Abstract
Platelet-rich plasma containing various growth factors can promote nerve regeneration. An inside-out vein graft can substitute nerve autograft to repair short nerve defects. It is hypothesized that an inside-out vein graft filled with platelet-rich plasma shows better effects in the repair of short sciatic nerve defects. In this study, an inside-out vein autograft filled with platelet-rich plasma was used to bridge a 10 mm-long sciatic nerve defect in rats. The sciatic nerve function of rats with an inside-out vein autograft filled with platelet-rich plasma was better improved than that of rats with a simple inside-out vein autograft. At 6 and 8 weeks, the sciatic nerve function of rats with an inside-out vein autograft filled with platelet-rich plasma was better than that of rats undergoing nerve autografting. Compared with the sciatic nerve repaired with a simple inside-out vein autograft, the number of myelinated axons was higher, axon diameter and myelin sheath were greater in the sciatic nerve repaired with an inside-out vein autograft filled with platelet-rich plasma and they were similar to those in the sciatic nerve repaired with nerve autograft. These findings suggest that an inside-out vein graft filled with platelet-rich plasma can substitute nerve autograft to repair short sciatic nerve defects.
Keywords: nerve regeneration, peripheral nerve injury, sciatic nerve, platelet-rich plasma, inside-out vein autograft, myelinated axons, axon diameter, myelin sheath thickness, histology, sciatic nerve index, neural regeneration
Introduction
Successful reconstruction of segmental nerve defects has been a challenging surgical hurdle for reconstructive surgeons. Although many surgical strategies have been attempted for the reconstruction of nerve defect, nerve autografts are still accepted as the most effective method when primary repair is impossible; however, donor site morbidity is an inevitable drawback. To eliminate or minimize the donor site morbidity, various nerve graft substitutes have been used, and nerve conduits made from autogenous tissues such as muscle, artery, or vein have been applied to a small nerve defect. Various synthetic nerve conduits have also been tested experimentally as alternatives to nerve autografts; however, these synthetic conduits have not been demonstrated to be clinically effective replacements for nerve autografts.
Recently, autogenous or synthetic conduits have been combined with cell-level therapy to replace damaged nerve cells, and research on a variety of factors that promote nerve regeneration has increased. As a part of these efforts, platelet-rich plasma (PRP) has been tested to promote regeneration of injured nerve, due to its high concentration of various growth factors such as platelet-derived epidermal growth factor, platelet-derived growth factor, transforming growth factor, insulin-like growth factor, vascular endothelial growth factor, endothelial cell growth factor, and basic fibroblast growth factor that aid tissue regeneration (Farrag et al., 2007; Elgazzar et al., 2008; Sariguney et al., 2008; Ding et al., 2009; Piskin et al., 2009; Cho et al., 2010; Kaplan et al., 2011). PRP is concentrated plasma with approximately five times the platelet concentration of normal plasma and has been used in various fields to regenerate damaged tissue (Everts et al., 2012). Accumulating data have revealed that the growth factors in PRP promote recovery of damaged target cells or tissues, such as blood vessels, fibroblasts, muscles, bone, and skin, resulting in cellular proliferation, differentiation, collagen synthesis, chemotaxis, and angiogenesis (Farrag et al., 2007; Foster et al., 2009; Smith and Smith, 2011; Taylor et al., 2011).
Though limited, early experimental data show promising results that PRP promotes nerve regeneration (Eccleston et al., 1993; Welch et al., 1997; Chen et al., 1999; Allamargot et al., 2001; Oya et al., 2002). Much experimental and clinical work has shown evidence that vein grafts, including inside-out vein graft, can be used as an alternative for a nerve autograft in short segment nerve defects (Wang et al., 1995; Ferrari et al., 1999; Gravvanis et al., 2004; Jeon et al., 2011).
The aim of this study was to investigate the effects of inside-out vein grafts filled with PRP on peripheral nerve regeneration in a rat sciatic nerve defect.
Materials and Methods
Animals
Thirty male Sprague-Dawley (SD) rats (provided by SLC, Inc.), weighing 250–300 g, aged 6–8 weeks, were randomly and evenly divided into three groups: nerve autograft group, vein autograft group, and vein autograft-PRP group. The animals were handled in compliance with the guidelines for the use and care of animals of KUMC-IACUC. The study protocol was approved by institutional animal care and use committee.
Surgical procedure
All rats were anesthetized with an intramuscular injection of ketamine hydrochloride (90 mg/kg, Ketalar; Bayer, Leverkusen, Germany) and xylazine (10 mg/kg Rompun; Bayer). The right sciatic nerve was exposed through an intergluteal approach, and a 10-mm sciatic nerve gap was made in the mid portion of the nerve. In the nerve autograft group, the removed segment of nerve was everted and grafted into the same nerve gap under an operating microscope (M650; Leica, Wetzlar, Germany) with 8 stiches of 10-0 monofilament nylon sutures (Ethicon Inc., Somerville, NJ, USA). In the vein autograft group, 15 mm of the left jugular vein was harvested, everted in an inside-out fashion, and grafted into the gap. Both the proximal and distal nerve stumps were inserted about 2–3 mm from the ends of the vein graft. In the vein autograft-PRP group, the grafted, inside-out vein was filled with approximately 0.15–0.2 mL of prepared PRP. PRP was injected into the cavity of vein after anastomosis through the space of stiches (Figure 1).
Figure 1.
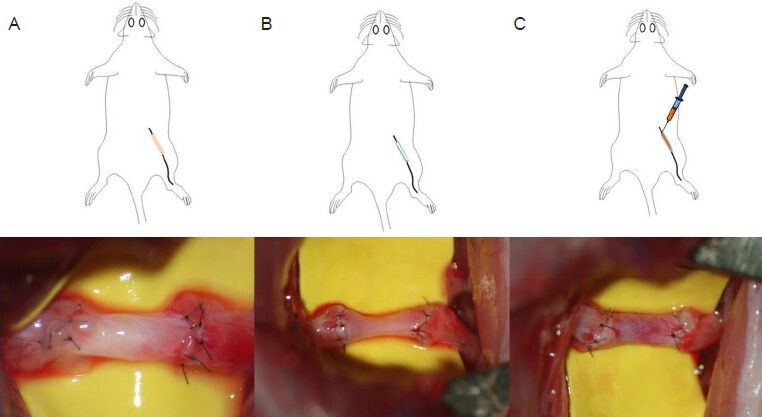
Schematic drawing of experimental groups and intra-operative photograph of a nerve autograft (A), inside-out vein autograft (B), and inside-out vein autograft filled with platelet-rich plasma (C).
In all groups, the skin was closed with 4-0 nylon sutures, and aminoglycosides (Netromycin; Merck & Co. Inc., Summit, NJ, USA) were injected intramuscularly to prevent wound infection. After the operation, rats were returned to their cage and raised in an environment with a 12 hour on-off light cycle. Food and water were allowed ad libitum.
PRP preparation
Three milliliters of whole blood was collected from each rat prior to the operation and treated with 0.35 mL of 3.2% sodium citrate to prevent coagulation. The blood was centrifuged at 160 × g at 22°C for 20 minutes. The blood was separated into three layers: red blood cells at the bottom; acellular plasma at the top; and platelets, called buffy coat, in the middle. The red blood cells in the lower layer were discarded, and the acellular plasma and platelets were centrifuged again at 400 × g at 22°C for 15 minutes. After the second centrifugation, approximately 0.3 mL of PRP was pipetted from the bottom of the tube. The PRP was mixed with 0.015 mL of 10% calcium chloride (activate the growth factors) prior to use.
Sciatic functional index (SFI)
The SFI was evaluated using the walking tract test biweekly for 24 weeks. Footprints from the experimental (E) and contralateral normal (N) sides were analyzed by measuring the lengths of the third toe to heel (PL), the first toe to the fifth toe (TS), and the second toe to the fourth toe (IT). The SFI was calculated using the following formula described by Bain et al. (1989).
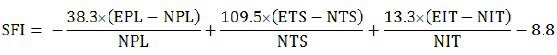
In general, an index of 0 indicates normal function and an index of –100 represents complete loss of function. Because three rats in each group were sacrificed at weeks 8, 16, and 24, SFI was measured from ten rats in each group at 2–8 weeks, seven rats at 10–16 weeks, and four rats at 18–24 weeks.
Histological evaluation
A 5 mm segment of graft was harvested from the midportion of the graft (graft level), and another 5 mm segment was taken 5 mm distal to the graft site (distal level). The nerve specimens were fixed with 2.5% glutaraldehyde in 0.1mol/L phosphate buffer (pH 7.4) for 48 hours at room temperature and post-fixed with 1% osmium tetroxide. The nerve specimens were embedded in epoxy resin, cut into 1-μm, semi-thin sections with an ultramicrotome (Leica, Ultracut, UCT, Austria), and stained with 1% toluidine blue for light microscopy (BX46, Olympus, Japan). Images were digitized with a charge-coupled device camera (DP21, Olympus, Japan) and analyzed by standard image processing at a magnification of × 200. The myelinated and unmyelinated axons were counted, myelin thickness and regenerated axon diameter were measured, and the number of regenerated vessels was counted at both the graft and distal levels every 8 weeks. Ten random fields of view from each semi-thin section were analyzed with imaging software (Image Pro Plus™, MediaCybernetics, Silver Spring, CA, USA). A nerve counting grid was overlaid on the photographs, and the sample area was chosen in a systemic, uniform, random manner ensuring that all locations in the nerve cross-section were equally represented. The observer was blinded to each group. The number of regenerated vessels was counted in the whole area of each specimen with appropriate digital magnification which is large enough to identify vessels from the other tissues. The histological evaluation was performed on three rats in each group at 8 and 16 weeks and on four rats at 24 weeks.
Wet weight of the gastrocnemius and soleus
The wet weights of the gastrocnemius and soleus were measured using Sartorius Entris® 8201-1S balance (Sartorius, Germany) at 24 weeks to determine the severity of denervated muscle atrophy in both lower limbs. Results are expressed as a percentage (%) of the muscle weight of the normal side.
Statistical analysis
All results are expressed as mean ± standard deviation (SD), and the statistical analyses were performed using SPSS 18.0 (SPSS, Chicago, IL, USA). Changes in SFI and histological results were analyzed by repeated-measures analysis of variance (ANOVA). The SFI, vessel count, and wet muscle weight were analyzed using the Kruskal-Wallis test. The numbers of myelinated and unmyelinated axons, regenerated axon diameter, and myelin thickness were analyzed using one-way ANOVA. The statistical significance level was at P < 0.05.
Results
SFI
Sciatic function of all groups progressively improved over 24 weeks (Figure 2). The SFI in the vein autograft-PRP group recovered faster than that in the vein autograft group at 6 (P = 0.002), 8 (P = 0.011), and 12 weeks (P = 0.043). The SFI at 24 weeks was highest in the nerve autograft group, however, and there was no statistically significant difference among groups (P = 0.181).
Figure 2.
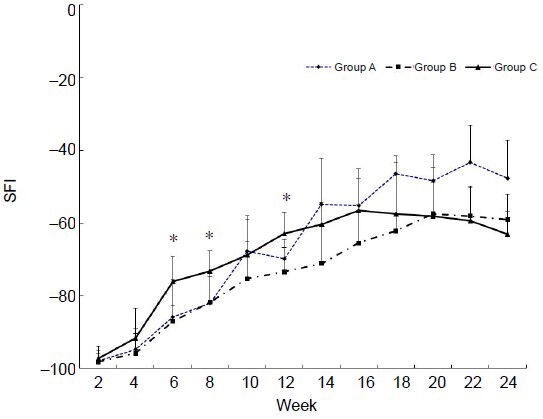
Recovery of sciatic functional index (SFI) through 24 weeks post-operation.
Group A: Nerve autograft; B: inside-out vein autograft; C: inside-out vein autograft-platelet-rich plasma (PRP). The values are expressed as mean ± SD. Asterisks indicate statistically significant differences between groups B and C (P < 0.05). Changes in SFI were analyzed by repeated-measures analysis of variance and the Kruskal-Wallis test.
Histological results
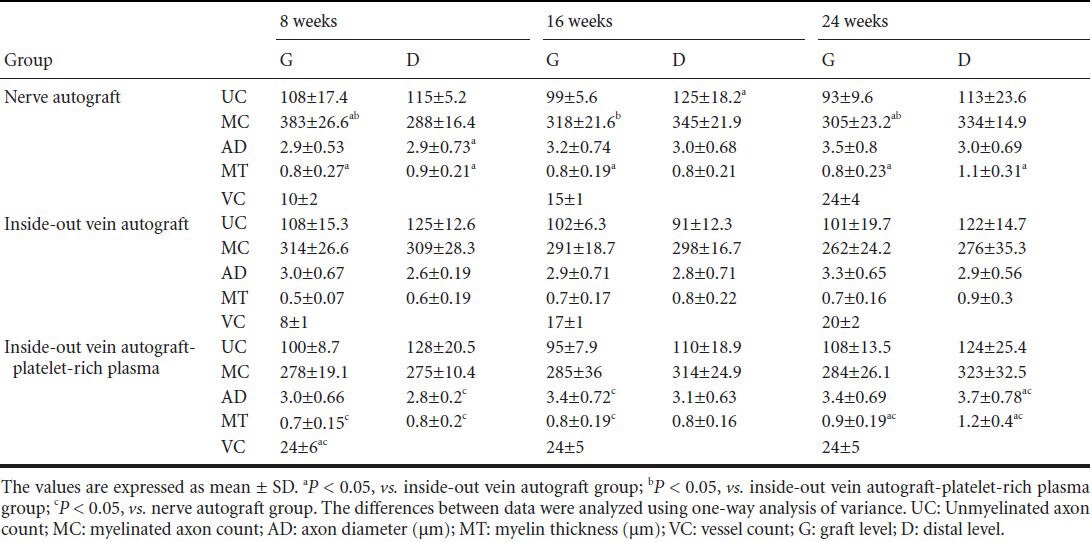
Histological results are shown in Figures 3, 4 and Table 1. Myelinated axons at the graft level in the nerve autograft group were more abundant than in the vein autograft group and vein autograft-PRP group at all time points (P < 0.05). However, the number of unmyelinated axons did not differ significantly among groups (P > 0.05). At the distal level, the number of myelinated and unmyelinated axons increased progressively over time in the nerve autograft group and vein autograft-PRP group but not in the vein autograft group (P > 0.05). Myelin in the nerve autograft group and vein autograft-PRP group was thicker than in the vein autograft group both at the graft level and at the distal level at all time points, but there was no significant difference between the nerve autograft group and vein autograft-PRP group. Regenerated axons became progressively thicker over time at both the graft level and distal level. The axon diameter at the graft level in the vein autograft-PRP group was greater than that in the vein autograft group at 16 weeks (P < 0.05). The axon diameter at the distal level in the nerve autograft group and vein autograft-PRP group was greater than in the vein autograft group, but there was no significant difference between nerve autograft group and vein autograft-PRP group. The neoangiogenesis was more active in the vein autograft-PRP group than that in the nerve autograft group and vein autograft group at 8 weeks (P < 0.05). The vessels in the vein autograft-PRP group were twice as many as those in the nerve autograft group and three times as many as those in the vein autograft group at 8 weeks. The number of regenerated vessels did not differ significantly among the groups at 16 and 24 weeks (P > 0.05).
Figure 3.
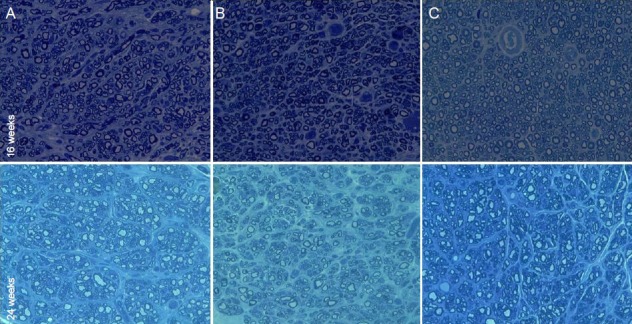
Histological change of the sciatic nerve (middle segment of graft level) regenerated with a nerve autograft (A), inside-out vein autograft (B), and inside-out vein autograft filled with platelet-rich plasma (PRP) (C) at 16 and 24 weeks post-operation (toluidine blue staining, × 400).
PRP-filled inside-out vein autograft has a larger axon diameter and better-organized fascicles than an inside-out vein autograft.
Figure 4.
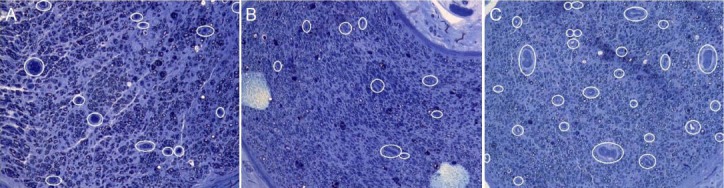
Neovessels at the midportion of the graft (graft level) at 8 weeks post-operation.
Neovessels are marked with white circles (toluidine blue staining, × 200). (A) A nerve autograft; (B) inside-out vein autograft, (C) and inside-out vein autograft filled with platelet-rich plasma.
Table 1.
Histological results of grafts
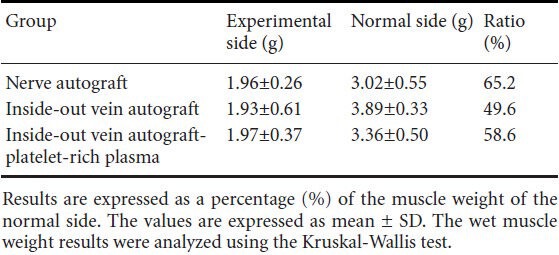
Wet weight of the gastrocnemius and soleus
Wet weight of the gastrocnemius and soleus was slightly, but not significantly, greater in the nerve autograft group and vein autograft-PRP group than in the vein autograft group at 24 weeks (Table 2).
Table 2.
The wet weight of gastrocnemius and soleus at 24 weeks post-operation
Discussion
This study showed that the inside-out vein autograft filled with PRP enhanced axonal regeneration compared with the inside-out vein autograft alone. We suggest that the earlier neoangiogenesis and faster regeneration of myelinated axons improved sciatic function.
PRP is now convenient to prepare for clinical use due to the number of commercially available kits. However, the optimum PRP concentration in experimental studies with rats has not been confirmed. To obtain an appropriate PRP concentration from the whole blood and establish our experimental protocol, we performed several pilot studies to achieve a PRP concentration of 4–5 times that of whole blood. By optimizing the speed and duration of centrifugation, we generated PRP with an average platelet count of 2.5 × 106 ± 5 × 105 /μL from 3 mL of whole blood, which was approximately 5–6 times the mean platelet count in whole rat blood (4 × 105 /μL) (Zheng et al., 2013).
Accumulative experimental and clinical data show that several growth factors in PRP have potential benefits in promoting nerve regeneration (Eccleston et al., 1993; Wang et al., 1995; Chen et al., 1999; Allamargot et al., 2001; Oya et al., 2002). Oya et al. (2002) showed a substantial increase in platelet-derived growth factor-β (PDGF-β) chain transcription in injured peripheral nerves. They also showed that augmented PDGF-β expression after nerve injury might contribute to peripheral nerve regeneration because PDGF-β is a Schwann cell mitogen and survival factor with trophic activity on neurons. Chen et al. (1999) also suggested that endogenous basic fibroblast growth factor not only facilitates angiogenesis in a transected facial nerve, but also acts as a neurotrophic agent during facial nerve regeneration. The healing cascade in nerve fibers is initiated and controlled by bioactive proteins in platelets. Increasing the concentration of these bioactive proteins may accelerate nerve fiber regeneration (Elgazzar et al., 2008). Although the neurotrophic growth factors in PRP have not yet been shown to act directly on nerve regeneration, much evidence indicates that PRP can affect nerve regeneration through various indirect routes.
Simply applying the PRP to injured or repaired nerve endings improves peripheral nerve remyelination (Farrag et al., 2007; Elgazzar et al., 2008; Sariguney et al., 2008; Ding et al., 2009; Cho et al., 2010). Farrag et al. (2007) transected the facial nerve in rats and performed direct neurorrhaphy. PRP infiltration at the neurorrhaphy site improved the functional outcome compared with the use of fibrin sealant or a non-bioactive agent. Sariguney et al. (2008) showed that applying PRP to a nerve repair site induced earlier remyelination of the sciatic nerve in rats.
Piskin et al. (2009), however, suggested that platelet gels do not improve axon regeneration after microsurgical reconstruction of a nerve gap using collagen tubes. They suggested that the synthetic collagen conduits might interfere with regenerative potential of platelet gels; thus, hindering its effect. We used inside-out autogenous vein grafts to bridge the nerve gap instead of a synthetic nerve conduit. After Chiu et al. (1982) reported the use of autogenous vein grafts as conduits for nerve regeneration in animals, a number of clinical trials demonstrated that short segments of autogenous vein can successfully serve as alternative conduits for nerve regeneration (Walton et al., 1989; Tang et al., 1993; Lee and Shieh, 2008). The vein graft provides a favorable environment for axonal regeneration, acts as a biological barrier to scar tissue invasion, and contributes to the local accumulation of neurite-promoting factors. However, contact between endothelial cells and regenerating axons causes grafts to constrict secondary to the development of fibrous connective tissue, which eventually impairs axonal regeneration (Heijke et al., 1993). Inside-out vein grafts emerge to promote axonal regeneration within vein grafts. Simple eversion of a harvested vein brings the laminin and collagen-rich adventitia layer in direct contact with nerve ends. The adventitia layer promotes axonal regeneration by generating abundant trophic and neurite-promoting factors (Wang et al., 1995; Ferrari et al., 1999; Gravvanis et al., 2004; Jeon et al., 2011). We also reported good clinical results with the use of inside-out vein grafts in patients who had short segment sensory nerve defects at various sites (Jeon et al., 2011).
Earlier reports show that PRP has been successfully used for the purpose of enhanced nerve regeneration in experimental trials (Farrag et al., 2007; Elgazzar et al., 2008; Sariguney et al., 2008; Ding et al., 2009; Piskin et al., 2009; Cho et al., 2010; Kaplan et al., 2011). Elgazzar et al. (2008) reported satisfactory results using a PRP to cover a cyanoacrylate reanastomosis. However, most of those studies just sprayed PRP over the nerve anastomoses (Farrag et al., 2007; Sariguney et al., 2008; Ding et al., 2009; Cho et al., 2010). Despite positive results from those studies, simple spraying of PRP may waste the efficient growth factors by allowing them to rapidly diffuse into surrounding fluid and tissues. To overcome these shortcomings, we injected PRP into the cavity of a grafted inside-out vein, which served as a PRP reservoir for sufficient time to allow growth factor release. Platelets begin to release the proteins and growth factors within 10 minutes after activation, and approximately 95% of the presynthesized growth factors are secreted within 1 hour (Kevy and Jacobson, 2004). After the initial burst of PRP-related growth factors, the platelets synthesize and secrete additional growth factors for the remaining 5 to 9 days of their life span (Kevy and Jacobson, 2004). We confirmed that PRP gel did not leak at all outside of venous conduit, and we think PRP remains and acts in the venous conduit for sufficient time with meaning amount.
In this study, neoangiogenesis was more prominent in the vein autograft-PRP group than in the nerve autograft group and vein autograft group in the early period of axonal regeneration. Faster recovery of function in SFI indicates earlier axonal regeneration in the vein autograft-PRP group is closely related with prominent neoangiogenesis. The SFI after 14 weeks did not show statistical difference in each group and relatively reached to plateau in the vein autograft group and vein autograft-PRP group after 20 weeks. However, more preserved gastrocnemius and soleus in the vein autograft-PRP group at 24 weeks indicates earlier regeneration of axons and decreased denervated muscle atrophy. The increased circulation during initial period of axonal regeneration might be positive effect in Schwann cell proliferation and axonal sprouting. A recent study also showed that PRP enhanced neurotrophic function and Schwann cell migration in peripheral nerve regeneration in rats (Zheng et al., 2013).
To the best of our knowledge, no studies have been reported that PRP promotes neoangiogenesis during peripheral nerve regeneration. We suggest that vascular endothelial growth factor and endothelial cell growth factor, which are major growth factors in PRP, promote neoangiogenesis in regenerating nerves and may contribute to early axonal sprouting and myelination (Sondell et al., 1999; Hobson et al., 2000; Pereira Lopes et al., 2011). We did not, however, find evidence of a direct relationship between the growth factors in PRP and neoangiogenesis in a regenerating nerve. Several studies have found meaningful evidence that PRP stimulates neovascularization during tendon and bone healing (de Mos et al., 2008; Yokota et al., 2008; Bir et al., 2009; Lyras et al., 2009; Lyras et al., 2010; Bosch et al., 2011). Additional immunohistochemical studies, including growth factor assays, would help clarify our hypothesis, the lack of which is a limitation in this study.
Recently, Konofaos and Ver Halen (2013) suggested that the most important developmental field from a future perspective will be tissue-engineered conduits enriched with either neuroprophic factors and/or support cells of nerve regeneration and the potential to release growth or trophic factors inside conduit lumen, to reduce nerve cell death, and to improve the outgrowth of axons after nerve injury. Our study seems to coincide with the future nerve repair by means of tissue-engineered conduits for substitution of nerve autografts.
There are some limitations of this study. Although this study includes long enough (up to 24 weeks) follow ups, serial follow up histological results are lacked in very early period of regeneration, i.e., within the first 8 weeks. The statistical power in the histologic evaluation is relatively low due to limited number of animals at each time point. Revealing the direct relationship between the growth factors in PRP and neoangiogenesis in a regenerating nerve is another limitation of this study and will be further studied.
In conclusion, this study showed that inside-out vein autografts filled with PRP promoted axon regeneration in rat sciatic nerve segmental defects. PRP is suggested to promote neoangiogenesis in the early period of axon regeneration based on evidence from morphometric analysis and sciatic functional assessment. We suggest that this strategy can be applied when a venous conduit, instead of a nerve autograft, is used to reconstruct a short segment nerve defect.
Footnotes
Funding: This study was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology, No. 2011-0010429.
Conflicts of interest: None declared.
Copyedited by Li CH, Song LP, Zhao M
References
- 1.Allamargot C, Pouplard-Barthelaix A, Fressinaud C. A single intracerebral microinjection of platelet-derived growth factor (PDGF) accelerates the rate of remyelination in vivo. Brain Res. 2001;918:28–39. doi: 10.1016/s0006-8993(01)02761-5. [DOI] [PubMed] [Google Scholar]
- 2.Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–138. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Bir SC, Esaki J, Marui A, Yamahara K, Tsubota H, Ikeda T, Sakata R. Angiogenic properties of sustained release platelet-rich plasma: characterization in-vitro and in the ischemic hind limb of the mouse. J Vasc Surg. 2009;50:870–879. doi: 10.1016/j.jvs.2009.06.016. e872. [DOI] [PubMed] [Google Scholar]
- 4.Bosch G, Moleman M, Barneveld A, van Weeren PR, van Schie HT. The effect of platelet-rich plasma on the neovascularization of surgically created equine superficial digital flexor tendon lesions. Scand J Med Sci Sport. 2011;21:554–561. doi: 10.1111/j.1600-0838.2009.01070.x. [DOI] [PubMed] [Google Scholar]
- 5.Chen YS, Murakami S, Gyo K, Wakisaka H, Matsuda S, Sakanaka M. Effects of basic fibroblast growth factor (bFGF)-neutralizing antibody and platelet factor 4 on facial nerve regeneration. Exp Neurol. 1999;155:274–283. doi: 10.1006/exnr.1998.6980. [DOI] [PubMed] [Google Scholar]
- 6.Chiu DT, Janecka I, Krizek TJ, Wolff M, Lovelace RE. Autogenous vein graft as a conduit for nerve regeneration. Surgery. 1982;91:226–233. [PubMed] [Google Scholar]
- 7.Cho HH, Jang S, Lee SC, Jeong HS, Park JS, Han JY, Lee KH, Cho YB. Effect of neural-induced mesenchymal stem cells and platelet-rich plasma on facial nerve regeneration in an acute nerve injury model. Laryngoscope. 2010;120:907–913. doi: 10.1002/lary.20860. [DOI] [PubMed] [Google Scholar]
- 8.de Mos M, van der Windt AE, Jahr H, van Schie HT, Weinans H, Verhaar JA, van Osch GJ. Can platelet-rich plasma enhance tendon repair? A cell culture study. Am J Sports Med. 2008;36:1171–1178. doi: 10.1177/0363546508314430. [DOI] [PubMed] [Google Scholar]
- 9.Ding XG, Li SW, Zheng XM, Hu LQ, Hu WL, Luo Y. The effect of platelet-rich plasma on cavernous nerve regeneration in a rat model. Asian J Androl. 2009;11:215–221. doi: 10.1038/aja.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eccleston PA, Funa K, Heldin CH. Expression of platelet-derived growth factor (PDGF) and PDGF alpha- and beta-receptors in the peripheral nervous system: an analysis of sciatic nerve and dorsal root ganglia. Dev Biol. 1993;155:459–470. doi: 10.1006/dbio.1993.1044. [DOI] [PubMed] [Google Scholar]
- 11.Elgazzar RF, Mutabagani MA, Abdelaal SE, Sadakah AA. Platelet rich plasma may enhance peripheral nerve regeneration after cyanoacrylate reanastomosis: a controlled blind study on rats. Int J Oral Max Surg. 2008;37:748–755. doi: 10.1016/j.ijom.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 12.Everts PA, Hoogbergen MM, Weber TA, Devilee RJ, van Monftort G, de Hingh IH. Is the use of autologous platelet-rich plasma gels in gynecologic, cardiac, and general, reconstructive surgery beneficial? Curr Pharm Biotechnol. 2012;13:1163–1172. doi: 10.2174/138920112800624346. [DOI] [PubMed] [Google Scholar]
- 13.Farrag TY, Lehar M, Verhaegen P, Carson KA, Byrne PJ. Effect of platelet rich plasma and fibrin sealant on facial nerve regeneration in a rat model. Laryngoscope. 2007;117:157–165. doi: 10.1097/01.mlg.0000249726.98801.77. [DOI] [PubMed] [Google Scholar]
- 14.Ferrari F, De Castro Rodrigues A, Malvezzi CK, Dal Pai Silva M, Padovani CR. Inside-out vs. standard vein graft to repair a sensory nerve in rats. Anat Rec. 1999;256:227–232. doi: 10.1002/(SICI)1097-0185(19991101)256:3<227::AID-AR2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 15.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 16.Gravvanis AI, Tsoutsos DA, Tagaris GA, Papalois AE, Patralexis CG, Iconomou TG, Panayotou PN, Ioannovich JD. Beneficial effect of nerve growth factor-7S on peripheral nerve regeneration through inside-out vein grafts: an experimental study. Microsurgery. 2004;24:408–415. doi: 10.1002/micr.20055. [DOI] [PubMed] [Google Scholar]
- 17.Heijke GC, Klopper PJ, Dutrieux RP. Vein graft conduits versus conventional suturing in peripheral nerve reconstructions. Microsurgery. 1993;14:584–588. doi: 10.1002/micr.1920140908. [DOI] [PubMed] [Google Scholar]
- 18.Hobson MI, Green CJ, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat 197 Pt. 2000;4:591–605. doi: 10.1046/j.1469-7580.2000.19740591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeon WJ, Kang JW, Park JH, Suh DH, Bae JH, Hong JY, Park JW. Clinical application of inside-out vein grafts for the treatment of sensory nerve segmental defect. Microsurgery. 2011;31:268–273. doi: 10.1002/micr.20850. discussion 274-265. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan S, Piskin A, Ayyildiz M, Aktas A, Koksal B, Ulkay MB, Turkmen AP, Bakan F, Geuna S. The effect of melatonin and platelet gel on sciatic nerve repair: an electrophysiological and stereological study. Microsurgery. 2011;31:306–313. doi: 10.1002/micr.20876. [DOI] [PubMed] [Google Scholar]
- 21.Kevy SV, Jacobson MS. Comparison of methods for point of care preparation of autologous platelet gel. J Extra Corpor Technol. 2004;36:28–35. [PubMed] [Google Scholar]
- 22.Konofaos P, Ver Halen JP. Nerve repair by means of tubulization: past, present, future. J Reconstr Microsurg. 2013;29:149–164. doi: 10.1055/s-0032-1333316. [DOI] [PubMed] [Google Scholar]
- 23.Lee YH, Shieh SJ. Secondary nerve reconstruction using vein conduit grafts for neglected digital nerve injuries. Microsurgery. 2008;28:436–440. doi: 10.1002/micr.20517. [DOI] [PubMed] [Google Scholar]
- 24.Lyras D, Kazakos K, Verettas D, Polychronidis A, Simopoulos C, Botaitis S, Agrogiannis G, Kokka A, Patsouris E. Immunohistochemical study of angiogenesis after local administration of platelet-rich plasma in a patellar tendon defect. Int Orthop. 2010;34:143–148. doi: 10.1007/s00264-009-0728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyras DN, Kazakos K, Verettas D, Polychronidis A, Tryfonidis M, Botaitis S, Agrogiannis G, Simopoulos C, Kokka A, Patsouris E. The influence of platelet-rich plasma on angiogenesis during the early phase of tendon healing. Foot Ankle Int. 2009;30:1101–1106. doi: 10.3113/FAI.2009.1101. [DOI] [PubMed] [Google Scholar]
- 26.Oya T, Zhao YL, Takagawa K, Kawaguchi M, Shirakawa K, Yamauchi T, Sasahara M. Platelet-derived growth factor-b expression induced after rat peripheral nerve injuries. Glia. 2002;38:303–312. doi: 10.1002/glia.10074. [DOI] [PubMed] [Google Scholar]
- 27.Pereira Lopes FR, Lisboa BC, Frattini F, Almeida FM, Tomaz MA, Matsumoto PK, Langone F, Lora S, Melo PA, Borojevic R, Han SW, Martinez AM. Enhancement of sciatic nerve regeneration after vascular endothelial growth factor (VEGF) gene therapy. Neuropathol Appl Neurobiol. 2011;37:600–612. doi: 10.1111/j.1365-2990.2011.01159.x. [DOI] [PubMed] [Google Scholar]
- 28.Piskin A, Kaplan S, Aktas A, Ayyildiz M, Raimondo S, Alic T, Bozkurt HH, Geuna S. Platelet gel does not improve peripheral nerve regeneration: an electrophysiological, stereological, and electron microscopic study. Microsurgery. 2009;29:144–153. doi: 10.1002/micr.20599. [DOI] [PubMed] [Google Scholar]
- 29.Sariguney Y, Yavuzer R, Elmas C, Yenicesu I, Bolay H, Atabay K. Effect of platelet-rich plasma on peripheral nerve regeneration. J Reconstr Microsurg. 2008;24:159–167. doi: 10.1055/s-2008-1076752. [DOI] [PubMed] [Google Scholar]
- 30.Smith MA, Smith WT. Emerging techniques in orthopaedics: platelet-rich plasma. Orthop Nurs. 2011;30:260–263. doi: 10.1097/NOR.0b013e3182247c42. [DOI] [PubMed] [Google Scholar]
- 31.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci. 1999;19:5731–5740. doi: 10.1523/JNEUROSCI.19-14-05731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang JB, Gu YQ, Song YS. Repair of digital nerve defect with autogenous vein graft during flexor tendon surgery in zone 2. J Hand Surg. 1993;18:449–453. doi: 10.1016/0266-7681(93)90144-5. [DOI] [PubMed] [Google Scholar]
- 33.Taylor DW, Petrera M, Hendry M, Theodoropoulos JS. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin J Sport Med. 2011;21:344–352. doi: 10.1097/JSM.0b013e31821d0f65. [DOI] [PubMed] [Google Scholar]
- 34.Walton RL, Brown RE, Matory WE, Jr, Borah GL, Dolph JL. Autogenous vein graft repair of digital nerve defects in the finger: a retrospective clinical study. Plast Reconstr Surg. 1989;84:944–949. [PubMed] [Google Scholar]
- 35.Wang KK, Costas PD, Bryan DJ, Eby PL, Seckel BR. Inside-out vein graft repair compared with nerve grafting for nerve regeneration in rats. Microsurgery. 1995;16:65–70. doi: 10.1002/micr.1920160205. [DOI] [PubMed] [Google Scholar]
- 36.Welch JA, Kraus KH, Wells MR, Blunt DG, Weremowitz J. Effect of combined administration of insulin-like growth factor and platelet-derived growth factor on the regeneration of transected and anastomosed sciatic nerve in rats. Am J Vet Res. 1997;58:1033–1037. [PubMed] [Google Scholar]
- 37.Yokota K, Ishida O, Sunagawa T, Suzuki O, Nakamae A, Ochi M. Platelet-rich plasma accelerated surgical angio-genesis in vascular-implanted necrotic bone: an experimental study in rabbits. Acta Orthop. 2008;79:106–110. doi: 10.1080/17453670710014842. [DOI] [PubMed] [Google Scholar]
- 38.Zheng C, Zhu Q, Liu X, Huang X, He C, Jiang L, Quan D, Zhou X, Zhu Z. Effect of platelet-rich plasma (PRP) concentration on proliferation, neurotrophic function and migration of Schwann cells in vitro. J Tissue Eng Regen Med. 2013 doi: 10.1002/term.1756. doi: 10.1002/term.1756. [DOI] [PubMed] [Google Scholar]


