Abstract
Background
The Totaled Health Risks in Vascular Events (THRIVE) score strongly predicts clinical outcome, mortality, and risk of thrombolytic haemorrhage in ischemic stroke patients, and performs similarly well in patients receiving intravenous tissue plasminogen activator, endovascular stroke treatment, or no acute treatment. It is not known if the THRIVE score predicts outcomes with the Solitaire endovascular stroke treatment device.
Aims
To validate the relationship between the THRIVE score and outcomes after treatment with the Solitaire endovascular stroke treatment device.
Methods
The study conducted a retrospective analysis of the prospective SWIFT and STAR trials to examine the relationship between THRIVE and outcomes after treatment with the Solitaire device. We examined the relationship between THRIVE and clinical outcomes (good outcome or death at 90 days) among patients in SWIFT and STAR. Receiver–operator characteristics curve analysis was used to compare THRIVE score performance with other stroke prediction scores. Multivariable modeling was used to confirm the independence of the THRIVE score from procedure-specific predictors (successful recanalization or device used) and other predictors of functional outcome.
Results
The THRIVE score strongly predicts good outcome and death among patients treated with the Solitaire device in SWIFT and STAR (Mantel-Haenszel chi-square test for trend P < 0·001 for good outcome, P = 0·01 for death). In receiver–operator characteristics (ROC) curve comparisons, totaled health risks in vascular events score is superior to Stroke Prognostication using Age and NIH Stroke Scale score-100 (P < 0·001) and performed similarly to Houston Intra-Arterial Therapy score (HIAT) (P = 0·98) and HIAT-2 (P = 0·54). In multivariable models, THRIVE's prediction of good outcome is not altered after controlling for recanalization or after controlling for device used. The THRIVE score remains a strong independent predictor after controlling for the above predictors together with time to procedure, rate of symptomatic haemorrhage, and use of general anesthesia. Of note, use of general anesthesia was not an independent predictor of outcome in SWIFT + STAR after controlling for totaled health risks in vascular events and other factors.
Conclusions
The THRIVE score strongly predicts clinical outcome and mortality in patients treated with the Solitaire device in the SWIFT and STAR trials. The lack of interaction between THRIVE and procedure-specific elements such as vessel recanalization or device choice makes the THRIVE score a reasonable candidate for use as a patient selection criterion in stroke clinical trials.
Keywords: acute, acute stroke therapy, cerebral infarction, ischemic stroke, reperfusion, treatment
Introduction
Several ischemic stroke outcome scores have been developed to predict outcomes among patients undergoing endovascular stroke treatment (EST) (1–3). The THRIVE score was originally developed in the context of EST, using data from the MERCI and Multi-MERCI trials (2). THRIVE has since been validated in EST using data from the Merci Registry (4) and using data from the TREVO-2 trial (5). We have also found that the THRIVE score performs equally well in patients receiving tissue plasminogen activator (tPA) or no acute stroke treatment, using data from the NINDS tPA trial (6) and the Virtual International Stroke Trials Archive (7).
We have found that the THRIVE score strongly predicts outcomes in the TREVO-2 trial, a randomized controlled trial (RCT) comparing the Merci device to the Trevo device, a type of retrievable stent (5). Two retrievable stent EST devices (the Trevo device and Solitaire device) are now available, with each device having been found to be superior to a previous generation device (the Merci device) for both recanalization and clinical outcomes in two RCTs (8,9).
Here, we examine the utility of the THRIVE score in patients undergoing EST with the Solitaire device in two recently published clinical trials, the SWIFT trial (9) and the STAR trial (10). We examine the relationship between THRIVE and outcomes in SWIFT and STAR, and then control for recanalization success and for treatment assignment (Solitaire vs. Merci device) in the SWIFT RCT. We then compare the performance of the THRIVE score with other clinical prediction scores in the context of the Solitaire device in acute ischemic stroke.
Aims
We sought to validate the relationship between the THRIVE score and outcomes after treatment with the Solitaire EST device.
Methods
Data source and subjects
We obtained demographic data, clinical data, three-month functional outcomes on the modified Rankin Scale (mRS), and three-month mortality from the SWIFT trial and the STAR trial (9,10). SWIFT was a 146-patient trial of EST for acute ischemic stroke with two components: a run-in phase with 32 patients nonrandomly allocated to treatment with the Solitaire EST device and an RCT component with 58 patients randomized to the Solitaire device and 56 patients randomized to the Merci device, as previously described in detail (9). The STAR trial was a prospective, single-arm trial of 202 acute stroke patients treated with the Solitaire device (10). Institutional review board and regulatory approvals were obtained by participating centers in SWIFT and STAR (9,10), and no primary data or protected health information was transmitted outside the trial databases to the investigators of this report. The SWIFT and STAR trials were both registered with ClinicalTrials.gov (registration numbers NCT01054560 and NCT01327989).
Measurements
To calculate the THRIVE score, we noted age, initial stroke severity on the National Institutes of Health Stroke Scale (NIHSS) score, and the presence or absence of hypertension (HTN), diabetes mellitus (DM), or atrial fibrillation (AF). The THRIVE score assigns 1 point for age 60–79 years, 2 points for age ≥ 80 years, 2 points for NIHSS score 11–20, 4 points for NIHSS score ≥ 21, and 1 point each for HTN, DM, and AF (2). Other clinical prediction scores [Houston Intra-Arterial Therapy score (HIAT), HIAT-2, and Stroke Prognostication using Age and NIH Stroke Scale score (SPAN)-100] were calculated as follows. HIAT assigns 1 point each for age > 75, NIHSS > 18, and glucose > 150 mg/dL (1). HIAT-2 assigns 2 points for age 60–79, 4 points for age ≥ 80 years, 1 point for NIHSS score 11–20, 2 points for NIHSS score ≥ 21, and 3 points for Alberta Stroke Program Early Computed Tomography Score of ≤7·3 The SPAN-100 is a single point score – the score is positive if the sum of the patient’s age and NIHSS is > 100 (11).
Outcome measures were functional outcome on the mRS at three-months (with good outcome defined as mRS = 0–2 and poor outcome defined as mRS = 3–6) and mortality by three-months. Successful recanalization was defined as a Thrombolysis in Cerebral Ischemia (TICI) score of 2b or 3 at the completion of the EST procedure.
Statistical analysis
Categorical data in contingency tables were analyzed with the Fisher’s exact test and the Mantel-Haenszel chi-square test for trend. Continuous data were analyzed with the nonparametric Wilcoxon rank-sum test. Multivariable logistic regression was performed using standard techniques to model good outcome (mRS 0–2). Multivariable generalized ordinal logistic regression was performed using standard techniques to model the full range of the mRS, trichotomized as good outcome (mRS 0–2) vs. intermediate outcome (mRS 3–4) vs. poor outcome (mRS 5–6). In generalized ordinal logistic regression, predictors not meeting the proportional odds assumption have two different odds ratios presented for each of the two outcome transitions, while predictors meeting the proportional odds assumption have the same odds ratio for both. Receiver–operator characteristics (ROC) curves were constructed by plotting test sensitivity against (1 –specificity). We compared pairwise score discrimination for three-month outcomes by comparing the area under the curve (AUC) for ROC curves using the chi-square statistic. In cases in which a particular score could only be calculated for a subset of patients in the total dataset, only that subset of patients was used to perform the pairwise ROC curve comparison. All statistical analyses were performed using sas version 9·2 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Patient characteristics are summarized in Table 1, which displays patient age, NIHSS, comorbidities (HTN, DM, and AF), and clinical prediction scores (THRIVE, HIAT, HIAT-2, and SPAN-100) according to each of three categories: all patients treated with the Solitaire device in SWIFT and STAR (n = 291), patients randomized to the Merci device in SWIFT (n = 58), and patients randomized to the Solitaire device in SWIFT (n = 55).
Table 1.
Patient characteristics
| All Solitaire SWIFT + STAR (n = 291) |
SWIFT randomized to Solitaire (n = 58) |
SWIFT randomized to Merci (n = 55) |
P value (Merci vs. Solitaire) |
|
|---|---|---|---|---|
| Age | 67·8 ± 12·6 | 67·1 ± 12·0 | 67·1 ± 12·1 | 0·99 |
| NIHSS | 17 (13–20) | 18 (14–19) | 18 (13–22) | 0·67 |
| HTN | 63·9% (186/291) | 72·4% (42/58) | 69·1% (38/55) | 0·84 |
| DM | 18·6% (54/291) | 24·1% (14/58) | 30·9% (17/55) | 0·53 |
| AF | 39·5% (115/291) | 44·8% (26/58) | 67·3% (37/55) | 0·02 |
| Female | 58·4% (170/291) | 51·7% (30/58) | 49·1% (27/55) | 0·85 |
| IV thrombolytics | 55·3% (161/291) | 32·8% (19/58) | 47·2% (25/55) | 0·17 |
| sICH | 1·4% (4/291) | 1·7% (1/58) | 10·9% (6/55) | 0·06 |
| Proximal clot | 86·5% (244/285) | 87·9% (51/58) | 77·4% (41/53) | 0·21 |
| THRIVE score | 4 (3–6) | 4·5 (3–6) | 5 (4–6) | 0·18 |
| HIAT score | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0·94 |
| HIAT-2 score | 2 (2–4) | 2 (1–4) | 3 (2–4) | 0·25 |
| SPAN-100 | 8·1% (23/285) | 8·6% (5/58) | 7·3% (4/55) | 1·00 |
Categories in columns are All Solitaire = all patients in Solitaire run-in phase of SWIFT + all patients randomized to Solitaire in SWIFT RCT + all patients in STAR; SWIFT-Merci = all patients randomized to Merci in SWIFT RCT; SWIFT-Solitaire = all patients randomized to Solitaire in SWIFT RCT. Age is presented as mean ± SD. NIHSSand other ordinal scores are presented as median [interquartile range (IQR)]. Dichotomous values (comorbidities, gender, and SPAN-100) are presented as % (number out of total). Between the categories of SWIFT-Merci and SWIFT-Solitaire, continuous data were compared by the nonparametric Wilcoxon rank-sum test, and categorical data were compared by Fisher's exact test. IV thrombolytics = use of IV thrombolytics prior to the procedure. sICH = occurrence of symptomatic ICH within 24 h of the procedure. Proximal clot = occlusion of intracranial ICA, proximal MCA (M1 segment), basilar artery.
AF, atrial fibrillation; DM, diabetes mellitus; HIAT, Houston Intra-Arterial Therapy score; HTN, hypertension; ICA, internal carotid artery; ICH, intracerebral haemorrhage; IV, intravenous; MCA, middle cerebral artery; NIHSS, National Institutes of Health Stroke Scale; RCT, randomized controlled trial; SD, standard deviation; sICH, symptomatic intracerebral haemorrhage; SPAN, Stroke Prognostication using Age and NIH Stroke Scale score; THRIVE, totaled health risks in vascular events.
THRIVE score and clinical outcomes in Solitaire-treated patients in SWIFT and STAR
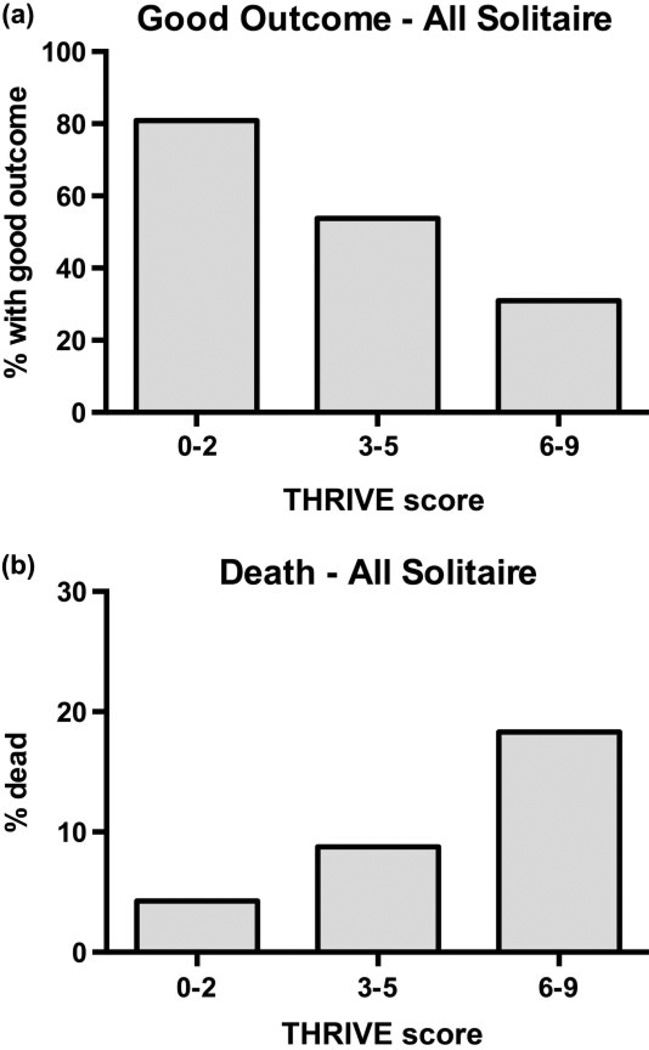
Increasing THRIVE score strongly predicts a decreasing chance of good outcome (mRS of 0–2 at three-months, Fig. 1a; P < 0·001, Mantel-Haenszel chi-square test for trend) and increased chance of death by three-months (Fig. 1b; P < 0·001, Mantel-Haenszel chi-square test for trend) among all patients treated with Solitaire in SWIFT and STAR.
Fig. 1.
Relationship between THRIVE score and outcomes among patients treated with Solitaire in SWIFT and STAR. (a) Decreasing chances of good outcome (mRS 0–2 at three-months) with increasing levels of trichotomized THRIVE score (0–2, 3–5, 6–9). The good outcomes at each level are THRIVE 0–2: 80·9% (38/47), THRIVE 3–5: 53·8% (86/160), and THRIVE 6–9: 31·0% (22/71). (P < 0·001, Mantel-Haenszel test for trend). (b) Increasing chances of death by three-months with increasing levels of trichotomized THRIVE score. The chance of death at each level is THRIVE 0–2: 4·3% (2/47), THRIVE 3–5: 8·8% (14/160), and THRIVE 6–9: 18·3% (13/71). (P < 0·001, Mantel-Haenszel test for trend). mRS, modified Rankin Scale; THRIVE, Totaled Health Risks in Vascular Events.
THRIVE score and clinical outcomes in the SWIFT RCT
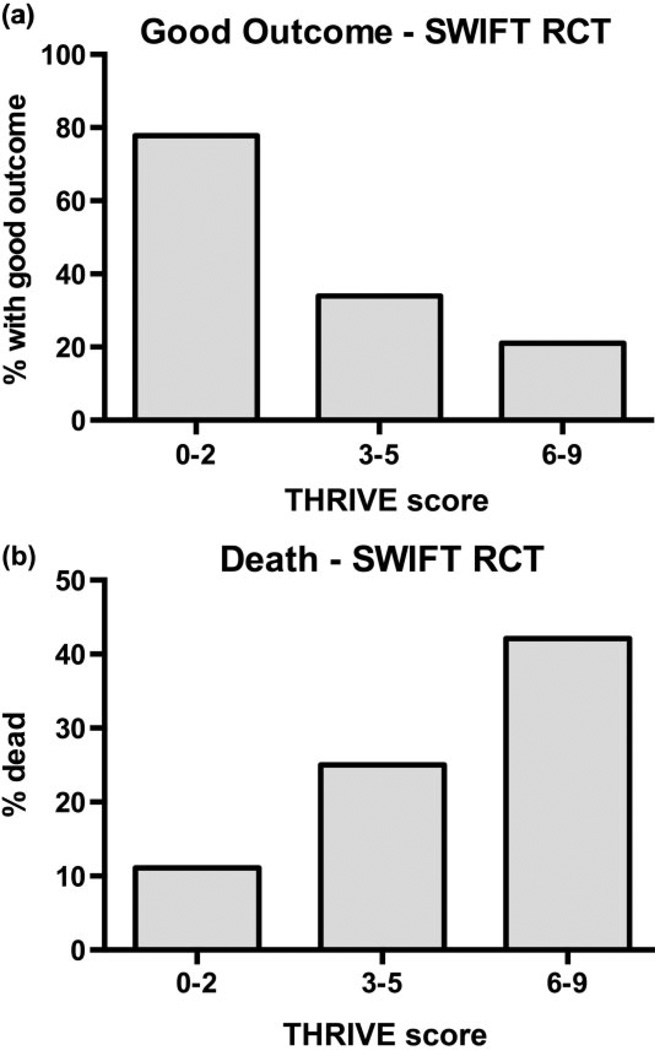
Increasing THRIVE score strongly predicts a decreasing chance of good outcome (mRS of 0–2 at 3 months, Fig. 2a; P < 0·001, Mantel-Haenszel chi-square test for trend) and increased chance of death by three-months (Fig. 2b; P < 0·001, Mantel-Haenszel chi-square test for trend) among all patients in the RCT component of SWIFT (treatment with either the Merci device or the Solitaire device).
Fig. 2.
Relationship between THRIVE score and outcomes among all patients in the SWIFT randomized controlled trial (RCT) (patients randomized to Solitaire or Merci device). (a) Decreasing chances of good outcome (mRS 0–2 at three-months) with increasing levels of trichotomized THRIVE score (0–2, 3–5, 6–9). The good outcomes at each level are THRIVE 0–2: 77·8% (7/9), THRIVE 3–5: 33·9% (19/56), and THRIVE 6–9: 21·1% (8/38). (P < 0·001, Mantel-Haenszel test for trend). (b) Increasing chances of death by three-months with increasing levels of trichotomized THRIVE score. The chance of death at each level is THRIVE 0–2: 11·1% (1/9), THRIVE 3–5: 25·0% (14/56), and THRIVE 6–9: 41·1% (16/38). (P < 0·001, Mantel-Haenszel test for trend). mRS, modified Rankin Scale; THRIVE, Totaled Health Risks in Vascular Events.
Independence of THRIVE score and recanalization or treatment assignment
We have previously found that THRIVE score predicts outcomes independent of recanalization therapy with intravenous (IV) tPA (6,7) or recanalization success with EST (2,4,5). Similarly, EST treatment assignment in the TREVO-2 RCT did not alter the relationship between THRIVE and outcome (5). In other words, the relative impact of THRIVE score on outcomes is not modified by successful recanalization during EST or the type of recanalization therapy used, and the relative impact of recanalization therapy on clinical outcomes is not modified by the patient’s THRIVE score (2,4,5).
Among patients treated with the Solitaire device in SWIFT and STAR, THRIVE score and recanalization are similarly independent and without interaction (Table 2). Controlling for successful recanalization does not alter the relationship between THRIVE score and good outcome (Table 2, Models 1 and 2). The treatment assignment (Solitaire device vs. Merci device) in the SWIFT RCT also does not interact with the relationship between THRIVE score and outcome. Controlling for treatment arm (Solitaire device vs. Merci device) does not alter the relationship between THRIVE score and good outcome (Table 2, Models 3 and 4).
Table 2.
Logistic regression: independence of THRIVE score from recanalization or device
| Odds ratio |
95% CI | P value | |
|---|---|---|---|
| Model 1: good outcome (all Solitaire) | |||
| THRIVE score | 0·67 | 0·58–0·79 | <0·001 |
| Model 2: good outcome (all Solitaire) | |||
| THRIVE score | 0·68 | 0·58–0·80 | <0·001 |
| Successful recanalization (TICI 2b/3) | 3·1 | 1·54–6·10 | 0·001 |
| Model 3: good outcome (SWIFT RCT) | |||
| THRIVE score | 0·69 | 0·53–0·90 | 0·006 |
| Model 4: good outcome (SWIFT RCT) | |||
| THRIVE score | 0·63 | 0·50–0·79 | 0·007 |
| Randomized to Solitaire | 1·18 | 0·49–2·82 | 0·71 |
All models in the table are logistic regression models of good outcome (mRS 0–2) at 90 days.
In Model 1, THRIVE score predicts good outcome in all patients treated with the Solitaire device (SWIFT + STAR).
In Model 2, THRIVE score and successful recanalization predict good outcome in all patients treated with the Solitaire device (SWIFT + STAR). Addition of successful recanalization to the model does not alter the relationship of THRIVE score and outcome (Model 2 compared with Model 1).
In Model 3, THRIVE score predicts good outcome in all patients in the SWIFT RCT.
In Model 4, THRIVE score and device assignment (randomization to Solitaire) predict good outcome in all patients in the SWIFT RCT. Addition of device assignment to the model does not alter the relationship of THRIVE score and outcome (Model 4 compared with Model 3). Addition of device assignment to the model does not alter the relationship of THRIVE score and outcome (Model 4 compared with Model 3).
CI, confidence interval; mRS, modified Rankin Scale; RCT, randomized controlled trial; THRIVE, Totaled Health Risks in Vascular Events; TICI, Thrombolysis in Cerebral Ischemia.
ROC curve analysis comparing THRIVE with other outcome prediction scores
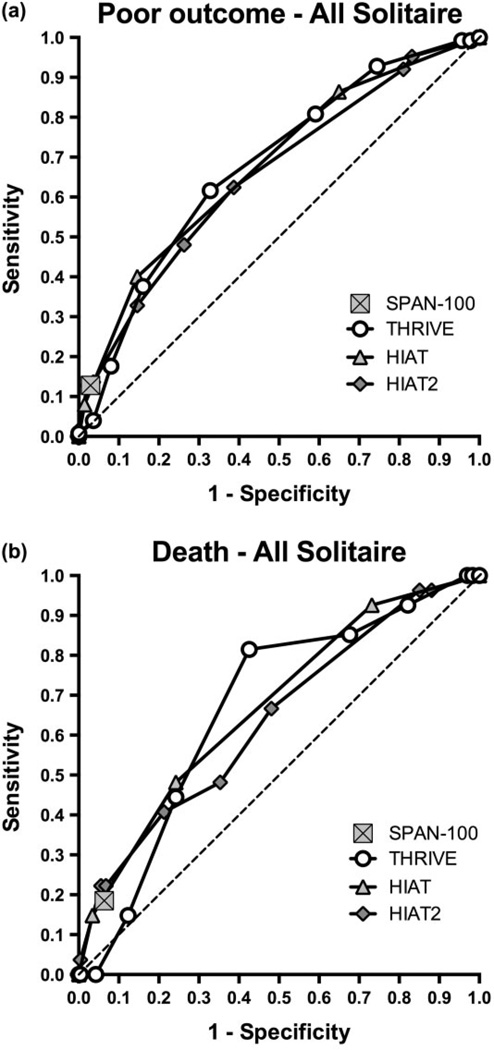
To better understand the relative utility of available ischemic stroke outcome prediction scores in the context of current generation EST devices, we used ROC curve analysis to compare the THRIVE score with other scores for which sufficient data were available for Solitaire-treated patients in SWIFT and STAR (HIAT, HIAT-2, and SPAN-100).
The ROC curves for the THRIVE score has a similar AUC in comparison with HIAT and HIAT-2 and is superior to the SPAN-100 (Fig. 3a,b). For prediction of poor outcome at three-months (mRS 3–6), the THRIVE ROC AUC is 0·68, compared with 0·68 for HIAT (P = 0·98), 0·66 for HIAT-2 (P = 0·54), and 0·55 for SPAN-100 (P < 0·001). For prediction of death by three-months, the THRIVE ROC AUC is 0·67 compared with 0·67 for HIAT (P = 0·94), 0·64 for HIAT-2 (P = 0·61), and 0·56 for SPAN-100 (P < 0·04). Similar results are found when the slightly different cut point for poor outcome used in the validation of HIAT (1) and HIAT-2 (3) is tested (mRS 4–6; Supporting Information Fig. S1).
Fig. 3.
Comparison of THRIVE to other outcome prediction scores by receiver–operator characteristics (ROC) curve analysis. (a) ROC curves for score prediction of poor outcome at three-months (mRS 3–6), comparing THRIVE, HIAT, HIAT2, and SPAN-100. SPAN-100 is represented by a single point because it is a dichotomous predictor. (b) ROC curves for score prediction of death by three-months (mRS 0–2), comparing THRIVE, HIAT, HIAT2, and SPAN-100. HIAT, Houston Intra-Arterial Therapy score; mRS, modified Rankin Scale; SPAN, Stroke Prognostication using Age and NIH Stroke Scale score; THRIVE, Totaled Health Risks in Vascular Events.
Modeling of multiple outcome predictors in SWIFT + STAR
To examine the relative impact of several known predictors of outcome (including THRIVE score, time to procedure, device used, successful recanalization, symptomatic intracerebral haemorrhage (sICH), and use of general anesthesia) on outcomes, we performed generalized ordinal logistic regression modeling the full range of the mRS in the complete SWIFT + STAR dataset. Because of significant interaction between the device used (Solitaire vs. Merci) and rate of successful recanalization (TICI 2b/3), two models are presented, one with device used and the other with successful recanalization (Table 3). Each predictor was found to independently predict outcome, with the notable exception of general anesthesia, which did not predict outcome in either model (Table 3). Of note, patients treated with the Solitaire device in the SWIFT + STAR trials were about twice as likely to have successful recanalization [221/271 (81·5%)] than patients treated with the Merci device [22/53 (41·5%)] (P < 0·001).
Table 3.
Generalized ordinal logistic regression: predictors of outcome in SWIFT + STAR
| Model 1 | Good outcome (mRS 0–2) |
Good to intermediate outcome (mRS 0–4) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | Coefficient | SE | P value | OR | 95% CI | Coefficient | SE | P value | |
| THRIVE score | 0·70 | 0·61–0·81 | −0·35 | 0·07 | <0·001 | 0·70* | 0·61–0·81 | −0·35 | 0·07 | <0·001 |
| Time to treatment (hours) | 0·67 | 0·56–0·80 | −0·40 | 0·09 | <0·001 | 0·87 | 0·71–1·06 | −0·14 | 0·10 | 0·18 |
| Treatment with Solitaire device |
1·4 | 0·65–3·15 | 0·36 | 0·40 | 0·37 | 4·81 | 2·22–10·4 | 1·57 | 0·39 | <0·001 |
| sICH | 0·07 | 0·01–0·43 | −2·60 | 0·89 | 0·004 | 0·07* | 0·01–0·43 | −2·60 | 0·89 | 0·004 |
| General anesthesia | 0·85 | 0·52–1·38 | −0·16 | 0·25 | 0·51 | 0·85* | 0·52–1·38 | −0·16 | 0·25 | 0·51 |
| Model 2 | Good outcome (mRS 0–2) |
Good to intermediate outcome (mRS 0–4) |
||||||||
| OR | 95% CI | Coefficient | SE | P value | OR | 95% CI | Coefficient | SE | P value | |
| THRIVE score | 0·69 | 0·60–0·80 | −0·37 | 0·07 | <0·001 | 0·69* | 0·60–0·80 | −0·37 | 0·07 | <0·001 |
| Time to treatment (hours) | 0·71 | 0·60–0·83 | −0·34 | 0·08 | <0·001 | 0·71* | 0·60–0·83 | −0·34 | 0·08 | <0·001 |
| Recanalization (TICI 2b/3) | 2·5 | 1·49–4·43 | 0·94 | 0·28 | <0·001 | 2·5* | 1·49–4·43 | 0·94 | 0·28 | <0·001 |
| sICH | 0·05 | 0·01–0·29 | −2·99 | 0·89 | <0·001 | 0·05* | 0·01–0·29 | −2·99 | 0·89 | <0·001 |
| General anesthesia | 0·80 | 0·49–1·31 | −0·22 | 0·25 | 0·38 | 0·80* | 0·49–1·31 | −0·22 | 0·25 | 0·38 |
Generalized ordinal logistic regression models showing the relative impact of predictors on outcome across the range of the mRS, trichotomized as good outcome (0–2) vs. intermediate outcome (3–4) vs. poor outcome (5–6). Odds ratios above 1 (with positive coefficients) represent a predictor that is associated with improved odds of a better outcome, and odds ratios below 1 (with negative coefficients) represent a predictor that is associated with reduced odds of a better outcome.
In each model, odds ratios are shown for good outcome (mRS 0–2, on the left) and for good to intermediate outcome (mRS 0–4, on the right). For predictors meeting the proportional odds assumption for ordinal logistic regression, the same odds ratio value is presented for both outcome levels, indicated by an asterisk. For predictors failing to meet the proportional odds assumption, two different odds ratio values are presented for the two outcome levels.
In Model 1, predictors are THRIVE score, time to treatment (onset to groin puncture in hours), treatment with the Solitaire device (vs. Merci device), occurrence of symptomatic intracerebral haemorrhage (sICH), and use of general anesthesia (vs. monitored anesthesia care without endotracheal intubation).
In Model 2, predictors are THRIVE score, time to treatment (onset to groin puncture in hours), successful recanalization (TICI 2b/3), occurrence of sICH, and use of general anesthesia (vs. monitored anesthesia care without endotracheal intubation).
Two separate models are shown because of significant interaction between use of the Solitaire device and the rate of successful recanalization. Patients treated with the Solitaire device were significantly more likely to have successful recanalization [(221/271 (81·5%)] than patients treated with the Merci device [22/53 (41·5%)] (P < 0·001).
CI, confidence interval; mRS, modified Rankin Scale; OR, odds ratio; SE, standard error; THRIVE, Totaled Health Risks in Vascular Events; TICI, Thrombolysis in Cerebral Ischemia.
Discussion
We find that the THRIVE score strongly predicts clinical outcomes among acute stroke patients treated with a third generation EST device (the Solitaire device) in the SWIFT and STAR trials. The performance of the THRIVE score in the TREVO-2 trial is similar to or superior to other predictive scores (HIAT, HIAT-2, and SPAN-100).
The THRIVE score has been serially validated with similar robust performance across all acute ischemic stroke treatment contexts: IV tPA treatment (6,7), EST (2,4,5), and no acute stroke treatment (6,7). In each of these contexts, including the present study, the THRIVE score has been found to predict outcomes independent of recanalization therapy, such that there is no interaction between the relationship between THRIVE and outcomes and the relationship between recanalization therapy and outcomes. It is likely that this consistent lack of interaction arises from the fact that the THRIVE score is composed of a set of non-modifiable predictors of outcome: the patient’s age, the clinical severity of the stroke itself (NIHSS), and three medical comorbidities (HTN, DM, and AF).
The lack of interaction between THRIVE and recanalization/ device used has important implications for the use of THRIVE as a potential a priori patient screening tool for participation in clinical trials. Various inclusion/exclusion criteria have been used in EST trials to attempt to enroll only patients with an anticipated potential to benefit from recanalization therapy with an acceptable risk profile. One proposed patient selection approach is the use of advanced neuroimaging to determine the balance of ischemic penumbra to the territory at risk (12). While advanced neuroimaging of magnetic resonance imaging diffusion and perfusion maps are predictive of final infarct volume (12), initial attempts to use such techniques for RCT patient selection have not yet led to positive results (13). Because a high THRIVE score (6 to 9) has been consistently found to predict poor outcomes in EST (2,4,5) and THRIVE consistently predicts outcomes in a fashion that does not interact with the relationship between recanalization and outcome, this means that at high THRIVE scores, the relative positive impact of successful recanalization only minimally impacts the absolute rate of good outcomes for such patients. It is therefore likely, based on these data, that screening using the easily determined THRIVE score in EST clinical trials should enrich such a trial’s population for patients with a better chance of a good outcome after EST.
While the THRIVE score has been found to predict the risk of haemorrhage after tPA (6,7), we have not found evidence for a similar relationship between THRIVE and sICH in the context of EST (5). However, because of the very low rate of sICH in both SWIFT and STAR (1·4%), we were not able to explore the relationship between THRIVE and sICH in the setting of Solitaire device use.
The THRIVE score continued to be a strong independent predictor of outcomes in well-specified models including other known predictors of outcome such as time to procedure, successful recanalization (TICI 2b/3), sICH, and use of general anesthesia (Table 3). Of note, although other observational studies have suggested an association between general anesthesia and poor outcome (14), we do not find such an association in the SWIFT + STAR trials after controlling for THRIVE score, time, successful recanalization, and sICH.
In summary, the THRIVE score is an easy-to-use predictive score to assess post-stroke functional outcome and mortality in EST, and has now been validated in the context of both clinically available retrievable stent devices, the Solitaire device and the Trevo device. The THRIVE score is in the public domain (Creative Commons license) – free web calculators are provided at http://www.thrivescore.org and http://www.mdcalc.com/thrive-score-for-stroke-outcome/
Supplementary Material
Acknowledgment
The authors thank Dr Scott Brown of Covidien, Plymouth, MN, for his extensive statistical work for this study. Dr Brown had full access to all the primary data in the study, and all analyses were performed at the direction of the first author, Dr Flint, who takes responsibility for the integrity of the data and the accuracy of the data analysis. There was no funding organization or sponsor for the present analysis.
The University of California (The Regents) receive funding for J. L. Saver’s services as a scientific consultant regarding trial design and conduct from Covidien/ev3, BrainsGate, CoAxia, Grifols/ Talecris, Ferrer, Mitsubishi, Genervon, Benechill, Asubio, and Sygnis. J. L. Saver is an investigator in the NIH FAST-MAG, MR RESCUE, ICES, CUFFS, CLEAR-ER, and IMS 3 multicenter clinical trials for which the UC Regents receive payments based on clinical trial performance. J. L. Saver has also served as an unpaid site investigator in multicenter trials run by Covidien/ev3, Genervon, Lundbeck, and Mitsubishi for which the UC Regents received payments based on the clinical trial contracts for the number of participants enrolled. J. L. Saver and R. Jahan are employees of the University of California, which holds a patent on retriever devices for stroke. The University of California Regents receive funding for R. Jahan’s services as a scientific consultant regarding trial design and conduct from Covidien/ev3 and Chestnut Medical. E. I. Levy serves as a scientific consultant for Covidien/ev3, Codman and Shurtleff Inc, and TheraSyn Sensors Inc; and receives fees for carotid stent training from Covidien/ev3 and Abbott Vascular. T. G. Jovin has served as a scientific consultant to Covidien/ev3, CoAxia, Concentric Medical, and Micrus. R. G. Nogueira has served as a scientific consultant to Covidien/ev3, CoAxia, and Concentric Medical. V. M. Pereira was global PI for the STAR trial and serves as a consultant to Covidien. D. S. Liebeskind serves as a consultant to Stryker and Covidien. R. G. Nogueira was on the steering committee for SWIFT and the core laboratory for STAR, serves as a consultant for Covidien, serves on physician advisory boards for Stryker and Penumbra.
Footnotes
Conflict of interest: Other authors report no financial or other potential conflicts of interest of relevance to the present study.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher’s website:
Figure S1 Comparison of THRIVE to other prediction scores by ROC curve analysis using an alternate definition of poor outcome (mRS 4–6). ROC curves for score prediction of poor outcome at three-months (mRS 4–6), comparing THRIVE, HIAT, HIAT-2, and SPAN-100. HIAT, Houston Intra-Arterial Therapy score; mRS, modified Rankin Scale; ROC, receiver–operator characteristics; SPAN, Stroke Prognostication using Age and NIH Stroke Scale score; THRIVE, Totaled Health Risks in Vascular Events.
References
- 1.Hallevi H, Barreto AD, Liebeskind DS, et al. Identifying patients at high risk for poor outcome after intra-arterial therapy for acute ischemic stroke. Stroke J Cereb Circ. 2009;40:1780–1785. doi: 10.1161/STROKEAHA.108.535146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flint AC, Cullen SP, Faigeles BS, Rao VA. Predicting long-term outcome after endovascular stroke treatment: the totaled health risks in vascular events score. AJNR Am J Neuroradiol. 2010;31:1192–1196. doi: 10.3174/ajnr.A2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarraj A, Albright K, Barreto AD, et al. Optimizing prediction scores for poor outcome after intra-arterial therapy in anterior circulation acute ischemic stroke. Stroke J Cereb Circ. 2013;44:3324–3330. doi: 10.1161/STROKEAHA.113.001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flint AC, Kamel H, Rao VA, Cullen SP, Faigeles BS, Smith WS. Validation of the Totaled Heath Risks in Vascular Events (THRIVE) score for outcome prediction in endovascular stroke treatment. Int J Stroke. 2014;9:32–39. doi: 10.1111/j.1747-4949.2012.00872.x. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 5.Flint AC, Xiang B, Gupta R, et al. THRIVE score predicts outcomes with a third-generation endovascular stroke treatment device in the TREVO-2 trial. Stroke J Cereb Circ. 2013;44:3370–3375. doi: 10.1161/STROKEAHA.113.002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kamel H, Patel N, Rao VA, et al. The THRIVE score predicts ischemic stroke outcomes independent of thrombolytic therapy in the NINDS tPA trial. J Stroke Cerebrovasc Dis. 2013;22:1111–1116. doi: 10.1016/j.jstrokecerebrovasdis.2012.08.017. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 7.Flint AC, Faigeles BS, Cullen SP, et al. THRIVE score predicts ischemic stroke outcomes and thrombolytic hemorrhage risk in VISTA. Stroke J Cereb Circ. 2013;44:3365–3369. doi: 10.1161/STROKEAHA.113.002794. [DOI] [PubMed] [Google Scholar]
- 8.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 10.Pereira VM, Gralla J, Davalos A, et al. Prospective, multicenter, single-arm study of mechanical thrombectomy using solitaire flow restoration in acute ischemic stroke. Stroke. 2013;44:2802–2807. doi: 10.1161/STROKEAHA.113.001232. STROKEAHA.113.001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saposnik G, Guzik AK, Reeves M, Ovbiagele B, Johnston SC. Stroke prognostication using age and NIH stroke scale: SPAN-100. Neurology. 2013;80:21–28. doi: 10.1212/WNL.0b013e31827b1ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wheeler HM, Mlynash M, Inoue M, et al. Early diffusion-weighted imaging and perfusion-weighted imaging lesion volumes forecast final infarct size in DEFUSE 2. Stroke J Cereb Circ. 2013;44:681–685. doi: 10.1161/STROKEAHA.111.000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;368:914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta R. Local is better than general anesthesia during endovascular acute stroke interventions. Stroke. 2010;41:2718–2719. doi: 10.1161/STROKEAHA.110.596015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.