Abstract
Electroacupuncture has traditionally been used to treat pain, but its effect on pain following brachial plexus injury is still unknown. In this study, rat models of an avulsion injury to the left brachial plexus root (associated with upper-limb chronic neuropathic pain) were given electroacupuncture stimulation at bilateral Quchi (LI11), Hegu (LI04), Zusanli (ST36) and Yanglingquan (GB34). After electroacupuncture therapy, chronic neuropathic pain in the rats’ upper limbs was significantly attenuated. Immunofluorescence staining showed that the expression of β-endorphins in the arcuate nucleus was significantly increased after therapy. Thus, experimental findings indicate that electroacupuncture can attenuate neuropathic pain after brachial plexus injury through upregulating β-endorphin expression.
Keywords: nerve regeneration, peripheral nerve injury, brachial plexus injury, neuropathic pain, electroacupuncture, β-endorphin, chronic neuropathic pain, brachial plexus avulsion, neural regeneration
Introduction
Brachial plexus injury is a serious peripheral nerve injury that severely disables upper limbs and affects patients’ daily life and work. Neurotmesis after brachial plexus injury also causes movement disorders of the enervated muscles and results in loss of sensory function in the skin (Parry, 1980; Wynn Parry, 1984; Carvalho et al., 1997; Berman et al., 1998; Anand and Birch, 2002; Sindou et al., 2005). In addition to these problems, the pain associated with this injury is often intolerable, and both patients and clinicians find it tricker than a non-functioning limb. Brachial plexus avulsion induces immediate or delayed pain that is described as crushing, squeezing, or burning, and is primarily treated through surgery. In preliminary studies, our group has treated 48 such patients with surgeries at different injury locations (Gu et al., 1997). After the first surgery, pain was effectively reduced in 70.8% of patients, which increased to 93.8% after a second surgery (Gu et al., 1997). While surgery is truly the most effective means to treat the pain after brachial plexus injury, the cost is exorbitant, and the long recovery time after surgery is extremely unpleasant.
The pain that occurs after brachial plexus injury is related to flaccidity syndrome, a condition described in traditional Chinese medicine. Li and colleagues have treated flaccidity syndrome using acupoints from the Yangming Meridian, and achieved good effect (Li, 1996; Zhong, 2004). Indeed, electroacupuncture has been used widely in the treatment of pain after brachial plexus injury because the cost is low and the treatment period is relatively short. In this study, a total brachial plexus-avulsion model was generated in rats through lesions of the cervicothoracic posterior route, and then used for studying the curative effects of electroacupuncture on chronic neuropathic pain of upper limbs.
Materials and Methods
Establishment of an animal model for chronic neuropathic pain
A total of 60 healthy and pathogen-free adult male Sprague- Dawley rats were used in the study (weight: 220 ± 1.38 g, Shanghai Slac Laboratory Animal Co., Ltd., Shanghai, China; license No. Shanghai ICP 05033115). The rats were housed in cages with free access to water and food in a temperature-controlled room (22 ± 1°C) and 12-hour/12-hour light-dark cycle. The study was approved by the Ethics Committee of Huashan Hospital, affiliated with Fudan University, Shanghai, China. The 60 rats were randomly and evenly divided into a control group and an electroacupuncture group.
The model for left brachial plexus avulsion was established in both the control group and the electroacupuncture group. After abdominal anesthesia with 1% pentobarbital sodium, the rats’ limbs and teeth were fixed in a prone position, followed by routine depilation, disinfection, and draping. A median incision was made at the back of the neck in a horizontal line from the occiput to the upper corner of shoulder (about 4 cm). Next, the T2 spine (1 cm higher than the T1 spine) was used for positioning. The operation was performed on the left side. The longissimus capitis, semispinalis cervicis, biventer cervicis, and abdominal muscle were pulled outside, and the small muscles adhering to spine and lamina were avulsed until the facet joint at C4–T1 was exposed. The left hemilamina at C4–T1 was removed using a special rongeur. A self-made microsurgical retractor was used to open the spinal cord to the right side and the C5–T1 dorsal roots were exposed. Under direct vision, the dorsal roots were avulsed from the spinal cord, exposing the ventral roots, which were also avulsed from the spinal cord. The operation was completed under a 10 × surgical microscope (Shanghai Medical Optics Instrument Factory, Shanghai, China) (Bertelli et al., 1995).
Electroacupuncture therapy
Acupuncture therapy started on day 2 after the operation. After ethyl-ether anesthesia, rats were immobilized on a table. Local skin was disinfected and filiform needles (diameter 0.30 mm, length 40 mm) were pierced 1–4 mm deep into the forepaw acupoints and 6–7 mm deep into the hindpaw acupoints. An electrode (Hwato SDZ-II electroacupuncture apparatus, Suzhou Hua Tuo Medical Instruments Co., Ltd., Suzhou, Jiangsu Province, China) was connected and a dilatational wave (8 mA, 2–100 Hz) was passed through the electrodes for 15–20 minutes. The optimal intensity was the intensity at which each rat's body started to quiver slightly. The electroacupuncture group received therapy at Quchi (LI11, in the hollow of the anterior-exterior of the elbow joint), Hegu (LI04, between the first and second metacarpal bones), Zusanli (ST36, 5 mm beneath the capitulum fibulae and located laterally and posterior to the knee-joint), and Yanglingquan (GB34, in the hollow of the exterior-inferior of the caput fibulae) alternating between the injured and uninjured sides on different days of the week. Each week included three sessions: day 1 (injured side), day 3 (uninjured side), and day 5 (injured side). Each rat received 2 weeks of treatment (day 1, day 3, day 5) × 2, totally 6 times in a course. The control group did not receive any electroacupuncture treatment.
Behavioral tests
Mechanically induced pain
Both groups were observed 1 day before the operation and on days 1, 4, 7, 14, 21, and 28 after the operation to determine their paw-withdrawal pain threshold. Rats were placed in a glass box on top of a metal screen for 30 minutes. A Von-Frey Filament (North Coast Medical, Inc., Morgan Hill, CA, USA) was used to vertically irritate the middle of the back of a hindfoot for 4 seconds. Foot lifting or licking was considered a positive reaction, and any other response was considered a negative reaction. The initial force was 2 g. If this force did not elicit a positive reaction, the force level was increased to 4, 6, 8, 10, and 15 g. This procedure was repeated at 30-second intervals until the force level (threshold) that resulted in 50% paw withdrawal was determined (Lombard et al., 1999; Willis, 1999; Maleki et al., 2000; Alstermark et al., 2004; Campbell and Meyer, 2006).
Autotomy scoring
On day 28, six rats from each group were checked for autotomy in the affected limbs and scored according to established quantitative criteria: autotomy involving two or more nails was scored as 1, and each half nail added another 1 for a maximum score of 11 (Barak et al., 2002; Vogel and Anderson, 2002; Devor, 2007).
Immunofluorescence staining of β-endorphin expression in rat arcuate nucleus
Six rat brains from each group were immunohistochemically stained 1 day before surgery and on days 7, 14, 21, and 28 post-surgery. Rats were first injected peritoneally with 1% pentobarbital sodium, and after anesthesia, chests were immediately opened and spiled via the left ventricle to an aorta cannula. Approximately 250 mL of normal saline was perfused to wash the blood away, followed by fixation with 4% paraformaldehyde (pH 7.4), first quickly and then slowly. The arcuate nucleus (Paxinos and Watson, 2005) was then collected and stored in 4% paraformaldehyde at 4°C overnight, and successively put into 10, 20, and 30% sucrose solutions for dehydration until the tissues settled down. Sections (40 μm thick) were crosswise frozen, and one of every five sections was selected and put into section-protection liquid. Then, for immunofluorescence staining, the brain sections were taken out from the protective liquid, bleached three times in 0.01 mol/L PBS (pH 7.4) for 10 minutes, and sealed in 10% sheep serum at 4°C overnight. Rabbit anti-β-endorphin polyclonal antibody (Abcam Biochemicals Company, Cambridge, UK) was then added at a concentration of 1:400, followed by incubation at 4°C for 48–72 hours. The liquid was then changed and goat anti-rabbit IgG-FITC (1:200; Abcam Biochemicals Company) was added, followed by incubation at 37°C for 2 hours. The sections were finally observed under a fluorescence microscope (Leica, Heidelberg, Germany) to detect β-endorphin expression in arcuate nucleus. For each section, five overlapping fields of view of the arcuate nucleus were selected at 200 × magnification. Cells showing β-endorphin expression were counted in each field of view, and the average from the five fields of view was calculated.
Statistical analysis
SPSS 10.0 software (SPSS, Chicago, IL, USA) was used for data processing. All data are expressed as mean ± SD. The difference between groups was compared using two-sample t-tests, and intragroup behavioral comparisons at different time points were performed with two-sample t-tests. P-values less than 0.05 were considered significant.
Results
General behavior observation
After the laminectomy, all rats were completely paralyzed in the left forepaw and unable to grip, grab, or stretch. The rats were still able to find food and move as before. No obvious instability in the neck appeared after hemilaminectomy at C5–T1.
Electroacupuncture therapy improved the behavior of rats with neuropathic pain after brachial plexus injury
Mechanical stimulation pain threshold
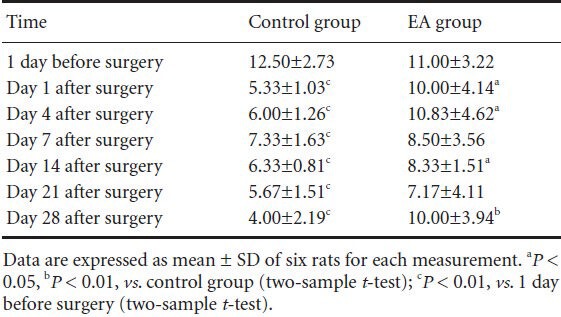
The pain threshold for mechanical stimulation of the left hindpaw was 12.50 ± 2.73 g and 11.00 ± 3.22 g (baseline) before operation in both groups, and the baseline thresholds did not differ between groups. At each time point after surgery, the threshold was significantly lower in the control group (range: 4–7.3 g, all P < 0.01). In contrast, pain thresh- olds did not go down in the electroacupuncture group, and were significantly higher on days 1, 4, 14 (all P < 0.05), and 28 (P < 0.01) after surgery (Table 1).
Table 1.
Effect of electroacupuncture (EA) therapy on the pain threshold (g) of left hindpaw mechanical stimulation in rats with neuropathic pain following brachial plexus injury
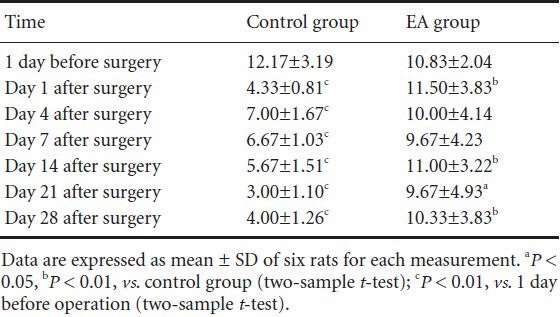
In the control group, the pain threshold for mechanical stimulation of the right hindpaw on days 1, 4, 7, 14, 21, and 28 after surgery was significantly lower than baseline (all P < 0.01). The pain threshold was higher in the electroacupuncture group than in the control group on days 1, 14, 28 (all P < 0.01), and 21 (P < 0.05) after surgery (Table 2).
Table 2.
Effect of electroacupuncture (EA) therapy on the pain threshold (g) of right hindpaw mechanical stimulation in rats with neuropathic pain following brachial plexus injury
Severe autotomy
On day 28 after surgery, rats in the control group exhibited autotomy in the affected paw. When not severe, autotomy involved several nails or half-nails, while in a severe case, all four nails were affected. The incidence rate of any autotomy on day 28 was 83.3% (5 out of 6 rats) (Figure 1). In contrast, only three rats (50%) expressed autotomy in the electroacupuncture group, and all three cases were mild. When the quantified amount of autotomy was compared between groups, analysis showed that the electroacupuncture group scored significantly lower than the control group (electroacupuncture group: 0.56 ± 0.68; control group: 1.81 ± 1.19; P < 0.05).
Figure 1.
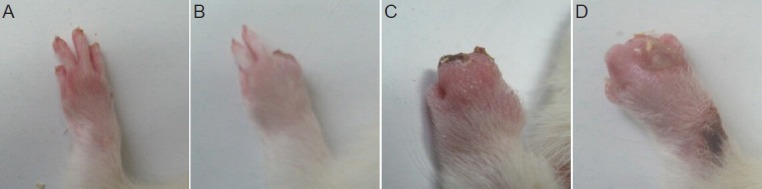
Varying degrees of autotomy in the injured paw on day 28 after total brachial plexus avulsion.
(A–D) Autotomy scores of 0, 3, 11, and 11.
Electroacupuncture therapy increased β-endorphin expression in the arcuate nucleus of rats with neuropathic pain following brachial plexus injury
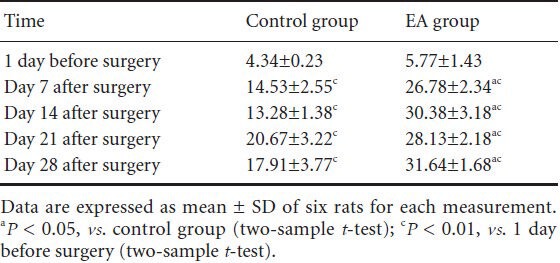
β-Endorphin is a peptide composed of 31 amino acid residues and is present in vertebrate neurons. When we experience pain, this endogenous opioid peptide is released and reduces the pain that we feel (Basbaum and Jessel, 2000; Fields et al., 2005). Immunofluorescence staining here revealed that β-endorphin was expressed at low levels in the arcuate nucleus of pre-surgery normal rats. After the avulsion surgery, the number of β-endorphin positive cells in the arcuate nucleus of rats increased significantly (all P < 0.01). Further, the number of β-endorphin positive cells was significantly higher in the electroacupuncture group than in the control group (all P < 0.05; Figure 2, Table 3).
Figure 2.
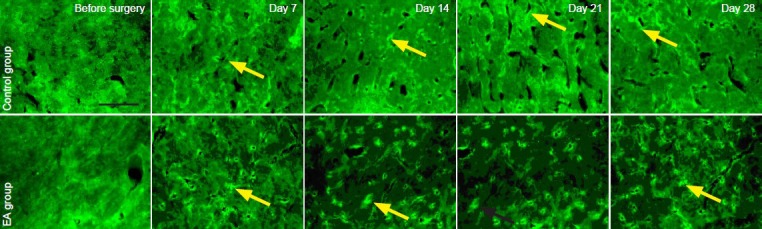
Effect of electroacupuncture (EA) therapy on β-endorphin expression in the arcuate nucleus of rats with neuropathic pain following brachial plexus injury.
Arrows refer to β-endorphin positive expression in the arcuate nucleus. Scale bar: 2 mm.
Table 3.
Effect of electroacupuncture (EA) therapy on the number of β-endorphin-positive cells (/200-fold field) in the arcuate nucleus of rats with neuropathic pain following brachial plexus injury
Discussion
This study aimed to observe the neuropathic pain, autotomy and β-endorphin expression in the rats with brachial plexus injury before and after electroacupuncture treatment. The findings indicate that, electroacupuncture can attenuate the neuropathic pain after brachial plexus injury.
Currently, neuropathic pain is evaluated primarily by observing and quantifying pain-related behavioral responses. For rats, this typically includes changes in pain thresholds for mechanically, heat-, or cold-induced pain in injured paws, as well as spontaneous pain and walking gait. However, these behaviors cannot be measured in rats with total brachial plexus avulsion because sensorimotor function in the affected regions is completely lost after avulsion. The loss of sensory function makes it impossible to observe the induced pains, and the loss of motor function makes it impossible to observe spontaneous pain or walking gait. Therefore, a new behavioral index is needed that can evaluate neuropathic pain in the injured paw (Campbell and Meyer, 2006). A rat model of anterior avulsion of the brachial plexus truncus caudalis has been established, and a series of behavioral observations show that pain thresholds in the hindpaw declined. However, pain thresholds also decreased in the control group (Rodrigues-Filho et al., 2003). We used a rat model with posterior total brachial plexus avulsion, which is closer to the type of damage found clinically than is the model with anterior brachial plexus truncus caudalis avulsion. The majority of pain caused by anterior avulsion to the brachial plexus truncus caudalis is from injury to the postganglionic brachial plexus nerve, which usually has nubs and does not approximate clinical brachial plexus avulsion, which is usually a preganglionic injury. This problem can be solved by posterior avulsion because it opens lamina and avulses ventral and dorsal roots under the naked eye. With our rat model with posterior total brachial plexus avulsion, we observed an obvious decline in mechanical pain thresholds that was blocked through electroacupuncture treatment. This proves that electroacupuncture treatment can increase pain thresholds after injury, and can thus be effective in treating chronic neuropathic pain after total brachial plexus avulsion. Among all the symptoms of neuropathological pain, spontaneous pain is the most unbearable, and thus it is very important to evaluate spontaneous pain in animal experiments. Unfortunately, few behavioral studies have done so, which greatly limits the ability to reveal the underlying mechanisms of spontaneous pain. Weight loss, abnormal gait, sleep disorders, decreased activity, and spontaneous paw lift are all considered as manifestations of spontaneous pain, but this has not been experimentally validated. For example, weight loss in infected animals may be caused by inflammatory mediators (rather than by pain) that act on the central system to control appetite. Autotomy is one manifestation of neuropathic pain that is similar to what is found in a clinical setting. Although social pressures lead autotomy in people to be much lower than in animals, it does occasionally occur (Barak et al., 2002). In this study, we found that electroacupuncture treatment significantly reduced autotomy in rats, further showing its effectiveness in reducing neuropathic pain after brachial plexus injury.
Opioid peptides can be divided into three types: β-endorphin, encephalin, and dynorphin, with μ, δ, and κ receptors being widely distributed in peripheral afferent terminals and in regions of the central nervous system related to tissue irritation and pain. They play very important roles in fighting central and peripheral and pain. The existence of endogenous opioid peptides encourages further exploration into their actions during electroanalgesia. Electroanalgesia was shown to work through endogenous opioids when naloxone, a specific opioid receptor blocker, was shown to partially reverse electroanalgesia (Mayer et al., 1977; Basbaum and Jessel, 2000; Fields et al., 2005). Extensive studies show that low- and high-frequency electroanalgesia are mediated by different central sites. Hypothalamic nuclei, particularly the arcuate nucleus, have been shown to respond well to low frequency electroacupuncture (2 Hz), but not to high frequency electroacupuncture. In electroanalgesia, the release of intracephalic opioid peptide increases, and low frequency (2 Hz) irritation may induce the release of enkephalin and endorphin, which act on μ and δ receptors and cause slow and persistent analgesia. High frequency (100 Hz) irritation may cause the release of dynorphin, which acts on κ-receptors and eases pains immediately. Additionally, endomorphin antibodies can block 2 Hz electroanalgesia, without affecting 100 Hz electroanalgesia, indicating that low frequency electroacupuncture promotes the release of endomorphin, while high frequency electroacupuncture may be not. All four opioid peptides can be released using 2/100 Hz alternating dilatational waves, achieving an optimal pain-easing effect (Han et al., 1991; Han, 2001; Gu et al., 2003). Many nuclei and areas in the brain are involved in the transfer of acupuncture signals, such as the caudate nucleus, septal area, arcuate nucleus, periaqueductal gray, and nucleus raphe magnus, all of which contain opioid peptides, and μ, δ, and κ receptors. In the endogenous opioid peptide system, the hypothalamic arcuate nucleus is an important structure, and a key site that mediates low frequency electroanalgesia, where the β-endorphin-rich neurons are concentrated. Their axons project to the nearby nucleus accumbens, septum, periaqueductal gray, and locus coeruleus, indicating that the arcuate nucleus may regulate electroanalgesia. In praxiology and electrophysiology, stimulating the arcuate nucleus will enhance electroanalgesic effects, increasing the activity of neurons in the dorsal raphe nucleus, and decreasing the activity of neurons in the locus coeruleus. These effects can be reversed through injection of naloxone. The excitability of neurons in the nucleus raphe magnus induced by stimulating the arcuate nucleus can be blocked by cutting off the β-endorphin gateway or by injection of trace naloxone into the periaqueductal gray. Studies indicate that an injured arcuate nucleus almost completely blocks the effect of electroanalgesia. These results indicate that the opioid-system network comprising the arcuate nucleus, periaqueductal gray, nucleus raphe magnus, and the dorsal horn plays an important role in electroanalgesia (Mayer et al., 1977; Han et al., 1991; Basbaum and Jessel, 2000; Fields et al., 2005). Our experiment shows that β-endorphin expression is very low in normal hypothalamic arcuate nuclei. At each time point postoperatively, the positive expression of β-endorphin was significantly higher in the control group than in normal rats, indicating that when the body is suffering pain, endogenous opioids are released. In the electroacupuncture group, this positive expression of β-endorphin was significantly higher than in the control group, indicating that electroacupuncture promotes the release of more β-endorphin and thus achieves a greater analgesic effect. This result is consistent with the behavioral observation that thresholds for mechanically induced pain were higher and instances of autotomy were less in the electroacupuncture group than in the control group, further indicating that electroacupuncture is an effective treatment for neuropathic pain following brachial plexus injury.
In conclusion, thresholds for mechanically induced pain remained unchanged and instances of autotomy were few in the electroacupuncture group. Further, positive expression of β-endorphin in the arcuate nucleus was higher in the electroacupuncture group than in controls. Taken together, these findings indicate that electroacupuncture can effectively relieve neuropathic pain following total brachial plexus avulsion. As electroacupuncture is safe and inexpensive, we recommend that it be used clinically when treating pain after total brachial plexus avulsion.
Footnotes
Funding: This work is supported by the Project of Ministry of Health (Comprehensive Research on Brachial Plexus Injury), No. 13D22270800 from the National Natural Science Foundation of China, and 2011 Shanghai Medical College Young Scientist Fund of Fudan University, No. 11L-24.
Conflicts of interest: None declared.
Copyedited by Phillips A, Norman C, Yu J, Yang Y, Li CH, Song LP, Zhao M
References
- 1.Alstermark B, Ogawa J, Isa T. Lack of monosynaptic corticomotoneuronal EPSPs in rats: disynaptic EPSPs mediated via reticulospinal neurons and polysynaptic EPSPs via segmental interneurons. J Neurophysiol. 2004;91:1832–1839. doi: 10.1152/jn.00820.2003. [DOI] [PubMed] [Google Scholar]
- 2.Anand P, Birch R. Restoration of sensory function and lack of long‐term chronic pain syndromes after brachial plexus injury in human neonates. Brain. 2002;125:113–122. doi: 10.1093/brain/awf017. [DOI] [PubMed] [Google Scholar]
- 3.Barak O, Weidenfeld J, Goshen I, Ben-Hur T, Taylor AN, Yirmiya R. Intracerebral HIV-1 glycoprotein 120 produces sickness behavior and pituitary-adrenal activation in rats: role of prostaglandins. Brain Behav Immun. 2002;16:720–735. doi: 10.1016/s0889-1591(02)00025-9. [DOI] [PubMed] [Google Scholar]
- 4.Basbaum AI, Jessel TM. The perception of pain. In: Kandel ER, Schwartz JH, Jessel TM, editors. Principles of Neural Science. New York: McGraw Hil; 2000. [Google Scholar]
- 5.Berman JS, Birch R, Anand P. Pain following human brachial plexus injury with spinal cord root avulsion and the effect of surgery. Pain. 1998;75:199–207. doi: 10.1016/s0304-3959(97)00220-0. [DOI] [PubMed] [Google Scholar]
- 6.Bertelli JA, Taleb M, Saadi A, Mira JC, Pecot‐-Dechavassine M. The rat brachial plexus and its terminal branches: an experimental model for the study of peripheral nerve regeneration. Microsurgery. 1995;16:77–85. doi: 10.1002/micr.1920160207. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JN, Meyer RA. Mechanisms of neuropathic pain. Neuron. 2006;52:77–92. doi: 10.1016/j.neuron.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho GA, Nikkhah G, Samii M. Pain management after post-traumatic brachial plexus lesions. Conservative and surgical therapy possibilities. Orthopade. 1997;26:621–625. doi: 10.1007/s001320050132. [DOI] [PubMed] [Google Scholar]
- 9.Devor M. Encyclopedia of pain. Berlin: Springer; 2007. Anesthesia dolorosa model, autotomy. [Google Scholar]
- 10.Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon SB, Koltzenburg M, editors. Wall and Melzack's Textbook of Pain. London: Churchill Livingstone; 2005. [Google Scholar]
- 11.Gu CY, Hu J, Cai YB. Advances of studies on stimulating parameters of electroacupuncture. Zhongguo Zhenjiu. 2003;23:489–491. [Google Scholar]
- 12.Gu YD, Chen DS, Cheng XM, Zhang LY, Cai PQ, Xu JG. The treatment of pain after brachial plexus injury. Zhonghua Xianwei Waike Zazhi. 1997;17:255–257. [Google Scholar]
- 13.Han JS. New evidence to substantiate the frequency specificity of acupuncture-induced analgesia. Zhenci Yanjiu. 2001;26:224. [Google Scholar]
- 14.Han JS, Chen XH, Sun SL, Xu XJ, Yuan Y, Yan SC, Hao JX, Terenius L. Effect of low-and high-frequency TENS on Met-enkephalin-Arg-Phe and dynorphin A immunoreactivity in human lumbar CSF. Pain. 1991;47:295–298. doi: 10.1016/0304-3959(91)90218-M. [DOI] [PubMed] [Google Scholar]
- 15.Li M. Clinical application of choosing Yangming meridian in treating flaccidity. Liaoning Zhongyi Zazhi. 1996;23:551. [Google Scholar]
- 16.Lombard MC, Weil-Fugazza J, Ries C, Allard M. Unilateral joint inflammation induces bilateral and time-dependent changes in neuropeptide FF binding in the superficial dorsal horn of the rat spinal cord: implication of supraspinal descending systems. Brain Res. 1999;816:598–608. doi: 10.1016/s0006-8993(98)01242-6. [DOI] [PubMed] [Google Scholar]
- 17.Maleki J, LeBel AA, Bennett GJ, Schwartzman RJ. Patterns of spread in complex regional pain syndrome, type I (reflex sympathetic dystrophy) Pain. 2000;88:259–266. doi: 10.1016/S0304-3959(00)00332-8. [DOI] [PubMed] [Google Scholar]
- 18.Mayer DJ, Price DD, Rafii A. Antagonism of acupuncture analgesia in man by the narcotic antagonist naloxone. Brain Res. 1977;121:368–372. doi: 10.1016/0006-8993(77)90161-5. [DOI] [PubMed] [Google Scholar]
- 19.Parry C. Pain in avulsion lesions of the brachial plexus. Pain. 1980;9:41–53. doi: 10.1016/0304-3959(80)90027-5. [DOI] [PubMed] [Google Scholar]
- 20.Paxinos G, Watson C. 5th ed. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- 21.Rodrigues-Filho R, Santos ARS, Bertelli JA, Calixto JB. Avulsion injury of the rat brachial plexus triggers hyperalgesia and allodynia in the hindpaws: a new model for the study of neuropathic pain. Brain Res. 2003;982:186–194. doi: 10.1016/s0006-8993(03)03007-5. [DOI] [PubMed] [Google Scholar]
- 22.Sindou MP, Blondet E, Emery E, Mertens P. Microsurgical lesioning in the dorsal root entry zone for pain due to brachial plexus avulsion: a prospective series of 55 patients. J Neurosurg. 2005;102:1018–1028. doi: 10.3171/jns.2005.102.6.1018. [DOI] [PubMed] [Google Scholar]
- 23.Vogel LC, Anderson CJ. Self-injurious behavior in children and adolescents with spinal cord injuries. Spinal Cord. 2002;40:666–668. doi: 10.1038/sj.sc.3101377. [DOI] [PubMed] [Google Scholar]
- 24.Willis WD. Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- 25.Wynn Parry CB. Brachial plexus injuries. (134-139).Br J Hosp Med. 1984;32:130–132. [PubMed] [Google Scholar]
- 26.Zhong H. Experience of treating flaccidity by using acupuncture and tuina. Zhenjiu Linchuang Zazhi. 2004;20:40–41. [Google Scholar]


