Abstract
Dendritic cells (DCs) initiate and shape both the innate and adaptive immune responses. Accordingly, recent evidence from clinical studies and experimental models implicates DCs in the pathogenesis of most autoimmune diseases. However, fundamental questions remain unanswered concerning the actual roles of DCs in autoimmunity, both in general and, in particular, in specific diseases. In this Review, we discuss the proposed roles of DCs in immunological tolerance, the effect of the gain or loss of DCs on autoimmunity and DC-intrinsic molecular regulators that help to prevent the development of autoimmunity. We also review the emerging roles of DCs in several autoimmune diseases, including autoimmune myocarditis, multiple sclerosis, psoriasis, type 1 diabetes and systemic lupus erythematosus.
In 2011, the late Ralph Steinman was awarded the Nobel Prize in Physiology or Medicine for his part in the discovery of dendritic cells (DCs) and the important role they have in initiating the adaptive immune response. DCs are specialized sentinel cells that bridge the innate and adaptive immune systems without directly engaging in effector activities such as pathogen killing. DCs recognize pathogens using pattern recognition receptors, including Toll-like receptors (TLRs), and then they migrate to T cell areas of lymphoid organs to present pathogen-derived antigens to antigen-specific T cells. Activated DCs upregulate co-stimulatory molecules and produce cytokines that drive T cell priming and effector differentiation, and they activate various types of immune cells. In the absence of activation, antigen presentation by steady-state DCs might lead to T cell unresponsiveness and might promote tolerance1.
DCs comprise two major classes: plasmacytoid DCs (pDCs) and conventional or classical DCs (cDCs). The pDCs rapidly produce type 1 interferon (IFN) following activation through nucleic acid-sensing TLRs, such as TLR7 and TLR9. cDCs are dedicated antigen-presenting cells (APCs) that have a characteristic dendritic morphology and express high levels of MHC class II molecules. Mouse cDCs can be broadly categorized into two distinct subsets: the CD8+ DCs (which are CD103+ in tissues) and the CD11b+ DCs (TABLE 1). The CD8+ DC subset is highly efficient at mediating antigen cross-presentation to cytotoxic CD8+ T cells. The full functional range of CD11b+ DCs remains to be determined, although they are believed to preferentially present MHC class II-restricted antigens to CD4+ T cells. There is substantial genetic and functional heterogeneity within each DC subset, especially in those subsets that are localized to barrier tissues such as the intestine2,3. Within each subset, some DCs may express higher levels of TLRs, may preferentially sense pathogens and may secrete pro-inflammatory cytokines, whereas other DCs may be more efficient at migration, antigen presentation and T cell priming. Such functional division into ‘detector’ and ‘presenter’ DCs might be a general feature of DC subsets in lymphoid organs and tissues4. Major advances have been made in the characterization of human DCs; these include the discovery of genetic DC deficiencies5 and the characterization of DCs in the lymph nodes, tissues and inflammatory fluids6,7,116,117. Collectively, these studies have shown that there is a strong evolutionary conservation of the major DC subsets between mice and humans (TABLE 1).
Table 1.
Major subsets of DCs in humans and mice*
| DC subset | Phenotype | Specific transcription factors |
Specific mediators produced upon activation |
Specific antigen- presentation capacities |
Location | Tissue condition |
|
|---|---|---|---|---|---|---|---|
| Mice | Humans | ||||||
| Plasmacytoid DC | CD11cint MHC class IIint B220, BST2 and SIGLEC-H | CD11c− MHC class IIint CD123, BDCA2 and BDCA4 | TCF4 (also known as E2-2) | Type 1 IFN | Present and Cross-present peptides only after activation | Lymphoid organs | Steady state |
| CD11b+ESAMhi cDC | CD11chi, MHC class IIhi, CD11b, CX3CR1low and ESAMhi | CD11chi MHC class IIhi CD11b and BDCA1 | Notch 2 | ND | Present peptides on MHC class II molecules to CD4+ T cells | Lymphoid organs | Steady state |
| CD11b+ESAMlow cDC‡ | CD11chi, MHC class IIhi, CD11b, CX3CR1hi and ESAMlow | CD11chi, MHC class IIhi, CD11b, CD16 and CD14 | ND | TNF and IL-12 | ND | Lymphoid organs | Steady state |
| CD8α+ cDC | CD11chi, MHC class IIhi, XCR1, CLEC9A and CD8α | CD11cint MHC class IIhi XCR1, CLEC9A and BDCA3 | BATF3 | IL-12 | Cross-present peptides on MHC class I molecules to CD8+ T cells | Lymphoid organs | Steady state |
| CD103+ cDC | CD11chi, MHC class IIhi, CD103, XCR1 and CLEC9A | CD11chi MHC class IIhi XCR1, CLEC9A and BDCA3 | BATF3 | ND | Cross-present peptides on MHC class I molecules to CD8+ T cells | Peripheral tissues | Steady state |
| CD11b+ cDC | CD11chi MHC class IIhi CD103§, CD11b and CD24 | CD11chi, MHC class IIhi, CD11b, SIRPα§ and BDCA1║ | IRF4 and Notch 2❡ | IL-6 and IL-23 | Present peptides on MHC class II molecules to CD4+ T cells | Lungs and gut | Steady state |
| Interstitial cDC | CD11chi, MHC class IIhi, X3CR1 and CD11b | CD11chi, MHC class IIhi, CD11b, CD16 and CD14 | ND | TNF and IL-12 | ND | Peripheral tissues | Steady state |
| Langerhans cell | CD11chi MHC class IIhi Langerin and CD205 | CD11chi MHC class IIhi CD1a, Langerin and CD205 | ND | ND | ND | Epidermis | Steady state |
| Monocyte-derived DC | CD11chi, MHC class IIhi, CD11b, CX3CR1 and CD209 | CD11chi MHC class IIhi and CD11b | ND | TNF and iNOS | Present peptides on MHC class II molecules to CD4+ T cells | Inflamed lymph nodes and tissues | Inflamed |
BATF3, basic leucine zipper transcription factor ATF-like 3; BDCA, blood dendritic cell antigen; BST2, bone marrow stromal antigen 2; cDC, classical dendritic cell; CLEC9A, C-type lectin domain family 9 member A; CX3CR1, CX3C-chemokine receptor 1; ESAM, endothelial cell-selective adhesion molecule; IFN, interferon; IL-12, interleukin-12; iNOS, inducible nitric oxide synthase; IRF4, interferon-regulatory factor 4; ND, not described; SIGLEC, salic acid-binding immunoglobulin-like lectin; SIRPα, signal-regulatory protein-α; TCF4, transcription factor 4; TNF, tumour necrosis factor; XCR1, XC-chemokine receptor 1.
The table (which is by no means all-inclusive) shows the main cell surface markers, subset-specific transcription factors and predominant functional features of the putative orthologous human and mouse dendritic cell subsets3–5,116,117.
It is unclear whether the human and mouse subsets of these DCs are orthologous.
Expression has been reported in the gut.
Expression has been reported in the lungs.
The expression of Notch 2 is ubiquitous; however, its function is only required in this DC subset in the gut and not in the lungs.
Three decades ago, pioneering studies in a rat model of induced autoimmune neural inflammation identified DCs that were in close contact with T cells in inflammatory brain lesions8. Strikingly, DCs transferred from mice in which autoimmune neural inflammation had been induced were able to induce the disease in naive recipients, showing their extraordinary potential as APCs9. Since then, the crucial involvement of DCs in virtually every aspect of autoimmunity has been documented in patients as well as in animal models. In particular, novel models of constitutive and inducible DC ablation, as well as DC-specific gene targeting, have facilitated direct analysis of the roles of DCs in autoimmune diseases. In this Review, we briefly discuss the current ideas concerning the role of DCs in immunological tolerance and the general aspects of their involvement in autoimmunity. In addition, we review the recent findings regarding the roles that DCs are thought to have in either promoting or inhibiting pathology in various different autoimmune diseases, both in patients and in the associated animal models.
The role of DCs in immune tolerance
DCs in central tolerance
Medullary thymic epithelial cells (mTECs) are primarily responsible for the negative selection of autoreactive T cells in the thymus. They express the transcription factor autoimmune regulator (AIRE), which drives the low-level expression of many tissue-specific self antigens for presentation to developing thymocytes. Thymic DCs have been shown to cross-present these self antigens that have been acquired from mTECs10,11. In addition, mTECs might recruit thymic DCs in an AIRE-dependent manner and might facilitate the generation of regulatory T (TReg) cells12. It is probable that the DC-dependent presentation of self antigens occurs in parallel with the direct presentation of these antigens by mTECs13 and that it might be secondary to their presentation by mTECs; this has been suggested because the negative selection of thymocytes that are specific for a model self antigen was shown to be unimpaired by DC ablation14. Finally, peripheral DCs might migrate into the thymus and might present peripheral self antigens to induce clonal deletion of T cells or to induce TReg cell generation15,16. However, it is unclear whether this mechanism of tolerance is relevant and whether it occurs in conditions other than artificial ones. Overall, the role of DCs in central tolerance seems to be fairly limited and might be restricted to promoting tolerance to a minor subset of self antigens.
DCs in peripheral tolerance
Pioneering studies in which antigens were delivered to DCs in vivo using DC-specific antibodies showed that DC-targeted model antigens induced profound T cell tolerance in the steady state17,18. In subsequent studies, antigens were delivered to DCs using Cre recombinase-induced expression of model antigens in DCs in vivo19. This genetic approach showed that steady-state antigen presentation by DCs induces a profound and irreversible unresponsiveness in CD8+ T cells; this includes the upregulation of the inhibitory molecules programmed cell death protein 1 (PD1) and cytotoxic T lymphocyte antigen 4 (CTLA4) on CD8+ T cells20. The tolerogenic capacity of DCs in this context was enforced by MHC class II-dependent interactions with TReg cells, and MHC class II-deficient DCs were proposed to induce CD8+ T cell-mediated autoimmunity21. Thus, DCs can induce peripheral T cell tolerance to immunodominant antigens that are expressed at high levels. However, the applicability of this model to the full range of endogenous self antigens remains to be investigated.
DCs were proposed to contribute to peripheral tolerance by facilitating the homeostasis of peripheral TReg cells. DCs can induce TReg cells in the presence of strong stimuli, such as transforming growth factor-β (TGFβ) and retinoic acid22,23, and can support the homeostatic proliferation of TReg cells after their depletion24. DCs seem to contribute to the maintenance of TReg cell populations in the peripheral blood, which requires the expression of the co-stimulatory molecules CD80 and/or CD86 on DCs25. It was recently suggested that migratory cDCs, but not resident cDCs, in the lymph nodes induce the development of TReg cells that are specific for a particular self antigen, even though that self antigen is expressed by both DC populations26. However, constitutive DC ablation causes only a small reduction in TReg cell numbers without any obvious functional con-sequences14,25. Conversely, the administration of the DC growth factor FMS-related tyrosine kinase 3 ligand (FLT3L) leads to an expansion of both the DC and the TReg cell populations27–29. This expansion of the TReg cell population was associated with reduced severity of intestinal inflammation and of graft-versus-host disease, showing that a tolerogenic environment had been induced. However, FLT3L treatment skews DC subset distribution, it expands myeloid non-DC populations and it might change the function of individual DCs. Thus, the observed expansion of the TReg cell population might not be a simple consequence of the increased numbers of DCs; this leaves the idea that there is a simple linear relationship between DCs and TReg cells open to question. Taken together, DCs seem to facilitate the induction and/or the maintenance of peripheral TReg cells; however, they are neither strictly required for TReg cell homeostasis nor do they seem to preferentially regulate TReg cells compared to effector T cells.
Tolerogenic DCs: subset or state?
The role of DCs in self-tolerance might be primarily determined by their functional state or it might be mediated through a distinct ‘tolerogenic’ DC subset. Although DCs that have distinctly tolerogenic properties have been shown to arise in artificial systems30, genetic evidence for a tolerogenic DC subset in the steady state is lacking. For example, an indispensable role for intestinal CD103+ DCs in TReg cell induction has not been supported by loss-of-function analyses31,32. DCs that express perforin and that can kill cognate T cells might be an exception, although the identity of these DCs in vivo has not yet been established33. Another interesting exception might be peripheral DCs that express AIRE and that therefore present an unusually broad range of self antigens, including antigens that are normally restricted to other cell types. These DCs might correspond to very rare cells that have hallmarks of the DC lineage (such as MHC class II expression) but that have an unconventional surface phenotype, such as low levels of expression of the haematopoietic marker CD45 (M. Anderson, personal communication). Although the exact role of these DCs in peripheral tolerance remains to be established, the tolerogenic properties of cDCs seem to be primarily mediated by their functional state (for example, by the absence of activation).
The role of DCs in autoimmunity: general aspects
The numbers game
One important question about the role of DCs in autoimmunity has been whether the actual size of the overall DC population contributes to the development of autoimmunity. It was initially reported that the constitutive ablation of cDCs in inbred mice resulted in normal T cell development, the establishment of a normal peripheral T cell compartment without overt hyperactivation and normal TReg cell numbers in most lymphoid organs14. However, DC-deficient mice were shown to develop an age-dependent myeloproliferative syndrome that was associated with increased serum concentrations of FLT3L. A subsequent study in mice of mixed backgrounds suggested that constitutive DC ablation does lead to autoimmune manifestations34. However, the nature and full course of the disease was not fully described nor was any evidence for T cell auto-reactivity provided in this study. Thus, the observed disease manifestations were apparently caused by myeloproliferation rather than by autoimmunity. Indeed, constitutive DC ablation in mice of an autoimmunity-prone background ameliorated rather than exacerbated the resulting disease35. Furthermore, the global constitutive ablation of specific DC subsets — including the CD8+ cDCs31 and pDCs36 — did not cause any detectable autoimmunity. Thus, DCs seem to be dispensible for peripheral tolerance even though they might contribute to the process. In addition, their loss does not cause overt autoimmunity. This might reflect both the redundancy of tolerogenic DC function and the essential role of DCs in the activation of autoreactive T cells4.
Increased DC numbers (for example, after cytokine treatment) have been associated with TReg cell induction and with the development of a tolerogenic environment27–29, as discussed above. Paradoxically, it was also suggested that impaired apoptosis of DCs might increase DC numbers and cause autoimmunity37–39. However, it remains unclear whether the in vivo blockade of apoptosis was limited to DCs in these studies; indeed, some of these studies used a Cre-deleter strain that has a broad deletion range (BOX 1). Taken together, the association between increased DC numbers and autoimmunity has not been conclusively shown and its potential relevance remains debatable.
Box 1. Advantages and limitations of DC-specific gene targeting.
Dendritic cell (DC)-specific gene targeting in mice uses conditional LoxP-flanked (floxed) alleles combined with ‘deleter’ strains that express Cre recombinase specifically in DCs. The available deleter strains have Cre transgenes controlled by the Itgax gene, which encodes the DC-specific marker CD11c. However, CD11c is also expressed at lower levels by several immune cell types, including natural killer (NK) cells and activated T cells. The mouse strain expressing Cre recombinase under the control of the 5 kilobase Itgax promoter38 was recently shown to mediate Cre recombination in a broad range of immune cells114. The strain expressing Cre recombinase under the control of the 160 kilobase genomic fragment of Itgax115 mediates Cre recombination in the majority of classical DCs and plasmacytoid DCs, but only in approximately 10% of non-DCs (including T cells and NK cells). In combination with Cre-inducible diphtheria toxin or diphtheria toxin receptor (DTR), this strain enables the efficient and specific ablation of the classical DC lineage. However, the DC-intrinsic nature of the observed phenotypes should be ascertained in every case, for example, through comparison with other deleter strains.
DC-intrinsic regulation of autoimmunity
Although the mere gain or loss of DCs does not seem to initiate autoimmunity, changes in DC functionality might induce inflammation and/or autoimmune manifestations. The advent of DC-specific gene targeting (BOX 1) has facilitated the direct genetic manipulation of DC functionality and the analysis of its effect on autoimmunity. Several genes have been identified that induce spontaneous autoimmune and/or inflammatory manifestations when they are deleted in DCs40–46 (TABLE 2). Most of these genes are negative regulators of pro-inflammatory signals (for example, A20 (also known as TNFAIP3) and SH2 domain-containing protein tyrosine phosphatase 1 (SHP1; also known as PTPN6)) or molecules that are upstream or downstream of anti-inflammatory signals (such as αVβ8 integrin and signal transducer and activator of transcription 3 (STAT3), respectively). Importantly, these are general negative regulators of immune activation and their deletion in other cell types also causes inflammation and/or autoimmunity. Thus, inappropriate DC activation is apparently sufficient to induce a broad range of autoimmune manifestations and it is actively opposed by multiple pathways and factors. However, cell-type specific negative regulators that prevent DC hyperactivation and autoimmunity remain mostly unknown. Given the powerful functionality and unique gene expression profile of DCs, we propose that such (unidentified) DC-specific regulators might exist and might have a crucial role in the maintenance of tolerance and in the prevention of autoimmunity.
Table 2.
Cell-intrinsic negative regulators of DC function in autoimmunity
| Protein deleted in DCs (gene targeted)* |
Disease phenotype | Mechanism of action | Refs |
|---|---|---|---|
| αVβ8 integrin (Itgb8) | Polyclonal immune activation, production of autoantibodies and development of colitis | DCs cannot activate latent TGFβ to induce regulatory T cell conversion | 42 |
| STAT3 (Stat3) | Cervical lymphadenopathy and ileocolitis | DCs are unresponsive to inhibitory signals delivered by IL-10 and they show enhanced induction of T cell priming and TH1 cell differentiation | 43 |
| BLIMP1 (Prdm1) | Increased germinal centre reaction, production of DNA-specific antibodies and development of SLE-like disease (females only) | Spontaneous production of IL-6 and the induction of T follicular helper cell differentiation by DCs | 44 |
| A20 (Tnfaip3) | Polyclonal immune activation, ankylosing arthritis and colitis | Spontaneous DC maturation, increased inflammatory cytokine production and enhanced T cell activation by DCs | 45 |
| Polyclonal immune activation and development of SLE-like disease | Reduced apoptosis and spontaneous maturation of DCs, increased inflammatory cytokine production by DCs, and increased uptake and presentation of apoptotic cells by DCs | 46 | |
| SHP1 (Ptpn6) | Polyclonal immune activation and development of SLE-like disease | Spontaneous DC maturation, and increased inflammatory cytokine production and induction of TH1 cell differentiation by DCs | 47,48 |
BLIMP1, B lymphocyte-induced maturation protein 1; DC, dendritic cell; IL, interleukin; SHP1, SH2 domain-containing protein tyrosine phosphatase 1; SLE, systemic lupus erythematosus; STAT3, signal transducer and activator of transcription 3; TGFβ; transforming growth factor-β; TH1, T helper 1.
Genes are shown that, when deleted in DCs using a DC-specific Cre-deleter mouse strain115, caused spontaneous inflammatory and/or autoimmune manifestations.
The role of DCs in autoimmune diseases
As discussed above, the involvement of DCs in tolerance and autoimmunity is complex and bidirectional (FIG. 1). Indeed, DCs might promote tolerance through multiple mechanisms, including through the generation and maintenance of TReg cells, as well as through the induction of T cell unresponsiveness. Conversely, the powerful antigen presentation capacity of DCs might promote the priming and/or the effector differentiation of self-reactive T cells, either because of inappropriate activation signals or because of a cell-intrinsic breakdown of negative regulation. Below, we review the current evidence for these mechanisms from several major autoimmune diseases, which were primarily chosen because of the availability of data. We consider a somewhat special case of cardiac autoimmunity that is associated with molecular mimicry, as well as tissue-specific diseases of the central nervous system and skin. We also review two major diseases for which multigenic spontaneous mouse models are available: type 1 diabetes (T1D) and systemic lupus erythematosus (SLE).
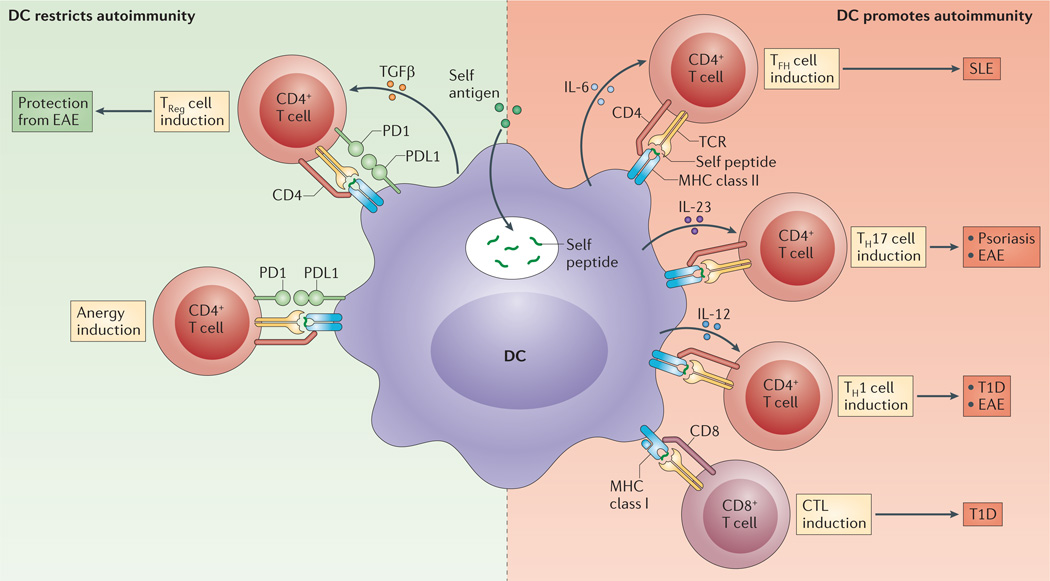
Figure 1. Potential roles of DCs in autoreactive T cell responses.
Depending on the inflammatory context and the expression of cell-intrinsic regulators, dendritic cell (DC)-mediated presentation of self antigens might promote or inhibit autoimmune responses; for example, the presentation of self antigens to T cells in the context of the programmed cell death protein 1 (PD1)—PD1 ligand 1 (PDL1) interaction and/or transforming growth factor-β (TGFβ) signalling can lead to anergy in self-reactive T cells or it can promote their development into regulatory T (TReg) cells (left panel). By contrast, if DCs take up and present self antigens to T cells in the context of pro-inflammatory mediators (such as interleukin-6 (IL-6), IL-12 and IL-23), they can promote the development of self-reactive effector CD4+ T cells and cytotoxic T lymphocytes (CTLs). These self-reactive T cells might contribute to pathological autoimmune responses, such as experimental autoimmune encephalomyelitis (EAE) in mice, or systemic lupus erythematosus (SLE), psoriasis and type 1 diabetes (T1D) in patients. TCR, T cell receptor; TFH, T follicular helper; TH, T helper.
DCs in cardiac autoimmunity
Autoimmune myocarditis often occurs after viral infections (for example, cox-sackievirus or adenovirus infections) and is associated with the production of autoantibodies against cardiac proteins, which suggests that there might be molecular mimicry between the pathogen-derived and the cardiac antigens. Similar cases of molecular mimicry have been documented in rheumatic pancarditis, in which infection with group A Streptococcus (also known as Streptococcus pyogenes) initiates an immune response to host antigens in the perivascular connective tissue and in the myocardium. Adoptive transfer of DCs that are loaded with cardiac antigens can induce the infiltration of CD4+ T cells into cardiac tissue and the development of experimental autoimmune myocarditis (EAM); this indicates that DCs are sufficient for inducing the disease47. The activation of DCs by the pro-inflammatory cytokines interleukin-1 (IL-1)48 and granulocyte–macrophage colony-stimulating factor (GM-CSF)49 was found to be crucial for efficient autoreactive T cell responses and EAM induction. Another study described the contribution of the RNA-sensing receptor TLR7 to EAM severity, suggesting that TLR7-expressing DCs (such as pDCs) have a role in driving the disease50. In a related model of pericardial inflammation caused by the injection of antigen-pulsed DCs, the cardiac proteoglycan biglycan was found to substitute for pathogen-associated stimuli and to induce cDC activation in the context of damaged cardiomyocytes51. Thus, in the inducible model of autoimmune myocarditis, the integration of cytokines and pathogen-derived or endogenous inflammatory stimuli by DCs is important for the development of the disease. However, it remains to be determined whether DCs are strictly necessary for the development of autoimmune myocarditis and whether they have a role in antigen mimicry-associated cardiac inflammation in patients.
The role of DCs in neurological autoimmunity
In multiple sclerosis and its associated animal models — such as experimental autoimmune encephalomyelitis (EAE) — T cells that are specific for the myelin antigen are crucial effector cells that drive inflammation and tissue damage. Importantly, the rare autoreactive T cells that are present in the natural T cell repertoire need to encounter their cognate antigen on APCs at least twice for the development of EAE. During the initial priming phase, the population of T cells expands and the cells become polarized encephalitogenic T helper 1 (TH1) or TH17 effector cells. Subsequent interactions, which occur in close proximity to or within the brain, confer a crucial restimulation signal.
It was shown that cDCs are highly efficient in priming and polarizing encephalitogenic T cells in EAE caused by a myelin-associated peptide antigen, as well as in demyelinating disease caused by Theiler’s murine encephalomyelitis virus52,53. Moreover, transgenic re-expression of MHC class II molecules specifically in the cDCs of MHC class II-deficient mice was sufficient to mediate peptide-induced EAE54. Notably, the subcutaneous delivery of the full-length protein antigen failed to achieve efficient T cell priming in this model, which suggests that in some situations DCs are insufficient as APCs. Furthermore, several models of DC lineage ablation showed that DCs are completely dispensable for encephalitogenic T cell priming in myelin protein-induced EAE55,56. Thus, DCs are involved in and might be sufficient for encephalitogenic T cell priming, but other types of APCs might substitute for DCs in inducible multiple sclerosis models.
DC-specific targeting approaches were recently used to identify several molecules that regulate the polarization capacity of DCs that prime TH cells; for instance, DC-specific ablation of mitogen-activated protein kinase p38α (p38α MAPK; also known as MAPK14) impaired the priming and the maintenance of TH17 responses and prevented disease in both actively and passively induced models of EAE57. Given the evidence discussed above55,56, it is probable that p38α MAPK-deficient DCs redirect T cells to a different effector cell fate rather than failing to prime them. In addition to such cell-intrinsic effects, DCs have a role in establishing the cytokine milieu that is required for T cell polarization. Thus, a DC-specific deficiency of αVβ8 integrin, which is required for the local activation of latent TGFβ, diminished the ability of cDCs to support TH17 cell responses and rendered mice resistant to peptide-induced — but not adoptively transferred — EAE58. Conversely, DC-intrinsic TGFβ signalling reduces the capacity of DCs to prime T cells and its specific blockade in DCs was shown to worsen EAE in immunization-induced and T cell receptor (TCR)-transgenic models59. Similarly, engagement of the surface-expressed receptor T cell immunoglobulin and mucin domain-containing protein 1 (TIM1; also known as HAVCR1) on DCs increases their capacity to drive effector T cell differentiation as opposed to TReg cell induction. The antibody-mediated ligation of TIM1 exacerbated EAE, which suggests that aberrant DC hyperactivation might strongly contribute to the breach of tolerance in this model60.
IFNβ is used as an effective therapy for multiple sclerosis, thus the modulation of DC function by type I IFN is of particular interest in this disease. The genetic blockade of type I IFN induction or signalling exacerbates active EAE, possibly by facilitating TH17 cell effector differentiation or maintenance61,62. The suppression of T H17 cell responses by type 1 IFN requires the expression of the IFNα/β receptor (IFNAR) by DCs but not by macrophages or microglia; this implicates DC-intrinsic IFNAR signalling in the control of TH cell differentiation62. In addition, administered IFNβ might directly inhibit DC functions, such as their migration, thereby reducing T cell priming63. Thus, DCs might represent important cellular targets of anti-inflammatory type I IFN signalling during the natural course of multiple sclerosis and of IFNβ therapy during the treatment of the disease.
Primed encephalitogenic effector T cells have to infiltrate the brain to induce autoimmune inflammation. However, the brain is a site of immune privilege that is protected by multiple mechanisms, including the blood–brain barrier. Notably, cDCs are present at all sites of immune cell entry to the brain, residing in the perivascular space of the blood–brain barrier and integrating into the physical barrier structures64,65. Thus, DCs might act as ‘gatekeepers’ that control T cell entry to the brain, presenting self antigen in situ to effector T cells but also inducing the TReg cell activity that reduces inflammation and that maintains immune privilege at this site.
In the model of active relapsing EAE, CD11b+ cDCs in the brain were proposed to drive and to maintain TH17 cell differentiation66. Indeed, extensive interactions between DCs and T cells were documented in the inflamed central nervous system (CNS) and were partially dependent on Cc-chemokine receptor 4 (CCR4) expression by DCs67. Both TH17 and TH1 cells in EAE secrete GM-CSF and this is essential for their effector functions68,69. GM-CSF activates the production of IL-23 by APCs, probably including DCs. Conversely, both constitutive and inducible ablation of DCs was shown to exacerbate EAE, which suggests that DCs might collectively ameliorate inflammation in the brain55. Furthermore, DC-specific expression of a myelin self antigen protected against the development of EAE and this was associated with TReg cell induction via the expression of inhibitory PD1 ligand 1 (PDL1) on DCs. Similarly, pDC depletion was shown to exacerbate acute and relapsing EAE and to increase the production of inflammatory cytokines by T cells70. Moreover, the loss of MHC class II expression specifically on pDCs was shown to exacerbate EAE, apparently by reducing the induction of TReg cells that were specific for myelin antigens after immunization71. Similarly, the constitutive ablation of pDCs increased EAE disease severity, which suggests that the pDCs have regulatory functions at all stages of EAE (S.H., unpublished observations).
Taken together, data from EAE models suggest that DCs are important — albeit not strictly required — for the T cell priming and the effector T cell polarization that sustains brain inflammation (FIG. 1). Conversely, strong genetic evidence suggests that DCs reduce T cell-mediated inflammation and promote TReg cell differentiation at later stages of the disease. A major challenge now lies in extrapolating these data to multiple sclerosis in humans, in which the identity of the initiating event, the self antigens that are involved and the sites of T cell priming remain unresolved. Evidence for pro-inflammatory DC activity in multiple sclerosis has been reported72, but whether DCs also have tolerogenic activity remains to be established.
DCs in psoriasis
Psoriasis is an inflammatory disease of a barrier tissue (the skin) and may not be primarily of autoimmune origin; however, it seems to have a strong autoimmune component. Keratinocytes in the psoriatic plaque are viewed as both ‘victims’ and ‘perpetrators’, as they drive the recruitment of immune cells by expressing antimicrobial peptides, chemokines and growth factors. Effector T cells that produce IL-17 and IL-22 — including CD4+ TH17 cells and potentially γδ T cells — have a major role in psoriatic inflammation73,74, which raises the question of which cells and signals they are primed by.
One emerging model of psoriasis-like inflammation is induced by painting the skin with the TLR7 ligand imiquimod75, although TLR7-independent effects that are induced by the vehicle might also contribute to plaque formation76. It was recently shown that DC depletion in CD11c–diphtheria toxin receptor (DTR) mice protected against disease development in this model77. This study suggested that the production of IL-36 by skin cells activates DCs to produce IL-23 and to drive TH17 cell differentiation, which indicates that DCs are necessary for the disease. Conversely, DC-specific deletion of the A20-binding inhibitor of NF-κB inhibitor activation 1 (ABIN1; also known as TNIP1) caused TLR-induced cDC activation and hyperproduction of IL-23 and greatly exacerbated imiquimod-induced psoriasis (A. Ma, personal communication). Notably, polymorphisms in the TNIP1 gene have been associated with psoriasis in patients. These observations suggest that the aberrant hyperactivation of cDCs might be sufficient for innate immune signals to induce psoriasis, and further investigation of cDC function in other models of the disease is warranted.
Type I IFN is sufficient to inadvertently induce flares of psoriasis in patients who have been treated for other conditions such as multiple sclerosis. Therefore, it has been postulated that type I IFN-producing pDCs have a major role in the initiation of psoriasis. Indeed, nascent psoriatic plaques show prominent infiltration of pDCs78 and express the chemotactic factor chemerin, which recruits pDCs to the skin through chemokine-like receptor 1 (CMKLR1; also known as CHEMR23)79. Furthermore, pDCs were shown to accumulate at the site of skin injury, to secrete type I IFN in a TLR-dependent manner and to promote either wound healing in normal mice or inflammation in mice from autoimmunity-prone backgrounds80,81. Using biochemical analysis of psoriatic plaques, Gilliet and colleagues82 showed that activated keratinocytes produce the antimicrobial peptide LL37, which can bind to extracellular DNA and RNA that is generated in the skin during normal cell turnover or during injury82. The resulting nanoscale LL37–nucleic acid particles are protected from nuclease degradation, and they can induce type I IFN production by pDCs and activate cDCs82,83 (FIG. 2). Unexpectedly, pDC ablation did not affect the severity of imiquimod-induced psoriatic plaque formation in mice118.
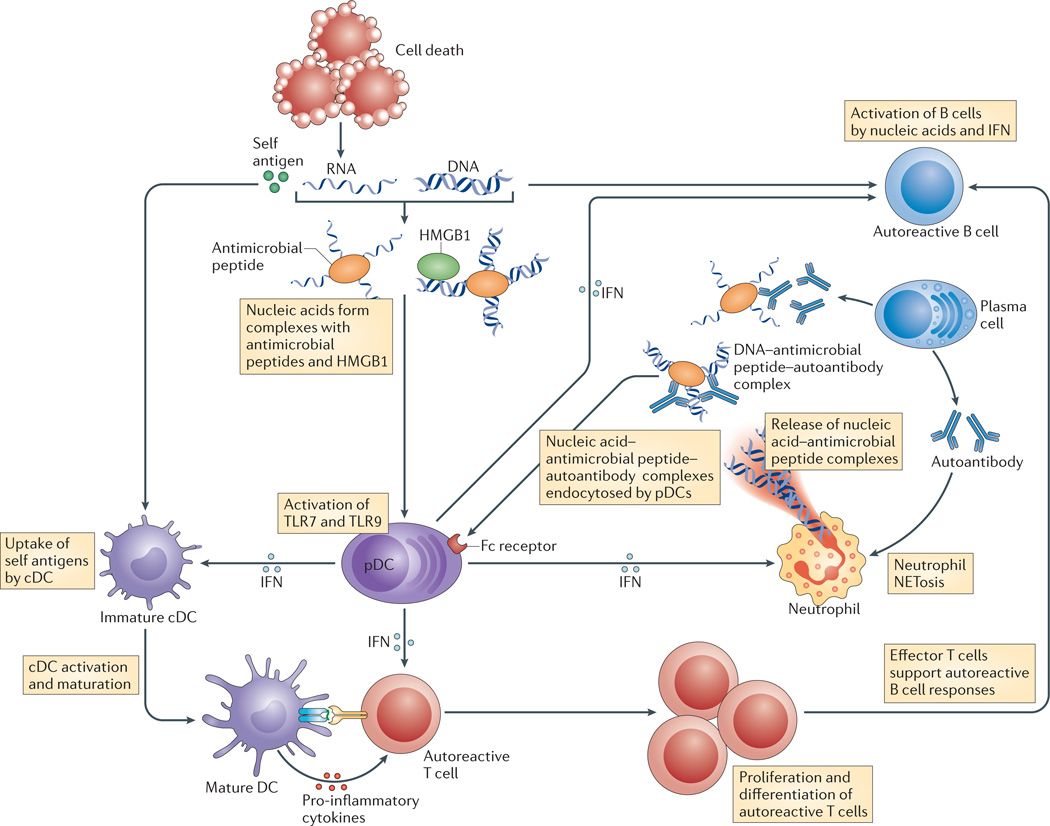
Figure 2. The production of type I IFN by pDCs as a common mechanism of pathogenesis.
The production of type I interferon (IFN) by plasmacytoid dendritic cells (pDCs) is a common mechanism of pathogenesis in type 1 diabetes, systemic lupus erythematosus and psoriasis. Dying tissue cells release nucleic acids; these form large complexes with antimicrobial peptides, such as LL37 in humans and cathelin-related antimicrobial peptide (CAMP) in mice, and with endogenous DNA-binding proteins, such as high mobility group protein B1 (HMGB1). These DNA and RNA complexes activate pDCs via Toll-like receptor 9 (TLR9) and TLR7, respectively, and they induce the production of type IIFN. In turn, type I IFN promotes T cell activation, autoantibody production by B cells and the release of neutrophil extracellular traps (NETs) that consist of immune complexes that are preferentially endocytosed by pDCs via Fc receptors. Self-nucleic acids also activate classical DCs (cDCs) and they promote the release of inflammatory cytokines and the priming of T cells that are specific for self antigens in a process that is also facilitated by type I IFN82,83,95,110,111.
Thus, the pDC–type I IFN axis seems to have a prominent role in the initiation of inflammation and in the transition to a chronic disease, but it might be redundant in actual psoriatic lesion formation. By contrast, cDCs might be essential for IL-23 production, which drives T cell effector differentiation and plaque formation118. The imiquimod-induced model of psoriasis provides useful insights into the course of the disease, but a better understanding of the roles of pDCs and cDCs in psoriasis will be gained by studying these cells in spontaneous genetic models of the disease.
DCs in T1D
Pancreatic β-islet cells are the source of the autoantigens that drive disease in T1D but they are unable to directly prime diabetogenic CD4+ or CD8+ T cell responses84, which suggests that cross-presenting APCs might be involved. Self antigens might be released after the death of β-islet cells, which occurs physiologically during pancreatic remodelling in non-obese diabetic (NOD) mice once they reach 2 weeks of age. Such β-islet cell-derived antigens can be captured by DCs and presented to autoreactive T cells after the migration of DCs to the pancreatic lymph nodes, thereby initiating T1D development85. Indeed, T1D development in NOD mice was shown to be prevented by inducible DC depletion and to be restored by the adoptive transfer of CD11b+ DCs86. However, the CD11c–DTR model of DC ablation used in these studies is not entirely DC-specific and was shown to deplete other potentially relevant cell types. Despite this caveat, these data suggest that β-islet cell death might initiate diabetogenic T cell responses via DCs. DCs are constitutively sampling and presenting β-islet cell-derived antigens to T cells in the pancreatic lymph nodes87, but why they induce active immune responses rather than tolerance in response to self antigens is not yet clearly understood. It was proposed that dying pancreatic β-islet cells release the DNA-binding protein high mobility group protein B1 (HMGB1), which is a known danger signal that can activate DCs through TLR2 and TLR4. Antibody-mediated blockade of HMGB1 reduced the incidence and the onset of T1D in NOD mice, and this was associated with a decreased expression of co-stimulatory molecules on CD11b+ DCs88. These results are consistent with a protective role of TLR2 deficiency in T1D pathogenesis in NOD mice89. In addition, inflammatory signals such as tumour necrosis factor (TNF)90 and IL-21 (REF. 91) were shown to promote the maturation of β-islet cell-associated DCs and their migration to the pancreatic lymph nodes.
The expression of IFNα is detected in the pancreatic β-islet cells of patients with T1D and in experimental models of T1D, and the induction of a type I IFN response accelerates T1D development92,93. It was shown that pDCs infiltrate pancreatic islands and the pancreatic lymph nodes in NOD mice; moreover, antibody-mediated blockade of type I IFN-mediated signalling reduced T1D development in this model94. A recent study showed that pDCs in the β-islet cells produce type I IFN and that this is essential for the development of T1D95. According to the proposed model, dying β-islet cells release DNA that forms complexes with DNA-specific antibodies and with cathelin-related antimicrobial peptide (CAMP), which are produced by B1 cells and neutrophils, respectively. The resulting immune complexes activate type I IFN production by pDCs in a TLR9-dependent manner, possibly promoting cDC activation and autoreactive T cell priming95. In patients with T1D, pDCs were shown to present β-islet cell-derived antigens that were obtained by immune complex capture more efficiently than cDCs, thereby directly activating diabetogenic T cells96. Thus, aberrant pDC-mediated type I IFN production seems to be a common mechanism that leads to pathogenesis in several autoimmune diseases, including T1D (FIG. 2).
In line with the putative tolerogenic functions of DCs that are discussed above, these cells have also been proposed to protect NOD mice from developing T1D. The administration of FLT3L or G-CSF protected NOD mice from T1D development and was associated with an expansion of the DC population and an enhanced generation of TReg cells97–99. However, these treatments might modulate the function of the expanded DC population and/or they might affect multiple non-DC cell types (as discussed above). The depletion of DCs using the CD11c–DTR system in TCR-transgenic NOD mice that have progressed to insulitis was shown to exacerbate T1D and this was suggested to be due to the loss of pDCs86. However, the CD11c–DTR system is not known to appreciably deplete pDCs, which leaves this result open to alternative explanations. A recent study described the expression of the β-islet cell-derived antigen insulin in a distinct AIRE+ DC subset, and DC-specific deletion of insulin caused insulitis in wild-type mice100. The molecular and cellular identity of this DC subset, as well as its role in T1D development in NOD mice, remains to be established.
Taken together, DCs seem to have an essential role in the pathogenesis of T1D through their ability to take up and present β-cell antigens to pathogenic T cells in the pancreatic lymph nodes. This process might happen early in life — that is, during the remodelling of pancreatic tissue — and it might be promoted by genetic predisposition factors such as the impaired clearance of dead cells. Although DCs might induce immunological tolerance against β-cell antigens in the absence of such a predisposition or they might suppress autoreactive T cell responses, these DC functions remain to be proved in defined genetic systems.
DCs in SLE
SLE is often considered to be an antibody-driven autoimmune disease, unlike T1D or multiple sclerosis, which are thought to be primarily T cell-mediated. However, SLE shows a strong association with MHC haplotypes, which suggests that T cell responses are essential for the development of this disease. Indeed, even an SLE-like disease that is driven by TLR7 overexpression in mice, and that presumably involves an innate initiating stimulus, is completely MHC haplotype-dependent101. This circumstantial evidence suggests that DCs are possibly involved in the presentation of chromatin and RNA-associated proteins to self-reactive T cells. However, the role of DCs in SLE has only been definitively studied in a single model of SLE — one that is driven by a mutation in the death receptor CD95 (also known as FAS). The loss of CD95 in DCs was found to be sufficient to induce disease manifestations in C57BL/6 mice38, although the specificity of the CD95 deletion remains to be confirmed (BOX 1). Furthermore, the constitutive deletion of DCs in SLE-prone MRL–FasLpr mice reduced T cell population expansion, plasmablast differentiation and target organ damage, which ameliorated the disease35. T cell priming was not substantially affected in the absence of DCs in this model, probably because lymphoproliferation seems to be a key initiating event in this model.
Conversely, DC-specific deletion of several negative regulators is sufficient to induce an SLE-like disease in vivo (TABLE 2). The SLE manifestations that are caused by DC-specific ablation of B lymphocyte-induced maturation protein 1 (BLIMP1; also known as PRDM1), a transcriptional repressor that controls the differentiation of B and T cells, are of particular interest42. Polymorphisms in the PRDM1 gene have been associated with SLE in humans, which shows that it is a highly relevant candidate regulator. BLIMP1-deficient DCs were shown to induce T follicular helper cell differentiation via increased IL-6 production and, thus, they enhanced germinal centre reactions and humoral autoreactivity. Both the monoallelic and biallelic loss of PRDM1 in DCs induced this phenotype, which highlights the sensitivity of DCs to the gene dosage of PRDM1. Strikingly, the resulting disease occurred predominantly in female animals, which recapitulates the strong bias towards female patients with SLE. This study shows that the aberrant activation of DCs might be sufficient to drive the entire spectrum of cellular and humoral autoreactivity in SLE and that it might contribute to the characteristic sex bias that is associated with the disease.
A prominent role for type I IFNs in SLE pathogenesis has been known since the 1970s, when high serum levels of type I IFN were noted to be associated with the exacerbation of SLE disease parameters. Subsequent studies reported a gene expression signature of type I IFN signalling in leukocytes from SLE patients that correlated with disease severity102. Type I IFNs might have pleiotropic effects in promoting this disease, for example, by inducing the maturation of monocytes and the stimulation of autoantibody production by B cells. Indeed, genetic ablation of type I IFN signalling ameliorates SLE development in animal models, such as in NZB/NZW-derived lupus-prone mouse strains103,104. Given the powerful type I IFN-producing capacity of pDCs, these cells have been proposed to be an important source of aberrant type I IFN secretion and major drivers of SLE progression105.
In recent years, multiple studies have provided strong — albeit indirect — evidence that pDCs have a role in SLE propagation. Patients with SLE have reduced numbers of pDCs in the blood and an increased accumulation of pDCs in tissue lesions106. Furthermore, pDCs can be activated by self-nucleic acids that are in a complex with antibodies107,108, or with DNA- or RNA-binding proteins such as HMGB1 (REF. 109). It was recently shown that complexes of DNA and LL37 are released from activated neutrophils as components of neutrophil extracellular traps (NETs) in a process termed ‘NETosis’. These complexes induce pDCs to secrete type I IFN, which in turn activates monocytes and neutrophils and drives the vicious circle of immune activation that is seen in patients with SLE110,111 (FIG. 2). Moreover, activated pDCs become resistant to glucocorticoids, which is a possible reason for the limited efficacy of these drugs in the treatment of SLE112,113. Thus, pDCs might represent an important component of SLE pathogenesis and they might provide an attractive therapeutic target. Indeed, pDC-specific therapy would not affect type I IFN production by other cell types and therefore would cause less immunosuppression than a general type I IFN blockade. However, causal evidence for the role of pDCs in SLE is lacking and awaits further genetic studies in experimental models of pDC lineage ablation.
Conclusions and future directions
Although the experimental evidence reviewed above is obviously incomplete, some preliminary conclusions can be made. In each disease reviewed, substantial support exists for the pathogenic role of DCs, which drive the activation and effector differentiation of the relevant T cell populations (FIG. 1). In addition, some DC-based mechanisms, such as those involving type I IFN production by pDCs, might represent common mechanisms that lead to pathogenesis in autoimmune diseases as distinct as psoriasis, SLE and T1D (FIG. 2). Conversely, the evidence for the tolerogenic roles of DCs is less robust in most diseases other than EAE, which is an inducible model in which T cells are primed in the periphery but function in a unique, privileged tissue. Thus, the role of DCs in preventing autoimmunity might be confined to the earliest stages of spontaneous disease, at a time when this cannot be prospectively shown. One major challenge for the field is to extend the studies in inducible models, such as EAM, EAE and imiquimod-induced psoriasis, to spontaneous monogenic or (better yet) multigenic models (BOX 2). An even bigger challenge is to translate the findings in these models to the infinitely more complex human diseases. Although the possibility of DC-based therapies for autoimmune diseases does not appear to be close at hand, a better understanding of DC functions in autoimmunity would bring it closer to practice.
Box 2. Key questions and future directions for the field.
What is the specific role of individual dendritic cell (DC) subsets (as opposed to the general population of DCs) in human autoimmune disease and in spontaneous multigenic animal models of autoimmunity?
Are ‘tolerogenic DCs’ a developmentally and genetically distinct subset, a functional state or an artificial (albeit useful) cell type?
Is there a ‘special relationship’ between DCs and regulatory T (TReg) cells that differs from the relationship of DCs with effector T cells? What is the role of DCs in TReg cell induction and TReg cell-mediated suppression of T effector cell function?
What are the environmental factors and cell-extrinsic signals that lead to abnormal DC activation and to the ensuing breach of tolerance?
Are there DC-specific cell-intrinsic regulators that prevent DC activation and autoimmunity?
Does the self-nucleic-acid-induced type I interferon production by plasmacytoid DCs have a causative role in autoimmune diseases? Can it work in reverse to oppose autoimmunity — for example, in multiple sclerosis?
The answers to these questions would provide not only essential insights into the causes and mechanisms of autoimmunity, but also potential targets for rational therapeutic approaches.
Acknowledgements
The authors thank M. Anderson and A. Ma for communicating unpublished results. The authors apologize to many colleagues whose studies could not be cited because of space constraints. B.R. has been supported by the Lupus Research Institute, the New York State Department of Health IDEA award N09G-22 and the US National Institutes of Health grant AI072571; V.S. has been supported by the Cancer Research Institute; D.G. has been supported by the S.L.E. Lupus Foundation, USA; and S.H. has been supported by the Swiss National Science Foundation.
Glossary
- Type I interferon
(Type I IFN). A family of cytokines that comprises IFNβ and multiple subtypes of IFNα, which all signal through a common IFNα/β receptor (IFNAR). Type I IFNs are typically induced by viral infection and can confer an antiviral state growth arrest and/or apoptosis in host cells, as well as being able to recruit and to activate multiple immune cell types
- Cross-presentation
The ability of certain antigen-presenting cells to load peptides that are derived from exogenous antigens on MHC class I molecules. This property is uncommon, as most cells exclusively present peptides from their endogenous proteins on MHC class I molecules. Cross-presentation is essential for the initiation of immune responses to viruses that do not infect antigen-presenting cells
- DC ablation
The process by which denditic cells (DCs) are depleted; for example using diphtheria toxin expression or administration. Diphtheria toxin or the diphtheria toxin receptor can be specifically expressed in DCs, which facilitates constitutive or diphtheria toxin-inducible DC ablation, respectively. Limitations to this method include the potential depletion of other cell types, the nonspecific toxicity of the administered diphtheria toxin and secondary effects owing to substantial cell death
- FMS-related tyrosine kinase 3 ligand
(FLT3L). An important growth factor in dendritic cell (DC) development. Its receptor FLT3 is expressed on haematopoietic progenitor cells as well as on all DCs, and FLT3L administration causes uneven expansion of most DC subsets
- Type 1 diabetes
(T1D also known as insulin-dependent or juvenile diabetes). A disease that arises in children and young adults and that is caused by the destruction of pancreatic insulin-producing β-islet cells. It is thought that β-islet cell destruction is mediated by CD4+ and CD8+ T cells that are specific to β-islet cell autoantigens, such as insulin, zinc transporter 8 (ZNT8), islet-specific glucose-6-phospatase catalytic subunit-related protein (IGRP) and chromogranin A. Inbred non-obese diabetic (NOD) mice represent an excellent spontaneous model of T1D, a disease which is controlled by multiple genetic loci
- Systemic lupus erythematosus
(SLE). A disease characterized by the production of autoantibodies against self-DNA, chromatin, and RNA-associated proteins. The resulting immune complexes are deposited and induce inflammation in multiple tissues, particularly in kidney glomeruli (glomerulonephritis). Mouse models of spontaneous SLE include: the NZB/NZW strain and its derivative strains; MRL–FasLpr mice with a mutation in the death receptor CD95; and models with overexpression of Toll-like receptor 7 (TLR7; for example, Yaa locus-containing strains and Tlr7-transgenic mice)
- Autoimmune myocarditis
A form of heart inflammation that is commonly associated with dilated cardiomyopathy, which is the most common cause of heart failure in young adults. It can be modelled in mice with experimental autoimmune myocarditis (EAM), which is induced by immunization with cardiac proteins such as the α-myosin heavy chain
- Multiple sclerosis
A disease that is most frequent in young female adults as a relapsing-remitting disease. It involves inflammation and focal neurodegeneration of the white matter of the central nervous system, which results from an autoimmune response to the components of the myelin sheath
- Experimental autoimmune encephalomyelitis
(EAE). A classical induced model of multiple sclerosis. EAE can be either actively induced by immunization against proteins of the myelin sheath, or passively induced by the adoptive transfer of encephalitogenic T helper cells. Transgenic models of EAE expressing myelin-specific T cell receptors are also widely studied
- Psoriasis
A chronic inflammation of the skin that is characterized by vascular hyperplasia and keratinocyte hyperproliferation, and is accompanied by a local pro-inflammatory milieu and immune cell infiltration. Mouse models typically recapitulate only some aspects of the disease and include genetic manipulation or chemical activation of immune responses (for example, with the Toll-like receptor 7 agonist imiquimod)
Footnotes
Competing interests statement
The authors declare no competing financial interests.
References
- 1.Steinman RM. Decisions about dendritic cells: past, present, and future. Annu. Rev. Immunol. 2012;30:1–22. doi: 10.1146/annurev-immunol-100311-102839. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto D, Miller J, Merad M. Dendritic cell and macrophage heterogeneity in vivo . Immunity. 2011;35:323–335. doi: 10.1016/j.immuni.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satpathy AT, Wu X, Albring JC, Murphy KM. Re (de)fining the dendritic cell lineage. Nature Immunol. 2012;13:1145–1154. doi: 10.1038/ni.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lewis KL, Reizis B. Dendritic cells: arbiters of immunity and immunological tolerance. Cold Spring Harb. Perspect. Biol. 2012;4:a007401. doi: 10.1101/cshperspect.a007401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collin M, Bigley V, Haniffa M, Hambleton S. Human dendritic cell deficiency: the missing ID? Nature Rev. Immunol. 2011;11:575–583. doi: 10.1038/nri3046. [DOI] [PubMed] [Google Scholar]
- 6.Segura E, et al. Human inflammatory dendritic cells induce Th17 cell differentiation. Immunity. 2013;38:336–348. doi: 10.1016/j.immuni.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Segura E, et al. Characterization of resident and migratory dendritic cells in human lymph nodes. J. Exp. Med. 2012;209:653–660. doi: 10.1084/jem.20111457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olsson T, Holmdahl R, Klareskog L, Forsum U. la-expressing cells and T lymphocytes of different subsets in peripheral nerve tissue during experimental allergic neuritis in Lewis rats. Scand. J. Immunol. 1983;18:339–343. doi: 10.1111/j.1365-3083.1983.tb01805.x. [DOI] [PubMed] [Google Scholar]
- 9. Knight SC, Mertin J, Stackpoole A, Clark J. Induction of immune responses in vivo with small numbers of veiled (dendritic) cells. Proc. Natl Acad. Sci. USA. 1983;80:6032–6035. doi: 10.1073/pnas.80.19.6032. This paper shows that ‘veiled cells’ (that is, DCs) from animals with EAE could transfer the disease to naive recipients, which establishes the capacity of DCs to prime autoreactive T cell responses.
- 10.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J. Exp. Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hubert FX, et al. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–2472. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- 12.Lei Y, et al. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J. Exp. Med. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klein L, Hinterberger M, von Rohrscheidt J, Aichinger M. Autonomous versus dendritic cell-dependent contributions of medullary thymic epithelial cells to central tolerance. Trends Immunol. 2011;32:188–193. doi: 10.1016/j.it.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 14. Birnberg T, et al. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. This paper shows that the constitutive ablation of cDCs does not breach central or peripheral tolerance or induce overt autoimmunity.
- 15.Bonasio R, et al. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nature Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 16.Proietto AI, et al. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc. Natl Acad. Sci. USA. 2008;105:19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hawiger D, et al. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo . J. Exp. Med. 2001;194:769–779. doi: 10.1084/jem.194.6.769. This study pioneered antibody-mediated antigen targeting to demonstrate the capacity of DCs to induce peripheral T cell tolerance.
- 18.Hawiger D, Masilamani RF, Bettelli E, Kuchroo VK, Nussenzweig MC. Immunological unresponsiveness characterized by increased expression of CD5 on peripheral T cells induced by dendritic cells in vivo . Immunity. 2004;20:695–705. doi: 10.1016/j.immuni.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 19. Probst HC, Lagnel J, Kollias G, van den Broek M. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8+ T cell tolerance. Immunity. 2003;18:713–720. doi: 10.1016/s1074-7613(03)00120-1. This paper further shows the tolerogenic capacity of DCs in the steady state by genetically targeting antigens to DCs.
- 20.Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nature Immunol. 2005;6:280–286. doi: 10.1038/ni1165. [DOI] [PubMed] [Google Scholar]
- 21.Muth S, Schutze K, Schild H, Probst HC. Release of dendritic cells from cognate CD4+ T-cell recognition results in impaired peripheral tolerance and fatal cytotoxic T-cell mediated autoimmunity. Proc. Natl Acad. Sci. USA. 2012;109:9059–9064. doi: 10.1073/pnas.1110620109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamazaki S, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sela U, Olds P, Park A, Schlesinger SJ, Steinman RM. Dendritic cells induce antigen-specific regulatory T cells that prevent graft versus host disease and persist in mice. J. Exp. Med. 2011;208:2489–2496. doi: 10.1084/jem.20110466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suffner J, et al. Dendritic cells support homeostatic expansion of Foxp3+ regulatory T cells in Foxp3. LuciDTR mice. J. Immunol. 2010;184:1810–1820. doi: 10.4049/jimmunol.0902420. [DOI] [PubMed] [Google Scholar]
- 25.Bar-On L, Birnberg T, Kim KW, Jung S. Dendritic cell-restricted CD80/86 deficiency results in peripheral regulatory T-cell reduction but is not associated with lymphocyte hyperactivation. Eur. J. Immunol. 2011;41:291–298. doi: 10.1002/eji.201041169. [DOI] [PubMed] [Google Scholar]
- 26.Vitali C, et al. Migratory, and not lymphoid-resident, dendritic cells maintain peripheral self-tolerance and prevent autoimmunity via induction of iTreg cells. Blood. 2012;120:1237–1245. doi: 10.1182/blood-2011-09-379776. [DOI] [PubMed] [Google Scholar]
- 27.Darrasse-Jeze G, et al. Feedback control of regulatory T cell homeostasis by dendritic cells in vivo . J. Exp. Med. 2009;206:1853–1862. doi: 10.1084/jem.20090746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swee LK, Bosco N, Malissen B, Ceredig R, Rolink A. Expansion of peripheral naturally occurring T regulatory cells by Fms-like tyrosine kinase 3 ligand treatment. Blood. 2009;113:6277–6287. doi: 10.1182/blood-2008-06-161026. [DOI] [PubMed] [Google Scholar]
- 29.Collins CB, et al. Flt3 ligand expands CD103+ dendritic cells and FoxP3+ T regulatory cells, and attenuates Crohn’s-like murine ileitis. Gut. 2011;61:1154–1162. doi: 10.1136/gutjnl-2011-300820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kriegel MA, Rathinam C, Flavell RA. Pancreatic islet expression of chemokine CCL2 suppresses autoimmune diabetes via tolerogenic CD11c+ CD11b+ dendritic cells. Proc. Natl Acad. Sci. USA. 2012;109:3457–3462. doi: 10.1073/pnas.1115308109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edelson BT, et al. Peripheral CD103+ dendritic cells form a unified subset developmentally related to CD8α+ conventional dendritic cells. J. Exp. Med. 2010;207:823–836. doi: 10.1084/jem.20091627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis KL, et al. Notch2 receptor signaling controls functional differentiation of dendritic cells in the spleen and intestine. Immunity. 2011;35:780–791. doi: 10.1016/j.immuni.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zangi L, et al. Deletion of cognate CD8 T cells by immature dendritic cells: a novel role for perforin, granzyme A, TREM-1, and TLR7. Blood. 2012;120:1647–1657. doi: 10.1182/blood-2012-02-410803. [DOI] [PubMed] [Google Scholar]
- 34.Ohnmacht C, et al. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J. Exp. Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teichmann LL, et al. Dendritic cells in lupus are not required for activation of T and B cells but promote their expansion, resulting in tissue damage. Immunity. 2010;33:967–978. doi: 10.1016/j.immuni.2010.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cervantes-Barragan L, et al. Plasmacytoid dendritic cells control T-cell response to chronic viral infection. Proc. Natl Acad. Sci. USA. 2012;109:3012–3017. doi: 10.1073/pnas.1117359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen M, et al. Dendritic cell apoptosis in the maintenance of immune tolerance. Science. 2006;311:1160–1164. doi: 10.1126/science.1122545. [DOI] [PubMed] [Google Scholar]
- 38.Stranges PB, et al. Elimination of antigen-presenting cells and autoreactive T cells by Fas contributes to prevention of autoimmunity. Immunity. 2007;26:629–641. doi: 10.1016/j.immuni.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen M, Felix K, Wang J. Immune regulation through mitochondrion-dependent dendritic cell death induced by T regulatory cells. J. Immunol. 2011;187:5684–5692. doi: 10.4049/jimmunol.1101834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Travis MA, et al. Loss of integrin αvβ8 on dendritic cells causes autoimmunity and colitis in mice. Nature. 2007;449:361–365. doi: 10.1038/nature06110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melillo JA, et al. Dendritic cell (DC)-specific targeting reveals Stat3 as a negative regulator of DC function. J. Immunol. 2010;184:2638–2645. doi: 10.4049/jimmunol.0902960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim SJ, Zou YR, Goldstein J, Reizis B, Diamond B. Tolerogenic function of Blimp-1 in dendritic cells. J. Exp. Med. 2011;208:2193–2199. doi: 10.1084/jem.20110658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hammer GE, et al. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nature Immunol. 2011;12:1184–1193. doi: 10.1038/ni.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kool M, et al. The ubiquitin-editing protein A20 prevents dendritic cell activation, recognition of apoptotic cells, and systemic autoimmunity. Immunity. 2011;35:82–96. doi: 10.1016/j.immuni.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 45.Kaneko T, et al. Dendritic cell-specific ablation of the protein tyrosine phosphatase Shp1 promotes Th1 cell differentiation and induces autoimmunity. J. Immunol. 2012;188:5397–5407. doi: 10.4049/jimmunol.1103210. [DOI] [PubMed] [Google Scholar]
- 46.Abram CL, Roberge GL, Pao LI, Neel BG, Lowell CA. Distinct roles for neutrophils and dendritic cells in inflammation and autoimmunity in motheaten mice. Immunity. 2013;38:489–501. doi: 10.1016/j.immuni.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eriksson U, et al. Dendritic cell-induced autoimmune heart failure requires cooperation between adaptive and innate immunity. Nature Med. 2003;9:1484–1490. doi: 10.1038/nm960. [DOI] [PubMed] [Google Scholar]
- 48.Eriksson U, et al. Activation of dendritic cells through the interleukin 1 receptor 1 is critical for the induction of autoimmune myocarditis. J. Exp. Med. 2003;197:323–331. doi: 10.1084/jem.20021788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sonderegger I, et al. GM-CSF mediates autoimmunity by enhancing IL-6-dependent Th17 cell development and survival. J. Exp. Med. 2008;205:2281–2294. doi: 10.1084/jem.20071119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pagni PP, Traub S, Demaria O, Chasson L, Alexopoulou L. Contribution of TLR7 and TLR9 signaling to the susceptibility of MyD88-deficient mice to myocarditis. Autoimmunity. 2010;43:275–287. doi: 10.3109/08916930903509056. [DOI] [PubMed] [Google Scholar]
- 51.Popovic ZV, et al. The proteoglycan biglycan enhances antigen-specific T cell activation potentially via MyD88 and TRIF pathways and triggers autoimmune perimyocarditis. J. Immunol. 2011;187:6217–6226. doi: 10.4049/jimmunol.1003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nature Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 53.Greter M, et al. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nature Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 54.Wu GF, et al. Limited sufficiency of antigen presentation by dendritic cells in models of central nervous system autoimmunity. J. Autoimmun. 2011;36:56–64. doi: 10.1016/j.jaut.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yogev N, et al. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor+ regulatory T cells. Immunity. 2012;37:264–275. doi: 10.1016/j.immuni.2012.05.025. This paper shows the overall anti-inflammatory role of cDCs in EAE using various DC ablation methods.
- 56.Isaksson M, Lundgren BA, Ahlgren KM, Kampe O, Lobell A. Conditional DC depletion does not affect priming of encephalitogenic Th cells in EAE. Eur. J. Immunol. 2012;42:2555–2563. doi: 10.1002/eji.201142239. [DOI] [PubMed] [Google Scholar]
- 57.Huang G, et al. Signaling via the kinase p38α programs dendritic cells to drive TH17 differentiation and autoimmune inflammation. Nature Immunol. 2012;13:152–161. doi: 10.1038/ni.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melton AC, et al. Expression of αvβ8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J. Clin. Invest. 2010;120:4436–4444. doi: 10.1172/JCI43786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laouar Y, et al. TGF-β signaling in dendritic cells is a prerequisite for the control of autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA. 2008;105:10865–10870. doi: 10.1073/pnas.0805058105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao S, et al. Tim-1 stimulation of dendritic cells regulates the balance between effector and regulatory T cells. Eur. J. Immunol. 2011;41:1539–1549. doi: 10.1002/eji.201040993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. J. Clin. Invest. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dann A, et al. Cytosolic RIG-I-like helicases act as negative regulators of sterile inflammation in the CNS. Nature Neurosci. 2012;15:98–106. doi: 10.1038/nn.2964. [DOI] [PubMed] [Google Scholar]
- 63.Yen JH, Kong W, Ganea D. IFN-β inhibits dendritic cell migration through STAT-1-mediated transcriptional suppression of CCR7 and matrix metalloproteinase 9. J. Immunol. 2010;184:3478–3486. doi: 10.4049/jimmunol.0902542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Anandasabapathy N, et al. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J. Exp. Med. 2011;208:1695–1705. doi: 10.1084/jem.20102657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Prodinger C, et al. CD11c–expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol. 2011;121:445–458. doi: 10.1007/s00401-010-0774-y. [DOI] [PubMed] [Google Scholar]
- 66.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ TH-17 cells in relapsing EAE. Nature Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 67.Poppensieker K, et al. CC chemokine receptor 4 is required for experimental autoimmune encephalomyelitis by regulating GM-CSF and IL-23 production in dendritic cells. Proc. Natl Acad. Sci. USA. 2012;109:3897–3902. doi: 10.1073/pnas.1114153109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Codarri L, et al. RORyt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nature Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 69.El-Behi M, et al. The encephalitogenicity of TH17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nature Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bailey-Bucktrout SL, et al. Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis. J. Immunol. 2008;180:6457–6461. doi: 10.4049/jimmunol.180.10.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Irla M, et al. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J. Exp. Med. 2010;207:1891–1905. doi: 10.1084/jem.20092627. Genetic ablation of MHC class II expression specifically in pDCs shows that pDCs might promote TReg cell development and they might inhibit inflammation in EAE.
- 72.Karni A, et al. Innate immunity in multiple sclerosis: myeloid dendritic cells in secondary progressive multiple sclerosis are activated and drive a proinflammatory immune response. J. Immunol. 2006;177:4196–4202. doi: 10.4049/jimmunol.177.6.4196. [DOI] [PubMed] [Google Scholar]
- 73.Cai Y, et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pantelyushin S, et al. Roryt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J. Clin. Invest. 2012;122:2252–2256. doi: 10.1172/JCI61862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van der Fits L, et al. Imiquimod-induced psoriasislike skin inflammation in mice is mediated via the IL-23/IL-17 axis. J. Immunol. 2009;182:5836–5845. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 76.Walter A, et al. Aldara activates TLR7-independent immune defence. Nature Commun. 2013;4:1560. doi: 10.1038/ncomms2566. [DOI] [PubMed] [Google Scholar]
- 77.Tortola L, et al. Psoriasiform dermatitis is driven by IL-36-mediated DC-keratinocyte crosstalk. J. Clin. Invest. 2012;122:3965–3976. doi: 10.1172/JCI63451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nestle FO, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Albanesi C, et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J. Exp. Med. 2009;206:249–258. doi: 10.1084/jem.20080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Guiducci C, et al. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J. Exp. Med. 2010;207:2931–2942. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gregorio J, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J. Exp. Med. 2010;207:2921–2930. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lande R, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 83.Ganguly D, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009;206:1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Jersey J, et al. β-cells cannot directly prime diabetogenic CD8 T cells in nonobese diabetic mice. Proc. Natl Acad. Sci. USA. 2007;104:1295–1300. doi: 10.1073/pnas.0610057104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Turley S, Poirot L, Hattori M, Benoist C, Mathis D. Physiological β-cell death triggers priming of self-reactive T cells by dendritic cells in a type-1 diabetes model. J. Exp. Med. 2003;198:1527–1537. doi: 10.1084/jem.20030966. This paper established the role of DCs in the presentation of β-islet cell antigens to self-reactive T cells in experimental diabetes.
- 86.Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J. Immunol. 2007;179:5041–5053. doi: 10.4049/jimmunol.179.8.5041. [DOI] [PubMed] [Google Scholar]
- 87.Calderon B, Suri A, Miller MJ, Unanue ER. Dendritic cells in islets of Langerhans constitutively present β-cell-derived peptides bound to their class II MHC molecules. Proc. Natl Acad. Sci. USA. 2008;105:6121–6126. doi: 10.1073/pnas.0801973105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Han J, et al. Extracellular high-mobility group box 1 acts as an innate immune mediator to enhance autoimmune progression and diabetes onset in NOD mice. Diabetes. 2008;57:2118–2127. doi: 10.2337/db07-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim HS, et al. Toll-like receptor 2 senses β-cell death and contributes to the initiation of autoimmune diabetes. Immunity. 2007;27:321–333. doi: 10.1016/j.immuni.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 90.Lee LF, et al. The role of TNF-α in the pathogenesis of type 1 diabetes in the nonobese diabetic mouse: analysis of dendritic cell maturation. Proc. Natl Acad. Sci. USA. 2005;102:15995–16000. doi: 10.1073/pnas.0508122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Van Belle TL, Nierkens S, Arens R, von Herrath MG. Interleukin-21 receptor-mediated signals control autoreactive T cell infiltration in pancreatic islets. Immunity. 2012;36:1060–1072. doi: 10.1016/j.immuni.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huang X, Hultgren B, Dybdal N, Stewart TA. Islet expression of interferon-α precedes diabetes in both the BB rat and streptozotocin-treated mice. Immunity. 1994;1:469–478. doi: 10.1016/1074-7613(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 93.Huang X, et al. Interferon expression in the pancreases of patients with type I diabetes. Diabetes. 1995;44:658–664. doi: 10.2337/diab.44.6.658. [DOI] [PubMed] [Google Scholar]
- 94.Li Q, et al. Interferon- α initiates type 1 diabetes in nonobese diabetic mice. Proc. Natl Acad. Sci. USA. 2008;105:12439–12444. doi: 10.1073/pnas.0806439105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Diana J, et al. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nature Med. 2013;19:65–73. doi: 10.1038/nm.3042. [DOI] [PubMed] [Google Scholar]
- 96.Allen JS, et al. Plasmacytoid dendritic cells are proportionally expanded at diagnosis of type 1 diabetes and enhance islet autoantigen presentation to T-cells through immune complex capture. Diabetes. 2009;58:138–145. doi: 10.2337/db08-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chilton PM, et al. Flt3-ligand treatment prevents diabetes in NOD mice. Diabetes. 2004;53:1995–2002. doi: 10.2337/diabetes.53.8.1995. [DOI] [PubMed] [Google Scholar]
- 98.O’Keeffe M, et al. Fms-like tyrosine kinase 3 ligand administration overcomes a genetically determined dendritic cell deficiency in NOD mice and protects against diabetes development. Int. Immunol. 2005;17:307–314. doi: 10.1093/intimm/dxh210. [DOI] [PubMed] [Google Scholar]
- 99.Kared H, et al. Treatment with granulocyte colony-stimulating factor prevents diabetes in NOD mice by recruiting plasmacytoid dendritic cells and functional CD4+CD25+ regulatory T-cells. Diabetes. 2005;54:78–84. doi: 10.2337/diabetes.54.1.78. [DOI] [PubMed] [Google Scholar]
- 100.Grupillo M, et al. Essential roles of insulin expression in Aire+ tolerogenic dendritic cells in maintaining peripheral self-tolerance of islet β-cells. Cell. Immunol. 2012;273:115–123. doi: 10.1016/j.cellimm.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 101.Walsh ER, et al. Dual signaling by innate and adaptive immune receptors is required for TLR7-induced B-cell-mediated autoimmunity. Proc. Natl Acad. Sci. USA. 2012;109:16276–16281. doi: 10.1073/pnas.1209372109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bennett L, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santiago-Raber ML, et al. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J. Exp. Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jorgensen TN, Roper E, Thurman JM, Marrack P, Kotzin BL. Type I interferon signaling is involved in the spontaneous development of lupuslike disease in B6.Nba2 and (B6.Nba2 × NZW)F(1) mice. Genes Immun. 2007;8:653–662. doi: 10.1038/sj.gene.6364430. [DOI] [PubMed] [Google Scholar]
- 105.Ronnblom L, Alm GV. A pivotal role for the natural interferon-a-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J. Exp. Med. 2001;194:F59–F63. doi: 10.1084/jem.194.12.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blomberg S, et al. Presence of cutaneous interferon-α producing cells in patients with systemic lupus erythematosus. Lupus. 2001;10:484–490. doi: 10.1191/096120301678416042. [DOI] [PubMed] [Google Scholar]
- 107.Bave U, et al. Fcy RIIa is expressed on natural IFN-α-producing cells (plasmacytoid dendritic cells) and is required for the IFN-α production induced by apoptotic cells combined with lupus IgG. J. Immunol. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 108.Means TK, et al. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tian J, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nature Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 110.Lande R, et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001180. 73ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Garcia-Romo GS, et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011;3 doi: 10.1126/scitranslmed.3001201. 73ra20. References 110 and 111 provide evidence that pDC activation by neutrophil-derived self-DNA molecules promotes the development of SLE in humans.
- 112.Guiducci C, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lepelletier Y, et al. Toll-like receptor control of glucocorticoid-induced apoptosis in human plasmacytoid predendritic cells (pDCs) Blood. 2010;116:3389–3397. doi: 10.1182/blood-2010-05-282913. [DOI] [PubMed] [Google Scholar]
- 114.Zhu XJ, Yang ZF, Chen Y, Wang J, Rosmarin AG. PU.1 is essential for CD11c expression in CD8+/CD8− lymphoid and monocyte-derived dendritic cells during GM-CSF or FLT3L–induced differentiation. PLoS ONE. 2013;7:e52141. doi: 10.1371/journal.pone.0052141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8 dendritic cells in the spleen. J. Exp. Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Persson EK, et al. IRF4 transcription-factor-dependent CD103+CD11b+ dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38:958–969. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 117.Schlitzer A, et al. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wohn C, et al. Langerinneg conventional dendritic cells produce IL-23 to drive psoriatic plaque formation in mice. Proc. Natl Acad. Sci. USA. 2013;110:10723–10728. doi: 10.1073/pnas.1307569110. [DOI] [PMC free article] [PubMed] [Google Scholar]