Abstract
It remains unclear whether autophagy affects hippocampal neuronal injury in vascular dementia. In the present study, we investigated the effects of autophagy blockade on hippocampal neuronal injury in a rat model of vascular dementia. In model rats, hippocampal CA1 neurons were severely damaged, and expression of the autophagy-related proteins beclin-1, cathepsin B and microtubule-associated protein 1 light chain 3 was elevated compared with that in sham-operated animals. These responses were suppressed in animals that received a single intraperitoneal injection of wortmannin, an autophagy inhibitor, prior to model establishment. The present results confirm that autophagy and autophagy-related proteins are involved in the pathological changes of vascular dementia, and that inhibition of autophagy has neuroprotective effects.
Keywords: nerve regeneration, vascular dementia, autophagy, beclin-1, cathepsin B, microtbuleassociated protein 1 light chain 3, autophagosomes, lysosomes, wortmannin, neural regeneration
Introduction
Autophagy is a basic catabolic mechanism by which unnecessary or dysfunctional cellular components are degraded by lysosomes; it is a specific, vital phenomenon of eukaryotic cells (Yorimitsu and Klionsky, 2005; Voigt and Pöggeler, 2013). Damaged organelles such as mitochondria are scavenged by autophagic processes to maintain the stability of nerve cells (Inoue et al., 2006; Bharadwaj et al., 2012). Excessive activation of autophagy leads to cell death (Kiffin et al., 2006; Chen et al., 2012). Previous studies have shown that autophagy is associated with senescence, malignant tumor growth, infection with pathogenic microorganisms, neurodegenerative disease, and diseases of the muscles and cerebrovascular system (Rubinsztein et al., 2005; Koike et al., 2008; Levine and Kroemer, 2008; Mizushima et al., 2008; Wen et al., 2008; Henriques-Pons and Nagaraju, 2009; Banerjee et al., 2010; Alger et al., 2011; Chang et al., 2012; Jia et al., 2012; Lynch-Day et al., 2012; Tung et al., 2012). However, few studies have explored the role of autophagy in the onset of vascular dementia. The autophagy inhibitor wortmannin is highly lipophilic, passing readily through the blood-brain barrier and cell membrane (Wipf and Halter, 2005). Cell culture experiments showed that wortmannin dose-dependently inhibits autophagy by blocking lysosomal transport substrates (Powis et al., 1994) with high selectivity (Fruman et al., 1998). In the present study, we sought to observe the changes in autophagy in the hippocampal CA1 region of a rat model of vascular dementia, and to investigate the effects of autophagy blockade on the onset of vascular dementia symptoms.
Materials and Methods
Animals
225 healthy male Sprague-Dawley rats, weighing 250–280 g (6–8 weeks old), were provided by Beijing HFK Bioscience Co., Ltd., Beijing, China (certificate No. SCXK (Jing) 2012-0013). All rats were housed in the Environmental Barrier Animal Laboratory, Hebei United University, China, at 23 ± 2°C, with natural illumination and free access to food and water. Animals were habituated to the housing conditions for 2 weeks prior to experimental procedures. This study was approved by the Ethics Committee of Hebei United University, China.
The 225 rats were randomly and equally divided into a sham surgery group, a vascular dementia model group and a wortmannin group. Each group was randomly subdivided according to time points after model induction, i.e. 1, 2, 4, 8 and 12 weeks (n = 15 per subgroup). Within each subgroup, six rats were used for immunohistochemistry, six for western blot assay, and three for electron microscopy.
Wortmannin treatment
Rats in the wortmannin group received an intraperitoneal injection of an autophagy inhibitor wortmanninian (0.5 mg/kg; Beijing Biosynthesis Biotechnology Co., Ltd.). One hour after drug injection, surgery was performed to establish the vascular dementia model.
Vascular dementia model preparation
A rat model of vascular dementia was established using a modification of the Pulsinelli four-vessel occlusion method (Sibolt et al., 2013). The rats were fasted for 12 hours before surgery, then anesthetized intraperitoneally with 10% chloral hydrate (350 mg/kg; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing, China). A median incision was made in the dorsal neck region to expose both transverse foramina of the first cervical vertebra. An electrocoagulation needle (0.5 mm in diameter) was inserted into each foramen for bilateral cauterization of the vertebral artery, resulting in permanent occlusion. The rats were fixed in the supine position, and a median ventral neck incision was made to isolate the common carotid artery bilaterally. The wound was sutured and the rats remained under anesthesia for 24 hours, before the artery was occluded three times, by clamping for 5 minutes at hourly intervals. The surgical site was sprayed with gentamicin to prevent infection and the skin was sutured. The sham-operated group underwent the same procedures as the model groups, but ischemia was not carried out. After surgery, all rats were treated with gentamicin for 3 consecutive days to prevent infection.
Behavioral assessment of learning and memory
A Morris water maze (Huaibei Zhenghua Biologic Apparatus Facilities Co., Ltd., Huaibei, Anhui Province, China), 120 cm in diameter, was used. A circular platform (23 cm high and 10 cm in diameter) was placed in the center of a quadrant, 2 cm below the surface of the water. The water temperature was 24 ± 2°C and the room temperature was 24°C. Behavioral assessment was carried out at 1, 2, 4, 8 and 12 weeks after modeling. Five days of navigation tests were performed before a probe test on the sixth day. Navigation tests consisted of two sessions per day (morning and afternoon), each with four trials. Each trial began when the rat was released into the water at one of four release points with its face toward the pool wall. The time taken for the rat to find and climb the platform was recorded as the escape latency. When the rat found the platform within 120 seconds, it was allowed to remain on the platform for 30 seconds, before a 60 second break in the home cage. If the rat failed to climb onto the platform within 120 seconds, it was manually guided onto the platform and allowed to stay there for 30 seconds before the break. The escape latency was recorded as 120 seconds in such cases. In the spatial probe test on day 6, the platform was removed from the pool. The rat was released into the water with its face toward the pool wall and the number of times it crossed the area in which the platform was previously located was recorded over a period of 120 seconds.
Transmission electron microscopy of the hippocampus
Three rats per group were deeply anesthetized with 10% chloral hydrate (0.3 mL/100 g), and the tissue was fixed with 4% paraformaldehyde and 2.5% glutaral. The rats were decapitated, the brains removed, and the hippocampi cut coronally into blocks (1 mm3). Specimens were post-fixed in 2.5% glutaral, and sliced into ultrathin sections (50–70 nm). Ultrastructure changes were observed under a transmission electron microscope (H-7650; Hitachi, Tokyo, Japan).
Immunohistochemistry of beclin-1 and cathepsin B in the hippocampal CA1 region
Rats were deeply anesthetized with 10% chloral hydrate (0.3 mL/100 g) and decapitated. Brains were removed on ice and placed in 4% paraformaldehyde in phosphate buffer for at least 24 hours after fixation. The portion of the brain containing the hippocampus was cut coronally into slices approximately 2 mm thick. The slices were embedded in paraffin and sectioned using a microtome (Jung Histocut, model 820-II, Leica, Wetzlar, Germany), dewaxed in xylene, dehydrated through an alcohol gradient, and immersed in pH 6.0 sodium citrate buffer and boiled for antigen retrieval. After cooling to room temperature, the sections were washed three times with PBS (5 minutes each wash), and incubated with rabbit anti-beclin-1 (1:500; Beijing Biosynthesis Biotechnology Co., Ltd.) and anti-cathepsin B (1:500; Beijing Biosynthesis Biotechnology Co., Ltd.) antibodies at 4°C overnight. The sections were then washed three times with PBS as before, incubated with goat anti-rabbit IgG (1:2,000; Beijing Biosynthesis Biotechnology Co., Ltd.) at 37°C for 30 minutes, washed again three times with PBS, and visualized with freshly prepared 3,3′-diaminobenzidine. They were then counterstained with hematoxylin, permeabilized, and mounted. The number of immunopositive cells in the hippocampal CA1 region was counted in six non-overlapping visual fields observed under a high-power lens (200 × magnification; Hitachi, Japan). A cell was counted as positive if its nucleus was stained royal blue and its cytoplasm was stained yellow or brown.
Western blot assay of light chain 3 protein expression in the hippocampus
Light chain 3 is a protein marker of autophagy (Kanno et al., 2011). Rats were deeply anesthetized with 10% chloral hydrate (0.3 mL/100 g), and decapitated. Brains were removed on ice and washed with cold PBS. Hippocampi were isolated immediately, placed in a 15 mL centrifuge tube and homogenized at 12,000 r/min (r = 3 cm) for 15 seconds using a rotary homogenizer. Each subsequent stage was performed at 4°C unless stated otherwise. The samples were treated with lysis buffer, shaken, and incubated for 30 minutes. The cell suspension was centrifuged at 12,000 r/min for 10 minutes and the supernatant was kept. After quantitation, protein concentration was adjusted to 750 μg/mL with PBS and an equal volume of loading buffer was added. The specimens were boiled in water for at least 5 minutes. A bicinchoninic acid kit (Beijing Biosynthesis Biotechnology Co., Ltd.) was used to determine protein concentrations against a standard curve, according to the manufacturer's instructions. Samples (50 μg total protein in each lane) were electrophoresed in sodium dodecyl sulfate-polyacrylamide gel, and electrically transferred to polyvinylidene fluoride membranes using the wet method (Hahn et al., 2011). The transfer buffer contained glycine 5.8 g, Tris 2.9 g and sodium dodecyl sulfate 0.37 g dissolved in 800 mL redistilled water and 200 mL methanol. Subsequently, the membranes were blocked in 5% non-fat dry milk, incubated with rabbit anti-rat light chain 3 monoclonal antibody (1:1,000; Beyotime Institute of Biotechnology, Beijing, China) at 4°C overnight, washed with PBS, incubated with goat anti-rabbit IgG (1:2,000; Beijing Biosynthesis Biotechnology Co., Ltd.) at 37°C for 1 hour, and washed again with PBS. Protein bands were visualized using enhanced chemiluminescence and film exposure (Pierce, Rockford, IL, USA). Results were analyzed using Image J software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data were collected with Excel 2007 software (Microsoft Corporation, Redmond, WA, USA), analyzed using SPSS 17.0 software (SPSS, Chicago, IL, USA), and expressed as mean ± SD. One-way analysis of variance was used to identify differences between multiple groups, and two-sample t-tests were used to compare means between two groups. A value of P < 0.05 was considered statistically significant.
Results
Effect of autophagy blockade on learning and memory in vascular dementia model rats
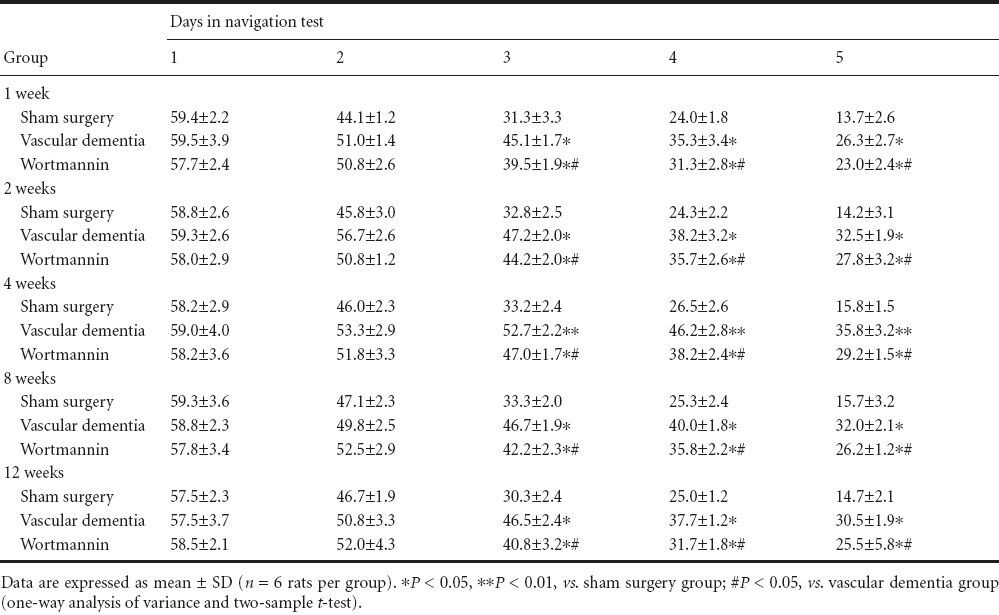
In the Morris water navigation test (Table 1), no significant differences in the time to reach the platform (average escape latency) were observed between the groups at 1 or 2 days (P > 0.05). However, from day 3, the average escape latency was longer in the vascular dementia group than in the sham surgery group (P < 0.05 or P < 0.01). Furthermore, escape latency in the wortmannin group was significantly shorter than that in the vascular dementia model group (P < 0.05).
Table 1.
Effect of autophagy blockade on the escape latency (second) of vascular dementia model rats in the Morris water navigation task
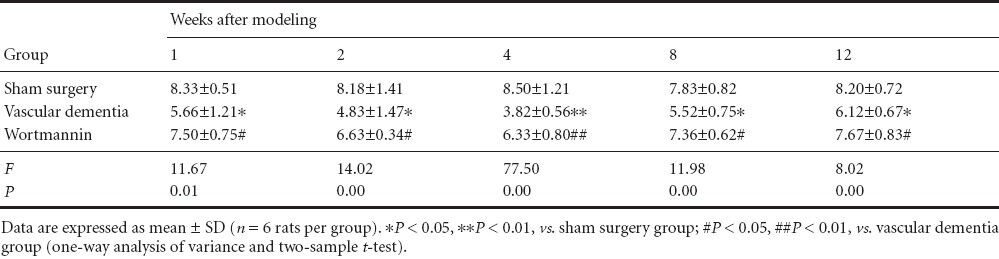
In the spatial probe test (Table 2), rats in the vascular dementia model group made significantly fewer crossings of the platform area than rats in the sham surgery group (P < 0.05 or P < 0.01), whereas rats in the wortmannin group crossed the platform area significantly more times than those in the vascular dementia group (P < 0.05 or P < 0.01).
Table 2.
Effect of autophagy blockade on the number of platform area crossings (/120 seconds) made by vascular dementia model rats in the probe test of the Morris water navigation task
Effect of autophagy blockade on the ultrastructure of the hippocampal CA1 region in vascular dementia model rats
Under the transmission electron microscope, neurons in the hippocampal CA1 region of rats in the sham surgery group (Figure 1A) showed evident nuclei with uniformly distributed chromatin. Cytoplasmic organelles were abundant and their structures were intact. Mitochondria were not swollen. No autophagosomes were visible. In rats in the vascular dementia model group, at 1 and 2 weeks (Figure 1B, C), electron density of the neuronal cytoplasm was greater than that in sham-operated rats. Nuclear membrane collapse and nuclear condensation were visible. There were few normal mitochondria, with most exhibiting vacuolar degeneration. The rough endoplasmic reticulum was expanded, vacuolization appeared around nuclei, and polysome depolymerization and a high degree of swelling were visible. Cellular organelles were sparse. Round autophagosomes were located next to nuclei and there were a large number of primary lysosomes. At 4 and 8 weeks (Figure 1D, E), autophagosomes and lysosomes were abundant. Some residual mitochondria coated by autophagosomes were detectable, and autophagy was evident. Autophagosomes could also be seen at 12 weeks (Figure 1F). In the hippocampal CA1 region of rats in the wortmannin group (Figure 1G–K), degeneration occurred in some nerve cells but there was less neuronal injury than in the model group at all time points. Staining was weak. Nuclear shrinkage, edema and mitochondrial swelling were not apparent. Rough endoplasmic reticulum was normal. Autophagy occurred, but the number of autophagosomes was less than that in the model group.
Figure 1.
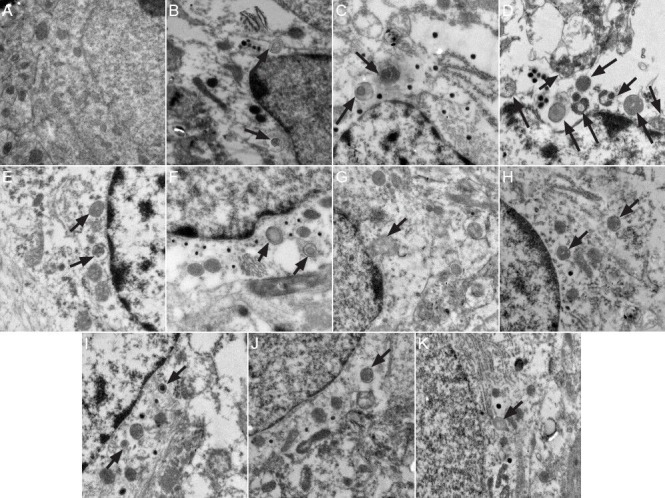
Effect of autophagy blockade on the ultrastructure of hippocampal CA1 neurons in vascular dementia model rats (transmission electron microscopy, × 25,000).
(A) Sham surgery group, no observable autophagosomes. (B–F) Vascular dementia group, autophagosomes (arrows) appeared at 1, 2, 4, 8 and 12 weeks after modeling. (G–K) Wortmannin group, fewer autophagosomes (arrows) were visible than in the vascular dementia group at 1, 2, 4, 8 and 12 weeks after model induction.
Effect of autophagy blockade on beclin-1 and cathepsin B expression in the hippocampal CA1 region of vascular dementia model rats
Immunohistochemistry showed that beclin-1 and cathepsin B expression was low at the various time points in the hippocampal CA1 region of rats in the sham surgery group. However, in the vascular dementia group, expression of both proteins was greater than that in sham-operated animals at 1 week. Expression levels peaked at 4 weeks and began to decrease at 8 weeks but were still elevated at 12 weeks compared with the levels observed in sham-operated rats (P < 0.05 or P < 0.01). Beclin-1 protein and cathepsin B protein expression was lower in the wortmannin group than in the vascular dementia group at all time points (P < 0.05 or P < 0.01; Figure 2, Tables 3, 4).
Figure 2.

Effect of autophagy blockade on beclin-1 (A) and cathepsin B (B) expression in the hippocampal CA1 region of vascular dementia model rats (immunohistochemistry, × 200).
Sham surgery group (Sham), few beclin-1- and cathepsin B-positive cells; vascular dementia group (VD), more beclin-1- and cathepsin B-positive cells, peaking at 4 weeks after model induction; wortmannin group (WM), fewer beclin-1- and cathepsin B-positive cells than in the vascular dementia group. Arrows show immunopositive cells.
Table 3.
Effect of autophagy blockade on beclin-1 expression (number of immunopositive cells) in the hippocampal CA1 region of vascular dementia model rats
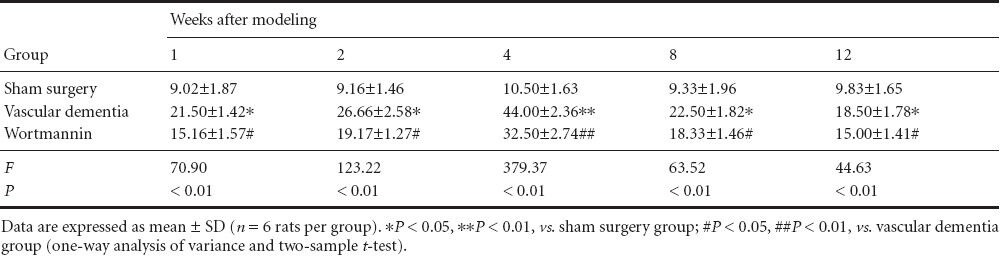
Table 4.
Effect of autophagy blockade on cathepsin B expression (number of immunopositive cells) in the hippocampal CA1 region of vascular dementia model rats
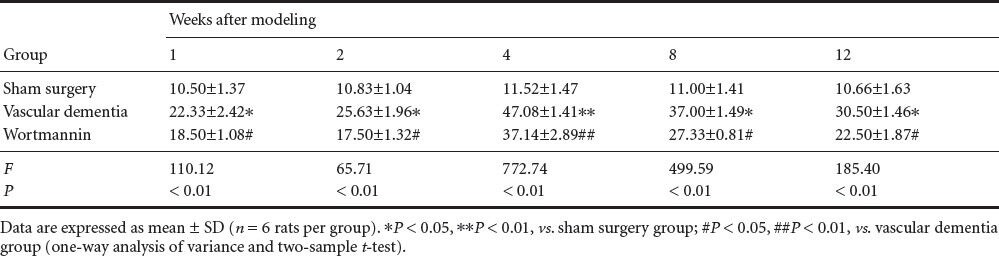
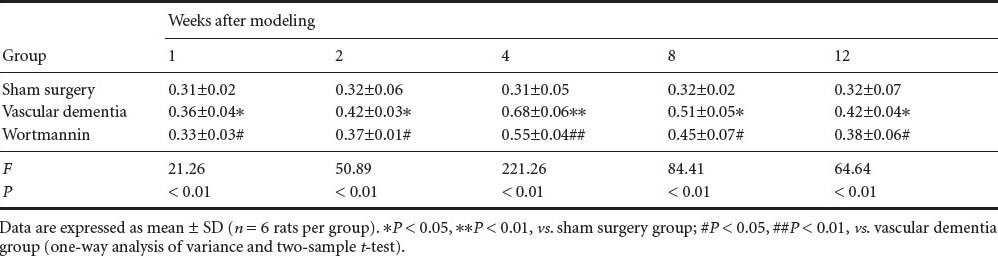
Effect of autophagy blockade on microtubule-associated protein 1 light chain 3 (light chain 3-II/light chain 3-I ratio) in the hippocampal CA1 region of vascular dementia model rats
Western blot assay revealed that a small quantity of light chain 3 protein was present in the hippocampal CA1 region of rats in the sham surgery group, and the light chain 3-II/ light chain 3-I ratio was low. In the vascular dementia group, the light chain 3-II/light chain 3-I ratio was greater than that in the sham-operated group at 1 week, peaked at 4 weeks, began to diminish at 8 weeks, and was still elevated at 12 weeks compared with the sham-operated group (P < 0.05 or P < 0.01). In the wortmannin group, the light chain 3-II/ light chain 3-I ratio was lower than that in the vascular dementia group at all time points (P < 0.05 or P < 0.01; Figure 3, Table 5).
Figure 3.
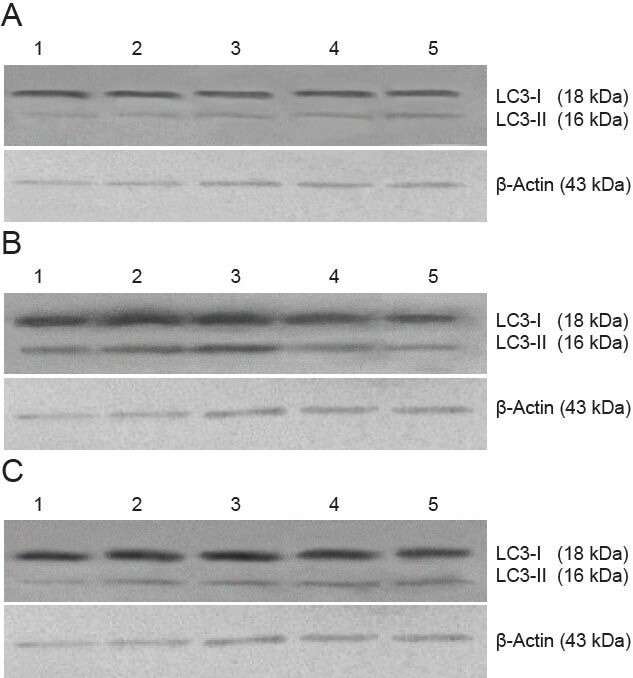
Effect of autophagy blockade on the expression of microtubule-associated protein 1 light chain 3 (LC3) in the hippocampal CA1 region of vascular dementia model rats (western blot assay).
(A) Sham surgery group; (B) vascular dementia group; (C) wortmannin group. 1–5: 1, 2, 4, 8 and 12 weeks after modeling.
Table 5.
Effect of autophagy on the expression of microtubule-associated protein 1 light chain 3 (LC3) (LC3-II/LC3-I ratio) in the hippocampal CA1 region of vascular dementia model rats
Discussion
Vascular dementia is primarily caused by cerebrovascular disease, specifically recurrent cerebral ischemia (Pulsinelli et al., 1982). In the present study, we established a rat model of vascular dementia using a modification of the Pulsinelli four-vessel occlusion method, which severely reduced the blood supply to the brain, simulating the pathogenic process of human vascular dementia and resulting in a decrease in nerve cell functions and impaired learning and memory. The Pulsinelli method is an established technique for producing vascular dementia models. A previous study demonstrated that neurons in different cerebral regions had different sensitivities to ischemia, with the hippocampus being the most sensitive (Hölscher, 2003). The CA1 region of the hippocampus is strongly associated with spatial discrimination, learning and memory (Hölscher, 2003; Lin et al., 2014). Neuronal injury in the CA1 region affects individual learning and memory functions (Vorhees and Williams, 2006; Foster, 2012). In the present study, we tested learning and memory in rats and showed that performance in the Morris water maze was significantly impaired in vascular dementia model rats. Thus, the model rats used in this study met the criteria for vascular dementia models, confirming the success of the model. After injection of wortmannin, an inhibitor of autophagy, escape latency was shorter in the navigation tests, and rats crossed the platform area more times in the probe test, than rats in the vascular dementia model group, indicating that inhibition of autophagy prevented the impairment of learning and memory seen in the vascular dementia model rats.
Autophagy is a self-degradative process that is important for balancing sources of energy. It involves lysosomal-dependent recycling, synthesis and degradation of intracellular components (Reggiori and Klionsky, 2002; Jung and Lee, 2010), thus maintaining the stability of the internal environment (Kanki and Klionsky, 2010; Novak and Dikic, 2011; Lee et al., 2012). During this process, targeted cytoplasmic constituents are isolated from the rest of the cell within the autophagosomes, which are then fused with lysosomes and degraded or recycled (Reggiori and Klionsky, 2002). The formation of the autophagosome is essential in the detection of autophagy, and electron microscopy is the gold standard method by which to observe autophagosomes (Mizushima, 2004). In the present study, electron microscope images revealed ultrastructural changes in the hippocampal CA1 neurons of rats in the sham surgery group, with the presence of autophagy. However, in the wortmannin group, there was less neuronal injury, and although autophagy occurred, there were fewer autophagosomes than in the model group. These results demonstrate that autophagy and significant changes in neuronal ultrastructure occur in the hippocampal CA1 region of vascular dementia model rats.
Beclin-1, highly homologous with yeast autophagy-related gene 6 (Apg6/Vps30), is essential to the induction and regulation of autophagy (Aita et al., 1999; Liang et al., 2001). It is the earliest autophagic gene found in mammals, and can regulate autophagic activity by binding to phosphatidylinositol 3-kinase type 3 (Edinger and Thompson, 2003; Scarlatti et al., 2008). Previous studies have demonstrated that upregulation of beclin-1 promotes autophagic activity (Liang et al., 1999; Qu et al., 2003; Yue et al., 2003). Autophagic activity is reduced in cells with low beclin-1 expression (Yue et al., 2002; Yue et al., 2003). In the adult mammal brain, beclin-1 is expressed in neurons and astrocytes (Liang et al., 1998) in the cerebral cortex, hippocampus and cerebellum (Yue et al., 2002). Another study showed that beclin-1 was strongly associated with the anti-apoptotic factor Bcl-2 (Kim et al., 2006). The binding of beclin-1 to Bcl-2 functions as a defense system, suppressing cell autophagy and reducing apoptosis in the central nervous system. Moreover, enzymes in the lysosomes of eukaryotic cells are involved in cell metabolism (Qin et al., 2008). For example, cellular organelles surrounded by autophagosomes are degraded by lysosomal enzymes. Cathepsin is a major lysosomal enzyme (Diskin et al., 2005), comprising three forms of protease: cysteine protease (including cathepsin B, F, H and K), aspartate protease (cathepsin D and E), and serine protease (cathepsin A and G). Cathepsin is associated with the occurrence and development of various nervous system diseases (Kikuchi et al., 2003; Pišlar and Kos, 2014). A previous study reported that cathepsin B and cathepsin L are important for maintaining the normal function of the central nervous system (Turk et al., 2002). Results from the present study showed that protein expression of beclin-1 and cathepsin B was significantly greater in the vascular dementia group than in the sham surgery group, whereas expression of both proteins was lower in the wortmannin group than in the vascular dementia group. These results indicate that hippocampal expression of autophagy-related beclin-1 and cathepsin B proteins is increased following ischemic injury, and may play a role in the onset and development of vascular dementia.
Autophagy is an adaptation reaction of cells to stress produced by alterations in the internal and external environments. Stimulating factors trigger autophagic genes and induce an increase in the expression of related protein products. This results in the formation of autophagosomes and lysosomes and an increase in the expression of proteins related to autophagiclysosomal pathways (Levine and Klionsky, 2004; Meijer and Codogno, 2004; Todde et al., 2009). Light chain 3 is the first autophagosome membrane protein found in eukaryotic cells and is a characteristic marker of autophagy (Kanno et al., 2011). It exists as light chain 3-I and light chain 3-II. Light chain 3-I is soluble, with a molecular weight of 18 kDa. It is the form of light chain 3 found before autophagy, and is mainly distributed in the cytoplasm (Tanida et al., 2004). The molecular weight of light chain 3-II is 16 kDa. Light chain 3-II is produced by binding light chain 3-I after ubiquitin-like modification to phosphatidylethanolamine on the surface of autophagic membranes. Light chain 3-II is located in the preautophagosome and autophagosome, and serves as an autophagosomal marker. The light chain 3-II/ light chain 3-I ratio is associated with the number of autophagic vacuoles (Alonso et al., 2007). Western blot assay of light chain 3-II or light chain 3-II/light chain 3-I ratio is a simple and relatively quantitative method for evaluating autophagic activity (Cherra et al., 2010). Results from the present study demonstrated that the light chain 3-II/light chain 3-I ratio was greater in the hippocampal CA1 region of rats in the vascular dementia group than in the sham-operated group, but that this difference was reduced in rats that had received wortmannin. These results indicate that autophagic processes were activated in the hippocampus of vascular dementia model rats after ischemic injury, and suggest that autophagy is involved in the onset of vascular dementia.
The autophagy inhibitor wortmannin is a specific inhibitor of phosphatidylinositol 3-kinase, blocking the phosphatidylinositol 3-kinase/Akt signaling pathway inside the cell and reducing the occurrence of autophagy (Carloni et al., 2008). Our results confirmed that autophagy is activated in the hippocampus of vascular dementia model rats, suggesting its involvement in the onset of vascular dementia. Wortmannin reduced vascular dementia-induced hippocampal injury by suppressing autophagic activity, and showed neuroprotective effects. Our results suggest a novel pathway and target for drug treatment of vascular dementia.
Footnotes
Funding: This study was supported by the Scientific Technology Research Project of Hebei Provincial Higher Learning Schools in China, No. ZH2012046; and the Major Medical Research Program of Hebei Province in China, No. ZD2013087.
Conflicts of interest: None declared.
Copyedited by de Souza M, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Aita VM, Liang XH, Murty VV, Pincus DL, Yu W, Cayanis E, Kalachikov S, Gilliam TC, Levine B. Cloning and genomic organization of beclin 1, a candidate tumor suppressor gene on chromosome 17q21. Genomics. 1999;59:59–65. doi: 10.1006/geno.1999.5851. [DOI] [PubMed] [Google Scholar]
- Alger HM, Raben N, Pistilli E, Francia DL, Rawat R, Getnet D, Ghimbovschi S, Chen YW, Lundberg IE, Nagaraju K. The role of TRAIL in mediating autophagy in myositis skeletal muscle: a potential nonimmune mechanism of muscle damage. Arthritis Rheum. 2011;63:3448–3457. doi: 10.1002/art.30530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso S, Pethe K, Russell DG, Purdy GE. Lysosomal killing of Mycobacterium mediated by ubiquitin-derived peptides is enhanced by autophagy. Proc Natl Acad Sci U S A. 2007;104:6031–6036. doi: 10.1073/pnas.0700036104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, Beal MF, Thomas B. Autophagy in neurodegenerative disorders: pathogenic roles and therapeutic implications. Trends Neurosci. 2010;33:541–549. doi: 10.1016/j.tins.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj PR, Verdile G, Barr RK, Gupta V, Steele JW, Lachenmayer ML, Yue Z, Ehrlich ME, Petsko G, Ju S, Ringe D, Sankovich SE, Caine JM, Macreadie IG, Gandy S, Martins RN. Latrepirdine (dimebon) enhances autophagy and reduces intracellular GFP-A 42 levels in yeast. J Alzheimers Dis. 2012;32:949–967. doi: 10.3233/JAD-2012-120178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32:329–339. doi: 10.1016/j.nbd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Chang SH, Minai-Tehrani A, Shin JY, Park S, Kim JE, Yu KN, Hong SH, Hong CM, Lee KH, Beck GR, Jr, Cho MH. Beclin1-induced autophagy abrogates radioresistance of lung cancer cells by suppressing osteopontin. J Radiat Res. 2012;53:422–432. doi: 10.1269/jrr.11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Fong TH, Lee AW, Chiu WT. Autophagy is activated in injured neurons and inhibited by methylprednisolone after experimental spinal cord injury. Spine (Phila Pa 1976) 2012;37:470–475. doi: 10.1097/BRS.0b013e318221e859. [DOI] [PubMed] [Google Scholar]
- Cherra SJ, Kulich SM, Uechi G, Balasubramani M, Mountzouris J, Day BW, Chu CT. Regulation of the autophagy protein LC3 by phosphorylation. J Cell Biol. 2010;190:533–539. doi: 10.1083/jcb.201002108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diskin T, Tal-Or P, Erlich S, Mizrachy L, Alexandrovich A, Shohami E, Pinkas-Kramarski R. Closed head injury induces upregulation of Beclin 1 at the cortical site of injury. J Neurotrauma. 2005;22:750–762. doi: 10.1089/neu.2005.22.750. [DOI] [PubMed] [Google Scholar]
- Edinger AL, Thompson CB. Defective autophagy leads to cancer. Cancer Cell. 2003;4:422–424. doi: 10.1016/s1535-6108(03)00306-4. [DOI] [PubMed] [Google Scholar]
- Foster TC. Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca 2+ channels in senescent synaptic plasticity. Prog Neurobiol. 2012;96:283–303. doi: 10.1016/j.pneurobio.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- Hahn T, Münchow G, Hardt S. Electrophoretic transport of biomolecules across liquid-liquid interfaces. J Phys Condens Matter. 2011;23:184107. doi: 10.1088/0953-8984/23/18/184107. [DOI] [PubMed] [Google Scholar]
- Henriques-Pons A, Nagaraju K. Nonimmune mechanisms of muscle damage in myositis: role of the endoplasmic reticulum stress response and autophagy in the disease pathogenesis. Curr Opin Rheumatol. 2009;21:581–587. doi: 10.1097/BOR.0b013e3283319265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölscher C. Time space and hippocampal functions. Rev Neurosci. 2003;14:253–284. doi: 10.1515/revneuro.2003.14.3.253. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Suzuki T, Hattori M, Yoshimoto K, Ohsumi Y, Moriyasu Y. AtATG genes, homologs of yeast autophagy genes, are involved in constitutive autophagy in Arabidopsis root tip cells. Plant Cell Physiol. 2006;47:1641–1652. doi: 10.1093/pcp/pcl031. [DOI] [PubMed] [Google Scholar]
- Jia L, Gopinathan G, Sukumar JT, Gribben JG. Blocking autophagy prevents bortezomib-induced NF-κB activation by reducing I-κBα degradation in lymphomacells. PLoS One. 2012;7:e32584. doi: 10.1371/journal.pone.0032584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HS, Lee MS. Role of autophagy in diabetes and mitochondria. Ann N Y Acad Sci. 2010;1201:79–83. doi: 10.1111/j.1749-6632.2010.05614.x. [DOI] [PubMed] [Google Scholar]
- Kanki T, Klionsky DJ. Te molecular mechanism of mitochondria autophagy in yeast. Mol Microbiol. 2010;75:795–800. doi: 10.1111/j.1365-2958.2009.07035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno H, Ozawa H, Sekiguchi A, Yamaya S, Itoi E. Induction of autophagy and autophagic cell death in damaged neural tissue after acute spinal cord injury in mice. Spine (Phila Pa 1976) 2011;36:E1427–E1434. doi: 10.1097/BRS.0b013e3182028c3a. [DOI] [PubMed] [Google Scholar]
- Kiffin R, Bandyopadhyay U, Cuervo AM. Oxidative stress and autophagy. Antioxid Redox Signal. 2006;8:152–162. doi: 10.1089/ars.2006.8.152. [DOI] [PubMed] [Google Scholar]
- Kikuchi H, Yamada T, Furuya H, Doh-ura K, Ohyagi Y, Iwaki T, Kira J. Involvement of cathepsin B in the motor neuron degeneration of amyotrophic lateral sclerosis. Acta Neuropathol. 2003;105:462–468. doi: 10.1007/s00401-002-0667-9. [DOI] [PubMed] [Google Scholar]
- Kim R, Emi M, Matsuura K, Tanabe K. Antisense and nonantisense efects of antisense Bcl-2 on multiple roles of Bcl-2 as a chemosensitizer in cancer therapy. Cancer Gene Ther. 2006;14:1–11. doi: 10.1038/sj.cgt.7700986. [DOI] [PubMed] [Google Scholar]
- Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, Kawahara N, Kuida K, Nagata S, Kominami E, Tanaka K, Uchiyama Y. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol. 2008;172:454–469. doi: 10.2353/ajpath.2008.070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Giordano S, Zhang J. Autophagy mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6:463–477. doi: 10.1016/s1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Kleeman LK, Jiang HH, Gordon G, Goldman JE, Berry G, Herman B, Levine B. Protection against fatal Sindbis virus encephalitis by beclin a novel Bcl-2-interacting protein. J Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Liang XH, Yu J, Brown K, Levine B. Beclin 1 contains a leucinerich nuclear export signal that is required for its autophagy and tumor suppressor function. Cancer Res. 2001;61:3443–3449. [PubMed] [Google Scholar]
- Lin N, Pan XD, Chen AQ, Zhu YG, Wu M, Zhang J, Chen XC. Tripchlorolide improves age-associated cognitive deficits by reversing hippocampal synaptic plasticity impairment and NMDA receptor dysfunction in SAMP8 mice. Behav Brain Res. 2014;258:8–18. doi: 10.1016/j.bbr.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ. Te role of autophagy in Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2:a009357. doi: 10.1101/cshperspect.a009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer AJ, Codogno P. Regulation and role of autophagy in mammalian cells. Int J Biochem Cell Biol. 2004;36:2445–2462. doi: 10.1016/j.biocel.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Methods for monitoring autophagy. Int J Biochem Cell Biol. 2004;36:2491–2502. doi: 10.1016/j.biocel.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak I, Dikic I. Autophagy receptors in developmental clearance of mitochondria. Autophagy. 2011;7:301–303. doi: 10.4161/auto.7.3.14509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pišlar A, Kos J. Cysteine cathepsins in neurological disorders. Mol Neurobiol. 2014;49:1017–1030. doi: 10.1007/s12035-013-8576-6. [DOI] [PubMed] [Google Scholar]
- Powis G, Bonjouklian R, Berggren MM, Gallegos A, Abraham R, Ashendel C, Zalkow L, Matter WF, Dodge J, Grindey G, Vlahos CJ. Wortmannin, a potent and selective inhibitor of phosphatidylinositol-3-kinase. Cancer Res. 1994;54:2419–2423. [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Qin AP, Zhang HL, Qin ZH. Mechanisms of lysosomal proteases participating in cerebral ischemia-induced neuronal death. Neurosci Bull. 2008;24:117–123. doi: 10.1007/s12264-008-0117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Yu J, Bhagat G, Furuya N, Hibshoosh H, Troxel A, Rosen J, Eskelinen EL, Mizushima N, Ohsumi Y, Cattoretti G, Levine B. Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest. 2003;112:1809–1820. doi: 10.1172/JCI20039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reggiori F, Klionsky DJ. Autophagy in the eukaryotic cell. Eukaryot Cell. 2002;1:11–21. doi: 10.1128/EC.01.1.11-21.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- Scarlatti F, Maffei R, Beau I, Codogno P, Ghidoni R. Role of noncanonical Beclin 1-independent autophagy in cell death induced by resveratrol in human breast cancer cells. Cell Death Differ. 2008;15:1318–1329. doi: 10.1038/cdd.2008.51. [DOI] [PubMed] [Google Scholar]
- Sibolt G, Curtze S, Melkas S, Putaala J, Pohjasvaara T, Kaste M, Karhunen PJ, Oksala NKJ, Erkinjuntti T. Poststroke dementia is associated with recurrent ischaemic stroke. J Neurol Neurosurg Psychiatry. 2013;84:722–726. doi: 10.1136/jnnp-2012-304084. [DOI] [PubMed] [Google Scholar]
- Tanida I, Ueno T, Kominami E. LC3 conjugation system in mammalian autophagy. Int J Biochem Cell Biol. 2004;36:2503–2518. doi: 10.1016/j.biocel.2004.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todde V, Veenhuis M, van der Klei IJ. Autophagy: Principles and significance in health and disease. Biochim Biophys Acta. 2009;1792:3–13. doi: 10.1016/j.bbadis.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Tung YT, Wang BJ, Hu MK, Hsu WM, Lee H, Huang WP, Liao YF. Autophagy: a double-edged sword in Alzheimer'sdisease. J Biosci. 2012;37:157–165. doi: 10.1007/s12038-011-9176-0. [DOI] [PubMed] [Google Scholar]
- Turk V, Turk B, Gunčar G, Turk D, Kos J. Lysosomal cathepsins: structure, role in antigen processing and presentation, and cancer. Adv Enzyme Regul. 2002;42:285–303. doi: 10.1016/s0065-2571(01)00034-6. [DOI] [PubMed] [Google Scholar]
- Voigt O, Pöggeler S. Self-eating to grow and kill: autophagy in filamentous ascomycetes. Appl Microbiol Biot. 2013;97:9277–9290. doi: 10.1007/s00253-013-5221-2. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, Han F, Fukunaga K, Qin ZH. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 2008;4:762–769. doi: 10.4161/auto.6412. [DOI] [PubMed] [Google Scholar]
- Wipf P, Halter RJ. Chemistry and biology of wortmannin. Org Biomol Chem. 2005;3:2053–2061. doi: 10.1039/b504418a. [DOI] [PubMed] [Google Scholar]
- Yorimitsu T, Klionsky DJ. Autophagy: molecular machinery for self-eating. Cell Death Differ. 2005;12(Suppl 2):1542–1552. doi: 10.1038/sj.cdd.4401765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Horton A, Bravin M, DeJager PL, Selimi F, Heintz N. A novel protein complex linking the delta 2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice. Neuron. 2002;35:921–933. doi: 10.1016/s0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]
- Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]


