Abstract
Background
Spontaneous intracerebral hemorrhage (ICH) is a devastating disease with high morbidity and mortality. ICH lacks an effective medical or surgical treatment despite the acknowledged pathophysiological benefits of achieved hemostasis and clot removal. Image guided stereotactic endoscopic hematoma evacuation is a promising minimally invasive approach designed to limit operative injury and maximize hematoma removal.
Methods
A single center randomized controlled trial was designed to assess the safety and efficacy of stereotactic hematoma evacuation compared to best medical management. Patients were randomized within 24 hours of hemorrhage in a 3:2 fashion to best medical management plus endoscopic hematoma evacuation or best medical management alone. Data was collected to assess efficacy and safety of hematoma evacuation and to identify procedural components requiring technical improvement.
Results
10 patients have been enrolled and randomized to treatment. Six patients underwent endoscopic evacuation with a hematoma volume reduction of 80% +/−13 at 24 hours post procedure. The medical arm demonstrated a hematoma enlargement of 78% +/−142 during this same period. Rehemorrhage rates and deterioration rates were similar in the two groups. Mortality was 20% in the endoscopic group and 50% in the medical treatment cohort. The endoscopic technique was shown to be effective in identification and evacuation of hematomas while reduction in the number of endoscopic passes and maintenance of hemostasis require further study.
Conclusion
Image guided stereotactic endoscopic hematoma removal is a promising minimally invasive technique that is effective in immediate hematoma evacuation. This technique deserves further investigation to determine its role in ICH management.
Keywords: Intracerebral hemorrhage, endoscopy, minimally invasive surgery, hemorrhagic stroke
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a devastating disease with historically poor outcomes. Medical management of these patients results in unacceptable morbidity and mortality. Only 48-65% of ICH victims are alive at one-month follow up and only 10% of these patients are living independently (4-6,9). The natural course of acute ICH is not static (10). After initial irreversible tissue injury is suffered near the hemorrhage nidus, a progressive cascade of elevated local pressures, edema and excito-toxicity cause additional secondary injury to surrounding brain. (12,16,17) Much of this secondary process is thought to be attributable to the mass effect of the new hemorrhage, as well as the toxicity associated with hematoma decomposition and release of inflammatory and free radical mediators.
As a result, there has been great interest in the potential benefits of acute hematoma evacuation. In the United States, over 7,000 patients with ICH undergo evacuation procedures each year (8). This enthusiasm has been tempered by the lack of supportive clinical data. The efficacy of surgical evacuation was most recently studied in the iSTICH trial (11). This large multi-center international randomized trial evaluated the efficacy of early (<24 hour after randomization) surgical therapy versus best medical management in the treatment of spontaneous intracerebral hemorrhages. Though this study has received criticism for the subjectivity of its inclusion criteria, frequency of treatment group crossover, and diversity of surgical approaches, it remains the most powerful study of hematoma evacuation to date. This trial corroborated the results of prior smaller studies that failed to show a survival or morbidity benefit of conventional surgery.
The surprising failure of conventional hematoma evacuation may be attributable to the type of surgical approach. While standard open craniotomy is routinely effective in complete hematoma evacuation and maintenance of hemostasis, the approach commonly causes damage to uninjured brain overlying the hematoma. Minimally invasive surgical strategies have been devised to minimize this risk. The safety of image guided and frameless stereotactic procedures has been reported in numerous small trials (7,15). These approaches commonly necessitate use of thrombolytic therapy and require increased evacuation times. Furthermore, these methods are limited in their ability to achieve hemostasis and completely evacuate the hematoma.
Despite the success of early studies, endoscopic assisted evacuation of ICH has received little attention as a minimally invasive technique. The endoscopic approach has been shown to be effective in achieving immediate and complete hematoma evacuation (2,14). This technique also has the benefit of improved visualization and hemostasis with electric cautery (13). Auer and colleagues have published the most promising surgical data related to ICH evacuation (1). They have reported a significant survival benefit of endoscopic evacuation over medical therapy with hematomas greater than 50 cc. While there was no improvement in mortality with smaller lesions, patients undergoing endoscopic evacuation were more likely to have a favorable functional recovery at 6 months.
The lack of an effective surgical treatment for ICH, despite the persistence of a sound pathophysiological argument for hematoma evacuation, has led us to initiate a single center randomized controlled trial of acute endoscopic assisted hematoma evacuation. This phase two study, comparing image guided endoscopic evacuation versus aggressive medical management, was designed to assess the feasibility and potential benefit of this minimally invasive technique. The percent volume of hematoma evacuated at 24 hours was set as the primary efficacy outcome measure. The rate of rehemorrhage, deterioration of the GCS by 2 points during the intensive care unit (ICU) stay, and fall in the National Institute of Health Stroke Scale (NIHSS) by 4 points at 30 days was used to determine the safety of endoscopic evacuation. This manuscript will report the current safety and efficacy data of this on-going trial and investigate the technical challenges associated with this minimally invasive approach.
Methods
The University of California Institutional Review Board approved this study. Subjects were considered for enrollment if they presented to UCLA Medical Center within 24 hours of onset of symptoms for acute intracerebral hemorrhage. Patients were eligible for the study if they were 18 years of age or older, had an ICH volume >15 cc, and had significant neurological symptoms resulting from the acute hematoma. Patients with a Glasgow Coma Scale (GCS) <5, posterior fossa hemorrhage, ICH secondary to an ischemic infarction or large vessel rupture, coagulopathy, significant prior disability, allergy to Magnetic Resonance (MR) contrast agents, or active participation in another clinical trial were restricted from enrollment. Pregnant women were also excluded from this study. Informed consent was obtained from the patient or their legally authorized representative prior to randomization.
Patients were randomized to endoscopic and medical management or medical management in a 3:2 ratio. For all surgical patients, one mm slice Computed Tomography (CT) images were acquired and loaded into the BrainLab® frameless neuronavigational software to enable identification of image-guided burr hole location, endoscopic trajectory and depth of endoscope insertion. The patients were taken to the operative suite and underwent standard general anesthesia. The patient's head was appropriately positioned in a Mayfield headholder (Codman, Inc., Raynham, MA) and the scalp was shaved and prepared in the routine sterile fashion. Cranial landmarks were registered with the BrainLab® Navigation system and a 4 cm incision was made in the scalp, consistent with the planned endoscopic trajectory. A 1.8 cm burr hole was created utilizing a Midas Rex Instrument (Medtronic, Inc., Minneapolis, MN). The dura was then coagulated and opened and the pia was dissected to allow entrance of the endoscope sheath. The sheath was guided transcortically into the hematoma bed with stereotactic assistance. The trocar was then removed and replaced by the Frazee Ridged Endoscope® (Karl Storz Endoscopy, Inc., Culver City, CA). In each case, the hematoma was extracted utilizing a suction port on the endoscope and collected into a Luken's trap. Direct endoscopic guidance was used to ensure maximal hematoma removal. The hematoma cavity was irrigated with saline and hemostasis was inspected via endoscope.
Conventional microsurgical bipolar cautery was used as needed. Standard closure procedures were performed and a ventriculostomy catheter was left within the hematoma cavity for 48-72 hours to evacuate early recurrent bleeding.
Surgical and medically treated patients received standard neurocritical care management which included intracranial pressure management, deep venous thrombosis prophylaxis, swallowing evaluation, physical and occupational therapy consultation, prophylactic anticonvulsant treatment, and routine chemistries. Mean arterial pressure goals were targeted at 70-100 mm Hg. Neurological examination was obtained at baseline, 8 hours post-randomization, 24 hours, and at day 30 and day 90. National Institute of Health Stroke Scale (NIHSS), Glasgow Coma Score (GCS), Modified Rankin Scale (mRS), and Barthel Index (BI) measures were completed. CT scans were obtained at 24 hours, 72 hours, and on day 30 and ICH volumes were measured. Hematoma volumes were determined using the L X W X H divided by 2 formula (3) and confirmed by planar volumetry using the volumetric CT images.
Rebleeding rates, hematoma expansion, neurological worsening within the first 72 hours and conversion of treatment to open surgery were used to track the safety of each treatment. Patients whose hematoma expanded >33% from baseline measurements were considered to have rebled or expanded. Neurological worsening was defined as a 2-point or greater decline in the GCS or a 4-point or greater worsening of the NIHSS. Treatment efficacy was determined by the volume reduction in hematoma size between 8 and 24 hours and the percentage of patients with a 90 day mRS less than or equal to two. Change in NIHSS between admission and day 90 was also used to assess clinical outcome.
For those patients randomized to surgery, six technical aims were individually scored for each endoscopic evacuation. Three aims were assessed using a qualitative score of satisfactory, needs improvement, or failed:
Was the hematoma easily localized with the stereotactic frameless approach?
Was the hematoma easily evacuated utilizing endoscopic suction removal?
Was hemostasis easily achieved endoscopically?
Three procedural aims were assessed quantitatively:
Was the hematoma entered with the first endoscopic pass?
How many endoscopic passes were required for maximal hematoma evacuation?
What portion of the hematoma was removed?
Results
Thirty-four total patients were screened for enrollment. Twenty-four patients did not meet inclusion criteria with stroke sub-type (6), lesion size (4), and preclusive admission GCS (4) being the most common limiting factors. Three patients were not enrolled due to refusal of consent by the legally authorized patient representative. One additional patient was randomized to the surgical arm (case 6) and required emergent conventional craniotomy as a result of brain herniation and clinical deterioration. This case is subsequently illustrated, but was not included in the statistical analysis of the endoscopic arm. Of the 10 enrolled patients, four were allocated to the medical treatment arm. The remaining six surgical patients are illustrated below. No patients underwent craniotomy after endoscopic evacuation. The demographic data for the medical and endoscopic treatment arms are shown in Table 1.
Table 1. Characteristics of Medical and Endoscopic Treatment Groups.
Demographic data for Medical and Endoscopic cohorts. IPH volume and time to enrollment/surgery data includes standard deviations.
| Medical Treatment | Endoscopy | |
|---|---|---|
| Age (years) (range) | 50 (37–59) | 65 (46–86) |
| Initial ICH volume (cc) | 35.5 +/−13.5 | 56.4 +/−30.7 |
| % IPH in subcortical location | 100% | 80% |
| Time to enrollment (hrs) | 17 +/−12 | N/A |
| Time to surgery (hrs) | N/A | 18 +/−9 |
| Sex - % male | 75% | 100% |
| % of patients with IVH | 50% | 67% |
ICH=intracerebral hemorrhage, cc = cubic centimeter, hrs = hours, IVH = intraventricular hemorrhage.
The safety and clinical efficacy for the two groups is shown in Table 2. Neither group had a patient who achieved the pre-specified outcome of mRS less than or equal to two. Both groups had one patient with a re-hemorrhage that resulted in a correlative decline in the NIHSS. One patient in the medical group (25%) had hemorrhagic expansion that met the pre-defined criteria. One patient (20%) in the surgical group had post-operative bleeding. This patient also experienced pre-operative bleeding. Excluding this patient, the average percent reduction in hematoma size was 80 % +/−13. The average change in hematoma size in the medical group showed an enlargement of 78% +/−142. This result was inflated by a single large hemorrhagic expansion, however, two of four medically treated patients did experience detectable hematoma enlargement (>33%). The mortality rates were 50% in the medical group and 20% in the endoscopic group. Despite disappointing 90 days mRS and BI averages, the 30 post-bleed day NIHSS average appeared to improve. The current study does not contain statistical power to comment on the significance of this improvement.
Table 2. Clinical Outcomes for Medical and Endoscopic Treatment.
Comparison of safety and clinical outcomes between medical and endoscopic cohorts. GCS decline is defined as a drop in GCS of 2 or greater points during the first 24 hours of hospitalization. NIHSS decline is defined as a drop in NIHSS by 4 or greater points during the first 24 hours of hospitalization. The % mean hematoma reduction is defined as the mean reduction in hematoma size between the pre and post operative CT scans. Data from a single patient in the surgical group who experienced massive hematoma expansion before endoscopy was not included in this analysis. Hematoma enlargement of >33% was required to meet criteria for rebleed/expansion.
| Medical | Endoscopic | |
|---|---|---|
| Admit GCS | 11+/−2 | 7 +/−1 |
| % with GCS decline | 0 | 40% |
| Mean admit NIHSS | 21+/−6 | 25+/−7 |
| 30 day NIHSS | 18+/−11 | 18+/−8 |
| % with NIHSS decline | 25% | 20% |
| % mRS <=2 at day 90 | 0 % | 0 % |
| Mean mRS at day 90 | 4.5 +/−1 | 4.6 +/−1 |
| Mean BI at day 90 | 25 +/−47 | 8 +/−18 |
| % mean ICH volume change | + 78%+/−142 | -80% +/−13 |
| % rebleed or expansion | 25% | 20% |
| 90 day mortality | 50% | 20% |
GCS = Glasgow Coma Scale, NIHSS = National Institute of Heath Stroke Scale, mRS = modified Rankin Score, BI = Barthel Index.
The scoring of the six separate technical aims was assessed by the surgeon for each of the cases. These results are summarized in Table 1. All hematomas were able to be accessed on the first pass with image guided stereotactic guidance. Maximal hematoma evacuation required an additional endoscopic pass for cases 1 and 2. Orientation of the endoscopic trajectory along the long axis of the hematoma was associated with improved efficiency of evacuation and a reduced number of required passes. An average of 80 % +/−13 of the hematoma was removed as determined by comparison of the pre-operative and 24 hour post-endoscopic CT scans. The CT guidance endoscopic system was used with considerable ease in all patients. Hematoma evacuation by endoscopic suction was considered satisfactory in 80% of the patients. Hemostasis was satisfactory in 3 of the 5 patients. One patient (case 1) had massive rebleeding while another (case 2) required additional blood products to achieve hemostasis.
This randomized study of minimally invasive hematoma evacuation was aborted prior to completion. Slow subject enrollment limited the study's capacity to address the designated specific aims. Furthermore, the investigators recognized the need for a single arm study of surgical optimization prior before initiating a comparative study with medically treated controls.
Discussion
In this study, we demonstrated that image guided endoscopic evacuation of primary intracerebral hemorrhage is feasible and safe. The primary results were: 1) The mean reduction in hematoma volume was 80 ±13%. 2) The 90-day mortality rate in the surgical group was less than ½ of that in the medical management group. 3) Endoscopic evacuation can be accomplished safely with minimal risk of clinical deterioration or hemorrhagic expansion. 4) Technical efficacy was assessed and needed improvements in selected technical features, including hemostasis and number of endoscopic passes. These results suggest to us that while promising, endoscopic surgery should undergo further technical refinement prior to embarking on a large phase 3 randomized controlled study. For these reasons, enrollment was suspended and this summary of data was provided.
The percentage reduction in hematoma volume compares favorably with open surgical evacuation in previously completed trials. This reduction was accomplished with a minimal number of endoscopic passes. Only two of the five patients required multiple passes for optimal evacuation. While these observations support the minimally invasive intent of the study, improved efficiency of evacuation can be expected with additional modification of this technique. Orientation of the endoscopic trajectory with the long axis of the hematoma appeared to result in fewer passes. This approach to the hematoma cavity will be prioritized in future endoscopic evacuations. Despite the difficulties encountered, our percentage of hematoma evacuated is similar to that reported by Auer. The percentage reduction in hematoma was not dependent upon hemorrhage location.
The incidence of rebleeding was difficult to assess given the small number of surgical patients. However, a favorable comparison can be made between our population and that studied by Morgenstern and Mendelow. The role of adjunctive hemostatic agents, such as 1-deamino-8-D-arginine vasopressin (DDAVP) and platelet transfusion, has yet to be defined in the endoscopic technique or other surgical approaches to hematoma evacuation. Our future study will aim to assess the effect of anti-platelet and anticoagulant medications upon initial hemostasis and clot expansion after surgery. Additionally, the pre-surgical management of these risks will be standardized. Hemostatic pledgets were used infrequently in the surgical cohort to assist control of bleeding. Whether these sponges may serve a more routine role in hemostasis deserves further study. Finally, while hemorrhagic expansion after ICH is known to commonly occur, the impact of surgical timing upon post-operative expansion will need to be further evaluated as our enrollment numbers increase.
Endoscopy holds promise as a therapy for acute intraparenchymal hemorrhage due to its ability to remove a large portion of the hemorrhage immediately, achieve hemostasis, and minimize perturbation of surrounding brain. These preliminary results add to previous literature supporting the concept that endoscopy can be safely performed in critically ill patients with large hematomas. With the recent failure of a large randomized study of conventional surgery to demonstrate improved clinical outcomes, minimally invasive techniques provide us with the greatest hope of reducing secondary deterioration after the sentinel hemorrhage. Refinement of surgical technique will need to be explored in small preliminary studies such as this one. Utilization of transparent introducer sheaths, adaptation of multifunctional endoscopes, and intra-operative neuro-imaging are just a few of the recent advances designed to improve the surgical outcome. Similarly, the volume of evacuation that optimizes clinical recovery and minimizes surgical risk is currently not known. It has been assumed that more complete evacuation of hematomas is preferred, though this notion is not supported by existing data. While the future of the minimally invasive approach remains unclear, an accepted sentiment persists regarding the inadequacy of current medical therapies and conservative management. We intend to adapt the lessons learned from this study to a revised single-arm protocol aimed at refining these surgical techniques. This new protocol will incorporate a standardized approach to hemostasis, emphasize the minimally invasive attributes of endoscopy, and better assess the complicating aspects of ICH care. It is our hope that this on-going project will bring us closer to randomized testing of an alternative treatment for spontaneous intraparenchymal hematomas.
Figure 1a. Brain CT scans of pre and post endoscopic hematoma evacuation.
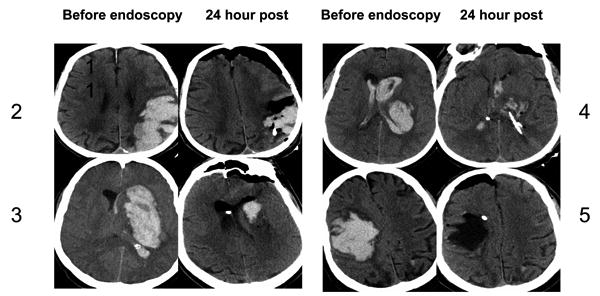
Non-contrast head computed tomography scan obtained immediately before and 24 hours after endoscopic evacuation of hematoma. Cases 2-5 shown.
Figure 1b. Head CT of patients in endoscopic arm suffering hemorrhagic expansion.
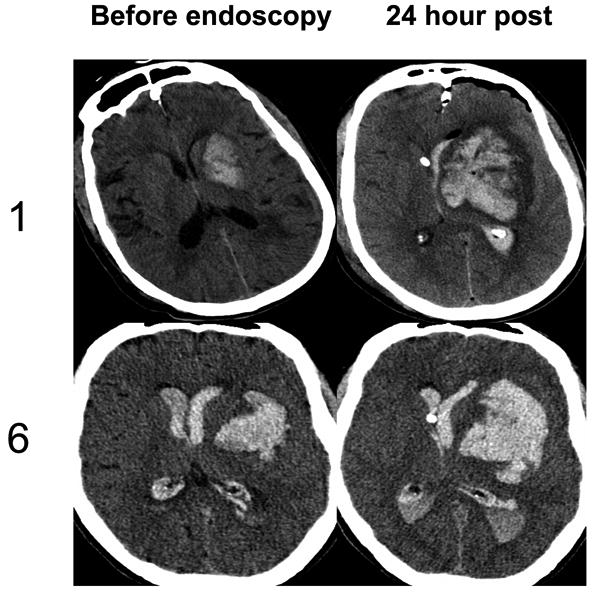
Non-contrast head computed tomography scan obtained immediately before and 24 hours after endoscopic evacuation of hematoma. Case 1 suffered hemorrhagic expansion after endoscopic evacuation. Case 6 required emergent craniotomy due to massive expansion of hemorrhage after randomization to endoscopy, but before the procedure could be initiated.
Figure 2. Endoscopic view of hematoma during evacuation.
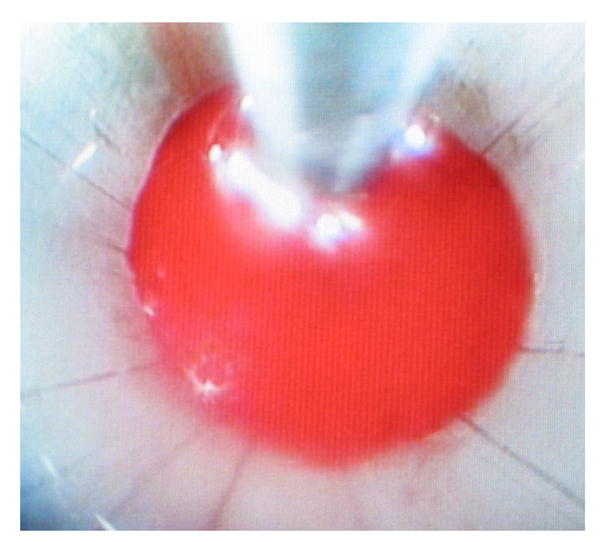
Endoscopic view of suction catheter approaching hematoma bed.
Table 3.
Summary of surgical technical aims. The numbers in the qualitative columns represent the number of cases receiving that descriptor as assessed by the operating neurosurgeon.
| Surgical Technical Aims | ||||
|---|---|---|---|---|
| Quantitative Measures | Hematoma entry on 1st pass | 5/5 | ||
| # of endoscopic passes | Ave 1.4 | |||
| % of hematoma evacuated | 80% +/- 13 | |||
| Qualitative Measures | Satisfactory | Needs Improvement | Failed | |
| Ease of Localization | 5 | 0 | 0 | |
| Ease of Suction | 4 | 1 | 0 | |
| Achieving Hemostasis | 3 | 1 | 1 | |
Acknowledgments
Funding: This work was supported by a grant from the Division of Extramural Research of the National Institutes of Health: P50-NS044378 (Alger, Vespa, Martin, Kidwell).
Abbreviations
- BI
Barthel Index ICH – Intracerebral Hemorrhage
- CT
Computed Tomography NIHSS – National Institute of Health Stroke Scale
- GCS
Glasgow Coma Scale
- IVH
Intraventricular Hemorrhage
- mRS
modified Rankin Score
- NIHSS
National Institute of Health Stroke Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Auer LM, Deinsberger W, Niederkorn K, et al. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neurosurg. 1989;70:530–5. doi: 10.3171/jns.1989.70.4.0530. [DOI] [PubMed] [Google Scholar]
- 2.Bakshi A, Asha MC, Banerji AK. Neuroendoscope-assisted evacuation of large intracerebral hematomas: introduction of a new, minimally invasive technique. Neurosurg Foc. 2004;16(6):1–5. doi: 10.3171/foc.2004.16.6.8. [DOI] [PubMed] [Google Scholar]
- 3.Broderick JP, Brott TG, Duldner JE, et al. Volume of intracerebral hemorrhage.A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 4.Counsell C, Boonyakarndul S, Dennis M, et al. Primary intracerebral hemorrhage in the Osfordshire Community Stroke Project. Cerebrovasc Dis. 1995;5:26–34. [Google Scholar]
- 5.Douglas MA, Haerer AF. Long-term prognosis of hypertensive intracerebral hemorrhage. Stroke. 1982;13:402–6. doi: 10.1161/01.str.13.4.488. [DOI] [PubMed] [Google Scholar]
- 6.Helweg-Larsen S, Sommer W, Strange P, et al. Prognosis for patients treated conservatively for spontaneous intracerebral hematomas. Stroke. 1984;15:1045–8. doi: 10.1161/01.str.15.6.1045. [DOI] [PubMed] [Google Scholar]
- 7.Hondo H. CT-guided stereotactic evacuation of hypertensive intracerebral hematomas. Tokushima J Exp Med. 1983;30:25–39. [PubMed] [Google Scholar]
- 8.Juvela S, Heiskanen O, Poranen A, et al. The treatment of spontaneous intracerebral hemorrhage: a prospective randomized trial of surgical and conservative treatment. J Neurosurg. 1989;70:755–758. doi: 10.3171/jns.1989.70.5.0755. [DOI] [PubMed] [Google Scholar]
- 9.Kojima S, Omura T, Wakamatsu W, et al. Prognosis and disability of stroke patients after 5 years in Akita, Japan. Stroke. 1990;21:71–77. doi: 10.1161/01.str.21.1.72. [DOI] [PubMed] [Google Scholar]
- 10.Mayer SA, Sacco RL, Shi T, et al. Neurologic deterioration in non-comatose patients with supratentorial intracerebral hemorrhage. Neurology. 1994;44:1379–1384. doi: 10.1212/wnl.44.8.1379. [DOI] [PubMed] [Google Scholar]
- 11.Mendelow D, Gregson BA, Fernandes HM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomized trial. Lancet. 2005;365:387–97. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 12.Miller CM, Vespa PM, McArthur, et al. Frameless Stereotactic Aspiration and Thrombolysis of Deep Intracerebral Hemorrhage is Associated With Reduced Levels of Extracellular Cerebral Glutamate and Unchanged Lactate Pyruvate Ratios. Neurocritical Care. 2007;6(1):22–9. doi: 10.1385/NCC:6:1:22. [DOI] [PubMed] [Google Scholar]
- 13.Nievas MNC, Haas E, Hollerhage HG, et al. Combined Minimal Invasive Techniques in Deep Supratentorial Intracerebral Haematomas. Minim Invas Neurosurg. 2004;47:294–298. doi: 10.1055/s-2004-830073. [DOI] [PubMed] [Google Scholar]
- 14.Nishihara T, Morita A, Teraoka A, et al. Endoscopic-guided removal of spontaneous intracerebral hemorrhage: comparison with computer tomography-guided stereotactic evacuation. Childs Nerv Syst. 2007;23:677–83. doi: 10.1007/s00381-007-0325-6. [DOI] [PubMed] [Google Scholar]
- 15.Vespa P, McArthur D, Miller CM, et al. Frameless stereotactic aspiration and Thrombolysis of deep intracerebral hemorrhage is associated with reduction of hemorrhage volume and neurological improvement. Neurocrit Care. 2005;2:274–81. doi: 10.1385/NCC:2:3:274. [DOI] [PubMed] [Google Scholar]
- 16.Wu J, Hua Y, Keep RF, et al. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34:1964–9. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- 17.Xi G, Keep RF, Hoff JT. Pathophysiology of brain edema formation. Neurosurg Clin N Am. 2002;13:371–83. doi: 10.1016/s1042-3680(02)00007-4. [DOI] [PubMed] [Google Scholar]


