Abstract
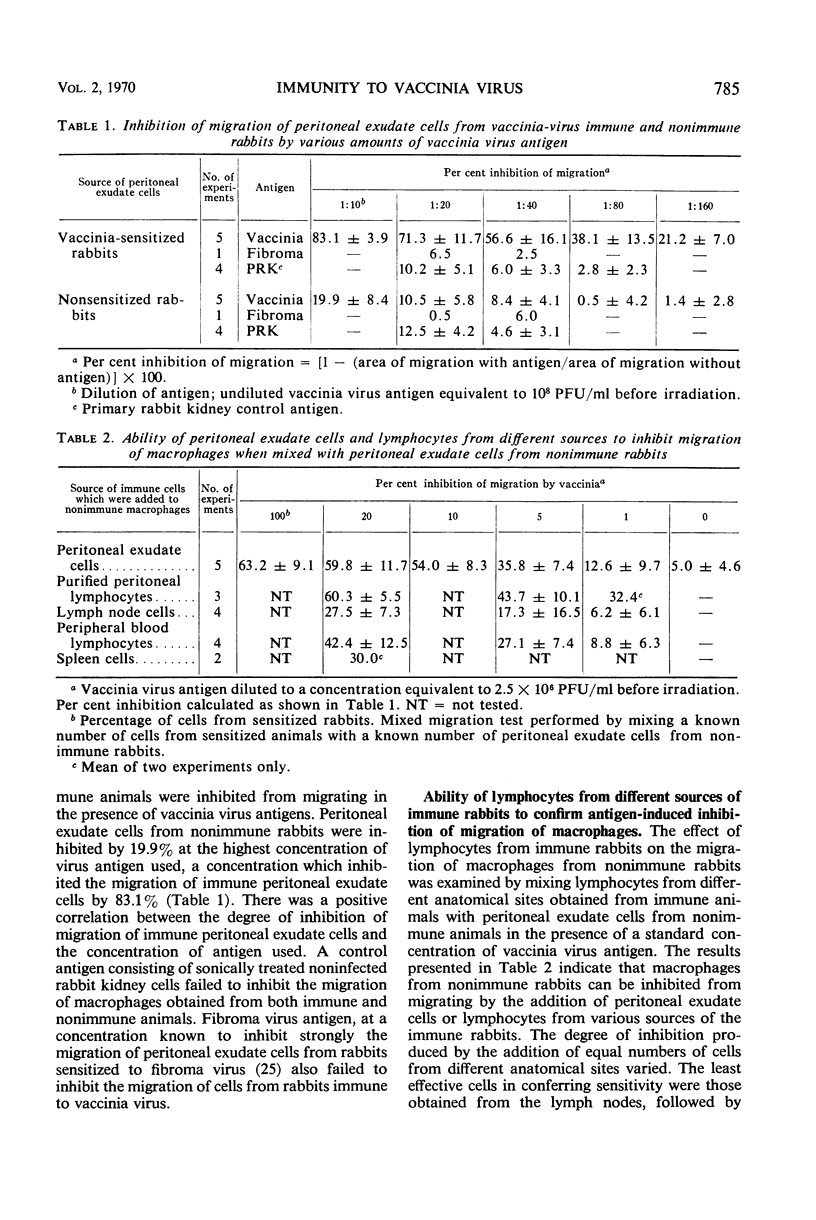
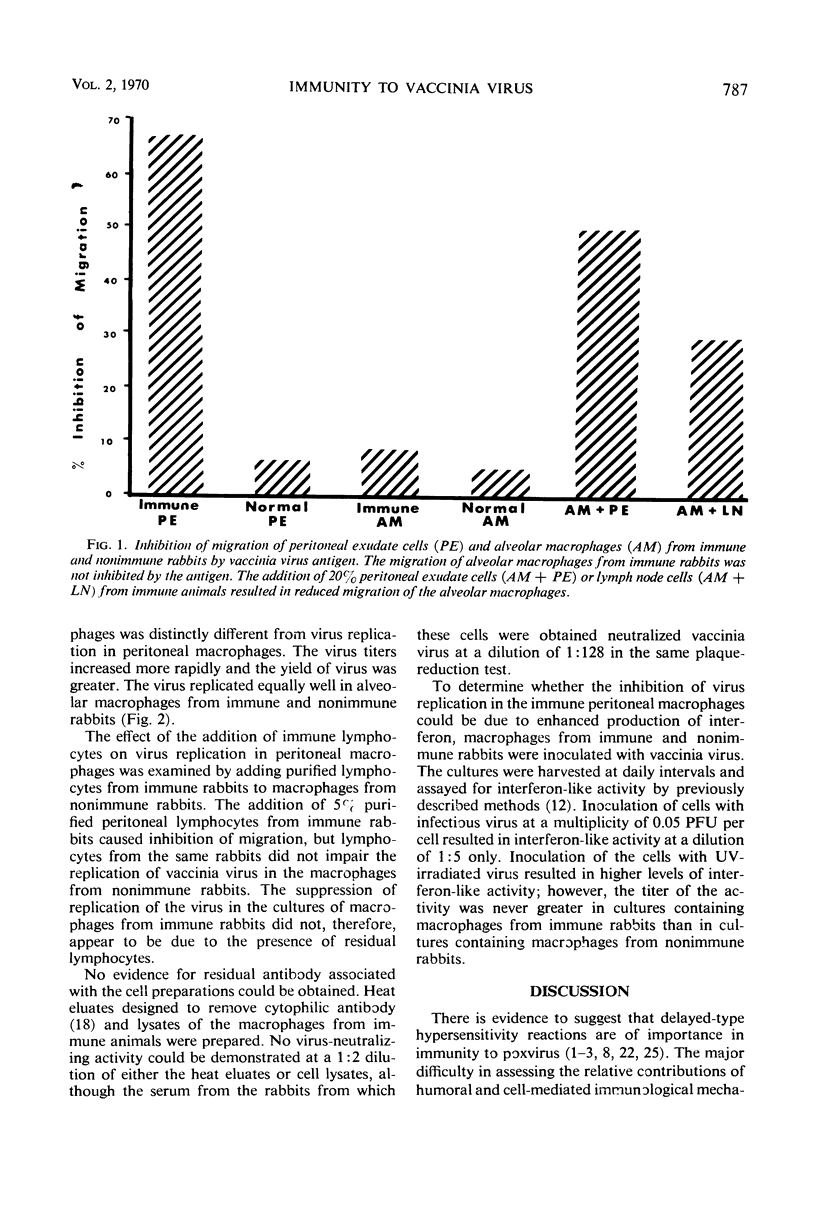
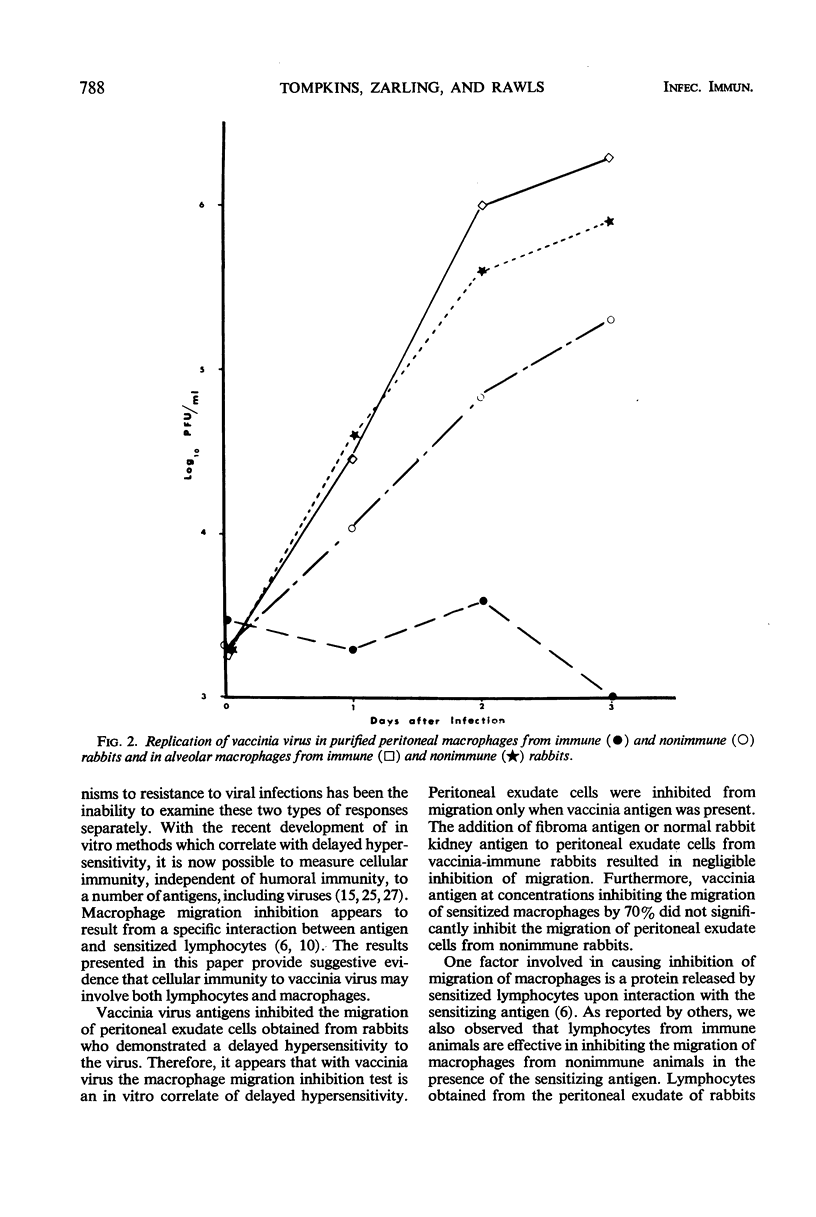
The possible contributions of lymphocytes and macrophages to immunity to vaccinia virus were examined in vitro by determination of antigen-specific inhibition of migration of macrophages and the replication of the virus in purified macrophages. Lymphocytes from various anatomic sites of vaccinia virus-immune rabbits mixed with macrophages from nonimmune rabbits resulted in antigen-specific inhibition of macrophage migration; purified lymphocytes of peritoneal exudates were found to be the most potent inhibitors. Macrophages from peritoneal exudates of immune rabbits purified to contain less than 1% lymphocytes were sensitive to inhibition of migration by vaccinia antigens. Virus grew in macrophages from nonimmune rabbits, but failed to replicate in macrophages from the peritoneal cavity of immune rabbits. Alveolar maracrophages from immune rabbits were not inhibited from migrating in the presence of the viral antigen, and the virus replicated in these cells. The data suggest, but do not prove, that cellular immunity to vaccinia virus in rabbits may be mediated both by lymphocytes and by macrophages.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Cell-mediated immune responses to virus infections and virus-induced tumours. Br Med Bull. 1967 Jan;23(1):60–65. doi: 10.1093/oxfordjournals.bmb.a070518. [DOI] [PubMed] [Google Scholar]
- Asherson G. L., Zembala M. Contact sensitivity in the mouse. IV. The role of lymphocytes and macrophages in passive transfer and the mechanism of their interaction. J Exp Med. 1970 Jul 1;132(1):1–15. doi: 10.1084/jem.132.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett B., Bloom B. R. Studies on the migration inhibitory factor associated with delayed-type hypersensitivity: cytodynamics and specificity. Transplantation. 1967 Jul;5(4 Suppl):996–1000. [PubMed] [Google Scholar]
- Bennett B. Specific suppression of tumor growth by isolated peritoneal macrophages from immunized mice. J Immunol. 1965 Oct;95(4):656–664. [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science. 1966 Jul 1;153(3731):80–82. doi: 10.1126/science.153.3731.80. [DOI] [PubMed] [Google Scholar]
- Boulter E. A. Protection against poxviruses. Proc R Soc Med. 1969 Mar 3;62(3):295–297. [PMC free article] [PubMed] [Google Scholar]
- Bowden D. H., Adamson I. Y., Grantham W. G., Wyatt J. P. Origin of the lung macrophage. Evidence derived from radiation injury. Arch Pathol. 1969 Nov;88(5):540–546. [PubMed] [Google Scholar]
- DAVID J. R., AL-ASKARI S., LAWRENCE H. S., THOMAS L. DELAYED HYPERSENSITIVITY IN VITRO. I. THE SPECIFICITY OF INHIBITION OF CELL MIGRATION BY ANTIGENS. J Immunol. 1964 Aug;93:264–273. [PubMed] [Google Scholar]
- David J. R. Delayed hypersensitivity in vitro: its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc Natl Acad Sci U S A. 1966 Jul;56(1):72–77. doi: 10.1073/pnas.56.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmyter J., Rawls W. E., Melnick J. L. A human interferon that crosses the species line. Proc Natl Acad Sci U S A. 1968 Jan;59(1):69–76. doi: 10.1073/pnas.59.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumonde D. C. The role of the macrophage in delayed hypersensitivity. Br Med Bull. 1967 Jan;23(1):9–14. doi: 10.1093/oxfordjournals.bmb.a070524. [DOI] [PubMed] [Google Scholar]
- Edelman R., Wheelock E. F. Specific role of each human leukocyte type in viral infections. II. Phytohemagglutinin-treated lymphocytes as host cells for vesicular stomatitis virus replication in vitro. J Virol. 1968 May;2(5):440–448. doi: 10.1128/jvi.2.5.440-448.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstone S. M., Beachey E. H., Rytel M. W. Induction of delayed hypersensitivity to influenza and mumps viruses in mice. J Immunol. 1969 Oct;103(4):844–849. [PubMed] [Google Scholar]
- Glasgow L. A. Leukocytes and interferon in the host response to viral infections. II. Enhanced interferon response of leukocytes from immune animals. J Bacteriol. 1966 Jun;91(6):2185–2191. doi: 10.1128/jb.91.6.2185-2191.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gresser I., Lang D. J. Relationships between viruses and leucocytes. Prog Med Virol. 1966;8:62–130. [PubMed] [Google Scholar]
- Heise E. R., Han S., Weiser R. S. In vitro studies on the mechanism of macrophage migration inhibition in tuberculin sensitivity. J Immunol. 1968 Nov;101(5):1004–1015. [PubMed] [Google Scholar]
- Klein G., Oettgen H. F. Immunologic factors involved in the growth of primary tumors in human or animal hosts. Cancer Res. 1969 Sep;29(9):1741–1746. [PubMed] [Google Scholar]
- MIMS C. A. ASPECTS OF THE PATHOGENESIS OF VIRUS DISEASES. Bacteriol Rev. 1964 Mar;28:30–71. doi: 10.1128/br.28.1.30-71.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MYRVIK Q., LEAKE E. S., FARISS B. Studies on pulmonary alveolar macrophages from the normal rabbit: a technique to procure them in a high state of purity. J Immunol. 1961 Feb;86:128–132. [PubMed] [Google Scholar]
- STEINBERGER A., RIGHTS F. L. EFFECT OF IMMUNIZATION ON TISSUE SUSCEPTIBILITY TO VACCINIA VIRUS IN VITRO. Virology. 1963 Nov;21:402–410. doi: 10.1016/0042-6822(63)90202-2. [DOI] [PubMed] [Google Scholar]
- Silverstein S. Macrophages and viral immunity. Semin Hematol. 1970 Apr;7(2):185–214. [PubMed] [Google Scholar]
- Subrahmanyan T. P., Mims C. A. Interferon production by mouse peritoneal cells. J Reticuloendothel Soc. 1970 Jan;7(1):32–42. [PubMed] [Google Scholar]
- Tompkins W. A., Adams C., Rawls W. E. An in vitro measure of cellular immunity to fibroma virus. J Immunol. 1970 Feb;104(2):502–510. [PubMed] [Google Scholar]
- Tompkins W. A., Walker D. L., Hinze H. C. Cellular deoxyribonucleic acid synthesis and loss of contact inhibition in irradiated and contact-inhibited cell cultures infected with fibroma virus. J Virol. 1969 Nov;4(5):603–609. doi: 10.1128/jvi.4.5.603-609.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremonti L. P., Jackson G. G. Study of delayed hypersensitivity to myxoviruses induced by vaccines. Appl Microbiol. 1969 Apr;17(4):577–583. doi: 10.1128/am.17.4.577-583.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems F. T., Melnick J. L., Rawls W. E. Replication of poliovirus in phytohemagglutinin-stimulated human lymphocytes. J Virol. 1969 May;3(5):451–457. doi: 10.1128/jvi.3.5.451-457.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]


