Abstract
The development of tyrosine kinase inhibitors (TKIs) has led to extended lifespans for many patients with chronic myelogenous leukemia (CML). However, 20% to 30% of patients fail to respond, respond suboptimally, or experience disease relapse after treatment with imatinib. A key factor is drug resistance. The molecular mechanisms implicated in this resistance include those that involve upregulation or mutation of BCR-ABL kinase and those that are BCR-ABL independent. The clinical consequences of these molecular mechanisms of resistance for disease pathogenesis remain open for debate. This review summarizes the molecular mechanisms and clinical consequences of TKI resistance and addresses the current and future treatment approaches for patients with TKI-resistant CML.
Keywords: BCR-ABL mutations, Molecular mechanisms, T315I
Introduction
Chronic myelogenous leukemia (CML) is a myeloproliferative hematologic neoplasm associated with a chromosomal translocation that gives rise to the Philadelphia (Ph) chromosome and the fusion BCR-ABL gene. Before the introduction of tyrosine kinase inhibitor (TKI) therapy, the disease was inevitably life-shortening. Understanding of the pathophysiology underlying CML has facilitated the development of targeted agents: TKIs such as imatinib, dasatinib, and nilotinib have succeeded in altering the course of the disease and extending life to potentially near-normal spans for many patients. Despite this remarkable achievement, the challenge of overcoming resistance to TKI therapy persists in the management of CML.
It is now known that approximately 20% to 30% of patients with CML fail to respond to imatinib or experience disease relapse after an initial response.1 Much progress has been made in the identification of the molecular mechanisms of resistance in vitro and their clinical impact.2–4 However, the clinical significance of certain mechanisms of resistance and the consequences for disease pathogenesis are ongoing matters for investigation and debate. We provide an overview of the BCR-ABL-independent and -dependent mechanisms of TKI resistance, including the clinical consequences of the more extensively studied BCR-ABL mutations. We also discuss identification of BCR-ABL mutations and other molecular indicators of drug response that may be used to predict treatment responses and influence clinical decision-making. Finally, we address the current approaches to the treatment of patients with TKI-resistant CML, agents in development, and the use of newer agents to ensure optimal clinical outcomes.
Defining the Lack of a Response to Therapy
Responses to TKI treatment are described in terms of hematologic, cytogenetic, and molecular outcomes.5,6 Suboptimal response and treatment failure have been defined in terms of these outcomes at certain time points (Table 1) by the European LeukemiaNet (ELN) guidelines.6,7 The ELN guidelines also provide warning signs, indicating possibility of treatment failure or suboptimal response.6 However, the applicability of the ELN milestones has been questioned, particularly in the case of newly diagnosed patients with chronic-phase CML receiving second-generation TKI therapy.8 There is increasing evidence for the use of molecular monitoring (i.e., measurement of BCR-ABL transcript level) to assess response and predict outcome early in the course of first-line treatment.9–13 The National Comprehensive Cancer Network® (NCCN® ) currently recommends molecular monitoring at 3 months and defines an inadequate molecular response as a BCR-ABL/ABL transcript level of > 10% (as determined by quantitative real-time polymerase chain reaction [PCR] using the international scale).5 This is likely to be refined in the future as more information is accrued on optimal timing and threshold transcript levels for different treatments and standardization of measurements.
Table 1.
European LeukemiaNet Proposed Criteria for Defining Treatment Failure and Suboptimal Response for Chronic Myelogenous Leukemia
| Evaluation Time (mo) | Failure of Response | Suboptimal Response | Warning Signs |
|---|---|---|---|
| 0 | NA | NA | High risk; CCA/Ph+a |
| 3 | Less than CHR | No CyR (Ph+ > 95%) | NA |
| 6 | No CyR (Ph+ > 95%) | Less than PCyR (Ph+ > 35%) | NA |
| 12 | Less than PCyR (Ph+ > 35%) | PCyR (Ph+ 1%−35%) | Less than MMolR |
| 18 | Less than CCyR | Less than MMolRb | NA |
| At any time | Loss of CHR; loss of CCyR; mutations poorly sensitive to imatinib; CCA/Ph+ | Loss of MMolR; mutations still sensitive to imatinib | Increase in transcript levels;c CCA/Ph− |
Abbreviations: CCA = clonal chromosome abnormalities; CCyR = complete cytogenetic response; CHR = complete hematologic response; CyR = cytogenetic response; MMolR = major molecular response; NA = not applicable; PCyR = partial cytogenetic response; Ph+ = Philadelphia chromosome positive; Ph− = Philadelphia chromosome negative.
CCA/Ph+ at diagnosis is considered to be a warning factor, and its occurrence during treatment is a marker of treatment failure.
MMolR indicates a ratio of BCR-ABL1:ABL1 or other housekeeping genes of ≤ 0.1% on the international scale.
Two consecutive cytogenetic tests should be performed, and these must show the same CCA in ≥ 2 Ph+ cells.
Reproduced with permission from Baccarani M et al. J Clin Oncol 27(35), 2009: 6041–6051.6 American Society of Clinical Oncology. All rights reserved.
How Many Patients Fail to Achieve the Response Milestones?
The management of CML has been transformed by the use of imatinib (STI571), with an estimated 5-year overall survival rate of 89% being achieved in patients receiving imatinib therapy alone during follow-up of the pivotal International Randomized Study of Interferon and STI571 (IRIS) clinical trial.14 However, for a proportion of patients, single-agent imatinib therapy is not sufficient to control their disease. Some patients may respond suboptimally, and others fail to respond at all.
The lack of hematologic response to imatinib is exceptional in newly diagnosed cases of Ph-positive chronic-phase CML2,5 and occurs in approximately 5% of patients in whom interferon-alfa therapy had previously failed.15 Failure to achieve a cytogenetic response within 18 months of initial imatinib therapy is more commonly observed; for example, in the IRIS trial, 23.8% of patients receiving imatinib failed to reach this milestone.16 In addition, clinical response in patients with chronic-phase CML decreases during the course of imatinib therapy. This is demonstrated by Alvarado et al.,17 who reported that the frequency of suboptimal response in patients with chronic-phase CML receiving first-line imatinib was 4%, 8%, and 40% after 6, 12, and 18 months of therapy, respectively.
Resistance to Imatinib Therapy
Patients respond suboptimally or fail to respond to imatinib for a variety of reasons, including lack of adherence to prescribed treatment because of toxicity, pharmacokinetic factors, and drug resistance due to molecular/cytogenetic mechanisms. In the published literature, there is little information on the relative contribution made by each of these factors to the lack of response in study populations; however, drug resistance is a key factor.
Resistance to imatinib is divided into 2 categories: primary (innate) resistance and secondary resistance (also referred to as “acquired resistance”). Patients with primary resistance display a lack of response from the start of therapy, whereas patients with secondary resistance achieve a degree of response for a variable length of time before the development of resistance. In describing poor response, investigators often refer to patients as being “resistant” even though the underlying cause of the poor response may not have clearly determined to be specifically drug resistance. The true incidence of drug resistance is thus unclear. In one study, Hochhaus et al.2 examined the underlying causes of poor responses to imatinib. In patients in different phases of CML progression, the authors found that 4% of those with primary resistance and 74% of those who relapsed after a good response had a detectable molecular/cytogenetic mechanism of resistance. In other studies, suboptimal response has mostly been reported in terms of the occurrence of point mutations in the BCR-ABL tyrosine kinase domain, which will be discussed later in the article.
BCR-ABL—Independent Mechanisms of Resistance
Pharmacokinetics and Oral Bioavailability
Analysis of imatinib pharmacokinetics and pharmacodynamics has revealed considerable interpatient variability in drug exposure. Failure to achieve a hematologic response has been observed in patients with low imatinib plasma levels, suggesting that at least some cases of primary resistance to imatinib may result from exposure to inadequate drug levels.18–20 The intrinsic variability of the cytochrome P450 (CYP) enzyme system 18,19 may also affect the response to imatinib. CYP isoenzyme 3A4 (CYP3A4) is the major isoenzyme that metabolizes imatinib, and its levels vary between individuals.21 The involvement of CYP3A4 also means that systemic imatinib concentrations are susceptible to change if the patient is concurrently receiving other drugs.
Another proposed mechanism underlying resistance to imatinib is the binding of a plasma acute phase protein, α1-acid glycoprotein (AGP), to imatinib. Binding to AGP interferes with imatinib’s biologic activity because only non—protein-bound imatinib is available for cellular uptake.22 However, the clinical relevance of this binding is a matter of debate.23
Inadequate Intracellular Concentrations
Intracellular concentrations of imatinib may be affected by transporters involved in drug influx and efflux.24,25 The human organic cation transporter (hOCT1) protein mediates imatinib influx, and single nucleotide polymorphisms in hOCT1 have been associated with in vitro resistance in the imatinib-sensitive human leukemic cell line (K562), the prediction of clinical response, treatment failure, and the need to increase imatinib doses.25–27 However, a clear correlation between hOCT1 expression and patient response to imatinib has not been fully elucidated.25,28
The adenosine triphosphate-binding cassette (ABC) transporter ABCB1 is a transmembrane protein that mediates multidrug resistance through the regulation of efflux of several chemotherapeutic agents and is overexpressed in patients with blast-phase CML.24 Overexpression of ABCB1 has also been identified in patients with accelerated-phase CML who have experienced disease progression 3 months after receiving imatinib, suggesting that ABCB1 expression could influence imatinib resistance when CML is not fully controlled.29 However, the activity of ABCB1 in imatinib efflux is low compared with that of other cytotoxic agents, and ABCB1 overexpression was not found to confer resistance in K562 cells.30
Activation of Alternative Signaling Pathways
Because imatinib does not completely eliminate BCR-ABL—expressing leukemic cells, researchers investigated alternative signaling pathways that may affect CML.31,32 The Src family kinases (SFKs) are nonreceptor, intracellular tyrosine kinases that regulate signal-transduction pathways involved in cell growth, differentiation, and survival.32 BCR-ABL kinase activates 3 SFKs (Lyn, Hck, and Fgr) resulting in cell cycle progression from G1 to S phase.1,33 Hck and Lyn kinase expression in specimens from patients with blast-phase CML has been associated with disease progression and resistance.34 Furthermore, in cells with high levels of Lyn expression, imatinib was not sufficient to fully disengage BCR-ABL—mediated signaling; for optimal treatment efficacy, inhibition of both BCR-ABL and Lyn kinase was required.35 The Src pathway seems to be a critical BCR-ABL—independent mechanism whereby leukemic cells survive imatinib treatment and for CML transition to lymphoid blast crisis, although the factors involved in the stimulation of this pathway are unclear.31
Clonal Evolution
Clonal evolution occurs when CML cells acquire additional chromosomal abnormalities, such as trisomy 8, del(20q), a second Ph chromosome, or aberrations of chromosome 17p.36–38 In patients with hematologic resistance/recurrence after imatinib treatment, Lahaye et al.37 reported the incidence of cytogenetic aberrations to be approximately 50% in patients with chronic and accelerated phases of disease and 73% in patients in blast crisis. At least some of the abnormalities are associated with poor response to imatinib.39–41 For example, chromosome 17 abnormalities are associated with a poor outcome.39 This is possibly because of changes to the TP53 gene, which encodes the tumor suppressor protein p53, because inactivation of p53 reduces sensitivity to imatinib.42 Other anomalies (e.g., trisomy 8) seem to have little impact on imatinib response.39
Epigenetic Modulation
Gene expression and subsequent protein function are affected by the action of epigenetic methylation and post-translation acetylation.43 Epigenetic methylation of nonhistone proteins can affect gene expression and support cellular proliferation and resistance to apoptosis.43 Lee and colleagues44 reported in vitro imatinib resistance in CML cell lines due to alterations in the regulation of histone deacetylases and histone acetyltransferases. An example of the impact of methylation was described by San José-Eneriz et al:45 Reduced expression of the pro-apoptotic B-cell lymphoma 2—interacting mediator was associated with poor response to imatinib in patients with CML, and downregulation of this apoptosis mediator was found to be controlled by hypermethylation. However, because clinical trials have reported only modest responses in patients with CML treated with hypomethylating agents alone or in combination with TKIs, it is unclear whether targeting this process is effective.46,47
Stem Cell Persistence
Some patients with undetectable levels of BCR-ABL transcripts may experience a recurrence of disease after discontinuation of imatinib therapy, which suggests that CML cells persist despite being below detectable limits.48 This observation and the biphasic nature of BCR-ABL transcript clearance during imatinib exposure indicate that there are differences in the susceptibility of CML cell subpopulations to imatinib.1,49 Current research indicates that differentiated cells are rapidly cleared by imatinib; however, CML stem cells are unaffected because of their quiescent nature, leading to disease relapse.1 Quiescent CML stem cells are inherently resistant to imatinib and make up approximately 5% of the CD34-positive cell population.50 Two investigations have shown that imatinib does not induce apoptosis in primitive CML cells; these cells express only a single copy of BCR-ABL but are capable of expressing considerably higher levels of BCR-ABL transcripts and BCR-ABL protein compared with more mature CML cells.51,52 Corbin et al.53 also reported that primitive CML cells are capable of BCR-ABL—independent survival and are not eliminated by imatinib. In the absence of BCR-ABL activity, cytokine support is thought to permit growth and subsequent survival to a level comparable to that of normal stem or progenitor cells.54
BCR-ABL—Dependent Resistance
BCR-ABL Duplication and Amplification
The upregulation of BCR-ABL kinase associated with the amplification of the BCR-ABL gene was first described in imatinib-resistant CML cell lines in the absence of BCR-ABL gene mutations.24 BCR-ABL kinase upregulation was subsequently reported in patients with blast-phase CML or acute lymphoblastic leukemia who developed resistance to imatinib.3 However, the importance of BCR-ABL amplification as a mechanism of resistance was questioned in a further study which found that only 3% of patients with imatinib-resistant CML had BCR-ABL gene amplification.2 Although BCR-ABL overexpression may account for only a small proportion of cases of resistance seen in patients with CML, BCR-ABL protein levels are related to the emergence and rate of emergence of imatinib-resistant subclones. This was demonstrated by Barnes et al.,55 who found that CD34-positive CML cells expressing large amounts of BCR-ABL had reduced sensitivity to imatinib and developed mutations at a higher rate compared with cells expressing BCR-ABL at lower levels.
BCR-ABL Mutations
The activation loop of the BCR-ABL kinase is the major regulatory component of the kinase domain and is able to assume an open/active or closed/inactive conformation.56 The closed/inactive conformation of the BCR-ABL kinase domain is necessary for imatinib binding.57 The anti-tumor activity of imatinib results from it binding to and stabilizing the BCR-ABL kinase domain in the closed/inactive conformation, inhibiting the enzyme activity of BCR-ABL.57 Resistance to imatinib occurs when mutations in the kinase domain of BCR-ABL alter amino acid residues needed for direct contact with the TKI, or prevent BCR-ABL from assuming the inactive conformation required for imatinib binding.56
Point mutations within the kinase domain of BCR-ABL are the most frequently reported reason for imatinib resistance. The reported incidence of BCR-ABL mutations in patients with imatinib-resistant disease varies from 40% to 90% depending on the detection methods used, the definition of resistance applied, and the disease phase examined.1,2,37,58 Recent analyses of patients resistant to imatinib therapy have identified more than 100 distinct point mutations in BCR-ABL.1 Most of the mutations do not occur with a high level of frequency in patients with CML, but 15 mutations comprise approximately 85% of all those detected.59,60 Table 2 shows the relative frequency of the most common mutations as a proportion of all mutations detected in a range of studies in patients receiving imatinib.58–68 Soverini et al.60 noted that amino acid substitutions at 7 residues (M244V, G250E, Y253F/H, E255K/V, T315I, M351T, and F359V) accounted for 85% of all resistance-associated mutations.
Table 2.
Frequencies of Selected Mutations as a Proportion of All Detected Mutations in Patients With Chronic Myelogenous Leukemia Treated With Imatinib
| Study Population | Proportion of Key Mutations (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phosphate-Binding Loop Residues | Imatinib-Binding Site Residues |
Catalytic Loop Residues |
Activation Loop Residues |
|||||||
| G250 | M244 | Y253 | E255 | Q252 | T315 | F317 | F359 | M351 | H396 | |
| Patients treated with imatinib61 | 9 | 7 | 8 | 8 | 3 | 11 | 4 | 9 | 9 | 5 |
| Patients with CML or Ph+ ALL and first evidence of hematologic or cytogenetic resistance to imatinib60 | 10 | 10 | 13 | 17 | 10 | 12 | 12 | 11 | 11 | 4 |
| Patients in all phases who failed to respond to imatinib58 | 15 | 9 | 6 | 3 | 2 | 5 | 11 | 5 | 3 | 6 |
| Patients in all phases who relapsed on imatinib therapy62 | NR | 10 | 12 | 12 | 2 | 16 | 2 | 2 | 12 | 9 |
| Patients in AP resistant to/intolerant of imatinib63 | 15 | 8 | 10 | 13 | NR | 8 | 7 | 15 | 10 | NR |
| Patients in CP resistant to/intolerant of imatinib64 | 18 | 10 | 6 | 7 | 4 | 4 | NR | 3 | NR | 14 |
| Patients in CP treated with imatinib65 | 5 | 18 | 8 | 3 | NR | 5 | 3 | 8 | 5 | 8 |
| Patients in all phases resistant to/intolerant of imatinib, mutations identified before nilotinib therapy66 | 10 | NR | NR | 20 | 10 | 10 | NR | NR | 10 | 10 |
| Patients in AP resistant to/intolerant of imatinib67 | 10 | 17 | 14 | NR | NR | 7 | 10 | NR | 17 | NR |
| Patients in CP resistant to/intolerant of IFN-alfa; < MCyR at 12 mo with imatinib68 | 11 | 11 | 21 | 16 | NR | NR | 5 | 5 | 5 | 11 |
Abbreviations: AP = accelerated phase; CP = chronic phase; IFN = interferon; MCyR = major cytogenetic response; NR = not reported.
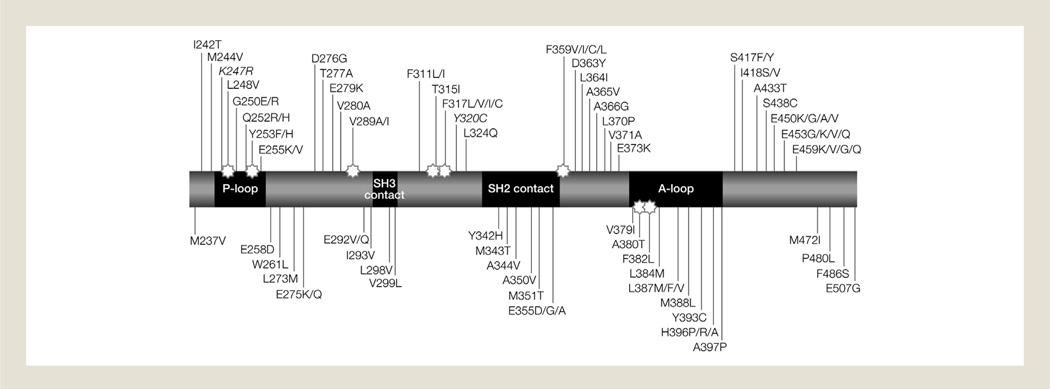
Four different categories of BCR-ABL kinase domain mutations have been described (Fig. 1)69 and include those that affect the imatinib-binding site,3,70 the P loop or adenosine triphosphate (ATP)-binding site,71 the activation (A) loop,59 and the catalytic loop.56
Figure 1.
Schematic Map of Some of the Most Common Amino Acid Substitutions Identified From Clinical Specimens in Patients Resistant to Imatinib. The Stars Highlight Regions Involved in Imatinib Binding. Mutations in Italics Have Been Found to be Single Nucleotide Polymorphismsa
Abbreviations: A loop = activation loop; P loop = phosphate-binding loop; SH2 = SRC homology 2; SH3 = SRC homology 3.
aReproduced with permission of the American Society of Hematology, from Soverini S, et al. Blood 118(5), 2011: 1208–1215,69 permission conveyed through Copyright Clearance Center, Inc.
Point mutations in the imatinib-binding site were first characterized by Gorre et al.,3 who reported that 6 of 9 patients in blast crisis possessed a single-nucleotide mutation resulting in a threonine to isoleucine substitution at amino acid 315 (T315I). The T315I mutation leads to a hydrogen bond being formed with imatinib that prevents imatinib localization within the ATP-binding pocket. This hydrogen bond and the addition of the bulkier side chain of the substituted isoleucine cause steric hindrance to the positioning/binding of imatinib.3,72 This mutation is one of the most frequently detected in patients receiving imatinib and confers resistance to currently available TKIs.73 In clinical practice, T315I comprises approximately 14% of detected mutations.21 Shortly after the identification of T315I, several other mutations were identified in adjacent residues, comprising some of the more common mutations that alter and prevent imatinib binding (e.g., D276G and F317C/L).73
Substitutions in residues 244 to 255 of BCR-ABL affect the P loop and are common in patients with imatinib resistance.71 Amino acid changes in the P loop/ATP-binding site cause conformational alterations in the BCR-ABL protein that lead to destabilization of the conformation needed for imatinib binding.50 Six P loop mutations (M244V, L248V, G250E, Q252H, Y253F/H, and E255K/V) are commonly found in patients with CML.21,74
Mutations affecting the A loop prevent the kinase achieving the inactive conformation.59 H396R is the most commonly identified mutation in this site.56 Catalytic-loop mutations affect tyrosine kinase activity and substitutions at 3 residues (M351, E355, and F359) are commonly found.56
The frequency and impact of mutations in patients with primary resistance are thought to be limited compared with patients who develop secondary resistance. Mutations have been detected with increased frequency in patients who acquired resistance during the course of therapy compared with patients in whom initial imatinib therapy failed.60 The frequency of mutations has been found to increase with sequential TKI therapy.75
The incidence of mutations has been examined in terms of treatment failure and suboptimal response. For example, mutations were detected in 24% of patients with early chronic-phase CML who failed and 13% of patients who had a suboptimal response to first-line imatinib therapy.76 Likewise, Kim and colleagues77 reported mutations in 25% of patients who failed and 13% of patients who responded suboptimally after 12 months of imatinib treatment. At 18 months, these incidences were 62% and 32%, respectively.
Several assessments have found that the degree of imatinib resistance is related to the type and location of the BCR-ABL mutation.78 For example, mutations in the P loop (Y253H and E255K/V) and T315I cause a high level of resistance to imatinib and are related to treatment failure,75 whereas substitutions at M244, F317, and M351 confer low-level resistance and correlate with suboptimal response.79 In addition, different amino acid substitutions occurring at the same position may result in varying degrees of resistance; for example, although high concentrations of imatinib were required to inhibit the proliferation of Ba/F3 pro-B cell-line cells with the T315I mutation, cells with the T315A mutation remained sensitive to the TKI.57 Several studies have observed varying sensitivities of kinase domain mutations to higher doses of imatinib.53,80,81
A few studies have shown that some patients are able to achieve and maintain a cytogenetic response to imatinib despite the presence of BCR-ABL mutations.82–84 For example, Sherbenou et al.84 identified mutations, including T315I, in 19% of patients with stable complete cytogenetic responses during imatinib therapy. On follow-up, half of the patients with mutations experienced an increase in BCR-ABL transcript levels that led to disease relapse, progression, or required an alternative treatment approach.84 Willis et al.85 also reported complete hematologic responses and major cytogenetic responses during first-line imatinib treatment in a small number of patients with BCR-ABL mutations.
The type of mutation within the BCR-ABL kinase domain may vary according to disease phase. Mutations at M244, M351, and G250 have frequently been detected in patients with chronic-phase CML, whereas mutations at T315, E255, and Y253 were often found in patients with accelerated or blast-phase disease.22,60,61 Soverini et al.60 reported that the rate of mutations leading to resistant phenotypes was lower in patients with chronic-phase CML compared with those in accelerated or blast phase.
Significant mutations have also been observed in BCR-ABL regions outside the kinase domain. The SH2, SH3, and Cap domains are involved in autoinhibition of the kinase; mutations in these regions may destabilize the inactive conformation of BCR-ABL.86 Several mutations in these regions have been detected in patients treated with imatinib,84,87 and at least 1 (T212R) has been shown to be associated with patient resistance to imatinib.84
Molecular Monitoring of Resistance
The widespread use of imatinib and the subsequent increase in the overall survival of patients with CML has led to an increase in the number of patients receiving imatinib and the duration of their treatment. As imatinib use increases, so does the number of patients who will require reassessment of their treatment strategy because of resistance issues.88 Therefore, this will lead to an increased requirement to monitor and characterize resistance in patients. Currently, there is no consensus on whether mutational analysis should be performed before treatment initiation or during second-line TKI therapy. However, both the European Treatment and Outcome Study Panel (part of the ELN) and the NCCN Clinical Practice Guidelines In Oncology (NCCN Guidelines® ) recommended that mutational tests should be performed in cases of disease progression and inadequate response to TKIs (Table 3).5,69
Table 3.
| European LeukemiaNet | NCCN |
|---|---|
At Diagnosis
|
In Patients Treated With TKIs
|
During First-line Imatinib Therapy
| |
During Second-line Dasatinib or Nilotinib Therapy
| |
Abbreviations: AP = accelerated phase; BP = blast phase; CCyR = complete cytogenetic response; IS = international scale; MMolR = major molecular response; NCCN = National Comprehensive Cancer Network; PCyR = partial cytogenetic response.
Reproduced with permission of the American Society of Hematology, from Soverini S, etal. Blood 118(5), 2011:1208–121569 permission conveyed through Copyright Clearance Center, Inc.; and adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Myelogenous Leukemia V.4.2013 © National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc.
Several assays currently are available for the detection of BCR-ABL kinase domain mutations, 89 which vary in terms of detection frequency, sensitivity, and the clinical utility of the results. Direct sequencing (DS) is the most commonly used (by approximately 80% of American and Canadian laboratories) and is recommended by the European Treatment and Outcome Study Panel.69,90 DS is the least sensitive assay, detecting a mutation in approximately 1 in 5 BCR-ABL transcripts, but this level of sensitivity is considered to be appropriate because the clinical significance of low-level mutations is a matter of debate.89,90 With the addition of denaturing-high performance liquid chromatography (D-HPLC), the sensitivity of DS can be improved to detecting a mutation in approximately 1 in 1000 BCR-ABL transcripts.91 D-HPLC is a cost-effective, high-throughput assay used to screen for sequence variations before DS, and it reduces the number of samples that need to be sequenced.69 The clinical utility of D-HPLC/DS was demonstrated by Ernst et al.,62 who reported that BCR-ABL mutations were detectable a median of 7.1 months before hematologic relapse. Other screening methods include mutation-specific PCR-based assays (fluorescence allele-specific PCR, nanofluidic digital arrays, and peptide nucleic acid clamping) and liquid bead array high-resolution melt-curve analysis. 89
Although there is no doubt that the PCR-based methods are highly sensitive, these techniques are specific for known mutations and cannot be used to detect novel mutations.92 However, they may increase the diagnostic window from 7.1 months to 10.1 months by detecting the known highly resistant mutations earlier than D-HPLC/DS.92 This may provide physicians with the opportunity to reassess the treatment strategy before the clinical impact of resistance is felt by the patient.92 It should be noted that the detection of low-level mutations via these highly sensitive assays may not correlate with treatment failure because these mutations may not represent clones that will survive and dominate unmutated cells.69
Additional factors, such as cost, specificity, labor/equipment requirements, turnaround time, and vulnerability to contamination, also affect the feasibility of using such assays in clinical practice.89 The increase in the number of highly sensitive assays available for BCR-ABL mutation analysis and the possible increase in their use have raised important issues regarding the standardization of these tests and the transferability of the assays to routine diagnostic procedure. When comparing the results of mutational assays, consideration must be given to the lack of standardization between laboratories with respect to the reagents and technology platforms used.90 This was demonstrated by a survey of American and Canadian accredited clinical laboratories performing routine BCR-ABL mutational analysis, which found that 3 different methods (DS, pyrosequencing, and microarray or liquid bead array) were used by 14 laboratories.90 Furthermore, only a small proportion of laboratories performed T315I analysis or reported whether the mutation(s) identified were known to confer resistance.90 Not only is there a need for standardization of BCR-ABL mutation assays, but also tools are required to aid physicians in the interpretation of assay findings.
Many physicians are unaware of the benefits of mutational analysis for the prediction of treatment failure/suboptimal response and the influence that mutational status should have when choosing a second-line TKI.88 A 2007 survey of American and European physicians who had treated at least 4 patients with CML reported that 60% of US-based respondents were unfamiliar with mutational testing or had never requested a test.93 This underuse of mutational analysis in the management of patients with CML in clinical practice was also highlighted by Morra et al.,94 who found that 57% of patients with imatinib-resistant disease had not been assessed for mutations.
In addition to assessing disease burden, BCR-ABL transcript levels may serve as a determinant of resistance.95 For example, Wang et al.96 reported that increases in transcript levels could be used to identify patients who develop BCR-ABL mutations. Other molecular predictors of response may also aid the selection of appropriate treatment strategies in patients with imatinib-resistant disease. As described earlier, hOCT1 activity is considered to be a determinant of resistance. Some studies have led to the suggestion that low hOCT1 levels may indicate patients who are prone to the development of resistance via BCR-ABL mutations.97
Available and Future Treatment Approaches to Overcome Resistance
In cases of treatment failure, CML treatment guidelines state that further imatinib therapy at the current dose is no longer the appropriate treatment strategy.5,6 Although patients with a suboptimal response to imatinib may benefit from the continuation of therapy, the long-term outcome for these patients is expected to be unfavorable if they remain on this treatment.98 It should be remembered that although the goal of therapy is achieving a complete cytogenetic response, when considering second-line therapy, patients often achieve lesser responses; however, patients achieving a partial or minor cytogenetic response still derive a survival benefit.99
Dose Escalation
Higher imatinib doses (up to 800 mg/day) may be considered to gain a response in patients with resistance.57,100–102 A retrospective analysis of the pivotal IRIS trial of patients who received the increased doses of imatinib concluded that dose escalation is an effective option in patients with chronic-phase disease who experience suboptimal cytogenetic response or cytogenetic relapse.100 More specifically, imatinib dose escalation may be an attractive therapeutic option in patients who have mutations that encode low-level resistance (e.g., M351T) or in patients whose resistance is due to insufficient plasma levels of imatinib or to BCR-ABL gene amplification.19,20 However, dose escalation is unlikely to have any benefit for patients who have never achieved a cytogenetic response during imatinib therapy.101
Imatinib and Interferon-Alfa Combination Therapy
In patients who respond to imatinib, indefinite imatinib therapy is recommended to prevent relapse and disease progression.103 However, this strategy raises concerns regarding the evolution of drug resistance.104 Considerable efforts are being made to develop treatment approaches that generate long-term remission and permit treatment discontinuation.105,106 One such approach is the use of imatinib in combination with interferon-alfa. Interferon-alfa is an effective treatment for CML because this agent stimulates an immune response to CML-specific antigens, such as proteinase-3.106 When interferon-alfa was combined with imatinib, the majority of patients with chronic-phase CML achieved long-term remission, enabling the discontinuation of imatinib therapy.105,106 The clinical effectiveness of this combination has been verified in patients with cytogenetic resistance and those with T315I mutations.107–109 Most interesting of these studies is the successful use of imatinib/dasatinib with pegylated interferon in 2 patients harboring the T315I mutation.107,109 Furthermore, Itonaga et al.109 reported the re-achievement of complete cytogenetic response and the disappearance of the T315I mutation in a patient after imatinib and interferon-alfa therapy.
Second-Generation TKIs
The “second-generation” TKIs dasatinib and nilotinib are approved for the treatment of imatinib-resistant patients. Dasatinib is a piperazinyl derivative that targets tyrosine kinase and is able to bind to BCR-ABL in both the active/open and closed/inactive conformation.6 This agent is also active against SFKs because dasatinib is able to bind to SFKs due to the similar geometry of SFKs and the BCR-ABL active conformation.43 In preclinical comparisons with imatinib, dasatinib was 325 times more potent than imatinib against cells expressing wild-type BCR-ABL.110 Three clinical trials (START-A, START-R, and START-C) demonstrated the superior activity of dasatinib compared with imatinib in patients with imatinib-resistant CML.111–113 Dasatinib has activity against a large proportion of imatinib-resistant BCR-ABL mutations, and during the START-A trial, responses were reported in patients despite the presence of mutations.111 However, Muller et al.114 reported that patients with T315I, F317L, or V299L mutations responded poorly to dasatinib. Low in vitro sensitivity to dasatinib is reported for cells with any of the aforementioned mutations.114 Furthermore, BCR-ABL mutational status may evolve during the course of second-line therapy; several studies have described the emergence of new BCR-ABL mutations in patients with imatinib-resistant disease receiving dasatinib.75,111,115–119 Therefore, when selecting dasatinib as a treatment approach for patients with imatinib-resistant disease, specific mutations present at baseline or arising during the course of dasatinib therapy must be considered. 5,69
Nilotinib is an orally active phenylaminopyrimidine derivative of imatinib and is approximately 30 times more potent than imatinib in inhibiting BCR-ABL.110,120 In vitro studies found that nilotinib inhibits 32 of 33 mutant forms of BCR-ABL resistant to imatinib at physiologically relevant concentrations.110,120 Nilotinib has been evaluated in several investigations in patients with imatinib-resistant chronic-phase CML.121–124 a considerable proportion of patients achieved major molecular response or complete cytogenetic response. However, in vitro analysis of nilotinib found that the agent is not active against cells with the T315I mutation and has low potency against cells with Y253F/H, E255K/V, or F359V/C/I mutations.57,125 The NCCN® and ELN recommend using an alternative to nilotinib in the presence of specific BCR-ABL mutations.5,69
Bosutinib (SKI-606) inhibits BCR-ABL with a 10- to 20-fold higher potency than imatinib, with additional activity against SFKs, c-kit, and the platelet-derived growth factor receptor.126,127 At the 2-year follow-up of a Phase I/II study, Cortes et al.128 observed responses in patients with chronic-phase imatinib-resistant or imatinib-intolerant CML with a range of BCR-ABL mutations except T315I. An analysis conducted after a minimum of 3 years of follow-up demonstrated durable responses and manageable toxicity in these patients (Table 4).130 Bosutinib has also proved effective in patients with chronic-phase CML who had been treated with imatinib followed by dasatinib and/or nilotinib.129,144 A safety analysis concluded that gastrointestinal, hematologic, and liver function adverse events were commonly associated with bosutinib treatment but could be readily managed.145 Bosutinib has recently been approved by the FDA for the treatment of Ph-positive CML in adult patients with resistance or intolerance to prior therapy.
Table 4.
Overview of Emerging Treatment Options for CML Showing Clinical Trial Outcome Data
| Agent | Clinical Trial | Outcomes |
|
|---|---|---|---|
| Efficacy | Safety | ||
| TKIs | |||
| Bosutinib (SKI-606) | Phase I/II study in CP-CML after imatinib only (2L CP cohort n = 286) and in CP-CML after imatinib plus dasatinib or nilotinib (3L CP n = 119). Patients received bosutinib 500 mg once daily129,130 |
3L CP cohort:129 MCyR in 41%, CCyR in 32%, CHR in 73% (median follow-up of 31.4 mo) At 2 years: PFS 75%, OS 84% (Kaplan—Meier estimates) |
Grade 3/4 hematologic AEs included thrombocytopenia (25%), neutropenia (19%) and lymphopenia (17%) Grade 3/4 nonhematologic AEs included diarrhea (8%), rash (3%), headache (3%) |
| 2L CP cohort:130 MCyR in 58% (IM-R) and 60% (IM-I), CCyR in 48% (IM-R) and 51% (IM-I) (minimum 36-mo follow-up) PFS at 3 years: 72% (IM-R), 89% (IM-I); OS at 2 years 88% (IM-R), 98% (IM-I) (Kaplan—Meier estimates) |
Grade 3/4 hematologic AEs included thrombocytopenia (25%), neutropenia (18%), lymphocytopenia (16%), and anemia (14%) Grade 3/4 nonhematologic AEs included diarrhea (10%), rash (9%), and vomiting (4%) |
||
| Ponatinib (AP24534) | Phase I open-label dose-escalation trial in patients with refractory CML or other malignancies (n = 81)131 |
Patients with CP-CML (n = 43): CHR in 98%, MCyR in 72%, CCyR in 63%, MMolR in 44%. MCyR in 92%, and MMolR in 67% with T315I (n = 12) Patients with AP- or BP-CML or Ph+ ALL (n = 22): MHR in 36%, MCyR in 32% |
DLTs included elevated lipase or amylase levels and pancreatitis Grade ≥ 3 AEs: 20% thrombocytopenia, 10% neutropenia, 2% anemia, 7% increased lipase, 5% pancreatitis |
| Phase II Ponatinib Ph+ ALL and CML Evaluation (PACE) trial:132,133 patients with refractory CML (CP, AP, or BP) or Ph+ ALL, resistant or intolerant to dasatinib or nilotinib, or with T315I received 45 mg ponatinib once daily |
Interim data: CP-CML (n = 267; median follow-up 11 mo): MCyR in 55%, CCyR in 46%, MMolR in 32%132 AP-CML (n = 83; median follow-up 13 mo): MCyR in 34% (R/I) and 56% (T315I), CCyR in 22% (R/I) and 33% (T315I), MMolR in 14%133 BP-CML (n = 62; median follow-up 6 mo): MCyR in 18% (R/I) and 29% (T315I), CCyR in 16% (R/I) and 21% (T315I)133 Ph+ ALL (n = 32; median follow-up 6 mo): MCyR in 60% (R/I) and 41% (T315I), CCyR in 50% (R/I) and 32% (T315I)133 |
36% thrombocytopenia, 33% rash, 31% dry skin133 | |
| XL228 | Phase I study in patients with Ph+ leukemias who failed multiple TKI therapies or have T315I mutation134 |
Interim data in patients with BCR-ABL mutations (n = 27): Hematologic responses of stable and decreasing white blood cell/platelet count within 2 mo in 52%, including 50% of patients with T315I. > 1 log reduction in BCR-ABL level within 3 mo in 11%, including 20% of patients with T315I |
DLT of grade 3 syncope and hyperglycemia observed at 10.8 mg/kg (once weekly) Treatment-related grade 2 AEs included hyperglycemia, fatigue, nausea, vomiting, bradycardia |
| Bafetinib (INNO-406) | Phase I study in patients with Ph+ CML or ALL and imatinib resistance/intolerance Bafetinib was administered to patients with resistance to (n = 40 [71%]) or intolerance of (n = 16 [29%]) imatinib (CP-CML n = 31, AP-CML n = 8, BP-CML n = 7).135 |
CML only (n = 46): MCyR in 19% of CP-CML No response in AP- or BP-CML |
DLT of elevated transaminases and thrombocytopenia at 480 mg BID 73% metabolic and laboratory abnormal findings, 64% gastrointestinal AEs, 59% pain, 48% constitutional symptoms, and 34% infection |
| Aurora Kinase Inhibitors | |||
| Danusertib (PHA-739358) |
Phase I study in patients with AP-CML or BP-CML and Ph+ ALL resistant or intolerant to imatinib or second-generation TKIs (n = 23; AP-CML n = 4, BP-CML n = 11)136 |
Interim data (all patients): Hematologic responses in 22% Cytogenetic responses in 13% |
DLT of grade 3 fainting at 90 mg/m2; 57% diarrhea, 50% pyrexia, 43% headache, 36% dyspnea, 36% nausea |
| Phase II study in patients with CML harboring T315I mutations (n = 7; CP-CML n = 1, AP-CML n = 1, BP-CML n = 5, T315I mutation n = 6); patients received danusertib 250 or 330 mg/m2/d137 |
CHR in 29% and CCyR in 14% (T315I–positive) | Grade 4 neutropenia in 14% No grade 3/4 nonhematologic AEs | |
| AT9283 | Phase I study in patients with refractory leukemia (n = 29, patients with refractory AML, ALL, high-risk MDS, and imatinib- and dasatinib-refractory CML)138 |
Hematologic response in 2 patients with refractory CML | DLTs: grade 4 elevation of serum aminotransferases, CK, and LDH at 162 mg/m2/d; TLS at 12 mg/m2/d |
| KW-2449 | A Phase I study in patients with relapsed/refractory AML, ALL, and MDS or resistant/intolerant CML (n = 37, CML n = 5, ALL n = 1, AML n = 31)139 |
1 patient with CML had 50% reduction in peripheral or bone marrow blasts 1 patient with T315I before treatment initiation lost T315I mutation during therapy |
DLTs of grade 3 atrial fibrillation (100 mg/d), nausea, and vomiting (500 mg/d) Grade 3/4 AEs: 24% febrile neutropenia, 11% pneumonia, 11% thrombocytopenia Most common AEs: 70% nausea, 49% vomiting, 46% fatigue, 32% diarrhea, 30% dyspnea, 30% febrile neutropenia, 30% pain in extremity, 27% arthralgia |
| Other | |||
| Omacetaxine | Phase II/III trial in patients with imatinib-resistant CML and T315I mutation; patients received omacetaxine 1.25 mg/m2 BID140,141 |
CP-CML (n = 66): CHR in 77%, MCyR in 23%, CCyR in 16%; median PFS 7.7 mo (median follow-up 19 mo)140 AP-CML (n = 16) and BP-CML (n = 10): CHR in 31% of AP-CML, 20% of BP-CML; CCyR in 6% of AP-CML (median follow-up 6.4 mo)141 |
In patients with CP-CML: grade 3/4 nonhematologic AEs: 8% infection, 5% fatigue; grade 3/4 hematologic AEs: 76% thrombocytopenia, 44% neutropenia, 39% anemia140 |
| Multicenter Phase II/III trial in patients resistant or intolerant to ≥ 2 TKIs (not carrying the T315I mutation) (n = 65; CP-CML n = 30, AP-CML n = 20, BP-CML n = 15); patients received omacetaxine 1.25 mg/m2 BID142 |
Interim data (4-mo follow-up): CHR: 80% of CP-CML, 60% of AP-CML, 40% of BP-CML CCyR: 5% of AP-CML MCyR: 20% of CP-CML MMolR: 10% of CP-CML |
1 treatment-related death (febrile neutropenia) Grade 3/4 AEs: 43% thrombocytopenia, 29% neutropenia, and 22% anemia |
|
| Phase II study of omacetaxine and imatinib in patients with advanced-stage CML or imatinib resistance or after imatinib failure (n = 15; CP-CML n = 3, AP-CML n = 3, BP-CML n = 9, including imatinib resistant n = 12); patients received omacetaxine 2.5 mg/m2/d and imatinib 400 or 600 mg/d143 |
CHR in 33% of BP-CML CyR in 28% of 14 patients, CCyR in 14%, PCyR in 7% |
Grade 3/4 nonhematologic AEs (all causes): 20% fatigue, 13% nausea and vomiting Grade 3/4 hematologic AEs: 93% thrombocytopenia, 80% anemia, 80% neutropenia |
|
Abbreviations: 2L = second line; 3L = third line; AE = adverse event; ALL = acute lymphocytic leukemia; AML = acute myelogenous leukemia; AP = accelerated phase; BID = twice daily; BP = blast phase; CCyR = complete cytogenetic response; CHR = complete hematologic response; CK = creatine kinase; CML = chronic myelogenous leukemia; CP = chronic phase; CyR = cytogenetic response; DLT = dose-limiting toxicity; IM-I = imatinib intolerance; IM-R = imatinib resistance; LDH = lactate dehydrogenase; MCyR = major cytogenetic response; MDS = myelodysplastic syndrome; MHR = major hematologic response; MMolR = major molecular response; OS = overall survival; PCyR = partial cytogenetic response; PFS = progression-free survival; Ph = Philadelphia chromosome; R/I = resistant or intolerant; T315I = patients with the T315I mutation; TKI = tyrosine kinase inhibitor; TLS = tumor lysis syndrome.
Third-Generation TKIs
A number of “third-generation” agents are being evaluated in clinical trials. These agents have similar 3-dimensional structures to the currently approved TKIs, and their main target is BCR-ABL kinase.43
Ponatinib was specifically designed to bind BCR-ABL with very high potency and to have activity against CML with any of the BCR-ABL mutations.146 Early data from Phase I and II trials indicate good responses in pretreated patients, including those with chronic-phase T315I-positive CML (Table 4).131–133 On the basis of response rates, the FDA approved ponatinib for the treatment of adult patients with chronic-, accelerated-, or blast-phase CML, or Ph-positive acute lymphoblastic leukemia that is resistant or intolerant to prior TKI therapy. Ponatinib is currently being assessed as first-line therapy in a Phase II clinical trial in patients with chronic-phase CML (NCT01570868). Although the early clinical trial data are promising, one group has reported both partial and full BCR-ABL—independent resistance to ponatinib in 4 BCR-ABL—positive cell lines grown in the presence of low ponatinib concentrations, which may have important implications for the future use of this agent.147
XL228 is a potent protein kinase inhibitor that has activity against fibroblast growth factor receptor, insulin-like growth factor receptor-1, Src, and Abl.148 In initial analyses of Phase I clinical trial data, this agent demonstrated the ability to induce hematologic or cytogenetic responses in 52% of patients, including 19% of those with the T315I mutation (Table 4).134
Bafetinib (INNO-406) is a dual BCR-ABL/Lyn kinase inhibitor with 25- to 55-fold higher potency than imatinib.43 Although this agent is not active against CML with the T315I mutation, it has demonstrated in vitro activity against CML with multiple P loop mutations.149 In a Phase I clinical trial of heavily pretreated patients with imatinib-resistant and imatinib-intolerant disease, responses were seen only in patients in the chronic phase (Table 4).135
Aurora Kinase Inhibitors
The aurora kinases are key mitotic regulators and control entry into mitosis, centrosome function, and chromosome assembly and segregation.150 They are frequently found to be aberrantly overexpressed in cancer cells, and inhibition of these proteins can result in mitotic catastrophe in leukemia cells.43 Danusertib, a small molecular inhibitor, exhibits activity against all known aurora kinases in addition to BCR-ABL tyrosine kinase, including the T315I variant.150 Preliminary results of a Phase I investigation assessing danusertib in a small number of patients with accelerated or blast-phase CML resistant/intolerant to imatinib or second-generation TKIs reported clinically important responses (Table 4).136 A Phase II study has reported hematologic and cytogenetic responses in a small number of patients with BCR-ABL T315I mutations.137
Two further aurora kinase inhibitors are in development: AT9283 and KW-2449, both of which have demonstrated in vitro activity against cells with the BCR-ABL T315I mutation.43 Clinical trials of these agents are ongoing in patients with TKI-resistant disease, but preliminary results with AT9283 indicate hematologic activity, and treatment with KW-2449 resulted in the disappearance of the T315I clone in a patient with blast-phase CML (Table 4).138,139
Omacetaxine
Omacetaxine mepesuccinate (“omacetaxine”) is a semisynthetic version of homoharringtonine, an agent that was originally recognized as having antitumor activity more than 35 years ago and that demonstrated efficacy in CML as a single agent and in combination with cytosine arabinoside or interferon-alfa. 151,152 Omacetaxine is a first-in-class cephalotaxine, a small molecule that inhibits protein synthesis in a BCR-ABL—independent manner by binding to the 80S ribosome and interfering with protein chain elongation.43,153 By blocking ribosomal function, omacetaxine decreases intracellular levels of several anti-apoptotic regulatory proteins, resulting in apoptosis.154 Allan et al.153 have reported that omacetaxine is also able to induce apoptosis in CML stem cells by inhibiting the synthesis of the anti-apoptotic protein Mcl-1 and that it can inhibit the function of surviving stem cells in a dose-dependent manner.
The clinical effectiveness of omacetaxine was demonstrated in several Phase II and III clinical trials in patients with the T315I mutation, patients with resistance to ≥ 2 TKIs, and patients unable to tolerate TKIs, with reports of sustained hematologic and cytogenetic responses (Table 4).140–143 Subset analyses of pooled data for heavily pretreated patients demonstrated a major cytogenetic response in 20% of those with chronic-phase CML, as well as hematologic responses in patients with accelerated-phase CML and hematologic improvement in a small proportion of patients with blast-phase CML.155–157
In an analysis of pooled safety data from 2 Phase II trials and a small pilot investigation assessing omacetaxine in patients in all phases of CML, the most commonly reported treatment-related grade 3/4 adverse event was thrombocytopenia, followed by neutropenia and anemia.158 The authors concluded that omacetaxine administration was associated with an acceptable toxicity profile.158
A further therapeutic approach is the combination of omacetaxine and a TKI. The combination of these agents has been found to inhibit the proliferation of imatinib-resistant CML cell lines in vitro in a synergistic manner.159 Dual imatinib and omacetaxine therapy in patients in all 3 phases of CML was evaluated by Ayoubi et al.,143 who reported that this combination was effective in patients with advanced disease. Another study reported suppression of CML cells with the T315I mutation and undetectable molecular residual disease in an imatinib-resistant patient treated with omacetaxine and nilotinib combination therapy after single-agent omacetaxine.160
Omacetaxine alone or in combination with other agents in patients with resistant disease, specifically those with T135I mutations, may represent an attractive future treatment approach. Omacetaxine has recently been approved by the FDA for the treatment of adult patients with chronic- or accelerated-phase CML with resistance or intolerance to 2 or more TKIs.
What Is the Feasibility of Treatment Selection Based on BCR-ABL Mutation Type?
The in vitro inhibitory effect (50% inhibitory concentration [IC50]) of all commercially available TKIs has been published for the most common mutations to help clinicians in their choice of TKI for patients known to harbor mutations.127 In vitro IC50 data for imatinib, dasatinib, and nilotinib show that the T315I mutation results in a high level of resistance to all 3 agents.57,127 These data also provide information regarding other mutations with differing levels of resistance to imatinib, dasatinib, and nilotinib. For example, CML cells with Y253F/H mutation are insensitive to imatinib, but display intermediate (moderate) resistance to nilotinib and are sensitive to dasatinib.57,127 Certain common mutations confer a high level of resistance to at least 1 of the 3 commercially available TKIs.127 For example, in cases in which T315I, V299L, T315A, Y253H, E255K/V, and F359V/C/I mutations are present, there is little doubt that a change in treatment strategy is warranted, and alternative therapeutic approaches have been recommended by the NCCN (Table 5) and ELN in such cases.5,69 However, in the case of substitutions at M244, F317, and M351, which correspond to moderate imatinib resistance in CML cells, patients may respond to an increased imatinib dose or another TKI.79 The use of IC50 values alone is insufficient to guide the choice of TKI therapy for many other mutations because the IC50 values determined in vitro do not necessarily correlate with the in vivo situation. For example, IC50 information does not account for the impact of important in vivo factors, such as pharmacokinetics, protein binding, and drug efflux.161 In cases of rare mutations, for which clinical trial evidence is lacking, in vitro data should be considered alongside evidence of treatment failure or suboptimal response.69 However, because the number of new rare kinase domain mutations is increasing for which the sensitivity to imatinib is unknown, the selection of effective therapeutic regimens for patients with uncharacterized mutations may be challenging.
Table 5.
National Comprehensive Cancer Network Recommendations for the Selection of Different Treatment Options Based on BCR-ABL Kinase Domain Mutation Status5
| Mutation | Therapeutic Options | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Imatinib | High-dose Imatinib |
HSCT | Nilotinib | Dasatinib | Ponatinib | Bosutinib | Omacetaxine | Clinical Trial |
|
| T315I | ✓ | ✓a | ✓ | ✓ | |||||
| V299L | ✓ | ✓ | ✓b | ||||||
| T315A | ✓c | ✓ | ✓ | ✓ | ✓b | ||||
| F317L/V/I/C | ✓ | ✓ | ✓ | ✓b | |||||
| Y253H,E255K/V,F359V/I/C | ✓ | ✓ | ✓ | ✓b | |||||
| Any other mutation | ✓d | ✓ | ✓ | ✓ | ✓ | ✓b | |||
Abbreviation: HSCT = hematopoietic stem cell transplantation.
Preferred option.
Omacetaxine is an option for patients with resistance or intolerance to ≥ 2 TKIs.
If mutation detected after dasatinib.
There are not sufficient data on dose escalation available to indicate if mutations with lower IC values are sensitive to high dose imatinib.
Adapted with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Myelogenous Leukemia V.4.2013 © National Comprehensive Cancer Network, Inc. All rights reserved. The NCCN Guidelines® and illustrations herein may not be reproduced in any form for any purpose without the express written permission of the NCCN. To view the most recent and complete version of the NCCN Guidelines, go online to NCCN.org. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc.
What Are the Nonpharmacotherapy Options If Pharmacologic Therapy Fails?
Before imatinib, CML was the most common reason for hematopoietic stem cell transplantation (HSCT).162 Since the introduction of imatinib, the number of patients with chronic-phase CML receiving HSCT has decreased dramatically.43 HSCT is now restricted to patients with advanced-phase CML complex comorbidities. However, HSCT remains the only means of achieving a cure for CML and is recommended by the ELN and NCCN Guidelines® for patients with TKI-resistant CML cells harboring the T315I mutation. 5,69 Stem cell trans-plantation is also an option for patients who have experienced prior hematologic resistance to imatinib and respond suboptimally during second-line TKI therapy.6 As new therapies that target the BCR-ABL T315I mutation continue to be developed, the role of stem cell transplantation in the management of CML will require reassessment.
Conclusions
The advent of TKI therapy for CML has changed the natural history of this hematologic neoplasm, and for many patients there is now the real possibility of long-term survival or even complete resolution. However, the management of patients with imatinib-resistant CML has become increasingly complex. There is no doubt that certain BCR-ABL mutations contribute to the development of resistance in patients with CML. However, TKI-resistant CML is more complex than indicated by the presence of mutations alone, and BCR-ABL—independent mechanisms of resistance also have a considerable clinical impact.
A range of new pharmacotherapies are in development for the treatment of CML. Although physicians are likely to have effective treatment options for patients with imatinib-resistant CML, they now face the challenge of assessing the significance of the large volume of preclinical and clinical trial data to incorporate emerging treatments into CML treatment algorithms. Physicians need to be aware of advances in mutational analysis, how best to identify reasons for resistance in clinical practice, and how to personalize therapy on the basis of mutation status. Challenges for the future include treatment optimization and development of strategies to meet the needs of patients with both primary and secondary resistance to current therapies.
Acknowledgments
Medical writing support was provided by Dr. Julia Duffey of Anthemis Consulting Ltd., and funded by Teva Pharmaceutical Industries (Frazer, PA). Teva Pharmaceutical Industries provided a single medical accuracy review of the final draft. The authors were not compensated and retained full editorial control over the content of the article. Dr. Jabbour has received honoraria from Bristol-Myers Squibb, Novartis, Pfizer, Ariad, and Teva Pharmaceutical Industries. Dr. Cortes is a consultant for Ariad, Teva Pharmaceutical Industries, and Pfizer and has received grant support from Bristol-Myers Squibb, Novartis, Ariad, Teva Pharmaceutical Industries, and Pfizer. Dr. Kantarjian has received research grants from Novartis, Bristol-Myers Squibb, Pfizer, Ariad, and ChemGenex.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quintas-Cardama A, Kantarjian HM, Cortes JE. Mechanisms of primary and secondary resistance to imatinib in chronic myeloid leukemia. Cancer Control. 2009;16:122–131. doi: 10.1177/107327480901600204. [DOI] [PubMed] [Google Scholar]
- 2.Hochhaus A, Kreil S, Corbin AS, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002;16:2190–2196. doi: 10.1038/sj.leu.2402741. [DOI] [PubMed] [Google Scholar]
- 3.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 4.le Coutre P, Tassi E, Varella-Garcia M, et al. Induction of resistance to the Abelson inhibitor STI571 in human leukemic cells through gene amplification. Blood. 2000;95:1758–1766. [PubMed] [Google Scholar]
- 5.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Chronic Myelogenous Leukemia V.4. 2013. © National Comprehensive Cancer Network, Inc 2013. All rights reserved. Accessed March 15, 2013. To view the most recent and complete version of the guideline, go online to www.nccn.org. NATIONAL COMPREHENSIVE CANCER NETWORK®, NCCN®, NCCN GUIDELINES®, and all other NCCN Content are trademarks owned by the National Comprehensive Cancer Network, Inc. Available at: http://www.nccn.org/professionals/physician_gls/pdf/cml.pdf.
- 6.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien S, Berman E, Moore JO, et al. NCCN Task Force report: tyrosine kinase inhibitor therapy selection in the management of patients with chronic myelogenous leukemia. J Natl Compr Canc Netw. 2011;9(Suppl 2):S1–S25. doi: 10.6004/jnccn.2011.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabbour E, Kantarjian HM, O’Brien S, et al. Front-line therapy with second-generation tyrosine kinase inhibitors in patients with early chronic phase chronic myeloid leukemia: what is the optimal response? J Clin Oncol. 2011;29:4260–4265. doi: 10.1200/JCO.2011.36.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marin D, Hedgley C, Clark RE, et al. The predictive value of early molecular response in chronic phase CML patients treated with dasatinib first line therapy. Blood. 2011;118(Suppl) doi: 10.1182/blood-2012-01-407486. Abstract No. 785. [DOI] [PubMed] [Google Scholar]
- 10.Hanfstein B, Muller MC, Hehlmann R, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML) Leukemia. 2012;26:2096–2102. doi: 10.1038/leu.2012.85. [DOI] [PubMed] [Google Scholar]
- 11.Saglio G, Kantarjian HM, Shah N, et al. Early response (molecular and cytogenetic) and long-term outcomes in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): exploratory analysis of DASISION 3-year data. Blood. 2012;;120(Suppl) Abstract No. 1675. [Google Scholar]
- 12.Hochhaus A, Hughes TP, Saglio G, et al. Outcome of patients with chronic myeloid leukemia in chronic phase (CML-CP) based on early molecular response and factors associated with early response: 4-year follow-up data from Enestnd (Evaluating Nilotinib Efficacy and Safety in Clinical Trials newly diagnosed patients) Blood. 2012;120(Suppl) Abstract No. 167. [Google Scholar]
- 13.Marin D, Ibrahim AR, Lucas C, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol. 2012;30:232–238. doi: 10.1200/JCO.2011.38.6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 15.Kantarjian H, Sawyers C, Hochhaus A, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645–652. doi: 10.1056/NEJMoa011573. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 17.Alvarado Y, Kantarjian H, O’Brien S, et al. Significance of suboptimal response to imatinib, as defined by the European LeukemiaNet, in the long-term outcome of patients with early chronic myeloid leukemia in chronic phase. Cancer. 2009;115:3709–3718. doi: 10.1002/cncr.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng B, Hayes M, Resta D, et al. Pharmacokinetics and pharmacodynamics of imatinib in a phase I trial with chronic myeloid leukemia patients. J Clin Oncol. 2004;22:935–942. doi: 10.1200/JCO.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 19.Larson RA, Druker BJ, Guilhot F, et al. Imatinib pharmacokinetics and its correlation with response and safety in chronic-phase chronic myeloid leukemia: a subanalysis of the IRIS study. Blood. 2008;111:4022–4028. doi: 10.1182/blood-2007-10-116475. [DOI] [PubMed] [Google Scholar]
- 20.Picard S, Titier K, Etienne G, et al. Trough imatinib plasma levels are associated with both cytogenetic and molecular responses to standard-dose imatinib in chronic myeloid leukemia. Blood. 2007;109:3496–3499. doi: 10.1182/blood-2006-07-036012. [DOI] [PubMed] [Google Scholar]
- 21.Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukaemia. Lancet Oncol. 2007;8:1018–1029. doi: 10.1016/S1470-2045(07)70342-X. [DOI] [PubMed] [Google Scholar]
- 22.Gambacorti-Passerini C, Zucchetti M, Russo D, et al. Alpha1 acid glycoprotein binds to imatinib (STI571) and substantially alters its pharmacokinetics in chronic myeloid leukemia patients. Clin Cancer Res. 2003;9:625–632. [PubMed] [Google Scholar]
- 23.Jorgensen HG, Elliott MA, Allan EK, et al. Alpha1-acid glycoprotein expressed in the plasma of chronic myeloid leukemia patients does not mediate significant in vitro resistance to STI571. Blood. 2002;99:713–715. doi: 10.1182/blood.v99.2.713. [DOI] [PubMed] [Google Scholar]
- 24.Mahon FX, Deininger MW, Schultheis B, et al. Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood. 2000;96:1070–1079. [PubMed] [Google Scholar]
- 25.Thomas J, Wang L, Clark RE, et al. Active transport of imatinib into and out of cells: implications for drug resistance. Blood. 2004;104:3739–3745. doi: 10.1182/blood-2003-12-4276. [DOI] [PubMed] [Google Scholar]
- 26.Skoglund K, Boiso Moreno S, Jönsson J-I, et al. Functional characterization of ABCG2 polymorphisms and their influence on tyrosine kinase inhibitor effects in chronic myeloid leukemia cells. Blood. 2011;118 Abstract No. 3495. [Google Scholar]
- 27.Kim DH, Sriharsha L, Xu W, et al. Clinical relevance of a pharmacogenetic approach using multiple candidate gene polymorphisms to predict response and resistance to imatinib mesylate therapy in chronic myeloid leukemia. Blood. 2007;110 doi: 10.1158/1078-0432.CCR-09-0145. Abstract No. 737. [DOI] [PubMed] [Google Scholar]
- 28.Crossman LC, Druker BJ, Deininger MW, et al. hOCT 1 and resistance to imatinib. Blood. 2005;106:1133–1134. doi: 10.1182/blood-2005-02-0694. [DOI] [PubMed] [Google Scholar]
- 29.Galimberti S, Cervetti G, Guerrini F, et al. Quantitative molecular monitoring of BCR-ABL and MDR1 transcripts in patients with chronic myeloid leukemia during imatinib treatment. Cancer Genet Cytogenet. 2005;162:57–62. doi: 10.1016/j.cancergencyto.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 30.Ferrao PT, Frost MJ, Siah SP, et al. Overexpression of P-glycoprotein in K562 cells does not confer resistance to the growth inhibitory effects of imatinib (STI571) in vitro. Blood. 2003;102:4499–4503. doi: 10.1182/blood-2003-01-0083. [DOI] [PubMed] [Google Scholar]
- 31.Hu Y, Swerdlow S, Duffy TM, et al. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc Natl Acad Sci U S A. 2006;103:16870–16875. doi: 10.1073/pnas.0606509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li S, Li D. Stem cell and kinase activity-independent pathway in resistance of leukaemia to BCR-ABL kinase inhibitors. J Cell Mol Med. 2007;11:1251–1262. doi: 10.1111/j.1582-4934.2007.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Danhauser-Riedl S, Warmuth M, Druker BJ, et al. Activation of Src kinases p53/56lyn and p59hck by p210bcr/abl in myeloid cells. Cancer Res. 1996;56:3589–3596. [PubMed] [Google Scholar]
- 34.Donato NJ, Wu JY, Stapley J, et al. BCR-ABL independence and LYN kinase overexpression in chronic myelogenous leukemia cells selected for resistance to STI571. Blood. 2003;101:690–68. doi: 10.1182/blood.V101.2.690. [DOI] [PubMed] [Google Scholar]
- 35.Wu J, Meng F, Lu H, et al. Lyn regulates BCR-ABL and Gab2 tyrosine phosphorylation and c-Cbl protein stability in imatinib-resistant chronic myelogenous leukemia cells. Blood. 2008;111:3821–3829. doi: 10.1182/blood-2007-08-109330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun J, Yin CC, Cui W, et al. Chromosome 20q deletion: a recurrent cytogenetic abnormality in patients with chronic myelogenous leukemia in remission. Am J Clin Pathol. 2011;135:391–397. doi: 10.1309/AJCPQFSC9ZJNMAZ6. [DOI] [PubMed] [Google Scholar]
- 37.Lahaye T, Riehm B, Berger U, et al. Response and resistance in 300 patients with BCR-ABL-positive leukemias treated with imatinib in a single center: a 4.5-year follow-up. Cancer. 2005;103:1659–1669. doi: 10.1002/cncr.20922. [DOI] [PubMed] [Google Scholar]
- 38.Gruber FX, Lundan T, Goll R, et al. BCR-ABL isoforms associated with intrinsic or acquired resistance to imatinib: more heterogeneous than just ABL kinase domain point mutations? Med Oncol. 2012;29:219–226. doi: 10.1007/s12032-010-9781-z. [DOI] [PubMed] [Google Scholar]
- 39.Cortes JE, Talpaz M, Giles F, et al. Prognostic significance of cytogenetic clonal evolution in patients with chronic myelogenous leukemia on imatinib mesylate therapy. Blood. 2003;101:3794–3800. doi: 10.1182/blood-2002-09-2790. [DOI] [PubMed] [Google Scholar]
- 40.O’Dwyer ME, Mauro MJ, Kurilik G, et al. The impact of clonal evolution on response to imatinib mesylate (STI571) in accelerated phase CML. Blood. 2002;100:1628–1633. doi: 10.1182/blood-2002-03-0777. [DOI] [PubMed] [Google Scholar]
- 41.Schoch C, Haferlach T, Kern W, et al. Occurrence of additional chromosome aberrations in chronic myeloid leukemia patients treated with imatinib mesylate. Leukemia. 2003;17:461–463. doi: 10.1038/sj.leu.2402813. [DOI] [PubMed] [Google Scholar]
- 42.Wendel HG, de Stanchina E, Cepero E, et al. Loss of p53 impedes the anti-leukemic response to BCR-ABL inhibition. Proc Natl Acad Sci U S A. 2006;103:7444–7449. doi: 10.1073/pnas.0602402103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bixby D, Talpaz M. Seeking the causes and solutions to imatinib-resistance in chronic myeloid leukemia. Leukemia. 2011;25:7–22. doi: 10.1038/leu.2010.238. [DOI] [PubMed] [Google Scholar]
- 44.Lee SM, Bae JH, Kim MJ, et al. Bcr-Abl-independent imatinib-resistant K562 cells show aberrant protein acetylation and increased sensitivity to histone deacetylase inhibitors. J Pharmacol Exp Ther. 2007;322:1084–1092. doi: 10.1124/jpet.107.124461. [DOI] [PubMed] [Google Scholar]
- 45.San José-Eneriz E, Agirre X, Jimenez-Velasco A, et al. Epigenetic down-regulation of BIM expression is associated with reduced optimal responses to imatinib treatment in chronic myeloid leukaemia. Eur J Cancer. 2009;45:1877–1889. doi: 10.1016/j.ejca.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 46.Oki Y, Kantarjian HM, Gharibyan V, et al. Phase II study of low-dose decitabine in combination with imatinib mesylate in patients with accelerated or myeloid blastic phase of chronic myelogenous leukemia. Cancer. 2007;109:899–906. doi: 10.1002/cncr.22470. [DOI] [PubMed] [Google Scholar]
- 47.Issa JP, Gharibyan V, Cortes J, et al. Phase II study of low-dose decitabine in patients with chronic myelogenous leukemia resistant to imatinib mesylate. J Clin Oncol. 2005;23:3948–3956. doi: 10.1200/JCO.2005.11.981. [DOI] [PubMed] [Google Scholar]
- 48.Rousselot P, Huguet F, Rea D, et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109:58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]
- 49.Corbin AS, Agarwal A, Loriaux M, et al. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vaidya S, Ghosh K, Vundinti BR. Recent developments in drug resistance mechanism in chronic myeloid leukemia: a review. Eur J Haematol. 2011;87:381–393. doi: 10.1111/j.1600-0609.2011.01689.x. [DOI] [PubMed] [Google Scholar]
- 51.Copland M, Hamilton A, Elrick LJ, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107:4532–459. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 52.Jorgensen HG, Holyoake TL. Characterization of cancer stem cells in chronic myeloid leukaemia. Biochem Soc Trans. 2007;35:1347–1351. doi: 10.1042/BST0351347. [DOI] [PubMed] [Google Scholar]
- 53.Corbin AS, La Rosee P, Stoffregen EP, et al. Several Bcr-Abl kinase domain mutants associated with imatinib mesylate resistance remain sensitive to imatinib. Blood. 2003;101:4611–464. doi: 10.1182/blood-2002-12-3659. [DOI] [PubMed] [Google Scholar]
- 54.La Rosee P, Deininger MW. Resistance to imatinib: mutations and beyond. Semin Hematol. 2010;47:335–343. doi: 10.1053/j.seminhematol.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Barnes DJ, Palaiologou D, Panousopoulou E, et al. Bcr-Abl expression levels determine the rate of development of resistance to imatinib mesylate in chronic myeloid leukemia. Cancer Res. 2005;65:8912–8919. doi: 10.1158/0008-5472.CAN-05-0076. [DOI] [PubMed] [Google Scholar]
- 56.Milojkovic D, Apperley J. Mechanisms of resistance to imatinib and second-generation tyrosine inhibitors in chronic myeloid leukemia. Clin Cancer Res. 2009;15:7519–7527. doi: 10.1158/1078-0432.CCR-09-1068. [DOI] [PubMed] [Google Scholar]
- 57.O’Hare T, Eide CA, Deininger MW. Bcr-Abl kinase domain mutations, drug resistance, and the road to a cure for chronic myeloid leukemia. Blood. 2007;110:2242–2249. doi: 10.1182/blood-2007-03-066936. [DOI] [PubMed] [Google Scholar]
- 58.Jabbour E, Kantarjian H, Jones D, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia. 2006;20:1767–1773. doi: 10.1038/sj.leu.2404318. [DOI] [PubMed] [Google Scholar]
- 59.Ernst T, Hochhaus A. Chronic myeloid leukemia: clinical impact of BCR-ABL1 mutations and other lesions associated with disease progression. Semin Oncol. 2012;39:58–66. doi: 10.1053/j.seminoncol.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Soverini S, Colarossi S, Gnani A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients The GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006;12:7374–7379. doi: 10.1158/1078-0432.CCR-06-1516. [DOI] [PubMed] [Google Scholar]
- 61.Branford S, Melo JV, Hughes TP. Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: does the BCR-ABL mutation status really matter? Blood. 2009;114:5426–5435. doi: 10.1182/blood-2009-08-215939. [DOI] [PubMed] [Google Scholar]
- 62.Ernst T, Erben P, Muller MC, et al. Dynamics of BCR-ABL mutated clones prior to hematologic or cytogenetic resistance to imatinib. Haematologica. 2008;93:186–192. doi: 10.3324/haematol.11993. [DOI] [PubMed] [Google Scholar]
- 63.Guilhot F, Apperley J, Kim DW, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood. 2007;109:4143–4150. doi: 10.1182/blood-2006-09-046839. [DOI] [PubMed] [Google Scholar]
- 64.Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:2303–2309. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- 65.Khorashad JS, de Lavallade H, Apperley JF, et al. Finding of kinase domain mutations in patients with chronic phase chronic myeloid leukemia responding to imatinib may identify those at high risk of disease progression. J Clin Oncol. 2008;26:4806–4813. doi: 10.1200/JCO.2008.16.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim TD, Turkmen S, Schwarz M, et al. Impact of additional chromosomal aberrations and BCR-ABL kinase domain mutations on the response to nilotinib in Philadelphia chromosome-positive chronic myeloid leukemia. Haematologica. 2010;95:582–588. doi: 10.3324/haematol.2009.014712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.le Coutre P, Ottmann OG, Giles F, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is active in patients with imatinib-resistant or -intolerant accelerated-phase chronic myelogenous leukemia. Blood. 2008;111:1834–1839. doi: 10.1182/blood-2007-04-083196. [DOI] [PubMed] [Google Scholar]
- 68.Soverini S, Martinelli G, Rosti G, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J Clin Oncol. 2005;23:4100–4109. doi: 10.1200/JCO.2005.05.531. [DOI] [PubMed] [Google Scholar]
- 69.Soverini S, Hochhaus A, Nicolini FE, et al. BCR-ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood. 2011;118:1208–1215. doi: 10.1182/blood-2010-12-326405. [DOI] [PubMed] [Google Scholar]
- 70.Schindler T, Bornmann W, Pellicena P, et al. Structural mechanism for STI-571 inhibition of abelson tyrosine kinase. Science. 2000;289:1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 71.Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102:276–283. doi: 10.1182/blood-2002-09-2896. [DOI] [PubMed] [Google Scholar]
- 72.Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 73.Tang C, Schafranek L, Watkins DB, et al. Tyrosine kinase inhibitor resistance in chronic myeloid leukemia cell lines: investigating resistance pathways. Leuk Lymphoma. 2011;52:2139–2147. doi: 10.3109/10428194.2011.591013. [DOI] [PubMed] [Google Scholar]
- 74.Griswold IJ, MacPartlin M, Bumm T, et al. Kinase domain mutants of Bcr-Abl exhibit altered transformation potency, kinase activity, and substrate utilization, irrespective of sensitivity to imatinib. Mol Cell Biol. 2006;26:6082–6093. doi: 10.1128/MCB.02202-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cortes J, Jabbour E, Kantarjian H, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood. 2007;110:4005–4011. doi: 10.1182/blood-2007-03-080838. [DOI] [PubMed] [Google Scholar]
- 76.Soverini S, Gnani A, Colarossi S, et al. In early-chronic phase chronic myeloid leukemia patients treated with imatinib, resistance is rarely mediated by Abl kinase domain mutations. Blood. 2007;;110 Abstract No. 1934. [Google Scholar]
- 77.Kim DW, Kim D, Kim SH, et al. Mutation analysis of BCR-ABL tyrosine kinase domain in new chronic phase-chronic myeloid leukemia patients with suboptimal response or treatment failure from imatinib treatment. Blood. 2010;;116 Abstract No. 3441. [Google Scholar]
- 78.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Branford S. Chronic myeloid leukemia: molecular monitoring in clinical practice. Hematology Am Soc Hematol Educ Program. 2007:376–383. doi: 10.1182/asheducation-2007.1.376. [DOI] [PubMed] [Google Scholar]
- 80.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 81.Corbin AS, Buchdunger E, Pascal F, et al. Analysis of the structural basis of specificity of inhibition of the Abl kinase by STI571. J Biol Chem. 2002;277:32214–32219. doi: 10.1074/jbc.M111525200. [DOI] [PubMed] [Google Scholar]
- 82.Sherbenou DW, Wong MJ, Humayun A, et al. Mutations of the BCR-ABL-kinase domain occur in a minority of patients with stable complete cytogenetic response to imatinib. Leukemia. 2007;21:489–493. doi: 10.1038/sj.leu.2404554. [DOI] [PubMed] [Google Scholar]
- 83.Chu S, Xu H, Shah NP, et al. Detection of BCR-ABL kinase mutations in CD34+ cells from chronic myelogenous leukemia patients in complete cytogenetic remission on imatinib mesylate treatment. Blood. 2005;;105:2093–2098. doi: 10.1182/blood-2004-03-1114. [DOI] [PubMed] [Google Scholar]
- 84.Sherbenou DW, Hantschel O, Kaupe I, et al. BCR-ABL SH3-SH2 domain mutations in chronic myeloid leukemia patients on imatinib. Blood. 2010;;116:3278–3285. doi: 10.1182/blood-2008-10-183665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Willis SG, Lange T, Demehri S, et al. High-sensitivity detection of BCR-ABL kinase domain mutations in imatinib-naive patients: correlation with clonal cytogenetic evolution but not response to therapy. Blood. 2005;;106:2128–2137. doi: 10.1182/blood-2005-03-1036. [DOI] [PubMed] [Google Scholar]
- 86.Azam M, Latek RR, Daley GQ. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell. 2003;112:831–843. doi: 10.1016/s0092-8674(03)00190-9. [DOI] [PubMed] [Google Scholar]
- 87.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 88.Terasawa T, Dahabreh I, Trikalinos TA. BCR-ABL mutation testing to predict response to tyrosine kinase inhibitors in patients with chronic myeloid leukemia. PLoS Curr. 2010;2 doi: 10.1371/currents.RRN1204. RRN1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alikian M, Gerrard G, Subramanian PG, et al. BCR-ABL1 kinase domain mutations: methodology and clinical evaluation. Am J Hematol. 2012;87:298–304. doi: 10.1002/ajh.22272. [DOI] [PubMed] [Google Scholar]
- 90.Jones D, Kamel-Reid S, Bahler D, et al. Laboratory practice guidelines for detecting and reporting BCR-ABL drug resistance mutations in chronic myelogenous leukemia and acute lymphoblastic leukemia: a report of the Association for Molecular Pathology. J Mol Diagn. 2009;11:4–11. doi: 10.2353/jmoldx.2009.080095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Branford S, Hughes TP. Mutational analysis in chronic myeloid leukemia: when and what to do? Curr Opin Hematol. 2011;18:111–116. doi: 10.1097/MOH.0b013e32834399ef. [DOI] [PubMed] [Google Scholar]
- 92.Ernst T, Gruber FX, Pelz-Ackermann O, et al. A co-operative evaluation of different methods of detecting BCR-ABL kinase domain mutations in patients with chronic myeloid leukemia on second-line dasatinib or nilotinib therapy after failure of imatinib. Haematologica. 2009;94:1227–1235. doi: 10.3324/haematol.2009.006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kantarjian HM, Cortes J, Guilhot F, et al. Diagnosis and management of chronic myeloid leukemia. Cancer. 2007;109:1365–1375. doi: 10.1002/cncr.22523. [DOI] [PubMed] [Google Scholar]
- 94.Morra E, Michallet M, Steegmann J, et al. Real-life rates of disease monitoring in clinical practice in Europe: the “unmet needs in CML and Ph+ALL” (UNIC) study. Blood. 2007;118 Abstract No. 1949. [Google Scholar]
- 95.Giles F. Molecular monitoring of BCR-ABL transcripts—standardization needed to properly use, and further investigate the value of, a critical surrogate marker for success in therapy of chronic myeloid leukemia. US Oncol Hematol. 2011;7:138–142. [Google Scholar]
- 96.Wang L, Knight K, Lucas C, et al. The role of serial BCR-ABL transcript monitoring in predicting the emergence of BCR-ABL kinase mutations in imatinib-treated patients with chronic myeloid leukemia. Haematologica. 2006;91:235–239. [PubMed] [Google Scholar]
- 97.White DL, Dang P, Engler J, et al. Functional activity of the OCT-1 protein is predictive of long-term outcome in patients with chronic-phase chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2010;28:2761–2767. doi: 10.1200/JCO.2009.26.5819. [DOI] [PubMed] [Google Scholar]
- 98.Assouline S, Lipton JH. Monitoring response and resistance to treatment in chronic myeloid leukemia. Curr Oncol. 2011;18:e71–e83. doi: 10.3747/co.v18i2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cortes J, Quintas-Cardama A, Jabbour E, et al. The clinical significance of achieving different levels of cytogenetic response in patients with chronic phase chronic myeloid leukemia after failure to front-line therapy: is complete cytogenetic response the only desirable endpoint? Clin Lymphoma Myeloma Leuk. 2011;11:421–426. doi: 10.1016/j.clml.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kantarjian HM, Larson RA, Guilhot F, et al. Efficacy of imatinib dose escalation in patients with chronic myeloid leukemia in chronic phase. Cancer. 2009;115:551–560. doi: 10.1002/cncr.24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jabbour E, Kantarjian HM, Jones D, et al. Imatinib mesylate dose escalation is associated with durable responses in patients with chronic myeloid leukemia after cytogenetic failure on standard-dose imatinib therapy. Blood. 2009;113:2154–2160. doi: 10.1182/blood-2008-04-154344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zonder JA, Pemberton P, Brandt H, et al. The effect of dose increase of imatinib mesylate in patients with chronic or accelerated phase chronic myelogenous leukemia with inadequate hematologic or cytogenetic response to initial treatment. Clin Cancer Res. 2003;9:2092–2097. [PubMed] [Google Scholar]
- 103.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 104.Deininger MW, Holyoake TL. Can we afford to let sleeping dogs lie? Blood. 2005;105:1840–1841. doi: 10.1182/blood-2004-12-4764. [DOI] [PubMed] [Google Scholar]
- 105.Preudhomme C, Guilhot J, Nicolini FE, et al. Imatinib plus peginterferon alfa-2a in chronic myeloid leukemia. N Engl J Med. 2010;363:2511–2521. doi: 10.1056/NEJMoa1004095. [DOI] [PubMed] [Google Scholar]
- 106.Burchert A, Muller MC, Kostrewa P, et al. Sustained molecular response with interferon alfa maintenance after induction therapy with imatinib plus interferon alfa in patients with chronic myeloid leukemia. J Clin Oncol. 2010;28:1429–1435. doi: 10.1200/JCO.2009.25.5075. [DOI] [PubMed] [Google Scholar]
- 107.Cornelison AM, Welch MA, Koller C, Jabbour E. Dasatinib combined with interferon-alfa induces a complete cytogenetic response and major molecular response in a patient with chronic myelogenous leukemia harboring the T315I BCR-ABL1 mutation. Clin Lymphoma Myeloma Leuk. 2011;11(Suppl 1):S111–S113. doi: 10.1016/j.clml.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 108.Nicolini FE, Hayette S, Legros L, et al. Pegylated IFN-alpha2a combined to imatinib mesylate 600mg daily can induce complete cytogenetic and molecular responses in a subset of chronic phase CML patients refractory to IFN alone or to imatinib 600mg daily alone. Leuk Res. 2011;35:80–86. doi: 10.1016/j.leukres.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 109.Itonaga H, Tsushima H, Hata T, et al. Successful treatment of a chronic-phase T-315I-mutated chronic myelogenous leukemia patient with a combination of imatinib and interferon-alfa. Int J Hematol. 2012;95:209–213. doi: 10.1007/s12185-012-1005-1. [DOI] [PubMed] [Google Scholar]
- 110.O’Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 111.Apperley JF, Cortes JE, Kim DW, et al. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: the START a trial. J Clin Oncol. 2009;27:3472–3479. doi: 10.1200/JCO.2007.14.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kantarjian H, Pasquini R, Levy V, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily: two-year follow-up of a randomized phase 2 study (START-R) Cancer. 2009;115:4136–4147. doi: 10.1002/cncr.24504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200–1206. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- 114.Muller MC, Cortes JE, Kim DW, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to preexisting BCR-ABL mutations. Blood. 2009;114:4944–4953. doi: 10.1182/blood-2009-04-214221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Soverini S, Gnani A, Colarossi S, et al. Philadelphia-positive patients who already harbor imatinib-resistant Bcr-Abl kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second- or third-line tyrosine kinase inhibitors. Blood. 2009;114:2168–2171. doi: 10.1182/blood-2009-01-197186. [DOI] [PubMed] [Google Scholar]
- 116.Soverini S, Colarossi S, Gnani A, et al. Resistance to dasatinib in Philadelphia-positive leukemia patients and the presence or the selection of mutations at residues 315 and 317 in the BCR-ABL kinase domain. Haematologica. 2007;92:401–404. doi: 10.3324/haematol.10822. [DOI] [PubMed] [Google Scholar]
- 117.Soverini S, Martinelli G, Colarossi S, et al. Second-line treatment with dasatinib in patients resistant to imatinib can select novel inhibitor-specific BCR-ABL mutants in Ph+ ALL. Lancet Oncol. 2007;8:273–274. doi: 10.1016/S1470-2045(07)70078-5. [DOI] [PubMed] [Google Scholar]
- 118.Khorashad JS, Milojkovic D, Mehta P, et al. In vivo kinetics of kinase domain mutations in CML patients treated with dasatinib after failing imatinib. Blood. 2008;111:2378–2381. doi: 10.1182/blood-2007-06-096396. [DOI] [PubMed] [Google Scholar]
- 119.Shah NP, Skaggs BJ, Branford S, et al. Sequential ABL kinase inhibitor therapy selects for compound drug-resistant BCR-ABL mutations with altered oncogenic potency. J Clin Invest. 2007;117:2562–2569. doi: 10.1172/JCI30890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Weisberg E, Manley P, Mestan J, et al. AMN107 (nilotinib): a novel and selective inhibitor of BCR-ABL. Br J Cancer. 2006;94:1765–1769. doi: 10.1038/sj.bjc.6603170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kantarjian HM, Giles F, Gattermann N, et al. Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood. 2007;110:3540–3546. doi: 10.1182/blood-2007-03-080689. [DOI] [PubMed] [Google Scholar]
- 122.Kantarjian HM, Giles FJ, Bhalla KN, et al. Update on imatinib-resistant chronic myeloid leukemia patients in chronic phase (CML-CP) on nilotinib therapy at 24 months: clinical response, safety, and long-term outcomes. Blood. 2009;114 Abstract No. 1129. [Google Scholar]
- 123.Kantarjian H, Giles F, Bhalla K, et al. Nilotinib in chronic myeloid leukemia patients in chronic phase (CML-CP) with imatinib (IM) resistance or intolerance: Longer follow-up results of a phase II study. J Clin Oncol. 2009;27(Suppl) Abstract No. 7029. [Google Scholar]
- 124.Giles F, le Coutre P, Bhalla K, et al. A phase II study of nilotinib administered to patients with imatinib resistant or intolerant chronic myelogenous leukemia (CML) in chronic phase (CP), accelerated phase (AP) or blast crisis (BC) who also failed dasatinib. J Clin Oncol. 2007;25(Suppl) Abstract No. 7038. [Google Scholar]
- 125.Bradeen HA, Eide CA, O’Hare T, et al. Comparison of imatinib mesylate, dasatinib (BMS-354825), and nilotinib (AMN107) in an N-ethyl-N-nitrosourea (ENU)-based mutagenesis screen: high efficacy of drug combinations. Blood. 2006;108:2332–2338. doi: 10.1182/blood-2006-02-004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Puttini M, Coluccia AM, Boschelli F, et al. In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl+ neoplastic cells. Cancer Res. 2006;66:11314–11322. doi: 10.1158/0008-5472.CAN-06-1199. [DOI] [PubMed] [Google Scholar]
- 127.Redaelli S, Piazza R, Rostagno R, et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR/ABL mutants. J Clin Oncol. 2009;27:469–471. doi: 10.1200/JCO.2008.19.8853. [DOI] [PubMed] [Google Scholar]
- 128.Cortes JE, Kantarjian HM, Brummendorf TH, et al. Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood. 2011;118:4567–4576. doi: 10.1182/blood-2011-05-355594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Khoury HJ, Gambacorti-Passerini C, Kantarjian HM, et al. Bosutinib as therapy for chronic phase chronic myeloid leukemia following failure with imatinib plus dasatinib and/or nilotinib: 24-month minimum follow-up update. Blood. 2012;120 Abstract No. 3785. [Google Scholar]
- 130.Cortes JE, Kantarjian HM, Kim D-W, et al. Bosutinib as therapy for chronic phase chronic myeloid leukemia following resistance or intolerance to imatinib: 36-month minimum follow-up update. Blood. 2012;120 Abstract No. 3779. [Google Scholar]
- 131.Cortes JE, Kantarjian HM, Shah N, et al. Ponatinib in refractory Philadelphia chromosome-positive leukemias. N Engl J Med. 2012;367:2075–2088. doi: 10.1056/NEJMoa1205127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cortes JE, Kim D-W, Pinilla-Ibarz J, et al. A pivotal phase 2 trial of ponatinib in patients with chronic myeloid leukemia (CML) and Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) resistant or intolerant to dasatinib or nilotinib, or with the T315I BCR-ABL mutation: 12-month follow-up of the PACE trial. Blood. 2012;120 Abstract No. 163. [Google Scholar]
- 133.Kantarjian HM, Kim D-W, Pinilla-Ibarz J, et al. Efficacy and safety of ponatinib in patients with accelerated phase or blast phase chronic myeloid leukemia (AP-CML or BP-CML) or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL): 12-month follow-up of the PACE trial. Blood. 2012;120 Abstract No. 915. [Google Scholar]
- 134.Cortes J, Paquette R, Talpaz M, et al. Preliminary clinical activity in a Phase I trial of the BCR-ABL/IGF- 1R/aurora kinase inhibitor XL228 in patients with Ph++ leukemias with either failure to multiple TKI therapies or with T315I mutation. Blood. 2008;112 Abstract No. 3232. [Google Scholar]
- 135.Kantarjian H, le Coutre P, Cortes J, et al. Phase 1 study of INNO-406, a dual Abl/Lyn kinase inhibitor, in Philadelphia chromosome-positive leukemias after imatinib resistance or intolerance. Cancer. 2010;116:2665–2672. doi: 10.1002/cncr.25079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cortes-Franco J, Dombret H, Schafhausen P, et al. Danusertib hydrochloride (PHA-739358), a multi-kinase aurora inhibitor, elicits clinical benefit in advanced chronic myeloid leukemia and Philadelphia chromosome positive acute lymphoblastic leukemia. Blood. 2009;114 Abstract No. 864. [Google Scholar]
- 137.Paquette RL, Shah NP, Sawyers CL, et al. PHA-739358, an aurora kinase inhibitor, induces clinical responses in chronic myeloid leukemia harboring T315I mutations of BCR-ABL. Blood. 2007;110 Abstract No. 1030. [Google Scholar]
- 138.Foran JM, Ravandi F, O’Brien S, et al. Phase I and pharmacodynamic trial of AT9283, an aurora kinase inhibitor, in patients with refractory leukemia. J Clin Oncol. 2008;26(Suppl) Abstract No. 2518. [Google Scholar]
- 139.Cortes J, Roboz GJ, Kantarjian HM, et al. A Phase I dose escalation study of KW-2449, an oral multi-kinase inhibitor against FLT3, Abl, FGFR1 and aurora in patients with relapsed/refractory AML, ALL and MDS or resistant/intolerant CML. Blood. 2008;112 Abstract No. 2967. [Google Scholar]
- 140.Cortes JE, Lipton JH, Rea D, et al. Phase 2 study of subcutaneous omacetaxine mepesuccinate after TKI failure in patients with chronic-phase CML with T315I mutation. Blood. 2012;120:2573–2580. doi: 10.1182/blood-2012-03-415307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cortes-Franco J, Khoury HJ, Nicolini FE, et al. Safety and efficacy of subcutaneous-administered omacetaxine mepesuccinate in imatinib-resistant chronic myeloid leukemia (CML) patients who harbor the Bcr-Abl T315I mutation-results of an ongoing multicenter Phase 2/3 study. Blood. 2009;114 Abstract No. 644. [Google Scholar]
- 142.Cortes-Franco J, Raghunadharao D, Parikh P, et al. Safety and efficacy of subcutaneous-administered omacetaxine mepesuccinate in chronic myeloid leukemia (CML) patients who are resistant or intolerant to two or more tyrosine kinase inhibitors - results of a multicenter Phase 2/3 study. Blood. 2009;114 Abstract No. 861. [Google Scholar]
- 143.Ayoubi M, Kantarjian HM, Wierda W, et al. A Phase 2 study of the combination of omacetaxine and imatinib in the treatment of patients with chronic myeloid leukemia (CML) in advanced stages or after failure to imatinib. Blood. 2009;114 Abstract No. 2193. [Google Scholar]
- 144.Khoury HJ, Cortes JE, Kantarjian HM, et al. Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood. 2012;119:3403–3412. doi: 10.1182/blood-2011-11-390120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kantarjian HM, Cortes JE, Kim DW, et al. Bosutinib safety profile and management of toxicities in leukemia patients with resistance or intolerance to imatinib and other tyrosine kinase inhibitors. Blood. 2011;118 doi: 10.1182/blood-2013-07-513937. Abstract No. 2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu Q, Eiring AM, Zabriskie MS, et al. Partially or fully BCR-ABL independent mechanisms of in vitro resistance to ponatinib. Blood. 2011;118 Abstract No. 2481. [Google Scholar]
- 148.Shah NP, Kasap C, Paquette R, et al. Targeting drug-resistant CML and Ph+- ALL with the spectrum selective protein kinase inhibitor XL228. Blood. 2007;110 Abstract No. 474. [Google Scholar]
- 149.Kimura S, Naito H, Segawa H, et al. NS-187, a potent and selective dual Bcr-Abl/Lyn tyrosine kinase inhibitor, is a novel agent for imatinib-resistant leukemia. Blood. 2005;106:3948–3954. doi: 10.1182/blood-2005-06-2209. [DOI] [PubMed] [Google Scholar]
- 150.Gontarewicz A, Brummendorf TH. Danusertib (formerly PHA-739358)—a novel combined pan-Aurora kinases and third generation Bcr-Abl tyrosine kinase inhibitor. Recent Results Cancer Res. 2010;184:199–214. doi: 10.1007/978-3-642-01222-8_14. [DOI] [PubMed] [Google Scholar]
- 151.Wetzler M, Segal D. Omacetaxine as an anticancer therapeutic: what is old is new again. Curr Pharm Des. 2011;17:59–64. doi: 10.2174/138161211795049778. [DOI] [PubMed] [Google Scholar]
- 152.Quintas-Cardama A, Kantarjian H, Cortes J. Homoharringtonine, omacetaxine mepesuccinate, and chronic myeloid leukemia circa 2009. Cancer. 2009;115:5382–5393. doi: 10.1002/cncr.24601. [DOI] [PubMed] [Google Scholar]
- 153.Allan EK, Holyoake TL, Craig AR, Jorgensen HG. Omacetaxine may have a role in chronic myeloid leukaemia eradication through downregulation of Mcl-1 and induction of apoptosis in stem/progenitor cells. Leukemia. 2011;25:985–994. doi: 10.1038/leu.2011.55. [DOI] [PubMed] [Google Scholar]
- 154.Quintas-Cardama A, Cortes J. Homoharringtonine for the treatment of chronic myelogenous leukemia. Expert Opin Pharmacother. 2008;9:1029–1037. doi: 10.1517/14656566.9.6.1029. [DOI] [PubMed] [Google Scholar]
- 155.Nicolini FE, Lipton J, Kantarjian H, et al. Subcutaneous omacetaxine mepesuccinate in patients with chronic phase (CP) or accelerated phase (AP) chronic myeloid leukemia (CML) resistant/intolerant to two or three approved tyrosine-kinase inhibitors (TKIs) J Clin Oncol. 2012;30(Suppl) Abstract No. 6513. [Google Scholar]
- 156.Akard LP, Kantarjian H, Nicolini FE, et al. Omacetaxine mepesuccinate in chronic-phase chronic myeloid leukemia (CML) in patients resistant, intolerant, or both to two or more tyrosine-kinase inhibitors (TKIs) J Clin Oncol. 2012;30(Suppl) Abstract No. 6596. [Google Scholar]
- 157.Khoury HJ, Cortes JE, Wetzler M, et al. Blast phase chronic myeloid leukemia: a pooled analysis of subcutaneous omacetaxine mepesuccinate in treatment-resistant patients. Blood. 2012;120 Abstract No. 3753. [Google Scholar]
- 158.Wetzler M, Kantarjian H, Nicolini FE, et al. Pooled safety analysis of omacetaxine mepesuccinate in patients with chronic myeloid leukemia (CML) resistant to tyrosine-kinase inhibitors (TKIs) J Clin Oncol. 2012;30(Suppl) Abstract No. 6604. [Google Scholar]
- 159.Tipping AJ, Mahon FX, Zafirides G, et al. Drug responses of imatinib mesylate-resistant cells: synergism of imatinib with other chemotherapeutic drugs. Leukemia. 2002;16:2349–2357. doi: 10.1038/sj.leu.2402775. [DOI] [PubMed] [Google Scholar]
- 160.Coude MM, Luycx O, Cariou ME, et al. Undetectable molecular residual disease after omacetaxine and nilotinib combination therapy in an imatinib-resistant chronic myeloid leukaemia patient harbouring the BCR-ABL1 T315I gatekeeper mutation. Br J Haematol. 2012;157:407–410. doi: 10.1111/j.1365-2141.2011.09016.x. [DOI] [PubMed] [Google Scholar]
- 161.Laneuville P, Dilea C, Yin OQ, et al. Comparative in vitro cellular data alone are insufficient to predict clinical responses and guide the choice of BCR-ABL inhibitor for treating imatinib-resistant chronic myeloid leukemia. J Clin Oncol. 2010;28:e169–e171. doi: 10.1200/JCO.2009.26.4945. [DOI] [PubMed] [Google Scholar]
- 162.Oyekunle A, Klyuchnikov E, Ocheni S, et al. Challenges for allogeneic stem cell transplantation in chronic myeloid leukemia in the era of tyrosine kinase inhibitors. Acta Haematol. 2011;126:30–39. doi: 10.1159/000323662. [DOI] [PubMed] [Google Scholar]