Abstract
Optic nerve transection increased the expression of heat shock protein 72 (HSP72) in the lateral geniculate body, indicating that this protein is involved in the prevention of neuronal injury. Zinc sulfate and quercetin induced and inhibited the expression of HSP72, respectively. Intraperitoneal injections of zinc sulfate, SP600125 (c-Jun N-terminal kinase inhibitor), or quercetin were performed on retinal ganglion cells in a Wistar rat model of chronic ocular hypertension. Our results showed that compared with the control group, the expression of HSP72 in retinal ganglion cells and the lateral geniculate body was increased after the injection of zinc sulfate, but was decreased after the injection of quercetin. The expression of phosphorylated c-Jun N-terminal kinases and phosphorylated c-Jun were visible 3 days after injection in the control group, and reached a peak at 7 days. Zinc sulfate and SP600125 significantly decreased the expression of p-c-Jun, whereas quercetin significantly enhanced the expression of this protein. These results suggest that HSP72 protects retinal ganglion cells and lateral geniculate body in a rat model of chronic ocular hypertension from injury by blocking the activation of the stress-activated kinase/c-Jun N-terminal kinase apoptotic pathway.
Keywords: neural regeneration, peripheral nerve injury, glaucoma, heat shock protein 72, retinal ganglion cells, lateral geniculate body, zinc sulfate, quercetin, SAPK/JNK pathway, neuroprotection, p-JNK, p-c-Jun, NSFC grant, neural regeneration
Introduction
Glaucoma is one of the world's leading causes of irreversible blindness and is characterized by progressive optic nerve damage and selective loss of retinal ganglion cells (RGCs) (Diekmann et al., 2013; Zhong et al., 2013). Current therapies have focused on lowering intraocular pressure and slowing disease progression (Baudouin et al., 2013). Although the RGC cell body is localized in the eye, the large part of its axon lies outside the eye, forming the optic nerve, chiasm, and optic tract. Ninety percent of the RGCs terminate in the lateral geniculate nucleus (LGN), the major relay station between the retina and visual cortex (Gupta et al., 2009; Ito et al., 2009). In experimental glaucoma with optic nerve fiber loss, the LGN undergoes degeneration, including overall LGN shrinkage and reduced neuronal size and number (Ito et al., 2011). These findings provide evidence that the pathology of glaucomatous may involve RGC loss and LGN degeneration.
Heat shock protein (HSP) is a well-conserved, ubiquitous protein found in all cells (Urbak et al., 2010; Lomiwes et al., 2014). Zinc sulfate significantly induces the expression of HSP72, whereas quercetin inhibits its expression. The inhibitor of c-Jun N-terminal kinase (JNK), SP600125, has been shown to specifically block the stress-activated kinase (SAPK)/JNK pathway reducing apoptosis of ganglion cells (Glushkova et al., 2013). HSPs are normally present at low levels, but their expression is rapidly increased in response to a variety of harmful stimuli, including hypoxia or ischemia (or other types of metabolic stress) and temperature elevation (O’Reilly et al., 2010). This stress response is thought to be vital in the intracellular chaperoning function to protect cells from death. Neurons of transgenic mice expressing HSP72 or those of rats injected with the herpes virus containing HSP72 genes have also been shown to be resistant to ischemia and seizures, which suggests that HSP72 is important for neuroprotection (Nagashima et al., 2011; Barreto et al., 2012; Abdi et al., 2013).
We have shown that HSP72 is induced in RGCs of rat acute glaucoma models with heat stress or zinc sulfate administration (Qing et al., 2004). Further, the dynamics of HSP72 induction between these two groups are quite different. In the present study, we investigated the expression of HSP72 in RGCs and LGN neurons in a rat model of chronic ocular hypertension, and explored the mechanism of HSP72-mediated neuroprotection in glaucoma. These findings may strengthen the current knowledge about the biological significance of HSP72 in behavior, and provide a novel therapeutic strategy against the progression of chronic glaucoma.
Materials and Methods
Animals
A total of 140 adult, healthy male Wistar rats, weighing 200–300 g, were supplied by the Animal Breeding Center, Second Xiangya Hospital, Central South University, China (license No. SYXK (Xiang) 2004-0013). The rats were maintained at 21 ± 2°C under a 12-hour light-dark cycle, and were matched for age and weight before each experiment. The pilot project was approved by the Ethics Committees of the Second Xiangya Hospital, Central South University, China.
Animal grouping
All experiments were performed on the right eye. A total of 140 male Wistar rats were divided into five groups: normal control (n = 12), damage control (n = 48), zinc sulfate (n = 30), quercetin (n = 30), or SP600125 (n = 20). Except normal control group, 128 rats were used to establish chronic glaucoma models. Among them, 48 model rats were killed at different intervals after laser treatment without drug injection, serving as damage controls. Thirty rats were treated with zinc sulfate (Sigma-Aldrich, St. Louis, MO, USA) via intraperitoneal injection 2 weeks after laser treatment. Thirty rats were treated with intraperitoneal injection of quercetin 6 hours after laser treatment. Twenty rats were treated with SP600125 (Calbiochem, Merck-Millipore, Darmstadt, Germany) after laser treatment. The treated model rats were sacrificed at specific intervals after treatment, and the right eye immediately enucleated.
Induction of chronic ocular hypertension
Rats were anesthetized (100 g/L chloral hydrate 3.8 mL/kg, intraperitoneal), then the bulbar conjunctiva was cut and two superficial venous tributaries were burnt by a viridis-lite laser (532 nm). The laser beam, which was directed towards all four episcleral veins and at 360° of the limbal plexus, was applied at 260–300 mW for 200 μs, producing a spot size of 200 µm at the episcleral veins. Intraocular pressure was also measured with Tonopen XL before the operation and 3, 7, 14, and 28 days after the operation. Intraocular pressure that was 40% beyond the preoperative value (9–16 mmHg, 1 mmHg = 0.133 kPa) indicated that the model was successfully established.
Retrograde labeling of regenerating RGCs
Rats were anesthetized and placed in a stereotactic device. Fluoro-Gold (5%) (Fluorochrome, Denver, CO, USA) injections into the bilateral superior colliculi were administered with the following coordinates: 6.3 and 6.8 mm posterior to bregma, 1.5 mm lateral to midline, and 3.6 and 4.0 mm from the skull. A total of 0.8 μL Fluoro-Gold was applied through a microinjector (Anting, Shanghai, China). Rats were sacrificed 3, 7, 14, or 28 days after intraperitoneal or intracerebroventricular injection. RGC levels were measured 3, 7, 14, and 28 days after treatments. Cells that contained Fluoro-Gold in the cytoplasm and possessed at least one dendrite, or an axon, were counted as axon-regenerating RGCs. Small Fluoro-Gold-labelled cells with identifiable dendrites or axon were also counted as axon-regenerating RGCs. To determine the total number of Fluoro-Gold-labelled RGCs per retina, the number of Fluoro-Gold-labelled RGCs in each field (0.25 × 0.25 mm) at a fixed distance from each other, in a pattern of grid intersections, was counted throughout the whole retina. A total of 70–80 fields (about 8–10% of the retinal area) were sampled per retina.
Immunohistochemistry for HSP72
The eye balls were immersed in a fixative consisting of 4% paraformaldehyde in 0.1 mol/L Sorensen's phosphate buffer (pH 7.2) for 4 hours at 4°C then washed (several rinses) in PBS. After the specimens were immersed in 10–20% sucrose (in 0.1 mol/L PBS), they were then embedded for cryo-sectioning. To reduce nonspecific staining, the sections were blocked in a solution containing 4% normal fetal calf serum. Sections were then incubated with the primary antibody, mouse anti-HSP72 polyclonal antibody (1:200; Santa Cruz Biotechnology, Santa Cruz, CA, USA), in blocking solution overnight at 4°C in a humidified chamber. The sections were rinsed three times with PBS and then incubated with the secondary antibody, rabbit anti-mouse IgG (1:500; Boster, Wuhan, Hubei Province, China), in blocking solution for 30 minutes at 37°C. Micrographs of immunolabeled sections were captured using a fluorescence microscope (Carl Zeiss Meditec, Jena, Germany).
Western blot analysis
Rats were anesthetized at the different time points followed by thoracotomy and left ventricular cannulation, then the dissection of the right atrial appendage, which was pre-cooled at 4°C in saline perfusion until the effluent became clear. The brains were then removed, the LGN tissue separated, and the retina peeled on ice rapidly (Paxinos and Watson, 1997, reprinted with permission from Elsevier). Retinal protein or LGN protein was extracted using RIPA lysis buffer (20 mmol/L Tris, pH 7.5, 150 mmol/L NaCl, 1% Triton X-100, 2.5 mmol/L sodium pyrophosphate, 1 mmol/L EDTA, 1% Na3VO4, 0.5 µg/mL leupeptin, and 1 mmol/L phenylmethanesulfonyl fluoride). After centrifugation for 10 minutes at 14,000 × g, the supernatant was collected. Cell supernatant was collected after centrifuging at 15,000 r/min for 15 minutes, frozen, and kept at –70°C for later usage. Proteins (5 µg/lane) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to nitrocellulose membranes (Hybond; GE Healthcare, Piscataway, NJ, USA). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline (TBS; 20 mmol/L Tris, 137 mmol/L NaCl [pH 7.4]) containing 0.02% Tween-20 (TBST) followed by the primary antibodies (diluted in TBST): mouse anti-HSP72 monoclonal antibody (1:2,000; Cell Signaling, USA), rabbit anti-p-JNK, or anti-p-c-Jun monoclonal antibodies (both at 1:2,000; Cell Signaling) for 24 hours at 4°C. Membranes were then washed three times with TBST. Rabbit anti-β-actin antibody (1:2,000; Sigma-Aldrich, St. Louis, MO, USA) was used as the loading control in all cases. Membranes were then exposed to horseradish peroxidase-conjugated anti-mouse or anti-rabbit antibody (BAbCo, Berkeley, CA, USA; 1:1,000 in TBST) for 1 hour at room temperature, followed by three washes with TBST. Immunoreactive proteins were visualized using chemiluminescence detection reagents (GE Healthcare) on autoradiograph films. The intensity of each band was quantified using the Scion Image program. Films were scanned and the target zone analyzed with the gel image processing system.
Statistical analysis
All data are expressed as mean ± SEM and were analyzed by the two-sample t-test to compare the differences between treated groups and control groups. All experiments were performed in triplicate. Statistical analysis was performed using SPSS 17.0 software (SPSS, Chicago, IL, USA). P < 0.05 was considered to be statistically significant.
Results
Effect of laser treatment on the survival of RGCs in rats following different drug injections
Fluoro-Gold retrograde labeling results showed the number of RGCs in the damage control, zinc sulfate, quercetin, SP600125 groups was significantly (P < 0.05) lower compared with the normal control group (Table 1). The number of RGCs was significantly (P < 0.05) higher in the zinc sulfate group compared with the damage control group at 3, 7, 14, and 28 days, and the number of RGCs was significantly (P < 0.05) lower in the quercetin group than that in the damage control group (Table 1).
Table 1.
Number (n/200-fold field of view) of retinal ganglion cells in rats before and after laser treatment
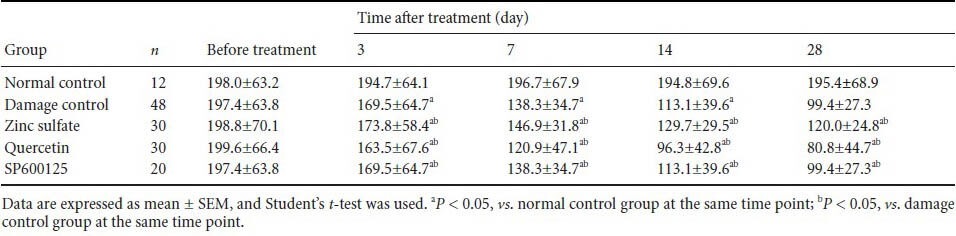
Effect of laser treatment on the expression of HSP72 in RGCs and the LGN of rats following different drug injections
Immunohistochemical staining and western blot analysis showed no presence and no expression, respectively of HSP72 in RGCs and the LGN of normal control rats (Figure 2A, C). At 7 days after laser treatment, both immunostaining and western blot were performed to detect the expression of HSP72 in the retinal ganglion cell layer, nerve fiber layer, outer plexiform layer and LGN (Figure 2B). Moreover, HSP72 expression reached the peak at 7 days in LGN (Figure 2D). In ocular hypertension for 28 days group, the expression of HSP72 in both retina and LGN was significantly reduced (Figure 2E). These indicated that HSP72, after chronic elevated IOP, was expressed effectively.
Figure 2.
Effect of laser treatment on the presence and expression of heat shock protein 72 (HSP72) in retinal ganglion cells (RGCs) and the lateral geniculate nucleus (LGN) of rats following different drug injections.
(A–D) Immunohistochemistry for HSP72. (A) HSP72 is not detected in the normal retina. (B) Compared with the normal control group, the pres-ence of HSP72 (brown staining) is less prominent at 7 days in the retinal ganglion cell layer (thin short arrow), nerve fiber layer (thick arrow) and outer plexiform layer (slender arrow) of the retina. (C) HSP72 is not detected in the normal LGN. (D) Seven days after laser treatment, LGN neurons are detected in tissues that label HSP72 (thin short arrow), vacuolated cells, nuclear condensation (thin arrow), and capillaries (thick arrow). West-ern immunoblot and quantitative analysis of HSP72 protein expression in the RGCs (E, F) and LGN (E, G) at different time points. At 3, 7, 14, and 28 days after laser treatment (II–V), HSP72 protein expression in the retina and LGN is significantly different at each time point (P < 0.05). I: Before treatment (damage control group).
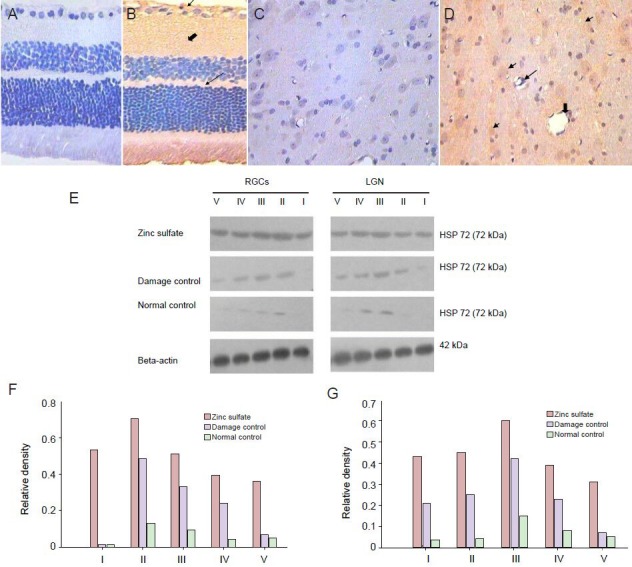
Figure 1.
Retinal ganglion cells (RGCs) in rats 28 days after laser treatment (Fluoro-Gold staining, × 200).
Fluoro-Gold retrograde labeling showing that at 28 days after laser treatment, more RGCs are found in the zinc sulfate group (C) compared with the normal control group (A), and fewer RGCs in the quercetin group (D) was found compared with the damage control group (B–D). (E) SP600125 (c-Jun N-terminal kinase inhibitor) had fewer RGCs compared with normal control group (A).
Effect of laser treatment on protein expression of phosphorylated c-Jun N-terminal kinases (p-JNK) and phosphorylated c-Jun (p-c-Jun) in the rat retina and the LGN
Three days after laser treatment, p-JNK and p-c-Jun proteins were mildly expressed in both the retina and LGN of rats from the damage control group. The expression levels reached a peak at 7 days and then maintained a high level. At each time point, the expression of p-JNK and p-c-Jun in the LGN was increased after laser treatment, reached the peak at 14 days, and remained at a high level during the observation time (Figure 3).
Figure 3.
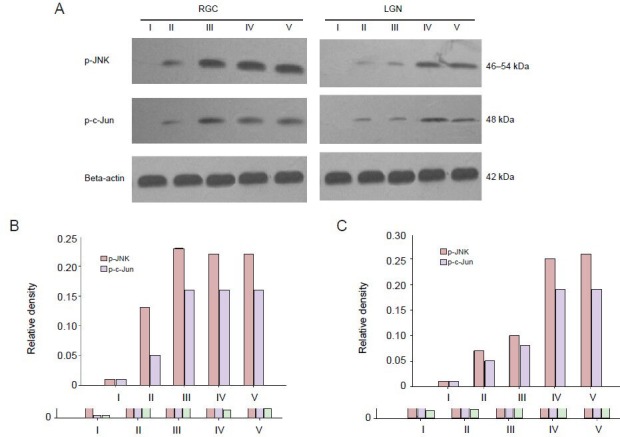
Protein expression of phosphorylated c-Jun N-terminal kinases (p-JNK) and phosphorylated c-Jun (p-c-Jun) in retinal ganglion cells (RGCs) and the lateral geniculate nucleus (LGN) of rats following laser treatment.
Western immunoblot and quantitative analysis of p-JNK and p-c-Jun in the RGCs (A, B) and LGN (A, C) in the damage control group at different time points. Three days after laser treatment, p-JNK and p-c-Jun proteins are mildly expressed in the RGCs and the LGN. The expression levels reach a peak at 7 days and are maintained at a high level. At each time point, the expression of p-JNK and p-c-Jun protein in the LGN increases af-ter laser treatment, reaches a peak at 14 days, and remains at a high level during the observation time. I–V: Before treatment, 3, 7, 14, 28 days after treatment in damage control group.
Effects of laser treatment on the protein expression of p-JNK and p-c-Jun in the retina and LGN after drug intervention
After 7–28 days of laser treatment, the expression of p-JNK peaked in the retina of the damage control group (Figure 4). Expression of p-JNK in the zinc sulfate, quercetin, and SP600125 groups was significantly increased (P < 0.01; Figure 4). The expression of p-JNK was not significantly different between these four groups (Figure 4). The expression of p-JNK was not significantly increased in the normal control (Figure 4). The expression of p-c-Jun (occurring downstream of p-JNK) was significantly (P < 0.05) different between the damage control, zinc sulfate, quercetin, and SP600125 groups (Figure 4). Its level of expression was lowest in the SP600125 group, followed by the zinc sulfate group and then the damage control group, with the highest expression occurring in the quercetin group (P < 0.05; Figure 4). The expression of p-c-Jun in the normal control group was almost undetectable (Figure 4). In the LGN, the expression of p-JNK and p-c-Jun reached its peak at 14 days in the damage control group (Figure 4). The expression of p-JNK was significantly higher in SP600125, zinc sulfate, and quercetin groups (P < 0.01; Figure 4). The expression of p-JNK and p-c-Jun in the LGN was the same compared with that of 7 days in the retina from the damage control group (Figure 4).
Figure 4.
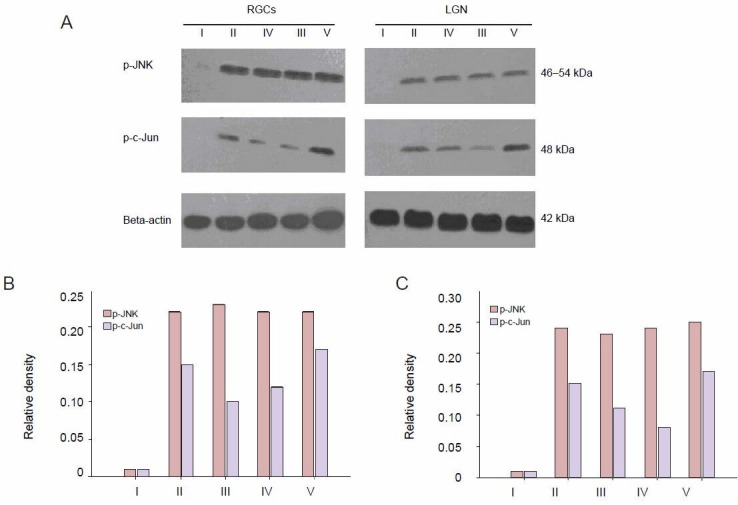
Effects of laser treatment on the protein expression of phosphorylated c-Jun N-terminal kinases (p-JNK) and phosphorylated c-Jun (p-c-Jun) in retinal ganglion cells (RGCs) and the lateral geniculate nucleus (LGN) of rats after drug intervention.
(A, B) The expression of p-JNK and p-c-Jun with different drug treatment groups in the retina at 7 days. (A, C) The expression of p-JNK and p-c-Jun with different drug treatment groups in the LGN at 14 days. The expression of p-c-Jun is significantly different between all four groups, 7 and 14 days after treatment. P < 0.05, damage control group vs. the other three groups. I: Normal control group; II: damage control group; IV: zinc sulfate group; III: SP600125 group; V: quercetin group.
Discussion
The preinduction of HSPs in RGCs enhances the survival of RGCs under hypoxic, excitotoxic, and glaucomatous stress (Windisch et al., 2009; Yamashima, 2012). In this study, we showed that the major inducible chaperone within the HSP70 family, HSP72, protects RGCs and LGN neurons in rat models of chronic ocular hypertension from injury. We further found that the protection was related to the inhibition of SAPK/JNK pathway. This is the first report to demonstrate that chronic ocular hypertension induces HSP72 in retinal neurons, and it provides evidence of a neuroprotective effect for RGCs and LGN neurons in a chronic ocular hypertensive rat model.
Protective effect of HSP72 activation on RGCs and LGN neurons
Studies have shown that cells synthesize HSPs under physiological or environmental stress, such as hyperthermia and hypoxia, and in the presence of cytokines and other toxics (Tanaka et al., 2009; Lunz et al., 2013). HSPs enhance cell survival under conditions of further severe stress. HSP72 is expressed during preconditioning stress, and is known to be essential for neuroprotection (Yao et al., 2007; Carmeli et al., 2010). The upregulation of HSP72 enhances the survival of RGCs and LGN (Ebrahimi et al., 2010; Sohn et al., 2010). The expression level correlates positively with the extent and duration of stress (Ortega et al., 2012).
In the present study, we found that the expression of HSP72 in RGCs and LGN was greatly upregulated when glaucoma model rats were treated with laser photocoagulation. Ischemic-mediated stress and mechanical compression occurred in RGCs. HSP72 was induced in the cells. The expression level reached its peak at 3 days and disappeared at 28 days. Further, the expression of HSP72 in RGCs and LGN was elevated when rats were treated with zinc administration. However, administration with quercetin inhibited the expression of HSP72 and blocked the protection for RGCs and LGN neurons.
The rat glaucoma model used here showed mild to moderate loss of RGCs and LGN (indicating cell death) and mild axonal damage in chronic intraocular hypertension models.
Inhibition of SAPK/JNK pathway enhances the survival of RGCs and LGN neurons
SAPK/JNK belongs to the MAP kinase superfamily and regulates many cellular events (You et al., 2013). The SAPK/JNK signal transduction pathway is involved in the expression of interferon and participates in cell proliferation, differentiation, apoptosis, and immune regulation process (Pereira et al., 2012; Kondo et al., 2013). Numerous studies have confirmed that p-c-Jun levels are significantly decreased by the SAPK/JNK pathway inhibitor, SP600125 (Yao et al., 2010).
In the present study, zinc sulfate increased the expression of HSP72 and decreased c-Jun activation of the SAPK/JNK pathway. However, quercetin inhibited the expression of HSP72 and increased p-c-Jun. These results suggest that HSP72 blocks the apoptotic events associated with the SAPK/JNK pathway like SP600125. HSP72 also enhanced RGC and LGN neuron survival through SAPK/JNK pathway.
This study shows HSP72 and SP600125 protects RGCs and LGN from injury by blocking the activation of the SAPK/JNK pathway. Quercetin inhibited the expression of HSP72 and increased the activation of c-Jun in the LGN. Results from the present study provide a better understanding of the mechanisms underlying chronic glaucoma.
These findings suggest that gradually increasing the intraocular pressure, and intraperitoneal injection of zinc sulfate, contribute to increase of the expression of HSP72. By blocking the apoptotic SAPK/JNK pathway, HSP72 may play a protective role on RGC and the LGN in chronic ocular hypertensive rats.
Footnotes
Funding: This study was financially supported by the National Natural Science Foundation of China, No. 81170843, 81370913; the Natural Science Foundation of Hunan Province, China, No. 5JJ30051; New Century Excellent Talents in University from the Ministry of Education of China, No. NCET-06-0677; the Natural Science Foundation of Anhui Province, China, No. 1408085QH158; the First Affiliated Hospital of Anhui Medical University, Incubation Program of the National Natural Science Foundation for Young Scholars of China, No. 2012KJ19.
Conflicts of interest: None declared.
Copyedited by Mark F, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- 1.Abdi Hamzehkolaei H, Dabidi Roshan V, Hosseinzadeh M. The interactive effects of exercise type and environment temperature on HSP72 in active females. J Sports Med Phys Fitness. 2013;53:80–87. [PubMed] [Google Scholar]
- 2.Barreto GE, White RE, Xu L, Palm CJ, Palm CJ, Giffard RG. Effects of heat shock protein 72 (Hsp72) on evolution of astrocyte activation following stroke in the mouse. Exp Neurol. 2012;238:284–296. doi: 10.1016/j.expneurol.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudouin C, Denoyer A, Rostène W. Glaucoma today: detection and therapeutic progress. Biol Aujourdhu. 2013;207:87–95. doi: 10.1051/jbio/2013009. [DOI] [PubMed] [Google Scholar]
- 4.Carmeli E, Beiker R, Maor M, Kodesh E. Increased iNOS, MMP- 2, and HSP-72 in skeletal muscle following high-intensity exercise training. J Basic Clin Physiol Pharmacol. 2010;21:127–146. doi: 10.1515/jbcpp.2010.21.2.127. [DOI] [PubMed] [Google Scholar]
- 5.Diekmann H, Fischer D. Glaucoma and optic nerve repair. Cell Tissue Res. 2013;353:327–337. doi: 10.1007/s00441-013-1596-8. [DOI] [PubMed] [Google Scholar]
- 6.Ebrahimi M, Mohammadi P, Daryadel A, Baharvand H. Assessment of heat shock protein (HSP60, HSP72, HSP90, and HSC70) expression in cultured limbal stem cells following air lifting. Mol Vis. 2010;16:1680–1688. [PMC free article] [PubMed] [Google Scholar]
- 7.Glushkova OV, Parfenyuk SB, Khrenov MO, Novoselova TV, Lunin SM, Fesenko EE, Novoselova EG. Inhibitors of TLR-4, NF-κB, and SAPK/JNK signaling reduce the toxic effect of lipopolysaccharide on RAW 264.7 cells. J Immunotoxicol. 2013;10:133–140. doi: 10.3109/1547691X.2012.700652. [DOI] [PubMed] [Google Scholar]
- 8.Gupta N, Greenberg G, Tilly LN, Gray B, Polemidiotis M, Yücel YH. Atrophy of the lateral geniculate nucleus in human glaucoma detected by magnetic resonance imaging. Br J Ophthalmol. 2009;93:56–60. doi: 10.1136/bjo.2008.138172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito Y, Shimazawa M, Chen YN, Tsuruma K, Yamashima T, Araie M, Hara H. Morphological changes in the visual pathway induced by experimental glaucoma in Japanese monkeys. Exp Eye Res. 2009;89:246–255. doi: 10.1016/j.exer.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Ito Y, Shimazawa M, Inokuchi Y, Yamanaka H, Tsuruma K, Imamura K, Onoe H, Watanabe Y, Aihara M, Araie M, Hara H. Involvement of endoplasmic reticulum stress on neuronal cell death in the lateral geniculate nucleus in the monkey glaucoma model. Eur J Neurosci. 2011;33:843–855. doi: 10.1111/j.1460-9568.2010.07578.x. [DOI] [PubMed] [Google Scholar]
- 11.Kondo A, Otsuka T, Matsushima-Nishiwaki R, Kuroyanagi G, Mizutani J, Wada I, Kozawa O, Tokuda H. Inhibition of SAPK/JNK leads to enhanced IL-1-induced IL-6 synthesis in osteoblasts. Arch Biochem Biophys. 2013;535:227–233. doi: 10.1016/j.abb.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Liu Q, Wan Y, Zhao Z, Yu H, Luo H, Tang Z. Osteopontin promotes the progression of gastric cancer through the NF-κB pathway regulated by the MAPK and PI3K. Int J Oncol. 2014;45:282–290. doi: 10.3892/ijo.2014.2393. [DOI] [PubMed] [Google Scholar]
- 13.Lomiwes D, Hurst SM, Dobbie P, Frost DA, Hurst RD, Young OA, Farouk MM. The protection of bovine skeletal myofibrils from proteolytic damage post mortem by small heat shock proteins. Meat Sci. 2014;97:548–557. doi: 10.1016/j.meatsci.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 14.Lunz W, Capettini LS, Davel AP, Munhoz CD, da Silva JF, Rossoni LV, Lemos VS, Baldo MP, Carneiro-Junior MA, Natali AJ, de Lacerda LH, Mill JG. L-NAME treatment enhances exercise-induced content of myocardial heat shock protein 72 (Hsp72) in rats. Cell Physiol Biochem. 2013;27:479–486. doi: 10.1159/000329969. [DOI] [PubMed] [Google Scholar]
- 15.Nagashima M, Fujikawa C, Mawatari K, Mori Y, Kato S. HSP70, the earliest-induced gene in the zebrafish retina during optic nerve regeneration: its role in cell survival. Neurochem Int. 2011;58:888–895. doi: 10.1016/j.neuint.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 16.O’Reilly AM, Currie RW, Clarke DB. HspB1 (Hsp 27) expression and neuroprotection in the retina. Mol Neurobiol. 2010;42:124–132. doi: 10.1007/s12035-010-8143-3. [DOI] [PubMed] [Google Scholar]
- 17.Ortega E, Bote ME, Besedovsky HO, del Rey A. Hsp72, inflammation, and aging: causes, consequences, and perspectives. Ann N Y Acad Sci. 2012;1261:64–71. doi: 10.1111/j.1749-6632.2012.06619.x. [DOI] [PubMed] [Google Scholar]
- 18.Pereira AC, Leite FG, Brasil BS, Soares-Martins JA, Torres AA, Pimenta PF, Souto-Padrón T, Traktman P, Ferreira PC, Kroon EG, Bonjardim CA. A vaccinia virus-driven interplay between the MKK4/7-JNK1/2 pathway and cytoskeleton reorganization. J Virol. 2012;86:172–184. doi: 10.1128/JVI.05638-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qing G, Duan X, Jiang Y. Induction of heat shock protein 72 in RGCs of rat acute glaucoma model after heat stress or zinc administration. Yan Ke Xue Bao. 2004;20:30–33. 51. [PubMed] [Google Scholar]
- 20.Sohn S, Im JE, Kim TE, Kee C. Effect of heat shock protein 72 expression on etoposide-induced cell death of rat retinal ganglion cells. Korean J Ophthalmol. 2010;27:48–51. doi: 10.3341/kjo.2013.27.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka K, Mizushima T. Protective role of HSF1 and HSP70 against gastrointestinal diseases. Int J Hyperthermia. 2009;25:668–676. doi: 10.3109/02656730903213366. [DOI] [PubMed] [Google Scholar]
- 22.Urbak L, Vorum H. Heat shock proteins in the human eye. Int J Proteomics 2010. 2010:479571. doi: 10.1155/2010/479571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Windisch BK, LeVatte TL, Archibald ML, Chauhan BC. Induction of heat shock proteins 27 and 72 in retinal ganglion cells after acute pressure-induced ischaemia. Clin Exp Ophthalmol. 2009;37:299–307. doi: 10.1111/j.1442-9071.2009.02032.x. [DOI] [PubMed] [Google Scholar]
- 24.Yamashima T. Hsp70.1 and related lysosomal factors for necrotic neuronal death. J Neurochem. 2012;120:477–494. doi: 10.1111/j.1471-4159.2011.07596.x. [DOI] [PubMed] [Google Scholar]
- 25.Yao C, Purwanti N, Karabasil MR, Azlina A, Javkhlan P, Hasegawa T, Akamatsu T, Hosoi T, Ozawa K, Hosoi K. Potential down-regulation of salivary gland AQP5 by LPS via cross-coupling of NF-kappaB and p-c-Jun/c-Fos. Am J Pathol. 2010;177:724–734. doi: 10.2353/ajpath.2010.090282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao S, Peng M, Zhu X, Cheng M, Qi X. Heat shock protein 72 protects hippocampal neurons from apoptosis induced by chronic psychological stress. Int J Neurosci. 2007;117:1551–1564. doi: 10.1080/00207450701239285. [DOI] [PubMed] [Google Scholar]
- 27.You H, Padmashali RM, Ranganathan A, Lei P, Girnius N, Davis RJ, Andreadis ST. JNK regulates compliance-induced adherens junctions formation in epithelial cells and tissues. J Cell Sci. 2013;126:2718–2729. doi: 10.1242/jcs.122903. [DOI] [PubMed] [Google Scholar]
- 28.Zhong YS, Wang J, Liu WM, Zhu YH. Potassium ion channels in retinal ganglion cells (review) Mol Med Rep. 2013;8:311–319. doi: 10.3892/mmr.2013.1508. [DOI] [PubMed] [Google Scholar]


