Abstract
Transgenic hypertensive (mRen2)27 rats overexpress the murine Ren2 gene and have impaired baroreflex sensitivity (BRS) for control of the heart rate. Removal of endogenous angiotensin (Ang)-(1–7) tone using a receptor blocker does not further lower BRS. Therefore, we assessed whether blockade of Ang II with a receptor antagonist or combined reduction in Ang II and restoration of endogenous Ang-(1-7) levels with Ang-converting enzyme (ACE) inhibition will improve BRS in these animals. Bilateral solitary tract nucleus (nTS) microinjections of the AT1 receptor blocker, candesartan (CAN, 24 pmol in 120 nl, n = 9), or a peptidic ACE inhibitor, bradykinin (BK) potentiating nonapeptide (Pyr–Trp–Pro–Arg–Pro–Gln–Ile–Pro–Pro; BPP9α, 9 nmol in 60 nl, n=12), in anesthetized male (mRen2)27 rats (15–25 weeks of age) show that AT1 receptor blockade had no significant effect on BRS, whereas microinjection of BPP9α improved BRS over 60–120 min. To determine whether Ang-(1-7) or BK contribute to the increase in BRS, separate experiments using the Ang-(1-7) receptor antagonist d-Ala7-Ang-(1-7) or the BK antagonist HOE-140 showed that only the Ang-(1-7) receptor blocker completely reversed the BRS improvement. Thus, acute AT1 blockade is unable to reverse the effects of long-term Ang II overexpression on BRS, whereas ACE inhibition restores BRS over this same time frame. As the BPP9α potentiation of BK actions is a rapid phenomenon, the likely mechanism for the observed delayed increase in BRS is through ACE inhibition and elevation of endogenous Ang-(1-7).
Keywords: angiotensin-(1-7), angiotensin II, BPP9α, brain, dorsal medulla oblongata
INTRODUCTION
Transgenic hypertensive (mRen2)27 rats overexpress the murine Ren2 gene, exhibit hypertension and have impaired baroreflex sensitivity (BRS) for control of heart rate (HR).1,2 Functionally, there is evidence for low angiotensin (Ang)-(1-7) expression in the medulla oblongata contributing to the impaired BRS in these rats as an Ang-(1-7) receptor blocker in the solitary tract nucleus (nTS) does not further lower BRS.2 Indeed, replacement of Ang-(1-7) by fusion-protein gene transfer into the cistern magna or by intracerebroventricular infusion of the peptide for 3–7 days corrects the impairments in BRS and lowers the mean arterial pressure (MAP) in (mRen2)27 rats.2–5 The reduction in MAP was substantial, almost completely returning resting pressure to that of normotensive animals. These findings suggest that replacement of Ang-(1-7) centrally is important to restoration of normal cardiovascular function.
Ang-converting enzyme (ACE) inhibition prevents formation of Ang II and inactivation of bradykinin (BK).6,7 ACE also metabolizes Ang-(1-7)8 and the concentration of Ang-(1-7) in the circulation increases following ACE inhibition.9–11 Ang-(1-7) also potentiates the actions of BK at the BK–B2 receptor.12,13 All of these factors may contribute to the blood pressure lowering effects of ACE inhibitors.14,15 Bomtempo et al.,16 show that intracerebroventricular infusion of Ang-(1-7), at a subeffective rate, combined with BK, also at a subeffective rate,17 produces a significant facilitation of the baroreflex bradycardia. Moreover, an intracerebroventricular infusion of either the BK–B2 receptor antagonist, HOE-140 or the Ang-(1-7) antagonist, d-Ala7-Ang-(1-7) (d-Ala), completely blocked the facilitatory effect on baroreflex modulation. The interaction of these two peptides centrally may represent a significant portion of the mechanism of action involved in the cardiovascular effects of ACE inhibition,18 in addition to any reduction in Ang II.
Our hypothesis was that ACE inhibition in the nTS will restore endogenous Ang-(1-7) levels within the nTS to improve the BRS for control of HR in hypertensive (mRen2)27 rats, a model of hypertension functionally deficient in Ang-(1-7). Our study was designed to compare the effects of Ang II receptor blockade vs. ACE inhibition on BRS for control of HR within the nTS of transgenic (mRen2)27 rats and the subsequent role of Ang-(1-7) or BK to the effects observed.
METHODS
Animals
The Institutional Animal Care and Use Committee approved the following procedures. Experiments were performed in 15–25 week-old male transgenic (mRen2)27 rats, 540±11 g body weight, from the Hypertension and Vascular Research Center Colony, Wake Forest University School of Medicine, Winston-Salem, NC, USA. Animals were housed in humidity- and temperature-controlled rooms in group cages (12-h light/dark cycle) with free access to standard rat chow and water.
Surgical procedures
Rats were anesthetized with the combination urethane–chloralose (750 mg and 35 mg kg 1) via intraperitoneal injections. Supplemental doses i.v. were given as required.19,20 Animals were instrumented with polyethylene femoral artery and vein catheters (PE-50 tubing, Clay Adams, Parsippany, NJ, USA) for measurement of cardiovascular indices and delivery of drugs, respectively. Animals were placed in a stereotaxic frame with the head tilted downward at 451 for surgical exposure of the dorsal medulla oblongata by incision of the atlanto-occipital membrane. Body temperature was maintained and rats breathed a 70:30% mixture of room air and oxygen. A stabilization period of ≥30 min was allowed before baseline measurements were recorded.
Measurement of BRS, arterial pressure (AP) and HR
As described previously,19–21 pulsatile AP and MAP were monitored by a strain gauge transducer connected to the femoral artery. Measurements were recorded and digitized using a Data Acquisition System (BIOPAC System; Acknowledge software Version 3.8.1; Santa Barbara, CA) and HR was determined from the AP wave. Baseline values of the BRS for bradycardia were established by bolus i.v. administration of three doses of phenylephrine (PE; 2, 5 and 10 μg kg−1 in 0.9% NaCl).19–21 We focused on the bradycardia in response to increases in MAP as the effects of Ang II or Ang-(1-7) are primarily on this limb of the reflex without altering the tachycardia in response to lowering of MAP.19–21 A period of 5 min between PE doses allowed MAP and HR to return to baseline levels before testing the next dose and all reflex testing was completed within 15 min. Maximum MAP responses (ΔMAP, mm Hg) and the associated reflex changes in HR (ΔHR, b.p.m.) were recorded at each dose of PE. ΔHR was converted to changes in pulse interval (ΔPI, ms) by the formula: 60 000/HR. The slope of the line fit through the ΔMAP and corresponding ΔPI in response to graded doses of PE, which is a positive relationship, was used as an index of BRS for control of HR as reported previously.19–21
Microinjections
At least 30 min was allowed after baseline measurements before commencing microinjections. Multi-barreled glass pipettes with an outer diameter of 30– 50 μm were used as described previously.19–21 For ACE inhibition, the BK potentiating nonapeptide (Pyr–Trp–Pro–Arg–Pro–Gln–Ile–Pro–Pro; BPP9α, BACHEM, Torrance, CA, USA, MW¼1101.27) was used at a dose of 9 nmol in 60 nl. This peptidic inhibitor should inhibit membrane-bound extracellular-facing ACE and will potentiate BK through a separate receptor sensitizing action.22 The AT1 receptor blocker, candesartan (CAN, 24 pmol in 120 nl), was also used. To address the mechanism of BRS changes following bilateral nTS microinjection of BPP9α, the Ang-(1-7) receptor antagonist d-Ala7-Ang-(1-7) (d-Ala, 144 fmol in 120 nl) or BK–B2 receptor antagonist HOE-140 (8 pmol in 120 nl, n=1 or 80 pmol in 120 nl, n=4) was microinjected bilaterally 90–120 min following nTS microinjection of BPP9α. Doses and volumes of BPP9α, CAN, d-Ala and HOE-140 were similar to those found functionally effective in previous studies.19–21,23,24 The concentration of BPP9α for nTS microinjection was three times higher than that of a previous study for hypothalamic microinjection 21,24to adequately block all afferent fibers and neurons receiving baroreceptor input. All drugs were dissolved in artificial cerebrospinal fluid (pH 7.4) and there is no effect of artificial cerebrospinal fluid on BRS, MAP or HR over the time course of study.19–21,25,26 Each drug was bilaterally micro-injected into the nTS (0.4 mm rostral, 0.4 mm lateral to the calamus scriptorius (caudal tip of the area postrema) and 0.4 mm below the dorsal surface) using a glass micropipette connected via PE 50 tubing to a syringe (1 ml; Becton, Dickinson and Company, Parsippany, NJ, USA). Air pressure was generated by pushing on the syringe to displace the desired amount of drug from the pipette into the nTS. This was visualized by movement of the fluid meniscus across the calibration line of the pipette barrel. Peak changes in MAP and HR were measured in response to each antagonist and the BRS was retested within 10 min of nTS microinjections therefore each animal was used as its own control. At the end of the study, rats were decapitated while they were anesthetized for brain removal and verification of injected sites. All injection sites were localized to the nTS within the rostro-caudal level from −13.3 to −14.0 mm caudal to bregma as illustrated previously.25
Analysis of data
Values are presented as mean±s.e.m. Comparisons of changes in BRS and resting MAP and HR in response to BPP9α or CAN and d-Ala or HOE-140 were compared with baseline using the Wilcoxon rank-sum test or a one-sample paired t-test or repeated measures analysis of variance. The criterion for statistical significance was P<0.05 and power for detecting the differences with a β error of 80% was sufficient for all comparisons. Tests were performed using Prism 4.0 and InStat 3 or JMP 5.0.1J software (GraphPad Software, San Diego, CA, USA, or SAS Institute, Cary, NC, USA, respectively).
RESULTS
Effect of nTS microinjection of the AT1 receptor blocker, CAN, and the peptidic ACE inhibitor, BPP9α, in male (mRen2)27 rats
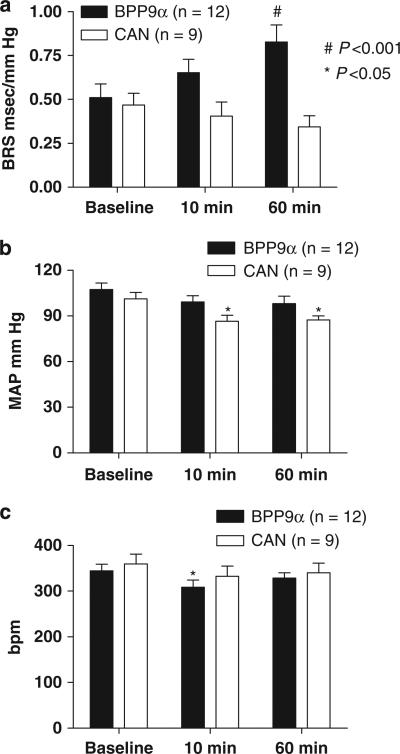
Bilateral nTS microinjection of CAN had no significant effect on BRS for control of HR in response to increases in AP, in spite of a modest reduction in MAP (101±4 baseline, 86±4 at 10 min, 87±3 mm Hg at 60 min, P<0. 05 vs. baseline, n = 9; Figures 1a and b). In a subset of these animals (n=4) studied again at 120 min CAN was still without effect on the BRS. In contrast, bilateral nTS micro-injection of the an ACE inhibitor BPP9α increased BRS for control of HR B60% over baseline at ~60 min (P<0.001, n = 12), without lowering MAP significantly (Figures 1a and b). BPP9α significantly lowered HR at 10 min (Figure 1c).
Figure 1.
The time course of changes in mean arterial pressure (MAP) and baroreflex sensitivity (BRS) after solitary tract nucleus (nTS) microinjection of AT1 receptor blocker, candesartan (CAN), or the peptidic angiotensin-converting enzyme (ACE) inhibitor, BPP9α, in male (mRen2)27 rats. (a) BRS for control of heart rate, (b) MAP and (c): heart rate (HR). CAN microinjected bilaterally into the nTS had no significant effect on BRS at 60 min after the microinjection. On the other hand, bradykinin potentiating nonapeptide, (BPP9α) microinjected bilaterally into the nTS improved BRS over time, requiring 60 min for the action to become significant. There was a significant lowering of MAP in the CAN but not BPP9α treatment group over this time frame. BPP9α significantly lowered HR at 10 min. *P<0.05 and #P<0.001 vs. baseline.
Effects of the Ang-(1-7) receptor antagonist, d-Ala, or the BK–B2 receptor antagonist, HOE-140, on the increase in BRS elicited by BPP9α in male (mRen2)27 rats
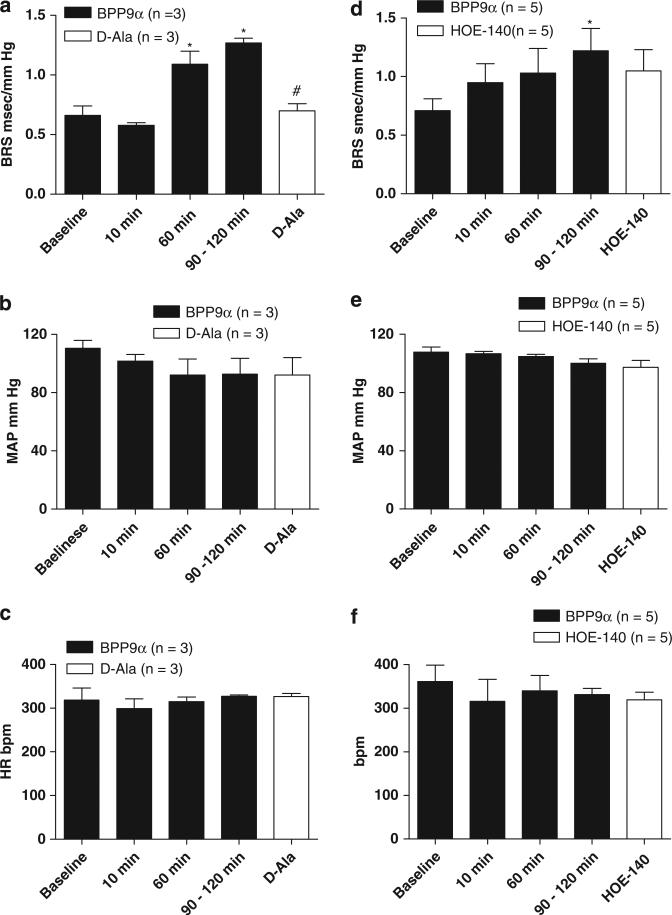
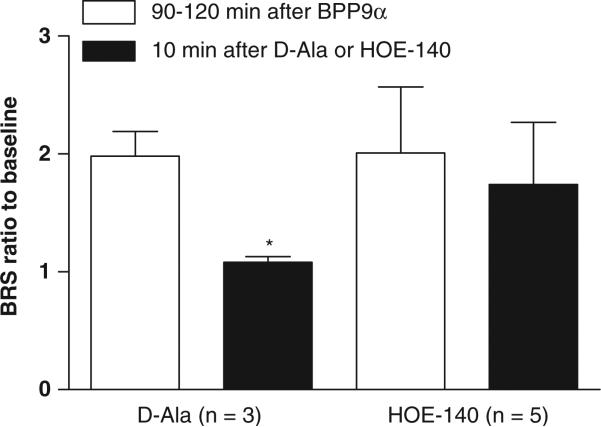
The BPP9α-treated animals shown in Figure 1 were allowed to progress to 90–120 min after which nTS injections of either the Ang-(1-7) receptor antagonist d-Ala or the BK–B2 receptor blocker HOE-140 were given. The BRS improvement elicited over time by BPP9α was reversed by bilateral nTS microinjection of the d-Ala (n=3, Figure 2a). On the other hand, the HOE-140 did not significantly attenuate the BRS improvement after nTS microinjection of BPP9α (n=5, Figure 2d). There was no change in MAP or HR after the d-Ala or the HOE-140 microinjection into nTS (Figures 2b–f). The ratio of the BRS increase to BPP9α relative to baseline was approximately double before injection of either antagonist at the 90–120-min time point and subsequent microinjection of d-Ala reversed BRS ratio completely back to baseline, whereas HOE-140 did not (Figure 3).
Figure 2.
Effects on improved baroreflex sensitivity (BRS) elicited by bradykinin potentiating nonapeptide (BPP)9a of the Ang-(1–7) receptor antagonist, d-Ala or the bradykinin (BK)–B2 receptor antagonist, HOE-140, in male (mRen2)27 rats. BRS changes (a, d), MAP changes (b, e) and HR changes (c, f) before and after microinjection of d-Ala7-Ang-(1–7) (d-Ala; a–c) or HOE-140 (d–f). The BRS improvement elicited by BPP9α was reversed completely by nTS microinjection of d-Ala. Microinjection of the BK–B2 receptor blocker HOE-140 into the nTS did not significantly alter the improvement in BRS elicited by BPP9α. Neither d-Ala nor HOE-140 significantly altered MAP and HR. *P<0.05 vs. baseline, #P<0.05 vs. 60 and 90–120 min.
Figure 3.
Baroreflex sensitivity (BRS) in response to bradykinin potentiating nonapeptide (BPP)9α expressed as the ratio to baseline 2 h and after solitary tract nucleus (nTS) microinjection of d-Ala or HOE-140. Microinjection of BPP9α approximately doubled BRS and this effect was reversed by d-Ala back to baseline, but HOE-140 did not significantly alter the effect of the BPP9α.
DISCUSSION
The data show that acute AT1 receptor blockade in the nTS of (mRen2)27 transgenic hypertensive rats is unable to reverse the effects of long-term Ang II overexpression in terms of impaired BRS for control of HR, whereas ACE inhibition improves BRS over this same time frame. That blockade with d-Ala completely prevents these beneficial effects suggests a primarily Ang-(1-7)-dependent mechanism of action. Furthermore, these data along with previous studies clearly indicate that the autonomic dysfunction in (mRen2)27 rats represents, at least acutely, a greater functional deficit of Ang-(1-7) centrally as opposed to overexpression of Ang II in these animals. In contrast, AT1 blockade with CAN injections into the nTS slightly but significantly lowered MAP in the (mRen2)27 rats in this study.
MAP tended to be higher in our study than in previous reports,2 perhaps accounting for the reduction in resting MAP in these experiments by the AT1 receptor blockade. The reduction in MAP did not result in improvement in the BRS. The present findings suggest that the elevation of Ang-(1-7) locally via ACE inhibition can restore BRS over a time frame of several hours. We do not know what contributes to the original functional deficit in Ang-(1-7) in the (mRen2)27 rats—less formation or more metabolism of the peptide, or loss of the receptor are all possibilities. Previous reports indicate that there are no differences in levels of mRNA for ACE, Ang-(1-7) processing enzymes (ACE2, neprilysin), or the Ang-(1-7) mas receptor in dorsal medullary tissue of normotensive Sprague-Dawley (SD) vs. hypertensive (mRen2)27 rats.2 Although we may argue from the above findings that enzymatic processing of the peptide and the receptor expression within the nTS of (mRen2)27 rats are intact, protein expression or activity levels of these components have not been assessed and may not parallel the mRNA changes.
Lower ACE2 activity in the nTS or in pathways with projections to the nTS 2,27 in (mRen2)27 rats would favor a greater Ang II to Ang-(1-7) ratio. Local nTS injections of an ACE2 inhibitor reduce BRS in SD rats with no additive effect of Ang-(1-7) receptor blockade with d-Ala, suggesting that local generation of Ang-(1-7) can occur in normotensive animals through conversion of Ang II.28 However, an ACE inhibitor would tend to lower both peptides if the sole pathway for Ang-(1-7) formation was dependent upon ACE2 conversion of Ang II to Ang-(1-7) within the nTS. Moreover, the source of Ang II for suppression of BRS at the level of the nTS has been linked to glial cells, whereas the endogenous source of Ang-(1-7) within the nTS providing facilitation of the BRS does not appear to be of glial origin.19,20,29 Thus, current data support independent cellular sources of the two peptides and Ang-(1-7) may be formed independently of Ang II by the endopeptidases neprilysin or prolyl-endopeptidase28,30–33 from Ang I either intracellularly in neurons from the PVN27 or reach the extracellular fluid of the nTS by diffusion from remote neuronal sources or from the circulation.34,35 We used BPP9α to inhibit ACE because it is a small-molecular-weight peptide (MW=1101 Da) that is not expected to enter neurons or glia after microinjection, thereby having primarily an extracellular mode of action. To increase endogenous Ang-(1-7) in the nTS of (mRen2)27 rats using this ACE inhibitor, the protection from metabolism likely occurred in the extracellular space. Finally, it is unlikely that loss of the Ang-(1-7) mas receptor in (mRen2)27 rats accounts for the impaired BRS in the (mRen2)27 rats as previous studies show the effect of Ang-(1-7) injections into nTS produced the same depressor and bradycardic actions as seen in SD rats.2 However, that an effect of the ACE inhibitor on expression or function of the Ang-(1-7) receptor contributed to the improvements in BRS over the time course of the study cannot be ruled out.
Paula et al.36 show that bolus i.v. or intra-arterial administration of Ang-(1-7) potentiated the hypotensive effect of BK. The mechanism of BK potentiation by Ang-(1-7) is complex involving binding to its receptor,37,38 ACE,7,13 and the release of nitric oxide13,36 and/or prostaglandins.36,38 In Wistar rats, the BK-potentiating activity of Ang-(1-7) was completely blocked by d-Ala in the whole animal.37 In these animals ACE inhibition facilitates the BK-potentiating activity.36–38 The effects of ACE inhibition on BRS in our study appear to be mediated by Ang-(1-7), as the Ang-(1–7) receptor blocker d-Ala, but not the BK–B2 receptor blocker, reversed the effect. We used a 10-fold higher concentration of HOE-140 than a previous study23 that showed functional BK–B2 blockade. The fact that the time course for improvement in the BRS required 1–2 h, and the BPP9α potentiation of BK actions is a rapid phenomenon,22 also tends to rule out BPP9α potentiation of BK actions as the primary mechanism of action.
It was surprising that direct blockade of AT1 receptors did not improve the BRS for control of HR, whereas the apparent increase in Ang-(1–7) had beneficial effects over the time course studied. Long-term Ang II overexpression in (mRen2)27 rats is associated with activation of the PI3 kinase pathway in nTS given that inhibition of this pathway lowers resting MAP and restores BRS in (mRen2)27 but not SD rats.39 This effect occurs over ~90 min and is similar to observations in the rostral ventral lateral medulla of spontaneously hypertensive but not normotensive animals.40,41 If Ang II was tonically driving this pathway, then AT1 blockade should have produced similar effects as PI3 kinase blockade. However, a decrease in function of the counterbalancing phosphatase PTP1b also is implied in (mRen2)27 rats, because a PTP1b inhibitor microinjected into nTS of SD rats impaired BRS, but had no effect in the (mRen2)27 rats.42 Our preliminary findings suggest upregulation of mitogen-activated protein kinase signaling pathways relative to the counterbalancing phosphatase MKP-1 (also known as DUSP-1) in the medulla of (mRen2)27 rats.43 If Ang II regulates these kinase and phosphatase pathways over the long-term, acute AT1 receptor blockade may not interrupt these pathways which may explain the hyposensitivity of these animals to AT1 receptor blockade. Finally, it is of interest that the ACE inhibition caused a delayed time course of improvement in the BRS. Although this may be explained by the time required to accumulate sufficient Ang-(1–7) to improve the reflex function, because Ang-(1–7) counteracts the effects of Ang II at least in part through actions of mitogen-activated protein kinase phosphatases,44 an alternate explanation is the time required for their upregulation. Whether the upregulation or activation of these phosphatases by Ang-(1–7) occurs over the time frame of our studies is not known, but this possibility would counteract the kinase activity independent of a direct action of Ang II.
Perspectives and significance
This study demonstrated that acute AT1 receptor blockade in the nTS is unable to reverse the effects of long-term Ang II overexpression on BRS, whereas ACE inhibition improves BRS over this same time frame in a manner that could be reversed completely by an Ang-(1–7), but not BK–B2, antagonist. These studies were carried out under anesthesia, a situation where the resting MAP in the (mRen2)27 rats is greatly reduced relative to conscious levels; however, baseline BRS remains lower than in SD even though baroreceptor reflex resetting likely occurs.2 Therefore, we propose that the main mechanism for the observed delayed increase in BRS following BPP9α is through ACE inhibition to restore endogenous Ang-(1–7) levels within the nTS. The data imply that replacement of Ang-(1–7), either in combination with or instead of a reduction of Ang II, is important for restoration of normal neural control of HR at this brain site. Moreover, the improved BRS occurs independently of the resting MAP.
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health grants HL51952 and HL56973 and the Farley-Hudson Foundation. Katsunori Isa was supported by a post-doctoral award (#0825482E) from the American Heart Association Mid-Atlantic Affiliation. We appreciate Ellen N Tommasi for her technical support.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Mullins JJ, Peters J, Ganten D. Fulminant hypertension in transgenic rats harbouring the mouse Ren-2 gene. Nature. 1990;344:541–544. doi: 10.1038/344541a0. [DOI] [PubMed] [Google Scholar]
- 2.Diz DI, Garcia-Espinosa MA, Gallagher PE, Ganten D, Ferrario CM, Averill DB. Angiotensin-(1-7) and baroreflex function in nucleus tractus solitarii of (mRen2)27 transgenic rats. J Cardiovasc Pharmacol. 2008;51:542–548. doi: 10.1097/FJC.0b013e3181734a54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Espinosa MA, Gallagher PE, Ganten D, Ferrario CM, Reudelhuber T, Diz DI. Intracisternal injection of angiotensin-(1-7)-producing fusion protein plasmid lowers blood pressure in hypertensive (mren2)27 rats. Hypertension. 2006;48:e87. [Google Scholar]
- 4.Dobruch J, Paczwa P, Lon S, Khosla MC, Szczepanska-Sadowska E. Hypotensive function of the brain angiotensin-(1-7) in Sprague Dawley and renin transgenic rats. J Physio Pharm. 2003;54:371–381. [PubMed] [Google Scholar]
- 5.Garcia-Espinosa MA, Shaltout HA, Chappell MC, Gallagher PE, Ferrario CM, Ganten D, Reudelhuber TL, Diz DI. Angiotensin-(1-7) replacement gene transfer in the cisterna magna lowers blood pressure and improves baroreflex function in transgenic (mRen2)27 rats. Hyperternsion. 2008;52:e34. [Google Scholar]
- 6.Wei L, Clauser E, henc-Gelas F, Corvol P. The two homologous domains of human angiotensin I-converting enzyme interact differently with competitive inhibitors. J Biol Chem. 1992;267:13398–13405. [PubMed] [Google Scholar]
- 7.Deddish PA, Marcic B, Jackman HL, Wang HZ, Skidgel RA, Erdos EG. N-domain-specific substrate and C-domain inhibitors of angiotensin-converting enzyme: angiotensin-(1-7) and keto-ACE. Hypertension. 1998;31:912–917. doi: 10.1161/01.hyp.31.4.912. [DOI] [PubMed] [Google Scholar]
- 8.Chappell MC, Pirro NT, Sykes A, Ferrario CM. Metabolism of angiotensin-(1-7) by angiotensin converting enzyme. Hypertension. 1998;31:362–367. doi: 10.1161/01.hyp.31.1.362. [DOI] [PubMed] [Google Scholar]
- 9.Kohara K, Brosnihan KB, Chappell MC, Khosla MC, Ferrario CM. Angiotensin-(1-7): a member of circulating angiotensin peptides. Hypertension. 1991;17:131–138. doi: 10.1161/01.hyp.17.2.131. [DOI] [PubMed] [Google Scholar]
- 10.Kohara K, Brosnihan KB, Ferrario CM. Angiotensin-(1-7) in the spontaneously hyper-tensive rat. Peptides. 1993;14:883–891. doi: 10.1016/0196-9781(93)90063-m. [DOI] [PubMed] [Google Scholar]
- 11.Luque M, Martin P, Martell N, Fernandez C, Brosnihan KB, Ferrario CM. Effects of captopril related to increased levels of prostacyclin and angiotensin-(1-7) in essential hypertension. J Hypertens. 1996;14:799–805. doi: 10.1097/00004872-199606000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Patchett AA, Harris E, Tristram EW, Wyvratt MJ, Wu MT, Taub D, Peterson ER, Ikeler TJ, ten Broeke J, Payne LG, Ondeyka DL, Thorsett ED, Greenlee WJ, Lohr NS, Hoffsommer RD, Joshua H, Ruyle WV, Rothrock JW, Aster SD, Maycock AL, Robinson FM, Hirschmann R, Sweet CS, Ulm EH, Gross DM, Vassil TC, Stone CA. A new class of angiotensin-converting enzyme inhibitors. Nature. 1980;288:280–283. doi: 10.1038/288280a0. [DOI] [PubMed] [Google Scholar]
- 13.Li P, Chappell MC, Ferrario CM, Brosnihan KB. Angiotensin-(1-7) potentiates vasodilation of bradykinin in canine coronary artery by local inhibition of angiotensin converting enzyme and release of nitric oxide. Hypertension. 1997;29:394–400. doi: 10.1161/01.hyp.29.1.394. [DOI] [PubMed] [Google Scholar]
- 14.Chappell MC. Emerging evidence for a functional angiotensin-converting enzyme 2-angiotensin-(1-7)-mas receptor axis: more than regulation of blood pressure? Hyper-tension. 2007;50:596–599. doi: 10.1161/HYPERTENSIONAHA.106.076216. [DOI] [PubMed] [Google Scholar]
- 15.Iyer SN, Ferrario CM, Chappell MC. Angiotensin-(1-7) contributes to the antihypertensive effects of blockade of the renin-angiotensin system. Hypertension. 1998;31:356–361. doi: 10.1161/01.hyp.31.1.356. [DOI] [PubMed] [Google Scholar]
- 16.Bomtempo CA, Santos GF, Santos RA, Campagnole-Santos MJ. Interaction of brady-kinin and angiotensin-(1-7) in the central modulation of the baroreflex control of the heart rate. J Hypertens. 1998;16:1797–1804. doi: 10.1097/00004872-199816120-00013. [DOI] [PubMed] [Google Scholar]
- 17.Gerken VM, Santos RA. Centrally infused bradykinin increases baroreceptor reflex sensitivity. Hypertension. 1992;19:II176–II181. doi: 10.1161/01.hyp.19.2_suppl.ii176. [DOI] [PubMed] [Google Scholar]
- 18.Santos RA, Campagnole-Santos MJ, Andrade SP. Angiotensin-(1-7): an update. Regul Pept. 2000;91:45–62. doi: 10.1016/s0167-0115(00)00138-5. [DOI] [PubMed] [Google Scholar]
- 19.Arnold AC, Sakima A, Ganten D, Ferrario CM, Diz DI. Modulation of reflex function by endogenous angiotensins in older transgenic rats with low glial angiotensinogen. Hypertension. 2008;51:1326–1331. doi: 10.1161/HYPERTENSIONAHA.107.106005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakima A, Averill DB, Kasper SO, Jackson L, Ganten D, Ferrario CM, Gallagher PE, Diz DI. Baroreceptor reflex regulation in anesthetized transgenic rats with low glial-derived angiotensinogen. Am J Physiol Heart Circ Physiol. 2007;292:H1412–H1419. doi: 10.1152/ajpheart.00984.2006. [DOI] [PubMed] [Google Scholar]
- 21.Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM, Diz DI. Impaired heart rate baroreflex in older rats. Role of endogenous angiotensin-(1-7) at the nucleus tractus solitarii. Hypertension. 2005;46:333–340. doi: 10.1161/01.HYP.0000178157.70142.33. [DOI] [PubMed] [Google Scholar]
- 22.Mueller S, Gothe R, Siems WD, Vietinghoff G, Paegelow I, Reissmann S. Potentiation of bradykinin actions by analogues of the bradykinin potentiating nonapeptide BPP9alpha. Peptides. 2005;26:1235–1247. doi: 10.1016/j.peptides.2005.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caligiorne SM, Santos RA, Campagnole-Santos MJ. Cardiovascular effects produced by bradykinin microinjection into the nucleus tractus solitarii of anesthetized rats. Brain Res. 1996;720:183–190. doi: 10.1016/0006-8993(95)01498-5. [DOI] [PubMed] [Google Scholar]
- 24.Diz DI, Jacobowitz DM. Cardiovascular effects of discrete intrahypothalamic and preoptic injections of bradykinin. Brain Res Bull. 1984;12:409–417. doi: 10.1016/0361-9230(84)90113-8. [DOI] [PubMed] [Google Scholar]
- 25.Arnold AC, Shaltout HA, Gallagher PE, Diz DI. Leptin impairs baroreflex sensitivity at the solitary tract nucleus: Evidence for up-regulation of leptin actions in animals with low brain angiotensin. Hypertension. 2009;54:1001–1008. doi: 10.1161/HYPERTENSIONAHA.109.138065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid IA. The use of saralasin to evaluate the function of the brain renin-angiotensin system. Prog Biochem Pharmacol. 1976;12:117–134. [PubMed] [Google Scholar]
- 27.Krob HA, Vinsant SL, Ferrario CM, Friedman DP. Angiotensin-(1-7) immunoreactivity in the hypothalamus of the (mRen-2d)27 transgenic rat. Brain Res. 1998;798:36–45. doi: 10.1016/s0006-8993(98)00384-9. [DOI] [PubMed] [Google Scholar]
- 28.Diz DI, Garcia-Espinosa MA, Gegick S, Tommasi EN, Ferrario CM, Tallant EA, Chappell MC, Gallagher PE. Injections of angiotensin-converting enzyme 2 inhibitor MLN4760 into nucleus tractus solitarii reduce baroreceptor reflex sensitivity for heart rate control in rats. Exp Physiol. 2008;93:694–700. doi: 10.1113/expphysiol.2007.040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakai K, Chapleau MW, Morimoto S, Cassell MD, Sigmund CD. Differential modulation of baroreflex control of heart rate by neuron-vs glia-derived angiotensin II. Physiol Genomics. 2004;20:66–72. doi: 10.1152/physiolgenomics.00168.2004. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto K, Chappell MC, Brosnihan KB, Ferrario CM. In vivo metabolism of angiotensin I by neutral endopeptidase (EC 3.4.24.11) in spontaneously hypertensive rats. Hypertension. 1992;19:692–696. doi: 10.1161/01.hyp.19.6.692. [DOI] [PubMed] [Google Scholar]
- 31.Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa J, Diz DI, Rose JC, Chappell MC. Angiotensin metabolism in renal proximal tubules, urine and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol. 2007;292:F82–F91. doi: 10.1152/ajprenal.00139.2006. [DOI] [PubMed] [Google Scholar]
- 32.Neves LA, Almeida AP, Khosla MC, Santos RA. Metabolism of angiotensin I in isolated rat hearts. Effect of angiotensin converting enzyme inhibitors. Biochem Pharmacol. 1995;50:1451–1459. doi: 10.1016/0006-2952(95)02049-7. [DOI] [PubMed] [Google Scholar]
- 33.Greene LJ, Spadaro ACC, Martins AR, Perussi DeJesus WD, Camargo ACM. Brain endooligopeptidase B: a post-proline cleaving enzyme that inactivates angiotensin I and II. Hypertension. 1982;4:178–184. doi: 10.1161/01.hyp.4.2.178. [DOI] [PubMed] [Google Scholar]
- 34.Tan PSP, Killinger S, Horiuchi J, Dampney RAL. Baroreceptor reflex modulation by circulating angiotensin II is mediated by AT1 receptors in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2267–R2278. doi: 10.1152/ajpregu.00267.2007. [DOI] [PubMed] [Google Scholar]
- 35.Benter IF, Diz DI, Ferrario CM. Pressor and reflex sensitivity is altered in spontaneously hypertensive rats treated with angiotensin-(1-7). Hypertension. 1995;26:1138–1144. doi: 10.1161/01.hyp.26.6.1138. [DOI] [PubMed] [Google Scholar]
- 36.Paula RD, Lima CV, Khosla MC, Santos RA. Angiotensin-(1-7) potentiates the hypotensive effect of bradykinin in conscious rats. Hypertension. 1995;26:1154–1159. doi: 10.1161/01.hyp.26.6.1154. [DOI] [PubMed] [Google Scholar]
- 37.Lima CV, Paula RD, Resende FL, Khosla MC, Santos RA. Potentiation of the hypotensive effect of bradykinin by short-term infusion of angiotensin-(1-7) in normotensive and hypertensive rats. Hypertension. 1997;30:542–548. doi: 10.1161/01.hyp.30.3.542. [DOI] [PubMed] [Google Scholar]
- 38.Aparecida Oliveira M, Bruno Fortes Z, Santos RAS, Kosla MC, De Carvalho MH. Synergistic effect of angiotensin-(1-7) on bradykinin arteriolar dilation in vivo. Peptides. 1999;20:1195–1201. doi: 10.1016/s0196-9781(99)00123-0. [DOI] [PubMed] [Google Scholar]
- 39.Logan E, Diz D, Ganten D, Ferrario C, Averill D. Inhibition of the PI3 kinase signal transduction pathway in nucleus tractus solitarii of (mRen2)27 transgenic rats improves baroreceptor sensitivity. FASEB J. 2008;22:737. [Google Scholar]
- 40.Seyedabadi M, Goodchild AK, Pilowsky PM. Differential role of kinases in brain stem of hypertensive and normotensive rats. Hypertension. 2001;38:1087–1092. doi: 10.1161/hy1101.096054. [DOI] [PubMed] [Google Scholar]
- 41.Veerasingham SJ, Yamazato M, Berecek KH, Wyss JM, Raizada MK. Increased PI3-kinase in presympathetic brain areas of the spontaneously hypertensive rat. Circ Res. 2005;96:277–279. doi: 10.1161/01.RES.0000156275.06641.b2. [DOI] [PubMed] [Google Scholar]
- 42.Arnold AC, Shaltout HA, Gallagher PE, Diz DI. Protein phosphatase 1b activity in the solitary tract nucleus is necessary for normal baroreflex function. Hypertension. 2009;54:e100–e127. [Google Scholar]
- 43.Nautiyal M, Tallant EA, Chappel MC, Diz DI. Mitogen-activated protein kinase phosphatase-1 (MKP-1) is low in dorsal medulla of hypertensive (mRen2)27 transgenic rats. FASEB J. 2010;24:955, 11. [Google Scholar]
- 44.Gallagher PE, Ferrario CM, Tallant EA. MAP kinase/phosphatase pathway mediates the regulation of ACE2 by angiotensin peptides. Am J Physiol Cell Physiol. 2008;295:C1169–C1174. doi: 10.1152/ajpcell.00145.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]