Abstract
Plasmacytoid dendritic cells (pDCs) are specialized in rapid and massive secretion of type I interferon (IFN-α/β) in response to foreign nucleic acids. Combined with their antigen presentation capacity, this powerful functionality enables pDCs to orchestrate innate and adaptive immune responses. pDCs combine features of both lymphocytes and classical dendritic cells and display unique molecular adaptations to nucleic acid sensing and IFN production. In the decade since the identification of the pDC as a distinct immune cell type, our understanding of its molecular underpinnings and role in immunity has progressed rapidly. Here we review select aspects of pDC biology including cell fate establishment and plasticity, specific molecular mechanisms of pDC function, and the role of pDCs in T cell responses, antiviral immunity, and autoimmune diseases. Important unresolved questions remain in these areas, promising exciting times in pDC research for years to come.
Keywords: type I interferon, dendritic cells, innate immunity, antigen presentation
NOTE FROM THE AUTHORS
Plasmacytoid dendritic cells (pDCs) are one of the most recent additions to the palette of immune cell types. Since the first unequivocal characterization of pDCs in 1999, our knowledge about these cells and the associated volume of publications increased dramatically (from 41 references in 2001 to 728 in 2009). The first and only review on pDCs in this series was published in 2005 (1), and nearly all key aspects of pDC biology have been recently reviewed elsewhere (2–7). Given the vastness of material, the availability of recent reviews, and the extraordinary pace of progress, a comprehensive overview of the field is neither feasible nor necessary. Instead, we highlight only several key aspects of pDC biology and function that are incompletely understood and require further elucidation. More than anything else, we would like this review to provide grounds for discussion and future investigation. As an unfortunate side effect of this selective task, we are unable to cover many important primary contributions, for which we apologize to their authors.
INTRODUCTION
Brief Historical Perspective
The primary function and unique property of pDCs is the secretion of type I interferon (IFN-α/β) in response to viruses and/or virus-derived nucleic acids. Pioneering studies by Alm and colleagues (8, 9), Fitzgerald-Bocarsly and colleagues (10, 11), and Trinchieri and colleagues (12, 13) identified and partially characterized a specific minor subset of human peripheral blood leukocytes responsible for high-level IFN production. Independently, Facchetti et al. (14, 15) built on the early observations by Lennert (16) to characterize secretory cells termed plasmacytoid mono-cytes, which accumulated in human reactive lymph nodes and sites of inflammation. In 1997, Liu et al. (17) showed that these plasma-cytoid cells efficiently generated dendritic cells (DCs) in vitro, designating them as type 2 DC precursors, or pre-DC2. Finally, in 1999 the Liu (18) and Colonna (19) groups demonstrated that plasmacytoid monocytes, pre-DC2, and natural IFN-producing cells were in fact the same cellular entity, the pDC. These converging lines of investigation reflect the key properties of pDCs: powerful IFN production, secretory plasmacytoid (i.e., plasma cell–like) morphology, and the ability to differentiate into conventional or classical DCs (cDCs). Subsequently, the murine counterparts of human pDCs were identified (20–22), and in vitro derivation of pDCs from human and murine hematopoietic progenitors was established (23, 24). These advances led to deep genetic and mechanistic insights into pDC development and function, including the most recent genome-wide expression (25) and proteomic (26) analyses.
Essential Features of pDCs
Despite certain molecular differences, the function, overall phenotype, and core gene expression program (25) of the murine and human pDCs are conserved. pDCs are rare (0.3–0.5% of the human peripheral blood or of murine lymphoid organs) cells that develop in the bone marrow and reside primarily in the lymphoid organs in the steady state, entering the lymph nodes from the blood (7, 27). pDCs have the round morphology of a secretory lymphocyte, turn over relatively slowly (28, 29), and express low levels of MHC class II and costimulatory molecules. pDCs are low (mouse) or negative (human) for the integrin CD11c but positive for the B cell marker B220/CD45RA. Notably, these features of steady-state pDCs are similar to those of lymphocytes but are distinct from those of cDCs [see sidebar, Classical or Conventional DCs (cDCs)]. Several relatively pDC-specific surface markers have been established, such as human blood dendritic cell antigen (BDCA)-2 and ILT7 (immunoglobulin-like transcript 7) and murine SiglecH and Bst2; other useful (albeit less specific) markers include human IL-3Rα (CD123) and BDCA-4 and murine Ly6C and Ly49Q.
pDCs express endosomal nucleic acid– sensing Toll-like receptors (TLRs) TLR7 and TLR9 and respond to the respective ligands, single-stranded RNA, and unmethylated CpG-containing DNA (CpG). The most distinct pDC response to these stimuli is rapid and abundant IFN secretion, which can be up to 1,000-fold more potent than in other cell types (1). In fact, IFN-α secretion in response to CpG challenge in vivo is mediated exclusively by pDCs, as suggested by antibody-mediated (31) and genetic ablation (32) and IFN reporter strain analysis (33). Other consequences of TLR-induced pDC activation include the secretion of cytokines such as TNF-α and (in the mouse) IL-12 and the acquisition of antigen presentation ability. Altogether, these powerful immunostimulatory functions of pDCs contribute to the recruitment and/or activation of nearly all immune cell types [e.g., natural killer (NK) cells (34) and plasma cells (35)], establishing pDCs as a key link between innate and adaptive immunity.
Practical Aspects of pDC Study
Given the rarity of pDCs and the complexity of their phenotype, caution is needed in their definition and functional analysis. For example, the definition of pDCs as CD11clow B220+ is clearly misleading, as it includes multiple additional cell types such as NK-like cells (36, 37) and cDC progenitors (38). Conversely, the use of a single pDC-specific marker may be equally problematic. For instance, the widely used murine pDC marker Bst2/mPDCA-1 is also expressed on plasma cells and is broadly inducible upon activation (39). Furthermore, the isolation of human pDCs based on their specific marker BDCA-2 (an inhibitory receptor) impairs their IFN production capacity. To boost the frustratingly low pDC numbers in mice, many studies inject a Flt3 ligand (Flt3L)-expressing melanoma cell line (40). This approach should be discouraged because the resulting gross tumor and supraphysiological Flt3L levels likely affect pDC ground state and functionality. Another practical consideration involves gene targeting in mice: Some Crerecombinase–expressing deleter strains such as the T cell–specific CD4-Cre strain mediate efficient off-target recombination in pDCs (41). Thus, a careful analysis of recombination in pDCs is required to exclude its potential contribution to the phenotype in conditional targeting experiments.
THE DEVELOPMENT OF pDCs
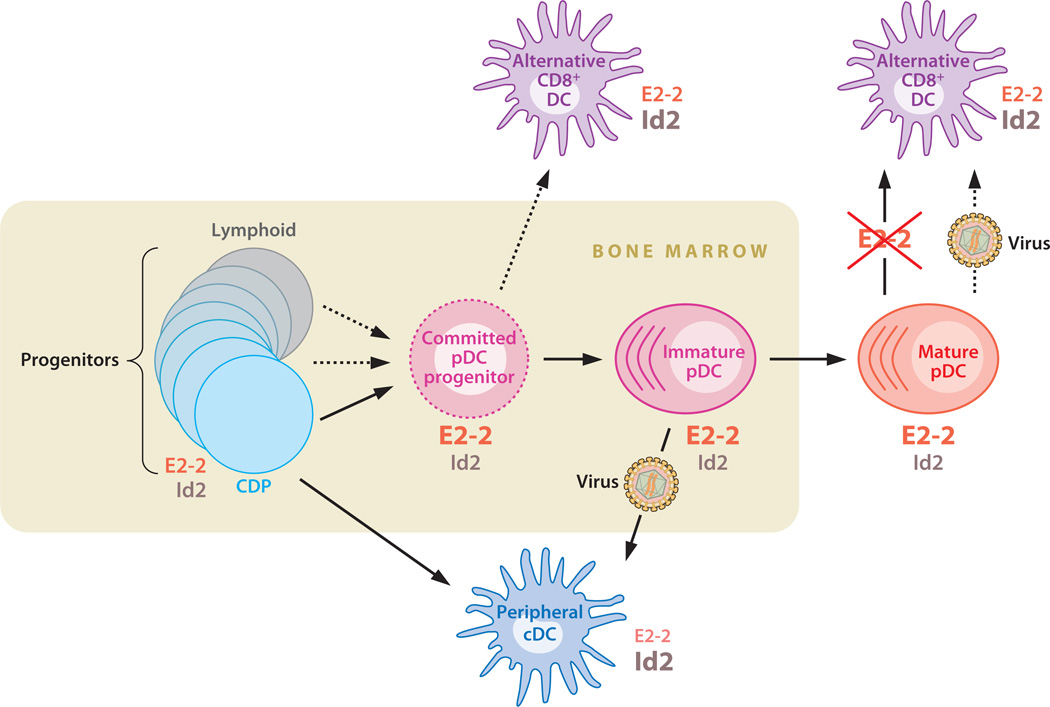
We have recently reviewed the developmental origin and transcriptional control of the pDC lineage (6). Here we provide a brief update and highlight several issues that await resolution in future studies. Some of the points discussed below are incorporated into the proposed scheme of pDC development (Figure 1).
Figure 1.
The proposed scheme of pDC development and relationship with cDCs. pDCs develop in the bone marrow from a continuum of Flt3+ c-Kitlow progenitors including lymphoid progenitors (dark gray) and common DC progenitors (CDPs, light blue). The development proceeds through the putative committed pDC progenitor (dashed magenta) and immature pDCs in the bone marrow toward the mature peripheral pDCs. The CDP gives rise to peripheral cDCs (dark blue), whereas immature bone marrow pDCs can differentiate into CD8 cDCs upon virus-induced activation. The alternative CD8+ DC ( purple) develops from pDC progenitors in the steady state; similar cells can be generated from mature pDCs after induced E2–2 deletion and possibly upon activation. The inferred relative amounts of E protein E2–2 and its inhibitor Id2 are indicated for each cell type.
pDC Progenitors
Most pDCs develop from common DC progenitors (CDP, or pro-DCs), a distinct progenitor type in the bone marrow that expresses cytokine receptors Flt3 (CD135), M-CSFR (CD115), and low levels of c-Kit (CD117) (42, 43). It is noteworthy that Flt3+ c-Kitlow is a broad definition of lymphoid progenitors, including the canonical IL-7Rα+ common lymphoid progenitor (CLP) (44); moreover, CLP can give rise to pDCs upon adoptive transfer (45). Indeed, at least a fraction of pDCs show evidence of lymphoid derivation (46, 47), as confirmed recently by single-cell genetic tracing for prior Rag1 expression (48). In a reverse tracing system, the DC/pDC-specific CD11c–Credeleter strain mediates deletion in 10–15% of lymphocytes; this fraction is similar in all lymphoid cell types (T, B, and NK cells) and in their earliest progenitors (49; B. Reizis, unpublished observations). We have located the origin of this recombination in a minor subset of CD11clow Flt3+ c-Kitlow progenitors, which appear to overlap with CDP and/or pre-DCs (50) but also contribute to lymphopoiesis as suggested by the Cre reporter tracing. Thus, pDCs may develop in a less linear fashion than suggested (51) and originate from a continuum of progenitors with DC and/or lymphoid potential. A fully committed pDC progenitor (i.e., lacking the cDC potential) remains to be identified but may reside within the Flt3+ CD11c+ Ly-6C+ population in the murine bone marrow (32).
The Role and Mechanism of Flt3L Signaling
The cytokine Flt3 ligand (Flt3L) and its receptor Flt3 are essential signals for DC and pDC development, controlling the expansion of common progenitors and peripheral DC homeostasis (52). Notably, pDCs are more dependent on Flt3 signaling than most cDCs, as the pDC population is selectively diminished in the lymphoid organs of mice with targeted Flt3 deletion (53) or with mutated nonfunctional Flt3 (54). This suggests that Flt3 signaling has a specific roleinp DCs after the common progenitor stage, possibly promoting terminal pDC differentiation or mature pDC survival. One important unresolved question has been the molecular pathway transducing Flt3L signals in pDC development. Our recent studies indicate that phosphoinositide 3-kinase (PI3K)-dependent activation of mammalian target of rapamycin (mTOR) mediates Flt3L signaling in DCs. In particular, pDC development in Flt3L–supplemented bone marrow cultures was exquisitely sensitive to the mTOR inhibitor, rapamycin, even when it was added at low concentrations or at late time points that largely spared cDC development. Conversely, PI3K hyperactivation by deletion of Pten in the bone marrow greatly accelerated Flt3L–driven cDC and pDC development in culture (55). Thus, PI3K/mTOR activation may represent a key signaling event downstream of Flt3L in DC progenitors and in the differentiating pDC.
Whereas Flt3L induces proliferative expansion of common DC/pDC progenitors (42), little or no proliferation appears to occur during and after pDC commitment. This likely explains the technical challenges of boosting pDC development in vivo: indeed, Flt3L administration expands the population of cDCs (especially the CD8+ cDC subset) to a much greater extent than pDCs (56). Similarly, Pten deletion in the bone marrow increases cDC but not pDC numbers in vivo (55). This apparently permanent quiescence distinguishes pDCs from lymphocytes and cDCs, which proliferate during development or in situ (29), respectively.
The Longevity of pDCs
Based on the relatively slow BrdU incorporation rate (28, 29), pDCs are thought to be long-lived. However, this slow uptake may be due to the above-mentioned quiescence of mature pDCs; moreover, in parabiosis experiments parabiotic partner-derived splenic pDCs disappeared very rapidly after surgical separation (29). Thus, the true life span of peripheral pDCs remains to be established through a combination of approaches. In general, the regulation of pDC survival and quiescence is poorly understood and would provide an interesting topic of future studies.
Molecular Basis of pDC Lineage Commitment
The upregulation of the basic helix-loop-helix transcription factor (E protein) E2–2 serves as a key lineage commitment event in pDC development, as E2–2-deficient hematopoietic progenitors fail to produce pDCs (32). The exact cellular stage of E2–2 induction is unknown and awaits the analysis of single-cell reporter strains for E2–2 expression. More importantly, the signals for E2–2 induction in pDCs remain obscure and may represent soluble factors, cell-associated ligands, and/or upstream transcription factors. It is noteworthy that E2–2 is expressed at significant levels in hematopoietic stem/progenitor cells and B lymphocytes; thus, a merely quantitative several-fold upregulation of E2–2 underlies pDC commitment. Importantly, pDCs show particularly low expression of the E protein inhibitor Id2, in contrast to abundant Id2 expression in T,NK, and myeloid cells (25, 32). The absence of Id2 expression likely serves as an important counterpart of E2–2 upregulation, increasing the effective E2–2 concentration in pDCs (Figure 1).
Every lineage commitment step involves the establishment of a lineage-specific gene expression program, as well as the suppression of alternative lineage programs. Our recent genome-wide analysis of E2–2 binding to promoters in the human pDC cell line (57) suggests that E2–2 is directly involved in both activities. We found that E2–2 binds to a large fraction of pDC-enriched genes, including highly pDC-specific genes such as LILRA4 (ILT7) (58) and PACSIN1 (59). In addition, E2–2 appears to bind directly to and repress several hallmark genes of the alternative cDC fate, such as ITGAX (CD11C) and ID2 itself. The transcriptional repression of Id2 by E2–2 and the post-translational inhibition of E2–2 by Id2 suggest that the two factors represent a pair of reciprocally antagonistic cell fate determinants, typical for many lineage bifurcations (e.g., Bcl6 and Blimp-1 in effector/memory lymphocyte differentiation). In addition to E/Id protein antagonism, bona fide transcriptional repressors likely modulate the decision between pDC and alternative (e.g., cDC) cell fates. One likely candidate is Bcl11a, a repressor that is prominently expressed in pDCs and required for pDC development (P. Tucker, personal communication). We found that BCL11A is a direct target of E2–2 (57), suggesting that E2–2 may promote pDC commitment in part through Bcl11a–mediated repression of cDC differentiation.
However, E2–2 likely represents only one of several transcription factors and other regulatory molecules (e.g., microRNAs) that collectively regulate different aspects of the pDC development and expression program. Some of the players are known, including pDC-enriched transcription factors Irf8 and SpiB. Both genes represent direct targets of E2–2 (32), although their own binding targets and precise role in pDC development and/or function remain to be established. Others, such as the highly pDC-enriched transcription factor Runx2 (25) and other candidates, must be characterized from scratch. Finally, it should be of interest to elucidate the epigenetic events (e.g., chromatin modifications) that accompany pDC commitment, maintenance, and activation.
A Novel pDC-Related Dendritic Cell Subset
A simple linear view of E2–2-driven pDC development recently became more complicated, as a novel pDC-related cDC subset in mice was identified using Cx3cr1 as a marker (60). Unlike the classical CD8+ cDCs, the alternative CD8-expressing Cx3cr1+ cDCs develop independently of transcription factor Batf3, do not cross-present antigen, and cannot be expanded by Flt3L. Instead, these cells carry IgH D→J rearrangements typical of pDCs, express many pDC-related genes including E2–2, and indeed fail to develop from E2–2-deficient bone marrow. On the other hand, Cx3cr1+ CD8+ cDCs do not secrete IFN and express significantly lower levels of E2–2 and other pDC-specific genes than do pDCs. Therefore, the alternative CD8+ cDCs likely split off the pDC lineage after E2–2-dependent lineage commitment and may arise from immature pDCs that failed terminal differentiation (Figure 1). This may represent either an accidental by-product of pDC development or a regulated switch between pDC and cDC lineages. Be that as it may, these data emphasize the intimate connection between pDC and cDC development and identify the likely default outcome of pDC differentiation.
The Plasticity of pDC Cell Fate
It has recently become clear that the terminally differentiated state of many cell types is actively enforced by genetic mechanisms. Thus, the removal of a single transcription factor may cause the loss of lineage identity and the transdifferentiation into an alternative cell fate. A dramatic example is the conversion of adult ovaries into testes after somatic loss of the ovary-specific transcription factor FoxL2 (61). The ultimately differentiated immune cell lineage, T cells, were recently shown to convert into NK-like cells after the deletion of Bcl11b (62). We have tested whether the pDC lineage shows similar plasticity by deleting E2–2 from mature pDCs in vivo (57). We found that E2–2-deficient mature pDCs spontaneously differentiate into cDC-like cells, acquiring a cDC phenotype, morphology, antigen-presenting capacity, and expression profile. Such differentiation is consistent with the rapid (within one week) disappearance of peripheral pDCs after E2–2 deletion (32). A similar but less pronounced drift toward the cDC phenotype was observed in E2–2+/− pDCs, likely underlying the depletion, aberrant phenotype, and impaired function of pDCs in E2–2+/− mice and human patients (32). Thus, continuous E2–2 expression is required to maintain the lineage identity of pDCs and to prevent their spontaneous differentiation into cDC-like cells.
These results demonstrate that the pDC cell fate is reversible in principle and raise the question of whether such lineage plasticity may occur in real life. Notably, the cDC-like cells derived from E2–2-deficient pDCs express CD8 yet preferentially upregulate CD8− cDC-enriched genes, resembling the alternative CD8+ cDC subset (60). Although the latter cDC subset is likely derived from early stages of pDC development rather than from mature peripheral pDCs, it may represent a common outcome of a natural or genetically induced pDC-to-cDC conversion (Figure 1). Another potential instance of such conversion may occur after pDC activation and is discussed below.
The Lineage Affiliation of pDCs
The constantly expanding variety of immune cell types can no longer be described by the simple lymphoid-versus-myeloid dichotomy, but rather comprise a continuous spectrum between the two sides. For instance, novel innate effector cell types have been described that appear similar to hematopoietic stem/progenitor cells, thus defying a conventional lineage assignment (63, 64). In particular, the development of DCs has now been recognized as a unique pathway, sharing early progenitors with monocytes and macrophages but distinct from canonical myelopoiesis (50, 51).
The close affiliation of pDCs with cDCs is supported by similar innate functions, common progenitors, a closely related gene-expression profile (59), and activation-induced differentiation into cDCs (see below). The spontaneous conversion of E2–2-deficient pDCs to cDC-like cells provides additional genetic evidence for their kinship with cDCs. Given that E proteins are key regulators of lymphopoiesis, the conspicuous lymphoid features of pDCs (such as morphology) likely result from the ongoing activity of E2–2 (6). In particular, many genes shared between pDCs and B cells, including important transcription factors SpiB, Bcl11a, and CIIta, are directly activated by E2–2 in the pDC. Thus, pDCs can be viewed as “the DCs in lymphocyte’s clothing,” exemplifying a truly complex lineage identity that evades simple definitions.
ASPECTS OF pDC FUNCTION
Pathogen-Induced Interferon Secretion
One of the most exciting questions of pDC biology is a seemingly simple one: What is the molecular basis of their uniquely powerful IFN secretion capacity? Although several plausible answers have been advanced over the years, they are likely to cover only part of the picture, and fundamental new insights can be expected. Given the recent extensive reviews on the subject (3, 65), we briefly discuss only several factors contributing to the unique pDC functionality.
Secretory morphology
Whereas human pDCs demonstrate plasmacytoid morphology with extensive rough endoplasmic reticulum (17), the latter appears less prominent in murine (20) or sheep (66) pDCs. Furthermore, murine cDCs lack secretory morphology but can produce IFN levels comparable to those of pDCs after stimulation through cytoplasmic RNA sensors (67). Indeed, XBP-1, the master regulator of secretory function, is constitutively active and required in pDCs and cDCs in mice (68). Thus, secretory morphology may be a useful adaptation for, but not the cause of, high-level IFN secretion capacity.
Expression of IRF7
Early studies using virus infection in mice proposed that IFN secretion in pDCs is independent of the IFN-α receptor (IFNAR) (69), which is required in other cell types to induce the expression of IRF7, the master regulator of IFN expression. Indeed, subsequent studies showed that naive pDCs express IRF7 in the steady state (70, 71). However, the IFNAR-mediated positive feedback is still operative in pDCs (70, 72); in particular, it is required for IFN secretion in the absence of viral replication (73). Notably, IRF7 transcript levels are only several-fold higher in murine and human pDCs than in other cell types (25). The rapid activation of IRF7 in pDCs may be facilitated by post-transcriptional mechanisms, such as the interaction with TLR signaling adaptor MyD88 (74). Thus, baseline IRF7 expression undoubtedly primes pDCs for rapid IFN secretion but appears insufficient to explain its pDC-specific nature.
TLR signaling
The TLR7/9-dependent pathway appears to be a predominant mode of nucleic acid sensing in pDCs, although additional DNA sensors such as DHX9/DHX36 have been proposed recently (75). TLR9 signaling by IFN-inducing large multimeric (type A) CpG oligonucleotides occurs in early endosomes, whereas monomeric (type B) CpG are transferred to endolysosomes and fail to induce IFN (74, 76). pDCs are uniquely capable of retaining type A CpG in the early endocytic compartment, thereby enabling IFN induction through TLR9/MyD88/IRF7 complexes (74). Notably, CpG A complexes with lipids are retained in endosomes and enable IFN secretion by bone marrow–derived cDCs in vitro (74), although pDCs remain the only IFN producers in response to these complexes in vivo (33). Therefore, a distinct endosomal trafficking/retention mechanism likely plays a critical role in the IFN secretion capacity of pDCs.
Recently, TLR9 signals that induce IFN (as opposed to interleukin-12) were shown to originate in a distinct endosomal compartment, the lysosome-related organelles (77). The trafficking of TLR9 to this compartment requires the adaptor protein 3 (AP-3) complex, and indeed CpG- or virus-induced IFN induction is abolished in AP-3-deficient pDCs (72, 77). It remains to be elucidated whether the lysosome-related organelles in pDCs are preformed and/or have unique molecular features. Transmembrane protein Slc15a4, which harbors a predicted AP-3 targeting motif, was identified by forward genetic screening as an essential regulator of cytokine secretion by pDCs but not by cDCs (72). Slc15a4 is required for the production of IFN and other cytokines (including IL-12) in response to TLR9 and TLR7 ligands, suggesting that Slc15a4 has a broader role in pDC function than does AP-3. Because both Slc15a4 and AP-3 components appear broadly expressed in immune cells, their function may involve additional pDC-enriched molecular regulators, such as pDC-specific endocytic adaptor Pacsin1 (59).
The PI3K signaling induced by TLR ligands in myeloid cells and cDCs is thought to promote antiinflammatory responses (78). In contrast, PI3K (79) and mTOR in particular (80) are essential for TLR9-induced IFN production by pDCs. PI3K/mTOR specifically promotes the activation and nuclear localization of IRF7, but not other consequences of TLR signaling (79). Thus, a distinct PI3K/mTOR-dependent pathway couples TLR signaling to IRF7 activation in pDCs. Again, the molecular components of this pathway are not known but may involve known PI3K pathway adaptors BCAP and TCL1 that are expressed in pDCs. The analysis of the mechanism and candidate regulators of the pDC-specific endocytic and PI3K/mTOR/IRF7 pathways should provide an exciting area of research in the near future.
pDC-specific receptors
Several membrane proteins with relatively pDC-specific expression have been shown to promote IFN secretion by pDCs. A C-type lectin receptor Ly49Q is specifically expressed on naive peripheral pDCs in mice and is required for optimal IFN production in vitro and in vivo (81). Ly49Q is homologous to NK cell receptors, binds to the MHC class I molecule H2-Kb, and contains an immunoreceptor tyrosine-based inhibitory motif; thus, the mechanism of its activating function in pDCs is unclear. It is possible that Ly49Q acts in an entirely different manner in the IFN response, e.g., by regulating the distribution of TLR ligands in the endosomal compartment (82). The mechanism and a possible functional ortholog of Ly49Q in the human pDCs remain to be characterized. Another receptor, pDC-TREM, is induced in pDCs upon activation and was proposed to facilitate IFN secretion based on RNAi results (83). pDC-TREM was found in a complex with Plexin A1, a cell guidance receptor implicated in DC function and trafficking. Whether pDC-TREM has a specific ligand (other than Plexin A1 ligand semaphorin) and a human ortholog and how it is integrated in the TLR/IRF7 signaling cascade are the questions to be elucidated. Together, these data highlight an emerging new world of novel pDC-specific receptors that act in completely unexpected ways to facilitate IFN secretion by pDCs.
Consistent with the potential danger of unchecked IFN secretion, multiple pDC-specific receptors inhibit pDC function. These include murine SiglecH and human BDCA-2 and ILT7, which signal through a common pathway involving FcεRIγ or DAP12, SYK, and BLNK (3, 84). This signaling attenuates TLR-induced production of IFN and other cytokines by an unknown mechanism. Recently, the significance of negative pDC regulation has been revealed through the identification of BST2 as a ligand of ILT7 (58). BST2 is an IFN-inducible membrane protein that directly restricts viral replication; thus, its expression provides negative feedback to pDCs by confirming a successful IFN signal. BST2 is also expressed in many tumors, suggesting a likely mechanism where by these tumors prevent pDC activation and escape tumor surveillance. Interestingly, murine pDCs lack an ILT7 ortholog but instead specifically express Bst2 (39), possibly providing a short-circuit or an inverted version of the same signaling pathway. The endogenous ligands for other inhibitory receptors remain unknown, although viruses such as human immunodeficiency virus (HIV) and hepatitis B virus may hijack the pathway and inhibit pDC function by binding to BDCA-2 (85, 86). Further characterization of inhibitory pDC receptors, their ligands, and their mechanism of interference with pDC activation should yield important insights into pDC biology.
Other Consequences of pDC Activation
In addition to cytokine secretion, activated pDCs undergo a characteristic DC maturation program involving the upregulation of co-stimulatory molecules and the acquisition of T cell stimulation capacity. This program can be selectively engaged by type B CpG oligonucleotides that induce pDC maturation at the expense of IFN secretion (70). The maturation program of pDCs is specifically mediated by NF-κB signaling, as NF-κB1/c-Rel double-deficient pDCs secrete IFN but fail to undergo maturation and die in response to CpG (87). These data further illustrate the two distinct molecular pathways leading to IFN secretion versus maturation in pDCs.
The antigen-presenting properties of pDCs have been reviewed in detail (2); the emerging conclusion is that activated pDCs can efficiently prime and cross-prime T lymphocytes. Unlike the cDCs isolated ex vivo from naive animals, pDCs absolutely require TLR-mediated activation for efficient antigen presentation and T cell activation (88, 89). Some of the antigen-presentation pathways may operate specifically in pDCs but not in cDCs, such as the proteasome-independent, endosomal pathway of viral antigen cross-presentation to cy-totoxic T cells (90). In addition, pDCs differ from cDCs (but are similar to B cells) in the continuous synthesis of MHC class II after activation, possibly to facilitate the presentation of virus-derived peptides (89, 91). The functional consequences of pDC-mediated antigen presentation for T cell responses are briefly discussed below.
The Fate of Activated pDCs
Antigen receptor triggering of T and Blymphocytes not only causes activation and its immediate effects (proliferation, cytokine secretion, surface phenotype change), but also launches a prolonged and tightly regulated differentiation program. This program ultimately leads to the acquisition of a new cell fate that may require a thorough erasure of the original cell identity, e.g., during B cell differentiation into plasma cells. Thus, lymphocytes are preprogrammed for lineage plasticity, in contrast to myeloid cells and cDCs which are not known to switch cell fate after activation. An important question is whether activated pDCs undergo full cell fate conversion to cDCs, as opposed to mere functional maturation. Benchmarks of full conversion would be indicated by phenotypic and functional changes, i.e., a switch from plasmacytoid to dendritic morphology, the loss of pDC markers and upregulation of cDC markers, and acquisition of naive T cell priming capacity for exogenous antigens.
pDCs were originally identified as efficient type 2 precursors of cDCs from the human peripheral blood (17). Indeed, mature human pDCs cultured with cytokines and/or activation stimuli (IL-3, CD40L, viruses, TLR ligands) exhibit full phenotypic and functional differentiation to cDCs (92). Although this provides a proof of principle for pDC-to-cDC conversion, the evidence for this event in vivo has been scarce. Lindstedt et al. (93) characterized human DC populations in resected tonsils and in the peripheral blood. Notably, the tonsils appear to contain a minor intermediate population between CD123+ pDCs and BDCA-3+ cDCs; moreover, increased expression of pDC-specific genes was observed in BDCA-3+ cDCs from the tonsils. These results may reflect ongoing pDC differentiation into BDCA-3+ cDCs, the human counterpart of mouse CD8+ cDCs, at the site of active chronic infection. In mice, phenotypic changes consistent with CD8+ cDC differentiation (loss of B220, increase in CD11c and CD8) were described in adoptively transferred splenic pDCs 10 h after inactivated virus injection (28). However, the pDC purification scheme used at the time (CD11clow B220+) likely included other cell types such as B220+ cDC precursors unrelated to pDCs (38). Subsequently, Zuniga and colleagues provided a compelling case for IFN-dependent pDC conversion to CD8− cDCs following lymphocytic choriomeningitis virus (LCMV) infection in vitro (94) and invivo (95). Importantly, only pDCs from the bone marrow but not from the spleen were capable of such conversion, suggesting that fully mature peripheral pDCs have lost this capacity.
Teleologically, the differentiation of activated pDCs to cDCs would appear to be both desirable and plausible. It would automatically terminate high-level IFN secretion and shorten the life span of an infected pDC; at the same time, it would facilitate T cell priming to viral antigens. In addition, pDCs can differentiate into an alternative CD8+ cDC subset during development or after E2–2 deletion (discussed above), suggesting their possible fate after activation. In fact, E2–2 expression is reduced several-fold after pDC activation (32), raising the possibility that this event may drive the conversion to cDCs. Thus, the cell fate conversion of activated pDCs appears likely but awaits clear demonstration in a natural infection model.
pDCs IN THE IMMUNE RESPONSE
As a major effector cell type in immunity, the pDC has been implicated in nearly all normal and pathological immune responses. For example, important roles of pDCs have been suggested in allergy and asthma (96), antitumor immunity (97), and responses to nonviral pathogens (98, 99). Here we briefly review only three related aspects of pDC function in immunity: their roles in T cell responses versus tolerance, in antiviral immune responses, and in autoimmunity.
The Role of pDCs in T Cell Responses: Activation or Tolerance?
The capacity of pDCs to prime productive T cell responses after infection or immunization is well documented. For instance, pDCs can efficiently prime CD4+ T cell responses in the lymph nodes (100), induce Th1 polarization (101, 102), and prime (103) and cross-prime (88, 90, 104) CD8+ T cell responses. These results are consistent with the postulated role of pDCs as key sensors and immune system activators during viral infections.
Intriguingly, the opposite tolerogenic role has been proposed for pDCs in several systems, mostly associated with the induction of regulatory T cells (Tregs). Thus, human pDCs induce T cell differentiation into IL-10-producing Tregs in vitro (105, 106). In vivo, pDCs were proposed to promote Treg differentiation in the human thymus (107) and to induce Treg-mediated tolerance in tumor-bearing mice (108), during tolerization to cardiac allografts (109), in experimental autoimmune encephalomyelitis (EAE) (110), and in graft-versus-host disease (111). In some of these studies, however, the phenotypic definition of pDCs can be questioned; for instance, the indoleamine 2,3-dioxygenase-expressing pDCs in tumor-draining lymph nodes (108) likely correspond to B cells with a similar phenotype (112). In other models, such as graft-versus-host disease, the T cell stimulatory function of pDCs has been subsequently demonstrated (113). An interesting recent study suggested an important role for liver pDCs in oral tolerance induction, largely through T cell clonal deletion (114). Thus, the tolerogenic and/or Treg-inducing properties of pDCs may be relevant in certain immune responses, likely in an organ-dependent fashion. However, they appear far from universal or conclusively proven at present, and thus a broad definition of steady-state pDCs as tolerogenic would be premature and misleading.
The Role of pDCs in Human Infections
In humans, pDCs have been most extensively studied during HIV and chronic viral hepatitis, particularly hepatitis C virus (HCV) infections. The emerging picture suggests an important role for pDCs in these infections, although the exact mechanism and consequences of pDC activity are controversial at present (115). It has been shown recently that pDCs can respond to HCV and particularly to HCV-infected hepatocytes through TLR7 (116). HCV may specifically impair pDC activity (117), thereby compromising T cell responses against it; however, other studies demonstrated normal pDC functionality on a per cell basis in chronic HCV (118). The resolution of this controversy would establish pDCs either as a weak link of anti-HCV immune response or as a potentially powerful effector type that can be harnessed for immunotherapy of chronic HCV.
In HIV (recently reviewed in 119), it is clear that pDCs can be infected with the virus and/or respond to it with robust IFN secretion. Furthermore, pDCs are progressively depleted from the blood of infected patients, either through infection-induced death or due to redistribution to lymphoid organs. The key unresolved question is whether HIV-induced pDC activation is beneficial or harmful for the host. On one hand, IFN secretion by pDCs was shown to inhibit viral replication in T cells and promote pDC and cDC maturation, leading to the killing of infected T cells. Indeed, HIV may have evolved mechanisms to suppress pDC activation, e.g., through BDCA-2 ligation (85). On the other hand, the same functions of pDCs may exacerbate T cell depletion, e.g., by disseminating HIV to uninfected CD4+ T cells or by bystander T cell killing. Most importantly, elevated IFN response by pDCs may contribute to chronic immune activation and faster T cell depletion, e.g., in female compared to male patients (120) or in primate species susceptible to simian immunodeficiency virus (121).
It is plausible that the function of pDCs in HIV infection changes from protective to pathogenic as the disease progresses. At the early stages of infection, IFN production and virus cross-presentation by pDCs may help limit virus spread and mount cytotoxic T cell responses. As the virus replication escapes control, IFN secretion may drive polyclonal T cell hyperactivation and depletion. The eventual loss, redistribution, or functional impairment of pDCs at the late stages of infection would contribute to immunodeficiency. Thus, the role of pDCs in HIV infection highlights the power and the danger of pDC activation and reveals yet another strategy of immune system subversion by HIV.
The Role of pDCs in Viral Infections: Mouse Models
Given the powerful pDC response to nearly all enveloped viruses, their indispensable role in antiviral immune responses would be anticipated; however, it appears surprisingly difficult to demonstrate. For example, pDCs produce IFN in response to vesicular stomatitis virus and influenza virus yet appear dispensable for their control in vivo (5, 122). Similarly, pDCs are primary IFN producers during LCMV infection (123) but are not required for optimal IFN production, virus clearance, or T cell response in acute LCMV infection (5, 124). The depletion of pDCs impairs the IFN response to murine cytomegalovirus but does not affect the net NK cell response or overall survival (34, 124). In topical viral infections such as respiratory syncytial virus (125, 126) in the lung and herpes simplex virus in the vagina (127), pDC depletion leads to several-fold increases in viral titers and exacerbated tissue pathology.
Perhaps the best-documented model of pDC-dependent antiviral immune response is mouse hepatitis virus (MHV) infection, an acute disease that requires Tlr7 and IFN for virus clearance. Ludewig and colleagues (128) showed that pDC depletion nearly abolishes IFN response to MHV, increases viral replication in the spleen ~1000-fold, causes virus spread to normally protected tissues, and severely exacerbates liver damage. Macrophages and cDCs were shown to be the major beneficiaries of protection conferred by pDC-secreted IFN (41), and it has been estimated that a single splenic pDC can protect 103–104 macrophages (129). Thus, the requirement for pDCs in antiviral responses appears variable, with only a single model of primarily pDC-dependent protection described to date. This is likely because antiviral responses are robust and therefore involve multiple redundant mechanisms of virus sensing and IFN secretion. However, in addition to acute IFN response to viruses, the role of pDCs in long-term protective T cell responses remains to be investigated. Interestingly, chronic LCMV infection impairs the IFN secretion capacity of pDCs through unknown mechanisms (130, 131). This suggests that normal pDC function would be detrimental to LCMV persistence, possibly by facilitating cytotoxic T cell responses. Studies in this direction would require more specific and long-term approaches to pDC depletion, such as diphtheria toxin–based transgenic models (5).
The Role of pDCs in Human Autoimmune Diseases
With the emergence of elevated IFN levels as a pathogenesis factor in several autoimmune diseases, the potentially important role of pDCs in autoimmunity has been recognized (132). To date, the strongest evidence for pDC involvement has been accumulated from the study of two diseases: psoriasis and systemic lupus erythematosus (reviewed in 3, 133). In psoriasis, early skin lesions are highly infiltrated by activated pDCs, corresponding with decreased numbers of circulating pDCs in the blood (134). Blocking IFN production by pDCs using anti-BDCA-2 antibody inhibited the development of skin lesions in a xenograft model, providing causal proof of pDC function in the disease (134). Subsequently, Gilliet et al. (135) identified the activating stimulus for pDCs as complexes of self-DNA with the antimicrobial peptide LL-37. This and possibly other homologous proteins promote the aggregation of released cellular DNA and RNA into large complexes that efficiently activate pDCs (135, 136). Although the origin of these immunostimulatory complexes and the consequences of pDC activation remain to be elucidated, the major role of pDCs in psoriasis appears well established.
Similarly, lupus patients show a decrease in circulating pDCs and the accumulation of activated, IFN-producing pDCs in affected tissues such as the skin (137). The hallmark of lupus is the production of antinuclear antibodies, and immune complexes of such antibodies with endogenous nucleic acids were shown to activate pDCs through TLR7/9 (138, 139). These complexes may be delivered into the endosomal compartment of pDCs via Fc receptor FcγRII (138, 140), and their stimulatory capacity can be augmented by the nuclear DNA-binding protein HMGB1 (141). In addition, self-DNA forms complexes with LL-37 and other antimicrobial peptides released by neutrophils, and the resulting complexes induce IFN secretion in pDCs through TLR9 (M. Gilliet, personal communication). Notably, TLR-activated pDCs become resistant to glucocorticoids, which could underlie the limited efficacy of these drugs in lupus (142, 143). The direct causal relationship between pDC-derived IFN and lupus progression/severity is hard to establish in the human system and should await elucidation in animal models. Nevertheless, the likely connection between the formation of nucleic acid–containing immune complexes, pDC activation, and IFN secretion and the pronounced IFN signature of the disease make a strong case for the pDC as a major player in lupus pathogenesis (133). Overall, the aberrant conversion of self-nucleic acids into ligands for TLR7/9 on pDCs (via immune complex formation, antimicrobial peptide binding, and other mechanisms to be discovered) may represent a common patho-genesis step in psoriasis, lupus, and possibly other autoimmune diseases such as Sjögren’s syndrome (144).
The Role of pDCs in Autoimmunity: Mouse Models
The role of pDCs in experimental autoimmunity models is poorly understood at present. For instance, studies using antibody-mediated pDC ablation yielded conflicting results in EAE (145, 146). Animals with selective loss of MHC class II expression on several cell types were used to demonstrate the anti-inflammatory role of pDCs (110); however, a strictly pDC-specific genetic loss of MHC expression and/or function is required to fully elucidate the issue. Similarly, the results of pDC ablation in the NOD model of type I diabetes (147) remain to be reconciled with other studies in the field (148). These conflicting data may reflect the specificity and efficiency issues of the transient pDC ablation and emphasize the necessity to deplete or functionally manipulate pDCs in the long term. This ability would be a prerequisite for the analysis of pDCs in spontaneous, chronic autoimmune diseases such as lupus in susceptible mouse strains. As Tlr7 and Tlr9 appear to play opposing roles in lupus pathogenesis (149), it would be interesting to dissect the net contribution of the Tlr7/Tlr9 double-edged cell type.
CONCLUSIONS AND FUTURE DIRECTIONS
The coming years should bring important insights into the key open questions of pDC biology, such as the regulation of pDC lineage commitment, homeostasis, and plasticity; the molecular basis of the unique IFN-secreting capacity of pDCs; tissue- and immune response– specific roles of pDCs in T cell activation and tolerance; and the precise function of pDCs in infectious immunity and in autoimmune diseases. Approaches involving cross-species gene expression (59) and functional analysis (32), forward genetic screening (72), and in vivo targeting and ablation (5) should facilitate the research in these directions.
With the growing evidence for the importance of IFN production in all aspects of immunity, pDCs are emerging as prime targets for immunotherapy. In some cases, such as chronic viral infections and/or immunodeficiency, controlled enhancement of pDC function would appear beneficial. One possible approach is to combine TLR stimuli and growth factors that preferentially engage pDCs (e.g., CpG and Flt3L, respectively) as vaccine adjuvants (150). Another future direction is the engagement of activating pDC-specific receptors suchasp DC-TREM (83). Conversely, autoimmune diseases such as lupus and psoriasis may benefit from targeted inhibition of chronic pDC activation. In addition to broader approaches targeting IFN signaling (151) or TLR7/9-mediated IFN production (152), one might envisage therapies targeted specifically at pDCs, such as the ligation of pDC-specific inhibitory receptors or targeting the unique endocytic machinery of pDCs. In any case, the insights into basic pDC biology are likely to fuel translational research and pave the way to novel immunotherapies.
CLASSICAL OR CONVENTIONAL DCs (cDCs).
cDCs, the original DC type identified by Steinman & Cohn (30) as a powerful stimulator of T cell responses, have the following characteristics:
-
■
possess dendritic morphology with prominent cytoplasmic veils and protrusions;
-
■
differentiate and reside in tissues and lymphoid organs in the steady state;
-
■
turn over rapidly and undergo proliferation in situ;
-
■
express a broad range of pattern-recognition receptors;
-
■
express high levels of MHC class II and of the integrin CD11c (a specific marker in the mouse), but not B220;
-
■
have a unique capacity for naive T cell priming. cDCs comprise two main subsets:
-
■
CD8− (CD11b+, also called myeloid) cDCs showing high capacity for MHC class II-mediated presentation of exogenous antigen;
-
■
CD8+ (CD103+ in tissues) cDCs capable of antigen cross-presentation to cytotoxic T cells. (Human BDCA-3+ cDCs have recently been identified as the genetic and functional counterpart of murine CD8+/CD103+ cDCs.)
ACKNOWLEDGMENTS
We are grateful to D. Ganguly, M. Gilliet, and P. Tucker for communicating unpublished data and to many colleagues in the field for insightful discussions. The work in our lab has been supported by the Lupus Research Institute and NIH grants AI072571 and AI085439.
Glossary
- pDC
plasmacytoid dendritic cell
- IFN
type I interferon, interferon-α/β
- cDC
classical or conventional dendritic cell
- TLR
Toll-like receptor
- CpG
unmethylated CpG-containing DNA
- CDP
common dendritic cell progenitor
- HIV
human immunodeficiency virus
- Treg
regulatory T cell
- EAE
experimental autoimmune encephalomyelitis
- HCV
hepatitis C virus
- MHV
murine hepatitis virus
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Liu YJ. IPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu. Rev. Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 2.Villadangos JA, Young L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity. 2008;29:352–361. doi: 10.1016/j.immuni.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Gilliet M, Cao W, Liu YJ. Plasmacytoid dendritic cells: sensing nucleic acids in viral infection and autoimmune diseases. Nat. Rev. Immunol. 2008;8:594–606. doi: 10.1038/nri2358. [DOI] [PubMed] [Google Scholar]
- 4.Jegalian AG, Facchetti F, Jaffe ES. Plasmacytoid dendritic cells: physiologic roles and pathologic states. Adv. Anat. Pathol. 2009;16:392–404. doi: 10.1097/PAP.0b013e3181bb6bc2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol. Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reizis B. Regulation of plasmacytoid dendritic cell development. Curr. Opin. Immunol. 2010;22:206–211. doi: 10.1016/j.coi.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sozzani S, Vermi W, Del Prete A, Facchetti F. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends Immunol. 2010;31:270–277. doi: 10.1016/j.it.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Ronnblom L, Ramstedt U, Alm GV. Properties of human natural interferon-producing cells stimulated by tumor cell lines. Eur. J. Immunol. 1983;13:471–476. doi: 10.1002/eji.1830130608. [DOI] [PubMed] [Google Scholar]
- 9.Sandberg K, Eloranta ML, Johannisson A, Alm GV. Flow cytometric analysisof natural interferon-αproducing cells. Scand. J. Immunol. 1991;34:565–576. doi: 10.1111/j.1365-3083.1991.tb01580.x. [DOI] [PubMed] [Google Scholar]
- 10.Feldman M, Fitzgerald-Bocarsly P. Sequential enrichment and immunocytochemical visualization of human interferon-α-producing cells. J. Interferon Res. 1990;10:435–446. doi: 10.1089/jir.1990.10.435. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald-Bocarsly P. Human natural interferon-α producing cells. Pharmacol. Ther. 1993;60:39–62. doi: 10.1016/0163-7258(93)90021-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chehimi J, Starr SE, Kawashima H, Miller DS, Trinchieri G, et al. Dendritic cells and IFN-α-producing cells are two functionally distinct non-B, non-monocytic HLA-DR+ cell subsets in human peripheral blood. Immunology. 1989;68:486–490. [PMC free article] [PubMed] [Google Scholar]
- 13.Starr SE, Bandyopadhyay S, Shanmugam V, Hassan N, Douglas S, et al. Morphological and functional differences between HLA-DR+ peripheral blood dendritic cells and HLA-DR+ IFN-αproducing cells. Adv. Exp. Med. Biol. 1993;329:173–178. doi: 10.1007/978-1-4615-2930-9_29. [DOI] [PubMed] [Google Scholar]
- 14.Facchetti F, de Wolf-Peeters C, Mason DY, Pulford K, van den Oord JJ, Desmet VJ. Plasmacytoid T cells. Immunohistochemical evidence for their monocyte/macrophage origin. Am. J. Pathol. 1988;133:15–21. [PMC free article] [PubMed] [Google Scholar]
- 15.Facchetti F, De Wolf-Peeters C, Kennes C, Rossi G, De Vos R, et al. Leukemia-associated lymph node infiltrates of plasmacytoid monocytes (so-called plasmacytoid T-cells). Evidence for two distinct histological and immunophenotypical patterns. Am. J. Surg. Pathol. 1990;14:101–112. doi: 10.1097/00000478-199002000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Vollenweider R, Lennert K. Plasmacytoid T-cell clusters in non-specific lymphadenitis. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1983;44:1–14. doi: 10.1007/BF02890155. [DOI] [PubMed] [Google Scholar]
- 17.Grouard G, Rissoan MC, Filgueira L, Durand I, Banchereau J, Liu YJ. The enigmatic plasmacytoid T cells develop into dendritic cells with interleukin (IL)-3 and CD40-ligand. J. Exp. Med. 1997;185:1101–1111. doi: 10.1084/jem.185.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegal FP, Kadowaki N, Shodell M, Fitzgerald-Bocarsly PA, Shah K, et al. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284:1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 19.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 20.Bjorck P. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood. 2001;98:3520–3526. doi: 10.1182/blood.v98.13.3520. [DOI] [PubMed] [Google Scholar]
- 21.Nakano H, Yanagita M, Gunn MD. CD11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 2001;194:1171–1178. doi: 10.1084/jem.194.8.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Asselin-Paturel C, Boonstra A, Dalod M, Durand I, Yessaad N, et al. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2001;2:1144–1150. doi: 10.1038/ni736. [DOI] [PubMed] [Google Scholar]
- 23.Blom B, Ho S, Antonenko S, Liu YJ. Generation of interferon α-producing predendritic cell (pre-DC)2 from human CD34+ hematopoietic stem cells. J. Exp. Med. 2000;192:1785–1796. doi: 10.1084/jem.192.12.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilliet M, Boonstra A, Paturel C, Antonenko S, Xu XL, et al. The development of murine plasma-cytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 2002;195:953–958. doi: 10.1084/jem.20020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crozat K, Guiton R, Guilliams M, Henri S, Baranek T, et al. Comparative genomics as a tool to reveal functional equivalences between human and mouse dendritic cell subsets. Immunol. Rev. 2010;234:177–198. doi: 10.1111/j.0105-2896.2009.00868.x. [DOI] [PubMed] [Google Scholar]
- 26.Luber CA, Cox J, Lauterbach H, Fancke B, Selbach M, et al. Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity. 2010;32:279–289. doi: 10.1016/j.immuni.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Randolph GJ, Ochando J, Partida-Sanchez S. Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 2008;26:293–316. doi: 10.1146/annurev.immunol.26.021607.090254. [DOI] [PubMed] [Google Scholar]
- 28.O’Keeffe M, Hochrein H, Vremec D, Caminschi I, Miller JL, et al. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8+ dendritic cells only after microbial stimulus. J. Exp. Med. 2002;196:1307–1319. doi: 10.1084/jem.20021031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu K, Waskow C, Liu X, Yao K, Hoh J, Nussenzweig M. Origin of dendritic cells in peripheral lymphoid organs of mice. Nat. Immunol. 2007;8:578–583. doi: 10.1038/ni1462. [DOI] [PubMed] [Google Scholar]
- 30.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J. Exp. Med. 1973;137:1142–1162. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Asselin-Paturel C, Brizard G, Pin JJ, Briere F, Trinchieri G. Mouse strain differences in plasmacytoid dendritic cell frequency and function revealed by a novel monoclonal antibody. J. Immunol. 2003;171:6466–6477. doi: 10.4049/jimmunol.171.12.6466. [DOI] [PubMed] [Google Scholar]
- 32.Cisse B, Caton ML, Lehner M, Maeda T, Scheu S, et al. Transcription factor E2–2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell. 2008;135:37–48. doi: 10.1016/j.cell.2008.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumagai Y, Takeuchi O, Kato H, Kumar H, Matsui K, et al. Alveolar macrophages are the primary interferon-αproducer in pulmonary infection with RNA viruses. Immunity. 2007;27:240–252. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 34.Krug A, French AR, Barchet W, Fischer JA, Dzionek A, et al. TLR9-dependent recognition of MCMV by IPC and DC generates coordinated cytokine responses that activate antiviral NK cell function. Immunity. 2004;21:107–119. doi: 10.1016/j.immuni.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 36.Caminschi I, Ahmet F, Heger K, Brady J, Nutt SL, et al. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. J. Exp. Med. 2007;204:2579–2590. doi: 10.1084/jem.20071351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blasius AL, Barchet W, Cella M, Colonna M. Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells. J. Exp. Med. 2007;204:2561–2568. doi: 10.1084/jem.20070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Segura E, Wong J, Villadangos JA. Cutting edge: B220+CCR9- dendritic cells are not plasmacytoid dendritic cells but are precursors of conventional dendritic cells. J. Immunol. 2009;183:1514–1517. doi: 10.4049/jimmunol.0901524. [DOI] [PubMed] [Google Scholar]
- 39.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J. Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 40.Mach N, Gillessen S, Wilson SB, Sheehan C, Mihm M, Dranoff G. Differences in dendritic cells stimulated in vivo by tumors engineered to secrete granulocyte-macrophage colony-stimulating factor or Flt3-ligand. Cancer Res. 2000;60:3239–3246. [PubMed] [Google Scholar]
- 41.Cervantes-Barragan L, Kalinke U, Zust R, Konig M, Reizis B, et al. Type I IFN-mediated protection of macrophages and dendritic cells secures control of murine coronavirus infection. J. Immunol. 2009;182:1099–1106. doi: 10.4049/jimmunol.182.2.1099. [DOI] [PubMed] [Google Scholar]
- 42.Naik SH, Sathe P, Park HY, Metcalf D, Proietto AI, et al. Development of plasmacytoid and conventional dendritic cell subtypes from single precursor cells derived in vitro and in vivo. Nat. Immunol. 2007;8:1217–1226. doi: 10.1038/ni1522. [DOI] [PubMed] [Google Scholar]
- 43.Onai N, Obata-Onai A, Schmid MA, Ohteki T, Jarrossay D, Manz MG. Identification of clono-genic common Flt3+M-CSFR+ plasmacytoid and conventional dendritic cell progenitors in mouse bone marrow. Nat. Immunol. 2007;8:1207–1216. doi: 10.1038/ni1518. [DOI] [PubMed] [Google Scholar]
- 44.Sitnicka E, Bryder D, Theilgaard-Monch K, Buza-Vidas N, Adolfsson J, Jacobsen SE. Key role of flt3 ligand inregulation of the common lymphoid progenitor but notin maintenance of the hematopoietic stem cell pool. Immunity. 2002;17:463–472. doi: 10.1016/s1074-7613(02)00419-3. [DOI] [PubMed] [Google Scholar]
- 45.Shigematsu H, Reizis B, Iwasaki H, Mizuno S, Hu D, et al. Plasmacytoid dendritic cells activate lymphoid-specific genetic programs irrespective of their cellular origin. Immunity. 2004;21:43–53. doi: 10.1016/j.immuni.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Corcoran L, Ferrero I, Vremec D, Lucas K, Waithman J, et al. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J. Immunol. 2003;170:4926–4932. doi: 10.4049/jimmunol.170.10.4926. [DOI] [PubMed] [Google Scholar]
- 47.Pelayo R, Hirose J, Huang J, Garrett KP, Delogu A, et al. Derivation of 2 categories of plasmacytoid dendritic cells in murine bone marrow. Blood. 2005;105:4407–4415. doi: 10.1182/blood-2004-07-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Welner RS, Esplin BL, Garrett KP, Pelayo R, Luche H, et al. Asynchronous RAG-1 expression during B lymphopoiesis. J. Immunol. 2009;183:7768–7777. doi: 10.4049/jimmunol.0902333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8dendritic cells in the spleen. J. Exp. Med. 2007;204:1653–1664. doi: 10.1084/jem.20062648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu K, Victora GD, Schwickert TA, Guermonprez P, Meredith MM, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324:392–397. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmid MA, Kingston D, Boddupalli S, Manz MG. Instructive cytokine signals in dendritic cell lineage commitment. Immunol. Rev. 2010;234:32–44. doi: 10.1111/j.0105-2896.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- 53.Waskow C, Liu K, Darrasse-Jeze G, Guermonprez P, Ginhoux F, et al. The receptor tyrosine kinase Flt3 is required for dendritic cell development in peripheral lymphoid tissues. Nat. Immunol. 2008;9:676–683. doi: 10.1038/ni.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Eidenschenk C, Crozat K, Krebs P, Arens R, Popkin D, et al. Flt3 permits survival during infection by rendering dendritic cells competent to activate NK cells. Proc. Natl. Acad. Sci. USA. 2010;107:9759–9764. doi: 10.1073/pnas.1005186107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sathaliyawala T, O’Gorman WE, Greter M, Bogunovic M, Konjufca V, et al. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33:597–606. doi: 10.1016/j.immuni.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vollstedt S, O’Keeffe M, Odermatt B, Beat R, Glanzmann B, et al. Treatment of neonatal mice with Flt3 ligand leads to changes in dendritic cell subpopulations associated with enhanced IL-12 and IFN-α production. Eur. J. Immunol. 2004;34:1849–1860. doi: 10.1002/eji.200324443. [DOI] [PubMed] [Google Scholar]
- 57.Ghosh HS, Cisse B, Bunin A, Lewis KL, Reizis B. Continuous expression of E2–2 maintains the cell fate of mature plasmacytoid dendritic cells. Immunity. 2010;33:905–916. doi: 10.1016/j.immuni.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cao W, Bover L, Cho M, Wen X, Hanabuchi S, et al. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J. Exp. Med. 2009;206:1603–1614. doi: 10.1084/jem.20090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robbins SH, Walzer T, Dembele D, Thibault C, Defays A, et al. Novel insights into the relationships between dendritic cell subsets in human and mouse revealed by genome-wide expression profiling. Genome Biol. 2008;9:R17. doi: 10.1186/gb-2008-9-1-r17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bar-On L, Birnberg T, Kewis KL, Edelson B, Bruder D, et al. CX3CR1+ CD8α+ dendritic cells: a steady state population related to plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA. 2010;107:14745–14750. doi: 10.1073/pnas.1001562107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, et al. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell. 2009;139:1130–1142. doi: 10.1016/j.cell.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 62.Li P, Burke S, Wang J, Chen X, Ortiz M, et al. Reprogramming of T cells to natural killer-like cells upon Bcl11b deletion. Science. 2010;329:85–89. doi: 10.1126/science.1188063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, et al. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takeuchi O, Akira S. Innate immunity to virus infection. Immunol. Rev. 2009;227:75–86. doi: 10.1111/j.1600-065X.2008.00737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pascale F, Contreras V, Bonneau M, Courbet A, Chilmonczyk S, et al. Plasmacytoid dendritic cells migrate in afferent skin lymph. J. Immunol. 2008;180:5963–5972. doi: 10.4049/jimmunol.180.9.5963. [DOI] [PubMed] [Google Scholar]
- 67.Diebold SS, Montoya M, Unger H, Alexopoulou L, Roy P, et al. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature. 2003;424:324–328. doi: 10.1038/nature01783. [DOI] [PubMed] [Google Scholar]
- 68.Iwakoshi NN, Pypaert M, Glimcher LH. The transcription factor XBP-1 is essential for the development and survival of dendritic cells. J. Exp. Med. 2007;204:2267–2275. doi: 10.1084/jem.20070525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barchet W, Cella M, Odermatt B, Asselin-Paturel C, Colonna M, Kalinke U. Virus-induced interferon α production by a dendritic cell subset in the absence of feedback signaling in vivo. J. Exp. Med. 2002;195:507–516. doi: 10.1084/jem.20011666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kerkmann M, Rothenfusser S, Hornung V, Towarowski A, Wagner M, et al. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J. Immunol. 2003;170:4465–4474. doi: 10.4049/jimmunol.170.9.4465. [DOI] [PubMed] [Google Scholar]
- 71.Izaguirre A, Barnes BJ, Amrute S, Yeow WS, Megjugorac N, et al. Comparative analysis of IRF and IFN-α expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 2003;74:1125–1138. doi: 10.1189/jlb.0603255. [DOI] [PubMed] [Google Scholar]
- 72.Blasius AL, Arnold CN, Georgel P, Rutschmann S, Xia Y, et al. Slc15a4, AP-3, and Hermansky-Pudlak syndrome proteins are required for Toll-like receptor signaling in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA. 2010;107:19973–19978. doi: 10.1073/pnas.1014051107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kumagai Y, Kumar H, Koyama S, Kawai T, Takeuchi O, Akira S. Cutting edge: TLR-dependent viral recognition along with type I IFN positive feedback signaling masks the requirement of viral replication for IFN-α production in plasmacytoid dendritic cells. J. Immunol. 2009;182:3960–3964. doi: 10.4049/jimmunol.0804315. [DOI] [PubMed] [Google Scholar]
- 74.Honda K, Ohba Y, Yanai H, Negishi H, Mizutani T, et al. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature. 2005;434:1035–1040. doi: 10.1038/nature03547. [DOI] [PubMed] [Google Scholar]
- 75.Kim T, Pazhoor S, Bao M, Zhang Z, Hanabuchi S, et al. Aspartate-glutamate-alanine-histidine box motif (DEAH)/RNA helicase A helicases sense microbial DNA in human plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA. 2010;107:15181–15186. doi: 10.1073/pnas.1006539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guiducci C, Ott G, Chan JH, Damon E, Calacsan C, et al. Properties regulating the nature of the plasmacytoid dendritic cell response to Toll-like receptor 9 activation. J. Exp. Med. 2006;203:1999–2008. doi: 10.1084/jem.20060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weichhart T, Saemann MD. The multiple facets of mTOR in immunity. Trends Immunol. 2009;30:218–226. doi: 10.1016/j.it.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 79.Guiducci C, Ghirelli C, Marloie-Provost MA, Matray T, Coffman RL, et al. PI3K is critical for the nuclear translocation of IRF-7 and type I IFN production by human plasmacytoid predendritic cells in response to TLR activation. J. Exp. Med. 2008;205:315–322. doi: 10.1084/jem.20070763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao W, Manicassamy S, Tang H, Kasturi SP, Pirani A, et al. Toll-like receptor-mediated induction of type I interferon in plasmacytoid dendritic cells requires the rapamycin-sensitive PI(3)K-mTOR-p70S6K pathway. Nat. Immunol. 2008;9:1157–1164. doi: 10.1038/ni.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tai LH, Goulet ML, Belanger S, Toyama-Sorimachi N, Fodil-Cornu N, et al. Positive regulation of plasmacytoid dendritic cell function via Ly49Q recognition of class I MHC. J. Exp. Med. 2008;205:3187–3199. doi: 10.1084/jem.20080718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoshizaki M, Tazawa A, Kasumi E, Sasawatari S, Itoh K, et al. Spatiotemporal regulation of intracellular trafficking of Toll-like receptor 9 by an inhibitory receptor, Ly49Q. Blood. 2009;114:1518–1527. doi: 10.1182/blood-2008-12-192344. [DOI] [PubMed] [Google Scholar]
- 83.Watarai H, Sekine E, Inoue S, Nakagawa R, Kaisho T, Taniguchi M. PDC-TREM, aplasmacytoid dendritic cell-specific receptor, is responsible for augmented production of type I interferon. Proc. Natl. Acad. Sci. USA. 2008;105:2993–2998. doi: 10.1073/pnas.0710351105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Blasius AL, Colonna M. Sampling and signaling in plasmacytoid dendritic cells: the potential roles of Siglec-H. Trends Immunol. 2006;27:255–260. doi: 10.1016/j.it.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 85.Martinelli E, Cicala C, Van Ryk D, Goode DJ, Macleod K, et al. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-α secretion in plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA. 2007;104:3396–3401. doi: 10.1073/pnas.0611353104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu Y, Hu Y, Shi B, Zhang X, Wang J, et al. HBsAg inhibits TLR9-mediated activation and IFN-α production in plasmacytoid dendritic cells. Mol. Immunol. 2009;46:2640–2646. doi: 10.1016/j.molimm.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 87.O’Keeffe M, Grumont RJ, Hochrein H, Fuchsberger M, Gugasyan R, et al. Distinct roles for the NF-κB1 and c-Rel transcription factors in the differentiation and survival of plasmacytoid and conventional dendritic cells activated by TLR-9 signals. Blood. 2005;106:3457–3464. doi: 10.1182/blood-2004-12-4965. [DOI] [PubMed] [Google Scholar]
- 88.Mouries J, Moron G, Schlecht G, Escriou N, Dadaglio G, Leclerc C. Plasmacytoid dendritic cells efficiently cross-prime naive T cells in vivo after TLR activation. Blood. 2008;112:3713–3722. doi: 10.1182/blood-2008-03-146290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasma-cytoid dendritic cells. Nat. Immunol. 2008;9:1244–1252. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- 90.Di Pucchio T, Chatterjee B, Smed-Sorensen A, Clayton S, Palazzo A, et al. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat. Immunol. 2008;9:551–557. doi: 10.1038/ni.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sadaka C, Marloie-Provost MA, Soumelis V, Benaroch P. Developmental regulation of MHC II expression and transport in human plasmacytoid-derived dendritic cells. Blood. 2009;113:2127–2135. doi: 10.1182/blood-2008-10-178152. [DOI] [PubMed] [Google Scholar]
- 92.Soumelis V, Liu YJ. From plasmacytoid to dendritic cell: morphological and functional switches during plasmacytoid predendritic cell differentiation. Eur. J. Immunol. 2006;36:2286–2292. doi: 10.1002/eji.200636026. [DOI] [PubMed] [Google Scholar]
- 93.Lindstedt M, Lundberg K, Borrebaeck CA. Gene family clustering identifies functionally associated subsets of human in vivo blood and tonsillar dendritic cells. J. Immunol. 2005;175:4839–4846. doi: 10.4049/jimmunol.175.8.4839. [DOI] [PubMed] [Google Scholar]
- 94.Zuniga EI, McGavern DB, Pruneda-Paz JL, Teng C, Oldstone MB. Bone marrow plasmacytoid dendritic cells can differentiate into myeloid dendritic cells upon virus infection. Nat. Immunol. 2004;5:1227–1234. doi: 10.1038/ni1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liou LY, Blasius AL, Welch MJ, Colonna M, Oldstone MB, Zuniga EI. In vivo conversion of BM plasmacytoid DC into CD11b+ conventional DC during virus infection. Eur. J. Immunol. 2008;38:3388–3394. doi: 10.1002/eji.200838282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kool M, van Nimwegen M, Willart MA, Muskens F, Boon L, et al. An anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J. Immunol. 2009;183:1074–1082. doi: 10.4049/jimmunol.0900471. [DOI] [PubMed] [Google Scholar]
- 97.Liu C, Lou Y, Lizee G, Qin H, Liu S, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J. Clin. Investig. 2008;118:1165–1175. doi: 10.1172/JCI33583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pepper M, Dzierszinski F, Wilson E, Tait E, Fang Q, et al. Plasmacytoid dendritic cells are activated by Toxoplasma gondii to present antigen and produce cytokines. J. Immunol. 2008;180:6229–6236. doi: 10.4049/jimmunol.180.9.6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ang DK, Oates CV, Schuelein R, Kelly M, Sansom FM, et al. Cutting edge: pulmonary Legionella pneumophila is controlled by plasmacytoid dendritic cells but not type I IFN. J. Immunol. 2010;184:5429–5433. doi: 10.4049/jimmunol.1000128. [DOI] [PubMed] [Google Scholar]
- 100.Sapoznikov A, Fischer JA, Zaft T, Krauthgamer R, Dzionek A, Jung S. Organ-dependent in vivo priming of naive CD4+, but not CD8+, T cells by plasmacytoid dendritic cells. J. Exp. Med. 2007;204:1923–1933. doi: 10.1084/jem.20062373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cella M, Facchetti F, Lanzavecchia A, Colonna M. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 2000;1:305–310. doi: 10.1038/79747. [DOI] [PubMed] [Google Scholar]
- 102.Boonstra A, Asselin-Paturel C, Gilliet M, Crain C, Trinchieri G, et al. Flexibility of mouse classical and plasmacytoid-derived dendritic cells in directing T helper type 1 and 2 cell development: dependency on antigen dose and differential Toll-like receptor ligation. J. Exp. Med. 2003;197:101–109. doi: 10.1084/jem.20021908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Salio M, Palmowski MJ, Atzberger A, Hermans IF, Cerundolo V. CpG-matured murine plasma-cytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J. Exp. Med. 2004;199:567–579. doi: 10.1084/jem.20031059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoeffel G, Ripoche AC, Matheoud D, Nascimbeni M, Escriou N, et al. Antigen crosspresentation by human plasmacytoid dendritic cells. Immunity. 2007;27:481–492. doi: 10.1016/j.immuni.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 105.Moseman EA, Liang X, Dawson AJ, Panoskaltsis-Mortari A, Krieg AM, et al. Human plasma-cytoid dendritic cells activated by CpG oligodeoxynucleotides induce the generation of CD4+CD25+ regulatory T cells. J. Immunol. 2004;173:4433–4442. doi: 10.4049/jimmunol.173.7.4433. [DOI] [PubMed] [Google Scholar]
- 106.Ito T, Yang M, Wang YH, Lande R, Gregorio J, et al. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J. Exp. Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martin-Gayo E, Sierra-Filardi E, Corbi AL, Toribio ML. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood. 2010;115:5366–5375. doi: 10.1182/blood-2009-10-248260. [DOI] [PubMed] [Google Scholar]
- 108.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J. Clin. Investig. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat. Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- 110.Irla M, Küpfer N, Suter T, Lissilaa R, Benkhoucha M, et al. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J. Exp. Med. 2010;207:1891–1905. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hadeiba H, Sato T, Habtezion A, Oderup C, Pan J, Butcher EC. CCR9 expression defines tolerogenic plasmacytoid dendritic cells able to suppress acute graft-versus-host disease. Nat. Immunol. 2008;9:1253–1260. doi: 10.1038/ni.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Johnson BA3rd, Kahler DJ, Baban B, Chandler PR, Kang B, et al. B-lymphoid cells with attributes of dendritic cells regulate T cells via indoleamine 2,3-dioxygenase. Proc. Natl. Acad. Sci. USA. 2010;107:10644–10648. doi: 10.1073/pnas.0914347107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koyama M, Hashimoto D, Aoyama K, Matsuoka K, Karube K, et al. Plasmacytoid dendritic cells prime alloreactive T cells to mediate graft-versus-host disease as antigen-presenting cells. Blood. 2009;113:2088–2095. doi: 10.1182/blood-2008-07-168609. [DOI] [PubMed] [Google Scholar]
- 114.Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, et al. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Albert ML, Decalf J, Pol S. Plasmacytoid dendritic cells move down on the list of suspects: in search of the immune pathogenesis of chronic hepatitis C. J. Hepatol. 2008;49:1069–1078. doi: 10.1016/j.jhep.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 116.Takahashi K, Asabe S, Wieland S, Garaigorta U, Gastaminza P, et al. Plasmacytoid dendritic cells sense hepatitis C virus-infected cells, produce interferon, and inhibit infection. Proc. Natl. Acad. Sci. USA. 2010;107:7431–7436. doi: 10.1073/pnas.1002301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yonkers NL, Rodriguez B, Milkovich KA, Asaad R, Lederman MM, et al. TLR ligand-dependent activation of naive CD4 T cells by plasmacytoid dendritic cells is impaired in hepatitis C virus infection. J. Immunol. 2007;178:4436–4444. doi: 10.4049/jimmunol.178.7.4436. [DOI] [PubMed] [Google Scholar]
- 118.Decalf J, Fernandes S, Longman R, Ahloulay M, Audat F, et al. Plasmacytoid dendritic cells initiate a complex chemokine and cytokine network and are a viable drug target in chronic HCV patients. J. Exp. Med. 2007;204:2423–2437. doi: 10.1084/jem.20070814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fitzgerald-Bocarsly P, Jacobs ES. Plasmacytoid dendritic cells in HIV infection: striking a delicate balance. J. Leukoc. Biol. 2010;87:609–620. doi: 10.1189/jlb.0909635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meier A, Chang JJ, Chan ES, Pollard RB, Sidhu HK, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat. Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mandl JN, Barry AP, Vanderford TH, Kozyr N, Chavan R, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat. Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 122.Wolf AI, Buehler D, Hensley SE, Cavanagh LL, Wherry EJ, et al. Plasmacytoid dendritic cells are dispensable during primary influenza virus infection. J. Immunol. 2009;182:871–879. doi: 10.4049/jimmunol.182.2.871. [DOI] [PubMed] [Google Scholar]
- 123.Jung A, Kato H, Kumagai Y, Kumar H, Kawai T, et al. Lymphocytoid choriomeningitis virus activates plasmacytoid dendritic cells and inducesacytotoxic T-cell response via MyD88. J. Virol. 2008;82:196–206. doi: 10.1128/JVI.01640-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dalod M, Salazar-Mather TP, Malmgaard L, Lewis C, Asselin-Paturel C, et al. Interferon α/β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 2002;195:517–528. doi: 10.1084/jem.20011672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang H, Peters N, Schwarze J. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J. Immunol. 2006;177:6263–6270. doi: 10.4049/jimmunol.177.9.6263. [DOI] [PubMed] [Google Scholar]
- 126.Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J. Exp. Med. 2006;203:1153–1159. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting edge: plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J. Immunol. 2006;177:7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- 128.Cervantes-Barragan L, Zust R, Weber F, Spiegel M, Lang KS, et al. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109:1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bocharov G, Zust R, Cervantes-Barragan L, Luzyanina T, Chiglintsev E, et al. A systems immunology approach to plasmacytoid dendritic cell function in cytopathic virus infections. PLoS Pathog. 2010;6:e1001017. doi: 10.1371/journal.ppat.1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zuniga EI, Liou LY, Mack L, Mendoza M, Oldstone MB. Persistent virus infection inhibits type I interferon production by plasmacytoid dendritic cells to facilitate opportunistic infections. Cell Host Microbe. 2008;4:374–386. doi: 10.1016/j.chom.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lee LN, Burke S, Montoya M, Borrow P. Multiple mechanisms contribute to impairment of type 1 interferon production during chronic lymphocytic choriomeningitis virus infection of mice. J. Immunol. 2009;182:7178–7189. doi: 10.4049/jimmunol.0802526. [DOI] [PubMed] [Google Scholar]
- 132.Ronnblom L, Alm GV. A pivotal role for the natural interferon a-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J. Exp. Med. 2001;194:F59–F63. doi: 10.1084/jem.194.12.f59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ronnblom L, Alm GV, Eloranta ML. Type I interferon and lupus. Curr. Opin. Rheumatol. 2009;21:471–477. doi: 10.1097/BOR.0b013e32832e089e. [DOI] [PubMed] [Google Scholar]
- 134.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-α production. J. Exp. Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 136.Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, et al. Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 2009;206:1983–1994. doi: 10.1084/jem.20090480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon-α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 2001;159:237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. FcγRIIais expressed on natural IFN- α-producing cells (plasmacytoid dendritic cells) and is required for the IFN- α production induced by apoptotic cells combined with lupus IgG. J. Immunol. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 139.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Investig. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007;8:487–496. doi: 10.1038/ni1457. [DOI] [PubMed] [Google Scholar]
- 142.Lepelletier Y, Zollinger R, Ghirelli C, Raynaud F, Hadj-Slimane R, et al. Toll-like receptor control of glucocorticoid-induced apoptosis in human plasmacytoid pre-dendritic cells (pDC) Blood. 2010;107:15181–15186. doi: 10.1182/blood-2010-05-282913. [DOI] [PubMed] [Google Scholar]
- 143.Guiducci C, Gong M, Xu Z, Gill M, Chaussabel D, et al. TLR recognition of self nucleic acids hampers glucocorticoid activity in lupus. Nature. 2010;465:937–941. doi: 10.1038/nature09102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bave U, Nordmark G, Lovgren T, Ronnelid J, Cajander S, et al. Activation of the type I interferon system in primary Sjogren’s syndrome: a possible etiopathogenic mechanism. Arthritis Rheum. 2005;52:1185–1195. doi: 10.1002/art.20998. [DOI] [PubMed] [Google Scholar]
- 145.Isaksson M, Ardesjo B, Ronnblom L, Kampe O, Lassmann H, et al. Plasmacytoid DC promote priming of autoimmune Th17 cells and EAE. Eur. J. Immunol. 2009;39:2925–2935. doi: 10.1002/eji.200839179. [DOI] [PubMed] [Google Scholar]
- 146.Bailey-Bucktrout SL, Caulkins SC, Goings G, Fischer JA, Dzionek A, Miller SD. Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis. J. Immunol. 2008;180:6457–6461. doi: 10.4049/jimmunol.180.10.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J. Immunol. 2007;179:5041–5053. doi: 10.4049/jimmunol.179.8.5041. [DOI] [PubMed] [Google Scholar]
- 148.Tisch R, Wang B. Role of plasmacytoid dendritic cells in type 1 diabetes: friend or foe? Diabetes. 2009;58:12–13. doi: 10.2337/db08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shlomchik MJ. Activating systemic autoimmunity: B’s, T’s, and tolls. Curr. Opin. Immunol. 2009;21:626–633. doi: 10.1016/j.coi.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kwissa M, Amara RR, Robinson HL, Moss B, Alkan S, et al. Adjuvanting a DNA vaccine with a TLR9 ligand plus Flt3 ligand results in enhanced cellular immunity against the simian immunodeficiency virus. J. Exp. Med. 2007;204:2733–2746. doi: 10.1084/jem.20071211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yao Y, Richman L, Higgs BW, Morehouse CA, de los Reyes M, et al. Neutralization of interferon-α/β-inducible genes and downstream effect in a phase I trial of an anti-interferon-αmonoclonal antibody in systemic lupus erythematosus. Arthritis Rheum. 2009;60:1785–1796. doi: 10.1002/art.24557. [DOI] [PubMed] [Google Scholar]
- 152.Barrat FJ, Meeker T, Chan JH, Guiducci C, Coffman RL. Treatment of lupus-prone mice with a dual inhibitor of TLR7 and TLR9 leads to reduction of autoantibody production and amelioration of disease symptoms. Eur. J. Immunol. 2007;37:3582–3586. doi: 10.1002/eji.200737815. [DOI] [PubMed] [Google Scholar]