Abstract
Severe combined immunodeficiency (SCID) is an inherited disease with profoundly defective T cells, B cells, and natural killer (NK) cells. X-linked SCID (X-SCID) is its most common form. In this report, we describe a 4-month-old male with X-SCID who presented invagination and also showed hemophagocytic lymphohistiocytosis (HLH). The patient was admitted to our hospital with fever, cough, vomiting, monoliasis, and hepatosplenomegaly in postoperative period at the age of 3 months. The laboratory finding revealed no detectable T cells and hypogammaglobulinemia despite normal B-cell counts. Diagnosis of X-SCID was established by DNA analysis of the interleukin (IL)-2 receptor gamma chain gene (IL2RG); namely, we detected the novel mutation in the splice-site of exon 5 (c.595-1G>T). The patient died due to infection at the age of 4 months. Also, this case is the first report that describes the patient with X-SCID with presented invagination.
Keywords: hemophagocytic lymphohistiocytosis, invagination, X-linked severe combined immunodeficiency
Introduction
X-linked severe combined immunodeficiency (X-SCID) is a rare, life-threatening disease that is characterized by marked impairment of both cellular and humoral immunity [1]. X-SCID is the most common form and accounts for approximately half of the patients with SCID; patients have complete or marked deficiency of T cells but carry a normal or slightly increased number of B cells [2]. X‑SCID is caused by mutations in IL2RG gene [3]. In the absence of a functional γc gene, early lymphoid progenitor cells are unable to respond to the cytokine signals of interleukin (IL)-2, IL-4, IL-7, IL-9, IL-15, and IL-21 that are crucial for the normal development of T cells, natural killer (NK) cells, and late-stage B cells [4]. Clinically, X-SCID form is characterized by severe and persistent infections starting in the first months of life typically accompanied by diarrhea and failure to thrive [5]. Here we reported that a 4-month-old boy with X-SCID, who presented invagination and also showed hemophagocytic lymphohistiocytosis (HLH), carried a novel mutation in exon 5 of the IL2RG gene.
Case report
A boy, who is the first child of unrelated parents, was admitted to pediatric emergency unit, because of fever and vomiting. From his medical history, it was learned that he had undergone operation of invagination 2 weeks ago. Routine immunizations including bacille Calmette-Guérin (BCG) had been performed until 3 months of age. Initial physical examination revealed severe ill appearance: fever (38.9 °C), pulse of 128/minute, and respiratory rate of 42/minute. Height, weight, and head circumference were between 25–50th percentiles. Severe oral monaliasis and hepatosplenomegaly 3–4 cm below costal margins were observed, and no tonsils could be seen. Laboratory findings at the time of admission revealed a hemoglobin of 11.5 g/dl, leukocyte count of 9140/mm3 (80% neutrophil, 12% [1100/mm3] lymphocytes), and platelet count of 163,000/mm3. Chest X-ray revealed perihilar and peribronchial infiltrates and absence of a thymic shadow. Serum immunoglobulin profile was as follows: IgG, 145 mg/dl (345–1236 mg/dl); IgM, 17 mg/dl (41–173 mg/dl); and IgA, 6.5 mg/dl (14–159 mg/dl). Flow cytometric analysis of lymphocyte subsets showed the following: CD3 T cells, 18/mm3 (2400–8100); CD4 T cells, 16/mm3 (1400–5200); CD8 T cells, 0 (600–300); CD19 B cells, 990 mm3 (300–1400); and CD16/CD56 NK cells, 10/mm3 (200–1800). The clinical and laboratory findings were consistent with T−B+NK− SCID. Diagnosis of X-SCID was established by DNA analysis of the IL2RG gene; the novel mutation in exon 5 (c.595-1G>T) was detected. Intravenous gammaglobulins (IVIG), broad-spectrum antibiotics, anti-tuberculosis, and prophylactic antifungal agents were started. In microbiologic evaluations, Candida albicans grew in the blood culture. Clinical improvement was seen in 2 weeks with this intensive treatment. Shortly thereafter, the patient was transferred to the pediatric intensive care unit with fever, cough, dyspnea, low saturation, jaundice, elevated transaminases, and a much more enlarged liver and spleen in third week of hospitalization. Blood count and biochemistry tests revealed pancytopenia, elevated liver enzymes and bilirubin, high triglycerides and ferritin, low fibrinogen, and abnormal coagulation (Table 1). A bone marrow aspiration showed lack of lymphocytes and no morphological evidence of malignancy and hemophagocytic histiocytes (Fig. 1). Thus, the clinical, laboratory, and histopathological criteria of HLH was fulfilled. Polymerase chain reaction (PCR) examination of blood for human immunodefiency virus (HIV), Epstein–Barr virus (EBV), cytomegalovirus (CMV), herpes simplex viruses 1 and 2 (HSV I and II), human herpes viruses 6, 7, and 8 (HHV 6, 7, and 8), enteroviruses, varicella zoster virus (VZV), respiratory syncytial virus (RSV), hepatitis B virus, and adenovirus were negative. Pseudomonas aeroginosa was cultured in the aspirated tracheal fluid. Hematopoietic stem cell transplantation (HSCT) was planned; however, the patient died before transplantation at the age of 4 months.
Table 1.
Laboratory findings of patient at the HLH time
| Patient | Reference values | |
|---|---|---|
| White blood cell (/mm3) | 6640 | 4800–10,800 |
| Hemoglobin (g/dl) | 8.8 | 12–18 |
| Lymphocytes (/mm3) | 780 | 1300–2900 |
| Neutrophil (/mm3) | 5490 | 2200–2800 |
| Platelet count (/mm3) | 103,000 | 150,000–400,000 |
| AST (U/L) | 1324 | 0–40 |
| ALT (U/L) | 394 | 0–55 |
| Total bilirubin (mg/dl) | 7.6 | 0.2–1.2 |
| Conjugated bilirubin (mg/dl) | 5.4 | 0–0.5 |
| Fasting triglycerides (mg/dl) | 524 | 35–150 |
| aPTT (s) | 62 | 25–35 |
| PT (s) | 21 | 10–15 |
| Fibrinogen (mg/l) | 110 | 180–350 |
| Ferritin (ng/ml) | 9973 | 18.5–306.5 |
| aPTT: activated partial thromboplastin time, s: second, PT: prothrombin time | ||
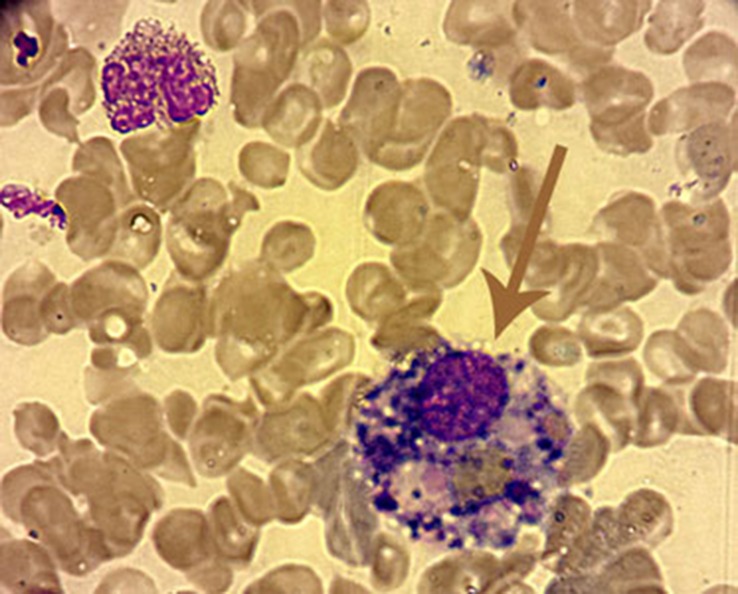
Fig. 1.
Light microscopy showing hemophagocytosis in bone marrow (erythrocyte and platelets were phagocyted by histiocyte) (Wright–Giemsa stain, ×100)
Discussion
We here report a novel mutation of the IL2RG gene in a patient, who presented invagination and also showed HLH, with X-SCID. A diagnosis of SCID should be suspected in patients with persistent infection and absolute lymphopenia in early infancy, as occurred in our patient. The X‑SCID patient in the present study had a classical, severe phenotype, and laboratory data showed low numbers of T cells, relatively well-preserved B cells, and reduced NK cell numbers. X-SCID is a disease that is characterized by severe lymphopenia and recurring persistent infections in the first months of life, as in presented patient. Affected infants lack T cells and NK cells and show hypogammaglobulinemia despite normal B-cell counts. Without HSCT, the disease is usually fatal within the first year of life, as in the presented patient [6].
Invagination (intussusception) is the most common cause of intestinal obstruction in infants, with 80% of cases occurring before 2 years of age [7]. Although the pathogenic mechanism of intussusception without leading points has not yet been clarified, its major cause is suggested to be swelling and lymph node hyperplasia of Peyer’s patch in the ileum secondary to infection [8]. Viral infections play a pivotal role in the etiology of invagination [9]. Invagination was seen after prolonged diarrhea in presented patient and any etiologic agent, such as adenovirus and rotavirus, was not detected.
HLH is clinically defined as a combination of fever, liver dysfunction, coagulation abnormalities, pancytopenia, progressive macrophage proliferation throughout the reticuloendothelial system, and cytokine over-production and may be primary or secondary to infectious, autoimmune, and tumoral diseases. Characteristic histopathological findings include diffuse infiltration of the bone marrow, spleen, or lymph nodes by activated histiocytes that phagocytose various blood cells, as in our patient [10]. The genetic defect in lymphocyte cytotoxicity predisposes patients to HLH. Immunodeficiency syndromes such as Griscelli syndrome 2, Chediak–Higashi syndrome, and Hermansky–Pudlak syndrome 2, associated with albinism, affect the transport, processing, and function of cytotoxic granules in NK cells and cytotoxic T lymphocytes. These diseases lead to defective killing of target cells and a failure to contract the immune response [11]. There were no manifestations compatible with Chediak–Higashi syndrome, Griscelli syndrome 2, or X-linked lymphoproliferative syndrome which another immune deficiency can predispose HLH which is associated with HLH in the presented patient. Grunebaum et al. [12] reported HLH associated with X-SCID first time in medical literature.
To our knowledge, the reported patient is the first reported case with X-SCID which presented with invagination and also showed HLH. This case demonstrated that detailed immunological evaluation is required in patients presenting with intussusception and HLH.
Contributor Information
Turkan Patiroglu, 1Department of Pediatric Immunology, Erciyes University School of Medicine, Kayseri, Turkey; 2Department of Pediatric Hematology and Oncology, Erciyes University School of Medicine, Kayseri, Turkey.
H. Haluk Akar, 1Department of Pediatric Immunology, Erciyes University School of Medicine, Kayseri, Turkey.
Mirjam van den Burg, 3Department of Erasmus MC, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Ekrem Unal, 2Department of Pediatric Hematology and Oncology, Erciyes University School of Medicine, Kayseri, Turkey.
Basak N. Akyildiz, 4Department of Pediatric Intensive Care Unit, Erciyes University Medical Faculty, Kayseri, Turkey.
Nazan U. Tekerek, 4Department of Pediatric Intensive Care Unit, Erciyes University Medical Faculty, Kayseri, Turkey.
Ebru Yilmaz, 2Department of Pediatric Hematology and Oncology, Erciyes University School of Medicine, Kayseri, Turkey.
References
- 1.Speckmann C, Pannicke U, Wiech E, Schwarz K, Fisch P, Friedrich W, Niehues T, Gilmour K, Buiting K, Schlesier M, Eibel H, Rohr J, Superti-Furga A, Gross-Wieltsch U, Ehl S. Clinical and immunologic consequences of a somatic reversion in a patient with X-linked severe combined immunodeficiency. Blood. 2008 Nov 15;112(10):4090–4097. doi: 10.1182/blood-2008-04-153361. [DOI] [PubMed] [Google Scholar]
- 2.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. The interleukin-2 receptor gamma chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996 Xxx;14(000):179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 3.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993 Apr 9;73(1):147–157. doi: 10.1016/0092-8674(93)90167-O. [DOI] [PubMed] [Google Scholar]
- 4.Leonard WJ, Shores EW, Love PE. Role of the common cytokine receptor gamma chain in cytokine signaling and lymphoid development. Immunol Rev. 1995 Dec;148(000):97–114. doi: 10.1111/j.1600-065X.1995.tb00095.x. [DOI] [PubMed] [Google Scholar]
- 5.Buckley RH, Schiff RI, Schiff SE, Markert ML, Williams LW, Harville TO, Roberts JL, Puck JM. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997 Mar;130(3):378–387. doi: 10.1016/S0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- 6.Allenspach E, Rawlings DJ, Scharenberg AM. X-Linked severe combined immunodeficiency. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Stephens K, editors. University of Washington, Seattle, 1993–2013. Seattle: University of Washington; 2013. [updated Jan 24, 2013] [PubMed] [Google Scholar]
- 7.Fischer TK, Bihrmann K, Perch M, Koch A, Wohlfahrt J, Kåre M, Melbye M. Intussusception in early childhood: a cohort study of 1.7 million children. Pediatrics. 2004 Sep;114(3):782–785. doi: 10.1542/peds.2004-0390. [DOI] [PubMed] [Google Scholar]
- 8.Kombo LA, Gerber MA, Pickering LK, Atreya CD, Breiman RF. Intussusception, infection, and immunization: summary of a workshop on rotavirus. Pediatrics. 2001 Aug;108(2):E37–000. doi: 10.1542/peds.108.2.e37. [DOI] [PubMed] [Google Scholar]
- 9.Lee YW, Yang SI, Kim JM, Kim JY. Clinical features and role of viral isolates from stool samples of intussuception in children. Pediatr Gastroenterol Hepatol Nutr. 2013 Sep;16(3):162–170. doi: 10.5223/pghn.2013.16.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egeler RM, Shapiro R, Loechelt B, Filipovich A. Characteristic immune abnormalities in hemophagocytic lymphohistiocytosis. J Pediatr Hematol Oncol. 1996 Nov;18(4):340–345. doi: 10.1097/00043426-199611000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Ansuini V, Rigante D, Esposito S. Debate around infection-dependent hemophagocytic syndrome in paediatrics. BMC Infect Dis. 2013 Jan 16;13(000):15–000. doi: 10.1186/1471-2334-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunebaum E, Zhang J, Dadi H, Roifman CM. Haemophagocytic lymphohistiocytosis in X-linked severe combined immunodeficiency. Br J Haematol. 2000 Mar;108(4):834–837. doi: 10.1046/j.1365-2141.2000.01923.x. [DOI] [PubMed] [Google Scholar]