Abstract
BACKGROUND
Symptomatic burden from constitutional symptoms, anemia, and splenomegaly-related symptoms are common and morbidity inducing in patients with myelofibrosis (MF). The authors previously developed a MF-specific instrument for capturing the burden of MF-associated disease-related symptoms, the Myelofibrosis Symptom Assessment Form.
METHODS
The authors evaluated the usefulness of serial administration of the Myelofibrosis Symptom Assessment Form as an instrument for the assessment of symptomatic burden and improvement in conjunction with the therapeutic clinical trial of the open label phase 2 trial of the JAK1 and JAK2 inhibitor INCB018424 in patients with MF.
RESULTS
The analysis cohort of 87 patients treated in this trial demonstrated that the instrument was comprehensive and sensitive to symptoms present at trial enrollment. In addition, baseline Myelofibrosis Symptom Assessment Form symptom scores correlated well with objective parameters such as splenomegaly and impaired performance status assessed by the 6-minute walk test. Serial administration while on therapy with INCB018424 demonstrated the instrument to be sensitive to symptomatic change, and that improvements in symptoms correlated well with objective improvements in both weight loss and performance status (6-minute walk test).
CONCLUSIONS
The use of the Myelofibrosis Symptom Assessment Form in this phase 2 trial helped characterize the symptomatic improvements observed with use of INCB018424 in MF patients. In an era of many targeted therapies undergoing testing for MF with potential symptomatic benefit, the Myelofibrosis Symptom Assessment Form may provide a useful tool for objective symptomatic assessment and potentially allow some non-randomized comparison between therapeutic agents.
Keywords: myelofibrosis, Myelofibrosis Symptom Assessment Form, myeloproliferative neoplasms, JAK2 inhibitor, symptoms
Myelofibrosis (MF) is a myeloproliferative neoplasm that is characterized by a series of consequences from the clonal disease.1 Included among the consequences of MF are the development of ineffective hematopoiesis (and thus potentially anemia and other cytopenias), leukoerythroblastosis, splenomegaly through perhaps a variety of mechanisms including (but not limited to) ineffective hematopoiesis and splenic sequestration of immature myeloid cells, significant constitutional symptoms (night sweats, fevers, weight loss), pruritus, risk of blastic transformation, and premature death.2 We have previously demonstrated that the symptomatic burden among patients with MF is significant both from directly observable effects of disease (ie, from anemia and/or splenomegaly) and by self-reported outcomes from MF patients for less quantifiable symptoms such as fatigue, night sweats, and pruritus.3 We have also reported that the prevalence of these symptoms is relatively uniform across the 3 main subtypes of MF (namely, primary MF, post-polycythemia vera MF [post-PV MF], and post-essential thrombocythemia MF [post-ET MF]).4 In addition, the presence of significant constitutional symptoms is not only bothersome for the individual patient, but has been found to be prognostically detrimental and is included as an adverse prognostic factor in the International Working Group for Myelofibrosis Research and Treatment prognostic score for MF.5
Historically, therapies for MF have been largely palliative, with very limited ability to significantly impact the symptomatic burden of patients afflicted with the disease. However, the discovery of the JAK2-V617F mutation in 20056 (and subsequent discovery of related myeloproliferative neoplasm-associated mutations7,8) has ushered in an era of targeted therapeutic approaches for MF including JAK inhibitors,9 which down-modulate the dysregulated activity of JAKs that is characteristic in the myeloproliferative neoplasms. Initial results of these targeted trials demonstrated a profound ability of these medications to decrease MF-associated splenomegaly and symptoms.10,11 The difficulty was that no current instrument of patient-reported outcomes adequately and concisely assessed the spectrum of MF-associated symptoms. Therefore, we developed, and validated in a single time point validation study, the Myelofibrosis Symptom Assessment Form.4 This instrument captured the spectrum of MF-associated constitutional symptoms (fatigue, night sweats, fevers, pruritus, weight loss) and splenomegaly-associated symptoms (abdominal pain, early satiety, mechanical effects from the spleen). This prior validation of the Myelofibrosis Symptom Assessment Form used a series of established instruments of patient-reported outcomes to validate the questions and results obtained from the Myelofibrosis Symptom Assessment Form. In addition, a separate question is asked relating to an overall assessment of quality of life.
We sought to evaluate the use of the Myelofibrosis Symptom Assessment Form for measurement of baseline symptoms and sensitivity to detect changes when used serially in clinical trials. Therefore, we used the Myelofibrosis Symptom Assessment Form in serial administrations in conjunction with the largest phase 2 trial ever conducted for that disorder, the open label phase 2 trial of the selective JAK1 and JAK2 inhibitor INCB018424 (Trial 251).10
MATERIALS AND METHODS
Patients
MF patients eligible for the INCB018424-251 trial10 (both primary MF and post-PV/post-ET MF) were required to have adequate organ function, to have sufficient hematopoietic reserves (ie, platelet count >100 × 109/L, absolute neutrophil count >1.0 × 109/L), and to require therapy. Therapy was administered as an open label phase 2 trial of INCB018424, where all patients received the investigational agent, although a range of doses was used based on the ability to tolerate the main dose-limiting toxicity of the agent, which is thrombocytopenia. Patients enrolled in the therapeutic trial completed the Myelofibrosis Symptom Assessment Form at screening, after 2 weeks on therapy, at the completion of cycles 1, 2, and 3, and then every 3 cycles (28-day cycles). The instrument was used after the initiation of the trial so these results are sequential but represent only approximately the latter half of patients enrolled in the trial (n = 87).
Myelofibrosis Symptom Assessment Form
The Myelofibrosis Symptom Assessment Form used in this trial was slightly modified from our prior publication.4 Specifically for ease of administration, fatigue was measured in a single item in which patients graded their fatigue on a simple 0 to 10 scale, where a score of 0 indicates absence of the symptom, and a score of 10 indicates the symptom with worst imaginable severity. Additional items were included exactly as we have analyzed previously. Please see Table 1 for a summary of Myelofibrosis Symptom Assessment Form as administered in the context of this trial.
Table 1.
Modified MFSAF Administered Serially to 87 Patients With Myelofibrosis in an Open Label Phase 2 Trial of INCB018424
| Circle the 1 Number That Describes How Much Difficulty You Have Had With Each of the Following Symptoms During the Past Week | |
|---|---|
| Original MFSAF items | |
| General fatigue | (Absent) 0 1 2 3 4 5 6 7 8 9 10 (worst imaginable) |
| Abdominal pain (and discomfort) | (Absent) 0 1 2 3 4 5 6 7 8 9 10 (worst imaginable) |
| Inactivity (ability to move and walk around) | (Absent) 0 1 2 3 4 5 6 7 8 9 10 (worst imaginable) |
| Cough | (Absent) 0 1 2 3 4 5 6 7 8 9 10 (worst imaginable) |
| Night sweats | (Absent) 0 1 2 3 4 5 6 7 8 9 10 (worst imaginable) |
| Itching (pruritus) | (Absent) 0 1 2 3 4 5 6 7 8 9 10 (worst imaginable) |
| Bone pain (diffuse not joint pain or arthritis) | (Absent) 0 1 2 3 4 5 6 7 8 9 10 (worst imaginable) |
| Fever (>100 F) | (Absent) 0 1 2 3 4 5 6 7 8 9 10 (daily) |
| Change in appetite/unintentional weight loss (or gain) in past 6 months | (Absent) 0 1 2 3 4 5 6 7 8 9 10 (worst imaginable) |
| What is your overall quality of life? | (As good as it can be) 0 1 2 3 4 5 6 7 8 9 10 (as bad as it can be) |
| Exploratory items | |
| Ability to bend down including to tie shoes | (Absent) 0 1 2 3 4 5 6 7 8 9 10 (worst imaginable) |
| Altered bowel movement and/or difficult or painful urination | (Absent) 0 1 2 3 4 5 6 7 8 9 10 (worst imaginable) |
| Body image and hindrance to perform daily activities | (Absent) 0 1 2 3 4 5 6 7 8 9 10 (worst imaginable) |
| Difficulty sleeping | (Absent) 0 1 2 3 4 5 6 7 8 9 10 (worst imaginable) |
| Swelling of extremities (arms and legs) | (Absent) 0 1 2 3 4 5 6 7 8 9 10 (worst imaginable) |
Abbreviation: MFSAF, Myelofibrosis Symptom Assessment Form.
Exploratory Questions
Additional exploratory questions were asked in addition to the Myelofibrosis Symptom Assessment Form, based on the prevalence of certain symptoms and complaints reported anecdotally among the first half of the MF patients enrolled on the therapeutic clinical trial. Specifically included were single line item questions on insomnia, ability to bend, altered self-image, swelling, and altered bowel or bladder habits (see Table 1).
Comparison Measurements
Where possible, objective measurements were made in conjunction with the therapeutic trial to allow for further evaluation of the sensitivity of the Myelofibrosis Symptom Assessment Form to assess change in clinical variables. Those variables amenable to such measurements included serial objective weights, spleen size, and the presence of anemia. In addition, the 6-minute walk test was used for assessment of overall ability to ambulate and was performed in standard fashion reported in cardiovascular and pulmonary disease-related trials (American Thoracic Society: observed laps of a 30-35 m course, indoors, level, without encouragement). The 6-minute walk test was performed twice at baseline (before first dose of therapy), and then after 1, 3, and 6 28-day cycles of therapy.
Analysis
Single line items of the modified Myelofibrosis Symptom Assessment Form were tracked over the course of the study to see their consistency in change over the duration of the trial. All 87 subjects who completed the Myelofib rosis Symptom Assessment Form were included in the initial analysis of change in symptom score from baseline. On the basis of initial responses and incidence of thrombocytopenia, doses of 10 mg twice daily to 25 mg twice daily were found to be most effective.10 Baseline symptom scores for subjects with doses of 10 mg twice daily to 25 mg twice daily (n = 66) were then further assessed for associations and correlations with baseline parameters that might impact MF-associated symptoms (anemia, degree of splenomegaly, and ability to ambulate as measured by the 6-minute walk test) (Table 2). Subsequently, changes in each Myelofibrosis Symptom Assessment Form parameter were correlated with the responses at 3 and 6 months to other objective measurements of response, including change in weight, decrease in spleen size, and improvements in the 6-minute walk test. Finally, changes in the Myelofibrosis Symptom Assessment Form were assessed in comparison to the main objective toxicity seen on the trial that might impact symptoms, specifically, worsening of anemia while on the trial.
Table 2.
Correlation Analysis Cohorts
| Factor | Baseline Features | Toxicity (Hb Drop After 6 Cycles) | Response (6 Cycles) |
|---|---|---|---|
| Anemia | <10 g/dL (or Tx dependent) (53%) | <0.5 g/dL (37%) | Not applicable |
| >10 g/dL (47%) | 0.5-2 g/dL (41%) | ||
| >2 g/dL (22%) | |||
| Weight | Underweight 1.8% (BMI <18.5) | Not applicable | >10% loss (7.9%) |
| Normal weight 50.9% (BMI 18.5-24.9) | |||
| Overweight 34.5% (BMI 25.0-29.9) | <10% change (36.5%) | ||
| Obese 12.7% (BMI >30) | >10% gain (55.6%) | ||
| 6MWT | Impaired (80.6%) | Not applicable | <10 m gain (39.4%) |
| Normal (19.4%) | 10-69 m gain (27.3%) | ||
| >70 m gain (33.3%) |
Abbreviations: 6MWT, 6-minute walk test; BMI, body mass index; Hb, hemoglobin; Tx, treatment.
RESULTS
Patients and Baseline Myelofibrosis Symptom Assessment Form Responses
Complete therapeutic results of the 153 patients enrolled in the INCB018424-251 trial are reported separately.10 The final 87 MF patients enrolled in that trial who had 2 or more Myelofibrosis Symptom Assessment Form instruments completed are included in this analysis.
Included patients were of a median age (65 years), sex distribution (35 % women), and disease subtype (53% primary MF) typical for a clinical trial in MF. Baseline assessment at trial enrollment demonstrated that among the patients enrolled in this clinical trial, each of the 15 items assessed in the Myelofibrosis Symptom Assessment Form was present in >50% of patients except for fever (46%), cough (45%), altered bowel movements (48%), and pruritus (45%) (Table 3). The most common symptoms were fatigue, night sweats, difficulty sleeping, and altered quality of life, all with median scores of 4.0 (of the 10-point scale) with 91% having some degree of fatigue (score 1). Ninety-five percent of patients had at least 2 symptoms present on the Myelofibrosis Symptom Assessment Form.
Table 3.
Serial Change Over Time of Individual Symptoms in 87 Patients in a Clinical Trial of INCB018424 Assessed With the Myelofibrosis Symptom Assessment Form
| Parameter | No.a | Baseline ≥1 | Median Baseline Scoreb | Improved at 6 Monthsb,c,d | Median Score at 6 Monthsb,c | Median Change From Baseline at 6 Monthsb,c |
|---|---|---|---|---|---|---|
| General fatigue | 87 | 91% | 4 | 40/79 (51%) | 2 | –1 |
| Abdominal pain/discomfort | 87 | 76% | 3 | 43/66 (65%) | 1 | –2 |
| Night sweats | 87 | 54% | 4 | 33/47 (70%) | 0 | –2 |
| Pruritus | 87 | 46% | 2 | 30/40 (75%) | 0 | –1 |
| Bone or muscle pain | 87 | 64% | 3 | 20/56 (36%) | 2 | 0 |
| Fever | 87 | 46% | 2 | 28/40 (70%) | 0 | –2 |
| Cough | 87 | 45% | 2 | 23/39 (59%) | 0.5 | –1 |
| Appetite, weight loss or gain | 87 | 75% | 2 | 36/65 (55%) | 1 | –1 |
| Move or walk around | 87 | 83% | 3 | 39/72 (54%) | 2 | –1 |
| QOL | 87 | 91% | 4 | 39/79 (49%) | 2 | –1 |
| Bend down to tie shoes | 87 | 66% | 2 | 36/57 (63%) | 1 | –1 |
| Altered bowel or urination | 87 | 48% | 2.5 | 28/42 (67%) | 0 | –1 |
| Altered body image | 87 | 79% | 3 | 41/69 (59%) | 1 | –2 |
| Insomnia | 87 | 77% | 4 | 44/67 (66%) | 1 | –2 |
| Swelling arms or legs | 87 | 57% | 3 | 30/50 (60%) | 1 | –1 |
Abbreviation: QOL, quality of life.
Subjects with no baseline scores are excluded from all analyses.
Subjects with 0 for baseline score for a given symptom are excluded from analysis for that symptom.
Subjects with missing C7D1 data, but subsequent follow-up date, have the last observation prior to C7D1 carried forward.
Subjects who drop out prior to C7D1 are considered as not having shown improvement at C7D1.
Comparison of Baseline Myelofibrosis Symptom Assessment Form Responses to Baseline Disease Features
A direct comparison of patient baseline disease features, including splenomegaly, anemia, body mass index (BMI), age, and 6-minute walk test performance, with individual Myelofibrosis Symptom Assessment Form items was performed. We found the clearest statistical correlations between baseline splenomegaly and Myelofibrosis Symptom Assessment Form responses (Table 4). We found massive splenomegaly (>20 cm below costal margin) to be positively associated with worse fatigue item scores (P = .01), decreased ability to walk around and exercise (P = .0001), decreased ability to bend (P = .03), hindrance to perform daily activities (P = .004), a worse quality of life (P = .01). Consistent our prior observations, the degree of anemia did not correlate with worsening fatigue scores (possibly because anemia was so common among enrolled patients). Neither the age of subjects nor baseline weight (by BMI) were associated with differences in baseline symptoms. Assessments of the 6-minute walk test results measured at baseline showed that 81% of participants in the 6-minute walk test were impaired in the distance walked compared with age-matched published controls, and these individuals has worse baseline fatigue and decreased ability to exercise (Table 4).
Table 4.
Correlation of MFSAF-Assessed Symptoms and Corresponding Objective Parameters
| Item | Baseline Features | Toxicity | Response After 6 Cycles of Therapy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Anemia | Spleen | 6MWT | BMI | Anemia | Spleen | Weight | 6MWT | |
| Original MFSAF items | |||||||||
| General fatigue | NS | NS | .01a | .05a | NS | NS | NS | NS | NS |
| Filling up quickly when you eat (early satiety) | NS | NS | NS | NS | NS | NS | NS | .05a | NS |
| Abdominal pain | NS | NS | NS | NS | NS | NS | NS | .05a | .03a |
| Abdominal discomfort | NS | NS | NS | NS | NS | NS | NS | NS | .03a |
| Inactivity | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Cough | NS | NS | NS | NS | NS | NS | NS | .05a | NS |
| Night sweats | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Itching (pruritus) | NS | .03a | NS | NS | NS | NS | NS | NS | NS |
| Bone pain (diffuse not joint pain or arthritis) | NS | NS | NS | NS | NS | NS | NS | .06a | NS |
| Fever (>100 F) | NS | .05a | NS | NS | NS | NS | NS | .02a | NS |
| Unintentional weight loss past 6 months | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| What is your overall quality of life? | NS | NS | .01a | NS | NS | NS | NS | NS | .05a |
| Exploratory items | |||||||||
| Ability to bend down or tie your shoes | NS | NS | .03a | NS | NS | NS | NS | NS | NS |
| Ability to move around or exercise | NS | NS | .0001a | .03a | NS | NS | NS | NS | NS |
| Altered bowel function or urination | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Appetite (weight loss or gain) | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Altered body image | NS | NS | .0004a | NS | NS | NS | NS | NS | NS |
| Difficulty sleeping | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| Swelling of the arms or legs | NS | NS | NS | NS | NS | NS | NS | NS | NS |
Abbreviations: 6MWT, 6-minute walk test; BMI, body mass index; MFSAF, Myelofibrosis Symptom Assessment Form; NS, not significant.
Statistically significant.
Serial Myelofibrosis Symptom Assessment Form Changes in Response to Therapy With INCB018424
Therapy with INCB018424 resulted in rapid reduction in MF-associated symptoms, with 46% to 85% of patients experiencing improvement in each individual item assessed by the Myelofibrosis Symptom Assessment Form. The greatest improvements in Myelofibrosis Symptom Assessment Form score improvements were reported by patients experiencing abdominal discomfort, night sweats, pruritus, altered body image, and fever (see Table 3 and Fig. 1) and corresponded to improvements in the individual MF symptom scales as well as the patients’ overall assessment of quality of life. A net improvement was noted over time in all symptoms except bone pain. Median improvement in individual symptom scores ranged from 1 to 2 points, given baseline severity scores of 2 to 4 across most symptoms, these changes were both valid and consistent. Although anemia was a toxicity encountered with INCB018424 therapy, we did not see a significant correlation between either developing anemia or the degree of anemia on the symptomatic improvements seen and recorded by the Myelofibrosis Symptom Assessment Form (Table 4).
Figure 1.
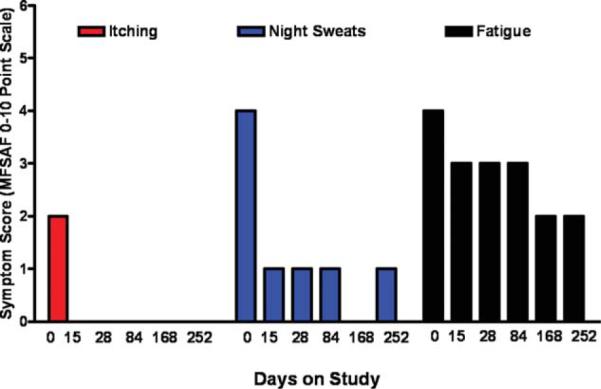
Median serial change over time is shown in cytokine-dependent symptoms in 87 patients with myelofibrosis treated with INCB018424. Abbreviation: MFSAF, Myelofibrosis Symptom Assessment Form.
Serial Myelofibrosis Symptom Assessment Form Changes in Response to Splenomegaly and Other Responses
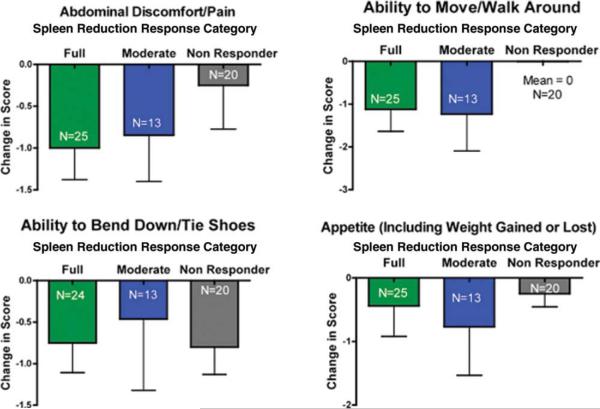
Of subjects who initiated dosing at 10 mg twice daily, 15 mg twice daily, or 25 mg twice daily, 77.6% achieved at least a 25% reduction in palpable spleen length after 6 cycles of therapy. Patients with reduction in spleen size were collated into 3 groups according to the response after 6 cycles of therapy (approximately 6 months); full responders were defined as subjects having achieved ≥35% reduction in spleen volume measured by magnetic resonance imaging (MRI) or ≥50% measured by palpable spleen length (an International Working Group for Myelofibrosis Research and Treatment defined response12), moderate responders were defined as subjects having achieved 25% to 49% reduction in palpable spleen length or 15% to 34% reduction in MRI-measured spleen volume, and nonresponders were subjects who did not meet the criteria for full or partial response. Graphical comparison shows that the relative improvements in Myelofibrosis Symptom Assessment Form responses for improvement in abdominal discomfort/pain, ability to move around, and appetite were associated with the level of spleen response (Fig. 2). Interestingly, all subjects reported improvement in ability to bend down/tie shoes, regardless of spleen reduction response. General fatigue improvement also tracked with spleen response, with mean changes in Myelofibrosis Symptom Assessment Form fatigue score of – 1.6, – 0.77, and – 0.2 for the full, moderate, and nonresponder groups, respectively. However, these associations did not reach the level of statistical correlations, most likely because of the small size of the analysis groups (Table 4).
Figure 2.
Median change is shown in 4 splenomegaly-associated symptoms (abdominal discomfort/pain, ability to move/walk around, ability to bend/tie shoes, changed appetite) based on the level of spleen response after 6 cycles of therapy (ie, full, International Working Group for Myelofibrosis Research and Treatment [IWG-MRT] 50% reduction; partial, some splenic reduction but less than IWG-MRT threshold; and no response). Subjects with palpable baseline spleen and on-study responses after 6 cycles and who initiated therapy at doses of 10 mg twice daily, 15 mg twice daily, or 25 mg twice daily are included (n = 58).
Weight loss resulting from poor appetite and illness contributes to the MF symptom burden. Subjects receiving INCB018424 therapy gained weight.10 The degree of weight gain was statistically significantly correlated with improvement in Myelofibrosis Symptom Assessment Form-based assessments of early satiety and abdominal pain. Interestingly, weight gain was also statistically significantly correlated with improvements in cough, bone pain, and recurrent fevers.
Correlation of Myelofibrosis Symptom Assessment Form With Improvement in the 6-Minute Walk Test
Improvement in the 6-minute walk test by >50 m was associated with a 2-fold greater improvement in inactivity score on the Myelofibrosis Symptom Assessment Form compared with subjects who improved 6-minute walk test performance by <50 m. In addition, improvements in 6-minute walk test were statistically significantly correlated with improvements in abdominal pain, discomfort, and quality of life scores (Table 4).
DISCUSSION
We report the first successful serial use of a MF-specific instrument for measuring baseline symptoms, and subsequent change, in the context of a therapeutic clinical trial. The process of validation of an instrument of patient-reported outcomes is never complete, and further validation of the Myelofibrosis Symptom Assessment Form will occur in the setting of randomized placebo-controlled trials (ongoing). The need for such an instrument for MF is that current instruments assessing patient-reported outcomes for cancer trials do not adequately address the needs of patients with MF or myeloproliferative neoplasms as a whole. Broadly used instruments for patients with malignant disease operate on 2 different paradigms. The first is that patients will be diagnosed with a malignancy (ie, breast cancer), receive therapy (ie, surgery ± adjuvant radiotherapy or chemotherapy), and then be cured. The second paradigm is for the patient with metastatic disease, in which death is likely inevitable, with therapy hopefully prolonging survival. The spectrum of symptoms in currently used instruments deal with symptoms likely to arise in these circumstances (ie, anemia, fatigue, and pain from disease, nausea from therapy or disease, hormonal changes from therapy). No current general instrument of measuring symptoms in cancer patients (ie, Memorial Symptom Assessment Score,13 The University of Texas MD Anderson Cancer Center Symptom Assessment Score14 or European Organization for Research and Treatment of Cancer QLQ-C3015) incorporates all the key aspects of symptoms an MF patient experiences in a solitary instrument (specifically, fatigue, splenomegaly-related symptoms, bone pain, night sweats, pruritus, and cough). Given this latter finding, no large MF clinical trial has previously incorporated any of these traditional cancer instruments into their assessments of enrolled patients.
The baseline values obtained in this trial further validate our previous observations that the Myelofibrosis Symptom Assessment Form is easy to administer,4 and that symptoms that were assessed were quite prevalent, with the symptoms present in >50% of patients in 11 of 15 items assessed. In addition, the severity of the symptoms at baseline corresponded well with the symptomatic burden expected in the cohort of advanced MF patients who were eligible for this trial. Correlations between expected burden of certain symptoms and their objective benchmarks (ie, the degree of splenomegaly) further validate the sensitivity of the instrument in assessing symptom burden. The exploratory items also were quite common, such as insomnia, and further added to the utility of the information provided by the instrument.
A key component to validation of an instrument for use in clinical trials is whether it is able to detect a change in symptoms in response to therapy. We note that not only was a change in individual symptoms identified by the Myelofibrosis Symptom Assessment Form, but the changes over time were consistent and stable. In addition, even with relatively small numbers of patients we were able to correlate levels of symptomatic response with objective markers of response such as improvements in weight loss, reduction in splenomegaly, and improvements in performance status as grossly assessed by 6-minute walk test. Although we saw trends for detection of certain symptomatic responses and the degree of splenic reduction, these were not statistically significant.
None of the objective response parameters assessed (6-minute walk test, spleen size, body weight) showed associations or statistically significant correlations with symptoms such as pruritus or night sweats. Although a mechanism for this lack of correlation is unproven, it is likely that improvement in many MF symptoms is multifactorial, and thus symptomatic benefit is not tied solely to the degree of splenic reduction. Indeed, we have recently reported the elevation in several inflammatory cytokines in MF patients compared with healthy volunteer controls.10 More importantly, the decrease in cytokine levels (interleukin-1ra, interleukin-6, macrophage inflammatory protein-1β, tumor necrosis factor-α, and C-reactive protein) was associated with improvements in a composite symptom score comprised of the Myelofibrosis Symptom Assessment Form elements night sweats, pruritus, abdominal pain/ discomfort, and bone/muscle pain for subjects who were treated with the JAK1/JAK2 inhibitor, INCB018424. However, in the end we must emphasize that the mechanism of symptomatic improvement in patients with MF, either with INCB18424 or with other JAK2 inhibitors, remains without a definitive explanation or mechanism.
The therapy of MF has entered an era of rapid change, with multiple lines of therapies in clinical development. Although in the past we have seen palliative benefit with agents for improving anemia (such as thalidomide16 [alone or in combination17] or androgens18), splenomegaly (splenectomy, splenic radiotherapy, hydroxyurea, or cladribine),19 or both (lenalidomide), none has ever had a significant impact on symptoms. Even if these agents had impacted symptoms, no MF instruments were available to assess these changes. The current era has multiple lines of therapeutic investigations, including JAK inhibitors in addition to INCB018424 (SB151820 and TG10134811 among others), histone deacetylase inhibitors (LBH589),21 mammalian target of rapamycin inhibitors (RAD001),22 and even more potent and better tolerated immunomodulatory drugs (pomalidomide).23 Pilot data on most of these mechanistic approaches suggest that there will be differential impact on MF symptoms associated with splenomegaly, anemia, and disease features such as pruritus and night sweats. Individual assessment of each of these agents on baseline MF symptoms, and some comparison of the agents by serial measurement of impact on MF symptoms (assessed by Myelofibrosis Symptom Assessment Form) may now be possible. Future development and validation of instruments applicable across the spectrum of myeloproliferative neoplasms, as well as availability in multiple languages, are planned.
Acknowledgments
FUNDING SOURCES
No specific funding was disclosed.
Footnotes
R.A.M. and A.T. designed the Myelofibrosis Symptom Assessment Form. R.A.M., A.D., and S.E.-V. analyzed the serial performance of the Myelofibrosis Symptom Assessment Form in the context of this clinical trial. R.A.M., H.K., A.T., R.L., K.V., D.A.T., J.C., G.B., A.D.P., Z.E., S.E.-V., and S.V. were involved in the design and conduct of the clinical trial.
CONFLICT OF INTEREST DISCLOSURES
R.L., S.E.-V., and K.V. are employees of trial sponsor Incyte Corporation (Wilmington, Del). No other conflicts of interest for the remaining authors are present.
Performance in 87 Myelofibrosis Patients on a JAK1 and JAK2 Inhibitor (INCB018424) Clinical Trial
REFERENCES
- 1.Vardiman JW, Thiele J, Arber DA, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009;114:937–951. doi: 10.1182/blood-2009-03-209262. [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A. Myelofibrosis with myeloid metaplasia. N Engl J Med. 2000;342:1255–1265. doi: 10.1056/NEJM200004273421706. [DOI] [PubMed] [Google Scholar]
- 3.Mesa RA, Niblack J, Wadleigh M, et al. The burden of fatigue and quality of life in myeloproliferative disorders (MPDs): an international Internet-based survey of 1179 MPD patients. Cancer. 2007;109:68–76. doi: 10.1002/cncr.22365. [DOI] [PubMed] [Google Scholar]
- 4.Mesa RA, Schwager S, Radia D, et al. The Myelofibrosis Symptom Assessment Form (MFSAF): an evidence-based brief inventory to measure quality of life and symptomatic response to treatment in myelofibrosis. Leuk Res. 2009;33:1199–1203. doi: 10.1016/j.leukres.2009.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113:2895–2901. doi: 10.1182/blood-2008-07-170449. [DOI] [PubMed] [Google Scholar]
- 6.James C, Ugo V, Le Couedic JP, et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature. 2005;434:1144–1148. doi: 10.1038/nature03546. [DOI] [PubMed] [Google Scholar]
- 7.Scott LM, Tong W, Levine RL, et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med. 2007;356:459–468. doi: 10.1056/NEJMoa065202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pikman Y, Lee BH, Mercher T, et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med. 2006;3:e270. doi: 10.1371/journal.pmed.0030270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardanani A. JAK2 inhibitor therapy in myeloproliferative disorders: rationale, preclinical studies and ongoing clinical trials. Leukemia. 2008;22:23–30. doi: 10.1038/sj.leu.2404948. [DOI] [PubMed] [Google Scholar]
- 10.Verstovsek S, Kantarjian H, Mesa RA, et al. Safety and efficacy of INCB018424, a JAK1 and JAK2 inhibitor, in myelofibrosis. N Engl J Med. 2010;363:1117–1127. doi: 10.1056/NEJMoa1002028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardanani AD, Jason R, Gotlib M, et al. A phase I evaluation of TG101348, a selective JAK2 inhibitor, in myelofibrosis: clinical response is accompanied by significant reduction in JAK2V617F allele burden. Blood. 2009;114:a755. [Google Scholar]
- 12.Tefferi A, Barosi G, Mesa RA, et al. International Working Group (IWG) consensus criteria for treatment response in myelofibrosis with myeloid metaplasia, for the IWG for Myelofibrosis Research and Treatment (IWG-MRT). Blood. 2006;108:1497–1503. doi: 10.1182/blood-2006-03-009746. [DOI] [PubMed] [Google Scholar]
- 13.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 14.Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 16.Barosi G, Grossi A, Comotti B, Musto P, Gamba G, Marchetti M. Safety and efficacy of thalidomide in patients with myelofibrosis with myeloid metaplasia. Br J Haematol. 2001;114:78–83. doi: 10.1046/j.1365-2141.2001.02918.x. [DOI] [PubMed] [Google Scholar]
- 17.Mesa RA, Steensma DP, Pardanani A, et al. A phase 2 trial of combination low-dose thalidomide and prednisone for the treatment of myelofibrosis with myeloid metaplasia. Blood. 2003;101:2534–2541. doi: 10.1182/blood-2002-09-2928. [DOI] [PubMed] [Google Scholar]
- 18.Cervantes F, Hernandez-Boluda JC, Alvarez A, Nadal E, Montserrat E. Danazol treatment of idiopathic myelofibrosis with severe anemia. Haematologica. 2000;85:595–599. [PubMed] [Google Scholar]
- 19.Mesa RA. How I treat symptomatic splenomegaly in patients with myelofibrosis. Blood. 2009;113:5394–5400. doi: 10.1182/blood-2009-02-195974. [DOI] [PubMed] [Google Scholar]
- 20.Verstovsek S, Odenike O, Scott B, et al. Phase I dose-escalation trial of SB1518, a novel JAK2/FLT3 inhibitor, in acute and chronic myeloid diseases, including primary or post-essential thrombocythemia/polycythemia vera myelofibrosis. Blood. 2009;114:a3905. [Google Scholar]
- 21.Mascarenhas J, Wang X, Rodriguez A, et al. A phase I study of LBH589, a novel histone deacetylase inhibitor in patients with primary myelofibrosis (PMF) and post-polycythemia/essential thrombocythemia myelofibrosis (post-PV/ET MF). Blood. 2009;114:a308. [Google Scholar]
- 22.Vannucchi AM, Guglielmelli P, Gattoni E, et al. RAD001, an inhibitor of mTOR, shows clinical activity in a phase I/II study in patients with primary myelofibrosis (PMF) and post polycythemia vera/essential thrombocythemia myelofibrosis (PPV/PET MF). Blood. 2009;114:a307. [Google Scholar]
- 23.Mesa RA, Pardanani AD, Hussein K, et al. Phase 1/2 dose finding study of pomalidomide in myelofibrosis. Blood. 2009;114:a2911. [Google Scholar]