Abstract
Background and Purpose
High revascularization rates in large-vessel occlusion strokes treated by mechanical thrombectomy are not always associated with good clinical outcomes. We evaluated predictors of functional dependence despite successful revascularization among patients with acute ischemic stroke treated with thrombectomy.
Methods
We analyzed the pooled data from the Multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI), Thrombectomy Revascularization of Large Vessel Occlusions in Acute Ischemic Stroke (TREVO), and TREVO 2 trials. Successful revascularization was defined as thrombolysis in cerebral infarction score 2b or 3. Functional dependence was defined as a score of 3 to 6 on the modified Rankin Scale at 3 months. We assessed relationship of demographic, clinical, angiographic characteristics, and hemorrhage with functional dependence despite successful revascularization.
Results
Two hundred and twenty-eight patients with successful revascularization had clinical outcome follow-up. The rates of functional dependence with endovascular success were 48.6% for Trevo thrombectomy and 58.0% for Merci thrombectomy. Age (odds ratio, 1.04; 95% confidence interval, 1.02–1.06 per 1-year increase), National Institutes of Health Stroke Scale score (odds ratio, 1.08; 95% confidence interval, 1.02–1.15 per 1-point increase), and symptom onset to endovascular treatment time (odds ratio, 1.11; 95% confidence interval, 1.01–1.22 per 30-minute delay) were predictors of functional dependence despite successful revascularization. Symptom onset to reperfusion time beyond 5 hours was associated with functional dependence. All subjects with symptomatic intracranial hemorrhage had functional dependence.
Conclusions
One half of patients with successful mechanical thrombectomy do not have good outcomes. Age, severe neurological deficits, and delayed endovascular treatment were associated with functional dependence despite successful revascularization. Our data support efforts to minimize delays to endovascular therapy in patients with acute ischemic stroke to improve outcomes.
Keywords: stroke
Although intravenous (IV) tissue-type plasminogen activator (tPA) is the recommended treatment for eligible patients with acute ischemic stroke within 4.5 hours after symptom onset, the recanalization rate is low for patients with large intracranial vessel occlusions. Nonresponse to IV tPA is associated with poor clinical outcomes in patients with moderate-to-severe stroke.1,2 Endovascular intervention with intra-arterial (IA) thrombolysis or mechanical thrombectomy offers an alternative treatment for patients with large-vessel occlusion strokes who are ineligible for or refractory to IV tPA ≤8 hours after symptom onset. Greater than 80% revascularization rates can be achieved with mechanical thrombectomy, particularly with stent retrievers. 3–8 Although studies have reported a strong association between better outcomes and successful revascularization, especially when tissue-level reperfusion is considered,4–6,9 and randomized trials between devices support better outcomes in the cohorts treated with more efficacious devices,6,7 the high revascularization rates with mechanical thrombectomy have not translated into better outcome in randomized trials against IV tPA or in combination with IV tPA.10,11 The lack of clinical benefit with endovascular treatment in 2 recent trials may be related to a substantially delayed time to endovascular therapy and less use of contemporary technologies such as stent retriever.10,11 Even in the stent retriever studies, no more than 60% of the patients had an independent neurological outcome.6–8,12
The factors leading to functional dependence despite successful revascularization remain unknown. Ideally, focusing the endovascular efforts to those patients who are not already destined to poor outcomes would improve the efficacy of treatment. We pooled data from the 3 prospective mechanical thrombectomy trials for acute ischemic stroke, namely the Multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI), Thrombectomy Revascularization of Large Vessel Occlusions in Acute Ischemic Stroke (TREVO), and TREVO 2 trials, to identify the clinical features associated with functional dependence despite successful revascularization.
Methods
Trial Inclusion and Patients
We analyzed data from the Multi MERCI, TREVO, and TREVO 2 trials, in which the Merci Retriever (Stryker Neurovascular, Mountain View, CA) and the Trevo Retriever (Stryker Neurovascular) were used.4,6,8 The databases of all 3 trials were maintained at Stryker Neurovascular. Appropriate institutional review board or ethics committees approvals were obtained by participating centers in all 3 trials. All 3 trials enrolled patients with acute ischemic stroke who were either ineligible for or refractory to IV tPA. Endovascular thrombectomy therapy was initiated within 8 hours of symptom onset.
All 3 trials included patients with angiographically confirmed intracranial vessel occlusion in both anterior and posterior circulations. All patients were older than 18 years with National Institutes of Health Stroke Scale (NIHSS) score of greater than 8. The TREVO and TREVO 2 trials had age limit of 85 years and NIHSS score upper limit of 30 and 29, respectively. In the Multi MERCI trial, IV tPA was allowed within 3 hours after symptom onset. A 4.5-hour time window was used for IV tPA in the TREVO and TREVO 2 trials.
Procedures
Details of the trial protocol and primary results of the 3 trials have been reported previously.4,6,8 Different revascularization scores were used for these trials. For our pooled analysis, digital subtraction angiography of subjects in the Multi MERCI trial was revaluated by the imaging core laboratory to derive thrombolysis in cerebral infarction (TICI) score.6 Successful revascularization was redefined as TICI 2b (major partial reperfusion of two thirds or more of the vascular distribution of the occluded artery) or 3 reperfusion flow in the target territory documented on the final angiogram after endovascular treatment.
Functional dependence was defined as a score of 3 to 6 on the modified Rankin Scale at 90 days. Functional independence was defined as a score of 0 to 2 on the modified Rankin Scale at 90 days.
Statistical Analysis
We compared patient demographic, angiographic characteristics, intracranial hemorrhage, and clinical outcome among the 3 trials. We analyzed continuous variables with the Wilcoxon rank-sum test or 2-sample t test and categorical variables with the Fisher’s exact test. The calculation of odds ratio (OR) and 95% confidence intervals (CI) was assessed in all tests.
The key management times were documented in all 3 trials. We defined time from stroke onset to procedure termination as onset to reperfusion time in subjects with successful revascularization. We tested time interval variables as continuous and categorical data for the analysis, including symptom onset to reperfusion time, onset to groin puncture, groin puncture to first device pass, onset to first device pass, and groin puncture to reperfusion time. We determined the cutoff points at different values and dichotomizations for variables based on clinical judgment and previous literature. Full details about variables are provided in the Methods in the online-only Data Supplement.
The association of patient characteristics with functional dependence despite successful revascularization was analyzed with univariate and multivariate logistic regression models. The prespecified variables as potential predictors of functional dependence despite successful revascularization included age, baseline NIHSS score, sex, internal carotid artery occlusion, onset to reperfusion time, onset to groin puncture time, onset to first device pass time, groin puncture to reperfusion time, and number of pass with study device. A separate multivariate logistic regression analysis was performed, using the stratified continuous variables of age, NIHSS score, and onset to reperfusion time. We analyzed the relationship of hemorrhage and device-related serious adverse events with functional dependence despite successful revascularization. We further assessed the association of age, stroke severity, and time intervals with functional dependence despite successful revascularization by categorization into quartiles or tertiles. We used SAS statistical software, version 9.2.
Results
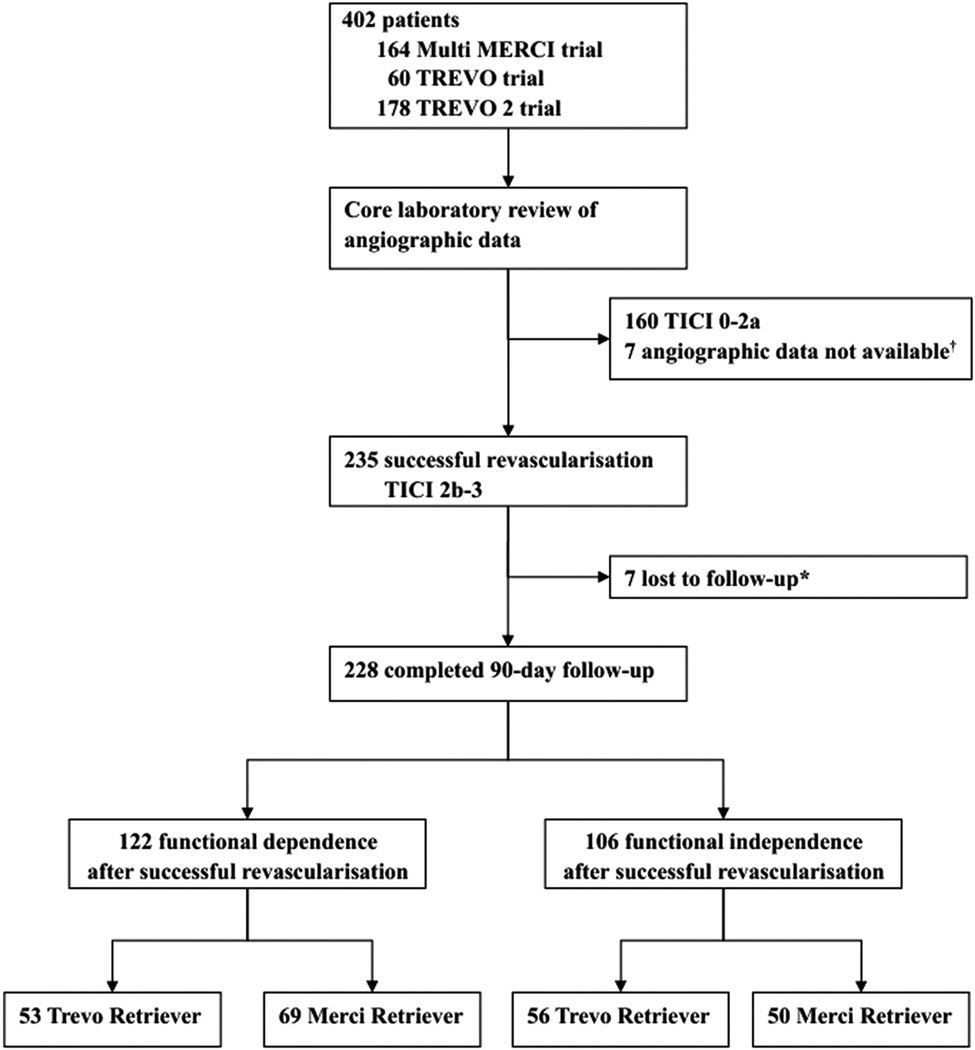
Trial participants were enrolled from 43 centers in 6 countries between January 2004 and December 2011. Among 402 subjects enrolled in the 3 trials, 235 subjects achieved successful revascularization with a TICI score of 2b or 3. The successful revascularization rate was higher in the primary Trevo thrombectomy than the primary Merci thrombectomy (75.0% versus 48.8%; P<0.0001). A total of 228 subjects were included in outcome analysis. The pooled analysis population consisted of 73 subjects from Multi MERCI trial, 47 from TREVO trial, and 108 from TREVO 2 trial. The study profile is shown in Figure 1.
Figure. Study profile.
TICI indicates thrombolysis in cerebral infarction. *Modified Rankin scores were not obtained for 7 patients at 90 days. †Angio-graphic images forwarded to the core laboratory were insufficient to allow TICI outcome assessment in 5 patients in Multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial. Technical reasons precluded the transfer of the angiographic images to the core laboratory in 2 patients in Thrombectomy Revascularization of Large Vessel Occlusions in Acute Ischemic Stroke (TREVO) trial.
Baseline and angiographic characteristics of 109 subjects treated primarily with Trevo devices and 119 subjects with Merci devices are shown in Table I in the online-only Data Supplement. Intracranial hemorrhage, device-related serious adverse events, and 90-day mortality were similar between treatment groups.
Functional dependence despite successful revascularization was observed in 122 of 228 (53.5%) subjects. The rates of functional dependence with endovascular success were 48.6% for Trevo thrombectomy and 58.0% for Merci thrombectomy. Baseline, angiographic characteristics, and hemorrhage between subjects with functional dependence and those with functional independence are shown in Table 1. In the entire cohort and Trevo thrombectomy group, subjects with functional dependence were older, had higher baseline NIHSS score, had higher systolic blood pressures and blood glucose, and more often had a cardioembolic stroke source and comorbidities (most common with hypertension and diabetes mellitus) than those with functional independence. In both Trevo and Merci thrombectomy groups, delay in revascularization contributes to functional dependence (Table 1 and Table II in the online-only Data Supplement). Symptom onset to groin puncture time, onset to first device pass, and onset to reperfusion time were all longer in subjects with functional dependence than functional independence. In the entire cohort, symptom onset to reperfusion time beyond 5 hours is associated with functional dependence (OR, 1.83; 95% CI, 1.07–3.12; P=0.0306). The same association is found in the Trevo group as well (OR, 2.48; 95% CI, 1.13–5.45; P=0.0319). In the entire cohort, functional dependence is related to intracranial hemorrhage and device-related serious adverse events, but not associated with number of device pass, rescue therapy, and location of vessel occlusion.
Table 1.
Functional Dependence Despite Successful Revascularization With Trevo Retriever and Merci Retriever
| All Patients (n=228) |
Trevo Group (n=109) |
Merci Group (n=119) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | mRS 0–2 (n=106) |
mRS 3–6 (n=122) |
P Value | mRS 0–2 (n=56) |
mRS 3–6 (n=53) |
P Value | mRS 0–2 (n=50) |
mRS 3–6 (n=69) |
P Value |
| Age, y, mean (SD) | 62 (16) | 69 (13) | 0.0003 | 62 (15) | 71 (10) | 0.0007 | 62 (18) | 68 (15) | 0.0661 |
| Male sex | 42/106 (40%) | 57/122 (47%) | 0.2878 | 20/56 (36%) | 29/53 (55%) | 0.0555 | 22/50 (44%) | 28/69 (41%) | 0.7117 |
| White race | 65/79 (82%) | 85/102 (83%) | 0.8455 | 23/29 (79%) | 29/33 (88%) | 0.4932 | 42/50 (84%) | 56/69 (81%) | 0.8092 |
| NIHSS score, median | 17 (14–20) | 20 (16–22) | 0.0002 | 16 (12–20) | 20 (16–22) | 0.0048 | 17 (15–21) | 19 (17–22) | 0.0268 |
| Cardioembolic stroke source | 37/70 (53%) | 63/85 (74%) | 0.0071 | 29/56 (52%) | 41/53 (77%) | 0.0089 | 8/14 (57%) | 22/32 (69%) | 0.5115 |
| Systolic blood pressure, mmHg, median | 138 (120–156) | 153 (128–170) | 0.0033 | 142 (130–160) | 160 (140–177) | 0.0266 | 131 (119–155) | 150 (122–168) | 0.0284 |
| Diastolic blood pressure, mm Hg, median | 77 (64–85) | 76 (66–90) | 0.5298 | 84 (72–91) | 80 (74–92) | 0.9888 | 72 (62–80) | 72 (63–84) | 0.2317 |
| Blood glucose, mg/dL, mean (SD) | 118 (30) | 156 (70) | <0.0001 | 113 (28) | 159 (68) | 0.0010 | 121 (32) | 154 (72) | 0.0010 |
| Medical history | |||||||||
| Hypertension | 45/79 (57%) | 90/102 (88%) | <0.0001 | 18/29 (62%) | 29/33 (88%) | 0.0354 | 27/50 (54%) | 61/69 (88%) | <0.0001 |
| Diabetes mellitus | 8/79 (10%) | 42/100 (42%) | <0.0001 | 5/29 (17%) | 20/33 (61%) | 0.0007 | 3/50 (6%) | 22/67 (33%) | 0.0005 |
| Dyslipidemia | 32/79 (41%) | 56/99 (57%) | 0.0360 | 14/29 (48%) | 23/32 (72%) | 0.0716 | 18/50 (36%) | 33/67 (49%) | 0.1883 |
| Congestive heart failure | 4/79 (5%) | 27/96 (28%) | <0.0001 | 3/29 (10%) | 12/31 (39%) | 0.0164 | 1/50 (2%) | 15/65 (23%) | 0.0009 |
| Atrial fibrillation | 24/79 (30%) | 47/100 (47%) | 0.0311 | 12/29 (41%) | 15/32 (47%) | 0.7974 | 12/50 (24%) | 32/68 (47%) | 0.0126 |
| Coronary artery disease | 22/79 (28%) | 44/100 (44%) | 0.0296 | 5/29 (17%) | 15/33 (45%) | 0.0286 | 17/50 (34%) | 29/67 (43%) | 0.3433 |
| Most proximal occlusion site | |||||||||
| Internal carotid artery | 24/106 (23%) | 30/122 (25%) | 0.7570 | 9/56 (16%) | 10/53 (19%) | 0.8025 | 15/50 (30%) | 20/69 (29%) | 1.0000 |
| Middle cerebral artery M1 | 58/106 (55%) | 63/122 (52%) | 0.6906 | 35/56 (63%) | 31/53 (58%) | 0.6988 | 23/50 (46%) | 32/69 (46%) | 1.0000 |
| Middle cerebral artery M2 | 15/106 (14%) | 14/122 (11%) | 0.5571 | 6/56 (11%) | 8/53 (15%) | 0.5736 | 9/50 (18%) | 6/69 (9%) | 0.1652 |
| Vertebrobasilar artery | 9/106 (8%) | 15/122 (12%) | 0.3930 | 6/56 (11%) | 4/53 (8%) | 0.7429 | 3/50 (6%) | 11/69 (16%) | 0.1487 |
| Intravenous tPA failure | 53/106 (50%) | 60/122 (49%) | 1.0000 | 36/56 (64%) | 32/53 (60%) | 0.6969 | 17/50 (34%) | 28/69 (41%) | 0.5662 |
| Intubation | 49/80 (61%) | 70/93 (75%) | 0.0506 | 37/56 (66%) | 40/53 (75%) | 0.3011 | 12/24 (50%) | 30/40 (75%) | 0.0581 |
| Time intervals, min, median | |||||||||
| Onset to groin puncture | 221 (180–300) | 260 (200–334) | 0.1105 | 219 (175–302) | 270 (176–345) | 0.1870 | 245 (190–292) | 252 (206–316) | 0.4187 |
| Groin puncture to first device pass | 33 (23–43) | 32 (24–44) | 0.6371 | 33 (20–43) | 29 (22–40) | 0.7850 | 34 (27–42) | 35 (26–45) | 0.7900 |
| Onset to first device pass | 264 (206–347) | 292 (233–374) | 0.0962 | 257 (199–339) | 293 (220–380) | 0.1325 | 275 (222–353) | 292 (235–370) | 0.3833 |
| Groin puncture to reperfusion | 77 (57–101) | 82 (53–105) | 0.6702 | 80 (58–100) | 82 (60–115) | 0.7394 | 75 (56–104) | 85 (53–104) | 0.7692 |
| Onset to reperfusion | 315 (256–410) | 345 (281–422) | 0.0642 | 285 (244–401) | 374 (285–422) | 0.0717 | 326 (268–416) | 345 (280–420) | 0.4148 |
| Number of passes with | 2 (1–3) | 2 (1–3) | 0.5221 | 2 (1–3) | 2 (1–3) | 0.4785 | 2 (1–3) | 2 (1–3) | 0.7960 |
| Intra-arterial lytic use | 14/106 (13%) | 14/122 (11%) | 0.6922 | 3/56 (5%) | 2/53 (4%) | 1.0000 | 11/50 (22%) | 12/69 (17%) | 0.6393 |
| Use of rescue treatment | 22/106 (21%) | 32/122 (26%) | 0.3527 | 8/56 (14%) | 9/53 (17%) | 0.7943 | 14/50 (28%) | 23/69 (33%) | 0.5549 |
| Symptomatic ICH* | 0/106 (0%) | 17/122 (14%) | <0.0001 | 0/56 (0%) | 8/53 (15%) | 0.0023 | 0/50 (0%) | 9/69 (13%) | 0.0099 |
| Device-related serious adverse events | 1/106 (1%) | 8/122 (7%) | 0.0395 | 0/56 (0%) | 4/53 (8%) | 0.0526 | 1/50 (2%) | 4/69 (6%) | 0.3968 |
Functional dependence was defined as a score of 3 to 6 on the mRS at 90 days. The baseline data of race, medical history, blood pressure, and blood glucose were not available in the Thrombectomy Revascularization of Large Vessel Occlusions in Acute Ischemic Stroke (TREVO) trial. The data of suspected stroke cause and intubation were not available in the Multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial. ICH indicates intracranial hemorrhage; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; and tPA, tissue-type plasminogen activator.
Symptomatic ICH was defined as a point increase of ≥4 in the NIHSS within 24 hours with evidence of any blood in the brain or within the cranium identified on 24-hour head computed tomography/MRI scan, or any intracranial hemorrhage in which no further NIHSS scores were available beyond baseline and the patient died.
Factors independently associated with functional dependence despite successful revascularization are shown in Table 2. The risks of functional dependence increases with each year of age (OR, 1.04; 95% CI, 1.02–1.06), each point of NIHSS score (OR, 1.08; 95% CI, 1.02–1.15), and each 30-minute delayed symptom onset to groin puncture for endovascular treatment (OR, 1.11; 95% CI, 1.01–1.22) in the entire cohort. When dichotomized variables of age, NIHSS score, and symptom onset to reperfusion time were selected into the multivariate model, predictors of functional dependence in the entire cohort included NIHSS score ≥20 (OR, 2.31; 95% CI, 1.33–4.02; P=0.003) and onset to reperfusion >5 hours (OR, 1.83; 95% CI, 1.06–3.17; P=0.03). Predictors of functional dependence in the Trevo cohort included age >80 years (OR, 7.35; 95% CI, 1.31–41.12; P=0.02), NIHSS score ≥20 (OR, 2.66; 95% CI, 1.14–6.24; P=0.02), onset to reperfusion >5 hours (OR, 2.70; 95% CI, 1.14–6.39; P=0.02), and men (OR, 0.38; 95% CI, 0.16–0.90; P=0.03). There was no predictor of functional dependence in the Merci group. We found similar predictors of functional dependence when subjects with prestroke modified Rankin Scale ≥2 were excluded from the analysis.
Table 2.
Multivariate Analysis of Predictors of Functional Dependence Despite Successful Revascularization With Trevo Retriever and Merci Retriever
| All Patients (n=228) |
Trevo Group (n=109) |
Merci Group (n=119) |
||||
|---|---|---|---|---|---|---|
| Variables | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value |
| Age, per year increase | 1.04 (1.02–1.06) | 0.0005 | 1.06 (1.02–1.10) | 0.0026 | 1.02 (1.00–1.05) | 0.0405 |
| NIHSS score, per point increase | 1.08 (1.02–1.15) | 0.0055 | 1.13 (1.03–1.23) | 0.0074 | … | NS |
| Onset to groin puncture, per 30-min increase | 1.11 (1.01–1.22) | 0.0306 | … | NS | … | NS |
Variables with P≤0.15 in the univariate analysis were considered in multivariate logistic regression model-building process. Models were built using stepwise regression with variables entered into the model at the 0.15 significance level and removed at the 0.05 significance level. CI indicates confidence interval; NIHSS, National Institutes of Health Stroke Scale; and NS, not significant.
The association of age, NIHSS score, and time intervals with functional dependence despite successful revascularization are shown in Tables 3 and 4. In the entire cohort, the ORs of functional dependence for every 30-minute delay in symptom onset to groin puncture time, onset to first device pass time, and onset to reperfusion time were 1.12 (95% CI, 1.02–1.24), 1.10 (95% CI, 1.01–1.21), 1.11 (95% CI, 1.01–1.21), respectively (Table 3). In the Trevo thrombectomy, every 10-year age increase is associated with a 92% relative increase in the odds of functional dependence. Every 5-point baseline NIHSS score increase is associated with a 78% relative increase in the odds of functional dependence after Trevo thrombectomy (Table 4). All subjects with symptomatic intracranial hemorrhage or severe parenchymal hematoma had functional dependence in both Trevo thrombectomy and Merci thrombectomy groups (Table III in the online-only Data Supplement).
Table 3.
Functional Dependence Despite Successful Revascularization by Time Intervals
| All Patients (n=228) |
Trevo Group (n=109) |
|||||
|---|---|---|---|---|---|---|
| Time Tertiles | Patient/Total (%) | Adjusted OR (95% CI) | P Value | Patient/Total (%) | Adjusted OR (95% CI) | P Value |
| Onset to groin puncture, min | ||||||
| 75–210 | 40/85 (47) | Reference | … | 19/45 (42) | Reference | … |
| 211–345 | 56/103 (54) | 1.53 (0.83–2.83) | 0.1770 | 21/42 (50) | 1.45 (0.56–3.74) | 0.4427 |
| 346–480 | 25/39 (64) | 2.81 (1.20–6.55) | 0.0168 | 13/22 (59) | 2.76 (0.83–9.12) | 0.0963 |
| OR per 30 min or P for trend | … | 1.12 (1.02–1.24) | 0.0223 | … | 1.14 (0.99–1.32) | 0.0623 |
| Onset to first device pass, min | ||||||
| 105–240 | 36/79 (46) | Reference | … | 17/42 (41) | Reference | … |
| 241–375 | 55/100 (55) | 1.64 (0.87–3.07) | 0.1234 | 21/44 (48) | 1.49 (0.58–3.85) | 0.4069 |
| 376–510 | 28/44 (64) | 2.57 (1.14–5.79) | 0.0230 | 15/23 (65) | 3.23 (0.95–10.98) | 0.0597 |
| OR per 30 min or P for trend | … | 1.10 (1.01–1.21) | 0.0379 | … | 1.12 (0.97–1.28) | 0.1145 |
| Onset to reperfusion, min | ||||||
| 120–270 | 26/61 (43) | Reference | … | 10/32 (31) | Reference | … |
| 271–420 | 65/115 (57) | 1.83 (0.94–3.55) | 0.0745 | 29/53 (55) | 2.70 (0.98–7.42) | 0.0549 |
| 421–570 | 31/52 (60) | 2.79 (1.23–6.33) | 0.0141 | 14/24 (58) | 4.10 (1.17–14.42) | 0.0279 |
| OR per 30 min or P for trend | … | 1.11 (1.01–1.21) | 0.0228 | … | 1.13 (0.99–1.29) | 0.0676 |
Data were adjusted for age, sex, and baseline National Institutes of Health Stroke Scale score. CI indicates confidence interval; and OR, odds ratio.
Table 4.
Functional Dependence Despite Successful Revascularization by Age and Baseline NIHSS Score
| All Patients (n=228) |
Trevo Group (n=109) |
|||||
|---|---|---|---|---|---|---|
| Variables | Patient/Total (%) | Adjusted OR (95% CI) | P Value | Patient/Total (%) | Adjusted OR (95% CI) | P Value |
| Age* | ||||||
| ≤60 | 28/69 (41) | Reference | … | 8/29 (28) | Reference | … |
| 60–70 | 27/50 (54) | 1.85 (0.86–3.99) | 0.1158 | 16/32 (50) | 2.92 (0.92–9.30) | 0.0698 |
| 70–80 | 42/73 (58) | 2.08 (1.03–4.20) | 0.0414 | 20/37 (54) | 3.72 (1.20–11.51) | 0.0225 |
| >80 | 25/36 (69) | 3.32 (1.35–8.16) | 0.0089 | 9/11 (82) | 14.29 (2.14–95.69) | 0.0061 |
| OR per 10 y or P for trend | … | 1.41 (1.15–1.73) | 0.0008 | … | 1.92 (1.27–2.89) | 0.0019 |
| NIHSS score† | ||||||
| 8–10 | 7/16 (44) | Reference | … | 5/11 (46) | Reference | … |
| 11–19 | 54/119 (45) | 0.69 (0.22–2.17) | 0.5298 | 20/54 (37) | 0.31 (0.06–1.50) | 0.1455 |
| ≥20 | 61/93 (66) | 1.48 (0.46–4.70) | 0.5092 | 28/44 (64) | 1.09 (0.23–5.20) | 0.9128 |
| OR per 5 points or P for trend | … | 1.47 (1.11–1.96) | 0.0083 | … | 1.78 (1.12–2.81) | 0.0144 |
CI indicates confidence interval; NIHSS, National Institutes of Health Stroke Scale; and OR, odds ratio.
Data were adjusted for baseline NIHSS score, sex, and onset to reperfusion time.
Data were adjusted for age, sex, and onset to reperfusion time.
Discussion
Our study shows that half of the patients with large intracranial vessel occlusion had functional dependence despite successful revascularization in the 3 endovascular thrombectomy trials. Older age, higher NIHSS score, delayed endovascular treatment, and procedural complications are associated with increased frequency of functional dependence despite endovascular success.
Our analysis confirms the superiority of the stent retriever over the Merci device for successful revascularization using TICI 2b or greater as the threshold. TICI and modified TICI scales are superior to TIMI scale for evaluating the extent of tissue reperfusion and predicting clinical outcome after IA therapy.9,10,13 The 75% reperfusion rate (TICI≥2b) with Trevo devices in our study is higher than the 40% rate (modified TICI≥2b) from IMS III trial, in which almost half of subjects in the endovascular group were treated only with IA tPA and seldom received the stent retriever.10 The variant thresholds for major partial perfusion (ie, 2/3 versus 1/2) may result in different rates of successful reperfusion, with about 20% of subjects with modified TICI 2b (ie, partial perfusion with 50%-66% of the target downstream territory) were not graded as TICI 2b.13 The discrepancy of successful TICI 2b or greater reperfusion with good functional outcome may suggest that even 66% territorial reperfusion may be suboptimal.
Advanced age is associated with functional dependence despite endovascular success. The proportion of functional dependence increased from only 28% in people aged ≤60 years to 82% in those aged >80 years after Trevo thrombectomy. The association of advanced age with functional dependence is consistent with the results in 2 studies of endovascular intervention with or without stent retriever.14,15 However, our finding of less favorable outcomes with older age (>80 years) has not been shown in a meta-analysis of IV tPA trials.16
The association of severe stroke (NIHSS score ≥20) with functional dependence despite endovascular success in our study is supported by a multicenter study of endovascular treatment before stent retriever.15 In our study, stent retriever thrombectomy and Merci thrombectomy had similar rates of functional dependence in this subset of severe stroke. In contrast, results from the SYNTHESIS trial indicated that endovascular treatment was not superior to IV tPA for acute stroke with the median NIHSS score 13.11 This finding differs from a recent study which showed that strokes with NIHSS score ≥14 benefit most from endovascular therapy.17 These conflicting results may be related to the infrequent use of mechanical thrombectomy (33.3% versus 88%) and more than one third stroke with NIHSS score ≤10 in the SYNTHESIS trial.11 This subset of patients with minor to moderate stroke does not benefit from IA therapy, as shown in a previous randomized trial.3
Age and NIHSS score have been found as outcome predictors for endovascular interventions with IA thrombolysis and mechanical thrombectomy.18,19 Despite this, IMS III trial results suggest that endovascular interventions, including the Merci Retriever, trend toward benefit over IV tPA alone for severe stroke with NIHSS ≥20,10 probably because these patients do even worse with IV tPA or medical therapy. Advanced age and severe stroke should not be used as exclusion criteria for stent retriever thrombectomy.
Our pooled analysis provides new information about time-dependent benefits of mechanical thrombectomy on acute ischemic stroke. In our study, a 30-minute delay from stroke onset to endovascular treatment was associated with a 11% increase in the odds of functional dependence. Time delay from symptom onset to first device pass also trends toward functional dependence. These findings are consistent with prior studies of IV tPA in randomized trials and clinical practice that showed shorten delay in initiation of thrombolytic treatment to be associated with both increased benefit and reduced mortality.16,20 In the IMS III and SYNTHESIS trials, delayed onset to endovascular treatment may have contributed to the futility of endovascular therapy.10,11
In addition, our study shows that symptom onset to reperfusion time beyond 5 hours is associated with functional dependence despite endovascular success after mechanical thrombectomy, especially with Trevo Retriever. Moreover, this relationship occurs only in moderate-to-severe stroke with NIHSS 8 to 19 but not in critical stroke with NIHSS ≥20. These findings are consistent with prior studies of IA therapies, which revealed the association of onset to reperfusion time with favorable clinical outcome and survival.21,22 Reducing delay from image to groin puncture time for IA therapy is likely associated with improved clinical outcome.23 Accordingly, our data suggest that clinical effectiveness with mechanical thrombectomy for acute ischemic stroke is critically time dependent, similar to IV tPA and IA thrombolysis.
Although groin puncture to reperfusion time (ie, procedure time in our study) is not associated with functional dependence, faster procedure time and shorter treatment delays in a recent multicenter study with stent retriever than those observed in our study can lead to better clinical outcomes.12 These findings from our study emphasize the importance of minimizing delays of onset to treatment and onset to reperfusion times with mechanical thrombectomy for achieving the best clinical outcomes. Reduction time from symptom onset to reperfusion within 5 hours with stent retriever may improve favorable outcome.
This study has several limitations. Data with a small cohort size were retrospectively collected from 2 single-arm prospective trials and a randomized controlled trial with a post hoc analysis. We cannot exclude the possibility that unmeasured confounding variables may influence some of our findings. Physiological determinants of outcome including blood pressure and glucose as well as medical history were not analyzed in the main multivariate model because these variables were not collected in the TREVO trial. However, the associations of diabetes mellitus, hypertension, and atrial fibrillation with functional dependence despite endovascular success after mechanical thrombectomy were also found in model excluding the TREVO trial data. A recent study suggests that good collateral flow may correlate with favorable outcomes after stent retriever thrombectomy.12 Early ischemic change measured by the Alberta Stroke Program Early CT Score (ASPECTS) may predict the clinical outcome and reperfusion after endovascular treatment.5 However, favorable baseline ASPECTS in prediction for benefit from endovascular therapy was not shown in the IMS III trial, which had a significant delay between baseline computed tomography scan and reperfusion.24 The associations of collateral flow and ASPECTS with functional dependence despite endovascular success after mechanical thrombectomy have not been investigated in this analysis. The impact of imaging selection with modalities such as multimodal computed tomography or MRI on functional dependence despite successful revascularization also remains unknown.
Conclusions
Our findings show functional dependence despite successful revascularization is relatively frequent in subjects with large-vessel occlusion strokes after endovascular thrombectomy treatment, particularly among old patients with severe neurological deficits and delayed endovascular treatment. A 30-minute delay from stroke onset to endovascular treatment is associated with a 11% increase in the odds of functional dependence. Symptom onset to reperfusion time beyond 5 hours is associated with functional dependence despite endovascular success after mechanical thrombectomy. Our data support minimizing delays to reperfusion in randomized controlled trials of mechanical thrombectomy with stent retriever alone or as an adjunctive therapy against IV tPA alone.
Supplementary Material
Acknowledgments
We thank the other investigators, the staff, and the participants of the Multi MERCI, TREVO, and TREVO 2 studies for their valuable contributions.
Sources of Funding
The original Multi MERCI, TREVO, and TREVO 2 trials were funded by Stryker Neurovascular/Concentric Medical. There was no funding source for this study. Dr Shi is supported by National Natural Science Foundation of China (81371275, 81070949), Program for New Century Excellent Talents in University of China (NCET-11–0539), and Fundamental Research Funds for Central Universities, Sun Yat-sen University (2013ykzd12).
Footnotes
The online-only Data Supplement is available with this article at http://stroke.ahajoui-nals.Org/lookup/suppl/doi:10.1161/STROKEAHA.114.005603/−/DC1.
Disclosures
Drs Liebeskind, Smith, and Duckwiler were employed by the University of California, which holds a patent on endovascular devices for stroke. Dr Ge is an employee of Stryker. Dr Liebeskind: consultant—Stryker and Covidien. Dr Albers: consultant—Covidien; equity interest—iSchemaView. Dr Budzik: consultant—Stryker and Covidien. Dr Gupta: consultant—Stryker, Covidien and Rapid Medical; royalties—UpToDate. Dr Jansen: consultant—Stryker and Covidien. Dr Jovin: consultant—Silk Road Medical. Dr Lutsep: con-sultant—Stryker. Dr Nogueira: consultant—Stryker (Trevo-2 Trial PI, DAWN Trial PI), Covidien (SWIFT and SWIFT-PRIME Trials Steering Committee, STAR Trial Core Laboratory) and Penumbra (3-D Separator Trial Executive Committee). Dr Rymer: honoraria and speaking engagements—Stryker and Covidien. Dr Smith: consultant—Stryker and Covidien.
References
- 1.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 2.Alexandrov AV, Molina CA, Grotta JC, Garami Z, Ford SR, Alvarez-Sabin J, et al. CLOTBUST Investigators. Ultrasound-enhanced systemic thrombolysis for acute ischemic stroke. N Engl J Med. 2004;351:2170–2178. doi: 10.1056/NEJMoa041175. [DOI] [PubMed] [Google Scholar]
- 3.Furlan A, Higashida R, Wechsler L, Gent M, Rowley H, Kase C, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II study: a randomized controlled trial. Prolyse in Acute Cerebral Thromboembolism. JAMA. 1999;282:2003–2011. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
- 4.Smith WS, Sung G, Saver J, Budzik R, Duckwiler G, Liebeskind DS, et al. Multi MERCI Investigators. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205–1212. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Menon BK, Coutts SB, Hill MD, Demchuk AM. Penumbra Pivotal Stroke Trial Investigators, Calgary Stroke Program, and the Seaman MR Research Center. Effect of baseline CT scan appearance and time to recanalization on clinical outcomes in endovascular thrombectomy of acute ischemic strokes. Stroke. 2011;42:93–97. doi: 10.1161/STROKEAHA.110.594481. [DOI] [PubMed] [Google Scholar]
- 6.Nogueira RG, Lutsep HL, Gupta R, Jovin TG, Albers GW, Walker GA, et al. TREVO 2 Trialists. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–1240. doi: 10.1016/S0140-6736(12)61299-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, et al. SWIFT Trialists. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–1249. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 8.Jansen O, Macho JM, Killer-Oberpfalzer M, Liebeskind D, Wahlgren N. TREVO Study Group. Neurothrombectomy for the treatment of acute ischemic stroke: results from the TREVO study. Cerebrovasc Dis. 2013;36:218–225. doi: 10.1159/000353990. [DOI] [PubMed] [Google Scholar]
- 9.Yoo AJ, Simonsen CZ, Prabhakaran S, Chaudhry ZA, Issa MA, Fugate JE, et al. Cerebral Angiographic Revascularization Grading Collaborators. Refining angiographic biomarkers of revascularization: improving outcome prediction after intra-arterial therapy. Stroke. 2013;44:2509–2512. doi: 10.1161/STROKEAHA.113.001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Interventional Management of Stroke (IMS) III Investigators. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. SYNTHESIS Expansion Investigators. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–913. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira VM, Gralla J, Davalos A, Bonafé A, Castaño C, Chapot R, et al. Prospective, multicenter, single-arm study of mechanical thrombectomy using Solitaire Flow Restoration in acute ischemic stroke. Stroke. 2013;44:2802–2807. doi: 10.1161/STROKEAHA.113.001232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh SH, Cloft HJ, Fugate JE, Rabinstein AA, Liebeskind DS, Kallmes DF. Clarifying differences among thrombolysis in cerebral infarction scale variants: is the artery half open or half closed? Stroke. 2013;44:1166–1168. doi: 10.1161/STROKEAHA.111.000399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singer OC, Haring HP, Trenkler J, Nolte CH, Bohner G, Reich A, et al. Age dependency of successful recanalization in anterior circulation stroke: the ENDOSTROKE study. Cerebrovasc Dis. 2013;36:437–445. doi: 10.1159/000356213. [DOI] [PubMed] [Google Scholar]
- 15.Hussein HM, Georgiadis AL, Vazquez G, Miley JT, Memon MZ, Mohammad YM, et al. Occurrence and predictors of futile recanalization following endovascular treatment among patients with acute ischemic stroke: a multicenter study. AJNR Am J Neuroradiol. 2010;31:454–458. doi: 10.3174/ajnr.A2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rangaraju S, Owada K, Noorian AR, Nogueira RG, Nahab F, Glenn BA, et al. Comparison of final infarct volumes in patients who received endovascular therapy or intravenous thrombolysis for acute intracranial large-vessel occlusions. JAMA Neurol. 2013;70:831–836. doi: 10.1001/jamaneurol.2013.413. [DOI] [PubMed] [Google Scholar]
- 18.Sarraj A, Albright K, Barreto AD, Boehme AK, Sitton CW, Choi J, et al. Optimizing prediction scores for poor outcome after intra-arterial therapy in anterior circulation acute ischemic stroke. Stroke. 2013;44:3324–3330. doi: 10.1161/STROKEAHA.113.001050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Flint AC, Xiang B, Gupta R, Nogueira RG, Lutsep HL, Jovin TG, et al. TREVO-2 Trialists. THRIVE score predicts outcomes with a third-generation endovascular stroke treatment device in the TREVO-2 trial. Stroke. 2013;44:3370–3375. doi: 10.1161/STROKEAHA.113.002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saver JL, Fonarow GC, Smith EE, Reeves MJ, Grau-Sepulveda MV, Pan W, et al. Time to treatment with intravenous tissue plasminogen activator and outcome from acute ischemic stroke. JAMA. 2013;309:2480–2488. doi: 10.1001/jama.2013.6959. [DOI] [PubMed] [Google Scholar]
- 21.Khatri P, Abruzzo T, Yeatts SD, Nichols C, Broderick JP, Tomsick TA. IMS I and II Investigators. Good clinical outcome after ischemic stroke with successful revascularization is time-dependent. Neurology. 2009;73:1066–1072. doi: 10.1212/WNL.0b013e3181b9c847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazighi M, Chaudhry SA, Ribo M, Khatri P, Skoloudik D, Mokin M, et al. Impact of onset-to-reperfusion time on stroke mortality: a collaborative pooled analysis. Circulation. 2013;127:1980–1985. doi: 10.1161/CIRCULATIONAHA.112.000311. [DOI] [PubMed] [Google Scholar]
- 23.Sun CH, Nogueira RG, Glenn BA, Connelly K, Zimmermann S, Anda K, et al. “Picture to puncture”: a novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation. 2013;127:1139–1148. doi: 10.1161/CIRCULATIONAHA.112.000506. [DOI] [PubMed] [Google Scholar]
- 24.Hill MD, Demchuk AM, Goyal M, Jovin TG, Foster LD, Tomsick TA, et al. IMS3 Investigators. Alberta Stroke Program early computed tomography score to select patients for endovascular treatment: Interventional Management of Stroke (IMS)-III Trial. Stroke. 2014;45:444–449. doi: 10.1161/STROKEAHA.113.003580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.