Abstract
Melanocortin 4 receptor (MC4R), which is associated with inherited human obesity, is involoved in food intake and body weight of mammals. To study the relationships between MC4R gene polymorphism and body weight in Beagle dogs, we detected and compared the nucleotide sequence of the whole coding region and 3′- and 5′- flanking regions of the dog MC4R gene (1214 bp). In 120 Beagle dogs, two SNPs (A420C, C895T) were identified and their relation with body weight was analyzed with RFLP-PCR method. The results showed that the SNP at A420C was significantly associated with canine body weight trait when it changed amino acid 101 of the MC4R protein from asparagine to threonine,while canine body weight variations were significant in female dogs when MC4R nonsense mutation at C895T. It suggested that the two SNPs might affect the MC4R gene’s function which was relative to body weight in Beagle dogs. Therefore, MC4R was a candidate gene for selecting different size dogs with the MC4R SNPs (A420C, C895T) being potentially valuable as a genetic marker.
Keywords: beagle dogs, body weight, MC4R, SNP
Introduction
Melanocortin-4 receptor (MC4R) plays an important role in the regulation of feeding behavior, body weight and energy homeostasis [2]. As one of five receptors in the melanocortin system, it is encoded by a single exon and expressed in the brain, predominantly in the hypothalamus. Mouse and human genetic studies showedthat the mice of MC4R knockout displayed extreme obesity [8], and human MC4R gene mutations can cause childhood simple obesity [1, 14], which is the most common cause of hereditary human obesity [4]. Over 50 mutations affecting the amino acid sequence of the human MC4R gene have been recorded [12], and some mutations were related to obesity that affect body weight. Body weight is an important economic trait. It have been reported that many significant associations of MC4R genotypes with backfat, growth rates and food intake in a number of breeds and lines of the pigs [3, 5, 6, 13]. Recently, some single nucleotide polymorphisms (SNPs) in the canine MC4R gene were identified in red fox, Chinese raccoon dog and arctic fox [16], too. However, no SNP that affect the MC4R gene function was found in Beagle dogs. In this study, we found two SNPs in MC4R genes in the Beagle dogs, and analyzed their relation with dog’s body weight.
Materials and Methods
Animals
Six beagle dogs from litter mate were housed as a group (males or females) in an air conditioned room (space: 5×5 m, temperature: 22 ± 2°C, relative humidity: 55 ± 10%, light period: 7:00 AM to 7:00 PM) in Laboratory Animal Center of Academy of Military Medical Sciences. Feeding was conducted twice a day at 8:00 AM and at 5:00 PM by providing abundant dog foods. Heparinized blood of 120 beagles (60 females and 60 males, 12–13 months) was collected for isolation of genomic DNA that was used to amplify the MC4R gene. The body weight of the dogs was recorded as while as taking blood.
SNP identification
Primers were designed based on the canine MC4R DNA sequence (GenBank BD181636) and the canine MC4R gene sequence (GenBank DQ084210). Four sets of PCR primers were designed to cover the whole coding sequence and part of the 3’ and 5’flanking regions. The sequences and the length of PCR products were shown in the Table 1. Each PCR reaction contained 100 ng of genomic DNA, 0.2 μM of each primer, 0.2 mM dNTPs, 1× PCR buffer with 1.5 mM MgCl2 and 1 U Taq polymerase in a total volume of 20 μl. PCR was performed using a Biometra thermocycler. The amplification conditions were 5 min at 94°C, 35 cycles of 30 s at 94°C, 30 s at 52°C (primer pairs 1), 50°C (primer pairs 2), 57°C (primer pairs 3) and 48°C (primer pairs 4) respectively, and 1 min at 72°C, followed by a 10-min final extension at 72°C (Table 1).
Table 1. Primers used for PCR amplification of four canine MC4R fragments.
| Primer symbol | Primer sequence (5’-3’) | SNP | Amino acid change | Length |
|---|---|---|---|---|
| MC4R-1 | F: CCTAGACTAAAGTTAAGGTGGGA | NO | NO | 432 bp |
| R: AGGGACTCAGTGGCGTTGC | ||||
| MC4R-2 | F: ACGCCACTGAGTCCCT | A420C | N101T | 492 bp |
| R: TGTCCGAGTAAATGATGAAC | ||||
| MC4R-3 | F: CATCATTTACTCGGACAG | C895T | NO | 419 bp |
| R: CACCCAGAGGATAGCA | ||||
| MC4R-4 | F: ACTCCATCATCGACCCT | NO | NO | 232 bp |
| R: TCTCAACTCCTGCCTTAT |
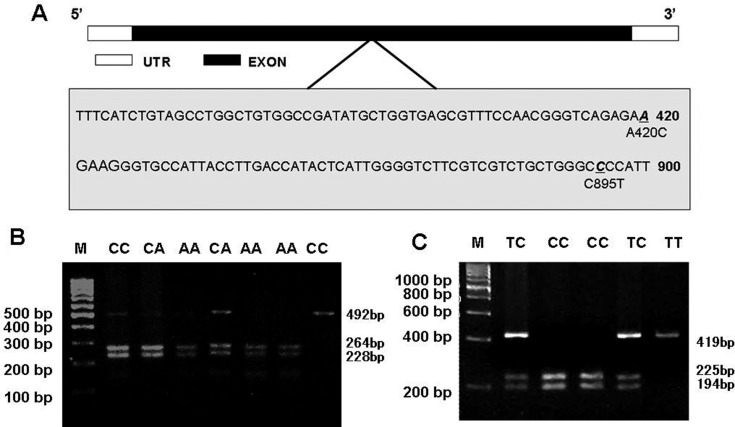
The PCR products in two individual dogs with 6.5 and 16 kg were isolated and purified from agarose gel with the use of a Agarose Gel DNA Fragment Recovery Kit (TaKaRa, DaLian, China) and then sequenced by Sangon Biotech Co., Ltd. (Shanghai, China). The two single nucleotide polymorphisms (SNPs) were identified in the sequenced fragments using BLAST, situated in Eco0109 І and PshA І restriction enzyme recognition site, respectively (Fig. 1).
Fig. 1.
Identification and PCR-RFLP analysis of two SNPs in canine MC4R gene. A. The site of two new SNPs in canine MC4R gene. B. The electrophoresis results of PCR-RFLP test in MC4R A420C site. After PshAI digestion, it divided into gene types with CC (492 bp, 1 bands), CA (228 bp, 264 bp, 492 bp, 3 bands) and AA (228 bp, 264 bp, 2 bands). C. The electrophoresis results of PCR-RFLP test in MC4R C895T site. Three genotypes with TT (419 bp, 1 band), TC (194 bp, 225 bp, 419 bp, 3 bands) and CC (194 bp, 225 bp, 2 bands) were identified by Eco0109 І restriction enzyme.
PCR-RFLP
To genotype all the studied animals at two polymorphic sites, PCR-RFLP analyses were performed. Eco0109 І and PshA І endonucleases were used. All restriction enzyme digestions were performed in 10 μl reaction mixture containing 4 μl of the PCR products, 1× digestion buffer and 2 U of the respective endonuclease. All digested products were detected by 2% agarose gel electrophoresis.
Statistical analyses
Allele and genotype frequencies were calculated by a simple allele counting method. The association between the genotypes of MC4R candidate gene and body weight traits was evaluated with the least square method (GLM procedure of the SAS software package; SAS Institute, USA) using the following statistical linear model:
| Yij=μ+Xj+bij+eij |
Yij is the observation of the carcass traits, μ is the mean for the trait, bij is the regression coefficients of the weight and eij is residual effect.
Results and Discussion
Identification of two SNPs in canine MC4R gene
Two SNPs were identified by DNA sequencing in the MC4R sequences from two individual beagles with minimum and maximum body weight (Table 2), a mis-sense substitution A420C that changes asparagine to threonine at position 101 of the amino acid sequence, and a silent substitution C895T (Fig.1A).
Table 2. Descriptive Statistics of dog body weight.
| Gender | Number | Body weight (kg) |
t-test* | ||
|---|---|---|---|---|---|
| Minimum | Maximum | Mean ± SD | |||
| Female | 60 | 6.5 | 14 | 8.76 ± 2.09 | |
| Male | 60 | 6.6 | 16 | 9.65 ± 2.84 | P=0.052 |
| Total | 120 | 6.5 | 16 | 9.21 ± 2.53 | |
*Difference in female and male dog’s body weight.
As one of G protein-coupled receptors (GPCRs), the highly conserved residues in MC4R protein may play important roles for ligand binding or intracellular signal transmission [11]. A multiple alignment of the amino acid sequences of the MC4R proteins from different species showed that the threonine found at the position 101 of the second transmembrane domain was very highly conserved in the MCR proteins (Fig. 2). Although a silent mutation doesn’t affect the amino-acid sequence of a protein, it can affect protein folding and function. Kimchi-Sarfaty et al. found a such silent mutation that was caused by a synonymous SNP in MDR1 (Multidrug Resistance 1), which encodes the protein pump P-gp (P-glycoprotein) [7]. Therefore, either mis-sense mutation or synonymous mutation in CDS (Coding DNA Sequence) region of MC4R gene may affect its function, which would present some variant traits among dogs.
Fig. 2.
Multiple amino acid alignments of the putative second transmembrane domain of canine MC4R with other MCRs. * represented the predicted sequence positions for canine MC4R. The other amino acid sequences were obtained from the GenBank database (Accession Numbers P32245, P56450, Q9GLJ8, O97504, P70596, AAT73771, AAP23196, AAH80622, AAO67714, P41968). The missense variant in canine MC4R substituted amino acid N (Asn) for T (Thr) in the position marked with an arrow. The Thr was highly conserved among different MC4R proteins, and it changed to Asn only in Human MC2R protein.
Association mis-sense SNP of MC4R with canine body weight trait
To investigate the relationships between the two SNPs of MC4R with canine body weight trait, we analyzed different MC4R genotypes in 120 Beagle dogs. Estimated MC4R alleles and genotype frequencies for the Beagles were shown in the Table 3. In A402C SNP site, the frequency for allele A was higher than that for allele C, and 11% and 55% displayed the CC and AA genotype. The C895T frequency for allele C was higher than that for allele T, and 3% and 67% displayed the TT and CC genotype, respectively. In general, body weight of females was lower than that of males in Beagle dogs. Therefore, statistical analysis in different sex group was also operated in this study, separately.
Table 3. Genotype frequencies and allele frequencies of MC4R gene.
| SNPs | Gender | % of genotype frequency (No. of samples) |
% of allele frequency |
|||
|---|---|---|---|---|---|---|
| A420C | CC | CA | AA | C | A | |
| Female | 8 (5) | 33 (20) | 58 (35) | 25 | 75 | |
| Male | 13 (8) | 35 (21) | 52 (31) | 31 | 69 | |
| Total | 11 (13) | 34 (41) | 55 (66) | 28 | 72 | |
| C895T | TT | TC | CC | T | C | |
| Female | 7 (4) | 30 (18) | 63 (38) | 22 | 78 | |
| Male | 30 (18) | 70 (42) | 15 | 85 | ||
| Total | 3 (4) | 30 (36) | 67 (80) | 18 | 82 | |
Association of different MC4R genotypes with body weight trait were evaluated with the least square method, and the overall means ± standard deviation of the analyzed traits were summarized in the Tables 4 and 5. In the overall analysis, the dogs with homozygous CC genotypes were on average significantly weightier than others, while insignificant differences between the CA and AA genotype dogs. The results suggested that the mis-sense substitution in A402C of MC4R gene affected the phenotype variation of body weight in 120 Beagle dogs. Meanwhile, insignificant differences for body weight were found between the TT, TC and CC genotype dogs on the whole for the C895T mutation although female dog with heterozygous gene have lighter body weight compared with homozygous gene. It indicated that silent mutation of MC4R genes in female dogs could affect the modulation of canine body weight, and the estrogen could be an important effective factor of MC4R function to regulate body weight of Beagle dogs. Recently, some SNPs of canine MC4R gene, such as G637T, T777C and C+33G, were found from 19 different dog breeds [16, 18]. For the SNPs G637T and T777C, no association of the variant with canine body weight, length, height, or body index score was found in 187 Golden Retrievers [18]. However, the associations between polymorphisms of MC4R with obesity factors [10, 15], and birth weight [17] have been verified in other animals. The studies suggested that the variations of body weight among dogs with MC4R mis-sense mutation may indicate functional defect of MC4R in vitro.
Table 4. Body weight of different MC4R (A420C) genotypes in Beagle dogs.
| Body weight (kg) | SNP Genotype |
P value | ||
|---|---|---|---|---|
| CC (mean ± SD) | CA (mean ± SD) | AA (mean ± SD) | ||
| Female | 7.52 ± 0.55B | 8.21 ± 1.81B | 9.26 ± 1.6A | 0.021 |
| Male | 7.40 ± 0.30B | 8.95 ± 2.08B | 10.72 ± 3.21A | 0.004 |
| Total | 7.44 ± 0.41B | 8.58 ± 1.96B | 9.94 ± 2.59A | 0.000 |
A, BDifferent superscripts within columns differ significantly (P<0.05).
Table 5. Body weight of different MC4R (C895T) genotypes in Beagle dogs.
| Body weight (kg) | SNP Genotype |
P value | ||
|---|---|---|---|---|
| TT (mean ± SD) | TC (mean ± SD) | CC (mean ± SD) | ||
| Female | 10.75 ± 2.30A | 7.76 ± 1.33B | 9.02 ± 2.16A | 0.013 |
| Male | 9.18 ± 3.49A | 9.86 ± 2.54A | ||
| Total | 10.75 ± 2.30A | 8.47 ± 2.72A | 9.46 ± 2.39A | |
A, BDifferent superscripts within columns differ significantly (P<0.05).
The change of amino acid in A402C sites might affected second transmembrane domain (Transmembrane 2, TM2) that construct a zinc finger structure of the human MC4R receptor [9]. However, a mutation does not always influence a gene function, Jin Yan et al found that the natural variant cMC4R V213F (G637T) did not have any functional defects by measuring ligand binding and signaling properties in Human embryonic kidney (HEK) 293T cells [19]. MC4R functional abnormality may induce excess food intake and lead to obesity. In our study, the dogs were on a condition they ate what they could because excess food was fed. Therefore, the difference in body weights observed in Beagle dogs might be addressed to food intake and the difference in body fat content although the relationship of MC4R genotypes with obesity was not directly indicated.
To explore the effect of the SNPs (A420C, C895T) in canine MC4R gene, the following researches should be done in the future. The difference of food intake and obesity should be analysised among the dogs with the different MC4R SNPs. Whether would the pharmacological characterization of canine melancortin-4 receptor in MDCK cells be influenced by the variant N101T receptor or C895T mutation? Whether does the estrogen affect canine MC4R gene function in vivo and in vitro?
In conclusion, the analysis of the MC4R gene polymorphism on the genotypes in beagle dogs showed the MC4R gene was a candidate modifier gene for canine body weight and the moderate MC4R SNP (A420C, C895T) might be applied in selecting different size dogs as a genetic marker.
References
- 1.Alfieri A., Pasanisi F., Salzano S., Esposito L., Martone D., Tafuri D., Daniele A., Contaldo F., Sacchetti L., Zagari A., Buono P.2010. Functional analysis of melanocortin-4-receptor mutants identified in severely obese subjects living in Southern Italy. Gene 457: 35–41. doi: 10.1016/j.gene.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 2.Butler A.A.2006. The melanocortin system and energy balance. Peptides 27: 281–290. doi: 10.1016/j.peptides.2005.02.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Davoli R., Braglia S., Valastro V., Annarratone C., Comella M., Zambonelli P., Nisi I., Gallo M., Buttazzoni L., Russo V.2012. Analysis of MC4R polymorphism in Italian Large White and Italian Duroc pigs: association with carcass traits. Meat Sci. 90: 887–892. doi: 10.1016/j.meatsci.2011.11.025 [DOI] [PubMed] [Google Scholar]
- 4.Gantz I., Miwa H., Konda Y., Shimoto Y., Tashiro T., Watson S.J., DelValle J., Yamada T.1993. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J. Biol. Chem. 268: 15174–15179. [PubMed] [Google Scholar]
- 5.Houston R.D., Cameron N.D., Rance K.A.2004. A melanocortin-4 receptor (MC4R) polymorphism is associated with performance traits in divergently selected Large White pig populations. Anim. Genet. 35: 386–390. doi: 10.1111/j.1365-2052.2004.01182.x [DOI] [PubMed] [Google Scholar]
- 6.Jokubka R., Maak S., Kerziene S., Swalve H.H.2006. Association of a melanocortin 4 receptor (MC4R) polymorphism with performance traits in Lithuanian White pigs. J. Anim. Breed. Genet. 123: 17–22. doi: 10.1111/j.1439-0388.2006.00559.x [DOI] [PubMed] [Google Scholar]
- 7.Kimchi-Sarfaty C., Oh J.M., Kim I.W., Sauna Z.E., Calcagno A.M., Ambudkar S.V., Gottesman M.M.2007. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 315: 525–528. doi: 10.1126/science.1135308 [DOI] [PubMed] [Google Scholar]
- 8.Kumar K.G., Sutton G.M., Dong J.Z., Roubert P., Plas P., Halem H.A., Culler M.D., Yang H., Dixit V.D., Butler A.A.2009. Analysis of the therapeutic functions of novel melanocortin receptor agonists in MC3R- and MC4R-deficient C57BL/6J mice. Peptides 30: 1892–1900. doi: 10.1016/j.peptides.2009.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagerstrom M.C., Klovins J., Fredriksson R., Fridmanis D., Haitina T., Ling M.K., Berglund M.M., Schioth H.B.2003. High affinity agonistic metal ion binding sites within the melanocortin 4 receptor illustrate conformational change of transmembrane region 3. J. Biol. Chem. 278: 51521–51526. doi: 10.1074/jbc.M307683200 [DOI] [PubMed] [Google Scholar]
- 10.Liu H., Tian W., Zan L., Wang H., Cui H.2010. Mutations of MC4R gene and its association with economic traits in Qinchuan cattle. Mol. Biol. Rep. 37: 535–540. doi: 10.1007/s11033-009-9706-0 [DOI] [PubMed] [Google Scholar]
- 11.Madala P.K., Fairlie D.P., Boden M.2012. Matching cavities in g protein-coupled receptors to infer ligand-binding sites. J. Chem. Inf. Model. 52: 1401–1410. doi: 10.1021/ci2005498 [DOI] [PubMed] [Google Scholar]
- 12.Perusse L., Rankinen T., Zuberi A., Chagnon Y.C., Weisnagel S.J., Argyropoulos G., Walts B., Snyder E.E., Bouchard C.2005. The human obesity gene map: the 2004 update. Obes. Res. 13: 381–490. doi: 10.1038/oby.2005.50 [DOI] [PubMed] [Google Scholar]
- 13.Piorkowska K., Tyra M., Rogoz M., Ropka-Molik K., Oczkowicz M., Rozycki M.2010. Association of the melanocortin-4 receptor (MC4R) with feed intake, growth, fatness and carcass composition in pigs raised in Poland. Meat Sci. 85: 297–301. doi: 10.1016/j.meatsci.2010.01.017 [DOI] [PubMed] [Google Scholar]
- 14.Roth C.L., Ludwig M., Woelfle J., Fan Z.C., Brumm H., Biebermann H., Tao Y.X.2009. A novel melanocortin-4 receptor gene mutation in a female patient with severe childhood obesity. Endocrine 36: 52–59. doi: 10.1007/s12020-009-9156-4 [DOI] [PubMed] [Google Scholar]
- 15.Seong J., Suh D.S., Park K.D., Lee H.K., Kong H.S.2012. Identification and analysis of MC4R polymorphisms and their association with economic traits of Korean cattle (Hanwoo). Mol. Biol. Rep. 39: 3597–3601. doi: 10.1007/s11033-011-1133-3 [DOI] [PubMed] [Google Scholar]
- 16.Skorczyk A., Stachowiak M., Szczerbal I., Klukowska-Roetzler J., Schelling C., Dolf G., Switonski M.2007. Polymorphism and chromosomal location of the MC4R (melanocortin-4 receptor) gene in the dog and red fox. Gene 392: 247–252. doi: 10.1016/j.gene.2006.12.027 [DOI] [PubMed] [Google Scholar]
- 17.Song X.M., Jiang J.F., Zhang G.Z., Shi F.X., Jiang Y.Q.2012. DNA polymorphisms of the Hu sheep melanocortin-4 receptor (MC4R) gene associated with birth weight and 45d-weaning weight. Genet. Mol. Res. 11: 4432–4441. doi: 10.4238/2012.September.27.3 [DOI] [PubMed] [Google Scholar]
- 18.van den Berg L., van den Berg S.M., Martens E.E., Hazewinkel H.A., Dijkshoorn N.A., Delemarre-van de Waal H.A., Heutink P., Leegwater P.A., Heuven H.C.2010. Analysis of variation in the melanocortin-4 receptor gene (mc4r) in Golden Retriever dogs. Anim. Genet. 41: 557. doi: 10.1111/j.1365-2052.2010.02049.x [DOI] [PubMed] [Google Scholar]
- 19.Yan J., Tao Y.X.2011. Pharmacological characterization of canine melancortin-4 receptor and its natural variant V213F. Domest. Anim. Endocrinol. 41: 91–97. doi: 10.1016/j.domaniend.2011.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]