Since 2003, there have been 573 World Health Organization (WHO)–documented cases of avian H5N1 influenza infections in humans reported from 15 countries (1). Of the WHO-confirmed cases, 58.6% have resulted in death (as of 15 December 2011) (1, 2). These severe H5N1 infections were diagnosed by using criteria developed by the WHO that are specific for H5N1 disease but that lack the sensitivity to identify the total number of human infections (table S1) (3).
Given the fact that most H5N1 infections in poultry and in humans occur in resource-poor areas where access to health care is often arduous and expensive to obtain, we hypothesized that many people with H5N1 virus infection would not have been examined by a health provider to allow formal H5N1 disease confirmation. In addition, persons who are seropositive for H5N1 infection often report no history of influenza-like illness, and subclinical or mild H5N1 infections are not recognized under the WHO criteria for confirmed cases (4–8).
We conducted a meta-analysis of studies that evaluated the serological evidence of H5N1 infections in humans (table S2). The study participants, by and large, reported no recent respiratory and/or febrile illness. Variation between studies was taken into account by using a random effects approach (9). We included in our primary analysis only studies that assessed serum samples on the basis of modified WHO guidelines (N = 19 study groups) or that presented data in such a way that modified WHO guidelines for H5N1 seropositivity could be applied [for modified WHO guidelines, see (10)]. In a secondary analysis, we compiled data from the remaining manuscripts on the basis of the authors’ criteria for positivity (N = 10). Study participants with confirmed H5N1 infection were excluded to allow an analysis of seroprevalence in the persons without WHO-documented infection; exposure to poultry or humans with confirmed or suspected H5N1 infection was not a criterion for exclusion.
The primary analysis using WHO criteria had its basis in 7304 study participants. All studies reported rates of seropositivity ranging from 0 to 5.3%, with the exception of one study reporting 11.7% positivity among household contacts of infected individuals (5). By using WHO criteria, meta-analysis revealed an overall seropositivity rate of 1.2% with a 95% confidence interval of 0.6 to 2.1% (Fig. 1A). Analysis of studies that could not be interpreted by WHO guidelines [(10), table S2, and references therein] included 6774 participants and yielded a seropositivity rate of 1.9% with a 95% confidence interval of 0.5 to 3.4% (Fig. 1B). With either criterion, the rate of human H5N1 infections within the study populations was about 1 to 2% (10).
Fig. 1.
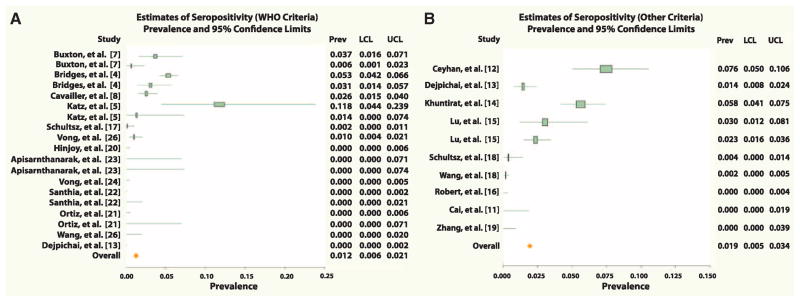
Forest plots of overall and study-specific seroprevalence estimates with 95% confidence limits. Analyses used WHO criteria (A) or other criteria (10) (B) for seropositivity. Individual values for prevalence (seropositivity); lower and upper confidence limits are shown at the right of each plot.
With WHO criteria, we performed subanalyses of study participants who were specifically employed as poultry workers (N = 2729) (4) (table S2). This analysis revealed a seropositivity rate of about 1.4%. If reports from the 1997 outbreak in Hong Kong are considered separately, the rate of seropositivity is about 3.2% (4, 5, 7). Studies after 1997 that use WHO criteria show an overall seropositivity rate of about 0.5% (8) (table S2).
The data were compiled from 12,677 study participants in 20 studies. They show that avian H5N1 viruses can cause a rate of mild or subclinical infections in humans that is not currently accounted for; thus, the true fatality rate for H5N1 influenza viruses is likely to be less than the frequently reported rate of more than 50%. Although it is not possible to determine an accurate fatality rate for H5N1 infections on the basis of the data presented here, if one assumes a 1 to 2% infection rate in exposed populations, this would likely translate into a large number of missed infections worldwide. It is possible that deaths caused by H5N1 infection, as documented by the WHO, are also underestimated. We suggest that further investigation, on a large scale and by a standardized approach, is warranted to better estimate the total number of H5N1 infections that have occurred in humans. This information is critical for calculation of a real fatality rate that is not solely based on hospitalized patients.
Supplementary Material
Acknowledgments
The authors thank N. Pica for generous assistance in the preparation of this manuscript and H. Jia for statistical analysis. This work was partially supported by NIH grants U54 AI057158-04 and HHSN2662000700010C. T.T.W. was supported by NIH training grant T32 AI007647 and Mount Sinai medical scientists training grant T32 GM007280.
Footnotes
References and Notes
- 1.WHO. Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO. 2011 www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html.
- 2.Abdel-Ghafar AN, et al. N Engl J Med. 2008;358:261. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 3.WHO. WHO case definitions for human infections with influenza A(H5N1) virus. 2006 www.who.int/influenza/resources/documents/case_definition2006_08_29/en/
- 4.Bridges CB, et al. J Infect Dis. 2002;185:1005. doi: 10.1086/340044. [DOI] [PubMed] [Google Scholar]
- 5.Katz JM, et al. J Infect Dis. 1999;180:1763. doi: 10.1086/315137. [DOI] [PubMed] [Google Scholar]
- 6.Buchy P, et al. PLoS ONE. 2010;5:e10864. doi: 10.1371/journal.pone.0010864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buxton Bridges C, et al. J Infect Dis. 2000;181:344. doi: 10.1086/315213. [DOI] [PubMed] [Google Scholar]
- 8.Cavailler P, et al. J Clin Virol. 2010;48:123. doi: 10.1016/j.jcv.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 9.DerSimonian R, Laird N. Control Clin Trials. 1986;7:177. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Materials and methods in supporting material on Science Online.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


