Abstract
Early diagnosis of diabetic peripheral neuropathy is important for the successful treatment of diabetes mellitus. In the present study, we recruited 500 diabetic patients from the Fourth Affiliated Hospital of Kunming Medical University in China from June 2008 to September 2013: 221 cases showed symptoms of peripheral neuropathy (symptomatic group) and 279 cases had no symptoms of peripheral impairment (asymptomatic group). One hundred healthy control subjects were also recruited. Nerve conduction studies revealed that distal motor latency was longer, sensory nerve conduction velocity was slower, and sensory nerve action potential and amplitude of compound muscle action potential were significantly lower in the median, ulnar, posterior tibial and common peroneal nerve in the diabetic groups compared with control subjects. Moreover, the alterations were more obvious in patients with symptoms of peripheral neuropathy. Of the 500 diabetic patients, neural conduction abnormalities were detected in 358 cases (71.6%), among which impairment of the common peroneal nerve was most prominent. Sensory nerve abnormality was more obvious than motor nerve abnormality in the diabetic groups. The amplitude of sensory nerve action potential was the most sensitive measure of peripheral neuropathy. Our results reveal that varying degrees of nerve conduction changes are present in the early, asymptomatic stage of diabetic peripheral neuropathy.
Keywords: nerve regeneration, peripheral nerve injury, diabetic peripheral neuropathy, neural conduction, electrophysiology, sensory nerve, motor nerve, early diagnosis, neural regeneration
Introduction
Diabetic peripheral neuropathy is the most common chronic complication of diabetes mellitus, with an incidence rate of about 50 % (Shi et al., 2013; Galuppo et al., 2014; O’Brien and Karem, 2014; Won et al., 2014; Zhong et al., 2014). Once symptoms appear, there are few effective therapeutic strategies (Won et al., 2014). Therefore, early discovery and diagnosis are extremely important. Nerve conduction studies are the most common method for diagnosis of peripheral neuropathy (An et al., 2007; Kincaid et al., 2007; Koçer et al., 2007; Severinsen and Andersen, 2007; Kiziltan and Benbir, 2008; Løseth et al., 2008, 2010; Uluc et al., 2008; Asad et al., 2009; Hemmi et al., 2009; Watanabe et al., 2009; Charles et al., 2010; Dyck et al., 2010; Lee et al., 2010; Suh et al., 2010; Watanabe et al., 2010; Altun et al., 2011; Dyck et al., 2011; Koytak et al., 2011; Shin et al., 2011; Heise et al., 2012; Mondal et al., 2012; Morimoto et al., 2012; Spadella et al., 2012; Arimura et al., 2013; Joa and Kim, 2013; Koo et al., 2013; Richardson et al., 2013; Chiles et al., 2014; McLellan et al., 2014). Heise et al. (2012) found that the combined index, comprising five parameters of nerve conduction, had greater sensitivity and equivalent specificity compared with individual parameters in the detection of diabetic polyneuropathy. In another study, Erdogan et al. (2011) found that the strength-duration time constant may be useful in the early stages of neuropathy, since most patients with diabetic neuropathy had predominant changes in this parameter in their lower extremities. In our previous electrophysiology studies, we used several indices that are routinely measured in diabetic and suspected diabetic patients, with the aim of facilitating early diagnosis of diabetic peripheral neuropathy, reducing treatment costs and improving therapeutic success. In the present study, we sought to establish a sensitive index for nerve conduction studies in the early diagnosis of peripheral neuropathy in 500 patients with diabetes mellitus.
Subjects and Methods
Subjects
500 diabetic patients who visited the doctor's office in the Fourth Affiliated Hospital of Kunming Medical University in China from June 2008 to November 2013 were recruited by advertisement. All patients’ conditions were consistent with the diagnostic criteria for diabetes, approved by the World Health Organization (Wendland et al., 2012). One hundred healthy control subjects who went to the doctor's office for other medical reasons were also recruited. Exclusion criteria: (1) peripheral neuropathy due to severe liver and kidney diseases, nutrition deficiency, connective tissue diseases and other metabolic or hereditary diseases; (2) radiculopathy due to cervical spondylosis and lumbar intervertebral disc protrusion; (3) history of long-term alcohol consumption or prolonged contact with poisonous substances (such as heavy metals) that could result in peripheral impairment; (4) history of drugs (such as isoniazid and furaxone) that may affect neural function. According to the symptoms of peripheral nerve impairment (such as numbness, pain, weakness, or burning cooling sensations in the limbs), the diabetic patients were divided into two groups, symptomatic and asymptomatic. One hundred healthy control subjects were also recruited by advertisement. There were no significant differences in disease course between the two groups of patients with diabetes, and no significant differences in gender, age, body height or weight among all three experimental groups (P > 0.05; Table 1).
Table 1.
Demographic information of diabetic patients and control subjects
The procedures were in accordance with institutional and regional ethical standards of responsible experimentation and with the Helsinki Declaration of 1975. The Chinese Ethics Committee approved the age range of adult research participants and obtained consent for children aged over 7 years participating in the trial. Subject approval was obtained for the electrophysiological test procedures, which were in accordance with the guidelines of the Chinese Ethics Committee.
Analysis of neural conduction
Each subject lay on a bed in a quiet room (22–25°C) with limbs relaxed. Skin temperature was maintained above 32°C. Electromyography/evoked potential apparatus (Dantec Dynamics, Skovlunde, Denmark) was employed in nerve conduction studies. Motor and sensory nerve conduction of unilateral or bilateral median nerve, ulnar nerve, posterior tibial nerve and common peroneal nerve were measured separately. In the motor nerve conduction test, the sellar stimulus electrode was placed in front of the stylomastoid foramen, with the negative pole of the electrode placed at the distal end of the nerve. The surface electrode was placed at the proximal end of the nerve, on the muscle belly of the frontalis, nasalis or orbicularis oris muscles. The groundwire was placed between the stimulus and recording electrodes. For sensory nerve conduction tests, a ring electrode was placed around the end of the fingers or toes, that is, the distal end of the sensory nerve, and used to stimulate the median, ulnar and posterior tibial nerves. A surface electrode was employed to stimulate the common peroneal nerve. The recording electrode was placed at the proximal end of the spinal cord using the antegrade method, described previously (Cui, 2006). Detection parameters were: amplitude of compound motor action potential, distal motor latency, amplitude of sensory nerve action potential, and sensory nerve conduction velocity. Control values corresponded to the standards of electromyography for age and gender in Peking Union Medical College Hospital in China (Zhang et al., 2013).
Statistical analysis
Data were analyzed using SPSS 11.5 software (SPSS, Chicago, IL, USA) and presented as mean ± SD. Paired t-tests were used to compare measurement data. Numeration data are expressed as numbers of nerve fibers (%). A chi-square test was used to compare rates between two samples. P < 0.05 was considered statistically significant (We used analysis of variance to compare the data of two experimental groups and control, and got similar outcome to those of paired t-tests, so we adopted the results of t-test as the final statistical outcome).
Results
Motor and sensory nerve conduction in patients with diabetic peripheral neuropathy
Distal latency of motor nerve conduction in the median, ulnar, posterior tibial and common peroneal nerve was longer in the asymptomatic diabetic group than in healthy controls, and the amplitude of compound motor action potential was significantly lower compared with that of the control group (P < 0.05; Figure 1A). Distal motor latency in the median and common peroneal nerves in the symptomatic group was markedly longer than in the asymptomatic group, and compound motor action potential amplitude in the median and common peroneal nerve was significantly lower in the symptomatic group than in asymptomatic patients (P < 0.05; Figure 1B).
Figure 1.
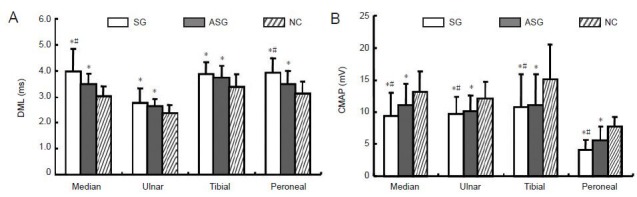
Motor nerve conduction in diabetic patients with/without peripheral neuropathy and in control subjects.
(A) Distal motor latency (DML); (B) compound muscle action potential (CMAP). Data are presented as mean ± SD. *P < 0.05, vs. NC; #P < 0.05, vs. ASG (paired t-test). SG: Symptomatic group (n = 221); ASG: asymptomatic group (n = 279); NC: normal control group (n = 100).
Similarly to the sensory nerve conduction comparison in the median, ulnar, posterior tibial and common peroneal nerve, sensory nerve action potential amplitude and sensory nerve conduction velocity in both diabetic groups was significantly lower than in healthy controls (P < 0.05). Importantly, sensory nerve action potential amplitude and sensory nerve conduction velocity of the median, ulnar, posterior tibial and common peroneal nerves in the symptomatic group was significantly lower than in the asymptomatic group (P < 0.05; Figure 2).
Figure 2.
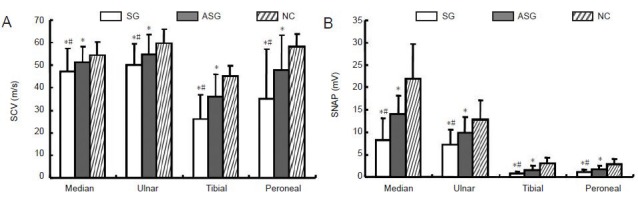
Sensory nerve conduction in diabetic patients with/without peripheral neuropathy and in control subjects.
(A) Sensory nerve conduction velocity (SCV); (B) sensory nerve action potential (SNAP). Data are presented as mean ± SD. *P < 0.05, vs. NC; #P < 0.05, vs. ASG (paired t -test). SG: Symptomatic group (n = 221); ASG: asymptomatic group (n = 279); NC: normal control group (n = 100).
Outcome analysis of nerve conduction studies
Of 500 diabetic patients, 358 (71.6%) exhibited nerve conduction abnormalities. The highest rate of abnormality was found in the common peroneal nerve, followed by the posterior tibial nerve and median nerve, with the ulnar nerve showing the lowest rate of abnormality (P < 0.05). In the motor nerve conduction tests, the common peroneal nerve showed the highest rate of absent waveforms, whereas in sensory nerve conduction tests, the posterior tibial nerve was found to have the highest rate of absent waveforms. The most sensitive index for nerve conduction studies in diabetic patients was sensory nerve action potential, followed by sensory nerve conduction velocity; compound motor action potential and distal motor latency had relatively low sensitivity. Among the nerve conduction indices, the highest rate of abnormality was found in median nerve sensory nerve action potential (Table 2).
Table 2.
Abnormal distribution (%) of motor and sensory nerve conduction in diabetic patients with/without peripheral neuropathy and in control subjects
Discussion
Diabetic peripheral neuropathy commonly develops insidiously, with various clinical manifestations. In the early stages, diagnosis is difficult as there are no symptoms. Fortunately, increasing use of electrophysiological techniques that allow the identification of sub-clinical pathological changes has made early diagnosis of diabetic peripheral neuropathy possible (Liu et al., 2005; Al-Geffari, 2012; Balbinot et al., 2012; Barriga et al., 2012; Gulichsen et al., 2012; Kuntzer et al., 2012; Calvet et al., 2013; Fang et al., 2013; Mete et al., 2013; Papanas et al., 2013; Sellers et al., 2013; Sun et al., 2013; Gordon Smith et al., 2014). Electrophysiology has the advantages of being objective and sensitive, allowing the scope and extent of affected peripheral nerves to be precisely ascertained and providing a conclusive diagnosis of diabetic peripheral neuropathy. Indeed, nerve conduction studies have become an indispensable tool in the discovery of peripheral nerve abnormalities. In patients with diabetic peripheral neuropathy, the main abnormality in nerve conduction studies manifests as reduced amplitude, slowed conductive velocity or prolonged latent phase, and in severe cases the waveform is eliminated entirely.
In the present study, we found that the total abnormal nerve conduction rate in both diabetic groups was 71.6%, which was in accordance with a previous report (Banthia et al., 2013). Distal motor latencies in the median, ulnar, posterior tibial and common peroneal nerves in the asymptomatic group were significantly prolonged compared with those in the control group. Sensory nerve conduction velocity and amplitudes of sensory nerve action potential and compound motor action potential were all markedly lower in the asymptomatic group than in controls, suggesting that impairment of sensory and motor axons and the myelin sheath at the distal end already exists in the early stages of diabetic peripheral neuropathy, before symptoms emerge. In the symptomatic group, sensory nerve conduction velocity was notably slower, distal motor latency in the median and common peroneal nerves was significantly prolonged and the amplitude of compound motor action potential was lower than in the asymptomatic group. These observations indicate that after symptomatic peripheral nerve impairment emerges in diabetic patients, lesions in the sensory fibers of the median, ulnar, posterior tibial and common peroneal nerve, and in the neuraxis and myelin sheath of motor fibers of the common peroneal nerve, are markedly aggravated compared with the asymptomatic stage. Importantly, we show for the first time that in both groups of diabetic patients, sensory nerves were affected to a greater extent than motor nerves, indicating that sensory nerves are more vulnerable to damage than motor nerves in diabetic peripheral neuropathy. In the diabetic groups, posterior tibial and common peroneal nerve conduction in the lower extremities had a markedly higher rate of absent waveforms than the median and ulnar nerves of the upper extremities, implying that the extent of nerve fiber lesions in the lower extremities is more severe than that in the upper extremities in diabetic patients. Importantly, we show for the first time that although diabetic peripheral neuropathy leads to diffuse conductive abnormality across whole peripheral nerves, the nearer the damage is to the distal end, the more severe the extent of impairment.
The total rate of abnormality detected in the median, ulnar, posterior tibial and common peroneal nerves in diabetic patients was 60%. The common peroneal nerve was the most prominently affected. Moreover, among all patients with diabetes, the common peroneal nerve showed the highest rate of absent waveforms, indicating that among the lesioned peripheral motor nerves in diabetic patients, the common peroneal nerve was most severely damaged. The posterior tibial nerve was most severely impaired from the sensory nerve tests. Comparing all indices of nerve conduction studies, we found that sensory nerve action potential was the most sensitive index. In the present study, the median nerve showed the highest rate of sensory nerve action potential abnormality, suggesting that this nerve is the first to be affected in diabetes. To our knowledge, we are the first to show a conclusive and simple relationship between peripheral nerve lesions and electrophysiological indices in different nerves commonly affected in diabetic peripheral neuropathy. In practical terms, this will enable earlier diagnosis of the condition.
Liu et al. (2005) studied 700 patients with diabetic peripheral neuropathy using nerve conduction studies and found that the longer the duration of the disease, the greater the possibility that abnormal electrophysiology will be observed. The electrophysiological abnormalities observed in the present study may be attributed to nutritional and metabolic disorders resulting from the diabetes itself, leading to impairment of axoplasmic transport in peripheral nerves, preventing distal axons from acquiring sufficient nutrition and ultimately leading to their degeneration. In a study by Bi et al. (2008), it was revealed that sensory nerve action potential amplitude is more sensitive than nerve conduction velocity in the diagnosis of mild or early diabetic peripheral neuropathy. Our study confirmed this notion. Importantly, we found that the abnormal rate of sensory nerve conduction velocity in diabetic patients was much higher than that of motor nerve conduction velocity, corresponding with previous studies (Tan and Tan, 2003; Kles and Bril, 2006; Vinik et al., 2006; Bi et al., 2008; Charles et al., 2013). These results indicate that nerve conduction studies should be used as an important index for the early diagnosis of diabetic peripheral neuropathy.
Taken together, our data demonstrate that various degrees of peripheral neuropathy occur in both sensory and motor nerves in the early stages of diabetes, before symptoms occur. The extent of impairment in the lower extremities is more severe than that in the upper extremities. Importantly, the extent of the lesion in sensory nerves is more severe than that in motor nerves. Symptoms and sensory nerve impairment will worsen with time; therefore, nerve conduction, especially sensory nerve action potential detection, should be routinely examined in diabetic patients so that diabetic peripheral neuropathy can be discovered and treated as early as possible.
Footnotes
Funding: This work is supported by the Science and Research Fund of Academic Department in Yunnan Province in China, No. 2011C08.
Conflicts of interest: None declared.
Copyedited by Slone-Murphy J, Yajima W, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- 1.Al-Geffari M. Comparison of different screening tests for diagnosis of diabetic peripheral neuropathy in Primary Health Care setting. Int J Health Sci (Qassim) 2012;6:127. doi: 10.12816/0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altun Y, Demirkol A, Tumay Y, Ekmekci K, Unsal I, Koyluoglu AC, Ozkul Y. The medial plantar and medial peroneal cutaneous nerve conduction studies for diabetic polyneuropathy. Neurol Sci. 2011;32:849–854. doi: 10.1007/s10072-011-0669-2. [DOI] [PubMed] [Google Scholar]
- 3.An JY, Park MS, Kim JS, Shon YM, Lee SJ, Kim YI, Lee KS, Kim BJ. Comparison of diabetic neuropathy symptom score and medial plantar sensory nerve conduction studies in diabetic patients showing normal routine nerve conduction studies. Intern Med. 2007;47:1395–1398. doi: 10.2169/internalmedicine.47.0901. [DOI] [PubMed] [Google Scholar]
- 4.Arimura A, Deguchi T, Sugimoto K, Uto T, Nakamura T, Arimura Y, Arimura K, Yagihashi S, Nishio Y, Takashima H. Intraepidermal nerve fiber density and nerve conduction study parameters correlate with clinical staging of diabetic polyneuropathy. Diabetes Res Clin Pract. 2013;99:24–29. doi: 10.1016/j.diabres.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Asad A, Hameed MA, Khan UA, Butt M, Ahmed N, Nadeem A. Comparison of nerve conduction studies with diabetic neuropathy symptom score and diabetic neuropathy examination score in type-2 diabetics for detection of sensorimotor polyneuropathy. J Pak Med Assoc. 2009;59:594–598. [PubMed] [Google Scholar]
- 6.Balbinot LF, Canani LH, Robinson CC, Achaval M, Zaro MA. Plantar thermography is useful in the early diagnosis of diabetic neuropathy. Clinics (Sao Paulo) 2012;67:1419–1425. doi: 10.6061/clinics/2012(12)12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banthia S, Bergner DW, Chicos AB, Ng J, Pelchovitz DJ, Subacius H, Kadish AH, Goldberger JJ. Detection of cardiovascular autonomic neuropathy using exercise testing in patients with type 2 diabetes mellitus. J Diabetes Complications. 2013;27:64–69. doi: 10.1016/j.jdiacomp.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Barriga ES, Chekh V, Carranza C, Burge M, Edwards A, McGrew E, Zamora G, Soliz P. Computational basis for risk stratification of peripheral neuropathy from thermal imaging. Conf Proc IEEE Eng Med Biol Soc 2012. 2012:1486–1489. doi: 10.1109/EMBC.2012.6346222. [DOI] [PubMed] [Google Scholar]
- 9.Bi J, Lu ZS, Chu H, Dong HJ. Diagnostic significance of sensory nerve action potential amplitude in early-stage diabetic neuropathy. Zhonghua Shenjing Ke Zazhi. 2008;41:657–660. [Google Scholar]
- 10.Calvet JH, Dupin J, Winiecki H, Schwarz PE. Assessment of small fiber neuropathy through a quick, simple and non invasive method in a German diabetes outpatient clinic. Exp Clin Endocrinol Diabetes. 2013;121:80–83. doi: 10.1055/s-0032-1323777. [DOI] [PubMed] [Google Scholar]
- 11.Charles M, Soedamah-Muthu SS, Tesfaye S, Fuller JH, Arezzo JC, Chaturvedi N, Witte DR. Low peripheral nerve conduction velocities and amplitudes are strongly related to diabetic microvascular complications in type 1 diabetes: the EURODIAB Prospective Complications Study. Diabetes Care. 2010;33:2648–2653. doi: 10.2337/dc10-0456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charles M, Fleischer J, Witte DR, Ejskjaer N, Borch-Johnsen K, Lauritzen T, Sandbaek A. Impact of early detection and treatment of diabetes on the 6-year prevalence of cardiac autonomic neuropathy in people with screen-detected diabetes: ADDITION-Denmark, a cluster-randomised study. Diabetologia. 2013;56:101–108. doi: 10.1007/s00125-012-2744-5. [DOI] [PubMed] [Google Scholar]
- 13.Chiles NS, Phillips CL, Volpato S, Bandinelli S, Ferrucci L, Guralnik JM, Patel KV. Diabetes, peripheral neuropathy, and lower-extremity function. J Diabetes Complications. 2014;28:91–95. doi: 10.1016/j.jdiacomp.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui LY. Beijing: Science Press; 2006. A Concise Handbook of Electromyography. [Google Scholar]
- 15.Dyck PJ, Overland CJ, Low PA, Litchy WJ, Davies JL, Dyck PJ, O’Brien PC. Signs and symptoms versus nerve conduction studies to diagnose diabetic sensorimotor polyneuropathy: Cl vs. NPhys trial. Muscle Nerve. 2010;42:157–164. doi: 10.1002/mus.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dyck PJ, Carter RE, Litchy WJ. Modeling nerve conduction criteria for diagnosis of diabetic polyneuropathy. Muscle Nerve. 2011;44:340–345. doi: 10.1002/mus.22074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Erdoğan Ç, Yücel M, Değirmenci E, Öz O, Akgün H, Odabaşı Z. Nerve excitability properties in early preclinical diabetic neuropathy. Diabetes Res Clin Pract. 2011;94:100–104. doi: 10.1016/j.diabres.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 18.Fang F, Wang YF, Gu MY, Chen H, Wang DM, Xiao K, Yan S, Yao LL, Li N, Zhen Q, Peng YD. Pedobarography-a novel screening tool for diabetic peripheral neuropathy? Eur Rev Med Pharmacol Sci. 2013;17:3206–3212. [PubMed] [Google Scholar]
- 19.Galuppo M, Giacoppo S, Bramanti P, Mazzon E. Use of natural compounds in the management of diabetic peripheral neuropathy. Molecules. 2014;19:2877–2895. doi: 10.3390/molecules19032877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gordon Smith A, Lessard M, Reyna S, Doudova M, Robinson Singleton J. The diagnostic utility of Sudoscan for distal symmetric peripheral neuropathy. J Diabetes Complications. 2014;28:511–516. doi: 10.1016/j.jdiacomp.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulichsen E, Fleischer J, Ejskjaer N, Eldrup E, Tarnow L. Screening for diabetic cardiac autonomic neuropathy using a new handheld device. J Diabetes Sci Technol. 2012;6:965–972. doi: 10.1177/193229681200600430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heise CO, Machado FC, Amorim SC, Toledo SM. Combined nerve conduction index in diabetic polyneuropathy. Arq Neuropsiquiatr. 2012;70:330–334. doi: 10.1590/s0004-282x2012000500005. [DOI] [PubMed] [Google Scholar]
- 23.Hemmi S, Inoue K, Murakami T, Sunada Y. Comparison of the sensitivities of plantar nerve conduction techniques for early detection of diabetic sensory polyneuropathy. Electromyogr Clin Neurophysiol. 2009;50:269–275. [PubMed] [Google Scholar]
- 24.Joa KL, Kim CH. Clinical significance of the double-peak sensory response in nerve conduction study of normal and diabetic patients. Am J Phys Med Rehabil. 2013;92:111–117. doi: 10.1097/PHM.0b013e318269eb60. [DOI] [PubMed] [Google Scholar]
- 25.Kincaid JC, Price KL, Jimenez MC, Skljarevski V. Correlation of vibratory quantitative sensory testing and nerve conduction studies in patients with diabetes. Muscle Nerve. 2007;36:821–827. doi: 10.1002/mus.20880. [DOI] [PubMed] [Google Scholar]
- 26.Kiziltan ME, Benbir G. Clinical and nerve conduction studies in female patients with diabetic dermopathy. Acta Diabetol. 2008;45:97–105. doi: 10.1007/s00592-008-0031-1. [DOI] [PubMed] [Google Scholar]
- 27.Kles KA, Bril V. Diagnostic tools for diabetic sensorimotor polyneuropathy. Curr Diabetes Rev. 2006;2:353–361. doi: 10.2174/157339906777950598. [DOI] [PubMed] [Google Scholar]
- 28.Koçer A, Domac FM, Boylu E, Us O, Tanridağ T. A comparison of sural nerve conduction studies in patients with impaired oral glucose tolerance test. Acta Neurol Scand. 2007;116:399–405. doi: 10.1111/j.1600-0404.2007.00886.x. [DOI] [PubMed] [Google Scholar]
- 29.Koo YS, Jung KY, Lee SH, Cho CS, Yang KS, Jang JH, Kim BJ. Multichannel surface electrodes increase the sensitivity of diagnosis of neuropathy in diabetic patients. J Electromyogr Kines. 2013;23:1057–1064. doi: 10.1016/j.jelekin.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Koytak PK, Isak B, Borucu D, Uluc K, Tanridag T, Us O. Assessment of symptomatic diabetic patients with normal nerve conduction studies: utility of cutaneous silent periods and autonomic tests. Muscle Nerve. 2011;43:317–323. doi: 10.1002/mus.21877. [DOI] [PubMed] [Google Scholar]
- 31.Kuntzer T, Medlin F, Burnand B, Camain JY. Diabetic neuropathies: clinical sub-types, early detection, and asking help from neurologist. Praxis (Bern 1994) 2012;101:1315–1319. doi: 10.1024/1661-8157/a001037. [DOI] [PubMed] [Google Scholar]
- 32.Løseth S, Stålberg E, Jorde R, Mellgren SI. Early diabetic neuropathy: thermal thresholds and intraepidermal nerve fibre density in patients with normal nerve conduction studies. J Neurol. 2008;255:1197–1202. doi: 10.1007/s00415-008-0872-0. [DOI] [PubMed] [Google Scholar]
- 33.Løseth S, Mellgren SI, Jorde R, Lindal S, Stålberg E. Polyneuropathy in type 1 and type 2 diabetes: comparison of nerve conduction studies, thermal perception thresholds and intraepidermal nerve fibre densities. Diabetes Metab Res Rev. 2010;26:100–106. doi: 10.1002/dmrr.1049. [DOI] [PubMed] [Google Scholar]
- 34.Lee SS, Han HS, Kim H. A 5‐yr follow‐up nerve conduction study for the detection of subclinical diabetic neuropathy in children with newly diagnosed insulin-dependent diabetes mellitus. Pediatr Diabetes. 2010;11:521–528. doi: 10.1111/j.1399-5448.2009.00636.x. [DOI] [PubMed] [Google Scholar]
- 35.Liu MS, Hu BL, Cui LY, Tang XF, Du H, Li BH. Clinical and neurophysiological features of 700 patients with diabetic peripheral neuropathy. Zhonghua Nei Ke Za Zhi. 2005;44:173–176. [PubMed] [Google Scholar]
- 36.McLellan KCP, Wyne K, Villagomez ET, Hsueh WA. Therapeutic interventions to reduce the risk of progression from prediabetes to type 2 diabetes mellitus. Ther Clin Risk Manag. 2014;10:173. doi: 10.2147/TCRM.S39564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mete T, Aydin Y, Saka M, Cinar Yavuz H, Bilen S, Yalcin Y, Arli B, Berker D, Guler S. Comparison of efficiencies of michigan neuropathy screening instrument, neurothesiometer, and electromyography for diagnosis of diabetic neuropathy. Int J Endocrinol 2013. 2013:821745. doi: 10.1155/2013/821745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mondal A, Sen S, Chanda D, Kundu S, Chatterjee M, Mukherjee S. Evaluation of diabetic polyneuropathy in type 2 diabetes mellitus by nerve conduction study and association of severity of neuropathy with serum sFasL level. Indian J Endocrinol Metab. 2012;16:S465–467. doi: 10.4103/2230-8210.104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morimoto J, Suzuki Y, Tada A, Akui M, Ozawa Y, Maruyama T. Time-course changes in nerve conduction velocity (NCV) in type 2 diabetes. J Diabetes Complications. 2012;26:237–240. doi: 10.1016/j.jdiacomp.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 40.O’Brien T, Karem J. An initial evaluation of a proof-of-concept 128-Hz electronic tuning fork in the detection of peripheral neuropathy. J Am Podiat Med Assn. 2014;104:134–140. doi: 10.7547/0003-0538-104.2.134. [DOI] [PubMed] [Google Scholar]
- 41.Papanas N, Boulton A, Malik R, Manes C, Schnell O, Spallone V, Tentolouris N, Tesfaye S, Valensi P, Ziegler D, Kempler P. A simple new non-invasive sweat indicator test for the diagnosis of diabetic neuropathy. Diabetic Med. 2013;30:525–534. doi: 10.1111/dme.12000. [DOI] [PubMed] [Google Scholar]
- 42.Richardson JK, Allet L, Kim H, Ashton-Miller JA. Fibular motor nerve conduction studies and ankle sensorimotor capacities. Muscle Nerve. 2013;47:497–503. doi: 10.1002/mus.23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sellers EA, Clark I, Tavakoli M, Dean HJ, McGavock J, Malik RA. The acceptability and feasibility of corneal confocal microscopy to detect early diabetic neuropathy in children: a pilot study. Diabetic Med. 2013;30:630–631. doi: 10.1111/dme.12125. [DOI] [PubMed] [Google Scholar]
- 44.Severinsen K, Andersen H. Evaluation of atrophy of foot muscles in diabetic neuropathy-a comparative study of nerve conduction studies and ultrasonography. Clin Neurophysiol. 2007;118:2172–2175. doi: 10.1016/j.clinph.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 45.Shi X, Chen Y, Nadeem L, Xu G. Beneficial effect of TNF-α inhibition on diabetic peripheral neuropathy. J Neuroinflammation. 2013;10 doi: 10.1186/1742-2094-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin TM, Bril V, Orszag A, Ahmed A, Perkins BA. How sensitive is the case definition for diabetic sensorimotor polyneuropathy to the use of different symptoms, signs, and nerve conduction parameters in type 1 diabetes? Diabetes Res Clin Pract. 2011;92:e16–19. doi: 10.1016/j.diabres.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 47.Spadella CT, Lucchesi AN, Alberti S, Resende LA. Early pancreas transplant improves motor nerve conduction in alloxan-induced diabetic rats. Exp Clin Endocrinol Diabetes. 2012;120:567–572. doi: 10.1055/s-0032-1321786. [DOI] [PubMed] [Google Scholar]
- 48.Suh BC, Chung PW, Moon HS, Kim YB, Yoon WT, Shim DS, Kim SB. Association between pulse wave velocity and nerve conduction study in diabetic patients. Eur Neurol. 2010;64:219–223. doi: 10.1159/000319605. [DOI] [PubMed] [Google Scholar]
- 49.Sun PC, Kuo CD, Chi LY, Lin HD, Wei SH, Chen CS. Microcirculatory vasomotor changes are associated with severity of peripheral neuropathy in patients with type 2 diabetes. Diab Vasc Dis Res. 2013;10:270–276. doi: 10.1177/1479164112465443. [DOI] [PubMed] [Google Scholar]
- 50.Tan M, Tan U. Early diagnosis of diabetic neuropathy using double-shock stimulation of peripheral nerves. Clin Neurophysiol. 2003;114:1419–1422. doi: 10.1016/s1388-2457(03)00154-8. [DOI] [PubMed] [Google Scholar]
- 51.Uluc K, Isak B, Borucu D, Temucin CM, Cetinkaya Y, Koytak PK, Tanridag T, Us O. Medial plantar and dorsal sural nerve conduction studies increase the sensitivity in the detection of neuropathy in diabetic patients. Clin Neurophysiol. 2008;119:880–885. doi: 10.1016/j.clinph.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 52.Vinik AI, Kong X, Megerian JT, Gozani SN. Diabetic nerve conduction abnormalities in the primary care setting. Diabetes Technol Ther. 2006;8:654–662. doi: 10.1089/dia.2006.8.654. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe T, Ito H, Sekine A, Katano Y, Nishimura T, Kato Y, Takeda J, Seishima M, Matsuoka T. Sonographic evaluation of the peripheral nerve in diabetic patients: the relationship between nerve conduction studies, echo intensity, and cross-sectional area. J Ultrasound Med. 2010;29:697–708. doi: 10.7863/jum.2010.29.5.697. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe T, Ito H, Morita A, Uno Y, Nishimura T, Kawase H, Kato Y, Matsuoka T, Takeda J, Seishima M. Sonographic evaluation of the median nerve in diabetic patients: comparison with nerve conduction studie. J Ultrasound Med. 2009;28:727–734. doi: 10.7863/jum.2009.28.6.727. [DOI] [PubMed] [Google Scholar]
- 55.Wendland EM, Torloni MR, Falavigna M, Trujillo J, Dode MA, Campos MA, Duncan BB, Schmidt MI. Gestational diabetes and pregnancy outcomes-a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12:23. doi: 10.1186/1471-2393-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Won JC, Kim SS, Ko KS, Cha BY. Current Status of Diabetic Peripheral Neuropathy in Korea: Report of a Hospital-Based Study of Type 2 Diabetic Patients in Korea by the Diabetic Neuropathy Study Group of the Korean Diabetes Association. Diabetes Metab J. 2014;38:25–31. doi: 10.4093/dmj.2014.38.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang YQ, Xu H, Cheng YQ, Zhou Y, Sheng XL, Fan YH. The value of multiple neurophysiological tests in the early diagnosis of diabetic peripheral neuropathy. Zhonghua Wuli Yixue yu Kangfu Zazhi. 2013;35:351–355. [Google Scholar]
- 58.Zhong W, Zhang W, Yang M, Li G, Ma Q, Yang X. Impact of diabetes mellitus duration on effect of lower extremity nerve decompression in 1,526 diabetic peripheral neuropathy patients. Acta Neurochir (Wien) 2014;156:1329–1333. doi: 10.1007/s00701-014-2087-8. [DOI] [PubMed] [Google Scholar]


