Abstract
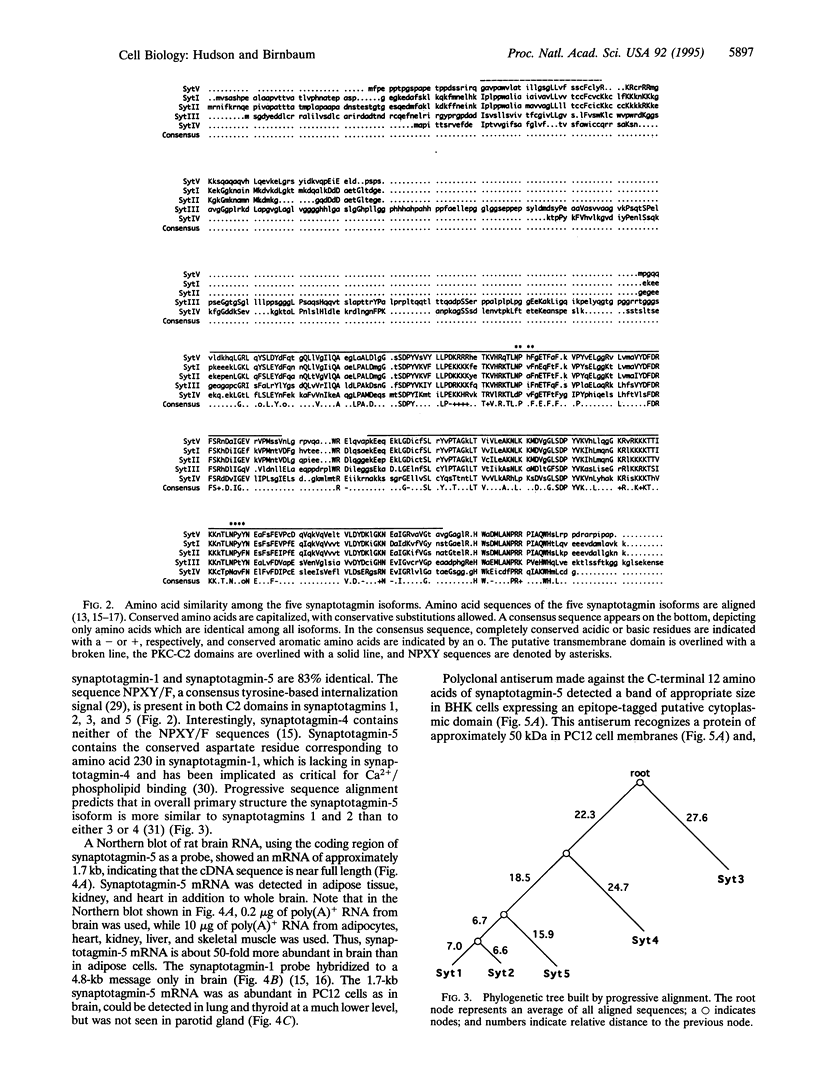
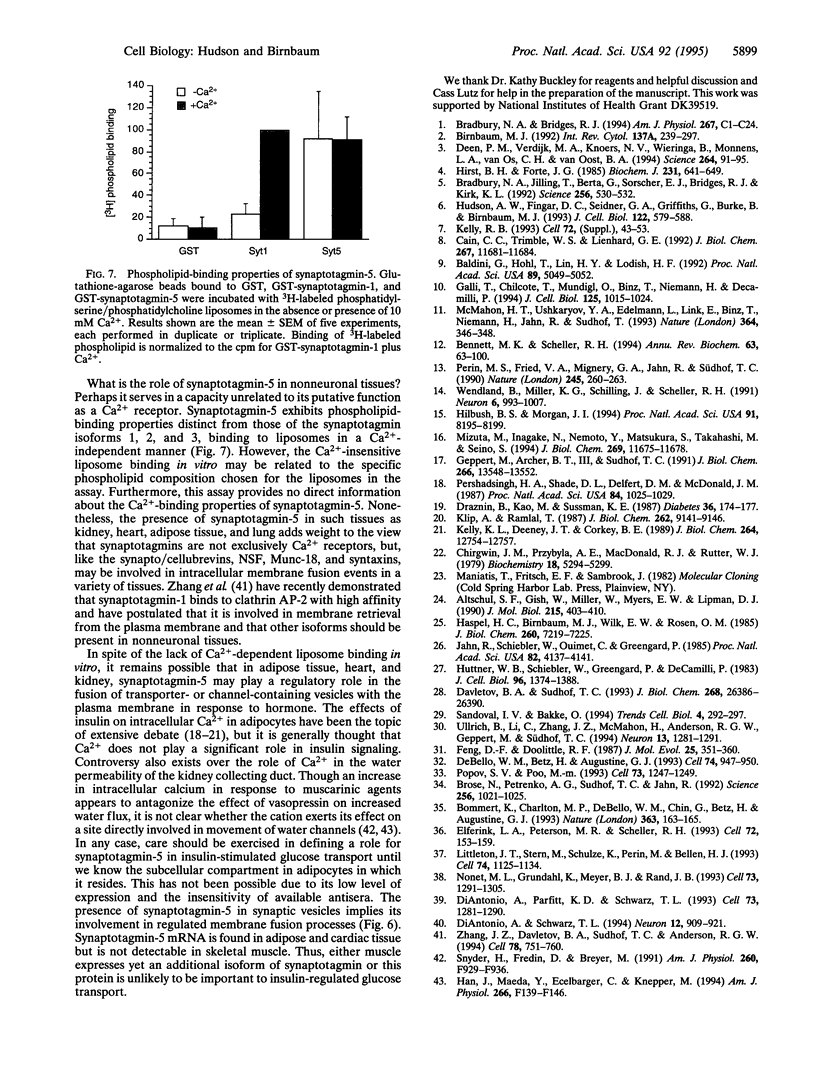
Synaptotagmins, which have been found exclusively in neuroendocrine or exocrine tissues, have been implicated in the regulation of secretory vesicle fusion with the plasma membrane. The present paper describes a synaptotagmin isoform (synaptotagmin-5) which exhibits 49% amino acid identity to synaptotagmin-1 and -2. Synaptotagmin-5 mRNA is expressed in rat kidney, adipose tissue, lung, and heart, as well as at higher levels in brain and PC12 cells. Antiserum specific for the synaptotagmin-5 isoform recognizes a protein of about 50 kDa which is about 6-fold more abundant in brain synaptic vesicles than in whole brain membranes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Baldini G., Hohl T., Lin H. Y., Lodish H. F. Cloning of a Rab3 isotype predominantly expressed in adipocytes. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5049–5052. doi: 10.1073/pnas.89.11.5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. K., Scheller R. H. A molecular description of synaptic vesicle membrane trafficking. Annu Rev Biochem. 1994;63:63–100. doi: 10.1146/annurev.bi.63.070194.000431. [DOI] [PubMed] [Google Scholar]
- Birnbaum M. J. The insulin-sensitive glucose transporter. Int Rev Cytol. 1992;137:239–297. [PubMed] [Google Scholar]
- Bommert K., Charlton M. P., DeBello W. M., Chin G. J., Betz H., Augustine G. J. Inhibition of neurotransmitter release by C2-domain peptides implicates synaptotagmin in exocytosis. Nature. 1993 May 13;363(6425):163–165. doi: 10.1038/363163a0. [DOI] [PubMed] [Google Scholar]
- Bradbury N. A., Bridges R. J. Role of membrane trafficking in plasma membrane solute transport. Am J Physiol. 1994 Jul;267(1 Pt 1):C1–24. doi: 10.1152/ajpcell.1994.267.1.C1. [DOI] [PubMed] [Google Scholar]
- Bradbury N. A., Jilling T., Berta G., Sorscher E. J., Bridges R. J., Kirk K. L. Regulation of plasma membrane recycling by CFTR. Science. 1992 Apr 24;256(5056):530–532. doi: 10.1126/science.1373908. [DOI] [PubMed] [Google Scholar]
- Brose N., Petrenko A. G., Südhof T. C., Jahn R. Synaptotagmin: a calcium sensor on the synaptic vesicle surface. Science. 1992 May 15;256(5059):1021–1025. doi: 10.1126/science.1589771. [DOI] [PubMed] [Google Scholar]
- Cain C. C., Trimble W. S., Lienhard G. E. Members of the VAMP family of synaptic vesicle proteins are components of glucose transporter-containing vesicles from rat adipocytes. J Biol Chem. 1992 Jun 15;267(17):11681–11684. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Davletov B. A., Südhof T. C. A single C2 domain from synaptotagmin I is sufficient for high affinity Ca2+/phospholipid binding. J Biol Chem. 1993 Dec 15;268(35):26386–26390. [PubMed] [Google Scholar]
- DeBello W. M., Betz H., Augustine G. J. Synaptotagmin and neurotransmitter release. Cell. 1993 Sep 24;74(6):947–950. doi: 10.1016/0092-8674(93)90716-4. [DOI] [PubMed] [Google Scholar]
- Deen P. M., Verdijk M. A., Knoers N. V., Wieringa B., Monnens L. A., van Os C. H., van Oost B. A. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994 Apr 1;264(5155):92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- DiAntonio A., Parfitt K. D., Schwarz T. L. Synaptic transmission persists in synaptotagmin mutants of Drosophila. Cell. 1993 Jul 2;73(7):1281–1290. doi: 10.1016/0092-8674(93)90356-u. [DOI] [PubMed] [Google Scholar]
- DiAntonio A., Schwarz T. L. The effect on synaptic physiology of synaptotagmin mutations in Drosophila. Neuron. 1994 Apr;12(4):909–920. doi: 10.1016/0896-6273(94)90342-5. [DOI] [PubMed] [Google Scholar]
- Draznin B., Kao M., Sussman K. E. Insulin and glyburide increase cytosolic free-Ca2+ concentration in isolated rat adipocytes. Diabetes. 1987 Feb;36(2):174–178. doi: 10.2337/diab.36.2.174. [DOI] [PubMed] [Google Scholar]
- Elferink L. A., Peterson M. R., Scheller R. H. A role for synaptotagmin (p65) in regulated exocytosis. Cell. 1993 Jan 15;72(1):153–159. doi: 10.1016/0092-8674(93)90059-y. [DOI] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Galli T., Chilcote T., Mundigl O., Binz T., Niemann H., De Camilli P. Tetanus toxin-mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor-containing vesicles in CHO cells. J Cell Biol. 1994 Jun;125(5):1015–1024. doi: 10.1083/jcb.125.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geppert M., Archer B. T., 3rd, Südhof T. C. Synaptotagmin II. A novel differentially distributed form of synaptotagmin. J Biol Chem. 1991 Jul 25;266(21):13548–13552. [PubMed] [Google Scholar]
- Han J. S., Maeda Y., Ecelbarger C., Knepper M. A. Vasopressin-independent regulation of collecting duct water permeability. Am J Physiol. 1994 Jan;266(1 Pt 2):F139–F146. doi: 10.1152/ajprenal.1994.266.1.F139. [DOI] [PubMed] [Google Scholar]
- Haspel H. C., Birnbaum M. J., Wilk E. W., Rosen O. M. Biosynthetic precursors and in vitro translation products of the glucose transporter of human hepatocarcinoma cells, human fibroblasts, and murine preadipocytes. J Biol Chem. 1985 Jun 25;260(12):7219–7225. [PubMed] [Google Scholar]
- Hilbush B. S., Morgan J. I. A third synaptotagmin gene, Syt3, in the mouse. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8195–8199. doi: 10.1073/pnas.91.17.8195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst B. H., Forte J. G. Redistribution and characterization of (H+ + K+)-ATPase membranes from resting and stimulated gastric parietal cells. Biochem J. 1985 Nov 1;231(3):641–649. doi: 10.1042/bj2310641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson A. W., Fingar D. C., Seidner G. A., Griffiths G., Burke B., Birnbaum M. J. Targeting of the "insulin-responsive" glucose transporter (GLUT4) to the regulated secretory pathway in PC12 cells. J Cell Biol. 1993 Aug;122(3):579–588. doi: 10.1083/jcb.122.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Ouimet C., Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K. L., Deeney J. T., Corkey B. E. Cytosolic free calcium in adipocytes. Distinct mechanisms of regulation and effects on insulin action. J Biol Chem. 1989 Aug 5;264(22):12754–12757. [PubMed] [Google Scholar]
- Kelly R. B. Storage and release of neurotransmitters. Cell. 1993 Jan;72 (Suppl):43–53. doi: 10.1016/s0092-8674(05)80027-3. [DOI] [PubMed] [Google Scholar]
- Klip A., Ramlal T. Cytoplasmic Ca2+ during differentiation of 3T3-L1 adipocytes. Effect of insulin and relation to glucose transport. J Biol Chem. 1987 Jul 5;262(19):9141–9146. [PubMed] [Google Scholar]
- Littleton J. T., Stern M., Schulze K., Perin M., Bellen H. J. Mutational analysis of Drosophila synaptotagmin demonstrates its essential role in Ca(2+)-activated neurotransmitter release. Cell. 1993 Sep 24;74(6):1125–1134. doi: 10.1016/0092-8674(93)90733-7. [DOI] [PubMed] [Google Scholar]
- McMahon H. T., Ushkaryov Y. A., Edelmann L., Link E., Binz T., Niemann H., Jahn R., Südhof T. C. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993 Jul 22;364(6435):346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- Mizuta M., Inagaki N., Nemoto Y., Matsukura S., Takahashi M., Seino S. Synaptotagmin III is a novel isoform of rat synaptotagmin expressed in endocrine and neuronal cells. J Biol Chem. 1994 Apr 22;269(16):11675–11678. [PubMed] [Google Scholar]
- Nonet M. L., Grundahl K., Meyer B. J., Rand J. B. Synaptic function is impaired but not eliminated in C. elegans mutants lacking synaptotagmin. Cell. 1993 Jul 2;73(7):1291–1305. doi: 10.1016/0092-8674(93)90357-v. [DOI] [PubMed] [Google Scholar]
- Perin M. S., Fried V. A., Mignery G. A., Jahn R., Südhof T. C. Phospholipid binding by a synaptic vesicle protein homologous to the regulatory region of protein kinase C. Nature. 1990 May 17;345(6272):260–263. doi: 10.1038/345260a0. [DOI] [PubMed] [Google Scholar]
- Pershadsingh H. A., Shade D. L., Delfert D. M., McDonald J. M. Chelation of intracellular calcium blocks insulin action in the adipocyte. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1025–1029. doi: 10.1073/pnas.84.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov S. V., Poo M. M. Synaptotagmin: a calcium-sensitive inhibitor of exocytosis? Cell. 1993 Jul 2;73(7):1247–1249. doi: 10.1016/0092-8674(93)90352-q. [DOI] [PubMed] [Google Scholar]
- Sandoval I. V., Bakke O. Targeting of membrane proteins to endosomes and lysosomes. Trends Cell Biol. 1994 Aug;4(8):292–297. doi: 10.1016/0962-8924(94)90220-8. [DOI] [PubMed] [Google Scholar]
- Snyder H. M., Fredin D. M., Breyer M. D. Muscarinic receptor activation inhibits AVP-induced water flow in rabbit cortical collecting ducts. Am J Physiol. 1991 Jun;260(6 Pt 2):F929–F936. doi: 10.1152/ajprenal.1991.260.6.F929. [DOI] [PubMed] [Google Scholar]
- Ullrich B., Li C., Zhang J. Z., McMahon H., Anderson R. G., Geppert M., Südhof T. C. Functional properties of multiple synaptotagmins in brain. Neuron. 1994 Dec;13(6):1281–1291. doi: 10.1016/0896-6273(94)90415-4. [DOI] [PubMed] [Google Scholar]
- Wendland B., Miller K. G., Schilling J., Scheller R. H. Differential expression of the p65 gene family. Neuron. 1991 Jun;6(6):993–1007. doi: 10.1016/0896-6273(91)90239-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. Z., Davletov B. A., Südhof T. C., Anderson R. G. Synaptotagmin I is a high affinity receptor for clathrin AP-2: implications for membrane recycling. Cell. 1994 Sep 9;78(5):751–760. doi: 10.1016/s0092-8674(94)90442-1. [DOI] [PubMed] [Google Scholar]







