Hypersalivation 5.1.1 Hypersalivation: 1. No effect/ not cured/ not markedly improved Significantly less people showed no improvement on doxepin than on astemizole (n=50, 1 RCT, RR 0.61 CI 0.42 to 0.88, NNT 5 CI 2.4 to 24.2).
Significantly more people failed to improve on doxepin (˜60%) compared with both oryzanol (n=104, 1 RCT, RR 2.21 CI 1.34 to 3.65, NNH 4 CI 2.0 to 6.8) and suo quan wan (n=70, 1 RCT, RR 3.27 CI 1.69‐66.31 NNH 3 CI 1.5 to 4).
There was no significant difference in those experiencing a clinically important change between doxepin and propantheline (n=80, 1 RCT, RR 1.10 CI 0.53 to 2.30).
5.1.2 Hypersalivation: 2. Average endpoint hypersalivation score No data were reported.
5.1.3 Hypersalivation: 3. Average change in hypersalivation scores No data were reported.
5.2 Adverse effects: Specific symptoms 5.2.1 Adverse effects: 1. Cardiac ‐ abnormal ECG There was no significant difference between the occurrence of abnormal ECG between those receiving doxepin (˜13%) and propantheline (10%) (n=80, 1 RCT, RR 1.25 CI 0.36 to 4.32), or between those receiving doxepin (˜15%) and suo quan wan (˜4%) (n=104, 1 RCT, RR 4.00 CI 0.89 to 17.95).
5.2.2 Adverse effects: 2. Gastric‐ constipation There was no significant difference in the incidence of constipation between doxepin (˜28%) and propantheline (25%) (n=80, 1 RCT, RR 1.10 CI 0.53 to 2.30).
Constipation occurred significantly more often with doxepin (˜17%) than with oryzanol (˜10%) (n=104, 1 RCT, RR 4.50 CI 1.02‐19.53, NNH 8 CI 4 to 52) and with doxepin (˜59%) compared with suo quan wan (0%) (n=70, 1 RCT, RR 46.90 CI 2.89 to 734.50, NNH 2 CI 1 to 2).
5.2.3 Adverse effects: 3. Hepatic ‐ abnormal liver function There was no significant difference in the frequency of abnormal liver function with doxepin (10%) compared with propantheline (˜8%) (n=80, 1 RCT, RR 1.33 CI 0.32 to 5.58).
5.2.4 Adverse effects: 4. Movement disorders‐ extrapyramidal There was no significant difference in the frequency of extrapyramidal side effects in participants on doxepin (5%) compared with those on propantheline (˜3%) (n=80, 1 RCT, RR 2.00 CI 0.19‐ to 21.18).
5.3 Leaving the study early No data were reported.
6. Comparison 5: RICE BRAN OIL DERIVATIVE: ORYZANOL vs CONTROL Two studies compared oryzanol with controls (14‐28 days duration, total N= 184). Doses of oryzanol used in these studies were 40 mg/day (Li 2004), and 30‐60 mg/day (Ren 2001). The dose of clozapine used was stated in both studies.
Within these studies oryzanol was compared with doxepin (an antimuscarinic) and placebo.
6.1 Hypersalivation 6.1.1 Hypersalivation: 1. No effect/ not cured/ not markedly improved Significantly less participants receiving oryzanol (˜27%) failed to improve clinically compared with doxepin (˜60%) (n=104, 1 RCT, RR 0.45 CI 0.27 to 0.45, NNT 4 CI 2 to 7).
6.1.2 Hypersalivation: 2. Average endpoint hypersalivation score (Scale: wet pillow diameter, high score= bad, skewed data) Li 2004 provided skewed data on average endpoint hypersalivation in the oryzanol and the placebo groups which showed significantly lower endpoint score in the oryzanol group compared to the placebo group (n=80, 1 RCT, WMD ‐1.00 CI ‐1.52 to ‐0.48).
6.1.3 Hypersalivation: 3. Average change in hypersalivation scores No data were reported.
6.2 Adverse effects: specific symptoms 6.2.1 Adverse effects: 1. Cardiac‐ abnormal ECG There was no significant difference in abnormal ECG between the oryzanol group (˜4%) and the doxepin group (˜15%) (n=102, 1 RCT, RR 0.22 CI 0.04 to 1.09).
6.2.2 Adverse effects: 2. Gastric ‐ constipation Constipation occurred significantly less with oryzanol (˜4%) compared with doxepin (˜17%) (n=104, 1 RCT, RR 0.19 CI 0.04 to 0.93, NNT 8 CI 4 to 52).
6.3 Leaving the study early No data were reported.
7. Comparison 6: TRADITIONAL CHINESE MEDICINE: SUO QUAN WAN vs CONTROL Two studies compared suo quan wan with controls (7‐28 days duration, total N= 110). The dose of suo quan wan was 18 g/day (Kang 1993) and 27 g/day (Yuan 2000). The dose of clozapine was stated in both trials.
Within these studies suo quan wan was compared with doxepin and placebo.
7.1 Hypersalivation 7.1.1 Hypersalivation: 1. No effect/ not cured/ not markedly improved Significantly less participants receiving suo quan wan failed to have clinically noticeable improvement compared with those on doxepin (n=70, 1 RCT, RR 0.31 CI 0.16 to 0.59, NNT 3 CI 1.5 to 4).
7.1.2 Hypersalivation: 2. Average endpoint hypersalivation score No data were reported.
7.1.3 Hypersalivation: 3. Average change in hypersalivation scores (Scale: wet pillow diameter, high score= good) There was significantly more change in the hypersalivation scores among the suo quan wan group than the placebo group (n=40, 1 RCT, WMD 1.98 CI 1.53 to 2.43, p<0.00001). The effect increased over the four weeks of the trial, the difference was greater at week four (n=40, 1 RCT, WMD 2.74 CI 1.81 to 3.67, p<0.00001).
7.2 Adverse effects: Specific symptom 7.2.1 Adverse effects: 1. Gastric ‐ constipation There were significantly lower incidences of constipation in participants on suo quan wan (0%) than those on doxepin (˜59%) (n=70, 1 RCT, RR 0.02 CI 0.00 to 0.35, NNT 2 CI 1 to 2).
7.3 Leaving the study early No data were reported.
8. Comparison 7: TRADITIONAL CHINESE MEDICINE: HUANG YUAN SAN vs NO TREATMENT There was one prevention study (Fan 1996) (28 days duration, N= 62). Participants were allocated to either huang yuan san (5‐15 g) or to no treatment at the same time as clozapine (150‐500 mg) was commenced.
8.1 Hypersalivation 8.1.1 Hypersalivation: 1. No effect/ not cured/ not markedly improved No data were reported.
8.1.2 Hypersalivation: 2. Average endpoint hypersalivation score (Scale: wet pillow diameter, high score= bad), skewed data There were lower hypersalivation scores in participants receiving huang yuan san than those with no treatment (n=62, WMD ‐0.81 CI ‐1.47 to ‐0.15) but the data were skewed.
8.1.3 Hypersalivation: 3. Average change in hypersalivation scores No data were reported.
8.2 Adverse effects No data were reported.
8.3 Leaving the study early No data were reported.
9. Comparison 8: TRADITIONAL CHINESE MEDICINE: WU DAN SAN paste applied to acupuncture points vs CONTROL Qian 1996 compared wu dan san with placebo, both applied to acupuncture points (28 days duration, N= 19).
9.1 Hypersalivation 9.1.1 Hypersalivation: 1. No effect/ not cured/ not markedly improved Results were not statistically significant between the wu dan san group (0%) and the placebo group (50%) (n=16, 1 RCT, OR 0.06 CI 0.00 to 1.36, p=0.08).
9.1.2 Hypersalivation: 2. Average endpoint hypersalivation score No data were reported.
9.1.3 Hypersalivation: 3. Average change in hypersalivation scores No data were reported.
9.2 Adverse effects No data were reported.
9.3 Leaving the study early No data were reported.
10 Comparison 9: ADJUNCTIVE ANTIPSYCHOTIC: AMISULPRIDE vs CONTROL Only one study measured effect of amisulpride 400 mg/day (Kreinin 2005) and compared it to a placebo, it was a cross‐over trial and had only one usable outcome.
10.1 Hypersalivation No usable data.
10.2 Adverse effects No usable data.
10.3 Leaving the study early There were people leaving early in either amisulpride group or the placebo group.
Discussion
1. Applicability of findings All but one of the studies took place in China, and all participants in the studies that mentioned setting (14/15) were in hospital. This has implications for the applicability of the findings both to other countries and to other settings.
1.1 Diagnoses Most studies (10/15) did not indicate the use of a diagnostic manual to confirm the diagnosis of schizophrenia, those that did used either the CCMD‐2 or CCMD‐2‐R. One study used the DSM‐IV. In most countries either the International Statistical Classification of Diseases tenth edition (ICD‐10) or the DSM‐IV are used to diagnose schizophrenia. The DSM‐III‐R has been found to be similar to the CCMD‐2 and CCMD‐2‐R in a previous study (Zheng 1994) and it is unlikely that the classification systems differ significantly. The lack of use diagnostic manuals to confirm diagnoses of schizophrenia in most of the studies may suggest we could be observing data that are relevant to a slightly diverse clinical population.
1.2 Setting Almost all studies (14/15) were conducted in a hospital setting. Although this ensured that participants did not drop‐out, it means that we are missing relevant data relevant to community settings. In many countries a large proportion of patients with schizophrenia are cared for in the community. In addition, in‐patients are likely to be more severely or chronically unwell than the general patient population. This restricts the applicability of the data from this review.
2. Limited data and confusing data 2.1 Collection and quality of reporting Overall the reporting of data were poor. The quality of Chinese trials has been questionable although appears to be improving (Wang 2007). In these studies blinding is mentioned in nine of the studies but it was not tested in any of them. Only one study described how the participants were randomised. Equally, concealment of allocation was not clearly described in any of the trials apart from Lu 1998 which was not attempting to conceal allocation. Poor reporting implies that these studies are likely to be biased and moreover to overestimate the effect size. Much outcome data could not be used due to poor reporting. Only one study used a validated tool to measure clozapine‐induced hypersalivation.
2.2 No data Unfortunately no usable outcomes were reported for many outcomes such as service use, behaviour, and engagement with services or satisfaction with care. Not only were measures of clozapine‐induced hypersalivation not validated, but neither were any other outcome data apart from the TESS.
We had predefined short term outcomes as being less than one month. No study measured outcomes after one month. This is a serious limitation as we cannot tell if any treatment effects are sustained. In addition clozapine‐induced hypersalivation may be worse over the initial stages of clozapine use. Having limited data regarding the length of schizophrenia diagnosis, duration of clozapine‐induced hypersalivation and the length of treatment with clozapine also limits the usefulness of the results of this review. However, it is likely that participants were a mixture of patients with variable lengths of diagnoses and clozapine treatment.
Of the interventions used in these trials only one, propantheline, is mentioned as an "examined" treatment in the Maudsley Guidelines (Taylor 2007). According to the same guidelines, hyoscine is widely used clinically but not investigated in these trials. Given that there is currently no accepted treatment for clozapine‐induced hypersalivation it seems reasonable to trial safe interventions for which there is a rationale for treatment. Only eight of the 15 trials had a placebo comparison. Arguably all of the trials should have involved a placebo control given that lack of an accepted treatment. The doses of clozapine used are mentioned in most (11/15) of the trials and are within ranges seen in clinical practice in the UK.
3. Comparison 1: ANTIMUSCARINIC: 1. ASTEMIZOLE versus CONTROL Astemizole was withdrawn from the UK market several years ago due to concerns regarding cardiac adverse effects. Although data are provided for 260 people, the studies had four different comparisons and the trials were all short (>28 days).
3.1 Hypersalivation 3.1.1 Hypersalivation: 1. No clinically important change in hypersalivation Based on the data of 97 participants from two studies, findings show that astemizole is significantly more effective at producing a clinically important improvement in clozapine‐induced hypersalivation when compared with placebo. Criterion used as clinically important improvement was a reduction of the diameter of the wet patch on the pillow by over one third. It is questionable how much this would be valued by patients. Although it was stated in both studies that they were randomised and double blind, neither of the studies tested blinding or described clearly the method of randomisation, or allocation concealment. These findings may, therefore, overestimate the effect size. It is also impossible to know if this effect would be sustained.
There is also no significant advantage of astemizole over diphenhydramine. However, these results are based on a small (N=68) short trial. Results from two studies showed that astemizole was significantly less effective than propantheline. Again this comparison is not robust as both studies are poorly reported. The results of a study of 50 people over one month show doxepin was significantly better than astemizole in terms of a clinically important improvement. This study, Wang 1998, did not report the use of a standard to confirm the diagnosis of schizophrenia, other methods were poorly reported and the impact on patients of the improvement of 1 point on a measure that we are not sure was ever validated, is questionable.
3.2 Adverse effects 3.2.1 Cardiac ‐ tachycardia It is important to note that astemizole was withdrawn from the UK market several years ago due to concerns regarding prolongation of the QTc interval. However, with regard to cardiac effects only tachycardia was measured. There is no significant difference between astemizole and propantheline in terms of tachycardia. This is a less common antimuscarinic effect and it was relatively uncommon (8% in astemizole group).
3.2.2 Gastric ‐ constipation Constipation is commonly seen even with the placebo group (19%). This is probably because is it a side effect of clozapine itself. There was no significant difference between astemizole and placebo. Even if there was a difference to find, there were probably insufficient numbers to highlight differences between astemizole and diphenhydramine (n=68) or astemizole and propantheline (n=67) in terms constipation.
In addition to constipation it might have been expected for studies to have investigated some of the other common antimuscarinic effects. These include blurred vision, urinary retention and hyperthermia.
3.2.3 Average endpoint TESS score There appears to be no significant difference between astemizole and placebo in terms of constipation or average end point TESS. Although the TESS is the only validated tool used for adverse effect, data for this outcome are limited, taking account of only 22 people, and being difficult to interpret.
4. Comparison 2: ANTIMUSCARINIC: 2. PROPANTHELINE versus CONTROL 4.1 Hypersalivation Propantheline demonstrated a significant advantage in terms of a clinically important improvement over both placebo and astemizole. Data are derived from two studies and are heterogeneous (I‐squared= 86.6%). There are many possible reasons for the heterogeneity. Neither study describes the criteria used to diagnose schizophrenia. Although both state that they are randomised and double blind, but there is no testing of the blinding and neither do they describe randomisation or allocation concealment. Lin 1999 does not describe the scale used to measure the curative effect, but does reference another study (Yung 1993) which we have not, as yet, acquired. Even though the findings are heterogeneous the data do both point to a beneficial effect of propantheline when compared with placebo or astemizole.
Propantheline has no significant advantage over diphenhydramine or doxepin. Given that these drugs are all antimuscarinics, and that even with the small sample sizes (163 and 80), there may genuinely be no difference to find between these compounds.
4.2 Adverse effects Some common antimuscarinic effects including blurred vision, urinary retention and hyperthermia have not been investigated.
4.2.1 Cardiac ‐ ECG changes ECG abnormalities are surprisingly common in both propantheline (10%) and doxepin groups (13%). It is difficult to interpret the significance of this as unfortunately this has not been investigated in a placebo group for comparison. Neither were the specific ECG abnormalities described. We might expect some ECG changes from clozapine treatment alone however it is unclear if antimuscarinic drugs exacerbate this. This is an important concern that is not well investigated.
4.2.2 Gastric ‐ constipation Propantheline was compared with placebo, diphenhydramine, doxepin and astemizole. There were no significant differences found. Rates of constipation are high (˜21% in propantheline group) but clozapine is also constipating and it is difficult to tease out effects of the antimuscarinic drugs.
4.2.3 Hepatic ‐ abnormal liver function We would not necessarily expect abnormal liver function tests from antimuscarinic interventions. The incidences are fairly high with both propantheline (3%) with doxepin (5%) although not different. Not only are numbers too small and the trial too short for us to be convinced that these rates are higher than would be expected but we have no placebo group with which to compare these findings.
4.2.4 Movement disorders ‐ extrapyramidal There are low incidences of extrapyramidal side effects with both propantheline and doxepin. Again it would have been useful to be able to compare these interventions with placebo.
5. Comparison 3: ANTIMUSCARINIC: 3. DIPHENHYDRAMINE versus CONTROL 5.1 Hypersalivation As with astemizole and propantheline significantly more participants have a clinically important improvement on diphenhydramine compared with placebo. This is based on data from 131 patients from two trials (Gong 1998, Lu 1998). Both trials stating that they are randomised and double blind but neither tested blindness nor clearly described the method of sequence allocation or allocation concealment. Both trials were short (<14 days). It is impossible to tell if the benefits would be sustained after this period. A clinically important improvement was described by Gong 1998 as a reduction of diameter of wet pillow surface by more than a third, and by Lu 1998 as an improvement in the hypersalivation score (non validated) by one point. The clinical value of these improvements is open to debate.
Diphenhydramine does not clearly have a significant advantage over either astemizole or propantheline. These results are discussed previously (see the relevant sections above).
5.2 Adverse effects: Gastric ‐ constipation Again constipation rates are high (26%) but not significantly higher than for placebo (23%). These high rates may well be an adverse effect of the underlying clozapine. When diphenhydramine was compared with propantheline and astemizole, there were no clear differences. These results are discussed in the relevant sections above.
6. Comparison 4: ANTIMUSCARINIC: 4. DOXEPIN versus CONTROL As well as being compared with other antimuscarinics (astemizole and propantheline) doxepin was also compared with oryzanol (rice bran oil derivative) and suo quan wan (traditional Chinese medicine). However it was not compared with placebo.
6.1 Hypersalivation Significantly more people had a clinically important improvement on doxepin compared with astemizole. This is discussed in the relevant section above. There was no difference between doxepin and propantheline. This too is discussed in the relevant section above.
Less people improved to a clinically significant extent on doxepin compared with oryzanol. These short‐term data are from a single poorly‐reported trial (Ren 2001). Randomisation was mentioned but the method of sequence generation was not described, blindness was stated as "double" but this was not tested, neither was the concealment of allocation clearly described. Even without bias, the definition of improvement of hypersalivation was not clearly described, and therefore the meaning of these results is unclear. It is impossible to tell if any benefits would be sustained after the first month of treatment.
Data suggest that suo quan wan has a significant advantage over doxepin. These results are from one study (Yuan 2000). This trial was a small (N=70), short (one week) and carries considerable risk of bias. There was no standardised method used to confirm the diagnosis of schizophrenia, randomisation was stated but not described, blinding was not mentioned at all, and allocation concealment was unclear.
6.2 Adverse effects 6.2.1 Cardiac ‐ abnormal ECG Doxepin is not usually recommended for use with clozapine. Both have been implicated in prolonging the QTc interval ‐ a potentially fatal adverse effect (Goodnick 2002). There is no comparison of doxepin with placebo. There was no significant difference between doxepin compared with propantheline (result discussed in the relevant section above) but both drugs did cause ECG abnormalities in about 10‐13% of people. There was no difference between doxepin (15%) and suo quan wan (4%), although the study was too small (N=70) to highlight even important differences with confidence.
6.2.2 Gastric ‐ constipation There was no significant difference of doxepin in comparison with propantheline, please see the relevant section above for a discussion of this result.
Doxepin causes constipation with rates of about 17% compared with oryzanol (10%) and 59% compared with suo quan wan (0%). Constipation is a common side effect in clozapine treatment. To find rates of 17 vs 59% suggests that there may be differences in how constipation was measured between trials. Oryzanol and suo quan wan may also treat constipation and, in this way, exaggerate the difference between the compounds.
6.2.3 Hepatic ‐ abnormal liver function There was no clear difference between doxepin (5%) and propantheline (3%). Please see discussion above.
6.2.4 Movement disorders ‐ extrapyramidal There are low incidences of extrapyramidal side effects with both propantheline and doxepin. This has been discussed above.
7. Comparison 5: RICE BRAN OIL DERIVATIVE: ORYZANOL vs CONTROL Oryzanol was compared with both doxepin and placebo. Unfortunately the data comparing oryzanol with placebo is largely unusable.
7.1 Hypersalivation The data relevant to this section comparing oryzanol with doxepin is discussed above. The skewed data reporting average endpoint hypersalivation score are difficult to interpret and could indicate an effect but should be replicated.
7.2 Adverse effects Cardiac and gastric data comparing oryzanol with doxepin are discussed in the relevant section above.
8. Comparison 6: TRADITIONAL CHINESE MEDICINE: SUO QUAN WAN vs CONTROL 8.1 Hypersalivation Data suggests that suo quan wan produces a significant and clinically important improvement over doxepin, these results are discussed in the doxepin section above. In comparing suo quan wan with placebo, the change data is likely to be skewed, however it suggests that people on suo quan wan improve more than those on placebo. Kang 1993 is small (N=40) and short term (four weeks); there was no standardised criteria for confirmation of diagnosis of schizophrenia. It is likely that these data contain considerable bias. The blinding is not mentioned at all, there is no explanation of how the sequence was generated for the randomisation and allocation concealment was not clearly reported. The change was measured on a non‐validated scale.
8.2 Adverse effects: Gastric ‐ constipation Data compares suo quan wan with doxepin, the results are discussed in the relevant section above.
9. Comparison 7: TRADITIONAL CHINESE MEDICINE: HUANG YUAN SAN vs CONTROL
9.1 Hypersalivation 9.1.1 Skewed data: Hypersalivation: 1. Average endpoint hypersalivation score Data comparing huang yuan san with no treatment in this prevention trial in which the intervention was started at the same time as clozapine treatment suggest the hypersalivation was less severe in the treated group (Fan 1996). However data are skewed and difficult to interpret with certainty, and the likelihood of bias in this small (N=62), short trial (one month) is high. Also diagnoses were not confirmed using manualised criteria, blinding was not mentioned, and although randomisation was mentioned, neither the method of sequence allocation or the concealment of allocation were clearly described. The scale used to measure the improvement was not validated and from its description, the actual impact on patients is questionable. This is one of several trials that generates more questions than it answers.
10. Comparison 8: TRADITIONAL CHINESE MEDICINE: WU DAN SAN PASTE APPLIED TO ACUPUNCTURE POINTS vs CONTROL
10.1 Hypersalivation: No clinically important change in hypersalivation Even though numbers not experiencing a clinically important improvement was very different between the wu dan san group (0%) and the placebo group (50%) the result was not statistically significant. A statistically significant result was always unlikely due to the small sample size (16). There is also a likelihood of bias in this study, Qian 1996, given that randomisation was implied and not stated, blinding was not tested and concealment of allocation was not clearly described. Again this is an interesting study from which future trialists in this area could learn.
11. Comparison 9: ADJUNCTIVE ANTIPSYCHOTIC: AMISULPIRIDE vs CONTROL 11.1 Leaving the study early The only usable outcome in Kreinin 2005 was drop outs of which there were none. This at least implied tolerability of the treatment, although the study was conducted in inpatients and therefore these results may not be applicable to community settings. Overall this study tells us little except that there is interest in this evaluation outside of Li 2008 and that there is a working theory that additional amisulpiride may be of value.
Authors' conclusions
Implications for practice.
1. For people with clozapine‐induced hypersalivation Despite the fact that clozapine‐induced hypersalivation is a troublesome adverse effect, the studies included in this review were not reported sufficiently well to provide clear evidence that any of the experimental drugs are adequately safe or that they reduce this effect to a meaningful extent for a reasonable length of time. This review illustrates that trials in this area are possible and quite numerous. Any suggested treatment for this troublesome adverse effect has not been adequately investigated within the context of a trial and this needs to be addressed. People with clozapine‐induced hypersalivation could encourage this investigation or support it by agreeing to be randomised to well designed and reported studies.
2. For clinicians Currently no drugs are licensed for the treatment of clozapine‐induced hypersalivation and no approach has been adequately investigated within randomised trials. Those that prescribe hyoscine are doing so supported by evidence that is fully open to biases with the potential harm that could do. Researchers and clinicians have undertaken studies but these studies fall well short of rigorous. Clinicians too should be working to complete relevant studies in this area.
3. For policymakers Clinical Practice Guidelines should include the best available evidence. Currently however there is insufficient evidence from trials on which to base guidelines. It could be suggested as policy that in such cases, clinical practice should take place within well‐designed trials.
Implications for research.
1. General The studies are of variable methodological quality despite most being published after CONSORT guidelines were available (Begg 1996). The methods regarding quality and bias minimisation must be reported clearly. Validated outcomes regarding the primary outcome and adverse effects should be used. The outcomes data should, at least, be presented as numbers. In addition, continuous data should be presented with means, standard deviations (or standard errors) and the number of participants. Data from graphs, 'p' values of differences and statements of significant or non‐significant differences are of limited value.
2. Specific This is an area of many unanswered questions, many of which could be addressed within trials. Pragmatic, real world, randomised controlled trials should be carried out to determine the value of possible clozapine‐induced hypersalivation treatments in standard clinical practice. Studies need to be of more than one month’s duration and involve people whose problems are clearly described, whether by manualised criteria or not. The methods should be very clearly described and tested and the interventions probably should involve use of placebo, but the best chosen experimental treatment may be one used or accepted locally. From this review an antimuscarinic may be indicated; from practice in the UK, it could be hyoscine. Studies need to include a validated method of measuring clozapine‐induced hypersalivation and some medium and long‐term outcomes including adverse events and satisfaction with treatment. We have suggested a design for a study in Table 1.
1. Suggested design for trial.
| Methods | Participants | Interventions | Outcomes | Notes |
| Allocation: centralised sequence generation with table of random numbers or computer generated code, stratified by severity of substance use. Sequence concealed until interventions assigned. Blinding: participants, those recruiting and assigning participants, those assessing outcomes will be blind to treatment allocation. Blinding can be tested by asking participants and raters to guess the treatment they were exposed to. Duration: minimum of 1 year. | Diagnosis: severe mental illness ‐ schizophrenia, schizoaffective disorder, other psychotic disorders for which longer term clozapine treatment is indicated and who are troubled by hypersalivation, rating > 2 on NHRS (nocturnal hypersalivation rating scale). N>1000. Age: adults. Sex: men and women. Setting: hospital and community. | 1. Hyocine: dose 0.3mg bd or tds. 2. Placebo: flexible dose. | Hypersalivation: NHRS (Nocturnal Hypersalivation Rating Scale) Global effect: days well, CGI. Adverse effects: checklist. Discontinuation of treatment. Behavior, social functioning. Satisfaction with treatment Cost. | * size of study to detect a 10% difference in improvement with 80% certainty. |
What's new
| Date | Event | Description |
|---|---|---|
| 18 January 2012 | Amended | Contact details updated. |
History
Protocol first published: Issue 1, 2006 Review first published: Issue 3, 2008
| Date | Event | Description |
|---|---|---|
| 10 November 2010 | Amended | Contact details updated. |
| 11 November 2009 | Amended | Contact details updated. |
| 17 November 2008 | Amended | Plain Language Summary added |
| 13 May 2008 | New citation required and conclusions have changed | Full review for publication |
| 3 May 2008 | Amended | Converted to new review format. |
| 30 April 2008 | New citation required and conclusions have changed | Substantive amendment |
Notes
None.
Acknowledgements
We thank Professor Clive Adams and Tessa Grant for their support during the development of the protocol and review. We are also grateful to Judith Wright for the search strategy and for conducting the electronic searches.
Data and analyses
Comparison 1. ANTIMUSCARINIC: 1. ASTEMIZOLE vs CONTROL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hypersalivation: 1. No Effect / not cured / not markedly improved | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 vs placebo | 2 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.47, 0.81] |
| 1.2 vs diphenpydramine (antimuscarinic) | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.98, 3.21] |
| 1.3 vs doxepin (anitmuscarinic) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.14, 2.37] |
| 1.4 vs propantheline (antimuscarinic) | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.46 [1.63, 3.72] |
| 2 Hypersalivation: 2. Average endpoint score (Scale: mixed clinical criteria, high score= bad, skewed data | Other data | No numeric data | ||
| 3 Hypersalivation: 3. Change in hypersalivation scores (Scale: mixed clinical criteria, high score=good) | 2 | 72 | Mean Difference (IV, Fixed, 95% CI) | 0.04 [‐0.34, 0.42] |
| 3.1 vs placebo | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [0.33, 1.47] |
| 3.2 vs propantheline (antimuscarinic) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐1.14, ‐0.14] |
| 4 Adverse effects: 1. Cardiac‐ tachycardia | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
| 4.1 vs doxepin (antimuscarinic) ‐ tachycardia | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
| 5 Adverse effects: 2. Gastric ‐ constipation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 vs placebo | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.42, 2.79] |
| 5.2 vs diphenpydramine (antimuscarinic) | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.44, 3.54] |
| 5.3 vs propantheline (antimuscarinic) | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.26, 1.39] |
| 6 Adverse effects: 3. Average endpoint score (TESS score, high score=bad) | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐1.73, 0.99] |
| 6.1 vs placebo | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐1.73, 0.99] |
1.1. Analysis.
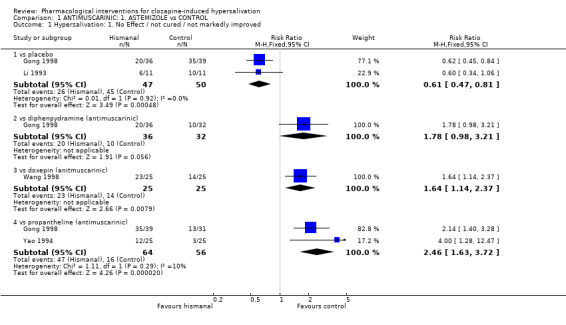
Comparison 1 ANTIMUSCARINIC: 1. ASTEMIZOLE vs CONTROL, Outcome 1 Hypersalivation: 1. No Effect / not cured / not markedly improved.
1.2. Analysis.
Comparison 1 ANTIMUSCARINIC: 1. ASTEMIZOLE vs CONTROL, Outcome 2 Hypersalivation: 2. Average endpoint score (Scale: mixed clinical criteria, high score= bad, skewed data.
| Hypersalivation: 2. Average endpoint score (Scale: mixed clinical criteria, high score= bad, skewed data | ||||
|---|---|---|---|---|
| Study | Intervention | Mean | sd | n |
| Li 1993 | Astemizole | 1.82 | 1.17 | 11 |
| Li 1993 | Placebo | 2.82 | 0.87 | 11 |
| Yao 1994 | Astemizole | 1.72 | 1.14 | 25 |
| Yao 1994 | Propantheline | 1 | 0.82 | 25 |
1.3. Analysis.
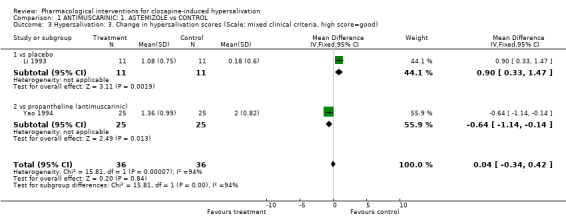
Comparison 1 ANTIMUSCARINIC: 1. ASTEMIZOLE vs CONTROL, Outcome 3 Hypersalivation: 3. Change in hypersalivation scores (Scale: mixed clinical criteria, high score=good).
1.4. Analysis.
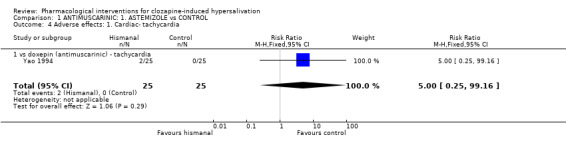
Comparison 1 ANTIMUSCARINIC: 1. ASTEMIZOLE vs CONTROL, Outcome 4 Adverse effects: 1. Cardiac‐ tachycardia.
1.5. Analysis.
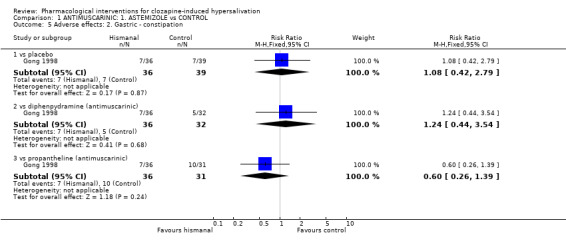
Comparison 1 ANTIMUSCARINIC: 1. ASTEMIZOLE vs CONTROL, Outcome 5 Adverse effects: 2. Gastric ‐ constipation.
1.6. Analysis.
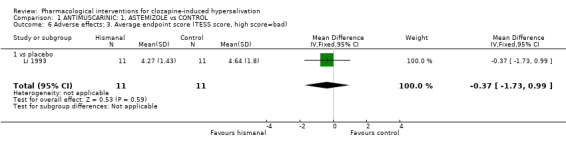
Comparison 1 ANTIMUSCARINIC: 1. ASTEMIZOLE vs CONTROL, Outcome 6 Adverse effects: 3. Average endpoint score (TESS score, high score=bad).
Comparison 2. ANTIMUSCARINIC: 2. PROPANTHELINE vs CONTROL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hypersalivation: 1. No effect / not cured / not markedly improved | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 vs placebo | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.45, 0.77] |
| 1.2 vs astemizole (antimuscarinic) | 2 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.35, 0.89] |
| 1.3 vs diphenhydramine (antimuscarinic) | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.88, 1.50] |
| 1.4 vs doxepin (antimuscarinic) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.44, 1.90] |
| 2 Hypersalivation: 2. Average endpoint score ( Scale: mixed clinical criteria, high score = bad, skewed data) | Other data | No numeric data | ||
| 3 Hypersalivation: 3. Change in hypersalivation scores (Scale: mixed clinical criteria, high score= good) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.64 [0.14, 1.14] |
| 3.1 vs astemizole (antimuscarinic) | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.64 [0.14, 1.14] |
| 4 Adverse effects: 1. cardiac‐abnormal ECG | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.20, 1.83] |
| 4.1 vs astemizole (antimuscarinic) ‐ tachycardia | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.97] |
| 4.2 vs doxepin (antimuscarinic) ‐ abnormal ECG | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 5 Adverse effects: 2. Gastric ‐ constipation | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 vs placebo | 2 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.77, 4.18] |
| 5.2 vs diphenpydramine (antimuscarinic) | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.80, 5.36] |
| 5.3 vs doxepin (antimuscarinic) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.44, 1.90] |
| 5.4 vs propantheline (antimuscarinic) | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [0.72, 3.84] |
| 6 Adverse effects: 3. Hepatic ‐ abnormal hepatic function | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.14] |
| 6.1 vs doxepin (antimuscarinic) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.18, 3.14] |
| 7 Adverse effects: 4. Movement disorders ‐ extrapyrimidal | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.30] |
| 7.1 vs doxepin (antimuscarinic) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.30] |
2.1. Analysis.
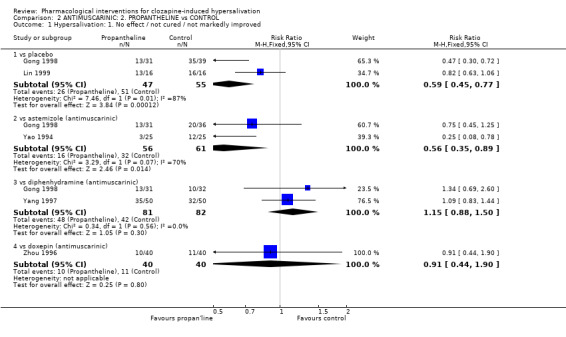
Comparison 2 ANTIMUSCARINIC: 2. PROPANTHELINE vs CONTROL, Outcome 1 Hypersalivation: 1. No effect / not cured / not markedly improved.
2.2. Analysis.
Comparison 2 ANTIMUSCARINIC: 2. PROPANTHELINE vs CONTROL, Outcome 2 Hypersalivation: 2. Average endpoint score ( Scale: mixed clinical criteria, high score = bad, skewed data).
| Hypersalivation: 2. Average endpoint score ( Scale: mixed clinical criteria, high score = bad, skewed data) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Notes |
| Yao 1994 | Hismanal | 1.72 | 1.14 | 25 | |
| Yao 1994 | Propantheline | 1.00 | 0.82 | 25 | |
2.3. Analysis.
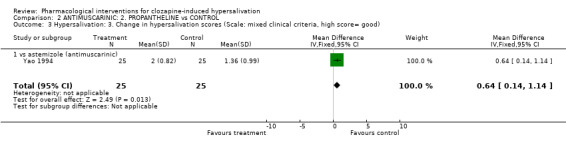
Comparison 2 ANTIMUSCARINIC: 2. PROPANTHELINE vs CONTROL, Outcome 3 Hypersalivation: 3. Change in hypersalivation scores (Scale: mixed clinical criteria, high score= good).
2.4. Analysis.
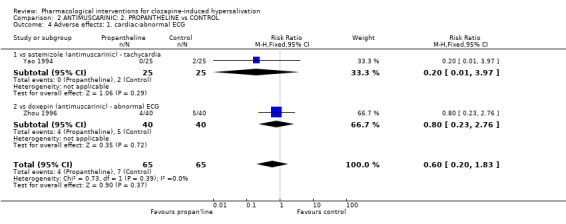
Comparison 2 ANTIMUSCARINIC: 2. PROPANTHELINE vs CONTROL, Outcome 4 Adverse effects: 1. cardiac‐abnormal ECG.
2.5. Analysis.
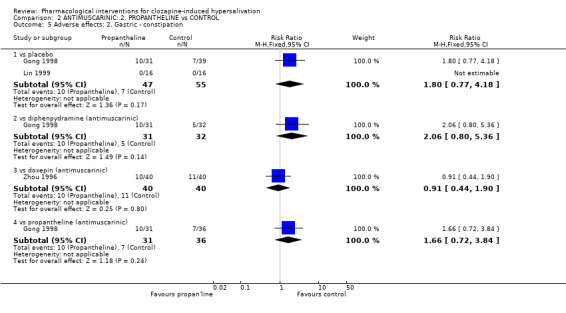
Comparison 2 ANTIMUSCARINIC: 2. PROPANTHELINE vs CONTROL, Outcome 5 Adverse effects: 2. Gastric ‐ constipation.
2.6. Analysis.
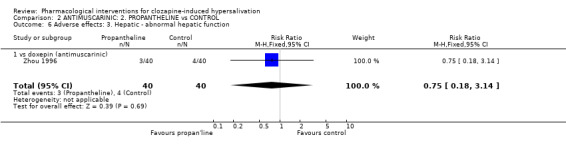
Comparison 2 ANTIMUSCARINIC: 2. PROPANTHELINE vs CONTROL, Outcome 6 Adverse effects: 3. Hepatic ‐ abnormal hepatic function.
2.7. Analysis.
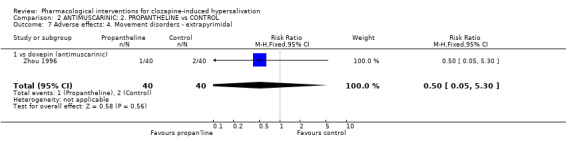
Comparison 2 ANTIMUSCARINIC: 2. PROPANTHELINE vs CONTROL, Outcome 7 Adverse effects: 4. Movement disorders ‐ extrapyrimidal.
Comparison 3. ANTIMUSCARINIC: 3. DIPHENHYDRAMINE vs CONTROL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hypersalivation: 1. No effect / not cured / not markedly improved | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 vs placebo | 2 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.31, 0.58] |
| 1.2 vs astemizole (antimuscarinic) | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.37, 1.32] |
| 1.3 vs propantheline (antimuscarinic) | 2 | 163 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.67, 1.13] |
| 2 Hypersalivation: 2. Average endpoint score (Scale: wet pillow diameter, high score = bad, skewed data) | Other data | No numeric data | ||
| 3 Adverse effects: Gastric ‐ constipation | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 vs placebo | 2 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.59, 1.95] |
| 3.2 vs hismanal (antimuscarinic) | 1 | 68 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.28, 2.28] |
| 3.3 vs propantheline (antimuscarinic) | 1 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.19, 1.26] |
3.1. Analysis.
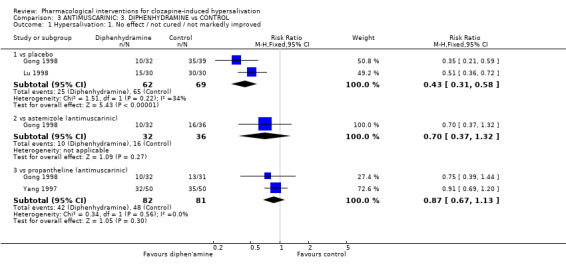
Comparison 3 ANTIMUSCARINIC: 3. DIPHENHYDRAMINE vs CONTROL, Outcome 1 Hypersalivation: 1. No effect / not cured / not markedly improved.
3.2. Analysis.
Comparison 3 ANTIMUSCARINIC: 3. DIPHENHYDRAMINE vs CONTROL, Outcome 2 Hypersalivation: 2. Average endpoint score (Scale: wet pillow diameter, high score = bad, skewed data).
| Hypersalivation: 2. Average endpoint score (Scale: wet pillow diameter, high score = bad, skewed data) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Notes |
| Lu 1998 | Diphenhydramine | 1.01 | 1.11 | 30 | |
| Lu 1998 | Placebo | 2.63 | 0.77 | 30 | |
3.3. Analysis.
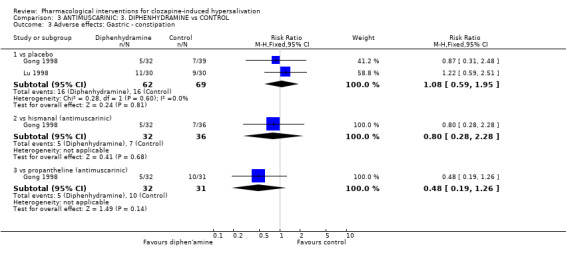
Comparison 3 ANTIMUSCARINIC: 3. DIPHENHYDRAMINE vs CONTROL, Outcome 3 Adverse effects: Gastric ‐ constipation.
Comparison 4. ANTIMUSCARINIC: 4. DOXEPIN vs CONTROL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hypersalivation: 1. No effect / not cured / not markedly improved | 4 | 304 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.14, 1.90] |
| 1.1 vs astemizole (antimuscarinic) | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.42, 0.88] |
| 1.2 vs oryzanolum (other: oryzanol) | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.34, 3.65] |
| 1.3 vs propantheline (antimuscarinic) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.53, 2.30] |
| 1.4 vs suoquanwan (other‐traditional chinese medicines) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [1.69, 6.31] |
| 2 Adverse effects: 1. Cardiac ‐ abnormal ECG | 2 | 184 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.86, 5.47] |
| 2.1 vs oryzanolum (other‐oryzanol) | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.89, 17.95] |
| 2.2 vs propantheline (antimuscarinic) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.36, 4.32] |
| 3 Adverse effects: 2. Gastric ‐ constipation | 3 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.30 [1.85, 5.91] |
| 3.1 vs oryzanolum (other‐oryzanol) | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.5 [1.02, 19.83] |
| 3.2 vs propantheline (antimuscarinic) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.53, 2.30] |
| 3.3 vs suoquanwan (other‐traditional Chinese medicines) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 46.09 [2.89, 734.50] |
| 4 Adverse effects: 3. Hepatic ‐ abnormal hepatic function | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.32, 5.58] |
| 4.1 vs propantheline (antimuscarinic) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.32, 5.58] |
| 5 Adverse effects: 4. Movement disorders ‐ extrapyrimidal | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 5.1 vs propantheline (antimuscarinic) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
4.1. Analysis.
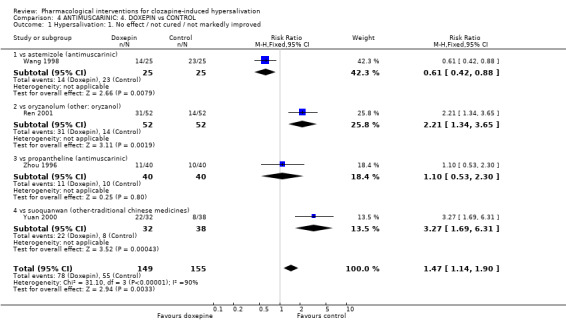
Comparison 4 ANTIMUSCARINIC: 4. DOXEPIN vs CONTROL, Outcome 1 Hypersalivation: 1. No effect / not cured / not markedly improved.
4.2. Analysis.
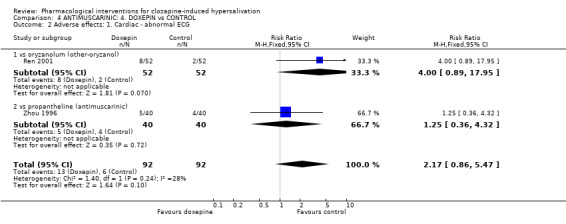
Comparison 4 ANTIMUSCARINIC: 4. DOXEPIN vs CONTROL, Outcome 2 Adverse effects: 1. Cardiac ‐ abnormal ECG.
4.3. Analysis.
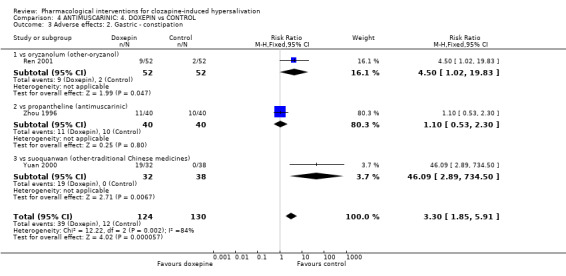
Comparison 4 ANTIMUSCARINIC: 4. DOXEPIN vs CONTROL, Outcome 3 Adverse effects: 2. Gastric ‐ constipation.
4.4. Analysis.
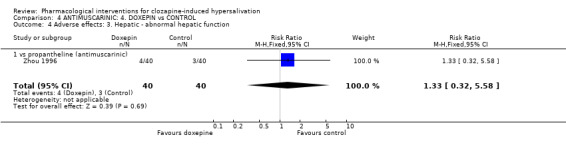
Comparison 4 ANTIMUSCARINIC: 4. DOXEPIN vs CONTROL, Outcome 4 Adverse effects: 3. Hepatic ‐ abnormal hepatic function.
4.5. Analysis.
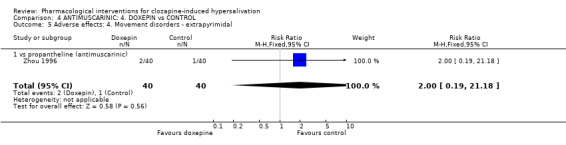
Comparison 4 ANTIMUSCARINIC: 4. DOXEPIN vs CONTROL, Outcome 5 Adverse effects: 4. Movement disorders ‐ extrapyrimidal.
Comparison 5. RICE BRAN OIL DERIVITAVE: ORYZANOL vs CONTROL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hypersalivation: 1. No effect / not cured / not markedly improved | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.27, 0.75] |
| 1.1 vs doxepin (antimuscarinic) | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.27, 0.75] |
| 2 Hypersalivation: 2. Average endpoint score (Scale: wet pillow diameter, high score = bad, skewed data) | Other data | No numeric data | ||
| 3 Adverse effects: 1. Cardiac ‐ abnormal ECG | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.12] |
| 3.1 vs doxepin (antimuscarinic) | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.06, 1.12] |
| 4 Adverse effects: 2. Gastric ‐ constipation | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.98] |
| 4.1 vs doxepin (antimuscarinic) | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.05, 0.98] |
5.1. Analysis.
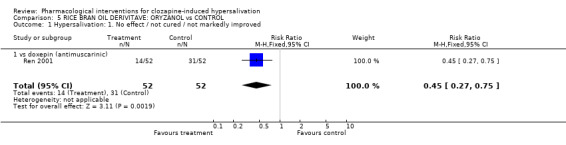
Comparison 5 RICE BRAN OIL DERIVITAVE: ORYZANOL vs CONTROL, Outcome 1 Hypersalivation: 1. No effect / not cured / not markedly improved.
5.2. Analysis.
Comparison 5 RICE BRAN OIL DERIVITAVE: ORYZANOL vs CONTROL, Outcome 2 Hypersalivation: 2. Average endpoint score (Scale: wet pillow diameter, high score = bad, skewed data).
| Hypersalivation: 2. Average endpoint score (Scale: wet pillow diameter, high score = bad, skewed data) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Notes |
| Li 2004 | Oryzanol | 1.90 | 1.46 | 40 | |
| Li 2004 | Placebo | 2.90 | 0.81 | 40 | |
5.3. Analysis.
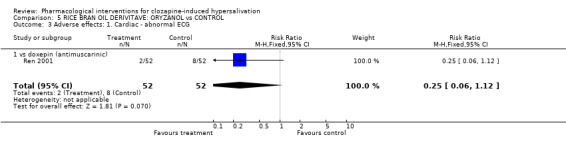
Comparison 5 RICE BRAN OIL DERIVITAVE: ORYZANOL vs CONTROL, Outcome 3 Adverse effects: 1. Cardiac ‐ abnormal ECG.
5.4. Analysis.
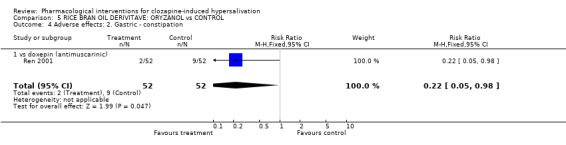
Comparison 5 RICE BRAN OIL DERIVITAVE: ORYZANOL vs CONTROL, Outcome 4 Adverse effects: 2. Gastric ‐ constipation.
Comparison 6. TRADITIONAL CHINESE MEDICINE: 1. SUOQUANWAN vs CONTROL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hypersalivation: 1. No effect / not cured / not markedly improved | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.16, 0.59] |
| 1.1 vs doxepin (antimuscarinic) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.16, 0.59] |
| 2 Hypersalivation: 2. Change in hypersalivation scores (Scale: wet pillow diameter, high score=good) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 After week 1 | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [0.23, 1.99] |
| 2.2 After week 2 | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.03 [1.04, 3.02] |
| 2.3 After week 3 | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.12 [1.29, 2.95] |
| 2.4 After week 4 | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.74 [1.81, 3.67] |
| 3 Adverse effects: Gastric ‐ constipation | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.35] |
| 3.1 vs doxepin (antimuscarinic) | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.02 [0.00, 0.35] |
6.1. Analysis.
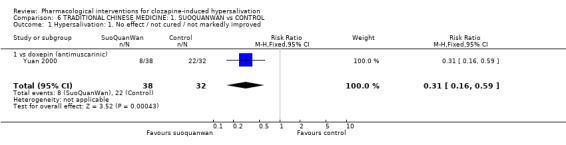
Comparison 6 TRADITIONAL CHINESE MEDICINE: 1. SUOQUANWAN vs CONTROL, Outcome 1 Hypersalivation: 1. No effect / not cured / not markedly improved.
6.2. Analysis.
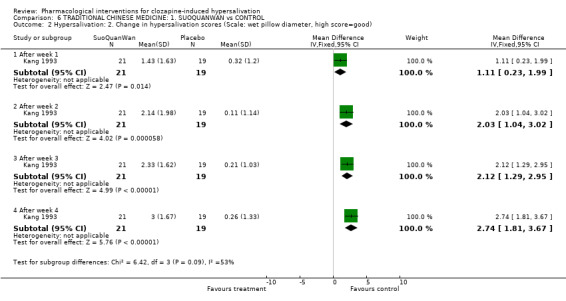
Comparison 6 TRADITIONAL CHINESE MEDICINE: 1. SUOQUANWAN vs CONTROL, Outcome 2 Hypersalivation: 2. Change in hypersalivation scores (Scale: wet pillow diameter, high score=good).
6.3. Analysis.
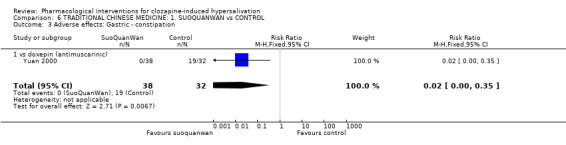
Comparison 6 TRADITIONAL CHINESE MEDICINE: 1. SUOQUANWAN vs CONTROL, Outcome 3 Adverse effects: Gastric ‐ constipation.
Comparison 7. TRADITIONAL CHINESE MEDICINE: 2. HUANGYUANSAN vs NO TREATMENT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hypersalivation: Average endpoint score (Scale: wet pillow diameter, high score = bad, skewed data) | Other data | No numeric data |
7.1. Analysis.
Comparison 7 TRADITIONAL CHINESE MEDICINE: 2. HUANGYUANSAN vs NO TREATMENT, Outcome 1 Hypersalivation: Average endpoint score (Scale: wet pillow diameter, high score = bad, skewed data).
| Hypersalivation: Average endpoint score (Scale: wet pillow diameter, high score = bad, skewed data) | |||||
|---|---|---|---|---|---|
| Study | Intervention | Mean | SD | N | Notes |
| Fan 1996 | Huang Yuang San | 0.94 | 1.58 | 31 | |
| Fan 1996 | No treatment | 1.75 | 1.03 | 31 | |
Comparison 8. TRADITIONAL CHINESE MEDICINE: 3. WUDANSAN PASTE APPLIED TO ACUPUNCTURE POINT vs PLACEBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hypersalivation: 1. No effect / not cured / not markedly improved | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 1.78] |
8.1. Analysis.
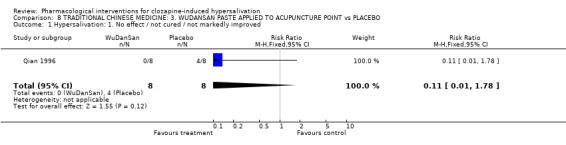
Comparison 8 TRADITIONAL CHINESE MEDICINE: 3. WUDANSAN PASTE APPLIED TO ACUPUNCTURE POINT vs PLACEBO, Outcome 1 Hypersalivation: 1. No effect / not cured / not markedly improved.
Comparison 9. ADJUCTIVE ANTIPSYCHOTIC: AMISULPRIDE vs PLACEBO.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
9.1. Analysis.
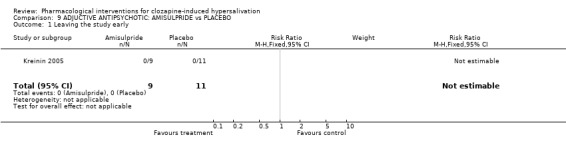
Comparison 9 ADJUCTIVE ANTIPSYCHOTIC: AMISULPRIDE vs PLACEBO, Outcome 1 Leaving the study early.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fan 1996.
| Methods | Allocation: randomised, method not described Blindness: not stated. Duration: 4 weeks. Loss: not described. Setting: inpatients, ChangZhou No.102 Military Hospital, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2). N=62. Age: 16‐55 years old. Sex: male. History: duration of schizophrenia‐ 3 months ‐ 20 years; no hypersalivation at beginning of trial*. Exclusion: patients with cardiac, hepatic or renal impairment, or organic mental disorder. | |
| Interventions | 1. HuangYuanSan (Chinese herbal medicine)** : 2.5‐5g bd/tds + Clozapine 175‐500mg. N=31. 2. Clozapine 150‐500mg. N=31. | |
| Outcomes | Measurement of salivation: average endpoint hypersalivation score***. | |
| Notes | *This is a prevention trial where clozapine and intervention were both started at beginning of trial. **contains: 1. ShengDaHuang (raw Rhubarb); 2. Sodium Sulphate (Anhydrate). Both ingredients are grinded into powder, mixed and divided into small packages of 5g each pack. ***score 0: no hypersalivation; score 1: mild hypersalivation, hypersalivate only during sleep, amount <50ml; score 2: increased hypersalivation, hypersalivate during daytime and drooling during speech occasionally, salivation amount 50‐100ml during sleep; score 3: serious hypersalivation, hypersalivate during daytime with drooling during speech, salivation amount >100ml during sleep. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Gong 1998.
| Methods | Allocation: randomised, method not described. Blindness: double. Duration: 10 days. Setting: inpatients, ZhaoYang Psychiatric Hospital, HuNan City, China. | |
| Participants | Diagnosis: schizophrenia (diagnosis standard not stated) + hypersalivation. N=130 (reported as 130, but actually it is 138). Age: not stated. Sex: not stated. History: duration of schizophrenia ‐ not stated; hypersalivation‐ wet pillow surface <10cm, 85/138; wet pillow surface 10‐20cm, 45/138; wet pillow surface >20cm, 8/138. Exclusion: patients concurrently using antidepressants, antihistamines or anticholinergics. | |
| Interventions | 1. Diphenhydramine: 100‐200mg od + Clozapine (dose not stated). N=32. 2. Propantheline: 60‐120mg od +Clozapine (dose not stated), N=31. 3. Astemizole: 10‐20mg od + Clozapine (dose not stated), N=36. 4. Placebo (Vitamin B1) + Clozapine (dose not stated): N=39. | |
| Outcomes | Measurement of salivation: curative effect* Adverse effects: number of cases with constipation. | |
| Notes | *cured: no hypersalivation; markedly improved: wet pillow surface diameter reduced by 2/3 compare to before treatment; improved: wet pillow surface diameter reduced by 1/3; no effect: wet pillow surface diameter reduced by less than 1/3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kang 1993.
| Methods | Allocation: randomised, method not described. Blinding: not stated. Duration: 4 weeks. Setting: inpatients. | |
| Participants | Diagnosis: schizophrenia (diagnosis standard not stated) + hypersalivation. N=40. Age: mean age in treatment group = 32.4; control group = 28.88. Sex: treatment group ‐ 13F, 8M; control group ‐ 14F, 5M. History: duration of schizophrenia‐ treatment group 1‐25 years, control group 1‐28 years; severity of hypersalivation not described. Exclusion: not described. | |
| Interventions | 1. SuoQuanWan* 9g bd + Clozapine 312mg (SD 71). N=21. 2. Placebo (activated carbon + starch) 9g bd + Clozapine 288mg (SD 69). N=19. | |
| Outcomes | Measurement of salivation: change in hypersalivation scores**. Unable to use ‐ Adverse effects: constipation, serum physiological changes, ECG changes (no data). |
|
| Notes | *Contains: 1. Shan Yao (Chinese yam <radix dioscoreae oppositae>); and 2. Yi Zhi Ren (bitter cardamom <Fructus Alpiniae Oxyphyllae>). Details of the dosage of each substance not stated. **Hypersalivation score: score 0, no hypersalivation; score 1, mild hypersalivation, wet pillow surface <10cm in diameter, or <10ml; score 2, moderate hypersalivation, wet pillow surface 16‐20cm in diameter, or 10‐20ml; score 3, severe hypersalivation, wet pillow surface >20cm, or 20ml. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kreinin 2005.
| Methods | Allocation: randomised, method not described. Blindness: not stated. Duration: 4 weeks. Setting: inpatients, ChangZhou No. 102 Military Hospital, China. | |
| Participants | Diagnosis: schizophrenia (inpatients, DSM IV) + hypersalivation. N=20 (cross over). Age: not stated. Sex: 10F, 10M. History: duration of schizophrenia not stated; hypersalivation with NHRS score 2 or above. Exclusion: unstable conditions eg unstable angina, uncontrolled DM, comorbid substance misuse. | |
| Interventions | 1. Amisulpride (400mg/day) + Clozapine (dose not stated). N=9. 2. Placebo + Clozapine (dose not stated). N=11. nb cross over so all crossed over to other intervention | |
| Outcomes | Leaving the study early. Unable to use ‐ Measurement of salivation: NHRS, continuous variable compared with control group (no data available pre‐crossover). Mental state: PANSS (no data available pre‐crossover). General functioning: CGI (no data available pre‐crossover). Adverse effects: SAS (no data available pre‐crossover). |
|
| Notes | Score of at least 2= mild hypersalivation, wakes the patient once during the night. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Li 1993.
| Methods | Allocation: randomised, method not described. Blindness: double. Duration: 2 weeks. Setting: inpatients, ChengDu Psychiatric Hospital, China. | |
| Participants | Diagnosis: schizophrenia (diagnosis standard not stated) + hypersalivation. N=22. Age: mean 40. Sex: 10F, 12M. History: duration of schizophrenia not stated; hypersalivation score* 2 or above. Exclusion: patients with cardiac, hepatic or renal impairment, or concurrently using anticholinergics or antihistamines. | |
| Interventions | 1. Astemizole: 10mg od + Clozapine (dose not stated). N=11. 2. Placebo (Vitamin C) + Clozapine (dose not stated), N=11. | |
| Outcomes | Measurement of salivation: average endpoint and change in hypersalivation scores*, curative effect**.
Adverse effects: TESS score. Unable to use ‐ Adverse effects: serum and urinary physiological measures, and ECG changes (no data). |
|
| Notes | * score 0: no hypersalivation; score 1: mild hypersalivation; score 2: noticeably increased hypersalivation; score 3: wet pillow; score 4: wet pillow and hypersalivation during daytime; score 5: drooling on standing. ** cured: no hypersalivation; markedly improved: symptoms significantly reduced; improved, symptoms slightly reduced; no effect: no improvement on symptoms, or worsened. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Li 2004.
| Methods | Allocation: randomised by tossing a coin. Blindness: not stated. Duration: 2 weeks. Setting: inpatients, BaiSe city, China. | |
| Participants | Diagnosis: schizophrenia (diagnosis standard not stated) + hypersalivation. N=80. Age: subject: 33.6 (SD 5.0); control: 35.8 (SD 4.8) Sex: 21F, 59M. History: duration of schizophrenia‐ treatment group 3.3 years (SD 1.2), control group 3.5 (SD 1.4); hypersalivation score* treatment group: 3.30 (SD 0.57); control group: 3.20 (SD 0.69). Exclusion: patients with cardiac, hepatic or renal impairment, or concurrently using anticholinergic or antihistamines. | |
| Interventions | 1. Oryzanol 10mg capsule, 2 capsules bd + Clozapine 340 mg (SD 112.0). N=40. 2. Placebo (starch) 2 capsules bd + Clozapine 350 mg (SD 98.0). N=40. | |
| Outcomes | Measurement of salivation: average endpoint hypersalivation scores*. | |
| Notes | *score 0: no hypersalivation; score 1: mild hypersalivation at daytime; score 2: mild hypersalivation with obvious moist tongue surface; score 3: wet pillow surface diameter <20cm during sleep; score 4: wet pillow surface diameter >20cm; score 5: obvious drooling. (derived from Yung 1993). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lin 1999.
| Methods | Allocation: randomised, method not described. Blindness: double. Duration: 10 days. Setting: inpatients, NanNing City, China. | |
| Participants | Diagnosis: schizophrenia (diagnosis standard not stated) + hypersalivation. N=32. Age: subject 37 (SD 7.2); control 39 (SD 6.8). Sex: male. History: duration of schizophrenia not stated, duration of clozapine treatment‐ treatment group 4.3 years (SD 1.6); control group 4.5 (SD 1.4); hypersalivation for over 1 week; mean hypersalivation score*, treatment group 3.25, control group 2.94. Exclusion: patients with cardiac, hepatic or renal impairment, or concurrently using anticholinergic or anti‐histamines. | |
| Interventions | 1. Propantheline 15mg bd + Clozapine 217mg (SD 94). N=16. 2. Placebo (vitamin B1) 10mg bd + Clozapine 244mg (SD 98.8). N=16. | |
| Outcomes | Measurement of salivation: curative effect**. Unable to use: Adverse effects: change in pulse rate (no data in control group) |
|
| Notes | *Hypersalivation score derived from Yung 1993. **Cured: no hypersalivation; Improved: hypersalivation reduced; no effect: no improvement on hypersalivation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lu 1998.
| Methods | Allocation: randomised, method not described. Blindness: double. Duration: 10 days. Setting: inpatients, GuangZhou Psychiatric Hospital, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R) + hypersalivation. N=60. Age: subject 26.0 (SD 11.0), control 27.0 (SD 5.0). Sex: female. History: duration of schizophrenia not stated; hypersalivation score* treatment group‐ score 2=8, score 3=17, score 4=5, control group‐ score 2=7, score 3=19, score 4=4. Exclusion: serious physical conditions. | |
| Interventions | 1. Diphenhydramin 25mg/capsule, 2 capsules od + Clozapine 335.2mg (SD103.7) od. N=30. 2. Placebo (not specified) 2 capsules od + Clozapine 352.7mg (SD105.2). N=30. | |
| Outcomes | Measurement of salivation: average change in hypersalivation scores* and curative effect**. Adverse effects: constipation. | |
| Notes | *Salivation score: score 0, no hypersalivation; score 1, occasional hypersalivation during sleep, wet surface diameter <10cm; score 2, hypersalivation during sleep, wet surface diameter 10‐20cm; score 3, hypersalivation whilst awake, wet surface diameter 10‐20cm during sleep; score 4, wet surface diameter >20cm. **Curative effect: cured; improved (score at least 1 point less); no effect. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Qian 1996.
| Methods | Allocation: not stated. Blindness: double. Duration: 4 weeks. Setting: inpatients, ShangHai city, China. | |
| Participants | Diagnosis: schizophrenia (diagnosis standard not stated) + hypersalivation. N=16. Age: not stated. Sex: male. History: duration of schizophrenia not stated; hypersalivation score* treatment group ‐ 4.25, control group ‐ 4.0. Exclusion: not stated. | |
| Interventions | 1. WuDanSan (Chinese herbal medicine)** applied to acupuncture point once daily + Clozapine 310.1mg (SD 30.4). N=8. 2. Placebo (flour + vinegar paste) applied to same acupuncuter point once daily + Clozapine 325.8mg (SD 4.5). N=8. | |
| Outcomes | Measurement of salivation: curative effect***.
Adverse effects: measure unclear. Unable to use ‐ Time to effect: no SD in control group. Adverse effects: no data provided. |
|
| Notes | *Hypersalivation score derived from Yung 1993. **Contains 1. WuZhuYu (Medicinal Evodia Fruit <Fructus Evodiae>; 2. DanNanXing (Arsaema Cum bile); 3. Rice Vinegar. Substances 1&2 are grinded and mixed at 3:1 ratio, then combined with rice vinegar to form paste to be applied to acupuncture point Yongquan. ***Curative effect: cured, markedly improved (wet pillow surface diameter 5cm), improved (6cm), no effect (>10cm). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Ren 2001.
| Methods | Allocation: randomised, method not described. Blindness: double. Duration: 4 weeks. Setting: inpatients, ZiBo Psychiatric Hospital, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2‐R) + hypersalivation. N=104. Age: Doxepin ‐ 15‐56 (32.13, SD 11.25); Oryzanolum 16‐58 (31.28, SD 10.59). Sex: Doxepin ‐ 23F, 29M; Oryzanolum 20F, 32M. History: duration of schizophrenia ‐ Doxepin 3m‐21y (5.60y, SD 5.00); Oryzanolum 3‐18y (5.20y, SD 5.10); severity of hypersalivation not described. Exclusion: not concurrently using any psychotropic medications. | |
| Interventions | 1. Doxepin: 25mg/capsule, 1‐2 capsules tds + Clozapine 50‐400mg (234.00, SD 159.00). N=52. 2. Oryzanolum: 10mg/capsule, 1‐2 capsules tds + Clozapine 50‐400mg (231.00, SD 105.00). N=52. | |
| Outcomes | Measurement of salivation: curative effect*.
Adverse effects: ECG changes and constipation. Unable to use: Adverse effects: blood pressure and serum physiological changes (no data). |
|
| Notes | *Cured: no hypersalivation; markedly improved: hypersalivation significantly, only hypersalivate during sleep and wet pillow surface diameter <10cm; improved: hypersalivation improved during sleep and daytime; No effect: no improvement on hypersalivation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wang 1998.
| Methods | Allocation: randomised (no further detail given). Blinding: not stated. Duration: 4 weeks. Setting: inpatients, Shanghai Mental Health Centre, China. | |
| Participants | Diagnosis: schizophrenia (diagnosis standard not stated) + hypersalivation. N=50. Age: Doxepin ‐ 33‐66 (47.3, SD 8.1); Hismanal ‐ 24‐68 (47.3, SD 10.2). Sex: male. History: Duration of schizophrenia: Doxepin ‐ 9‐40y (24.1, SD 9.6); Hismanal ‐ 2m‐39y (23.9, SD 9.3). Hypersalivation score*, Doxepin ‐ score 1=1, score 2=18, score 3=6; Hismanal ‐ score 1=6, score 2=13, score 3=6. | |
| Interventions | 1. Doxepin: 1st week 25mg od, 2nd week 25 mg bd, 3rd week 25mg noon, 50mg on + Clozapine 50‐500mg (204.3, SD 107.6). N=25. 2. Astemizole: 1st week 10 mg od, increased in the following 2 weeks (by similar increments as above, but exact increments not specified) + Clozapine 50‐500mg (202.8, SD 101.5). N=25. | |
| Outcomes | Measurement of salivation: curative effect** at week 1, 2, & 4. Unable to use: Adverse effects: TESS scores at week 0, 2 & 4 (no data). Mental state: BPRS scores at week 0, 2 & 4 (no data). |
|
| Notes | *Salivation score: score 0, no hypersalivation; score 1, occasional hypersalivation during sleep, wet surface diameter <10cm; score 2, hypersalivation during sleep, wet surface diameter 10‐20cm; score 3, hypersalivation whilst awake, wet surface diameter 10‐20cm during sleep; score 4, wet surface diameter >20cm. ** Markedly improved: hypersalivation score reduced by more than 2 points; improved: hypersalivation score reduced by 1 point; No effect: no improvement on hypersalivation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yang 1997.
| Methods | Allocation: randomised, method not described. Blindness: double. Duration: 4 weeks. Setting: inpatients, GuangZhou Psychiatric Hospital, China. | |
| Participants | Diagnosis: schizophrenia (diagnose standard not stated) + hypersalivation. N=100. Age: Diphenpydramine ‐ 18‐59 (26.7, SD 10.8); Probantheline ‐ 17‐60 (27.5, SD 9.8). Sex: female. History: Duration of schizophrenia: Diphenpydramine 6m‐40y (8.98, SD 9.67); Probanthine 5m‐37y (8.75, SD 9.52); hypersalivation score*: Diphenpydramine ‐ score 2=12, score 3=32, score 4=6, Probanthine ‐ score 2=14, score 3=31, 4=5. Inclusion: patients on Clozapine alone, has been hypersalivating for at least 1 week, with no other organic illnesses. Exclusion: patients who required to change dose of Clozapine, combination of other medications or deterioration of illness. | |
| Interventions | 1. Diphenhydramine: 50mg od + Clozapine 150‐525mg (325.67, SD 92.82). N=50. 2. Propantheline: 30mg od + Clozapine 150‐600mg (328.72, SD 91.34). N=50. | |
| Outcomes | Measurement of salivation: curative effect**. Unable to use: Adverse effects: ECG changes and serum physiological changes (no data). |
|
| Notes | *Salivation score: score 0, no hypersalivation; score 1, occasional hypersalivation during sleep, wet surface diameter <10cm; score 2, hypersalivation during sleep, wet surface diameter 10‐20cm; score 3, hypersalivation whilst awake, wet surface diameter 10‐20cm during sleep; score 4, wet surface diameter >20cm. **Cured: no hypersalivation; Improved: hypersalivation reduced by at least 1 point; no effect: no improvement on hypersalivation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yao 1994.
| Methods | Allocation: randomised, method not described. Blindness: double. Duration: 2 weeks. Setting: inpatients, No. 4 People's Hospital, ZhenJiang City, China. | |
| Participants | Diagnosis: schizophrenia (diagnosis standard not stated) + hypersalivation. N=50. Age: Probanthine 32.4y (SD 5.2); Hismanal 34.1 (Sd 7.3). Sex: Probanthine 14F, 11M; Hismanal 14F, 11M. History: duration of schizophrenia not stated; hypersalivation score*: Probantheline 3 (SD 0.866); Hismanal 3.08 (SD 0.76). Exclusion: patients with organic mental disorders, cardiac, renal or hepatic impairment, or concurrently using anticholinergics or antihistamines. | |
| Interventions | 1. Propantheline 30mg/capsule, 1 capsule bd + Clozapine (dose not stated). N=25. 2. Astemizole 10mg + vitamin C/capsule od + Vitamin C capsure on + Clozapine (dose not stated). N=25. | |
| Outcomes | Measurement of salivation: average endpoint hypersalivation scores*, average change in hypersalivation scores*, and curative effect**.
Adverse effects: tachycardia. Unable to use: Adverse effects: TESS score (no data). |
|
| Notes | *Hypersalivation score. Score 0: no hypersalivation; score 1: mild hypersalivation; score 2: noticeably increased hypersalivation; score 3: wet pillow; score 4: wet pillow and hypersalivation during daytime; score 5: drooling on standing. **Curative effect. Cured: 100% reduction; markedly improved: 50‐100% reduction; improved: 0‐50% reduction; no effect: 0% reduction. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yuan 2000.
| Methods | Allocation: randomised, method not described. Blindness: not stated. Duration: 7 days. Setting: inpatients, Mental health prevention hospital, JiNing city, China. | |
| Participants | Diagnosis: schizophrenia (diagnosis standard not stated) + hypersalivation. N=70. Age: SuoQuanWan 16‐52 (33, SD 10); Doxepin 16‐50 (32, SD 9). Sex: SuoQuanWan 15F, 23M; Doxepin 12F, 20M. History: duration of schizophrenia: SuoQuanWan 0.8‐7y (3.8, SD 1.9), Doxepin 0.6‐7y (3.6, SD 1.8); hypersalivation scale*: SuoQuanWan mild=18, intermediate=14, serious=6; Doxepine mild=15, intermediate=12, serious=5. Exclusion: not concurrently using antidepressants, antihistamines or other anitcholinergics. | |
| Interventions | 1.SuoQuanWan (Chinese herbal medicine)**: 9g/capsule, 1 capsule tds + Clozapine 150‐275mg (201.9, SD 44.9). N=38. 2. Doxepin: 25‐50mg tds + Clozapine 150‐275mg (205.7, SD 46.2). N=32. | |
| Outcomes | Measurement of salivation: curative effect***.
Adverse effects: constipation. Unable to use: Adverse effects: blood pressure, serum and urinary physiological changes, ECG changes and EEG changes (no data). |
|
| Notes | *Hypersalivation scale. mild: wet pillow surface diameter <10cm; intermediate: 10‐20cm; serious: >20cm. **Contains: 1. Wu Yao (combined spice bush root <radix linderae strychnifoliae>. Root of a shrub or small arbour plant Lindera strychnifolia Villar; 2. Shan Yao (Chinese yam <radix dioscoreae oppositae>. Tuber of dioscorea opposita thunb; 3. Yi Zhi Ren (bitter cardamom <Fructus Alpiniae Oxyphyllae>). All 3 ingredients are grinded and mixed at 1:1:1 ratio. Honey was added to the mixture to make it into round pills of 9g each. ***Curative effect. Markedly improved: no hypersalivation or diameter reduced by >2/3; improved: diameter reduced by 1/3‐2/3; no effect: diameter reduced by <1/3. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zhou 1996.
| Methods | Allocation: randomised, method not described. Blindness: double. Duration: not stated clearly, >2 weeks. Setting: GuangDong, China. | |
| Participants | Diagnosis: schizophrenia (CCMD‐2) + hypersalivation. N=80. Age: Doxepin 19‐50 (27.5, SD 8.3); Probanthine 20‐49 (28.3, SD 8.7). Sex: Doxepin 20F, 20M; Probanthine 19F, 21M. History: duration of schizophrenia: Doxepin 7m‐30y (7.3, SD 8.5), Probanthine 6m‐32y (7.2, SD 8.6); hypersalivation score*: Doxepin score 1=24, score 2=14, score 3=2; Probanthine score 1=25, score 2=13, score 3=2. Exclusion: patients with organic mental disorders, cardiac, renal or hepatic impairment, or concurrently using anticholinergics or antihistamines. | |
| Interventions | 1. Doxepin 25mg od + Clozapine 200‐550mg (375, SD 98). N=40. 2. Probantheline 30mg od + Clozapine 200‐550 mg (350, SD 94). N=40. | |
| Outcomes | Measurement of salivation: curative effect** Adverse effects: constipation, EPS, LFTs changes and ECG changes. | |
| Notes | *Hypersalivation score. score 1: hypersalivation during sleep, wet pillow surface diameter <10cm; score 2: mild hypersalivation whilst awake, wet pillow surface diameter during sleep 10‐20cm; score 3: hypersalivation whilst awake, wet pillow surface diameter during sleep >20cm. **Curative effect. Cured: no hypersalivation; improved: hypersalivation score reduced by 1 point; No effect. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Xu 1997 | Allocation: not described, no indication it was randomised. |
| Ya‐Mei 2001 | Allocation: randomised. Participants: people with clozapine‐induced hypersalivation (20). Interventions: pirenzepine versus placebo. Outcomes: diameter of wet patch on pillow, no data pre‐crossover ‐ unable to use. |
| Zhai 1992 | Allocation: not randomised, prospective cohort study. |
Characteristics of ongoing studies [ordered by study ID]
Kumar 2008.
| Trial name or title | Modafinil for clozapine related adverse effects in people with schizophrenia or schizoaffective disorders in remission: a randomized, placebo controlled trial |
| Methods | |
| Participants | Diagnosis: Schizophrenia or schizoaffective disorder on clozapine Age: Over 18 Sex: Both male and female History: In remission from psychotic and mood symptoms, who are experiencing troublesome side effects Exclusion: Active psychotic symptoms or adding modafinil poses unnecessary risk. Unstable general medical conditions. Prior trial of modafinil in the previous six weeks or hyper‐sensitivity to modafinil Pregnant or lactating women |
| Interventions | 1. Modafinil 2. Placebo |
| Outcomes | Primary; daytime sleepiness, nocturnal hypersalivation. Secondary; weight, BP changes, blood sugars and lipids, BPRS, PANSS, CGI, change in baseline clozapine blood levels, adverse effects |
| Starting date | Ongoing |
| Contact information | Sebind Kumar, Tutor, Dept Psychiatry, Unit II, Christian Medical College, Vellore 632002, Tamil Nadu, INDIA, sebind@cmcvellore.ac.in |
| Notes | Allocation: randomised, computer generated Blindness: Participants, asessors, investigators, data entry operators, and those providing the interventions will be blinded Duration: 9 weeks Loss: Intention to treat analysis Setting: Inpatients or outpatients Allocation Concealment: Pre‐numbered coded dispensers prepared by pharmacists and dispensed serially to study participants |
Li 2008.
| Trial name or title | A randomised, double blind, placebo controlled trial of tabellae belladonnae compositae for clozapine induced hypersalivation |
| Methods | |
| Participants | Inpatients diagnosed with schizophrenia CCMD‐3 N: 120 Age: Not known Sex: Not known History: Not known Exclusion: Not known |
| Interventions | 1. Tabellae belladonnae compositae 2. Placebo |
| Outcomes | The correlation between amount of hypersalivation and medication dosage, the correlation between amount of hypersalivation and length of medication, hypersalivation Using a scale: "Hypersalivation: 0, none; 1, mild ‐ only hypersalivation during sleep, wet pillow surface >10cm in diameter; 2, moderate ‐ slight hypersalivation during day time, wet pillow surface 10 ˜ 20cm; 3, severe ‐ constant hypersalivation, wet pillow surface >20 cm. |
| Starting date | Unstated, currently in planning stage |
| Contact information | Chunbo Li: licb@mail.tongji.edu.cn. Cochrane Schizophrenia Group: ceadams@cochrane‐sz.org |
| Notes | Allocation: randomised, using random number tables Blindness: "assessors are blinded". Duration: 8 weeks. Loss: not described. Setting: Inpatients Allocation Concealment: not mentioned |
Differences between protocol and review
None.
Contributions of authors
Katie Au ‐ writing of final report.
Caroline Cahill ‐ protocol writing and preparation of final report.
Lorna Duggan ‐ original idea, protocol writing.
Yanling He ‐ Translation, data extraction, input and preparation of final report.
Rebecca Syed ‐ data extraction and input, writing of final report.
Victor Udu ‐ protocol writing, data extraction and writing of final report.
Jun Xia ‐ Translation, data extraction, input and preparation of final report.
Sources of support
Internal sources
Institute of Psychiatry, UK.
St Andrew's Healthcare, Northampton, UK.
Kings College Hospital, London, UK.
Shanghai Mental Health Centre, China.
University of Nottingham, UK.
External sources
No sources of support supplied
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Fan 1996 {published data only}
- Fan QZ, Zhang LY, Duan H, Yu SW, Bu CG, Zhao HQ. Effectiveness of HuangYuanSan in the treatment of clozapine‐induced hypersalivation: A control study. Sichuan Mental Health 1996;9(3):169‐70. [Google Scholar]
Gong 1998 {published data only}
- Gong GQ, Yuan LX, Tan D, Li HJ, Zhao LX, Hu CF, Yang XT, Li XW, Lu CJ, Chen HM, Liu G, Yi WZ, Zhou SC, Zheng LB. Effectiveness of diphenhydramine, propantheline, hismanal and placebo in the treatment of clozapine‐induced hypersalivation: A double blind control study. Clinical Journal of Psychiatry 1998;31(3):138. [Google Scholar]
Kang 1993 {published data only}
- Kang B, Liu YC, Zhang YP, Han Y, Fan LZ, Zhou J. Effect of suo quan pill for reducing clozapine induced salivation. Chung Kuo Chung Hsi I Chieh Ho Tsa Chih 1993;13(6):347‐8, 325. [PubMed] [Google Scholar]
Kreinin 2005 {published data only}
- Kreinin A, Novitski D, Weizman A. Amisulpride treatment of clozapine‐induced hypersalivation in schizophrenia patients: a randomized, double‐blind, placebo‐controlled cross over study. International Clinical Psychopharmacology 2006;21(2):99‐03. [DOI] [PubMed] [Google Scholar]
Li 1993 {published data only}
- Li ZX, Song ZM, Cheng PF, Li CL, Xiang XY, Shi H. Hismanal treatment of clozapine‐induced hypersalivation: A double blind study. Journal of Clinical Psychiatry 1993;3(1):34‐35. [Google Scholar]
Li 2004 {published data only}
- Li Y. Effectiveness of oryzanol in the treatment of clozapine‐induced salivation reaction. Journal of Nursing Science 2004;19(21):72‐73. [116649] [Google Scholar]
Lin 1999 {published data only}
- Lin H. A double blind control study of propantheline and placebo in the treatment of clozapine‐induced hypersalivation. Chinese Journal of Nervous Mental Disorders 1999;25(5):316‐17. [Google Scholar]
Lu 1998 {published data only}
- Lu XB, Xue SJ, Yang BZ. A double‐blind comparative study of diphenhydramine and placebo in the treatment of sialorrhea caused by clozapine. Sichuan Mental Health 1998;11(1):35‐37. [Google Scholar]
Qian 1996 {published data only}
- Qian QK, Gao ZX. Application of WuDanSan in accupunture points for treatment of clozapine‐induced hypersalivation. Shanghai Archives of Psychiatry 1996;8(2):103. [Google Scholar]
Ren 2001 {published data only}
- Ren QT, Zhao CL, Ma XQ. The therapeutic efficacies of oryzanolum and doxepin on salivation caused by clozapine. Practical Clinical Medicine 2001;2(2):31‐32. [Google Scholar]
Wang 1998 {published data only}
- Wang SB, Song W, Gu WM. Doxepin and hismanal treament of clozapine‐induced hypersalivation. Shanghai Archives of Psychiatry 1998;10(3):176‐77. [Google Scholar]
Yang 1997 {published data only}
- Yang MZ, Zue SJ, Wen QQ. Effectiveness of diphenhydramine and propantheline in treatment of clozapine‐induced hypersalivation: A double blind control study. GuangDong Medicine 1997;18(11):731. [Google Scholar]
Yao 1994 {published data only}
- Yao HX, Qu HF, Li GH. Effectiveness of propantheline and hismanal in treatment of clozapine‐induced hypersalivation: a double blind control study. Journal of Clinical Psychiatry 1994;4(1):4. [Google Scholar]
Yuan 2000 {published data only}
- Yuan CM, Zhao XY, Han QY. A control study of the effectiveness of SuoQuanWan and doxepin in the treatment of clozapine‐induced hypersalivation. Chinese Journal of Psychiatry 2000;33(4):206. [Google Scholar]
Zhou 1996 {published data only}
- Zhou HH, Zhang Q. A double blinded control study of the effectiveness of doxepin and propatheline in the treatment of clozapine‐induced hypersalivation. Journal of Clinical Psychiatry 1996;6:300. [Google Scholar]
References to studies excluded from this review
Xu 1997 {published data only}
- Xu HD, Dong AZ, Zhang TT, Shu WJ. Diphenhydramine for clozapine‐induced salivation at various dosage levels. New Drugs and Clinical Remedies 1997;16(4):205‐06. [Google Scholar]
Ya‐Mei 2001 {published data only}
- Ya‐Mei B, Chao‐Cheng L, Jen‐Yeu C, Win‐Chien L. Therapeutic effect of pirenzepine for clozapine‐induced hypersalivation: A randomised, double‐blind, placebo‐controlled, cross‐over study. Journal of Clinical Psychopharmacology 2001;21(6):608‐11. [DOI] [PubMed] [Google Scholar]
Zhai 1992 {published data only}
- Zhai J. Hismal treatment for clozapine induced hypersalivation. Chinese Journal of Nervous and Mental Diseases 1992;4:202. [Google Scholar]
References to ongoing studies
Kumar 2008 {unpublished data only}
- Modafinil for clozapine related adverse effects in people with schizophrenia or schizoaffective disorders in remission: a randomized, placebo controlled trial. Ongoing study Ongoing.
Li 2008 {unpublished data only}
- A randomised, double blind, placebo controlled trial of tabellae belladonnae compositae for clozapine induced hypersalivation. Ongoing study Unstated, currently in planning stage.
Additional references
Altman 1996
- Altman DG, Bland JM. Detecing skewness from summary information. BMJ 1996;313:1200. [OLZ020600] [DOI] [PMC free article] [PubMed] [Google Scholar]
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637‐9. [DOI] [PubMed] [Google Scholar]
Ben‐Aryeh 1996
- Ben‐Aryeh H, Jungerman T, Szargel R, Klein E, Laufer D. Salivary flow‐rate and composition in schizophrenic patients on clozapine: subjective reports and laboratory data. Biological Psychiatry 1996;39(11):946‐9. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315:600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brodkin 1996
- Brodkin E, Pelton G, Price L. Treatment of clozapine‐induced parotid gland swelling. American Journal of Psychiatry 1996;152:445. [DOI] [PubMed] [Google Scholar]
Corrigan 1995
- Corrigan F, MacDonald S, Reynolds G. Clozapine‐induced hypersalivation and the alpha2 adrenoceptor. British Journal of Psychiatry 1995;167:412. [DOI] [PubMed] [Google Scholar]
Curtin 2002a
- Curtin F, Altman D, Elbourne D. Meta‐analysis combining parallel and cross‐over clinical trials. I: Continuous outcomes. Statistics in Medicine 2002;21(15):2131‐44. [DOI] [PubMed] [Google Scholar]
Curtin 2002b
- Curtin F, Elbourne D, Altman D. Meta‐analysis combining parallel and cross‐over clinical trials. II: Binary outcomes. Statistics in Medicine 2002;21(15):2145‐59. [DOI] [PubMed] [Google Scholar]
Curtin 2002c
- Curtin F, Elbourne D, Altman D. Meta‐analysis combining parallel and cross‐over clinical trials. III: The issue of carry‐over. Statistics in Medicine 2002;21(15):2161‐73. [DOI] [PubMed] [Google Scholar]
Davydov 2000
- Davydov L, Botts S. Clozapine‐induced hypersalivation. Annals of Pharmacotherapy 2000;34:662‐5. [DOI] [PubMed] [Google Scholar]
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623‐9. [DOI] [PubMed] [Google Scholar]
Donner 2002
- Donner A, Klar N. Issues in the meta‐analysis of cluster randomized trials. Statistics in Medicine 2002;21:2971‐80. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder CSO. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;13:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman D, Higgins J, Curtin F, Worthington H, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Fleiss 1984
- Fleiss JL. The crossover study. The design and analysis of clinical experiments. Chichester: John Wiley & Sons, 1984. [Google Scholar]
Goodnick 2002
- Goodnick PJ, Jerry J, Parra F. Psychotropic drugs and the ECG: focus on the QTc interval. Expert Opinion on Pharmacotherapy May 2002;3(5):479‐98. [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community‐based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149:876‐83. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5 [updated May 2005]. The Cochrane Collaboration. Chichester, UK: John Wiley & Sons, Ltd., 2005. [Google Scholar]
Hinkes 1996
- Hinkes R, et al. Aspiration pneumonia possibly secondary to clozapine‐induced sialorrhea. Journal of Clinical Psycho‐pharmacology 1996;16:462‐3. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249‐52. [DOI] [PubMed] [Google Scholar]
Rabinowitz 1996
- Rabinowitz T, Frankenburg F, Centorrino F, Kando J. The effect of clozapine on saliva flow rate: a pilot study. Biological Psychiatry 1996;40(11):1132‐4. [DOI] [PubMed] [Google Scholar]
Reinstein 1999
- Reinstein, M, Sirotovskya, L, Chasonov, M. Comparative efficacy and tolerability of benzatropine and terazosin in the treatment of hypersalivation secondary to clozapine. Journal of clinical drug investigation 1999;17:97‐02. [Google Scholar]
Robinson 1995
- Robinson D, Fenn H, Yesavage J. Possible association of parotitis with clozapine. American Journal of Psychiatry 1995;152:297‐8. [DOI] [PubMed] [Google Scholar]
Rogers 2000
- Rogers D, Shramko J. Therapeutic options in the treatment of clozapine‐induced sialorrhea. Pharmacotherapy 2000;20(9):1092‐5. [DOI] [PubMed] [Google Scholar]
Schmauss 1989
- Schmauss M, Wolff R, Erfurth A, Ruther E. Tolerability of long term clozapine treatment. Psychopharmacology (Berl) 1989;99 suppl:S105‐8. [DOI] [PubMed] [Google Scholar]
Spivak 1997
- Spivak B, Adlersberg S, Rosen L, Gonen N, Mester R, Weizman A. Trihexyphenidyl treatment of clozapine‐induced hypersalivation.. International Journal of Clinical Psychopharmacology 1997;12:213‐15. [DOI] [PubMed] [Google Scholar]
Taylor 2007
- Taylor D, Paton C, Kerwin R. The Maudsley Prescribing Guidelines. 9th Edition. Informa Healthcare, 2007. [Google Scholar]
Ukoumunne 1999
- Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area‐wide and organistation‐based intervention in health and health care: a systematic review. Health Technology Assessment 1999;3(5):1‐75. [PubMed] [Google Scholar]
Wahlbeck 1999
- Wahlbeck K, Cheine M, Essali MA, Rezk E. Clozapine versus 'typical' neuroleptic medication for schizophrenia. Cochrane Database of Systematic Reviews 1999, Issue 4. [DOI] [PubMed] [Google Scholar]
Wang 2007
- Wang G, Mao B, Xiong ZY, Fan T, Chen XD, Wang L, Lin GJ, Guo J, Chang J, Wu TX, Li TX. The quality of reporting of randomised trials of traditional chinese medicine: a survey of 13 randomly selected journals from mainland China. Clinical Therapeutics July 2007;29(7):1456‐67. [DOI] [PubMed] [Google Scholar]
Young 1998
- Young C, Bowers M, Mazure C. Management of the adverse effects of clozapine. Schizophrenia Bulletin 1998;24(3):381‐90. [DOI] [PubMed] [Google Scholar]
Yung 1993
- Yung CX. Effectiveness of applying WuDanSan to accupunture point in treatment of clozapine‐induced hypersalivation. Journal of NanTung Medical School 1993;13:23. [Google Scholar]
Zheng 1994
- Zheng YP, Lin KM, Zhao JP, Zhang MY, Yong D. Comparative study of diagnostic systems: Chinese Classificationof Mental Disorders‐Second Edition versus DSM‐III‐R. Comprehensive Psychiatry 1994;35(6):441‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zorn 1994
- Zorn S, Jones S, Ward K, Liston D. Clozapine is a potent and selective muscarinic M4 receptor agonist. European Journal of Pharmacology 1994;269:R1‐2. [DOI] [PubMed] [Google Scholar]


