Abstract
Long gap peripheral nerve injuries usually reulting in life-changing problems for patients. Skeletal muscle derived-multipotent stem cells (Sk-MSCs) can differentiate into Schwann and perineurial/endoneurial cells, vascular relating pericytes, and endothelial and smooth muscle cells in the damaged peripheral nerve niche. Application of the Sk-MSCs in the bridging conduit for repairing long nerve gap injury resulted favorable axonal regeneration, which showing superior effects than gold standard therapy--healthy nerve autograft. This means that it does not need to sacrifice of healthy nerves or loss of related functions for repairing peripheral nerve injury.
Keywords: peripheral nerve support cells, Schwann cells, perineurium, endoneurium, cytokine, paracrine effect, blood vessel formation
Introduction
Peripheral nerve injury is frequently caused by various traumatic injuries. Unavoidable tissue resections following surgical tumor excision can also lead to nerve damage. Although the peripheral nervous system has the ability to regenerate axons following injury, when nerve gaps are larger than 1–2 cm, bridging strategies are required for repair. In such cases, autologous nerve grafts have been used as the gold standard (Deumens et al., 2010; Pfister et al., 2011), although this requires the sacrifice of healthy nerves. However, there are limited numbers of suitable sites for harvesting nerve grafts, because of the adverse effects to donor sites, such as the loss of motor and sensory function.
Development of nerve bridging conduit
In order to overcome these issues, scaffold bridging materials that may be synthetic, absorbable or non-absorbable have been studied vigorously. Through these studies, several criteria were suggested to be necessary for better results, as follows: 1) the bridging conduit is necessary to maintain adequate mechanical support of separated nerve ends, in addition to preventing the diffusion of neurotrophic and neurotropic factors secreted by transected stumps (Deumens et al., 2010); 2) nerve scaffolds should be biocompatible in order to eliminate undesirable influences on neural cells/tissues, such as immune rejection (Avitable et al., 2001); 3) it should be degradable and/or absorbable in the appropriate duration in vivo (de Ruiter et al., 2009); 4) it must have sufficient permeability for nutrient and gas exchange (She et al., 2008); and 5) it must have a biomechanical flexibility to allow bending at the graft site, without kinking the transected nerve stumps (Gu et al., 2011). For longer nerve gap injuries, it is likely that scaffold structure will be another important factor. Scaffolds with a single hollow lumen represent the basic structure, but this has been refined using various biosynthetic materials in order to mimic the nerve-graft structure and to facilitate axonal re-extension. The multi-luminal substructure (de Ruiter et al., 2008b) and the filament-filled structure (Bunting et al., 2005; Cai et al., 2005; Hu et al., 2008) are typical cases; the former shows no significant benefit, but the latter shows improved nerve regeneration, as compared to the single lumen scaffold. Intraluminal surface coating with extracellular matrix (ECM) components, such as laminin, fibronectin and collagen, has been also attempted to promote active axonal re-growth. First, this was attempted using a single hollow conduit, and several positive effects were obtained (Kauppila et al., 1993; Whitworth et al.,1995). In this regard, the use of conduits of biological origin, such as acellular conduits, which have preserved ECM components, but are decellularized and immunosuppressed, was another approach (Frerichs et al., 2002; Hudson et al., 2004a). However, use of this hollow-type conduit shows non-significant effects on nerve regeneration across long gaps (Pfister et al., 2011). In contrast, conduits including laminin-coated collagen fibers were able to support the regeneration of long sciatic nerve gaps of over 80 mm in dogs (Matsumoto et al., 2000). At this point, the filament-filled conduit with laminin coating is likely to be the best alternative method. Importantly, these efforts have been performed to expect alternative effects of laminin-coated fibers in the conduit, similar to the basal lamina sheet in the individual endoneurium of the healthy nerve fibers. Laminin coating may exert supportive effects for the migration, proliferation and orientation of preserved Schwann cells, similarly to formation of Bands of Bungner, which normally occurs in the case of axonotmesis (Deumens et al., 2010). Furthermore, application of growth factors into the conduit is another method to mimic native conditions. For example, supply of several factors into the conduit, such as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), ciliary neurotrophic factor (CNTF), basic fibroblast growth factor (bFGF) and insulin-like growth factor-1 (IGF-1), has also been performed, and favorable effects on axonal growth and nerve regeneration have been confirmed (Rich et al., 1989; Nachemson et al., 1990; Fine et al., 2002; Timmer et al., 2003; Lewin et al., 2009).
Combination with cell transplantation
All of the applications described above were based on the notion of simulating the healthy nerve graft condition. In this regard, transplantation of Schwann cells with the conduit is considered to be the best method as the alternative for nerve grafting. Acellular conduits have thus been used with several cell sources, including Schwann cells and/or Schwann-like cells induced from cultivated bone morrow stromal cells (Dezawa et al., 2005), olfactory ensheathing cells (Radtke et al., 2011) and adipose tissue-derived cells (Kingham et al., 2007), but it is unlikely that these cells would match or exceed the performance of auto nerve grafts. Although Schwann cells play a central role in peripheral nerve regeneration, the formation of endoneurium and/or perineurium by endoneurial fibroblasts and perineurial cells is also important because of their protective role in axons with Schwann cells and myelin sheath. In particular, the perineurium plays an important role in preventing the passage of large molecules from the epineurium into perineurial fascicles, which is also known as the “blood-nerve-barrier” system (Weerasuriya and Mizisin, 2011). Furthermore, reconstitution of vascular networks is also an inevitable factor in tissue reconstruction. In this regard, cell sources that can give rise to Schwann cells and cells associated with the formation of perineurium/endoneurium and blood vessels are considered to be the best source for peripheral nerve regeneration.
Comprehensive regeneration with all nerve support cells/tissues
Recently, we reported preferential and comprehensive reconstitution of severely damaged sciatic nerve using 7-day cultivated murine skeletal muscle-derived multipotent stem cells (Sk-MSCs; Tamaki et al., 2014). In this treatment, engrafted donor cells preferentially differentiated into Schwann cells and perineurial/endoneurial cells, and formed myelin sheath and perineurium/endoneurium, encircling the regenerated axons. Donor cell-derived perineurium also had tight-junctions, which play a key role in the “blood-nerve-barrier” system; thus, suggesting functional reconstitution. Stem cells showing suitable multipotency to cover all peripheral nerve support cells in damaged nerve-specific niche have not been reported previously, although cultivated bone morrow stromal cells (MBCSs; Dezawa et al., 2005), olfactory ensheathing cells (Radtke et al., 2011) and adipose tissue-derived cells (Kingham et al., 2007) have been studied mainly expecting the differentiation of transplanted cells into Schwann cells. We directly compared the contribution to damaged nerve reconstitution among cultivated Sk-MSCs and BMSCs, and freshly isolated cells derived from damaged sciatic nerve (SNDC-D) (Tamaki et al., 2014). The results showed that Sk-MSCs have a significantly greater engraftment ratio when compared with the other two groups, and the values in the latter two groups were similar. In addition, there was no definitive formation of peripheral nerve supporting cells or incorporation into blood vessels in BMSC transplantation, whereas Sk-MSCs and SNDC-D showed typical differentiation into all support cells. In addition, engrafted Sk-MSCs contributed to increased vascular formation, which is favorable for blood supply and waste product excretion. Facilitation of blood vessel formation in Sk-MSCs was also confirmed in damaged skeletal muscle (Tamaki et al., 2005; 2007b; 2013) and urethra (Hoshi et al., 2008), as well as in and around the bladder wall (Nitta et al., 2010; Soeda et al., 2013). This trend appears to be typical of Sk-MSC transplantation. Expression of key neurotrophic and nerve/vascular growth factor mRNAs was also confirmed; in particular, NGF (Rich et al., 1989), BDNF (Lewin et al., 2009), GDNF (Fine et al., 2002), Galectin-1 (Horie et al., 1999), Ninjurin (Araki and Milbrandt, 1996), CNTF (Dubovy et al., 2011), LIF (Wang et al., 2009), Sox10 (Bremer et al., 2011; Britsch et al., 2001; Finzsch et al., 2010), bFGF (Timmer et al., 2003) and IGF-1 (Nachemson et al., 1990) are important for nerve regeneration, while VGEF (Ferrara, 2004) and HGF (Schroder et al., 2011) are important for blood vessels. Expression of these factors was retained for at least 4 weeks after transplantation. Some of these are redundant and/or primarily involved in the recipient nerve regeneration process (Campbell, 2008); however, sufficient expression of these factors in the damaged/transplantation site may induce paracrine effects on both recipient and donor cells as an adjuvant for nerve regeneration. Using Sk-MSCs, we examined the therapeutic capacity in a long transected nerve gap on the mouse sciatic nerve via an acellular conduit (1.5–2.0 × 106 cells/nerve), and demonstrated 94% reconstitution of the number of axons, 60% recovery of myelin, and a 9.1-fold increase in blood vessels through the conduit (Tamaki et al., 2014). Features and immunohistochemical properties of regenerated nerve at 8 weeks after bridging with Sk-MSCs transplantation are shown in Figure 1. Thick GFP+ tissues were evident throughout the conduit (Figure 1A), and a large number of GFP+ multi-luminal substructures, such as perineurium/endoneurium, were also apparent in histological sections (Figure 1B and C). Importantly, regenerated axons in the conduit were encircled by engrafted GFP+ perineurium/endoneurium (Figure 1B), having myelination (Figure 1C), thus suggesting that regenerating axons extended along the endoneurial/perineurial substructures with supporting Schwann cells. The bridging acellular conduit also includes a large number of blood vessels (Figure D); thus, favorable blood supply and waste product excretion are expected. These relationships between GFP+ tissues and regenerated axons were confirmed in longitudinal sections (Figure 1E), and GFP+ multi-luminal substructures also possessed the basal lamina sheath (Figure 1F and G). These results clearly indicated that our nerve treatment method using the Sk-MSCs with acellular conduits meet all of the above criteria, making it useful for nerve regeneration. In addition, we also demonstrated the greater therapeutic potential of Sk-MSC treatment as compared to case of healthy nerve grafts using the same mouse model (Tamaki et al., 2014).
Figure 1.
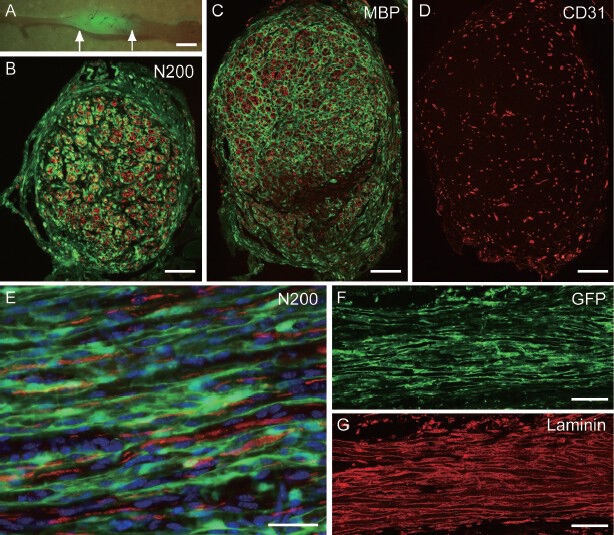
Typical structure of engrafted GFP+ skeletal muscle-derived multipotent stem cells (Sk-MSCs) in acellular bridging conduit at 8 weeks after transplantation.
(A) Macroscopic features of regenerated sciat-ic nerve with engrafted GFP+ tissues. Arrows indicate conduit portion. (B) Cross-sectional profile of the bridging conduit filled with donor-derived GFP+ perineurium/endoneu-rium. Red reactions are neurofilament-200 (N200)-positive axons. (C) Cross-sectional profile of bridging conduit filled with do-nor-derived GFP+ perineurium/endoneurium. Sections were obtained from different portions than in panel (B). Red reactions are myelin ba-sic protein (MBP)-positive myelin. (D) Same portion of panel (C) is stained with CD31 (vascular endothelial cell marker). (E) Longi-tudinal view of the bridging conduit at higher magnification. GFP+ perineurium/endoneu-rium show continuously elongated features having axons (red) inside. Blue nuclei staining = DAPI. (F) Longitudinal view of GFP+ peri-neurium/endoneurium in the conduit, and (G) laminin staining (red). GFP+ perineurium/en-doneurium also has basal lamina sheets, which are detected by anti-laminin staining. Bars in A = 2 mm, B–D= 200 μm, E = 20 μm, and F = 100 μm. GFP: Green fluorescence protein; MBP: myelin basic protein.
In conclusion, skeletal muscle is the largest organ in the body, comprising approximately 40–50% of total body mass, and presumably allowing donor cells to be obtained with relative ease and safety. Therefore, Sk-MSCs represent a novel/suitable alternative cell source for healthy nerve autografts. Skeletal muscle-derived stem cells have been used by many researchers for various purposes; however, our isolation method of Sk-MSCs differs from others (Tamaki et al., 2002; 2010), and this is potentially crucial to our results after transplantation (Tamaki et al., 2005, 2007a,b).
Future perspectives
In future research, the potential of human skeletal muscle-derived cells needs to be clarified. We are currently investigating the optimal isolation, fractionation and expansion culture conditions for human cells in order to maximize the nerve regeneration potential. In vivo evidence of the differentiation and reconstruction capacities of human Sk-MSCs is also important. In addition, suitable combinations of conduit materials remain to be clarified. We used the acellular conduit from the wild-type mice esophageal submucous membrane (mainly longitudinal muscle layer) separated after 3 days of 70% ethanol dehydration. In the human case, use of an appropriate size vein may be a candidate cellular or acellular conduit. However, from the viewpoint of minimizing invasiveness, biosynthetic tubes that are a good match for Sk-MSCs treatment need to be identified. Application of Sk-MSC treatment for the total regeneration of multi-branched nerve transections may also be useful in the case of surgical nerve damage.
Footnotes
Funding: This work was supported by a 2013 Tokai University School of Medicine, Project Research Grant.
References
- 1.Araki T, Milbrandt J. Ninjurin a novel adhesion molecule is induced by nerve injury and promotes axonal growth. Neuron. 1996;17:353–361. doi: 10.1016/s0896-6273(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 2.Avitabile T, Marano F, Castiglione F, Bucolo C, Cro M, Ambrosio L, Ferrauto C, Reibaldi A. Biocompatibility and biodegradation of intravitreal hyaluronan implants in rabbits. Biomaterials. 2001;22:195–200. doi: 10.1016/s0142-9612(00)00169-1. [DOI] [PubMed] [Google Scholar]
- 3.Bremer M, Frob F, Kichko T, Reeh P, Tamm ER, Suter U, Wegner M. Sox10 is required for Schwann-cell homeostasis and myelin maintenance in the adult peripheral nerve. Glia. 2011;59:1022–1032. doi: 10.1002/glia.21173. [DOI] [PubMed] [Google Scholar]
- 4.Britsch S, Goerich DE, Riethmacher D, Peirano RI, Rossner M, Nave KA, Birchmeier C, Wegner M. The transcription factor Sox10 is a key regulator of peripheral glial development. Genes Dev. 2001;15:66–78. doi: 10.1101/gad.186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunting S, Di Silvio L, Deb S, Hall S. Bioresorbable glass fibres facilitate peripheral nerve regeneration. J Hand Surg Br. 2005;30:242–247. doi: 10.1016/j.jhsb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Cai J, Peng X, Nelson KD, Eberhart R, Smith GM. Permeable guidance channels containing microfilament scaffolds enhance axon growth and maturation. J Biomed Mater Res A. 2005;75:374–386. doi: 10.1002/jbm.a.30432. [DOI] [PubMed] [Google Scholar]
- 7.Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008;119:1951–1965. doi: 10.1016/j.clinph.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 8.de Ruiter GC, Spinner RJ, Malessy MJA, Moore MJ, Sorenson EJ, Currier BL, Yaszemski MJ, Windebank AJ. Accuracy of motor axon regeneration across autograft, single-lumen, and multichannel poly(- lactic-co-glycolic acid) nerve tubes. Neurosurgery. 2008;63:144–153. doi: 10.1227/01.NEU.0000335081.47352.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Ruiter GC, Spinner RJ, Yaszemski MJ, Windebank AJ, Malessy MJ. Nerve tubes for peripheral nerve repair. Neurosurg Clin N Am. 2009;20:91–105. doi: 10.1016/j.nec.2008.08.001. vii. [DOI] [PubMed] [Google Scholar]
- 10.Deumens R, Bozkurt A, Meek MF, Marcus MA, Joosten EA, Weis J, Brook GA. Repairing injured peripheral nerves: Bridging the gap. Prog Neurobiol. 2010;92:245–276. doi: 10.1016/j.pneurobio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Dezawa M, Ishikawa H, Hoshino M, Itokazu Y, Nabeshima Y. Potential of bone marrow stromal cells in applications for neuro-degenerative, neuro-traumatic and muscle degenerative diseases. Curr Neuropharmacol. 2005;3:257–266. doi: 10.2174/157015905774322507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubovy P, Raska O, Klusakova I, Stejskal L, Celakovsky P, Haninec P. Ciliary neurotrophic factor promotes motor reinnervation of the musculocutaneous nerve in an experimental model of end-to-side neurorrhaphy. BMC Neurosci. 2011;12:58. doi: 10.1186/1471-2202-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25:581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 14.Fine EG, Decosterd I, Papaloı¨zos M, Zurn AD, Aebischer P. GDNF and NGF released by synthetic guidance channels support sciatic nerve regeneration across a long gap. Eur J Neurosci. 2002;15:589–601. doi: 10.1046/j.1460-9568.2002.01892.x. [DOI] [PubMed] [Google Scholar]
- 15.Finzsch M, Schreiner S, Kichko T, Reeh P, Tamm ER, Bosl MR, Meijer D, Wegner M. Sox10 is required for Schwann cell identity and progression beyond the immature Schwann cell stage. J Cell Biol. 2010;189:701–712. doi: 10.1083/jcb.200912142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frerichs O, Fansa H, Schicht C, Wolf G, Schneider W, Keilhoff G. Reconstruction of peripheral nerves using acellular nerve grafts with implanted cultured Schwann cells. Microsurgery. 2002;22:311–315. doi: 10.1002/micr.10056. [DOI] [PubMed] [Google Scholar]
- 17.Gu X, Ding F, Yang Y, Liu J. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 2011;93:204–30. doi: 10.1016/j.pneurobio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Horie H, Inagaki Y, Sohma Y, Nozawa R, Okawa K, Hasegawa M, Muramatsu N, Kawano H, Horie M, Koyama H, Sakai I, Takeshita K, Kowada Y, Takano M, Kadoya T. Galectin-1 regulates initial axonal growth in peripheral nerves after axotomy. J Neurosci. 1999;19:9964–9974. doi: 10.1523/JNEUROSCI.19-22-09964.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoshi A, Tamaki T, Tono K, Okada Y, Akatsuka A, Usui Y, Terachi T. Reconstruction of radical prostatectomy-induced urethral damage using skeletal muscle-derived multipotent stem cells. Transplantation. 2008;85:1617–1624. doi: 10.1097/TP.0b013e318170572b. [DOI] [PubMed] [Google Scholar]
- 20.Hu W, Gu J, Deng A, Gu X. Polyglycolic acid filaments guide Schwann cell migration in vitro and in vivo. Biotechnol Lett. 2008;30:1937–1942. doi: 10.1007/s10529-008-9795-1. [DOI] [PubMed] [Google Scholar]
- 21.Hudson TW, Liu SY, Schmidt CE. Engineering an improved acellular nerve graft via optimized chemical processing. Tissue Eng. 2004;10:1346–1358. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 22.Kauppila T, Jyvasjarvi E, Huopaniemi T, Hujanen E, Liesi P. A laminin graft replaces neurorrhaphy in the restorative surgery of the rat sciatic nerve. Exp Neurol. 1993;123:181–191. doi: 10.1006/exnr.1993.1151. [DOI] [PubMed] [Google Scholar]
- 23.Kingham PJ, Kalbermatten DF, Mahay D, Armstrong SJ, Wiberg M, Terenghi G. Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro. Exp Neurol. 2007;207:267–274. doi: 10.1016/j.expneurol.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 24.Lewin SL, Utley DS, Cheng ET, Verity AN, Terris DJ. Simultaneous treatment with BDNF and CNTF after peripheral nerve transection and repair enhances rate of functional recovery compared with BDNF treatment alone. Laryngoscope. 2009;107:992–999. doi: 10.1097/00005537-199707000-00029. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto K, Ohnishi K, Sekine T, Ueda H, Yamamoto Y, Kiyotani T, Nakamura T, Endo K, Shimizu Y. Use of a newly developed artificial nerve conduit to assist peripheral nerve regeneration across a long gap in dogs. ASAIO J. 2000;46:415–420. doi: 10.1097/00002480-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Nachemson AK, Lundborg G, Hansson HA. Insulin-like growth factor I promotes nerve regeneration: an experimental study on rat sciatic nerve. Growth Factors. 1990;3:309–314. doi: 10.3109/08977199009003673. [DOI] [PubMed] [Google Scholar]
- 27.Nitta M, Tamaki T, Tono K, Okada Y, Masuda M, Akatsuka A, Hoshi A, Usui Y, Terachi T. Reconstitution of experimental neurogenic bladder dysfunction using skeletal muscle-derived multipotent stem cells. Transplantation. 2010;89:1043–1049. doi: 10.1097/TP.0b013e3181d45a7f. [DOI] [PubMed] [Google Scholar]
- 28.Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE, Cullen DK. Biomedical engineering strategies for peripheral nerve repair: surgical applications, state of the art, and future challenges. Crit Rev Biomed Eng. 2011;39:81–124. doi: 10.1615/critrevbiomedeng.v39.i2.20. [DOI] [PubMed] [Google Scholar]
- 29.Radtke C, Wewetzer K, Reimers K, Vogt PM. Transplantation of olfactory ensheathing cells as adjunct cell therapy for peripheral nerve injury. Cell Transplant. 2011;20:145–152. doi: 10.3727/096368910X522081. [DOI] [PubMed] [Google Scholar]
- 30.Rich KM, Alexander TD, Pryor JC, Hollowell JP. Nerve growth factor enhances regeneration through silicone chambers. Exp Neurol. 1989;105:162–170. doi: 10.1016/0014-4886(89)90115-5. [DOI] [PubMed] [Google Scholar]
- 31.Schroder K, Schutz S, Schloffel I, Batz S, Takac I, Weissmann N, Michaelis UR, Koyanagi M, Brandes RP. Hepatocyte growth factor induces a proangiogenic phenotype and mobilizes endothelial progenitor cells by activating Nox2. Antioxid Redox Signal. 2011;15:915–923. doi: 10.1089/ars.2010.3533. [DOI] [PubMed] [Google Scholar]
- 32.She ZD, Zhang BF, Jin CR, Feng QL, Xu YX. Preparation and in vitro degradation of porous three-dimensional silk fibroin/chitosan scaffold. Polymer Degrad Stab. 2008;93:1316–1322. [Google Scholar]
- 33.Soeda S, Tamaki T, Hashimoto H, Saito K, Sakai A, Nakajima N, Nakazato K, Masuda M, Terachi T. Functional nerve-vascular reconstitution of the bladder-wall; application of patch transplantation of skeletal muscle-derived multipotent stem cell sheet-pellets. J Stem Cell Res Ther. 2013;3:142. [Google Scholar]
- 34.Tamaki T, Akatsuka A, Ando K, Nakamura Y, Matsuzawa H, Hotta T, Roy RR, Edgerton VR. Identification of myogenic-endothelial progenitor cells in the interstitial spaces of skeletal muscle. J Cell Biol. 2002;157:571–577. doi: 10.1083/jcb.200112106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tamaki T, Uchiyama Y, Okada Y, Ishikawa T, Sato M, Akatsuka A, Asahara T. Functional recovery of damaged skeletal muscle through synchronized vasculogenesis, myogenesis, and neurogenesis by muscle-derived stem cells. Circulation. 2005;112:2857–2866. doi: 10.1161/CIRCULATIONAHA.105.554832. [DOI] [PubMed] [Google Scholar]
- 36.Tamaki T, Okada Y, Uchiyama Y, Tono K, Masuda M, Wada M, Hoshi A, Ishikawa T, Akatsuka A. Clonal multipotency of skeletal muscle-derived stem cells between mesodermal and ectodermal lineage. Stem Cells. 2007a;25:2283–2290. doi: 10.1634/stemcells.2006-0746. [DOI] [PubMed] [Google Scholar]
- 37.Tamaki T, Okada Y, Uchiyama Y, Tono K, Masuda M, Wada M, Hoshi A, Akatsuka A. Synchronized reconstitution of muscle fibers, peripheral nerves and blood vessels by murine skeletal muscle-derived CD34(-)/45 (-) cells. Histochem Cell Biol. 2007b;128:349–360. doi: 10.1007/s00418-007-0331-5. [DOI] [PubMed] [Google Scholar]
- 38.Tamaki T, Uchiyama Y, Akatsuka A. Plasticity and physiological role of stem cells derived from skeletal muscle interstitium: contribution to muscle fiber hyperplasia and therapeutic use. Curr Pharm Des. 2010;16:956–967. doi: 10.2174/138161210790883408. [DOI] [PubMed] [Google Scholar]
- 39.Tamaki T, Soeda S, Hashimoto H, Saito K, Sakai A, Nakajima N, Masuda M, Fukunishi N, Uchiyama Y, Terachi T, Mochida J. 3D reconstitution of nerve-blood vessel networks using skeletal muscle-derived multipotent stem cell sheet pellets. Regen Med. 2013;8:437–451. doi: 10.2217/rme.13.30. [DOI] [PubMed] [Google Scholar]
- 40.Tamaki T, Hirata M, Soeda S, Nakajima N, Saito K, Nakazato K, Okada Y, Hashimoto H, Uchiyama Y, Mochida J. Preferential and comprehensive reconstitution of severely damaged sciatic nerve using murine skeletal muscle-derived multipotent stem cells. PLoS One. 2014;9:e91257. doi: 10.1371/journal.pone.0091257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Timmer M, Robben S, Muller-Ostermeyer F, Nikkhah G, Grothe C. Axonal regeneration across long gaps in silicone chambers filled with Schwann cells overexpressing high molecular weight FGF-2. Cell Transplant. 2003;12:265–277. doi: 10.3727/000000003108746821. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Lee HK, Seo IA, Shin YK, Lee KY, Park HT. Cell type-specific STAT3 activation by gp130-related cytokines in the peripheral nerves. Neuroreport. 2009;20:663–668. doi: 10.1097/WNR.0b013e32832a09f8. [DOI] [PubMed] [Google Scholar]
- 43.Weerasuriya A, Mizisin AP. The blood-nerve barrier: structure and functional significance. Methods Mol Biol. 2011;686:149–173. doi: 10.1007/978-1-60761-938-3_6. [DOI] [PubMed] [Google Scholar]
- 44.Whitworth IH, Brown RA, Dore C, Green CJ, Terenghi G. Orientated mats of fibronectin as a conduit material for use in peripheral nerve repair. J Hand Surg Br. 1995;20:429–436. doi: 10.1016/s0266-7681(05)80148-2. [DOI] [PubMed] [Google Scholar]


