Abstract
A chemically extracted acellular allogeneic nerve graft can reduce postoperative immune rejection, similar to an autologous nerve graft, and can guide neural regeneration. However, it remains poorly understood whether a chemically extracted acellular allogeneic nerve graft combined with neurotrophic factors provides a good local environment for neural regeneration. This study investigated the repair of injured rat sciatic nerve using a chemically extracted acellular allogeneic nerve graft combined with ciliary neurotrophic factor. An autologous nerve anastomosis group and a chemical acellular allogeneic nerve bridging group were prepared as controls. At 8 weeks after repair, sciatic functional index, evoked potential amplitude of the soleus muscle, triceps wet weight recovery rate, total number of myelinated nerve fibers and myelin sheath thickness were measured. For these indices, values in the three groups showed the autologous nerve anastomosis group > chemically extracted acellular nerve graft + ciliary neurotrophic factor group > chemical acellular allogeneic nerve bridging group. These results suggest that chemically extracted acellular nerve grafts combined with ciliary neurotrophic factor can repair sciatic nerve defects, and that this repair is inferior to autologous nerve anastomosis, but superior to chemically extracted acellular allogeneic nerve bridging alone.
Keywords: nerve regeneration, peripheral nerve injury, chemically extracted acellular allogeneic nerve, defect, repair, transplantation, ciliary neurotrophic factor, autologous nerve, neural regeneration
Introduction
Peripheral nerve injury is a frequently occurring condition. There are many restrictions on autologous nerve transplantation in the repair of peripheral nerve defects. Chemically extracted acellular allogeneic nerve, from which Schwann cells, myelin sheath and disintegrating fragments have been removed, reduced postoperative immune rejection. Simultaneously, chemically extracted acellular allogeneic nerve retains neural substrates and base materials, such as the bottom layer of Schwann cells, which can provide a good scaffold in the process of nerve regeneration. Chemically extracted acellular allogeneic nerve, similar to autologous nerve transplantation, can guide nerve regeneration and provide a favorable local environment for neural tube recanalization. Ciliary neurotrophic factor promotes neuronal survival and prevents nerve degeneration. Previous studies have demonstrated the potential of growth factors in peripheral nerve regeneration. A method was developed for sustained delivery of nerve growth factor for nerve repair with acellular nerve grafts to augment peripheral nerve regeneration. Nerve growth factor-containing polymeric microspheres were fixed with fibrin glue around chemically extracted acellular nerve allografts for prolonged, site-specific delivery of ciliary neurotrophic factor (Yu et al., 2009).
Peripheral nerves that are interrupted by trauma or surgical resection require re-approximation of their ends. When primary repair cannot be performed without undue tension, nerve grafting is required. Nerve repair with autograft is limited when there is an insufficient amount of autologous nerve available for large nerve defects. This encouraged the search for alternative means of reconstruction in extensive nerve injuries (Siemionow and Sonmez, 2007). Non-neuronal cells can exert protective effects in vitro and in vivo (Liu et al., 2014). When the trunk is lacerated, there may be a defect between the two nerve ends after the wound has been cleaned and necrotic tissue removed. Such a gap is usually bridged by an autologous nerve graft (Kvist et al., 2011). However, sometimes, there may be a shortage of graft material to bridge the defect, such as in lesions of the brachial plexus. The surgeon's reluctance to involve another extremity in the procedure, i.e. harvesting a sural nerve biopsy from the leg, necessitates alternatives to autologous nerve grafts (Hadlock et al., 2000). The treatment would in many cases be worse than the ill it was meant to cure. To overcome this problem, an acellular nerve graft is an appealing alternative (Lundborg, 1980; Liu et al., 2014), as they are thought not to induce rejection (Macmillan et al., 2009). Ciliary neurotrophic factor is a recently discovered neurotrophic factor, which inhibits apoptosis or the programmed death of nerve cells and can improve the survival rate of cultured nerve cells (Hadlock et al., 2000; He et al., 2002). Ciliary neurotrophic factor improved neurite outgrowth, neuronal migration, and growth-associated protein 43 expression inhibited by gp120. Ciliary neurotrophic factor also rescued neuronal apoptosis induced by gp120 (Liu et al., 2014). Acellular nerve grafts retain the nerve's internal structure with preserved extracellular matrix components, such as laminin (Lundborg, 1980; Caterina et al., 2006; Liu et al., 2014). Peripheral nerve fibers have been classified in relation to their conduction velocity, which, in general is proportional to size and function. However, it is not possible to make a precise estimation of function from mere size nor is it possible to designate function to individual fibers on the basis of structural features alone (Fitzgerald and Folan-Curran, 2002); however, selecting and matching a suitable combination of graft and host species may improve axonal outgrowth (Kvist et al, 2011). This study investigated the enhancement of rat sciatic nerve regeneration by ciliary neurotrophic factor, and provided evidence for clinical applications of ciliary neurotrophic factor.
Materials and Methods
Materials, animals and equipment
A total of 45 healthy, male, Sprague-Dawley rats aged 2 months, weighing 200–300 g were provided by Henan Provincial Experimental Animal Center, China (animal license No. Medical animal SCXK (Yu) 2010-0002). The protocols were approved by the Animal Ethics Committee of Southern Medical University, China.
Preparation of allogeneic nerve
After hair removal and disinfection of the lower edge of the piriformis, rats were anesthetized with 10% chloral hydrate under sterile conditions (Han et al., 2006). For graft preparation, 7 mm of bilateral sciatic nerve was collected (at the level of the lower edge of the piriformis) from 15 Sprague-Dawley rats under a dissecting microscope (ASOM-4, Photoelectric Technology Institute, Chinese Academy of Sciences, Chengdu, Sichuan Province, China). The remaining 30 rats were considered as experimental groups. Nerve samples were then rinsed in distilled water and then gently rocked in 3.0% Triton X-100 solution for 12 hours at room temperature. Sodium deoxycholate solution was then added and gentle rocking continued for 24 hours at room temperature. This operation was then repeated. After extraction of the sciatic nerve, the samples were rinsed in distilled water for 30 minutes (Sondell et al., 1999).
Preparation of diluted ciliary neurotrophic factor compound
Dichloromethane and polylactic acid/glycolic acid copolymer (Baosheng Biological Company, Dalian, Liaoning Province, China) formed an oily phase after concussion miscibility. 45 μg of ciliary neurotrophic factor and 100 mg of bovine serum albumin were added to the oily phase solution, and then 10 mL PBS containing 2% polyethylene glycol was added according to the method of Zhang et al. (2008). This method results in ciliary neurotrophic factor polymeric microspheres at a final concentration of 10 mg/mL.
Preparation of nerve defects in animal models and experimental groups
Thirty Sprague-Dawley rats were anesthetized with chloral hydrate for 2 hours to prepare models of 10 mm unilateral sciatic nerve defects under sterile conditions. Ciliary neurotrophic factor 0.1 mL was injected around the chemically extracted acellular nerve allograft with a microsyringe. The rats were divided into three groups randomly (n = 10): chemically extracted acellular nerve allograft + ciliary neurotrophic factor, autologous nerve anastomosis and chemically extracted acellular nerve allograft bridging.
Morphology
We daily observed the wound healing site and animals’ motor function for 8 weeks. Observations included mental state, wound healing, muscle atrophy, presence of ulcers or complications, and vestibular activity during walking.
Hematoxylin-eosin staining
Eight weeks after surgery sciatic nerve grafts 1 cm beyond anastomotic ends were collected from each experimental group. Samples were processed in 10% formalin and processed by conventional paraffin embedding and serial sectioning. Sections were observed under a light microscope (Olympus, Tokyo, Japan).
Determination of sciatic functional index
In the 2, 4, 6 and 8 weeks after surgery, in accordance with the method of Nakazato et al. (2007) the following parameters were measured: toe to toe distances of the 1st to 5th toe (toe spread, TS), from the front end of the 3rd toe to the trail end (print length, PL), and from the 2nd toe to 4th toe (inter-toes distance, IT). According to TS, PL and IT values, the rat sciatic index is calculated as, SSI = −38.3 (EPL–NPL/NPL) +109.5 (ETS–NTS/NTS) +13.3 (EIT–NIT/NIT) −8.8; SSI = 0 means normal neurological function and SSI = −100 corresponds to complete loss of nerve function (N: clear footprint of both normal feet; E: clear footprint of experimental feet).
Neurophysiological test and electromyography
Eight weeks after surgery, the rats were intraperitoneally anesthetized with 10% chloral hydrate. The sciatic nerve was exposed and gastrocnemius muscle surgery was performed using an SXP-1 operating microscope (Shanghai Medical Equipment Co., Shanghai, China). The electrodes were placed in the middle of the calf muscle to measure and record between the electrodes (NDI-200P EMG evoked potentiometer; Shanghai Haishen Medical Electronic Instrument Co., Shanghai, China).
Determination of wet weight recovery rate of rat triceps
Eight weeks after surgery, rats’ triceps muscles were taken; blood was absorbed, then their wet weight was recorded. Wet weight recovery rate was calculated by (triceps wet weight on the surgical side/triceps wet weight on the non-surgical side) × 100%.
Toluidine blue staining and electron microscopy
Eight weeks after the surgery, the distal anastomosis of the operated sciatic nerve was 3 mm, and nerve specimens (cut out) were also 3 mm long, and we used a specimen of the contralateral corresponding nerve as a control. After blood was washed away, samples were fixed with glutaraldehyde, embedded in epoxy resin 812, crosscut into 2-μm sections, and then processed for uranium-lead staining (Zhao et al., 2010). All samples were observed under an electron microscope (JEM-1200EX; Japanese Electronics Company, Tokyo, Japan). The Image-Pro Plus analysis system (Applied Biosystems ABI, Foster, CA, USA) was used to measure the thickness of the myelin sheath. Semi-thin slices were subjected to toluidine blue staining. The thickness of the myelin sheath and the total number of myelinated nerve fibers were measured.
Statistical analysis
SPSS 18.0 statistical software (SPSS, Chicago, IL, USA) was used to process and analyze the data. The data are expressed as mean ± SD. Data comparison between multiple groups was performed using one-way analysis of variance, pairwise comparisons using the least significant difference test. Inspection standards take α = 0.05.
Results
Overall condition of rats after transplantation
Eight weeks after surgery, wounds were well healed with no infection or significant differences in appearance; ulcers had completely subsided. In the chemically extracted acellular nerve allograft + ciliary neurotrophic factor group and the autologous nerve anastomosis group, toes could separate and there was no disability in walking. There was no significant difference in posture as compared with the left side of experimental animals. When attempting to walk, rats in the chemical acellular allogeneic nerve bridging group would stop and curl up.
Morphology of sciatic nerve of rats undergoing repair with chemically extracted acellular nerve allografts and ciliary neurotrophic factor
Eight weeks after surgery, uniformly myelinated nerve fibers were visible in the chemically extracted acellular nerve allograft + ciliary neurotrophic factor group and the autologous nerve anastomosis group. The density of myelinated nerve fibers in the chemically extracted acellular nerve allograft + ciliary neurotrophic factor group was higher than that of the chemically extracted acellular allogeneic nerve bridging group, which indicated that ciliary neurotrophic factor contributes to peripheral nerve defect repair (Figure 1).
Figure 1.
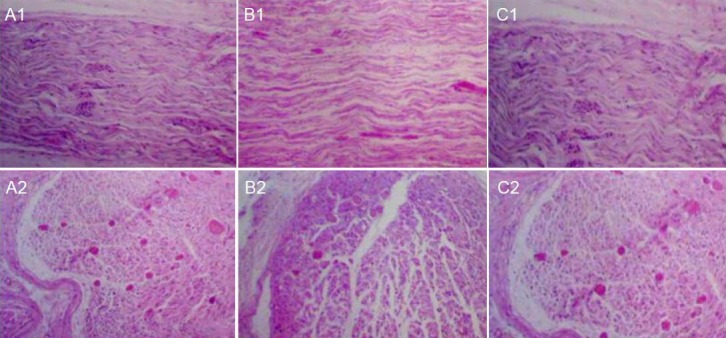
Morphology of injured sciatic nerve in rats after repair with CEANA and CNTF (hematoxylin-eosin staining, × 400).
(A1, A2) CEANA + CNTF group; (B1, B2) chemical acellular allogeneic nerve bridging group; (C1, C2) autologous nerve anastomosis group. My-elinated nerve fibers were uniform in the CEANA + CNTF group and the autologous nerve anastomosis group. The density of myelinated nerve fibers in the CEANA + CNTF group was lower than that in the autologous nerve anastomosis group, but higher than that in the chemical acellular allogeneic nerve bridging group. CEANA: Chemically extracted acellular nerve graft; CNTF: ciliary neurotrophic factor.
Effects of chemically extracted acellular nerve allografts combined with ciliary neurotrophic factor on sciatic nerve function in rats
In the 2, 4, 6 and 8 weeks after surgery, the sciatic functional index increased with time in the three groups: autologous nerve anastomosis group > chemically extracted acellular nerve allograft + ciliary neurotrophic factor group > chemical acellular allogeneic nerve bridging group (P < 0.05). These data indicated that ciliary neurotrophic factor contributes to peripheral nerve defect repair (Table 1).
Table 1.
Effects of CEANA combined with CNTF on sciatic functional index in rats

Effects of chemically extracted acellular nerve allografts combined with ciliary neurotrophic factor on soleus muscle function in rats
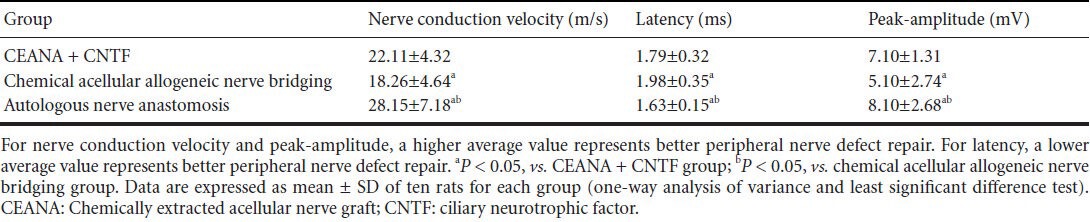
Eight weeks after surgery, nerve conduction velocity and peak-amplitude in the rat soleus muscle in the three groups showed autologous nerve anastomosis group > chemically extracted acellular nerve allograft + ciliary neurotrophic factor group > chemical acellular allogeneic nerve bridging group (P < 0.05). For latency: autologous nerve anastomosis group > chemically extracted acellular nerve allograft + ciliary neurotrophic factor group > chemical acellular allogeneic nerve bridging group (P < 0.05). These data indicate that the recovery was better in the chemically extracted acellular nerve allograft + ciliary neurotrophic factor group than in the chemically extracted acellular allogeneic nerve bridging group, indicating a promoting effect of ciliary neurotrophic factor on peripheral nerve defect repair (Table 2).
Table 2.
Effects of CEANA combined with CNTF on soleus muscle function in rats with sciatic nerve injury
Effects of chemically extracted acellular nerve allografts + ciliary neurotrophic factor on triceps wet weight recovery rate, total number of myelinated nerve fibers and myelin sheath thickness in rats with sciatic nerve injury
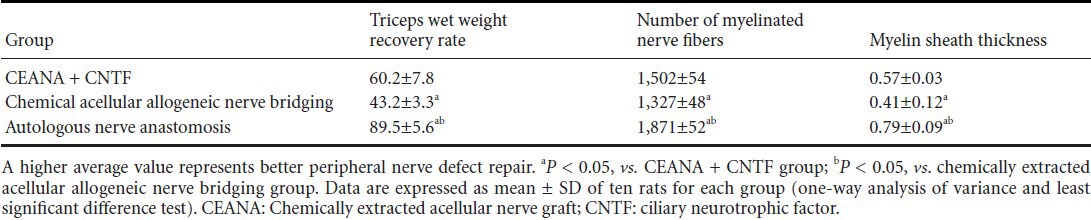
Eight weeks after surgery, values for triceps wet weight recovery rate, total number of myelinated nerve fibers and myelin sheath thickness in the three groups showed that autologous nerve anastomosis group > chemically extracted acellular nerve allograft + ciliary neurotrophic factor group > chemically extracted acellular allogeneic nerve bridging group (P < 0.05; Figure 2, Table 3).
Figure 2.
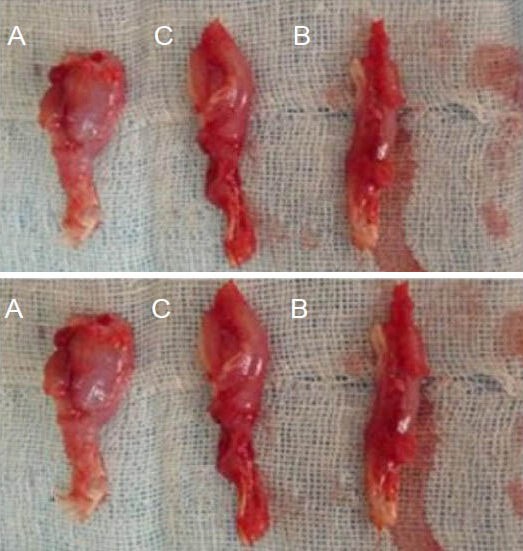
Morphology of triceps with sciatic nerve injury at 8 weeks after repair with CEANA and CNTF.
Upper panel: Operated triceps; lower panel: healthy triceps. (A) The chemical acellular allogeneic nerve anastomosis group; (B) CEANA + CNTF group; (C) autologous nerve anastomosis group. CEANA: Chem-ically extracted acellular nerve graft; CNTF: ciliary neurotrophic factor.
Table 3.
Effects of CEANA combined with CNTF on triceps wet weight recovery rate (%), total number of myelinated nerve fibers and myelin sheath thickness (μm) in rats with sciatic nerve injury
Discussion
In trauma centers, 5% of patients suffer from peripheral nerve injury. Peripheral nerve injury is frequently located in the upper extremities and is associated with a sub-optimal recovery of arm and hand function, loss of capacity to move fingers and other joints, and sometimes a loss of sensation in the entire limb (Svennigsen and Dahlin, 2013). The injuries often have severe consequences for the afflicted individuals, including loss of touch perception, impaired stereognosis, disturbed temperature perception, cold sensitivity, and although fortunately less frequently seen, pain, such as complex regional pain syndromes (Widerberg et al., 1997; Bruyns et al., 2003; Rosen et al., 2012). Peripheral nerve injury leads to both individual suffering and altered/degraded quality of life for the patient (Widerberg et al., 1997; Bruyns et al., 2003; Rosberg et al., 2005). During peripheral nerve regeneration, the microenvironment has a very important role and involves neurotrophins (Hobson, 2000; Hudson 2004). A large number of experimental studies have found that these soluble protein factors (Seckel, 1990) promote nerve regeneration by synthesizing certain enzymes and inhibiting neuronal apoptosis, thus resetting survival and morphology of neuronal cells. The drug microsphere technique is one method for the delivery of neurotrophic factors. This technology can maintain a sustained release of growth factors in the defective nerve area to improve the promoting effect of growth factors on peripheral nerve regeneration (Ide, 1993; Sun et al., 2013; Liu, 2014). Expression of selected neurotrophic factors is initiated to prepare the tissue for regeneration (Xu et al., 2013), and it has been reported that treatment with ciliary neurotrophic factor microspheres can produce a sustained effect for 10 weeks or more. Three months after its application for the repair of defective sciatic nerve, it was found that ciliary neurotrophic factor microspheres significantly increased the diameter and myelin thickness of regenerating nerve fibers (Mligiliche et al., 2002; Yetiser and Kahraman, 2008; Tormo et al., 2012). Microsphere technology with ciliary neurotrophic factor or other growth factors can promote peripheral nerve regeneration (Ard et al., 1987), and this could be a cheap and easy clinical treatment. The age of animals was not considered in this study; however, Cherqui et al. (2009) note that performance is better in pediatric patients; while Onne (1962) stated that there is good functional recovery up to the age of 50. However, there is some degree of disagreement concerning the optimal timing for suturing transected nerves (Mafi et al., 2012).
We considered the effect of the microenvironment because the outcome of peripheral nerve regeneration depends on the type of environment offered to the nerve fibers distal to the lesion. The distal nerve stump is an excellent growth substrate, superior to muscle or fat tissue (Weis and Schroder, 1989) and provides structural and trophic/tropic support during elongation of the axonal sprouts. The structural support has been ascribed to the endoneurial tubes (Young, 1942) in which laminin, the structural protein of the basal lamina, exerts distinct molecular interactions with the outgrowing axonal sprouts (Sanes and Tomaselli et al., 1986; Angelov et al., 1996; Fugleholm, 1998; Dohm et al., 2000).
Ducker (1969) recommended nerve repair within the first 10 days of injury, whereas Kleinert and Griffin (1973) and Holmes and Young (1942) believed that the nerve repair should be at the time of maximal axoplasmic synthesis. Cabaud et al. (1982) supported an early treatment to minimize scarring in the endoneurial space and to benefit from early axonal sprouting. Thus, we considered recording our observations from the first week postsurgery and our results were in agreement with the latter findings.
Seddon (1943) however, stated that fibrosis at 3 weeks offers a mechanical advantage for nerve suturing. Lenz-Scharf et al. (2004) argued that nerve regeneration was best within 6 weeks at the proximal end of the lesion as Wallerian degeneration was complete. Our studies also support these findings with good wound healing at 8 weeks. We observed no nerve regeneration difference between treatment groups after 10 days, in agreement with Sondell et al., (1999).
Ciliary neurotrophic factor is a cytoplasmic protein that has certain effects in the spinal cord and dorsal root sensory system and sympathetic neurons. Ciliary neurotrophic factor has noticeable effect on cell body development of both sensory and motor neurons and has considerable effect on the survival of sensory and motor neurons. Ciliary neurotrophic factor can promote the regeneration and the functional reconstruction of the peripheral nerve injury (Bechstein et al., 2012). In nerve injury, acid cholesterol ester hydrolase activity decreases significantly, while the activity of acid phosphatase increases significantly, and ciliary neurotrophic factor is able to reduce the magnitude of this exogenous increase or decrease by maintaining the biological activity of acid phosphatase and acid cholesterol ester hydrolase, thus reducing neuronal damage, and protecting the spinal cord neurons (He et al., 2002; Jason et al., 2013; Kang et al., 2013; Okano-Uchida et al., 2013). In ultra-thin sections examined by X-ray electron microscopy, we found that the anastomotic diameter and the number of nerve fibers were higher in the sciatic nerve end in the chemically extracted acellular nerve allograft + ciliary neurotrophic factor group compared with the chemically extracted acellular allogeneic nerve bridging group. The microscopic structure of myelin sheath in the chemically extracted acellular nerve allograft + ciliary neurotrophic factor group was clearer than that in the chemically extracted acellular allogeneic nerve bridging group, and these differences were statistically significant.
Although microsurgical techniques have been refined, functional recovery is usually unsatisfactory. It is conceivable that the approach to repair peripheral nerve injuries must be altered to improve functional recovery. An increased understanding of how neurons and supportive cells respond to trauma is needed as well as better knowledge regarding mechanisms regulating axonal outgrowth. One way to improve the results after nerve injury could be to use preconditioned nerves in nerve grafting situations. However, based on our present knowledge of graft repair, it seems that the surgical approaches must be supplemented with pharmacological treatment so as to optimize nerve regeneration (Annika, 2002).
Clinically useful injury grading systems have been developed that allow correlation of the microscopic changes occurring after nerve injury and patient symptomatology (Donoff, 1995). Perhaps the most widely accepted are those developed by Seddon (1943). Seddon divided nerve injuries by severity into three broad categories: neurapraxia, axonotmesis and neurotmesis. Neurapraxia, the mildest injury type, does not involve permanent loss of nerve and causes transient functional loss. This transience is thought to be due to a local ion-induced conduction block at the injury site, although subtle alterations in myelin structure have also been found. Axonotmesis occurs when there is complete interruption of the nerve axon and surrounding myelin, while the surrounding mesenchymal structures, including the perineurium and epineurium, are preserved. Axon and myelin degenerations occur distal to the point of injury, causing complete denervation. The prospect of recovery is excellent in such injuries because of the remaining uninjured mesenchymal latticework that provides a path for subsequent sprouting axons to reinnervate their target organ. Neurotmesis involves disconnection of a nerve. Functional loss is complete and recovery without surgical intervention does not usually occur because of scar formation and the loss of the mesenchymal guide that properly directs axonal regrowth (Burnett and Zager, 2004).
In this study, we applied a complete segmental nerve section (neurotmesis) and used drug microsphere technology in the repair of a rat sciatic nerve defect. Ciliary neurotrophic factor experiments showed that ciliary neurotrophic factor has a positive effect and improves triceps wet weight recovery rate; while nerve regeneration effects of diluted preparations in the chemically extracted acellular nerve allograft + ciliary neurotrophic factor group were superior to the chemically extracted acellular allogeneic nerve bridging group. Total myelinated nerve fibers observed under electron microscopy after toluidine blue staining had myelin sheath thickness that was higher in the chemically extracted acellular nerve allograft + ciliary neurotrophic factor group compared with that in the chemically extracted acellular allogeneic nerve bridging group. These results strongly suggest that exogenous ciliary neurotrophic factor improves the acellular nerve graft repair and the function of nerve conduction can be recovered, which is a good substitute of nerve grafts (Zhang et al., 2010). The present preliminary study has demonstrated that good nerve regeneration comparable with that of autografts is produced in the acellular allograft. We believe we will be able to further improve nerve regeneration with allografts using freeze-treated grafts with other neurotrophic factors without any immunosuppression (Ide et al., 1998) because immunosuppression was not performed in this study.
The regeneration process in peripheral nerves can be manipulated by different procedures using ciliary neurotrophic factor. However, we still need to understand Schwann cell proliferation and signal transduction pathways present in nerve cells to master the regeneration process because brain elasticity resets and interprets afferent signaling pathways of peripheral organs to avoid misdirection of nerve outgrowth after repair. However, from our results, we can conclude that ciliary neurotrophic factor favors nerve regeneration involving the Schwann cell surface or the extracellular matrix produced and assembled by Schwann cells. ciliary neurotrophic factor also promotes neurite outgrowth and guides outgrowth from several types of peripheral and central nervous system neurons. Despite our findings, we suggest that further research in this field is needed to identify all the neural components involved and to determine their specific functions.
Acknowledgments:
We are very grateful to the Institute of Clinical Anatomy, Southern Medical University in Guangzhou, Guangdong Province, China; and the Institute of International Studies and the Clinical Medicine of Zhengzhou University in Henan Province of China.
Footnotes
Conflicts of interest: None declared.
Copyedited by Allen J, Robens J, Wang J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Angelov DN, Neiss WF, Streppel M, Andermahr J, Mader K, Stennert E. Nimodipine accelerates axonal sprouting after surgical repair of rat facial nerve. J Neurosci. 1996;16:1041–1048. doi: 10.1523/JNEUROSCI.16-03-01041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ard MD, Bunge RP, Bunge MB. Comparison of the Schwann cell surface and Schwann cell extracellular matrix as promoters of neurite growth. J Neurocytol. 1987;16:539–555. doi: 10.1007/BF01668507. [DOI] [PubMed] [Google Scholar]
- 3.Askvig JM, Lo DY, Sudbeck AW, Behm KE, Leiphon LJ, Watt JA. Inhibition of the Jak-STAT pathway prevents CNTF-mediated survival of axotomized oxytocinergic magnocellular neurons in organotypic cultures of the rat supraoptic nucleus. Exp Neurol. 2013;240:75–87. doi: 10.1016/j.expneurol.2012.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechstein M, Häussler U, Neef M, Hofmann HD, Kirsch M, Haas CA. CNTF-mediated preactivation of astrocytes attenuates neuronal damage and epileptiform activity in experimental epilepsy. Exp Neurol. 2012;236:141–150. doi: 10.1016/j.expneurol.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Bruyns CN, Jaquet JB, Schreuders TA, Kalmijn S, Kuypers PD, Hovius SE. Predictors for return to work in patients with median and ulnar nerve injuries. J Hand Surg Am. 2003;28:28–34. doi: 10.1053/jhsu.2003.50026. [DOI] [PubMed] [Google Scholar]
- 6.Burnett MG, Zager EL. Pathophysiology of peripheral nerve injury: a brief review. Neurosurgical Focus. 2004;16:E1. doi: 10.3171/foc.2004.16.5.2. [DOI] [PubMed] [Google Scholar]
- 7.Cabaud HE, Rodkey WG, Nemeth TJ. Progressive ultrastructural changes after peripheral nerve transection and repair. J Hand Surg Am. 1982;7:353–365. doi: 10.1016/s0363-5023(82)80144-5. [DOI] [PubMed] [Google Scholar]
- 8.Cherqui A, Sulaiman WA, Kline DG. Resection and nerve grafting of a lipofibrohamartoma of the median nerve: case report. Neurosurgery. 2009;65:A229–235. doi: 10.1227/01.NEU.0000345353.21320.1F. [DOI] [PubMed] [Google Scholar]
- 9.Dohm S, Streppel M, Guntinas-Lichius O, Pesheva P, Probstmeier R, Walther M, Neiss WF, Stennert E, Angelov DN. Local application of extracellular matrix proteins fails to reduce the number of axonal branches after varying reconstructive surgery on rat facial nerve. Restor Neurol Neurosci. 2000;16:117–126. [PubMed] [Google Scholar]
- 10.Donoff RB. Nerve regeneration: basic and applied aspects. Rev Oral Biol Med. 1995;6:18–24. doi: 10.1177/10454411950060010201. [DOI] [PubMed] [Google Scholar]
- 11.Ducker TB, Kempe LG, Hayes GJ. The metabolic background for peripheral nerve surgery. J Neurosurg. 1969;30:270–280. doi: 10.3171/jns.1969.30.3part1.0270. [DOI] [PubMed] [Google Scholar]
- 12.Fugleholm K, Sørensen J, Schmalbruch H, Krarup C. Axonal elongation through acellular nerve segments of the cat tibial nerve: importance of the near-nerve environment. Brain Res. 1998;792:309–318. doi: 10.1016/s0006-8993(98)00160-7. [DOI] [PubMed] [Google Scholar]
- 13.Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000;6:119–127. doi: 10.1089/107632700320748. [DOI] [PubMed] [Google Scholar]
- 14.Han JB, Chen JW, Zhao BH, Zhang JC, Tian DH, Han JH. Preparation of acellular nerve grafts with triton X-100. Neural Regen Res. 2006;1:645–648. [Google Scholar]
- 15.He QY, Li CB, Wang ZB. Other experimental studies. Extracellular matrix and human amniotic fibroblasts in vitro. Zhonghua Zhengxing Waike Zazhi. 2002;18:229–231. [PubMed] [Google Scholar]
- 16.Hobson MI, Green CJ, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat. 2000;197:591–605. doi: 10.1046/j.1469-7580.2000.19740591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holmes W, Young JZ. Nerve regeneration after immediate and delayed suture. J Anat. 1942;77:63–96. [PMC free article] [PubMed] [Google Scholar]
- 18.Hudson TW, Zawko S, Deister C, Lundy S, Hu CY, Lee K, Schmidt CE. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004;10:1641–1651. doi: 10.1089/ten.2004.10.1641. [DOI] [PubMed] [Google Scholar]
- 19.Ide C. Nerve regeneration and Schwaan cell basal lamina: observation of the long-term regeneration. Arch Histol Jpn. 1993;46:243. doi: 10.1679/aohc.46.243. [DOI] [PubMed] [Google Scholar]
- 20.Ide C, Tohyama K, Tajima K, Endoh K, Sano K, Tamura M, Mizoguchi A, Kitada M, Morihara T, Shirasu M. Long acellular nerve transplants for allogeneic grafting and the effects of basic fibroblast growth factor on the growth of regenerating axons in dogs: a preliminary report. Exp Neurol. 1998;154:99–112. doi: 10.1006/exnr.1998.6921. [DOI] [PubMed] [Google Scholar]
- 21.Kang SS, Keasey MP, Arnold SA, Reid R, Geralds J, Hagg T. Endogenous CNTF mediates stroke-induced adult CNS neurogenesis in mice. Neurobiol Dis. 2013;49:68–78. doi: 10.1016/j.nbd.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleinert HE, Griffin JM. Technique of nerve anastomosis. Orthop Clin North Am. 1973;4:907–915. [PubMed] [Google Scholar]
- 23.Kvist M, Sondell M, Kanje M, Dahlin LB. Regeneration in, and properties of extracted peripheral nerve allo grafts and xenografts. J Plast Surg Hand Surg. 2011;45:122–128. doi: 10.3109/2000656X.2011.571847. [DOI] [PubMed] [Google Scholar]
- 24.Lenz-Scharf O, Fansa H, Galazky I, Schneider W. Secondary nerve reconstruction in the upper extremity in children--results with respect to number of motor units. Handchir Mikrochir Plast Chir. 2004;36:19–24. doi: 10.1055/s-2004-815810. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Liu G, Bi Y. CNTF regulates neurite outgrowth and neuronal migration through JAK2/STAT3 and PI3K/Akt signaling pathways of DRG explants with gp120-induced neurotoxicity in vitro. Neurosci Lett. 2014;569:110–115. doi: 10.1016/j.neulet.2014.03.071. [DOI] [PubMed] [Google Scholar]
- 26.Lundborg G. Nerve through performed tubes: a preliminary report of a new experimental model for studying the regeneration and reorganization of peripheral nerve tissue. J Handsurg. 1980;5:35–38. doi: 10.1016/s0363-5023(80)80041-4. [DOI] [PubMed] [Google Scholar]
- 27.Macmillan ML, Blazar BR, DeFor TE, Wagner JE. Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: results of a phase I–II clinical trial. Bone Marrow Transplant. 2009;43:447–454. doi: 10.1038/bmt.2008.348. [DOI] [PubMed] [Google Scholar]
- 28.Mafi P, Hindocha S, Dhital M, Saleh M. Advances of peripheral nerve repair techniques to improve hand function: a systematic review of literature. Open Orthop J. 2012;6:60–68. doi: 10.2174/1874325001206010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menovsky T, van der Bergh Weerman M, Kubista OL, Bartels RH, van Overbeeke JJ. Peripheral nerve graft repair of the oculomotor nerve in rats: a morphometric study. Microsurgery. 1999;19:392–400. doi: 10.1002/(sici)1098-2752(1999)19:8<392::aid-micr8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 30.Mligiliche N, Endo K, Okamoto K, Fujimoto E, Ide C. Extracellular matrix of human amnion manufactured into tubes as conduits for peripheral nerve regeneration. J Biomed Mater Res. 2002;63:591–600. doi: 10.1002/jbm.10349. [DOI] [PubMed] [Google Scholar]
- 31.Nakazato K, Song H, Waga T. Dietary apple polyphenols enhance gastrocnemius function in Wistar rats. Med Sci Sports Exerc. 2007;39:934–940. doi: 10.1249/mss.0b013e31803df4bc. [DOI] [PubMed] [Google Scholar]
- 32.Okano-Uchida T, Naruse M, Ikezawa T, Shibasaki K, Ishizaki Y. Cerebellar neural stem cells differentiate into two distinct types of astrocytes in response to CNTF and BMP2. Neurosci Lett. 2013;552:15–20. doi: 10.1016/j.neulet.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 33.Onne L. Recovery of sensibility and sudomotor activity in the hand after nerve suture. Acta Chir Scand Suppl. 1962;300:1–69. [PubMed] [Google Scholar]
- 34.Osawa T, Tohyama K, Ide C. Nerve grafts in rats and the role of Schwann cell basal laminae in nerve regeneration. J Neuroeytol. 1990;19:833–849. doi: 10.1007/BF01186814. [DOI] [PubMed] [Google Scholar]
- 35.Rosberg HE, Carlsson KS, Hojgard S, Lindgren B, Lundborg G, Dahlin LB. Injury to the human median and ulnar nerves in the forearm-analysis of costs for treatment and rehabilitation of 69 patients in southern sweden. J Hand Surg Br. 2005;30:35–39. doi: 10.1016/j.jhsb.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 36.Rosen B, Chemnitz A, Weibull A, Andersson G, Dahlin LB, Bjorkman A. Cerebral changes after injury to the median nerve: a long-term follow up. J Plast Surg Hand Surg. 2012;46:106–112. doi: 10.3109/2000656X.2011.653257. [DOI] [PubMed] [Google Scholar]
- 37.Seckel BR. Enhancement of peripheral nerve regeneration. Muscle Nerve. 1990;13:785–800. doi: 10.1002/mus.880130904. [DOI] [PubMed] [Google Scholar]
- 38.Seddon HJ. Three types of nerve injury. Brain. 1943;66:237. [Google Scholar]
- 39.Siemionow M, Sonmez E. Nerve allograft transplantation: a review. J Reconstr Microsurg. 2007;23:511–520. doi: 10.1055/s-2007-1022694. [DOI] [PubMed] [Google Scholar]
- 40.Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts acellular through chemical extraction. Brain Res. 1998;795:44–54. doi: 10.1016/s0006-8993(98)00251-0. [DOI] [PubMed] [Google Scholar]
- 41.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor stimulates Schwann cell invasion and neovascularization of acellular nerve grafts. Brain Res. 1999;846:219–228. doi: 10.1016/s0006-8993(99)02056-9. [DOI] [PubMed] [Google Scholar]
- 42.Sun H, Bénardais K, Stanslowsky N, Thau-Habermann N, Hensel N, Huang D, Claus P, Dengler R, Stangel M, Petri S. Therapeutic potential of mesenchymal stromal cells and MSC conditioned medium in Amyotrophic Lateral Sclerosis (ALS)--in vitro evidence from primary motor neuron cultures, NSC-34 cells, astrocytes and microglia. PLoS One. 2013;8:e72926. doi: 10.1371/journal.pone.0072926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Svennigsen AF, Dahlin LB. Review: Repair of the Peripheral Nerve-Remyelination that Works. Brain Sci. 2013;3:1182–1197. doi: 10.3390/brainsci3031182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomaselli KJ, Reichardt LF, Bixby JL. Distinct molecular interactions mediate neuronal process outgrowth on non-neuronal cell surfaces and extracellular matrices. J Cell Biol. 1986;103:2659–2672. doi: 10.1083/jcb.103.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tormo AJ, Letellier MC, Lissilaa R, Batraville LA, Sharma M, Ferlin W, Elson G, Crabé S, Gauchat JF. The cytokines cardiotrophin-like cytokine/cytokine-like factor-1 (CLC/CLF) and ciliary neurotrophic factor (CNTF) differ in their receptor specificities. Cytokine. 2012;60:653–660. doi: 10.1016/j.cyto.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 46.Wallquist W, Patarroyo M, Thams S, Carlstedt T, Stark B, Cullheim S, Hammarberg H. Human peripheral nerve: distribution and regulation during development and after axonal injury. J Comp Neurol. 2002;454:284–293. doi: 10.1002/cne.10434. [DOI] [PubMed] [Google Scholar]
- 47.Weis J, Schröder M. Differential effects of nerve, muscle, and fat tissue on regenerating nerve fibers in vivo. Muscle Nerve. 1989;12:723–734. doi: 10.1002/mus.880120905. [DOI] [PubMed] [Google Scholar]
- 48.Widerberg A, Bergman S, Danielsen N, Lundborg G, Dahlin LB. Nerve injury induced by vibration: Prevention of the effect of a conditioning lesion by D600, a Ca2+ channel blocker. Occup Environ Med. 1997;54:312–315. doi: 10.1136/oem.54.5.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu P, Rosen KM, Hedstrom K, Rey O, Guha S, Hart C, Corfas G. Nerve injury induces glial cell line-derived neurotrophic factor (GDNF) expression in schwann cells through purinergic signaling and the PKC-PKD pathway. Glia. 2013;61:1029–1040. doi: 10.1002/glia.22491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yetiser S, Kahraman E. An analysis of time-dependent changes of neurotrophic factors (BDNF, CNTF) in traumatic facial nerve injury of a nerve -cut and nerve-crush model in rats. Otol Neurotol. 2008;29:392–396. doi: 10.1097/mao.0b013e318161ab3e. [DOI] [PubMed] [Google Scholar]
- 51.Young JZ. The functional repair of nervous tissue. Physiol Rev. 1942;22:318–374. [Google Scholar]
- 52.Yu H, Peng J, Guo Q, Zhang L, Li Z, Zhao B, Sui X, Wang Y, Xu W, Lu S. Improvement of peripheral nerve regeneration in acellular nerve grafts with local release of nerve growth factor. Microsurgery. 2009;29:330. doi: 10.1002/micr.20635. [DOI] [PubMed] [Google Scholar]
- 53.Zhang DS, Zhong DJ, Song YM, Wu J. Preparation and in vitro properties of controlled release poly(lactic-co-glycolic acid) microspheres incorporating chondroitinase ABC. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2008;32:6247–6250. [Google Scholar]
- 54.Zhang L, Cao R, Zhu Y, Feng G, Zhang X, Huang F. Repair of the peripheral nerve defect with the combination of allogeneic nerve and autologous neuroma. Turk Neurosurg. 2010;4:470–479. doi: 10.5137/1019-5149.JTN.2942-10.3. [DOI] [PubMed] [Google Scholar]
- 55.Zhang YR, Na YJ, Wu ZM, Wang YS. Human placenta amniotic membrane wrap chemically extracted acellular nerve allograft repair of canine nerves. Zhengzhou Daxue Xuebao: Yixue Ban. 2012;4:508–512. [Google Scholar]
- 56.Zhao Z, Zhao B, Wang Y, Peng J, Zhang L, CHen Jifeng, Zhao Qing, Ren ZW, Liu Y, Xu WJ, LU SB. Functional evaluation of chemically extracted acellular nerve allograft supplement with different tissues of Schwann cells for peripheral nerve regeneration. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2010;11:1281–1287. [PubMed] [Google Scholar]


