Abstract
Duchenne muscular dystrophy (DMD) is an X-linked recessive progressive muscle degenerative disorder that causes dilated cardiomyopathy in the second decade of life in affected males. Dystrophin, the gene responsible for DMD, encodes full-length dystrophin and various short dystrophin isoforms. In the mouse heart, full-length dystrophin Dp427 and a short dystrophin isoform, Dp71, are expressed. In this study, we intended to clarify the functions of these dystrophin isoforms in DMD-related cardiomyopathy. We used two strains of mice: mdx mice, in which Dp427 was absent but Dp71 was present, and DMD-null mice, in which both were absent. By immunohistochemical staining and density-gradient centrifugation, we found that Dp427 was located in the cardiac sarcolemma and also at the T-tubules, whereas Dp71 was specifically located at the T-tubules. In order to determine whether T tubule-associated Dp71 was involved in DMD-related cardiac disruption, we compared the cardiac phenotypes between DMD-null mice and mdx mice. Both DMD-null mice and mdx mice exhibited severe necrosis, which was followed by fibrosis in cardiac muscle. However, we could not detect a significant difference in myocardial fibrosis between mdx mice and DMD-null mice. Based on the present results, we have shown that cardiac myopathy is caused predominantly by a deficiency of full-length dystrophin Dp427.
Keywords: cardiomyopathy, dp427, dp71, dystrophin, heart
Introduction
Mutations in the dystrophin gene cause Duchenne or Becker’s muscular dystrophy (DMD or BMD) as well as X-linked dilated cardiomyopathy [3]. The dystrophin gene has seven tissue-specific promoters, encoding at least seven protein isoforms: three 427 kDa full-length dystrophins (Dp427c, Dp427m, and Dp427p) and four N-terminus truncated short isoforms (Dp260, Dp140, Dp116, and Dp71). Dp427m is responsible for DMD and BMD, and is expressed mainly in skeletal and cardiac muscle, while other dystrophin isoforms are detected in various non-muscle tissues, mainly in neural tissues. Dp260 is expressed in the retina, and Dp140 in expressed in the brain, retina, and kidney. Dp116 is expressed in adult peripheral nerves, while Dp71 is ubiquitously detected in various tissues [10].
Cardiomyopathy in association with DMD and BMD has been well documented. During clinical progression of DMD, approximately 90% of patients develop a serious impairment of cardiac function, and cardiomyopathy is the cause of death in approximately 20% of cases [15]. Pathogenesis of cardiomyopathy has been examined using the mdx mouse, a naturally occurring animal model for DMD [14]. The mdx mouse has a point mutation in exon 23 of the dystrophin gene, which forms a premature stop codon. Therefore, mdx mice lack the expression of full-length dystrophin, but short dystrophin isoforms are expressed normally. These mice develop late onset and progressive cardiomyopathy that have some similarities with that observed in human dystrophic patients [11]. This makes it a suitable model system to study the pathogenetic mechanism of DMD-related cardiomyopathy.
Previously, we generated a new DMD model mouse by means of deleting the entire 2.4 Mb genomic region of the dystrophin gene on mouse chromosome X using a Cre-loxP recombination system [8]. Thus, the DMD-null mice produced are a suitable experimental animal for studying the function of the short dystrophin isoform. In cardiomyocytes, the dystrophin isoforms Dp427 and Dp71 are both expressed, as contrasted with skeletal myofibers, in which Dp427 is solely present. Dp71 has been reported to play a variety of unique roles. In PC12 cells, Dp71 is involved in cell-substrate interactions by regulating adhesion to the substrate [4]. In mice spermatozoa, absence of Dp71 has been reported to cause a significant increase in abnormal flagella morphology [6]. In addition, we have reported that loss of Dp71 in olfactory ensheathing cells causes vomeronasal nerve defasciculation in the mouse olfactory system [16]. Therefore, it is plausible that Dp71 expressed in cardiomyocytes may play a unique role. However, no study has reported thus far on the function of Dp71 in cardiac muscle. Involvement of Dp71 in cardiomyopathy can be assessed by comparing cardiac phenotypes of mdx mice and DMD-null mice. In the present study, we investigated the subcellular localization of Dp427 and Dp71 in cardiomyocytes and also examined the link between Dp71 and cardiomyopathy using mdx and DMD-null mice.
Materials and Methods
Mice
C57BL/6 and mdx mice (genetic background C57BL/10) were purchased from CLEA Japan, Inc. (Tokyo, Japan). The colony of DMD-null mice (genetic background: C57BL6/CBA hybrid) was maintained according to a previously described method [8]. The Institutional Animal Care and Use Committee of Kitasato University approved all experimental protocols.
Histology
Hematoxylin and eosin staining was conducted as described previously [8].
Masson trichrome staining was conducted on paraffin sections (7 µm) using Accustain trichrome stain and Weigert’s iron hematoxylin set (Sigma-Aldrich, Tokyo, Japan).
Isolation of cardiomyocytes
Single cardiomyocytes were prepared according to the partially modified method of Shioya [13] with some modifications. Isolated cells were plated on four-well chambers coated with poly-L lysine. For immunostaining, the cells were quickly fixed with 1% (w/v) paraformaldehyde and stained according to standard protocols.
Immunofluorescence procedures
Immunofluorescence procedures were essentially the same as those previously reported [16].
Western blot analysis
Tissues were extracted with Tris buffer containing 67.5 mM Tris–HCl (pH 6.8), 15% (w/v) sodium dodecyl sulfate, 20% (v/v) glycerol, 50 mM dithiothreitol, and protease inhibitor cocktail (Complete®, Roche, Tokyo, Japan). The soluble fraction after centrifugation for 15 min at 13,000 g (RT) was used according to a previously described method [16]. Primary antibodies against dystrophin (ab15277, Abcam, Tokyo, Japan), β-dystroglycan (Novocastra, Wetzlar, Germany), α1-syntrophin (AG-17,1; Sigma-Aldrich), neuronal nitric oxide synthase (nNOS; BD), vinculin (Sigma-Aldrich), utrophin (H-300, SantaCruz Biotechnology, Texas, U.S.A.), and GAPDH (3H12, MBL, Nagoya, Aichi, Japan) were used.
Subcellular fractionation
Plasma membranes and intercellular membranes of cardiomyocytes were separated using the method described by Biao Lei et al. [9], with some modifications. Homogenizing buffer (pH7.4) containing 0.1 M sucrose, 10 mM EDTA, 46 mM KCl, 100 mM Tris-HCl, and Complete® protease inhibitor cocktail (Roche Diagnostics, Tokyo, Japan) was used.
Results
Subcellular localization of dystrophin isoforms in cardiomyocytes
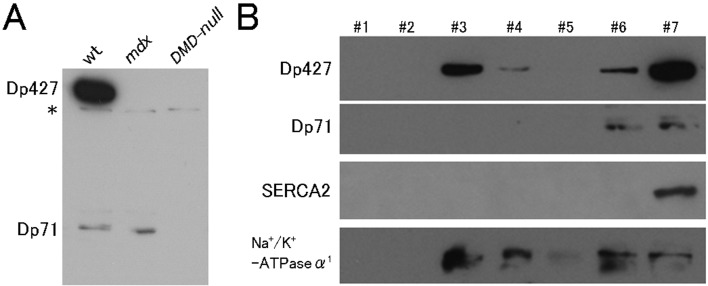
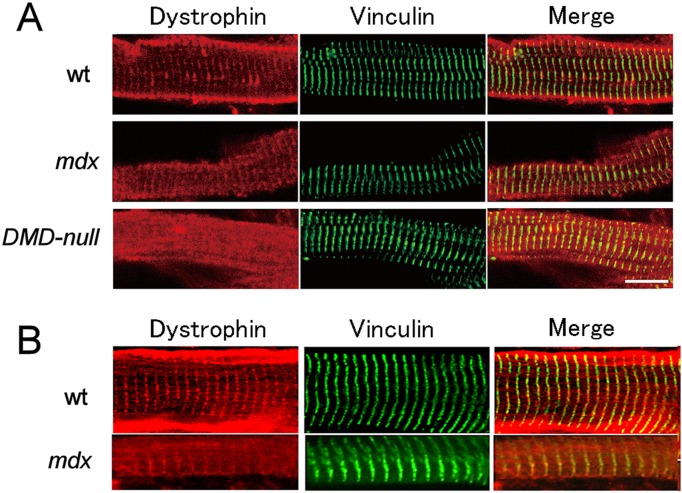
Western blot analysis confirmed that Dp427 and Dp71 were present in the cardiac muscle of wild-type (wt) mice, whereas Dp71 was only present in mdx mice. None of the isoforms were present in DMD-null mice (Fig. 1A). To examine subcellular localization of the dystrophin isoforms, immunostaining using an anti-dystrophin antibody was performed in ventricular cardiomyocytes isolated from wt, mdx, and DMD-null mice (Fig. 2A). In wt mice, dystrophin localized in the sarcolemma and also in the horizontally striped cytoplasmic area of the cytoplasm. The intense sarcolemmal immunostaining observed in wt mice was not found in mdx mice or DMD-null mice. Horizontally striated immunostaining in wt cardiomyocytes overlapped with that of the T-tubule marker vinculin, suggesting that cytoplasmic dystrophin was associated with T-tubules. Importantly, mdx cardiomyocytes exhibited a similar striated T-tubule-associated pattern. Because Dp71 was the only dystrophin isoform expressed in mdx cardiomyocytes, Dp71 was suggested to localize in T-tubules. The same results were obtained when immunostaining was conducted on frozen sections of the heart from wt, mdx, and DMD-null mice (Fig. 2B).
Fig. 1.
Western blot analysis. A: Immunoblots of heart extracts prepared from wt, mdx, and DMD-null mice. Note: Both Dp427 and Dp71 were present in the hearts of wt mice, only Dp71 was present in the mdx mice, and neither was present in the DMD-null mice. The asterisk indicates a nonspecific signal. B: Immunoblots of subcellular fractions after separating heart homogenates from wt mice by iodixanol density-gradient centrifugation. Note: Dp427 was present in the cardiac sarcolemmal (#3, 4) and intracellular membrane fractions (#6. 7), while Dp71 was present in intracellular membrane fractions (#6. 7).
Fig. 2.
Immunoconfocal images of cardiomyocytes labeled with anti-dystrophin (red) and anti-vinculin (green) antibodies. A: Cardiomyocytes isolated from either wt mice, mdx mice, or DMD-null mice. B: Longitudinal cryosections from either wt mice, mdx mice.
In order to know whether localization of Dp427 was restricted to the cardiac sarcolemma, we performed subcellular fractionation of wt cardiac homogenates. Using the discontinuous iodixanol density-gradient centrifugation method, the cardiac sarcolemmal and intracellular membranes were separated and used for Western blotting to check for the presence of dystrophin isoforms. As shown in Fig. 1B, we detected immunoreactivity for Dp427 and Dp71 in the intracellular membrane fraction, indicating that both Dp427 and Dp71 are associated with T-tubules.
Influence of loss of Dp71 in cardiomyocytes
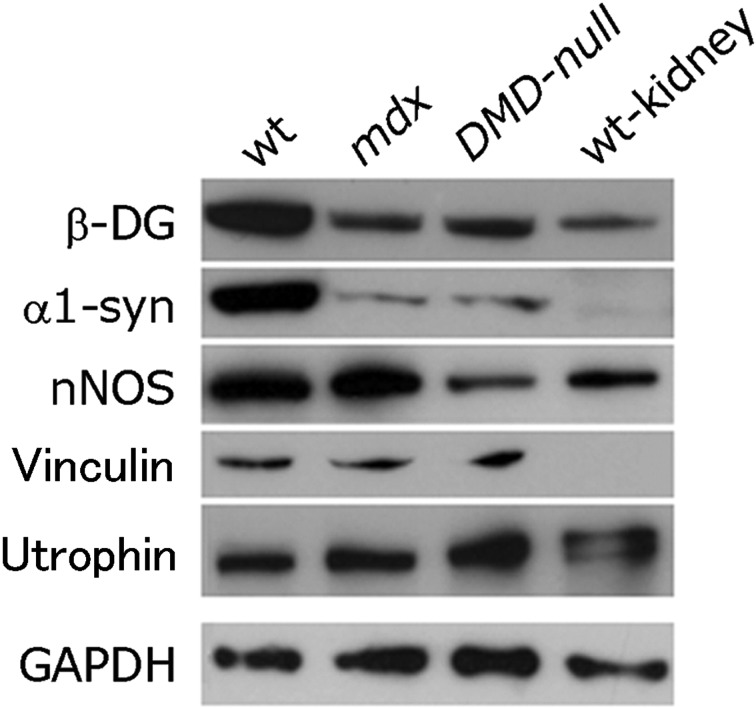
As shown in Fig. 2, the immunostaining pattern for vinculin in cardiomyocytes of DMD-null mice was essentially the same as that of mdx mice, suggesting that the structure of T-tubules was unaffected by the absence of Dp71. Moreover, no apparent difference could be observed in the ultrastructure of T-tubules between mdx mice and DMD-null mice (Supplemental Fig. 1). The amount of dystrophin-associated proteins in the heart was examined by Western blot analysis. As shown in Fig. 3, the amounts of β-dystroglycan and α1-syntrophin were equally low in mdx and DMD-null mice compared with wt mice. However, nNOS levels in the DMD-null mouse heart were lower than in the mdx mouse, suggesting that Dp71 may play some role in nNOS regulation. Additionally, we also immunohistochemically examined the expressions of some of the ion channels that have been known to locate in T-tubules and shown to be important for excitation–contraction coupling. The ion channels examined were Nav1.5, Kir2.1, and Cav1.2. All of them were found to locate at T-tubules as judged by their overlapped anti-vinculin immunostaining. No difference could be detected between DMD-null mice and mdx mice in the expression patterns of these ion channels (data not shown). Therefore, absence of Dp71 may have little influence on the expression of ion-channels located in T-tubules.
Fig. 3.
Western blot analysis. Immunoblots for dystrophin-related proteins in heart extracts. β-DG, β-dystroglycan; α1-syn, α1-syntrophin.
Analysis of cardiac muscle phenotype between mdx mice and DMD-null mice
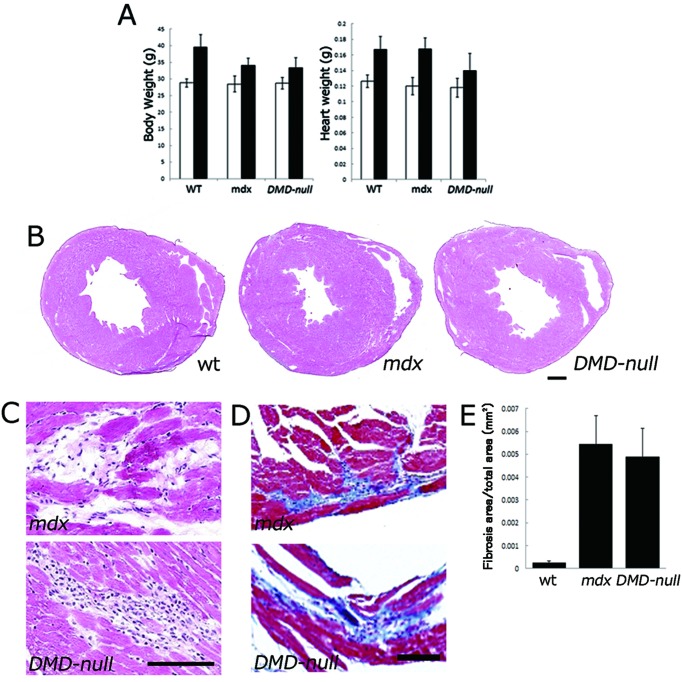
The body and heart weights of wt mice, mdx mice and DMD-null mice are shown in Fig. 4A. At 10–12 months of age, the heart weight of DMD-null mice was lower than that of mdx mice. Representative images of histological sections from 12-month-old wt, mdx, and DMD-null mice are shown in Fig. 4B. Focal necrotic lesions were frequently seen in mdx and DMD-null mice (Fig. 4C). Replacement of the necrotic myocardium with fibrotic tissue, assessed by Masson’s trichrome staining, was usually observed in the necrotic area (Fig. 4D). Such fibrosis was increasingly frequent with age. Importantly, the tissue area of fibrosis was not significantly different between mdx mice and DMD-null mice (Fig. 4E), suggesting that cardiac disruption occurred predominantly owing to the absence of Dp427 but not Dp71.
Fig. 4.
Phenotype analyses of cardiac muscle in mdx mice and DMD-null mice. A: Body weight (left panel) and heart weight (right panel) at 3 months (white bar) and 10–12 months (black bar) of age. B, C: Histological sections with hematoxylin-eosin staining. D: Histological sections with Masson’s trichrome staining. E: Graphic depiction of the ratio of connective tissue to normal myocardium in 12-month-old mdx, DMD-null mice and wt mice. The total area of blue-stained collagen was determined by digital image analysis. Note: No significant difference in necrotic area was observed between mdx and DMD-null mice (P=0.55).
Discussion
In the present study, we examined the subcellular distribution of the dystrophin isoforms Dp71 and Dp427 using two strains of mice: mdx mice and DMD-null mice. We showed that Dp427 was located in the cardiac sarcolemma and also at the T-tubules, whereas Dp71 was exclusively located at the T-tubules. Both DMD-null and mdx mice showed severe necrosis and fibrosis in the heart without significant differences. Based on these data, we concluded that cardiac myopathy is caused predominantly by a deficiency of full-length dystrophin Dp427.
The mdx mice, which develop progressive cardiomyopathy, have been widely used to study the molecular and cellular effects of dystrophin deficiency in cardiac tissues. The biochemical and physiological alterations observed in mdx mice have led to a number of hypotheses to explain the pathogenesis of cardiomyopathy. These include altered energetics [7], perturbation in L-type Ca2+ channel function [12], nitric oxide alterations [1, 18], altered function of the KATP channel complex [5], and increased oxidative damage [19]. Nevertheless, to date there is no evidence of a direct link between dystrophin and any of the aforementioned alterations in the heart.
Because Dp71 was exclusively located at the T-tubules of cardiomyocytes, Dp71 should play some role related to T-tubules. Because cardiomyopathy occurred equally in DMD-null and mdx mice, the function of Dp71 may not be linked to DMD-related cardiac disruption. Additionally, we found that utrophin, a protein homologous to dystrophin, was upregulated in the DMD-null mouse heart in our preliminary experiment. It is possible that the upregulation of utrophin may compensate for the absence of Dp71 in cardiac muscle.
As shown in Fig. 3, nNOS levels in the DMD-null mouse heart were lower than in the mdx mouse, suggesting the involvement of Dp71 in nNOS regulation. Interestingly, the decreased cardiac n-NOS might affect the conduction system in dystrophic mice. According to Bia et al. [1], the lack of dystrophin in the mdx mouse and also in the dystrophin/utrophin double-deficient mouse resulted in abnormal electrocardiograms that were associated with decreased myocardial nNOS and increased iNOS activities. Furthermore, it has been reported that Purkinje-fiber degeneration occurred prior to cardiac muscle degeneration, and Purkinje-fiber degeneration might be associated with the expression abnormality of Dp71, nNOS, and utrophin [17]. Therefore, it would be particularly important to investigate the cardiac conduction system pathologically and physiologically in DMD-null mice.
In the DMD-null mice examined in the present study, expression of dystrophin was completely lost in all skeletal myofibers and cardiac muscles [8]. This complete loss of dystrophin expression contrasted sharply with that of mdx mice. In mdx skeletal muscle, low but detectable levels of full-length dystrophin have been reported to be expressed, probably due to exon skipping [2]. Although the pathology of cardiac muscle in DMD-null mice was not essentially different from that of mdx mice, the DMD-null mice should be useful for studying pathological mechanisms, disease progression and therapy for cardiomyopathy due to dystrophin deficiency.
Supplementary
Acknowledgments
This work was supported in part by grants from the Ministry of Health, Labour and Welfare of Japan, and from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- 1.Bia B.L., Cassidy P.J., Young M.E., Rafael J.A., Leighton B., Davies K.E., Radda G.K., Clarke K.1999. Decreased Myocardial nNOS, Increased iNOS and Abnormal ECGs in Mouse Models of Duchenne Muscular Dystrophy. J. Mol. Cell. Cardiol. 31: 1857–1862. doi: 10.1006/jmcc.1999.1018 [DOI] [PubMed] [Google Scholar]
- 2.Crawford G.E., Lu Q.L., Partridge T.A., Chamberlain J.S.2001. Suppression of revertant fibers in mdx mice by expression of a functional dystrophin. Hum. Mol. Genet. 10: 2745–2750. doi: 10.1093/hmg/10.24.2745 [DOI] [PubMed] [Google Scholar]
- 3.Dalkilic I., Kunkel L.M.2003. Muscular dystrophies: genes to pathogenesis. Curr. Opin. Genet. Dev. 13: 231–238. doi: 10.1016/S0959-437X(03)00048-0 [DOI] [PubMed] [Google Scholar]
- 4.Enríquez-Aragón J.A., Cerna-Cortés J., Bermúdez L.M., García-Sierra F., González E., Mornet D., Cisneros B.2005. Dystrophin Dp71 in PC12 cell adhesion. Neuroreport. 16: 235–238. doi: 10.1097/00001756-200502280-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graciotti L., Becker J., Granata A.L., Procopio A.D., Tessarollo L., Fulgenzi G.2011. Dystrophin is required for the normal function of the cardio-protective K(ATP) channel in cardiomyocytes. PLoS ONE 6: e27034. doi: 10.1371/journal.pone.0027034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernández-González E.O., Mornet D., Rendon A., Martínez-Rojas D.2005. Absence of Dp71 in mdx3cv mouse spermatozoa alters flagellar morphology and the distribution of ion channels and nNOS. J. Cell. Sci. 118: 137–145. doi: 10.1242/jcs.01584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khairallah M., Khairallah R., Young M.E., Dyck J.R., Petrof B.J., Des Rosiers C.2007. Metabolic and signaling alterations in dystrophin-deficient hearts precede overt cardiomyopathy. J. Mol. Cell. Cardiol. 43: 119–129. doi: 10.1016/j.yjmcc.2007.05.015 [DOI] [PubMed] [Google Scholar]
- 8.Kudoh H., Ikeda H., Kakitani M., Ueda A., Hayasaka M., Tomizuka K., Hanaoka K.2005. A new model mouse for Duchenne muscular dystrophy produced by 2.4 Mb deletion of dystrophin gene using Cre-loxP recombination system. BBRC 328: 507–516. [DOI] [PubMed] [Google Scholar]
- 9.Lei B., Lionetti V., Young M.E., Chandler M.P., d’Agostino C., Kang E., Altarejos M., Matsuo K., Hintze T.H., Stanley W.C., Recchia F.A.2004. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. J. Mol. Cell. Cardiol. 36: 567–576. doi: 10.1016/j.yjmcc.2004.02.004 [DOI] [PubMed] [Google Scholar]
- 10.Leiden Muscular Dystrophy pages©: Dystrophin isoforms. (http://www.dmd.nl/isoforms.html:last modefied March 5, 2006).
- 11.Quinlan J.G., Hahn H.S., Wong B.L., Lorenz J.N., Wenisch A.S., Levin L.S.2004. Evolution of mdx mouse cardiomyopathy: philological and morphological finding. Neuromuscul. Disord. 14: 491–496. doi: 10.1016/j.nmd.2004.04.007 [DOI] [PubMed] [Google Scholar]
- 12.Sadeghi A., Doyle A.D., Johnson B.D.2002. Regulation of the cardiac L-type Ca2+channel by the actin-binding proteins α-actinin and dystrophin. Am. J. Physiol. Cell. Physiol. 282: C1502–C1511. [DOI] [PubMed] [Google Scholar]
- 13.Shioya T.2007. A Simple Technique for Isolating Healthy Heart Cells from Mouse Models. J. Physiol. Sci. 57: 327–335. doi: 10.2170/physiolsci.RP010107 [DOI] [PubMed] [Google Scholar]
- 14.Sicinski P., Geng Y., Ryder-Cook A.S., Barnard E.A., Darlison M.G., Barnard P.J.1989. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244: 1578–1580. doi: 10.1126/science.2662404 [DOI] [PubMed] [Google Scholar]
- 15.Spurney C.F.2011. Cardiomyopathy of Duchenne muscular dystrophy: current understanding and future directions. Muscle Nerve. 44: 8–19. doi: 10.1002/mus.22097 [DOI] [PubMed] [Google Scholar]
- 16.Takatoh J., Kudoh H., Kondo S., Hanaoka K.2008. Loss of short dystrophin isoform Dp71 in olfactory ensheathing cells causes vomeronasal nerve defasciculation in mouse olfactory system. Exp. Neurol. 213: 36–47. doi: 10.1016/j.expneurol.2008.04.041 [DOI] [PubMed] [Google Scholar]
- 17.Urasawa N., Wada M.R., Machida N., Yuasa K., Shimatsu Y., Wakao Y., Yuasa S., Sano T., Nonaka I., Nakamura A., Takeda S.2008. Selective vacuolar degeneration in dystrophin-deficient canine Purkinje fibers despite preservation of dystrophin-associated proteins with overexpression of Dp71. Circulation 117: 2437–2448. doi: 10.1161/CIRCULATIONAHA.107.739326 [DOI] [PubMed] [Google Scholar]
- 18.Wehling M., Spencer M.J., Tidball J.G.2001. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J. Cell. Biol. 155: 123–131. doi: 10.1083/jcb.200105110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams I.A., Allen D.G.2007. The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am. J. Physiol. Heart Circ. Physiol. 293: H1969–H1977. doi: 10.1152/ajpheart.00489.2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.